Abstract
Entry of human T-cell leukemia virus type 1 (HTLV-1) into cells is mediated by the viral envelope glycoproteins gp46 and gp21. The gp46 surface glycoprotein binds to a poorly characterized cell surface receptor, thereby promoting the gp21-dependent fusion of the viral and cellular membranes. Interestingly, a synthetic peptide (P-197) simulating amino acids 197 to 216 of gp46 strongly inhibits envelope-dependent membrane fusion with Molt-4 target cells. It has been suggested that this peptide acts by competitively binding to Hsc70, a putative cellular receptor for HTLV-1. We now demonstrate that P-197 inhibits membrane fusion among diverse HTLV-1-permissive target cells. Importantly, most of these cells lack detectable levels of Hsc70, indicating that P-197 inhibits membrane fusion by a mechanism that is Hsc70 independent. We now suggest that competition for primary receptor binding is unlikely to account for the inhibitory activity of P-197. Understanding the mechanism by which P-197 functions may reveal concepts of general relevance to antiretroviral chemotherapy.
Efficient entry into, and infection of, human cells by human T-cell leukemia virus type 1 (HTLV-1) is mediated by the viral envelope glycoproteins. The envelope proteins are expressed as a 68-kDa glycosylated protein precursor (gp68) that is posttranslationally cleaved by a cellular protease to yield the mature gp46 surface glycoprotein (SU) and the gp21 transmembrane protein (TM) (1, 4, 18). The surface glycoprotein remains associated with gp21 following precursor cleavage, and this SU-TM complex is anchored to the viral or infected-cell membrane by the membrane-spanning region within TM. The functionally important domains required for cellular recognition and receptor binding are contained within SU, while TM mediates fusion of the viral and target cell membranes (2, 4, 12, 17–21). By analogy to other retroviral systems, it is likely that binding of gp46 to one or more as-yet-uncharacterized cell surface receptors (12, 16, 26, 27, 32) brings the viral and cellular membranes into close proximity and induces a conformational change within the envelope glycoprotein complex. This alteration in envelope conformation activates the fusion domain within gp21 and promotes the TM-dependent fusion of the closely apposed viral and cellular membranes (2, 21, 24).
Recently, a peptide scanning approach was used to identify synthetic peptides derived from envelope that inhibit membrane fusion and syncytium formation between HTLV-1-infected cells and target Molt-4 T cells (22). Of the inhibitory peptides identified, one, P-400, was derived from amino acids 400 to 425 of TM, while another, P-197, was derived from amino acids 197 to 216 of the gp46 surface glycoprotein (22). Given that the inhibitory peptides map to distinct and nonoverlapping regions of envelope, it is likely that these peptides inhibit membrane fusion by functionally distinct mechanisms. In the case of P-197 it has been suggested that the SU-derived peptide inhibits membrane fusion by competitively binding to a primary cellular receptor for HTLV-1 (22), which was subsequently identified as heat shock cognate protein 70 (Hsc70) (23). This conclusion was based upon the observations that P-197 binds to Hsc70 in vitro and that Hsc70 is efficiently purified from cell lysates by affinity chromatography against immobilized peptide (23). In support of the view that P-197 inhibits membrane fusion by competing with SU for Hsc70 binding, it was reported that antibodies directed against Hsc70 antagonize HTLV-induced membrane fusion and block syncytium formation (23). Here, we have further examined the functional properties of P-197 and explored the requirement for Hsc70 in cell-to-cell membrane fusion.
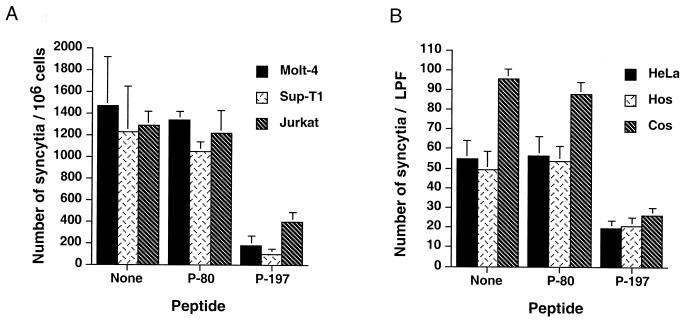
In our study, an inactive envelope-derived control peptide, P-80 (SLYLFPHWTKKPNRNGG; Mw, 2,118), and the inhibitory peptide P-197 (DHILEPSIPWKSKLLTLVQL; Mw, 2,331) were chemically synthesized by standard techniques. HTLV-1 envelope-mediated cell-to-cell fusion was monitored using a syncytium assay adapted from published protocols (10, 16). Molt-4, Jurkat, and SupT-1 T cells were resuspended at 2 × 106 cells/ml, and 0.5-ml aliquots were seeded into the wells of a 24-well dish. Subsequently, an approximately equal number of chronically HTLV-1-infected MT2 cells (0.5 ml) were added to each well, and the cocultures were incubated at 37°C for 18 to 21 h. Syncytia were scored by gently resuspending the cells, placing 40 μl from each culture onto a gridded tissue culture dish (Nunc), and counting the number of syncytia per sample by light microscopy using the high-power objective. Using this assay we routinely found that cocultures of MT2 cells and target lymphocytic cells contained 800 to 2,000 syncytia/106 target cells (Fig. 1A). We observed no overt differences in the abilities of Molt-4, Jurkat, or Sup-T1 cell lines to support syncytium formation, and syncytia were not observed when target lymphocytes were incubated in the absence of MT2 cells or when MT2 cells were incubated in the absence of target cells.
FIG. 1.
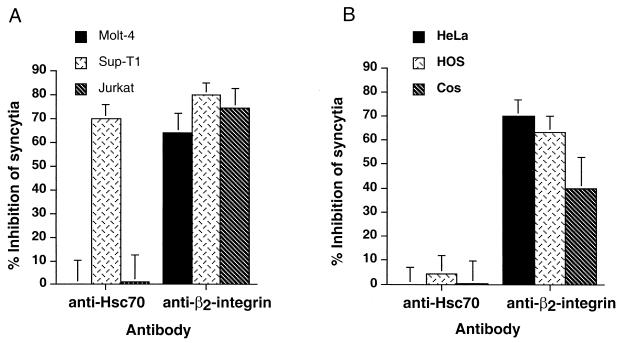
Peptide P-197 inhibits HTLV-1 envelope-mediated syncytium formation among diverse target cells. (A) Molt-4, Sup-T1, and Jurkat target cells were cocultured with HTLV-1-infected MT2 cells in the presence or absence of the indicated peptides (20 μg/ml). Control cocultures receiving no peptide were incubated in the presence of solvent only. The number of syncytia per 106 target cells was evaluated by high-power light microscopy. Data are means and standard deviations from six independent assays. (B) HeLa, HOS, or Cos target cells were cocultured with HTLV-1 envelope-expressing HeLa cells (transfected with the envelope expression vector pHTE-1) in the presence or absence of the indicated peptides. The number of syncytia per low-power field (LPF) was scored by light microscopy. Data are means and standard deviations from three fields from three independent assays.
P-197 inhibits HTLV-1 syncytium formation among diverse target cell populations.
To examine the inhibitory properties of the SU-derived peptides, the control peptide P-80, the inhibitory peptide P-197, or solvent only (dimethyl sulfoxide) was added to target cell suspensions and allowed to equilibrate at 37°C for 30 min. MT2 cells were then added to each well, and the cocultures were incubated for 18 to 20 h, whereupon the number of syncytia was scored as described above. Incubation of cocultures with the control peptide P-80 had little or no effect upon the fusogenic activity of the envelope-expressing MT2 cells, as cultures pretreated with P-80 contained as many syncytia as controls that received no peptide. In contrast, coculture of MT2 cells and target cells in the presence of P-197 resulted in a dramatic reduction in the number of syncytia formed (Fig. 1A), and the inhibitory activity of the synthetic peptide was observed for all the T-cell lines tested. These results confirm the findings of Sagara et al. (22) and further demonstrate that the inhibitory properties of P-197 extend to a range of lymphocytic cell lines in addition to the T-cell line Molt-4.
Since a variety of non-T-cell lines also support HTLV-1 envelope-mediated syncytium formation and viral entry (16, 32, 25), we wished to determine if P-197 would interfere with membrane fusion in heterologous mammalian cell types. In these assays, HeLa cells were transfected with the HTLV-1 envelope glycoprotein expression vector pHTE-1 (6), using Fugene 6 as directed by the manufacturer (Boehringer Mannheim); 24 h later the cells were lifted (phosphate-buffered saline [PBS], 1 mM EDTA) and resuspended at 1.5 × 105 cells/ml. The envelope-expressing HeLa cells (1 ml) were subsequently mixed and cocultured with a similar number of target cells in the wells of a six-well tissue culture dish. Target HeLa (human cervical carcinoma), HOS (human osteosarcoma), or Cos-1 (African green monkey kidney) cells were incubated in the presence of test peptide or in the presence of solvent only for 30 min prior to addition of the envelope-expressing effector cells. The following day, syncytia were scored by counting the number of multinucleate giant cells observed per low-power field by light microscopy.
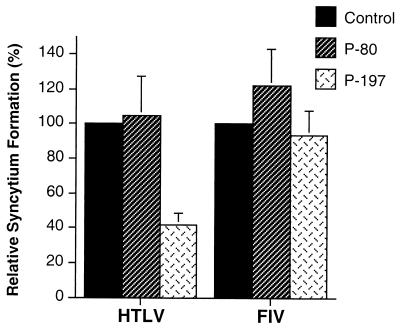
Coculture of envelope expressing HeLa cells with HeLa, HOS, or Cos-1 target cells resulted in rampant syncytium formation (Fig. 1B). We routinely found that the Cos-1 cell line produced slightly (1.5- to 2-fold) more syncytia with envelope-expressing HeLa cells than the human cell lines tested. No syncytia were observed in control cocultures using mock-transfected HeLa cells. Importantly, the control peptide P-80 did not inhibit syncytium formation in these assays (Fig. 1B). However, treatment of cocultured cells with the peptide P-197 resulted in a marked (60 to 70%) reduction in the number of syncytia formed (Fig. 1B) compared to control cocultures that had been treated with solvent only or with the inactive control peptide P-80. Moreover, in the presence of P-197 the sizes of the syncytia were greatly reduced, as judged by a decrease in the average number of nuclei within each syncytium (data not shown). This inhibitory effect of P197 appears to be specific for HTLV-1, since P197 does not inhibit syncytium formation induced by human immunodeficiency virus (22) or feline immunodeficiency virus (Fig. 2). Thus, the accumulating evidence indicates that inhibition of membrane fusion by P-197 is specific to HTLV-1 and is not confined to lymphocytic cells but is observed with a wide variety of HTLV-1-sensitive cell lines.
FIG. 2.
The peptide P-197 does not block syncytium formation by an unrelated retrovirus. HeLa cells transfected with the HTLV-1 envelope expression construct HTE-1 (HTLV) or the feline immunodeficiency virus proviral clone pFIV-34TF10 (FIV) were cocultured with untransfected target HeLa cells in the presence of the indicated peptides. Syncytium formation is expressed as a percentage relative to the number of syncytia obtained with each viral envelope in the absence of peptide (Control). Data are means and standard deviations from three low-power fields from three independent assays.
Most HTLV-1 sensitive cells do not express detectable levels of surface Hsc70.
Based upon the observation that P-197 binds to the cellular protein Hsc70, Sagara et al. (23) suggested that Hsc70 expressed on the cell surface acts as a receptor for HTLV-1 and that P-197 inhibits syncytium formation by competitively binding to this putative receptor. Since P-197 inhibits HTLV-1-induced syncytium formation for all cell lines tested, we examined these cells for surface expression of Hsc70. We wished to determine if surface expression of Hsc70 correlates with the ability of cells to support HTLV-1-induced syncytium formation and if inhibition of syncytium formation by P-197 requires surface expression of Hsc70.
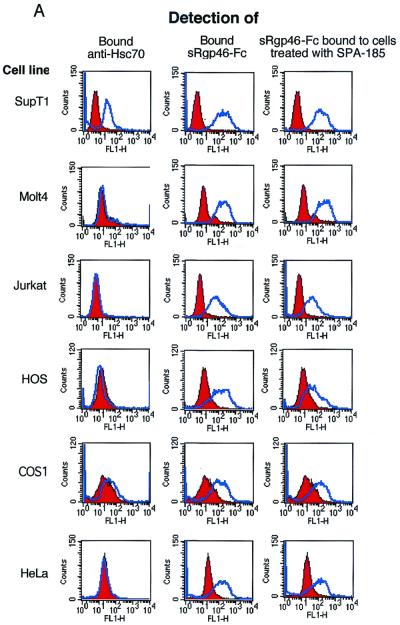
Expression of Hsc70 on the surface of HTLV-1 sensitive cell lines was examined by probing intact cells with the anti-Hsc70 monoclonal antibody SPA-815 (Stressgen Biotechnologies Corp.) and detecting the bound antibody using fluorescein isothiocyanate (FITC)-conjugated secondary antibody and flow cytometry. Importantly, the primary antibody SPA-815 used in our studies was also used by Sagara et al. (23) to demonstrate cell surface expression of Hsc70 on Molt-4 cells. Cells (2.5 × 105) were incubated with monoclonal antibody SPA-815 (1 μg/ml) in 1 ml of RPMI 1640 medium supplemented with 10% fetal bovine serum. Samples were incubated at room temperature on a rotary mixer for 1 h. The cells were spun down, washed in RPMI, and incubated with secondary antibody, FITC-conjugated anti-rat immunoglobulin G (IgG) in RPMI medium at room temperature for 30 min in the dark. The cells were washed (PBS, 0.1% sodium azide), fixed (0.5% paraformaldehyde in PBS, pH 7.4), and analyzed for antibody binding by flow cytometry (FACScan; Becton Dickinson).
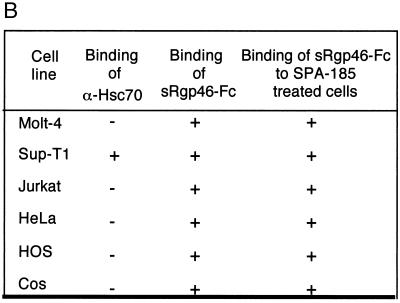
Surprisingly, we found that the vast majority of cell lines that support HTLV-1 infection or envelope-mediated syncytium formation did not express detectable levels of Hsc70 on the cell surface (Fig. 3). Moreover, in contrast to previously reported data (23), the Molt-4 cell line used in our studies (ATCC CRL-1582) did not express significant amounts of surface Hsc70. In fact, of all the cell lines examined, only the T-cell line SupT-1 expressed high levels of surface Hsc70, and these cells were no more prone to syncytium formation than the Hsc70-negative Jurkat cells (Fig. 1A and 3).
FIG. 3.
Most cell lines permissive for HTLV-1 syncytium formation do not express Hsc70 on the cell surface. Binding of the anti-Hsc70 antibody SPA-815 (1 μg/ml) and the recombinant HTLV-1 SU derivative sRgp46-Fc (0.6 μg/ml) to target cells was monitored by flow cytometry. Binding of sRgp46-Fc (0.6 μg/ml) to cells in the presence of SPA-815 (4 μg/ml) is also shown. In each case the blue histograms represent basal fluorescence in the presence of irrelevant control sera and the red histograms represent the fluorescence profile in the presence of the detected test ligand. The data are summarized in panel B.
Importantly, we also examined this panel of cell lines for receptors recognized by the HTLV-1 surface glycoprotein gp46. In these assays, we employed a functional form of soluble recombinant gp46 fused to the Fc region of human IgG (sRgp46-Fc). We have previously demonstrated that sRgp46-Fc (referred to as SU-Fc) (12) exhibits the biochemical and immunological properties of SU and that this recombinant protein retains the receptor binding specificity of the native viral glycoprotein (12). In particular, when added to target cells, sRgp46-Fc competitively inhibits HTLV-1-induced syncytium formation and viral entry into cells; demonstrating that sRgp46-Fc recognizes the primary cell surface receptor used by HTLV-1 (12). As anticipated, all of the HTLV-1-permissive syncytium-sensitive cell lines bound sRgp46-Fc, confirming that all of these cells express functional receptors for HTLV-1. Most importantly, all of the cell lines that are competent for syncytium formation and that lack detectable levels of surface Hsc70 bind sRgp46-Fc. Thus, from our study it would appear that cell surface expression of Hsc70 is not required for syncytium formation or for binding of HTLV-1 SU to cells. To explore these ideas further, we examined the ability of the anti-Hsc70 antibody SPA-815 to block binding of sRgp46-Fc to cells. We found that pretreatment of target cells with monoclonal antibody SPA-815 (4 μg/ml) did not prevent binding of sRgp46-Fc to cells (Fig. 3). Significantly, SPA-815 also failed to block binding of sRgp46-Fc to Sup-T1, a cell line which expresses high levels of surface Hsc70 (Fig. 3). Taken together, these results indicate that Hsc70 is unlikely to be the primary receptor recognized by HTLV-1 SU.
An anti-Hsc70 monoclonal antibody does not inhibit syncytium formation for most HTLV-1-sensitive cell lines.
It has been reported that exogenous addition of the anti-Hsc70 monoclonal antibody SPA-815 inhibits syncytium formation between cocultured HTLV-1-infected cells and the cell line Molt-4. However, our results indicate that SPA-815 is unable to block binding of sRgp46-Fc to cells, suggesting that Hsc70 is not the receptor recognized by gp46. Nevertheless, it is possible, though in our view unlikely, that the soluble SU fusion protein does not recognize the HTLV-1 primary receptor in the same way as the native virally expressed envelope protein. We therefore examined the ability of SPA-815 to interfere with syncytium formation between HTLV-1 envelope-expressing cells and diverse target cell populations. In the first assay, the lymphocytic cell lines Jurkat, Sup-T1, and Molt-4 were cocultured with the chronically HTLV-1-infected cell line MT2 in the presence or absence of SPA-815 or antibody directed against the cell adhesion molecule β2-integrin. Anti-β2-integrin was employed as a positive control for these syncytium interference assays, as β2-integrin supports envelope-mediated membrane fusion and antibodies recognizing this surface antigen block syncytium formation (3, 10). In keeping with previous reports (3), we found that treatment of cocultured cells with anti-β2-integrin severely disrupted membrane fusion, as revealed by a 40 to 60% reduction in the number of syncytia observed in cocultures treated with this antibody (Fig. 4A). In contrast, the anti-Hsc70 antibody SPA-815 had no effect on syncytium formation when either the Jurkat or Molt-4 cell lines were used as targets. However, when Sup-T1 cells were cocultured with MT2 cells, we observed a marked inhibitory effect of SPA-815 on syncytium formation (Fig. 4A), and the inhibitory activity of SPA-815 was similar to that obtained with the anti-β2-integrin antiserum.
FIG. 4.
Antibodies directed against Hsc70 do not block syncytium formation for most HTLV-1-permissive cells. (A) Molt-4, Sup-T1, and Jurkat cells were cocultured with HTLV-1-infected MT2 cells in the presence or absence of the indicated antibodies (2 μg/ml). The inhibition of syncytium formation relative to untreated control cocultures is indicated (means and standard deviations from four independent assays). (B) HeLa, HOS, and Cos target cells were cocultured with HTLV-1 envelope-expressing HeLa cells in the presence or absence of the indicated antibodies. The inhibition of syncytium formation relative to untreated control cocultures is indicated (means and standard deviations of triplicate determinations from three independent assays).
A second syncytium interference assay was performed in which HeLa cells were transfected with the envelope-expressing plasmid HTE-1 and the transfected cells were subsequently cocultured with HeLa, Cos, or HOS target cells in the presence or absence of the test antibodies. Once again, we found that the anti-Hsc70 monoclonal antibody had little or no effect upon syncytium formation, while the anti-β2-integrin antibody efficiently antagonized cell-to-cell fusion (Fig. 4B). These data correlate well with the flow cytometry results (Fig. 3). Thus, the accumulating evidence indicates that the vast majority of HTLV-1-permissive cell lines do not express detectable levels of Hsc70 on the cell surface, that Hsc70-negative cell lines are competent for envelope-mediated cell-to-cell fusion, and that antibodies directed against Hsc70 do not inhibit syncytium formation for most target cell populations.
P-197 does not compete with sRgp46-Fc for cell surface binding.
It has been suggested that P-197 competes with HTLV-1 SU for binding to the putative primary receptor Hsc70, and that binding of P-197 to Hsc70 blocks recognition of this receptor by SU. Taken together, the data presented above indicate that Hsc70 is unlikely to be the primary receptor recognized by HTLV-1 SU. Nevertheless, it is possible that P-197 binds to some other cell surface antigen used as a receptor by HTLV-1. To test this hypothesis, we examined the ability of P-197 to block binding of sRgp46-Fc to HTLV-1-permissive target cells. Jurkat cells (2.5 × 105) were preincubated with or without P-197 (40 μg/ml) for 30 min at 25°C, whereupon sRgp46-Fc (0.6 μg/ml) was added to the cell suspensions and incubated for a further hour. The cells were washed to remove unbound sRgp46-Fc and probed for bound sRgp46-Fc using FITC-conjugated anti-Fc antisera; the cells were then washed and fixed, and the bound antibody was detected by flow cytometry.
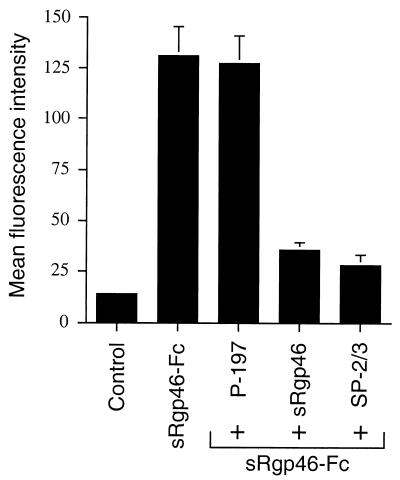
Compared with untreated cells, Jurkat cells incubated with sRgp46-Fc demonstrated a marked increase in FITC-specific fluorescence, consistent with the binding of sRgp46-Fc to the cell surface (Fig. 5). Surprisingly, we found that the synthetic peptide P-197, even in vast excess, did not block binding of sRgp46-Fc to Jurkat cells (Fig. 5). In comparison, binding of the SU immunoadhesin sRgp46-Fc to cells was clearly blocked by preincubation of cells with untagged sRgp46 (0.6 μg/ml), indicating that these gp46 derivatives compete for the same primary cell surface receptor. Moreover, pretreatment of sRgp46-Fc with a neutralizing antiserum, SP2-3 (17), which is reactive against native viral gp46, also blocked binding of SRgp46-Fc to cells. These results indicate to us that the inhibitory peptide P-197 does not disrupt primary receptor binding by the HTLV-1 surface glycoprotein.
FIG. 5.
The peptide P-197 does not prevent binding of sRgp46-Fc to cells. Jurkat cells were treated with P-197 (40 μg/ml) and, for comparison, sRgp46 (0.6 μg/ml) or neutralizing anti-SU antiserum SP2-3 (1:1,000). sRgp46-Fc was added and incubated for 1 h with mixing. Bound sRgp46-Fc was detected using anti-Fc FITC-conjugated sera and flow cytometry. Basal fluorescence of control samples was determined from cells treated with irrelevant nonspecific IgG. Means and standard deviations from three independent assays are shown.
The unambiguous identification and characterization of the cellular receptor recognized by HTLV-1 has remained elusive. Consequently, there is considerable interest in defining the molecular events that promote envelope-mediated entry of HTLV-1 into cells. Recently, Sagara et al. (22) reported that a synthetic peptide derived from the HTLV-1 surface glycoprotein potently inhibits envelope-mediated membrane fusion and syncytium formation. It was suggested that the inhibitory peptide, P-197, competes with SU for binding to a cellular receptor required for HTLV-1 infection. Subsequently, a cellular P-197-binding protein, Hsc70, was identified and proposed as a candidate receptor for HTLV-1. These suggestions were intriguing, since many cell types constitutively express Hsc70 as a soluble cytoplasmic protein, and to our knowledge a signal sequence for membrane targeting has not been identified within Hsc70. Moreover, as a cellular chaperonin Hsc70 is thought to bind to nascent or incorrectly folded proteins (28, 33) and to peptides produced in vivo during antigen processing (15, 27). We have therefore reexamined the properties of P-197 and explored the contribution of Hsc70 to HTLV-1 envelope-induced membrane fusion.
As reported by Sagara et al. (22), we confirm that the surface glycoprotein-derived peptide P-197 efficiently interferes with HTLV-induced membrane fusion and syncytium formation. Importantly, we have extended those initial findings by demonstrating that the inhibitory properties of P-197 are not confined solely to Molt-4 T cell targets but are also observed with other T-cell and non-T-cell lines. In fact, P-197 inhibits syncytium formation among all the HTLV-1-permissive and syncytium-proficient cell lines tested. Most surprisingly, we have found that although P-197 potently inhibits syncytium formation, the peptide was unable to block direct binding of a recombinant HTLV-1 envelope protein to cells. Our results suggest to us that P-197 does not inhibit syncytium formation by blocking viral recognition of a cell surface receptor.
Based upon the data reported here and for the reasons given below, we suggest that Hsc70 is unlikely to be a critical receptor for HTLV-1 entry into cells. First, the majority of HTLV-1-permissive cells do not express detectable levels of Hsc70, or express only exceedingly low levels of this surface antigen. Second, compared to Hsc70-negative cells, cell lines that express high levels of surface Hsc70 do not exhibit increased sensitivity to syncytium formation or greater resistance to syncytium interference by P-197. Third, antibodies directed against Hsc70 do not block syncytium formation for the majority of permissive cells, and anti-Hsc70 antibodies do not prevent binding of recombinant surface glycoprotein to target cells. Taken together, these results are inconsistent with the view that Hsc70 acts as a critical receptor for HTLV-1 entry. Why should Hsc70 bind to isolated peptides derived from envelope or bind to envelope protein expressed in mammalian cells? The answer may lie in the normal physiological role of Hsc70. As a constitutively expressed member of the heat shock family of chaperonins, Hsc70 binds to nascent proteins facilitating protein folding and promoting oligomerization of multiprotein complexes (28, 33). Therefore, binding of Hsc70 to envelope may be a normal physiological event that promotes correct processing and assembly of envelope within cells. A precedent for this type of interaction has been observed for membrane proteins of both cellular and viral origin and the endoplasmic reticulum-resident chaperonin BiP (9, 13, 14). Alternatively, the immunoprecipitation techniques used to demonstrate interaction of Hsc70 with envelope may partially unfold envelope, thereby promoting association of Hsc70 with the partially denatured protein. In addition, Hsc70 also binds to the synthetic peptide P-197. However, it is well known that Hsc70 binds to many classes of peptide (30, 31, 33), and one suggested function for Hsc70 is to bind and deliver peptides for presentation in the immune response (15, 27). Therefore, Hsc70 may possess affinity for particular classes of peptide, and binding of P-197 to Hsc70 may merely reflect the intrinsic affinity of Hsc70 for peptides. These alternative explanations for the association of Hsc70 with P-197 have yet to be fully explored.
Although anti-Hsc70 antibodies do not interfere with membrane fusion for most target cells, inhibition of syncytium formation was observed with Sup-T1 cells. However, Sup-T1 cells express high levels of surface Hsc70. Consequently, inhibition of syncytium formation by anti-Hsc70 antibodies may be due to steric effects that occlude the HTLV-1 receptor and block receptor engagement by envelope. Such inhibitory mechanisms need not necessarily involve direct binding of antibody to the receptor, as similar inhibitory effects on HTLV-1 syncytium formation have been observed for antisera directed against molecules of the major histocompatibility complex (11). Thus, the proximity of a bulky surface-bound antibody molecule to a small and easily masked receptor epitope may be all that is required to block access of the virally expressed envelope protein to the receptor, thereby disrupting membrane fusion. In contrast, the sRgp46-Fc fusion protein used in this study may enjoy unrestricted access to the surface-bound receptor, even in the presence of anti-Hsc70 antibodies, since the recombinant protein is freely diffusible and unrestrained by presentation on a cellular membrane.
An important finding of this study is that, even at high concentrations, the peptide P-197 does not inhibit binding of recombinant SU to cells. These data indicate to us that P-197 does not inhibit membrane fusion by competitively binding to a primary cellular receptor that is recognized by HTLV-1. Although the mechanism by which P-197 disrupts membrane fusion is not yet clear, several models can be proposed for the observed inhibitory activity: One possibility is that the peptide disrupts an interaction with a secondary receptor required for HTLV-1 entry. If this is the case, it is unlikely that Hsc70 is the coreceptor, since most HTLV-1-sensitive cells lack detectable levels of this protein on the cell surface. Alternatively, P-197 may disrupt critical interactions between envelope subunits. Retroviral envelope glycoproteins form the characteristic “knobs” or “spikes” that have been observed on virions or infected cells. These spikes likely consist of a trimeric association of SU-TM complexes, held together by intersubunit interactions between TM-TM, SU-TM, and perhaps SU-SU subunits. We suspect that perturbation of the envelope glycoprotein complex by synthetic peptides would have a profound inhibitory effect on envelope-mediated membrane fusion. Finally, a growing body of evidence suggests that retroviral envelope glycoproteins undergo dramatic alterations in conformation upon receptor engagement (2, 8, 24, 34). These structural transitions represent critical events in envelope-catalyzed membrane fusion, and elegant studies have demonstrated that interfering with these molecular transitions can block membrane fusion (24). Thus, a particularly attractive model to account for the inhibitory activity of P-197 is that P-197 binds directly to HTLV-1 SU and interferes with a conformational change in SU that is a prerequisite for membrane fusion. We are now designing experimental strategies to explore the validity of these alternative models.
In conclusion, our studies indicate that the cellular factor Hsc70 is not the direct target of the fusion-inhibitory peptide P-197 and that Hsc70 is not the receptor recognized by HTLV-1. Importantly, we now suggest that the synthetic peptide P-197 inhibits envelope-mediated membrane fusion by a mechanism that does not involve competition for a primary cellular receptor. Understanding the mechanism by which synthetic peptides, such as P-197, inhibit membrane fusion may provide new opportunities for the rational development of clinically useful antagonists of HTLV-1 infection.
Acknowledgments
We thank the members of the HTLV-1 European Research Network (HERN) for reagents and helpful discussions and Richard Pöhler for review of the manuscript.
This work was funded in part by grants from the Leukemia Research Fund, Tenovus-Scotland, and the Misses Barrie Charitable Trust to D.W.B.
REFERENCES
- 1.Cann A J, Chen I S Y. Human T-cell leukemia virus type I and II. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1501–1527. [Google Scholar]
- 2.Center R J, Kobe B, Wilson K A, Teh T, Howlett G J, Kemp B E, Poumbourios P. Crystallization of a trimeric human T-cell leukemia virus type 1 gp21 ectodomain fragment as a chimera with maltose-binding protein. Protein Sci. 1998;7:1612–1619. doi: 10.1002/pro.5560070715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daenke S, McCracken S A, Booth S. Human T-cell leukaemia/lymphoma virus type 1 syncytium formation is regulated in a cell-specific manner by ICAM-1, ICAM-3 and VCAM-1 and can be inhibited by antibodies to integrin β2 or β7. J Gen Virol. 1999;80:1429–1436. doi: 10.1099/0022-1317-80-6-1429. [DOI] [PubMed] [Google Scholar]
- 4.Delamarre L, Pique C, Pham D, Tursz T, Dokhelar M C. Identification of functional regions in the human T-cell leukemia virus type I SU glycoprotein. J Virol. 1994;68:3544–3549. doi: 10.1128/jvi.68.6.3544-3549.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delamarre L, Rosenberg A R, Pique C, Pham D, Dokhelar M C. A novel human T-leukemia virus type 1 cell-to-cell transmission assay permits definition of SU glycoprotein amino acids important for infectivity. J Virol. 1997;71:259–266. doi: 10.1128/jvi.71.1.259-266.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dokhelar M C, Pickford H, Sodroski J, Haseltine W A. HTLV-1 p27Rex regulates gag and env protein expression. J Acquir Immune Defic Syndr. 1989;2:431–440. [PubMed] [Google Scholar]
- 7.Doms R W, Lamb R A, Rose J K, Helenius A. Folding and assembly of viral membrane proteins. Virology. 1993;193:545–562. doi: 10.1006/viro.1993.1164. [DOI] [PubMed] [Google Scholar]
- 8.Doms R W. Protein conformational changes in virus-cell fusion. Methods Enzymol. 1993;221:61–72. doi: 10.1016/0076-6879(93)21007-u. [DOI] [PubMed] [Google Scholar]
- 9.Hammond C, Helenius A. Folding of VSV G protein: sequential interaction with BiP and calnexin. Science. 1994;266:456–458. doi: 10.1126/science.7939687. [DOI] [PubMed] [Google Scholar]
- 10.Hildreth J E, Subramanium A, Hampton R A. Human T-cell lymphotropic virus type 1 (HTLV-1)-induced syncytium formation mediated by vascular cell adhesion molecule-1: evidence for involvement of cell adhesion molecules in HTLV-1 biology. J Virol. 1997;71:1173–1180. doi: 10.1128/jvi.71.2.1173-1180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hildreth J E. Syncytium-inhibiting monoclonal antibodies produced against human T-cell lymphotropic virus type 1-infected cells recognize class II major histocompatibility complex molecules and block by protein crowding. J Virol. 1998;72:9544–9552. doi: 10.1128/jvi.72.12.9544-9552.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jassal S R, Pöhler J R G, Brighty D W. Human T-cell leukemia virus type 1 receptor expression among syncytium-resistant cell lines revealed by a novel surface glycoprotein-immunoadhesin. J Virol. 2001;75:8317–8328. doi: 10.1128/JVI.75.17.8317-8328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melnick J, Dul J L, Argon Y. Sequential interaction of the chaperones BiP and GRP94 with immunoglobulin chains in the endoplasmic reticulum. Nature. 1994;370:373–375. doi: 10.1038/370373a0. [DOI] [PubMed] [Google Scholar]
- 14.Mulvey M, Brown D T. Assembly of the Sindbis virus spike protein complex. Virology. 1996;219:125–132. doi: 10.1006/viro.1996.0229. [DOI] [PubMed] [Google Scholar]
- 15.Nossner E, Goldberg J E, Naftzger C, Lyu S C, Clayberger C, Krensky A M. HLA-derived peptides which inhibit T cell function bind to members of the heat-shock protein 70 family. J Exp Med. 1996;183:339–348. doi: 10.1084/jem.183.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okuma K, Nakamura M, Nakano S, Niho Y, Matsuura Y. Host range of human T-cell leukemia virus type I analyzed by a cell fusion-dependent reporter gene activation assay. Virology. 1999;254:235–244. doi: 10.1006/viro.1998.9530. [DOI] [PubMed] [Google Scholar]
- 17.Palker T J, Riggs E R, Spragion D E, Muir A J, Scearce R M, Randall R R, McAdams M W, McKnight A, Clapham P R, Weiss R A. Mapping of homologous, amino-terminal neutralizing regions of human T-cell lymphotropic virus type I and II gp46 envelope glycoproteins. J Virol. 1992;66:5879–5889. doi: 10.1128/jvi.66.10.5879-5889.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pique C, Tursz T, Dokhelar M C. Mutations introduced along the HTLV-I envelope gene result in a non-functional protein: a basis for envelope conservation? EMBO J. 1990;9:4243–4248. doi: 10.1002/j.1460-2075.1990.tb07872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pique C, Pham D, Tursz T, Dokhelar M C. Human T-cell leukemia virus type I envelope protein maturation process: requirements for syncytium formation. J Virol. 1992;66:906–913. doi: 10.1128/jvi.66.2.906-913.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pique C, Pham D, Tursz T, Dokhelar M C. The cytoplasmic domain of the human T-cell leukemia virus type I envelope can modulate envelope functions in a cell type-dependent manner. J Virol. 1993;67:557–561. doi: 10.1128/jvi.67.1.557-561.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberg A R, Delamarre L, Preira A, Dokhelar M C. Analysis of functional conservation in the surface and transmembrane glycoprotein subunits of human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2. J Virol. 1998;72:7609–7614. doi: 10.1128/jvi.72.9.7609-7614.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sagara Y, Inoue Y, Shiraki H, Jinno A, Hoshino H, Maeda Y. Identification and mapping of functional domains on human T-cell lymphotropic virus type 1 envelope proteins by using synthetic peptides. J Virol. 1996;70:1564–1569. doi: 10.1128/jvi.70.3.1564-1569.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sagara Y, Ishida C, Inoue Y, Shiraki H, Maeda Y. 71-kilodalton heat shock cognate protein acts as a cellular receptor for syncytium formation induced by human T-cell lymphotropic virus type 1. J Virol. 1998;72:535–541. doi: 10.1128/jvi.72.1.535-541.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sodroski J G. HIV-1 inhibitors in the side pocket. Cell. 1999;99:243–246. doi: 10.1016/s0092-8674(00)81655-4. [DOI] [PubMed] [Google Scholar]
- 25.Sommerfelt M A, Weiss R A. Receptor interference groups of 20 retroviruses plating on human cells. Virology. 1990;176:58–69. doi: 10.1016/0042-6822(90)90230-o. [DOI] [PubMed] [Google Scholar]
- 26.Sommerfelt M A. Retrovirus receptors. J Gen Virol. 1999;80:3049–3064. doi: 10.1099/0022-1317-80-12-3049. [DOI] [PubMed] [Google Scholar]
- 27.Srivastava P K, Udono H, Blachere N E, Li Z. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics. 1994;39:93–98. doi: 10.1007/BF00188611. [DOI] [PubMed] [Google Scholar]
- 28.Strickland E, Qu B H, Millen L, Thomas P J. The molecular chaperone Hsc70 assists the in vitro folding of the N-terminal nucleotide-binding domain of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 1997;272:25421–25424. doi: 10.1074/jbc.272.41.25421. [DOI] [PubMed] [Google Scholar]
- 29.Sutton R E, Littman D R. Broad host range of human T-cell leukemia virus type 1 demonstrated with an improved pseudotyping system. J Virol. 1996;70:7322–7326. doi: 10.1128/jvi.70.10.7322-7326.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeda S, McKay D B. Kinetics of peptide binding to the bovine 70 kDa heat shock cognate protein, a molecular chaperone. Biochemistry. 1996;35:4636–4644. doi: 10.1021/bi952903o. [DOI] [PubMed] [Google Scholar]
- 31.Takenaka I M, Leung S M, McAndrew S J, Brown J P, Hightower L E. Hsc70-binding peptides selected from a phage display peptide library that resemble organellar targeting sequences. J Biol Chem. 1995;270:19839–19844. doi: 10.1074/jbc.270.34.19839. [DOI] [PubMed] [Google Scholar]
- 32.Trejo S R, Ratner L. The HTLV receptor is a widely expressed protein. Virology. 2000;268:41–48. doi: 10.1006/viro.2000.0143. [DOI] [PubMed] [Google Scholar]
- 33.Wu S J, Wang C. Binding of heptapeptides or unfolded proteins to the chimeric C-terminal domains of 70-kDa heat shock cognate protein. Eur J Biochem. 1999;259:449–455. doi: 10.1046/j.1432-1327.1999.00073.x. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W, Canziani G, Plugariu C, Wyatt R, Sodroski J, Sweet R, Kwong P, Hendrickson W, Chaiken I. Conformational changes of gp120 in epitopes near the CCR5 binding site are induced by CD4 and a CD4 miniprotein mimetic. Biochemistry. 1999;38:9405–9416. doi: 10.1021/bi990654o. [DOI] [PubMed] [Google Scholar]