Abstract
Purpose
Lipopolysaccharide (LPS)-type endotoxins are naturally found in the gut microbiota and there is emerging evidence linking gut microbiota and neuroinflammation leading to retinal neurodegeneration. Thinning of the retinal nerve fiber layer (RNFL) is a biomarker of retinal neurodegeneration, and a hallmark of glaucoma, the second leading cause of blindness worldwide. We assessed the association of a blood biomarker of LPS with peripapillary RNFL thickness (RNFLT) and its longitudinal evolution up to 11 years.
Design
The Alienor study is a single center prospective population-based cohort study.
Subjects
The studied sample of this study includes 1062 eyes of 548 participants receiving ≥1 gradable RNFL measurement.
Methods
Plasma esterified 3-hydroxy fatty acids (3-OH FAs) were measured as a proxy of LPS burden. Retinal nerve fiber layer thickness was acquired using spectral-domain OCT imaging every 2 years from 2009 to 2020 (up to 5 visits).
Main Outcome Measures
Associations of plasma esterified 3-OH FAs with RNFLT were assessed using linear mixed models.
Results
Mean age of the included 548 participants was 82.4 ± 4.3 years and 62.6% were women. Higher plasma esterified 3-OH FAs was significantly associated with thinner RNFLT at baseline (coefficient beta = −1.42 microns for 1 standard deviation-increase in 3-OH FAs, 95% confidence interval [−2.56; −0.28], P = 0.02). This association remained stable after multivariate adjustment for potential confounders. No statistically significant association was found between 3-OH FAs and longitudinal RNFLT change.
Conclusions
Higher plasma esterified 3-OH FAs were associated with thinner RNFLT at baseline, indicating an involvement of LPS in the early processes of optic nerve neurodegeneration and highlighting the potential importance of the human microbiota in preserving retinal health.
Financial Disclosure(s)
Proprietary or commercial disclosure may be found in the Footnotes and Disclosures at the end of this article.
Keywords: Clinical (human) or epidemiologic studies: risk factor assessment, LPS, Microbiota, Systemic inflammation
Glaucoma comprises a heterogeneous group of diseases characterized by irreversible progressive loss of retinal ganglion cells (RGCs). Data from population-based surveys indicate that globally >3.5% of adults aged 40 to 80 years are affected by glaucoma, which equates to >95 million people.1,2
All forms of glaucoma are characterized by RGC loss, retinal nerve fiber layer (RNFL) thinning, and cupping of the optic disc. The anatomy of the anterior chamber angle defines open-angle and angle-closure glaucoma. Glaucoma is further classified into primary or secondary, where primary cannot be contributed to any known cause and secondary glaucoma develops due to other disease or injury. Several other forms of glaucoma exist, including developmental glaucoma.
In people with European, Hispanic, and African ancestry, the most common form of glaucoma is primary open-angle glaucoma (POAG), whereas angle-closure glaucoma is more common in Asia.3 The most important risk factors (both for open-angle and angle-closure glaucoma) are age, elevated intraocular pressure, and positive family history. For open-angle glaucoma, myopia has been consistently reported as a risk factor.
As early-stage glaucoma is mostly asymptomatic, diagnosis is frequently delayed. In the last decades, spectral-domain OCT (SD-OCT) has facilitated noninvasively characterizing changes in the RNFL more distinctly, enabling quantification of progression already at a very early stage of disease by providing reproducible measurements of peripapillary RNFL thickness (RNFLT) with an axial resolution of 5 μm.4
Although to date many different types of medication and surgery exist for glaucoma, their common aim is to prevent further damage as no treatment for irreversible damage to the optic nerve exists. Indeed, the multifactorial nature of POAG so far prevents the modeling of this disease in vitro and in vivo and thus the exact pathogenesis remains unclear.
Several human and animal studies indicate inflammatory changes in the RGC layer and the optic nerve head. In particular, a wide range of oxidative stress-related makers were found in glaucomatous patients, which may contribute to damage in the optic nerve. In recent years the “gut-eye” axis, a link between gut microbiota, diet, and micronutrients, has been described in ophthalmic pathologies, including glaucoma.5,6 Growing evidence also indicates the role of oral microbiota in glaucoma pathophysiology. Altered microbiota may impair the integrity of surface barriers allowing pathogens, bacterial endotoxins like lipopolysaccharide (LPS)-type endotoxins, cytokines, and other proinflammatory substances to pass into circulation, resulting in systemic inflammation, potential impairment of the immune response, and/or local ocular inflammation.
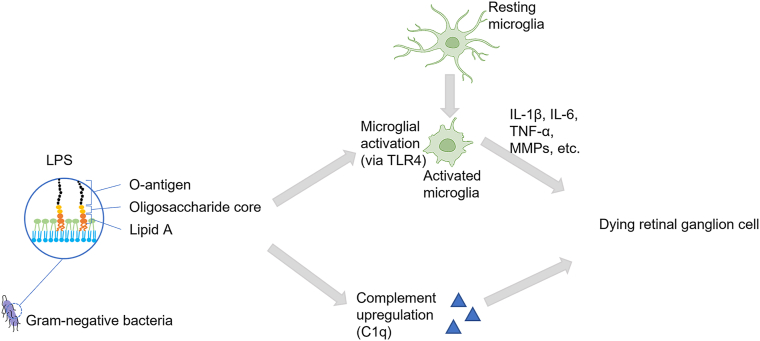
As component of the outer membrane of Gram-negative bacteria, LPS-type endotoxins are naturally found in the human microbiota. Mounting evidence indicates the involvement of LPS in the development of many chronic diseases, including glaucoma.7 In the eye, proinflammatory signaling via LPS occurs in microglia, perivascular macrophages, dendritic cells, photoreceptors, and retinal pigment epithelium cells and has been implicated in retinopathy of prematurity, diabetic retinopathy, age-related macular degeneration, and glaucoma (Fig 1). Low-dose LPS administration in glaucoma animal models resulted in enhancement of axonal degeneration and neuronal loss.8 Also, increased neuronal apoptosis and evidence of microglial activation after LPS exposure has been reported.9
Figure 1.
Potential impact of LPS on retinal ganglion cell death: impaired gut microbiota leads to translocation of proinflammatory signaling including bacterial LPS into systemic circulation. LPS induces an upregulation of TLR4 and the complement system in the retina. Microglia are among the first cells to be activated via TLR4, which in turn triggers the release of inflammatory cytokines activating apoptotic pathways leading to retinal ganglion cell death. IL-1β = interleukin 1β; IL-6 = interleukin 6; LPS = lipopolysaccharide; MMPs = matrix metalloproteinases; TLR4 = Toll-like receptor 4; TNF-α = tumor necrosis factor-α.
Thus, this study was conducted to further investigate the role of a serum biomarker of LPS exposure with peripapillary RNFLT in the framework of a population-based cohort study of older adults.
Methods
Study Cohort
The Alienor study is a prospective population-based cohort study initiated in 2006 to assess the epidemiology of age-related eye diseases. Subjects of the Alienor study were recruited from the population-based Three City (3C) study cohort of Bordeaux (France).10 The 3C study is a multicenter population-based prospective cohort study initiated in 1999 to assess the vascular risk factors of dementia. The 3C study included community-dwelling individuals aged >65 years randomly drawn from electoral rolls. Between 1999 and 2001, 9294 subjects were recruited in 3 French cities: Bordeaux (n = 2104), Dijon (n = 4931), and Montpellier (n = 2259).
During the third follow-up of the 3C study (2006–2008), an ophthalmic examination was offered to all subjects of the 3C Bordeaux cohort, which marks the baseline of the Alienor study. A total of 963 subjects were enrolled at baseline and have been examined every 2 years ophthalmically in parallel with the 3C study. In 2024, the sixth and final follow-up visit of the Alienor cohort was completed, which correlates to up to 18 years of ophthalmic follow-up http://www.alienor-study.com/langue-english-1.html, accessed on July 30, 2024). More details on both the 3C study cohort and the Alienor study cohort have been already published elsewhere.10,11
The Alienor study followed the tenets of the Declaration of Helsinki and was approved prospectively by the Ethical Committee of Bordeaux (Comité de Protection des Personnes Sud-Ouest et Outre-Mer III, code 2006/10) in May 2006. Informed consent of all participants of the Alienor study was obtained after explanation of the nature and possible consequences of the study.
Ophthalmic Examination and Imaging
The initial Alienor study protocol includes visual acuity, refraction, intraocular pressure, and 45° nonmydriatic high-resolution color retinal photographs (TRC NW6S).11 Starting from the first follow-up visit (2009–2011), SD-OCT examinations of the macula and the optic nerve were included to the Alienor study protocol (Spectralis; Heidelberg Engineering). Thus, SD-OCT examinations are available at 5 time points from 2009 to 2020. The same OCT device was used at all follow-up visits.
For the peripapillary SD-OCT RNFL acquisition, the high-resolution protocol was employed. Retinal nerve fiber layer examinations were performed using 1536 A-scans of low coherence near-infrared (840 nm) light beam. Thickness measurements of peripapillary RNFL were calculated using the circle scan provided by the machine, which consisted of a 3.45-mm diameter circle scan centered on the optic disc. Global peripapillary RNFLT was automatically segmented and calculated (Fig S2, available at www.ophthalmologyscience.org). All images were acquired and reviewed by specially trained technicians and manual corrections were performed if necessary. Correction for fovea-disc orientation is incorporated in the software and a real-time eye tracking system is used to compensate for eye movements. Signal strength <15 decibels (range, 0–40) or acquisitions with artifacts were excluded from the analysis. 12
Assessment of LPS-Type Endotoxins
All plasma measurements were assessed using blood samples collected during the 3C baseline visit (1999–2001).
Plasma esterified 3-hydroxy fatty acids (3-OH FAs) were quantified as a proxy of total plasma LPS burden using liquid chromatography tandem mass spectrometry. This method was previously described by Pais de Barros et al and quantifies plasma esterified 3-OH-FAs serve as a proxy for total plasma LPS burden.13 The detailed protocol can be found in Supplementary Methods 1 (available at www.ophthalmologyscience.org).
Covariates
Sociodemographic data, lifestyle characteristics, and medical variables were collected at 3C baseline.11
Medical variables including diabetes (fasting blood glucose ≥7 mmol/L or diabetes medication), hypertension (blood pressure >140/90 mm Hg or antihypertensive medication), self-reported cardiovascular history, and systemic lipid-lowering drugs were also assessed at 3C baseline. Fasting plasma lipids were measured at the Biochemistry Laboratory of the University Hospital of Dijon, France using routine enzymatic techniques.
Family history of glaucoma and axial length measurement were collected at Alienor baseline. Intraocular pressure was noted at time of first RNFL measurement.
The Mediterranean diet score is based on the MEDI-LITE score, which measures adherence to Mediterranean diet based on the literature and was developed by Sofi et al.14 This score is based on a 148-items validated food frequency questionnaire and reflects the level of adherence to the Mediterranean diet, ranging from 0 (poor adherence) to 18 (highest adherence).
The social deprivation index, a socioeconomic index based on the French European deprivation index adapted to elderly people, was calculated at the 3C baseline residential address.15
Statistical Analyses
Associations of plasma esterified 3-OH FAs with peripapillary RNFLT were estimated using linear mixed models, with the eye as the unit of analysis, as described previously.16 Interaction between 3-OH FAs and time was used to account for longitudinal effects.
All conditions for the application of the linear mixed model were verified. The linearity of quantitative variables was investigated using restricted cubic spline functions. Z score standardization was applied to the exposure variable 3-OH FAs.
Multivariate imputation by chained equations was performed to impute missing covariates, assuming that missing data were Missing At Random; more details can be found here.17,18 The fraction of missing/imputed data can be found in Table S1 and S2 (available at www.ophthalmologyscience.org). Multivariate adjustment was performed after imputation. Model 1 was adjusted for age and sex. Model 2 was further adjusted for the most relevant potential confounders identified from the literature and with a directed acyclic graph.
R software version 4.3.3 (R Foundation for Statistical Computing) was used for statistical analyses.
Results
Characteristics of the Studied Sample
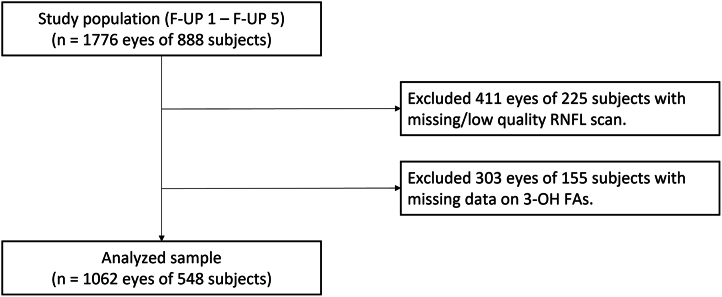
Peripapillary RNFL examinations were included to the Alienor study protocol at the first follow-up visit. Thus, the studied sample comprises all examinations beginning with the first follow-up visit of the Alienor study. Of the examined 888 participants, 411 eyes of 225 participants had no gradable RNFL scan (Fig 3). Another 303 eyes of 155 participants were excluded because of missing data for plasma esterified 3-OH-FA levels, leaving 1062 eyes of 548 subjects for further analyses. In total, 2497 RNFL examinations of 548 subjects were analyzed.
Figure 3.
Flow chart showing selection of participants for analyses. Data from the Alienor study, follow-up 1 to follow-up 5; 2009 to 2020. 3-OH FAs = 3-hydroxy fatty acids; F-UP = follow-up examination; RNFL = retinal nerve fiber layer.
Among the 548 included participants, mean age at first RNFL measurement was 82.4 years (±4.3), of which 343 (62.6%) were female (Table 3). Almost two-thirds of the study sample were never smokers, 15.3% were obese (body mass index >30 kg/m2), and the most common comorbidity was hypertension (72.3%). The average number of drugs consumed by each participant was 3.9 (±2.6). In particular, 15.5% of the participants took anti-inflammatory medication and 33.0% lipid-lowering medication, respectively. Included participants tended to be slightly younger and were less likely to have hypertension. No significant difference was observed for other sociodemographic, lifestyle, or medical data (Table 3). A total of 180 (32.8%) participants received 1 RNFL examination, 147 (26.8%) received 2, 103 (18.8%) received 3, 64 (11.7%) received 4, and 54 (9.9%) received 5. For the 368 participants receiving >1 RNFL examination, the average follow-up time was 4.7 years (range 1.0–10.3).
Table 3.
Description of the Study Population at Baseline RNFL Measurement (Mean ± SD or n [%])
| Characteristics | Included (n = 548) |
Nonincluded (n = 340) |
P Value |
|---|---|---|---|
| Mean ± SD or n (%) | |||
| Age | 82.4 ± 4.3 | 84.4 ± 5.0 | <0.001 |
| Sex | 0.05 | ||
| Male | 205 (37.4%) | 105 (30.9%) | |
| Female | 343 (62.6%) | 235 (69.1%) | |
| Smoking (pack-years) | n = 543 | n = 332 | 0.13 |
| Never smoked | 352 (64.8%) | 233 (70.2%) | |
| <20 | 98 (18.0%) | 48 (14.5%) | |
| ≥20 | 93 (17.1%) | 51 (15.4%) | |
| BMI (kg/m2) | n = 548 | n = 331 | 0.84 |
| [0, 18.5) | 3 (0.5%) | 5 (1.5%) | |
| [18.5,25) | 212 (38.7%) | 133 (40.2%) | |
| [25, 30) | 249 (45.4%) | 133 (40.2%) | |
| [30, 59] | 84 (15.3%) | 60 (18.1%) | |
| Social deprivation index | 0.6 ± 2.2 | 0.7 ± 2.2 | 0.57 |
| Plasma lipids (mmol/L) | |||
| LDL cholesterol | 3.7 ± 0.8 | 3.5 ± 0.8 | 0.08 |
| HDL cholesterol | 1.6 ± 0.4 | 1.6 ± 0.4 | 0.55 |
| Triglycerides | 1.2 ± 0.6 | 1.3 ± 0.7 | 0.27 |
| Diabetes | 40 (7.3%) | 30 (8.8%) | 0.12 |
| Hypertension | 396 (72.3%) | 267 (78.5%) | 0.03 |
| Cardiovascular diseases | 43 (7.8%) | 35 (10.3%) | 0.25 |
| Mediterranean diet score | 10.6 ± 2.1 | 10.6 ± 2.0 | 0.88 |
| Lipid-lowering medication | 181 (33.0%) | 102 (30.0%) | 0.40 |
| Family history of glaucoma | n = 537 | n = 203 | |
| 73.0 (13.6%) | 21.0 (10.3%) | 0.29 | |
| Axial length | n = 321 | n = 119 | |
| Right eye | 23.5 ± 1.2 | 23.8 ± 1.9 | 0.09 |
| Left eye | 23.4 ± 1.1 | 23.7 ± 1.7 | 0.16 |
| Intraocular pression | n = 542 | n = 330 | |
| Right eye | 13.4 ± 2.3 | 13.3 ± 2.3 | 0.40 |
| Left eye | 13. 6 ± 2.4 | 13.4 ± 2.2 | 0.21 |
| Global peripapillary RNFL thickness (μm) | n = 1062 eyes 89.8 ± 14.5 |
- | - |
BMI = body mass index; HDL = high-density lipoprotein; LDL = low-density lipoprotein; RNFL = retinal nerve fiber layer; SD = standard deviation.
Alienor study, 2009 to 2020.
Bold values represent statistically significant results.
Multivariate Analysis
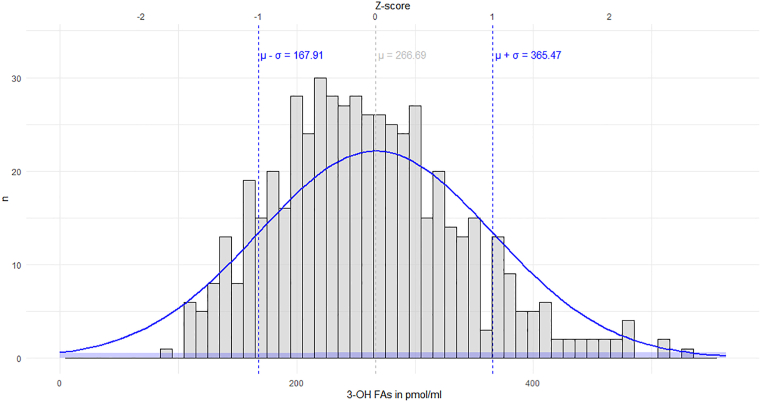
3-Hydroxy fatty acids were standardized using the formula Z = (x-μ)/σ, where Z is the standard score, μ the mean, and σ the standard deviation of 3-OH FAs, respectively. Measured concentration of 3-OH FAs as well as the standardized values together with a Gaussian overlay can be found in Figure 4.
Figure 4.
Distribution of measured concentration of the plasma esterified 3-OH FA with Gaussian curve overlay. 3-OH FA = 3-hydroxy fatty acid; Z-score = standard score, μ = mean, σ = standard deviation.
Associations between esterified 3-OH FAs and RNFLT are shown in Table 4. Using linear mixed model analysis, higher exposure to esterified 3-OH FAs was significantly associated with reduced RNFLT after adjustment for age and gender (model 1: coefficient beta [ß] = −1.42 microns for 1 SD-increase in 3-OH FAs, 95% confidence interval [CI] [−2.56; −0.28], P = 0.02).
Table 4.
Associations between Esterified 3-Hydroxy Fatty Acids and Peripapillary Retinal Nerve Fiber Layer Thickness
| Baseline |
Longitudinal Change (μm/yr) |
|||
|---|---|---|---|---|
| β (95% CI) | P value | β (95% CI) | P value | |
| Model 1∗ | −1.42 [−2.56; −0.28] | 0.02 | 0.03 [−0.08; 0.13] | 0.60 |
| Model 2† | −1.44 [−2.56; −0.33] | 0.01 | 0.03 [−0.08; 0.14] | 0.58 |
CI = confidence interval.
Alienor study, 2009 to 2020 (n = 548; 2497 examinations).
Model 1 was adjusted for age and sex.
Model 2 was adjusted for age, sex, social deprivation index, family history of glaucoma, axial length, intraocular pressure, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, diet quality, body mass index, and lipid-lowering medication (after imputation of missing covariates).
Further multivariate adjustment showed consistently a statistically significant association of exposure to 3-OH FAs with RNFLT (model 2: ß = −1.44, CI [−2.56; −0.33], P = 0.01).
Mean decrease of RNFLT over time derived from both model 1 and 2 was 0.56 ± 0.05 μm/year (P < 0.001). However, the longitudinal follow-up of participants with higher exposure to esterified 3-OH FAs did not show any statistical association to RNFLT change during the follow-up of the study (model 1: ß = 0.03, CI [−0.08; 0.13], P = 0.60, model 2: ß = 0.03, CI [−0.08; 0.14], P = 0.58).
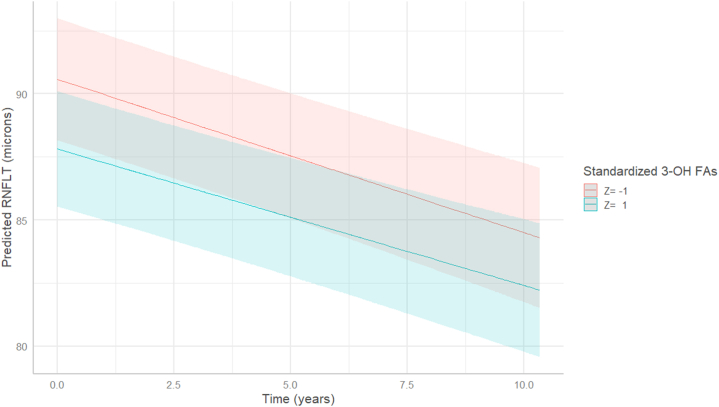
The evolution of RNFLT over the follow-up predicted by model 2 is presented in Figure 5. This graphical representation of model 2 shows a clear association of higher exposure of esterified 3-OH FAs with thinner RNFLT at baseline, comparing Z = 1 to Z = −1 (i.e., a difference of 2 standard deviations in 3-OH FAs). Figure 5 also illustrates the mean decrease of RNFLT over time and reveals an almost parallel RNFLT evolution of participants with and without higher exposure to esterified 3-OH FAs, corresponding to the lack of interaction with time.
Figure 5.
Longitudinal evolution of the mean RNFLT according to 3-OH FAs exposure, predicted by multivariate linear mixed model (model 2). 3-OH FAs were standardized using the formula Z = (x-μ)/σ, where Z is the standard score, μ the mean, and σ the standard deviation of 3-OH FAs, respectively. Data from the Alienor study, follow-up 1 to follow-up 5; 2009 to 2020. 3-OH FAs = 3-hydroxy fatty acids; RNFLT = retinal nerve fiber layer thickness.
Discussion
In this cohort, higher levels of 3-OH FAs, a proxy of plasma LPS exposure, were significantly associated with thinner peripapillary RNFLT at baseline. This significant association remained stable after multivariate-adjustment for potential confounders.
To our knowledge, this is the first study analyzing the association between plasma-endotoxin exposure and RNFLT. However, emerging research suggests a potential link between alterations in human microbiota composition, leading to higher plasma-endotoxin exposure, and glaucoma.7,8
Looking into human data, Collins et al found an association between POAG and mitochondrial DNA variants, which were also associated with alterations in the gut microbiome in an earlier association study of mitochondrial variation.19,20 Recently, differences in certain human gut microbial taxa between patients with POAG and controls have been described.21,22 Also a positive correlation of Streptococcus in fecal samples with RNFL has been reported.21 Recently, a Mendelian randomization analysis assessed the causal links between gut microbiota taxa and POAG.6 Using single-nucleotide polymorphisms associated with human gut microbiome from a multiethnic cohort of 18 340 individuals, and data for POAG including 133 492 cases and 90 939 controls, they reported that with high statistical powers of the causal estimates (power >0.8) Odoribacter genus, Ruminiclostridium9 genus, and Eubacterium rectale group, were associated with an increased risk of POAG.6,23 The authors found that intraocular pressure, central corneal thickness, and vertical cup-to-disc ratio played mediation effects between genus Ruminiclostridium9, genus Alloprevotella, phylum Euryarchaeota, and POAG, respectively, suggesting an impact of specific taxa on the pathogenesis of POAG.
Mechanistically, a mouse study showed that mice raised in absence of commensal microflora do not develop glaucomatous T-cell responses or associated neurodegeneration, indicating that glaucomatous neurodegeneration is mediated in part by T cells that are presensitized by exposure to commensal microflora.24 Another animal study showed that reducing glaucoma-specific gut microbiota significantly reduced RGC loss by alleviating the activation of retinal microglia cells and overproduction of inflammatory cytokines.22 Furthermore, they highlighted that fecal microbiome transplantation from glaucoma patients compared with healthy subjects significantly increased retinal inflammation in mice.
The oral cavity is another important habitat of human microbiota, and dysbiosis of oral microbiome has been described to affect glaucoma progression.25 In a case-control study, subjects with glaucoma showed significantly higher oral bacterial loads and fewer teeth.8 Using an animal model of glaucoma, low-dose LPS injected subcutaneously led to enhancement of axonal degeneration and neuronal loss and was accompanied by microglial activation in the optic nerve and retina as well as upregulation of Toll-like receptor 4 signaling and the complement system.8 Another case-control study by the same research group found Streptococci load to be higher in the saliva of patients with POAG than in controls.26 Using machine learning prediction models, Yoon et al revealed significant depletion of the genus Lactococcus, whereas the genus Faecalibacterium was enriched in the oral microbiome of patients with glaucoma.27 Taken together, the data of both human and animal studies underline that the oral and gut microbiome in patients with glaucoma was different from control participants, indicating an impact of microbiome dysbiosis on the pathogenesis of glaucoma.
The strengths of this study include a large and well-defined cohort, a detailed standardized ophthalmic examination, and long follow-up time. Our results show a cross-sectional association of higher plasma esterified 3-OH FAs and RNFLT, potentially linking human microbiota with pathophysiological processes leading to optic nerve degeneration. The lack of association on the longitudinal analysis might be related to the single blood draw conducted approximately 8 years before the initial RNFL examination and to the increasing age of the cohort. Another limitation concerns the quantification of LPS: to date, no standard exists and the commonly used limulus amebocyte lysate assay measures only the reactive portion of LPS and is not suitable for complex matrices as plasma. Here, we employed a proxy method to estimate LPS exposure by analyzing esterified 3-OH FAs through liquid chromatography tandem mass spectrometry. However, under specific conditions, esterified 3-OH FAs in plasma may indicate the presence of lipid A without acyloxyacyl structures, crucial for bioactive signaling. Therefore, the analysis of 3-OH FAs might have led to misclassification of the exposure. However, a differential misclassification of 3-OH FAs related to RNFLT is highly unlikely, thus misclassification of LPS exposure would tend to bias the associations with RNFLT toward the null. Moreover, a risk of selection bias cannot be excluded, as included and nonincluded participants differed in age and diagnosis of hypertension. However, included and nonincluded participants were comparable for all other characteristics.
Summarized, an association between higher plasma esterified 3-OH FAs and RNFLT at baseline was observed in our cohort, suggesting that LPS exposure may be involved in the pathophysiological processes of optic nerve neurodegeneration. This study adds to the available literature on RNFLT and LPS, and more generally oral and gut microbiomes, underlining the inflammatory component of neurodegeneration. However, future studies are warranted to explore this complex relation and to better understand the underlying mechanisms.
Manuscript no. XOPS-D-24-00220.
Footnotes
These data were presented in part at the ARVO Meeting in Seattle, WA, USA, in 2024.
Supplemental material available atwww.ophthalmologyscience.org.
Disclosures:
All authors have completed and submitted the ICMJE disclosures form.
The authors have made the following disclosures:
P.P.L.: Grants – German Research Foundation.
C.F.: Consultant – Synadiet, SYNPA; Travel expenses – Laboratoire Lescuyer, Société Française de Nutrition.
M.N.D.: Honoraria – AbbVie, Bayer, Novartis, Roche, Horus Pharma; Travel expenses – AbbVie, Bayer, Novartis, Roche, Horus Pharma; Consultant – Abbvie, Bayer, Horus Pharma, Novartis, Roche, Théa.
J.F.K.: Consultant – Abbvie, Allergan, Apellis, Bayer, Eyepoint Pharma, Janssen, NanoRetina, Ocuphire, Roche, Thea, Carl Zeiss Meditec; Participation on a Data Safety Monitoring Board or Advisory Board – Alexion, Novonordisk and Opthea.
C.S.: Consultant – Alcon, Elios, Glaukos, Horus, Nicox, Santen, Théa.
C.D.: Grants – Fondation de France; Consultant – Allergan, Bausch + Lomb, Laboratoires Théa, Novartis; Travel expenses – Laboratoires Théa; Patents planned, issued or pending – WO 2021/0589914 A1.
German Research Foundation grant PL 5077/1-1, Théa Pharma, Fondation Voir et Entendre, University of Bordeaux, Agence Nationale de la Recherche (ANR 2010-PRSP-011 VISA), Club Francophone des Spécialistes de la Rétine, French Ministry of Health (PHRC, 2012, PHRC12_157 ECLAIR), FRAILOMIC Initiative (FP7-HEALTH-2012-Proposal No. 305483–2), ITMO Santé Publique—Alliance nationale pour les sciences de la vie et de la santé (ISP05 2014).
HUMAN SUBJECTS: Human subjects were included in this study. The study was approved prospectively by the Ethical Committee of Bordeaux (Comité de Protection des Personnes Sud-Ouest et Outre-Mer III, code 2006/10) in May 2006. All research adhered to the tenets of the Declaration of Helsinki. All participants provided informed consent.
No animal subjects were used in this study.
Author Contributions:
Conception and design: Larsen, Delcourt
Data collection: Larsen, Féart, Pais de Barros, Gayraud, Delyfer, Korobelnik, Schweitzer, Delcourt
Analysis and interpretation: Larsen, Gayraud, Delcourt
Obtained funding: Larsen, Féart, Korobelnik, Delcourt
Overall responsibility: Larsen, Delcourt
Supplementary Data
References
- 1.Jayaram H., Kolko M., Friedman D.S., Gazzard G. Glaucoma: now and beyond. Lancet. 2023;402:1788–1801. doi: 10.1016/S0140-6736(23)01289-8. [DOI] [PubMed] [Google Scholar]
- 2.Tham Y.C., Li X., Wong T.Y., et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Jonas J.B., Aung T., Bourne R.R., et al. Glaucoma. Lancet. 2017;390:2183–2193. doi: 10.1016/S0140-6736(17)31469-1. [DOI] [PubMed] [Google Scholar]
- 4.van Velthoven M.E.J., Faber D.J., Verbraak F.D., et al. Recent developments in optical coherence tomography for imaging the retina. Prog Retin Eye Res. 2007;26:57–77. doi: 10.1016/j.preteyeres.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Chen J., Chen D.F., Cho K.S. The role of gut microbiota in glaucoma progression and other retinal diseases. Am J Pathol. 2023;193:1662–1668. doi: 10.1016/j.ajpath.2023.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou X., Xu J., Zhang X., et al. Causal relationships between Gut microbiota and primary open-angle Glaucoma: a Mendelian randomization and mediation analysis of Glaucoma endophenotypes. Exp Eye Res. 2024;240 doi: 10.1016/j.exer.2024.109788. [DOI] [PubMed] [Google Scholar]
- 7.Huang L., Hong Y., Fu X., et al. The role of the microbiota in glaucoma. Mol Aspects Med. 2023;94 doi: 10.1016/j.mam.2023.101221. [DOI] [PubMed] [Google Scholar]
- 8.Astafurov K., Elhawy E., Ren L., et al. Oral microbiome link to neurodegeneration in glaucoma. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer P.M., Zalis M.C., Abdshill H., et al. Inflamed in vitro retina: cytotoxic neuroinflammation and galectin-3 expression. PLoS One. 2016;11:1–20. doi: 10.1371/journal.pone.0161723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alpérovitch A., Amouyel P., Dartigues J.F., et al. Vascular factors and risk of dementia: design of the three-city study and baseline characteristics of the study population. Neuroepidemiology. 2003;22:316–325. doi: 10.1159/000072920. [DOI] [PubMed] [Google Scholar]
- 11.Delcourt C., Korobelnik J.F., Barberger-Gateau P., et al. Nutrition and age-related eye diseases: The Alienor (Antioxydants, lipides essentiels, nutrition et maladies oculaires) study. J Nutr Health Aging. 2010;14:854–861. doi: 10.1007/s12603-010-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schweitzer C., Korobelnik J.F., Le Goff M., et al. Diagnostic performance of peripapillary retinal nerve fiber layer thickness for detection of glaucoma in an elderly population: the ALIENOR study. Invest Ophthalmol Vis Sci. 2016;57:5882. doi: 10.1167/iovs.16-20104. [DOI] [PubMed] [Google Scholar]
- 13.Pais de Barros J.P., Gautier T., Sali W., et al. Quantitative lipopolysaccharide analysis using HPLC/MS/MS and its combination with the limulus amebocyte lysate assay. J Lipid Res. 2015;56:1363–1369. doi: 10.1194/jlr.D059725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sofi F., Dinu M., Pagliai G., et al. Validation of a literature-based adherence score to Mediterranean diet: the MEDI-LITE score. Int J Food Sci Nutr. 2017;68:757–762. doi: 10.1080/09637486.2017.1287884. [DOI] [PubMed] [Google Scholar]
- 15.Letellier N., Gutierrez L.A., Carrière I., et al. Sex-specific association between neighborhood characteristics and dementia: the Three-City cohort. Alzheimer's Dementia. 2018;14:473–482. doi: 10.1016/j.jalz.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Gayraud L., Mortamais M., Schweitzer C., et al. Association of long-term exposure to ambient air pollution with retinal neurodegeneration: the prospective Alienor study. Environ Res. 2023;232 doi: 10.1016/j.envres.2023.116364. [DOI] [PubMed] [Google Scholar]
- 17.Azur M.J., Stuart E.A., Frangakis C., Leaf P.J. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20:40–49. doi: 10.1002/mpr.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsen P.P., Féart C., Pais de Barros J.P., et al. Association of age-related macular degeneration with a blood biomarker of lipopolysaccharide, a gut bacterial proinflammatory toxin. Invest Ophthalmol Vis Sci. 2023;64:47. doi: 10.1167/iovs.64.14.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins D.W., Gudiseva H.V., Trachtman B., et al. Association of primary open-angle glaucoma with mitochondrial variants and haplogroups common in African Americans. Mol Vis. 2016;22:454–471. [PMC free article] [PubMed] [Google Scholar]
- 20.Ma J., Coarfa C., Qin X., et al. MtDNA haplogroup and single nucleotide polymorphisms structure human microbiome communities. BMC Genom. 2014;15:1–14. doi: 10.1186/1471-2164-15-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong H., Zhang S., Li Q., et al. Gut microbiota compositional profile and serum metabolic phenotype in patients with primary open-angle glaucoma. Exp Eye Res. 2020;191 doi: 10.1016/j.exer.2020.107921. [DOI] [PubMed] [Google Scholar]
- 22.Chen S., Wang Y., Liu Y., et al. Dysbiosis of gut microbiome contributes to glaucoma pathogenesis. MedComm. 2022;1 [Google Scholar]
- 23.Craig J.E., Han X., Qassim A., et al. Multitrait analysis of glaucoma identifies new risk loci and enables polygenic prediction of disease susceptibility and progression. Nat Genet. 2020;52:160–166. doi: 10.1038/s41588-019-0556-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen H., Cho K.S., Vu T.H.K., et al. Commensal microflora-induced T cell responses mediate progressive neurodegeneration in glaucoma. Nat Commun. 2018;9:3209. doi: 10.1038/s41467-018-05681-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou K., Wu Z.X., Chen X.Y., et al. Microbiota in health and diseases. Signal Transduct Target Ther. 2022;7 doi: 10.1038/s41392-022-00974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polla D., Astafurov K., Hawy E., et al. A pilot study to evaluate the oral microbiome and dental health in primary open-angle glaucoma. J Glaucoma. 2017;26:320–327. doi: 10.1097/IJG.0000000000000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoon B.W., Lim S.H., Shin J.H., et al. Analysis of oral microbiome in glaucoma patients using machine learning prediction models. J Oral Microbiol. 2021;13 doi: 10.1080/20002297.2021.1962125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.