Abstract
Background
People living with serious respiratory illness experience a high burden of distressing symptoms. Although opioids are prescribed for symptom management, they generate adverse events, and their benefits are unclear.
Methods
We examined the efficacy and safety of opioids for symptom management in people with serious respiratory illness. Embase, MEDLINE and the Cochrane Central Register of Controlled Trials were searched up to 11 July 2022. Reports of randomised controlled trials administering opioids to treat symptoms in people with serious respiratory illness were included. Key exclusion criteria included <80% of participants having a nonmalignant lung disease. Data were extracted regarding study characteristics, outcomes of breathlessness, cough, health-related quality of life (HRQoL) and adverse events. Treatment effects were pooled using a generic inverse variance model with random effects. Risk of bias was assessed using the Cochrane Risk of Bias tool version 1.
Results
Out of 17 included trials, six were laboratory-based exercise trials (n=70), 10 were home studies measuring breathlessness in daily life (n=788) and one (n=18) was conducted in both settings. Overall certainty of evidence was “very low” to “low”. Opioids reduced breathlessness intensity during laboratory exercise testing (standardised mean difference (SMD) −0.37, 95% CI −0.67– −0.07), but not breathlessness measured in daily life (SMD −0.10, 95% CI −0.64–0.44). No effects on HRQoL (SMD −0.42, 95% CI −0.98–0.13) or cough (SMD −1.42, 95% CI −3.99–1.16) were detected. In at-home studies, opioids led to increased frequency of nausea/vomiting (OR 3.32, 95% CI 1.70–6.51), constipation (OR 3.08, 95% CI 1.69–5.61) and drowsiness (OR 1.37, 95% CI 1.01–1.86), with serious adverse events including hospitalisation and death identified.
Conclusions
Opioids improved exertional breathlessness in laboratory exercise studies, but did not improve breathlessness, cough or HRQoL measured in daily life at home. There were significant adverse events, which may outweigh any benefits.
Shareable abstract
Opioids were shown to improve exertional breathlessness in exercise studies, but had no impact on breathlessness, cough or quality of life in daily life. Significant reported adverse events, including hospitalisation and death, may outweigh any benefits. https://bit.ly/3JTtD2A
Introduction
Serious illness is defined as a condition that carries a high risk of mortality, negatively impacts quality of life and daily function, and is burdensome in terms of symptoms, treatments or caregiver stress [1]. People living with nonmalignant serious respiratory illness, including, but not limited to COPD and interstitial lung disease (ILD) frequently experience a high burden of physical and psychosocial symptoms [2].
Breathlessness is the subjective experience of breathing discomfort, which can vary in intensity and quality [3]. Chronic breathlessness is often ranked by people with serious respiratory illness as their worst or most distressing symptom [4–7]. Management of chronic breathlessness is notoriously difficult; many people experience persisting breathlessness despite optimisation of all the underlying conditions contributing to the symptom [8]. Failure to adequately address chronic breathlessness is a key driver of reduced quality of life in people with serious respiratory illness [9–11] and a major contributor to costly unscheduled healthcare usage [12, 13]. In people with COPD, persisting chronic breathlessness is an important determinant of low physical and mental health [6, 7]. In people with ILD, breathlessness is highly prevalent and one of their major unmet palliative care needs [14, 15]. Fear of exertional breathlessness may result in avoiding exercise, leading to a downward spiral of deconditioning and social isolation with negative physical and emotional consequences [7]. There is therefore an immense need to better actively manage chronic breathlessness and other distressing symptoms in people with nonmalignant serious respiratory illness.
Opioid medications such as morphine have been recommended as a treatment option for chronic breathlessness when distressing breathlessness persists despite disease optimisation and utilisation of nonpharmacological approaches (such as breathing techniques and positions to ease breathlessness) [3, 16, 17]. A Cochrane review in 2016 revealed limited low-quality evidence regarding the effectiveness of opioids for the treatment of chronic breathlessness in people with serious respiratory illness [18]. Importantly, the mechanisms by which opioids may reduce breathlessness are poorly understood. It is theorised that opioids may alter the central perception of breathlessness via mechanisms similar to the central processing of pain stimuli [19–21]. Many clinicians and patients report a reluctance to prescribe or take opioids for chronic breathlessness, often compounded by concerns regarding known common side-effects, such as constipation, nausea, vomiting and drowsiness, and worries regarding serious adverse events such as respiratory depression [22–28]. Nevertheless, over the past few years some large clinical trials examining the efficacy of opioids for the treatment of chronic breathlessness in people with serious respiratory illness have been published [29–31]. Therefore, it is timely and crucial to reconsider the evidence base [32].
This systematic review and meta-analysis aimed to determine the effectiveness and safety of opioids administered systemically for the palliation of symptoms in people with nonmalignant serious respiratory illness, with the critical outcome being breathlessness. Other important outcomes included cough, health-related quality of life (HRQoL), arterial blood gas parameters and adverse events. This systematic review was conducted as part of the evidence synthesis for the European Respiratory Society (ERS) clinical practice guideline on symptom management for adults with serious respiratory illness [33].
Methods
The systematic review protocol was developed a priori, but was not published, due to the confidentiality requirements of the ERS clinical practice guideline development process. Instead, the protocol was submitted to European Respiratory Review editorial office in April 2023 to be held in confidence and made available to reviewers. The protocol can be found in the supplementary material.
Search strategy and study selection
A comprehensive search of Embase, MEDLINE and the Cochrane Central Register of Controlled Trials (CENTRAL) databases was conducted on 6 June 2022 to identify high-quality relevant systematic reviews. Prior systematic reviews were utilised to increase the efficiency of the search. A second comprehensive search of Embase, MEDLINE and CENTRAL databases was conducted on 8 July 2022 to identify any additional clinical trials published after those systematic reviews were completed (supplementary table S1), with inclusion dates varying according to when the preceding relevant systematic review was performed. Subject headings and keywords for illness related terms included “chronic obstructive pulmonary disease”, “interstitial lung disease”, “bronchiectasis”, “cystic fibrosis” and “pulmonary hypertension”. Subject headings for intervention related terms included “opioids” and related keywords, including, but not limited to “analgesic”, “narcotic”, “morphine”, “fentanyl”, “hydromorphone”, “oxycodone”, “pentazocine”, “methadone”, “codeine”, “dextromoramide”, “OTFC”, “diamorphine”, “dihydrocodeine”, “dextropropoxyphene”, “meptazinol”, “sufentanil”, “alfentanil”, “remifentanil”, “nalbuphine”, “meptazinol”, “dipipanone”, “pethidine”, “tramadol” and “buprenorphine”. Full search strategies are provided in the supplementary material.
Studies were included in our systematic review if they were randomised controlled trials (RCTs) or randomised crossover trials that investigated any opioid drug, given by intravenous, subcutaneous or oral routes in any dose, for the treatment of breathlessness or cough in adults with serious respiratory illness. The comparison was placebo or usual care, or any other pharmacological or nonpharmacological interventions that were directly compared with the opioid treatment. Serious respiratory illnesses included within this systematic review were asthma, bronchiectasis, COPD, cystic fibrosis, ILD, other obstructive lung diseases and pulmonary arterial hypertension (PAH). For studies with mixed cohorts of adults, only studies with ≥80% of participants having nonmalignant lung disease were included.
Outcomes
The primary outcome of interest for this review was breathlessness, measured using relevant, validated tools. This included measures taken during daily life at home or during exercise testing in a laboratory setting. Only exercise measures obtained at iso-workload before and after an intervention were included. In the interest of pragmatic study design, daily life measures included “right now” breathlessness measures, such as those recorded in symptom diaries. Important secondary outcomes included in this review were HRQoL, using any validated tool; cough, using any validated tool; arterial blood gas parameters (partial pressures of oxygen and carbon dioxide); and adverse events (specifically drowsiness, constipation, and nausea or vomiting), defined according to the investigators’ definition.
Data extraction
Two review authors (N.E. Smallwood and A. Pascoe) independently screened abstracts and full-text articles to determine eligibility for inclusion with disagreements resolved by discussion. Outcome data were extracted independently by two authors (N.E. Smallwood and A. Pascoe) for all included studies from source publications (not data reported in previous systematic reviews). Risk of bias for systematic reviews was appraised using the Assessing the Methodological Quality of Systematic Reviews (AMSTAR)-2 checklist. Risk of bias of included studies was assessed independently by two authors (N.E. Smallwood and A. Pascoe) using the Cochrane Risk of Bias tool version 1. The screening process was documented using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses study flow diagram [34].
Data synthesis
The administration of opioids was categorised into two conditions with associated outcomes synthesised and analysed separately: 1) opioids administered in a laboratory setting with participants completing a validated exercise test, with exercise-related outcomes measured in the laboratory at iso-time or iso-load to ensure standardised exertion [35]; or 2) opioids self-administered regularly for four or more consecutive days during daily life at home, with outcomes measured pragmatically.
Where studies used multiple doses of opioids in the intervention arm, data from the higher dose group were included. Some studies included both exercise and daily-life measures and therefore the appropriate outcome measures were included in both meta-analyses.
Treatment effects were pooled using a generic inverse variance model with random effects. Standardised mean differences (SMDs) for continuous data or odds ratios for dichotomous data (adverse events) were calculated with corresponding 95% confidence intervals. SMD estimates were interpreted using thresholds for effect sizes defined by Cohen [36], with SMD≈0.20: small effect; SMD≈0.50: moderate effect; SMD≈0.80: large effect. To determine clinical significance, the SMD was multiplied by the standard deviation of the baseline scores from a representative study so that the pooled effect could be expressed in the original units of that study [37]. Where data could not be combined in a meta-analysis, a narrative description of outcomes is provided. The unit of analysis was the individual participant, with no cluster RCTs included in the analysis. Heterogeneity was assessed using the I2 statistic. Sensitivity analyses were performed to examine the effects of methodological quality on the pooled estimates by removing studies that were at high or unclear risk of bias for the domains of blinding and incomplete outcome data. Overall certainty of evidence for each outcome was assessed and reported using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system (www.gradepro.org/). When ≥10 studies were pooled in a meta-analysis, we created and examined a funnel plot to explore potential publication bias.
Results
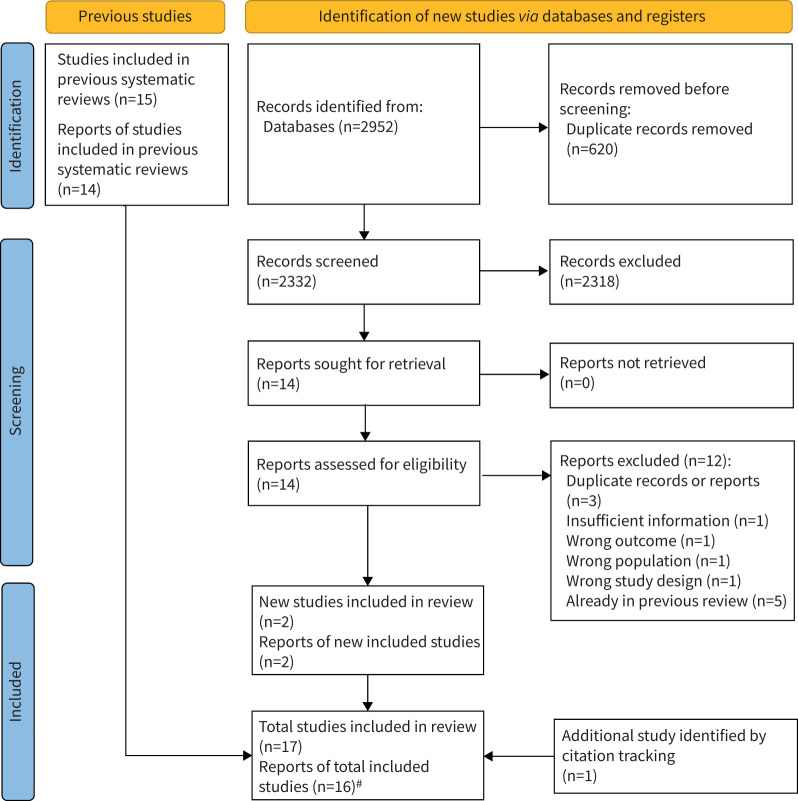
Of 2736 abstracts screened for the search for prior systematic reviews, 17 records were selected for full text review, with five systematic reviews included [18, 38–41] (supplementary table S2). From these five systematic reviews, 15 studies were included, which were described in 14 reports (with one report describing the findings from two separate studies). The subsequent search for clinical trials published after those five systematic reviews were completed yielded 2952 records. After removal of duplicates, 2332 records were screened and 14 were selected for full-text review, from which two new reports describing two RCTs were included [29, 42]. One record [29] was included (which was published after the search cut-off date, but prior to meta-analysis), having been identified from information within a qualitative study [43] identified in our search results (figure 1). Overall, a total of 16 reports describing 17 studies were included in the current systematic review (table 1).
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram. #: one report described the findings from two separate studies; hence 16 reports describe findings from 17 studies.
TABLE 1.
Characteristics of included studies
| First author, year [ref.] | Study design | Study setting | Population | Country | Sample size | Age years | Women % | Intervention | Comparator | Included outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Abdallah, 2017 [44] | Crossover | Laboratory-based exercise measures | COPD | Canada | 20 | 63.6±7.1 | 25.0 | Single oral dose immediate-release morphine sulphate (0.1 mg·kg−1) | Placebo | Breathlessness: Borg intensity at iso-time Self-reported nausea/vomiting |
| Abernethy, 2003 [45] | Crossover | At-home daily-life measures | Mixed (88% COPD) | Australia | 48 | 76±5 | 27.0 | 20 mg oral morphine sulphate daily for 4 days | Placebo | Breathlessness: 10 cm VAS intensity Self-reported constipation |
| Currow, 2020 [31] | Parallel | At-home daily-life measures | Mixed (58% COPD) | Australia | Morphine 145 Placebo 139 |
Morphine 74.0±9.6 Placebo 74.5±9.1 |
Morphine 35.9 Placebo 37.4 |
20 mg oral morphine daily for 7 days | Placebo | Breathlessness: 10 cm VAS intensity now HRQoL: EORTC-QLQ-C15 PAL Self-reported nausea/vomiting Self-reported drowsiness Self-reported constipation |
| Eiser, 1991 (A) [46] | Crossover | Laboratory-based exercise measures | COPD | United Kingdom | 10 | 49–79 | 30.0 | 5 mg oral diamorphine every 6 h for 2 weeks | Placebo | Breathlessness: 10 cm VAS post-6MWT Self-reported nausea/vomiting |
| Eiser, 1991 (B) [46] | Crossover | Laboratory-based exercise measures | COPD | United Kingdom | 8 | 49–79 | 40.0 | 2 doses of 7.5 mg oral diamorphine 5 h apart, for 2 days | Placebo | Breathlessness: 10 cm VAS post-6MWT PaCO2 1 h post-dose PaO2 1 h post-dose Self-reported nausea/vomiting |
| Ekström, 2022 [29] | Parallel | At-home daily-life measures | COPD | Australia | Morphine 51 Placebo 50 |
Morphine 73 (67–78) Placebo 72 (66–76) |
Morphine 51 Placebo 56 |
16 mg·day−1 oral extended-release morphine for week 1 | Placebo | Breathlessness: NRS intensity HRQoL: CAT Self-reported nausea/vomiting Self-reported drowsiness Self-reported constipation |
| Ferreira, 2018 [42] | Crossover | At-home daily-life measures | PAH | Australia | 19 | 64±11 | 70 | 20 mg oral kapanol daily for 7 days | Placebo | Breathlessness: 10 cm VAS intensity now Self-reported nausea/vomiting Self-reported drowsiness Self-reported constipation |
| Ferreira, 2020 [47] | Parallel | At-home daily-life measures | Mixed (60% COPD) | Australia | Morphine 74 Placebo 81 |
Morphine 74.49±8.35 Placebo 74.83±8.95 |
Morphine 35.1 Placebo 34.6 |
15 mg oral controlled-release oxycodone daily for 7 days | Placebo | Breathlessness: 10 cm VAS intensity now HRQoL: EORTC-QLQ-C15 PAL Self-reported nausea/vomiting Self-reported drowsiness Self-reported constipation |
| Johnson, 1983 [48] | Crossover | Laboratory-based exercise measures At-home daily-life measures |
Mixed (not specified) | United Kingdom | 18 | 64.9±9.1 | 16.7 | 15 mg dihydrocodeine for 1 week, taken 30 min before exercise up to 3 times per day | Placebo | Breathlessness: 10 cm VAS at iso-time Breathlessness: 10 cm VAS intensity (evening) |
| Kronborg-White, 2020 [49] | Parallel | At-home daily-life measures | ILD | Denmark | Morphine 18 Placebo 18 |
Morphine 72.5 (69.8–79.8) Placebo 75.2 (72.7–77.8) |
Morphine 16.6 Placebo 16.6 |
Oral morphine drops, 20 mg·mL−1 5 drops (equivalent to 5 mg) taken four times per day, i.e. 20 mg daily for 7 days Up to 5 extra drops per day up to four times per day permitted |
Placebo | Breathlessness: 10 cm VAS intensity HRQoL: KBILD Cough: LCS PaCO2 Self-reported nausea/vomiting Self-reported constipation |
| Light, 1989 [50] | Crossover | Laboratory-based exercise measures | COPD | USA | 13 | Mean 65.9, range 58–70 | 0.0 | Single dose of oral morphine 0.8 mg·kg−1 60 min before exercise test | Placebo | Breathlessness: Borg at iso-time PaCO2 at iso-time PaO2 at iso-time |
| Light, 1996 [51] | Crossover | Laboratory-based exercise measures | COPD | USA | 7 | 66.4±3.25 | 0.0 | 30 mg oral morphine | Placebo | Breathlessness: Borg at iso-time |
| Poole, 1998 [52] | Crossover | At-home daily-life measures | COPD | USA | 16 | 70.7±6.4 | 31.2 | 10 mg sustained-release morphine sulphate titrated to maximum of 2 tablets twice daily for 6 weeks: 10–40 mg | Placebo | Breathlessness: CRQ dyspnoea subscale HRQoL: CRQ Self-reported nausea/vomiting Self-reported drowsiness Self-reported constipation |
| Rice, 1987 [53] | Crossover | At-home daily-life measures | COPD | USA | 7 | 59–79 | 0.0 | 30 mg codeine taken 4 times daily for 1 month | 25 mg promethazine 4 times daily for 1 month | Breathlessness: 10 cm VAS past 24 h PaCO2 Self-reported drowsiness Self-reported constipation |
| Verberkt, 2020 [30] | Parallel | At-home daily-life measures | COPD | The Netherlands | Morphine 54 Placebo 57 |
Morphine 65.0±8.0 Placebo 65.7±8.0 |
Morphine 48 Placebo 44 |
10 mg oral SR morphine twice daily for 4 weeks: 20 mg Could be titrated to 3 times daily: 30 mg |
Placebo | Breathlessness: NRS past 24 h HRQoL: CAT Cough: CAT PaCO2 PaO2 Self-reported nausea/vomiting Self-reported drowsiness Self-reported constipation |
| Woodcock, 1981 [54] | Crossover | At-home exercise measures | COPD | United Kingdom | 12 | Mean 62 | 16.7 | Dihydrocodeine 1 mg·kg−1 in 200 mL bitter-lemon drink taken 45 min before a treadmill test | Placebo | Breathlessness: 10 cm VAS at iso-load Self-reported nausea/vomiting |
| Woodcock, 1982 [55] | Crossover | At-home daily-life measures | COPD | United Kingdom | 11 | Not reported | Not reported | 60 mg dihydrocodeine 3 times daily for 2 weeks | Placebo |
PaCO2 PaO2 Self-reported nausea/vomiting Self-reported drowsiness Self-reported constipation |
Data are presented as n, mean±sd, range or median (interquartile range), unless otherwise stated. VAS: visual analogue scale; HRQoL: health-related quality of life; EORTC QLQ-C15 PAL: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 15 Palliative Care; 6MWT: 6-min walk test; PaCO2: arterial carbon dioxide tension; PaO2: arterial oxygen tension; NRS: numerical rating scale; CAT: COPD Assessment Test; PAH: pulmonary arterial hypertension; ILD: interstitial lung disease; KBILD: King's Brief Interstitial Lung Disease; LCS: Leicester Cough Scale; CRQ: Chronic Respiratory Disease Questionnaire.
Study characteristics
Seven of the 17 included studies described laboratory-based exercise studies with 88 participants [44, 46, 48, 50, 51, 54 ] and 11 described at-home studies with 806 participants [30, 31, 40, 42, 45, 47–49, 52, 53, 55]. Notably, one study reported outcomes both in the laboratory and home settings, and therefore the data were included in both meta-analyses where appropriate [48].
All laboratory-based exercise studies were small crossover studies (with ≤20 participants), in which opioids were compared to an identically administered placebo. Six studies included only participants with COPD [44, 46, 50, 51, 54] and one study included a mixed cohort, but did not specify the primary conditions of the participants included [48].
Of the at-home studies, five were parallel-arm studies and six were crossover-design studies. All but one at-home study compared opioids to an identically administered placebo, with one study comparing opioids to promethazine [53]. At-home studies were a mix of small and large studies; seven had <50 participants [42, 45, 48, 49, 52, 53, 55] and the remaining four studies had >100 participants [29–31, 47]. Five studies included only participants with COPD [29, 30, 52, 53, 55]; one included only participants with ILD [49]; one included only participants with PAH [42]; and four studies included mixed cohorts [31, 45, 47, 48]. Of the mixed-cohort studies, most contained a majority of participants with COPD [31, 45, 47] and one did not specify the primary conditions of the included participants [48].
Interventions
Of the seven laboratory-based exercise studies, three studies administered a single dose of oral morphine ranging from 0.1 to 0.8 mg·kg−1, with maximum doses ranging from 10 to 30 mg [44, 50, 51]. Two studies administered a single oral dose of dihydrocodeine at either 1 mg·kg−1 [54] or a flat dose of 15 mg [48], and two studies that were reported together administered multiple oral doses of diamorphine, either 5 mg taken every 6 h for 2 weeks, or two doses of 7.5 mg taken 5 h apart on a single day [46].
The exercise tests administered varied. Three studies utilised cycle ergometers: two were incremental symptom-limited tests [50, 51] and one was a symptom-limited constant-load test at 75% peak power output [44]. Two studies administered 6-min walk tests (6MWT) [46], one of which included in addition a treadmill test. One study administered an incrementally increasing speed treadmill test [48] and one study utilised both a treadmill test and a cycle ergometer test [54] with the post-treadmill measure included in this meta-analysis.
Of the 11 at-home studies, seven administered morphine for a period of ≥4 days and up to 6 weeks [29–31, 42, 45, 49, 52]. In all but one of those seven studies, extended-release formulations of morphine were self-administered [49]. The typical dose of oral morphine was 20 mg per day, with two studies titrating up to 30 mg [30] or 40 mg [52] per day. Two studies administered oral dihydrocodeine up to either 45 mg per day for 1 week [48] or 180 mg per day for 2 weeks [55]. One study administered 15 mg per day oral extended-release oxycodone for 7 days [47], and one study administered 120 mg codeine per day for 1 month [53].
Risk of bias
Of the five included previous systematic reviews, no major sources of bias were identified using the AMSTAR-2 checklist. Of the 17 included studies, five had a high risk of bias in one domain and an additional 10 had an unclear risk of bias in up to three domains (supplementary figures S1 and S2). The domain of “selective reporting” was the most frequently downgraded domain, with two studies deemed high risk due to inconsistencies between pre-specified outcomes and reported results [30, 54], and 12 studies deemed unclear risk due to a lack of prospectively registered outcome reporting plans [31, 42, 45–48, 50–53, 55]. Half of the included studies had unclear risk of “selection bias” due to insufficient descriptions of random sequence generation and allocation concealment studies [46, 50–55]. Three studies had high risk of “other bias”; one study included participants that were not consistent with the reported criteria [53], and two studies were underpowered analyses stemming from discontinued arms of larger clinical trials [42, 47]. One study had an unclear risk for “detection bias” due to insufficient blinding of outcome assessments [44]. All included studies had a low risk of “performance bias” and “attrition bias”.
Two meta-analyses (at-home studies reporting breathlessness pragmatically during daily life including either evening (figure 2) or morning measures (supplementary figure S5)) contained ≥10 studies and were examined for publication bias using a funnel plot. No important asymmetry was identified on either funnel plot (supplementary figure S3 and supplementary figure S4).
FIGURE 2.
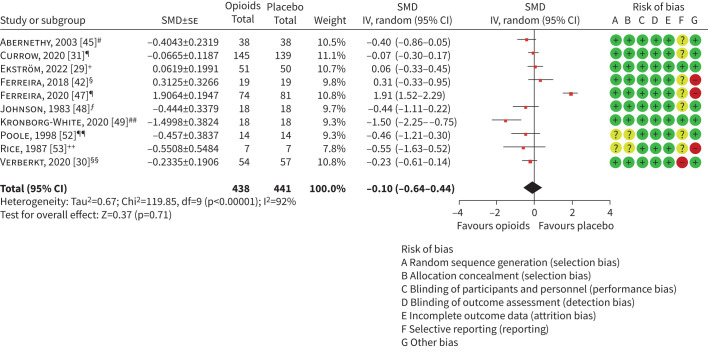
At-home studies reporting breathlessness pragmatically during daily life. SMD: standardised mean difference; IV: inverse variance. #: 10 cm visual analogue scale (VAS) breathlessness intensity (final evening relative to baseline); ¶: 10 cm VAS breathlessness intensity now (days 5–7 average of mean morning/evening scores relative to baseline; +: numerical rating scale (NRS) intensity of breathlessness (16 mg per day dose, days 5–7 average scores relative to days −3 to −1 average score; §: 10 cm VAS breathlessness intensity now (final evening score relative to baseline); ƒ: 10 cm VAS breathlessness (final early evening relative to baseline from alternating weeks’ period; ##: 10 cm VAS breathlessness during last hour (change from baseline to follow-up); ¶¶: Chronic Respiratory Disease Questionnaire (CRQ) dyspnoea subscale (change from baseline to 6 weeks); ++: 10 cm VAS breathlessness during past 24 h (change from baseline to follow-up); §§: NRS over past 24 h (change from baseline to follow-up).
Effects of interventions
Primary outcome: breathlessness
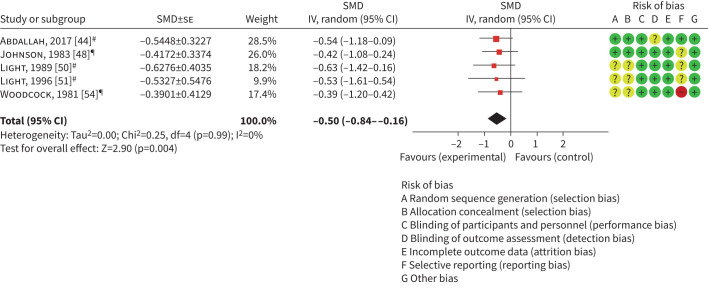
A meta-analysis of five laboratory-based exercise studies (n=70 participants) measuring breathlessness outcomes at iso-load or iso-time demonstrated a moderate and statistically significant treatment effect in favour of opioids (SMD −0.50, 95% CI −0.84– −0.16, I2=0%; figure 3). This translated to a ∼10 mm reduction in breathlessness on a 10 cm visual analogue scale (VAS), which suggests that this effect is clinically significant [56]. The remaining two laboratory-based exercise studies with 18 participants [46] measured breathlessness following a 6MWT and were excluded from the meta-analysis, as the exertion level could not be standardised. Similarly, one home-based study [49] reported a breathlessness measure following a 6MWT and was also excluded from the meta-analysis.
FIGURE 3.
Laboratory-based exercise studies reporting breathlessness at iso-time or iso-load. SMD: standardised mean difference; IV: inverse variance. #: Borg intensity at iso-time; ¶: 10 cm visual analogue scale breathlessness at iso-load.
10 at-home studies with 795 participants reported breathlessness intensity outcomes measured in daily life. Breathlessness was measured at morning, evening, both or at an unspecified time. For studies reporting both morning and evening scores separately, the evening score was included in the meta-analysis, which demonstrated no statistically or clinically significant treatment effect in favour of opioids compared to placebo or promethazine comparator, with considerable heterogeneity identified (SMD −0.10, 95% CI −0.64–0.44, I2=92%; figure 2). Given the considerable heterogeneity, we also conducted a narrative synthesis for this primary outcome. Of the 10 included studies, seven reported no statistically significant change in breathlessness; two showed statistically significant effects in favour of opioids [45, 48]; and one favoured the comparator [42]. Of the two that favoured opioid, one was below the clinically significant threshold [45]. A separate meta-analysis was conducted using morning scores for studies where both morning and evening measures were reported separately, which detected no significant treatment effect, and high heterogeneity (SMD −0.10, 95% CI −0.64–0.43, I2=92%; supplementary figure S5).
Secondary outcomes
Important outcome: HRQoL
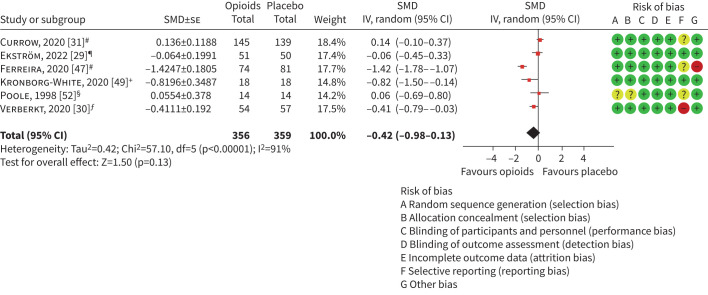
No laboratory-based exercise studies reported outcomes for HRQoL. Six at-home studies with 703 participants reported outcomes for HRQoL. Where higher scores represented better HRQoL (specifically, King's Brief Interstitial Lung Disease [57] and Chronic Respiratory Disease Questionnaire [58]), the direction of the data was reversed to reflect the direction of effect. The meta-analysis detected no statistically or clinically significant treatment effects and heterogeneity was considerable (SMD −0.42, 95% CI −0.98–0.13, I2=91%, figure 4).
FIGURE 4.
At-home studies reporting health-related quality of life. SMD: standardised mean difference; IV: inverse variance. #: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 15 Palliative Care (change from baseline); ¶: COPD Assessment Test (CAT) (16 mg per day dose, change from baseline); +: King's Brief Interstitial Lung Disease (change from baseline); §: Chronic Respiratory Disease Questionnaire (change from baseline); ƒ: CAT (change from baseline).
Important outcome: cough
No laboratory-based exercise studies reported outcomes for cough. Two at-home studies with a total of 147 participants reported cough outcomes using either the Leicester Cough Questionnaire (measuring chronic cough-related quality of life) or COPD Assessment Test cough subdomain (impact on daily life activities). In the meta-analysis, no statistically significant treatment effects were identified, but considerable heterogeneity was detected (SMD −1.42, 95% CI −3.99–1.16, I2=96%; supplementary figure S6).
Important outcome: arterial blood gases
Two laboratory-based exercise studies with 21 participants reported arterial carbon dioxide tension (PaCO2) (supplementary figure S7) and arterial oxygen tension (PaO2) (supplementary figure S8) measurements at any time during or after exercise. The direction of the treatment effect as plotted on the forest plot for PaO2 (supplementary figure S8) was reversed to reflect the direction of clinical improvement. The meta-analysis of PaCO2 did not find a significant effect, but was trending towards favouring placebo compared to opioids (SMD 0.63, 95% CI 0.00–1.26, I2=0%; supplementary table S3). This was equivalent to ∼5.2 mmHg rise in PaCO2 with opioids, which is clinically significant. A separate meta-analysis of PaO2 did not find any statistically or clinically significant effect (SMD −0.52, 95% CI −1.14–0.10, I2=0%, p=0.10).
Four at-home studies with 165 participants reported PaCO2 measurements (supplementary figure S9). The meta-analysis demonstrated a large and statistically significant effect in favour of placebo or promethazine comparator compared to opioids with considerable heterogeneity (SMD 0.86, 95% CI 0.03–1.69, I2=80%; supplementary table S4). This was equivalent to ∼2.2 mmHg rise in PaCO2 with opioids, which is not clinically significant. Two at-home studies with 122 participants reported PaO2 with no statistically or clinically significant treatment effects detected in the meta-analysis (SMD −0.22, 95% CI −0.56–0.12, I2=0%; supplementary figure S10).
Important outcome: adverse events
Treatment-emergent adverse events were commonly reported and were specifically cited as a cause for withdrawal of participants receiving active opioid interventions in 10 out of 11 at-home [30, 31, 42, 45, 47, 49, 52, 53] and three out of seven laboratory-based exercise studies [44, 46]. Adverse events experienced in opioid intervention arms were often higher grade and were a source of “moderate to severe distress” for participants [31, 45, 47]. Three studies specifically described treatment-emergent adverse events in opioid treatment arms that were serious in nature [29, 44, 53], including hospitalisation or death, in up to 33% of participants [29].
Four laboratory-based exercise studies with 60 participants reported frequency of nausea or vomiting adverse events with no statistically significant treatment effects detected in the meta-analysis (OR 3.79, 95% CI 0.75–19.18, I2=0%, p=0.11; supplementary figure S11). When studies reported nausea or vomiting as separate events, the events with the greatest frequency were included in the meta-analysis. No other adverse events of interest were reported in laboratory-based exercise studies.
Eight at-home studies with 733 participants reported frequency of nausea or vomiting as adverse events (supplementary figure S12). The meta-analysis detected a statistically significant increase in the frequency of nausea or vomiting events in people receiving opioids compared with placebo with substantial heterogeneity (OR 3.32, 95% CI 1.70–6.51, I2=54%). Eight at-home studies with 704 participants reported frequency of drowsiness (sometimes referred to as “somnolence”) as adverse events (supplementary figure S13). The meta-analysis detected a statistically significant increase in the frequency of drowsiness events in people receiving opioids compared with placebo or promethazine comparator (OR 1.37, 95% CI 1.01–1.86, I2=0%).
Nine at-home studies with 781 participants reported frequency of constipation as adverse events (supplementary figure S14). The meta-analysis detected a statistically significant increase in the frequency of constipation events in people receiving opioids compared with placebo with substantial heterogeneity (OR 3.08, 95% CI 1.69–5.61, I2=57%). Seven out of the 11 at-home studies included described prophylactic or as-needed prescription of laxatives alongside the blinded treatment, all of which contributed data to the constipation adverse event meta-analysis.
Certainty of evidence
The certainty of evidence for all included outcomes was assessed using the GRADE criteria and were found to be generally of “low” to “very low” quality (supplementary table S3 and supplementary table S4). The important outcomes of cough, HRQoL and nausea/vomiting adverse events in at-home studies were each graded as “low”. All other outcomes, including the critical outcome of breathlessness (as measured in both laboratory-based exercise studies and at-home studies) were graded as “very low”.
Key reasons for downgrading certainty of evidence included a high proportion of studies with unclear or high risk of bias in one or more domains, a high proportion of studies with small sample sizes and a lack of reported washout periods in crossover design studies. Washout periods between opioid and placebo exposures were either not described or were explicitly stated as not included in the study design for three out of seven included crossover laboratory-based exercise studies [46, 48] and three out of six included crossover community-based studies [45, 48, 55].
Sensitivity analyses
Sensitivity analyses were conducted as per protocol to examine the effects of methodological quality on the pooled estimates. Two meta-analyses (breathlessness at iso-time or iso-load, and nausea or vomiting data from laboratory-based exercise studies) were identified which each included one study with unclear risk of bias for the domains of blinding [44]. Removal of this study's data from both meta-analyses did not alter the estimates of the overall treatment effect. A further sensitivity analysis was performed excluding the one study which used promethazine as opposed to placebo for the comparator intervention [53]. Removal of this study's data from two out of three separate meta-analyses (breathlessness in daily life and drowsiness measures in at-home studies) did not alter the estimates of the overall treatment effect. For the third meta-analysis (PaCO2 measurements in arterial blood gases) from which this study was removed, the pooled estimate of the treatment effect continued to demonstrate a large effect in favour of placebo compared to opioids; however, this was no longer statistically significant (SMD 0.85, 95% CI −0.18–1.89, n=158, I2=86%).
Discussion
Main findings
This systematic review and meta-analysis identified that opioids, when compared to placebo, had no statistically or clinically significant effect on breathlessness outcomes measured pragmatically in daily life at home in people with nonmalignant serious respiratory illness. When administered in a laboratory-based setting, systemic opioids, compared to placebo, led to a statistically and clinically significant improvement in breathlessness outcomes measured under standardised exertion at iso-time or iso-load. No statistically or clinically significant effects of opioids on HRQoL or cough were detected when self-administered regularly at home. Importantly, when opioids were administered over some days at home, trial participants with serious respiratory illness experienced a significant increase in the frequency of adverse events, including drowsiness, constipation, nausea and vomiting. Adverse events were a considerable driver of withdrawal from studies, and in one RCT 33% of people treated with morphine experienced serious adverse events including hospitalisation or death [29]. Based on these results, the European Respiratory Society guideline taskforce made a conditional recommendation against the prescription of opioids for the treatment of chronic breathlessness in people with nonmalignant serious respiratory illness [33].
The findings from our systematic review and meta-analysis are consistent with the trend of previous systematic reviews, which have reported decreasing beneficial treatment effects with each subsequent review. It should be noted that some prior systematic reviews, including a recently published systematic review from Liu et al. [59], have included both nebulised and orally administered opioids with intentions to consider these as two separate groups [38, 40]. Nebulised opioids were omitted from this review and meta-analysis as the balance of evidence has previously demonstrated no beneficial treatment effect of nebulised opioids [60, 61].
The systematic review from Liu et al. [59] reported a beneficial treatment effect of short-acting opioids on breathlessness in crossover RCTs. However, their systematic review was limited to people with COPD and included both nebulised and systemic opioids. Additionally, Liu et al. [59] did not distinguish between studies where breathlessness was measured upon exertion and those where breathlessness was measured pragmatically during daily life, with most of the data (70% weighting) in their meta-analysis derived from studies measuring exertional breathlessness under laboratory conditions. This is consistent with our systematic review, which identified a small beneficial treatment effect of opioids on exertional breathlessness in the laboratory setting. Evidence regarding short-acting opioids, mostly administered as single doses with outcomes measured primarily under exercise conditions, cannot be extrapolated to make recommendations for the prophylactic treatment of breathlessness experienced in daily life. Indeed, Liu et al. [59] also reported a lack of beneficial treatment effect of sustained-release opioids on breathlessness for people with COPD, which is consistent with our findings.
Previous systematic reviews have highlighted the need for larger RCTs that are adequately powered to detect treatment effects [18, 40, 41]. Importantly, our systematic review and meta-analysis addresses this issue, as we included many recently published, large randomised controlled trials [29–31, 47], and it is increasingly clear that the beneficial treatment effects of opioids on breathlessness or HRQoL (as measured using validated tools in daily life) are minimal, while the adverse events are significant [29]. Despite this, the overall certainty of evidence included in this meta-analysis was graded “very low” to “low”, which reflects in part the high proportion of RCTs with small sample sizes.
The treatment-emergent adverse events reported in the included studies were often mild and self-limiting on withdrawal of opioids [45, 62], though it should be noted that even low-grade adverse events can be detrimental to quality of life and are important to patients’ perceptions of side-effects so should not be disregarded [63, 64]. Additionally, serious adverse events were not uncommon among larger trials; data from the Breathlessness Exertion and Morphine Sulphate study [29] indicated that one in three participants treated with morphine (46 out of 139) developed serious adverse events, including hospitalisation and death. Prior systematic reviews have similarly highlighted these safety risks and cautioned against recommendations for sustained-release opioid use for the treatment of breathlessness in people with COPD [59]. These risks should not be understated and are reflected in increased mortality associated with high-dose opioid use in population-level observational studies, which are more adequately powered to evaluate safety [65, 66]. Many consumers have negative perceptions of using opioids for the treatment of breathlessness and express concerns regarding safe use, respiratory depression, substance misuse, dependence and addiction, stigma and the association of opioids with death and dying [22–25, 67]. Additionally, opioids may affect capacity to drive, and cause many predictable adverse events, which are unacceptable or challenging for some patients [68]. The inability to recruit patients to some of the trials included in this systematic review also highlights negative community perceptions to opioids [30, 42, 47] and the need to offer and make more accessible nonpharmacological management approaches to breathlessness.
Strengths and limitations
This review utilised an approach of separating studies into two distinct settings for opioid delivery: regular daily use at home (with symptom score outcomes measured pragmatically) or acute dosing in a controlled exercise laboratory environment (with outcome measures taken specifically at iso-time). This is an important distinction, as breathlessness experienced in daily life and breathlessness during an incremental exercise test are related but distinct experiences, which may reflect differences in disease aetiology and severity, as well as deconditioning [69]. Several early studies examining opioids to treat breathlessness focused on exertional breathlessness under laboratory conditions only [46, 48, 50, 51, 54]. While the current meta-analysis indeed shows a small-to-moderate beneficial treatment effect from opioids used to treat exertional breathlessness in a laboratory setting, no benefit was found when opioids were taken regularly at home including no effect on HRQoL. This finding is crucial, as we prescribe treatments in the hope that they improve the symptoms that our patients experience in daily life.
An important limitation of this review is the relative homogeneity of included participants. Most studies primarily included people with COPD, with limited evidence regarding other nonmalignant serious respiratory illnesses. Participants were predominantly male and there was little to no reporting of other social determinants of health [70], such as ethnicity or socioeconomic status. Additionally, there are limited data on the use of opioids to treat breathlessness in people with nonmalignant serious respiratory illness who are at the very end of life. Given that many physicians consider prescribing opioids only in an end-of-life, palliative care setting [25–27, 71, 72], it is important to not rule out a potential beneficial treatment effect in this circumstance. Notably, RCTs focusing on people at the very end of life present a number of ethical and logistical challenges that limit the feasibility of conducting large-scale trials in such a cohort.
Additionally, considerable statistical heterogeneity was detected in several of the included meta-analyses within this systematic review owing in part due to variation in study designs. Calculation of 95% confidence intervals for the heterogeneity statistics was not possible within the RevMan5 software package. This heterogeneity was considered within the GRADE assessment of the certainty of the evidence and contributed to the overall “low” to “very low” certainty. For the primary outcome of breathlessness, we have included a narrative synthesis to supplement the meta-analysis which had high levels of heterogeneity.
Finally, there is a scarcity of evidence on the impact of opioids on other important symptoms experienced by people with nonmalignant serious respiratory illness, particularly cough. A recent crossover RCT (n=41 participants) published after the completion of the current meta-analysis, examining the effects of systemic opioids on cough in people with idiopathic pulmonary fibrosis (IPF), demonstrated a 75% decrease in daytime cough frequency with extended-release nalbuphine compared to a 23% decrease in people receiving placebo [73]. An additional crossover RCT protocol examining the effects of opioids on cough with a recruitment target of 44 participants with IPF has also been published [74]. These studies in conjunction with the existing evidence on cough may provide more robust evidence on the safety and efficacy of opioids for the treatment of cough in people with serious respiratory illness.
Conclusion
The systematic review and meta-analysis identified benefit from opioids on exertional breathlessness when induced in a laboratory setting only (very low certainty of evidence). Opioids administered regularly at home did not have any beneficial impact on breathlessness experienced in daily life in people with serious respiratory illness (very low certainty of evidence) and did not improve HRQoL. Treatment-emergent side-effects due to opioids were common, with serious adverse events occurring including hospitalisation and death. Data are lacking regarding any benefits that opioids may have on other symptoms (particularly cough), in people with illnesses other than COPD, and in people at the very end of life. While opioids may form part of an individualised, carefully monitored treatment plan for some people with nonmalignant serious respiratory illness, nonpharmacological approaches remain the mainstay of our treatment approach for persisting breathlessness.
Points for clinical practice
Opioids administered in a laboratory setting improved exertional breathlessness during standardised exercise testing; however, opioids administered regularly at home did not have any beneficial impact on breathlessness experienced in daily life in people with serious respiratory illness.
Treatment-emergent side-effects due to opioids were common, with serious adverse events including hospitalisation and death occurring.
Based on these results, the European Respiratory Society guideline taskforce made a conditional recommendation against the prescription of opioids for the treatment of chronic breathlessness in people with nonmalignant serious respiratory illness.
While opioids may form part of an individualised, carefully monitored treatment plan for some people with nonmalignant serious respiratory illness, nonpharmacological approaches remain the mainstay of our treatment approach for persisting breathlessness.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Protocol and full search strategy ERR-0265-2023.SUPPLEMENT (302.7KB, pdf)
Supplementary figures and tables ERR-0265-2023.SUPPLEMENT2 (516.3KB, pdf)
Footnotes
Provenance: Commissioned article, peer reviewed.
Number 1 in the Series “Symptom management for advanced lung disease” Edited by Anne E. Holland, Magnus Ekström and Natasha E. Smallwood
Conflict of interest: N.E. Smallwood reports grants from NHMRC, MRFF, Cancer Council Australia, Fisher & Paykel Healthcare (FPH), Windermere Foundation, Lung Foundation Australia, Lord Mayor's Foundation Melbourne and Bethlehem Griffiths Foundation, consulting fees from The Limbic and Orchard Consulting, lecture honoraria from GlaxoSmithKline, Boehringer Ingelheim, AstraZeneca, FPH and Health Ed, travel support from Chiesi and Boehringer Ingelheim, leadership roles as Board Director and past state president of the Thoracic Society of Australia and New Zealand, Board Director of Victorian Doctors’ Program and co-chair of the guidelines committee for European Respiratory Society; and receipt of equipment from FPH, outside the submitted work. M. Wijsenbeek reports grants from The Netherlands Organisation for Health Research and Development, The Dutch Lung Foundation, The Dutch Pulmonary Fibrosis organization, Sarcoidosis.nl, Boehringer Ingelheim, Hoffman la Roche and AstraZeneca-Daiichi, consulting fees from AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Galapagos, Galecto, GSK, Hoffman la Roche, Horizon therapeutics, Kinevant Sciences, Molecure, Nerre Therapeutics, Novartis, PureTech Health, Thyron, Trevi and Vicore, lecture honoraria from Boehringer Ingelheim, CSL Behring, Hoffman la Roche and Novartis, travel support from Boehringer Ingelheim, GSK, Hoffman la Roche and Galapagos, advisory board participation with Galapagos, and leadership roles as Chair of the Idiopathic Interstitial Pneumonia group of the European Respiratory Society, Member of the board of the Netherlands Respiratory Society, Member of the scientific advisory board of the European Idiopathic Pulmonary Fibrosis and related disorders federation, Chair of the educational committee of the European Reference Network for rare Lung Diseases, and membership of the advisory board of the Dutch Lungfibrosis and Sarcoidosis patient associations, outside the submitted work. A-M. Russell reports consulting fees from Boehringer Ingelheim, and lecture honoraria from Boehringer Ingelheim, Hoffman La Roche and the Irish Lung Fibrosis Association, outside the submitted work. A.E. Holland is President of Thoracic Society of Australia and New Zealand, an unpaid position unrelated to the present work. All other authors have nothing to disclose.
References
- 1.Kelley AS. Defining “serious illness”. J Palliat Med 2014; 17: 985. doi: 10.1089/jpm.2014.0164 [DOI] [PubMed] [Google Scholar]
- 2.Rantala HA, Leivo-Korpela S, Lehtimäki L, et al. . Assessing symptom burden and depression in subjects with chronic respiratory insufficiency. J Palliat Care 2022; 37: 134–141. doi: 10.1177/08258597211049592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Thoracic Society . Dyspnea. Mechanisms, assessment, and management: a consensus statement. Am J Respir Crit Care Med 1999; 159: 321–340. doi: 10.1164/ajrccm.159.1.ats898 [DOI] [PubMed] [Google Scholar]
- 4.Gysels MH, Higginson IJ. The lived experience of breathlessness and its implications for care: a qualitative comparison in cancer, COPD, heart failure and MND. BMC Palliat Care 2011; 10: 15. doi: 10.1186/1472-684X-10-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swetz KM, Shanafelt TD, Drozdowicz LB, et al. . Symptom burden, quality of life, and attitudes toward palliative care in patients with pulmonary arterial hypertension: results from a cross-sectional patient survey. J Heart Lung Transplant 2012; 31: 1102–1108. doi: 10.1016/j.healun.2012.08.010 [DOI] [PubMed] [Google Scholar]
- 6.Janson C, Marks G, Buist S, et al. . The impact of COPD on health status: findings from the BOLD study. Eur Respir J 2013; 42: 1472–1483. doi: 10.1183/09031936.00153712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Donnell DE, Milne KM, James MD, et al. . Dyspnea in COPD: new mechanistic insights and management implications. Adv Ther 2020; 37: 41–60. doi: 10.1007/s12325-019-01128-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson MJ, Yorke J, Hansen-Flaschen J, et al. . Towards an expert consensus to delineate a clinical syndrome of chronic breathlessness. Eur Respir J 2017; 49: 1602277. doi: 10.1183/13993003.02277-2016 [DOI] [PubMed] [Google Scholar]
- 9.Blinderman CD, Homel P, Billings JA, et al. . Symptom distress and quality of life in patients with advanced chronic obstructive pulmonary disease. J Pain Symptom Manage 2009; 38: 115–123. doi: 10.1016/j.jpainsymman.2008.07.006 [DOI] [PubMed] [Google Scholar]
- 10.Glaspole IN, Chapman SA, Cooper WA, et al. . Health-related quality of life in idiopathic pulmonary fibrosis: data from the Australian IPF Registry. Respirology 2017; 22: 950–956. doi: 10.1111/resp.12989 [DOI] [PubMed] [Google Scholar]
- 11.Kreuter M, Swigris J, Pittrow D, et al. . Health related quality of life in patients with idiopathic pulmonary fibrosis in clinical practice: insights-IPF registry. Respir Res 2017; 18: 139. doi: 10.1186/s12931-017-0621-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutchinson A, Pickering A, Williams P, et al. . Breathlessness and presentation to the emergency department: a survey and clinical record review. BMC Pulm Med 2017; 17: 53. doi: 10.1186/s12890-017-0396-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly AM, Keijzers G, Klim S, et al. . An observational study of dyspnea in emergency departments: the Asia, Australia, and New Zealand Dyspnea in Emergency Departments Study (AANZDEM). Acad Emerg Med 2017; 24: 328–336. doi: 10.1111/acem.13118 [DOI] [PubMed] [Google Scholar]
- 14.Bajwah S, Higginson IJ, Ross JR, et al. . The palliative care needs for fibrotic interstitial lung disease: a qualitative study of patients, informal caregivers and health professionals. Palliat Med 2013; 27: 869–876. doi: 10.1177/0269216313497226 [DOI] [PubMed] [Google Scholar]
- 15.Carvajalino S, Reigada C, Johnson MJ, et al. . Symptom prevalence of patients with fibrotic interstitial lung disease: a systematic literature review. BMC Pulm Med 2018; 18: 78. doi: 10.1186/s12890-018-0651-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahler DA. Opioids for refractory dyspnea. Expert Rev Respir Med 2013; 7: 123–134. doi: 10.1586/ers.13.5 [DOI] [PubMed] [Google Scholar]
- 17.Wiseman R, Rowett D, Allcroft P, et al. . Chronic refractory dyspnoea – evidence based management. Aust Fam Physician 2013; 42: 137–140. [PubMed] [Google Scholar]
- 18.Barnes H, McDonald J, Smallwood N, et al. . Opioids for the palliation of refractory breathlessness in adults with advanced disease and terminal illness. Cochrane Database Syst Rev 2016; 3: CD011008. doi: 10.1002/14651858.CD011008.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banzett RB, Mulnier HE, Murphy K, et al. . Breathlessness in humans activates insular cortex. Neuroreport 2000; 11: 2117–2120. doi: 10.1097/00001756-200007140-00012 [DOI] [PubMed] [Google Scholar]
- 20.Pattinson KTS, Governo RJ, MacIntosh BJ, et al. . Opioids depress cortical centers responsible for the volitional control of respiration. J Neurosci 2009; 29: 8177––8186.. doi: 10.1523/JNEUROSCI.1375-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrovic P, Kalso E, Petersson KM, et al. . Placebo and opioid analgesia – imaging a shared neuronal network. Science 2002; 295: 1737–1740. doi: 10.1126/science.1067176 [DOI] [PubMed] [Google Scholar]
- 22.Moran T, Zentner D, Wong J, et al. . Chronic breathlessness in advanced cardiorespiratory disease: patient perceptions of opioid use. BMJ Support Palliat Care 2023; 13: e334–e343. doi: 10.1136/bmjspcare-2020-002853 [DOI] [PubMed] [Google Scholar]
- 23.Politis J, Eastman P, Le B, et al. . Managing severe chronic breathlessness in chronic obstructive pulmonary disease is challenging for general practitioners. Am J Hosp Palliat Care 2021; 38: 472–479. doi: 10.1177/1049909120959061 [DOI] [PubMed] [Google Scholar]
- 24.Russo L, Willis K, Smallwood N. Assisting people with their living, not their dying: health professionals’ perspectives of palliative care and opioids in ILD. Am J Hosp Palliat Care 2022; 39: 211–219. doi: 10.1177/10499091211018664 [DOI] [PubMed] [Google Scholar]
- 25.Smallwood N, Currow D, Booth S, et al. . Differing approaches to managing the chronic breathlessness syndrome in advanced COPD: a multi-national survey of specialists. COPD 2018; 15: 294–302. doi: 10.1080/15412555.2018.1502264 [DOI] [PubMed] [Google Scholar]
- 26.Smallwood N, Currow D, Booth S, et al. . Attitudes to specialist palliative care and advance care planning in people with COPD: a multi-national survey of palliative and respiratory medicine specialists. BMC Palliat Care 2018; 17: 115. doi: 10.1186/s12904-018-0371-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smallwood N, Gaffney N, Gorelik A, et al. . Doctors’ attitudes to palliation and palliative care in patients with advanced chronic obstructive pulmonary disease. J Pain Symptom Manage 2018; 55: e9–e11. doi: 10.1016/j.jpainsymman.2017.11.020 [DOI] [PubMed] [Google Scholar]
- 28.Smallwood N, Gaffney N, Gorelik A, et al. . Junior doctors’ attitudes to opioids for refractory breathlessness in patients with advanced chronic obstructive pulmonary disease. Intern Med J 2017; 47: 1050–1056. doi: 10.1111/imj.13521 [DOI] [PubMed] [Google Scholar]
- 29.Ekström M, Ferreira D, Chang S, et al. . Effect of regular, low-dose, extended-release morphine on chronic breathlessness in chronic obstructive pulmonary disease: the BEAMS randomized clinical trial. JAMA 2022; 328: 2022–2032. doi: 10.1001/jama.2022.20206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verberkt CA, van den Beuken-van Everdingen MHJ, Schols JMGA, et al. . Effect of sustained-release morphine for refractory breathlessness in chronic obstructive pulmonary disease on health status: a randomized clinical trial. JAMA Intern Med 2020; 180: 1306–1314. doi: 10.1001/jamainternmed.2020.3134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Currow D, Louw S, McCloud P, et al. . Regular, sustained-release morphine for chronic breathlessness: a multicentre, double-blind, randomised, placebo-controlled trial. Thorax 2020; 75: 50–56. doi: 10.1136/thoraxjnl-2019-213681 [DOI] [PubMed] [Google Scholar]
- 32.Ekström M, Janssen DJA. Should opioids be used for breathlessness and in whom? A PRO and CON debate of the evidence. Curr Opin Support Palliat Care 2023; 17: 263–269. doi: 10.1097/SPC.0000000000000674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holland A, Spathis A, Marsaa K, et al. . European Respiratory Society clinical practice guideline on symptom management for adults with serious respiratory illness. Eur Respir J 2024; 63: 2400335. [DOI] [PubMed] [Google Scholar]
- 34.Liberati A, Altman DG, Tetzlaff J, et al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ekström M. Why treatment efficacy on breathlessness in laboratory but not daily life trials? The importance of standardized exertion. Curr Opin Support Palliat Care 2019; 13: 179–183. doi: 10.1097/SPC.0000000000000444 [DOI] [PubMed] [Google Scholar]
- 36.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Mahwah, NJ, L. Erlbaum Associates, 1988. [Google Scholar]
- 37.Schünemann H, Vist G, Higgins J, et al. eds. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, et al. eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from: www.training.cochrane.org/handbook [Google Scholar]
- 38.Jaiswal N, Singh M, Agarwal A, et al. . Palliative drug treatments for breathlessness in cystic fibrosis. Cochrane Database Syst Rev 2020; 4: CD011855. doi: 10.1002/14651858.CD011855.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bajwah S, Colquitt J, Loveman E, et al. . Pharmacological and nonpharmacological interventions to improve symptom control, functional exercise capacity and quality of life in interstitial lung disease: an evidence synthesis. ERJ Open Res 2021; 7: 00107-2020. doi: 10.1183/23120541.00107-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ekström M, Nilsson F, Abernethy AA, et al. . Effects of opioids on breathlessness and exercise capacity in chronic obstructive pulmonary disease. A systematic review. Ann Am Thorac Soc 2015; 12: 1079–1092. doi: 10.1513/AnnalsATS.201501-034OC [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi Y, Saif-Ur-Rahman KM, Nomura M, et al. . Opioid prescription method for breathlessness due to non-cancer chronic respiratory diseases: a systematic review. Int J Environ Res Public Health 2022; 19: 4907. doi: 10.3390/ijerph19084907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferreira DH, Ekström M, Sajkov D, et al. . Extended-release morphine for chronic breathlessness in pulmonary arterial hypertension-a randomized, double-blind, placebo-controlled, crossover study. J Pain Symptom Manage 2018; 56: 483–492. doi: 10.1016/j.jpainsymman.2018.07.010 [DOI] [PubMed] [Google Scholar]
- 43.Ferreira DH, Kochovska S, Honson A, et al. . Two faces of the same coin: a qualitative study of patients’ and carers’ coexistence with chronic breathlessness associated with chronic obstructive pulmonary disease (COPD). BMC Palliat Care 2020; 19: 64. doi: 10.1186/s12904-020-00572-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdallah SJ, Wilkinson-Maitland C, Saad N, et al. . Effect of morphine on breathlessness and exercise endurance in advanced COPD: a randomised crossover trial. Eur Respir J 2017; 50: 1701235. doi: 10.1183/13993003.01235-2017 [DOI] [PubMed] [Google Scholar]
- 45.Abernethy AP, Currow DC, Frith P, et al. . Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ 2003; 327: 523–528. doi: 10.1136/bmj.327.7414.523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eiser N, Denman WT, West C, et al. . Oral diamorphine: lack of effect on dyspnoea and exercise tolerance in the “pink puffer” syndrome. Eur Respir J 1991; 4: 926–931. doi: 10.1183/09031936.93.04080926 [DOI] [PubMed] [Google Scholar]
- 47.Ferreira DH, Louw S, McCloud P, et al. . Controlled-release oxycodone vs. placebo in the treatment of chronic breathlessness – a multisite randomized placebo controlled trial. J Pain Symptom Manage 2020; 59: 581–589. doi: 10.1016/j.jpainsymman.2019.10.017 [DOI] [PubMed] [Google Scholar]
- 48.Johnson MA, Woodcock AA, Geddes DM. Dihydrocodeine for breathlessness in “pink puffers”. Br Med J (Clin Res Ed) 1983; 286: 675–677. doi: 10.1136/bmj.286.6366.675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kronborg-White S, Andersen CU, Kohberg C, et al. Palliation of chronic breathlessness with morphine in patients with fibrotic interstitial lung disease – a randomised placebo-controlled trial. Respir Res 2020; 21: 793. 10.1186/s12931-020-01452-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Light RW, Muro JR, Sato RI, et al. . Effects of oral morphine on breathlessness and exercise tolerance in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1989; 139: 126–133. doi: 10.1164/ajrccm/139.1.126 [DOI] [PubMed] [Google Scholar]
- 51.Light RW, Stansbury DW, Webster JS. Effect of 30 mg of morphine alone or with promethazine or prochlorperazine on the exercise capacity of patients with COPD. Chest 1996; 109: 975–981. 10.1378/chest.109.4.975 [DOI] [PubMed] [Google Scholar]
- 52.Poole PJ, Veale AG, Black PN. The effect of sustained-release morphine on breathlessness and quality of life in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 157: 1877–1880. doi: 10.1164/ajrccm.157.6.9711061 [DOI] [PubMed] [Google Scholar]
- 53.Rice KL, Kronenberg RS, Hedemark LL, et al. . Effects of chronic administration of codeine and promethazine on breathlessness and exercise tolerance in patients with chronic airflow obstruction. Br J Dis Chest 1987; 81: 287–292. doi: 10.1016/0007-0971(87)90163-X [DOI] [PubMed] [Google Scholar]
- 54.Woodcock AA, Gross ER, Gellert A, et al. Effects of dihydrocodeine, alcohol, and caffeine on breathlessness and exercise tolerance in patients with chronic obstructive lung disease and normal blood gases. N Engl J Med 1981; 305: 1611–1616. 10.1056/NEJM198112313052703 [DOI] [PubMed] [Google Scholar]
- 55.Woodcock AA, Johnson MA, Geddes DM. Response to ‘Breathlessness , alcohol, and opiates'. N Engl J Med 1982; 306: 1363–1364. doi: 10.1056/NEJM198206033062214 [DOI] [PubMed] [Google Scholar]
- 56.Ekström M, Johnson MJ, Huang C, et al. . Minimal clinically important differences in average, best, worst and current intensity and unpleasantness of chronic breathlessness. Eur Respir J 2020; 56: 1902202. doi: 10.1183/13993003.02202-2019 [DOI] [PubMed] [Google Scholar]
- 57.Patel AS, Siegert RJ, Brignall K, et al. . The development and validation of the King's Brief Interstitial Lung Disease (K-BILD) health status questionnaire. Thorax 2012; 67: 804-810. doi: 10.1136/thoraxjnl-2012-201581 [DOI] [PubMed] [Google Scholar]
- 58.Guyatt GH, Berman LB, Townsend M, et al. . A measure of quality of life for clinical trials in chronic lung disease. Thorax 1987; 42: 773–778. doi: 10.1136/thx.42.10.773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu M, Xiao W, Du L, et al. . Effectiveness and safety of opioids on breathlessness and exercise endurance in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis of randomised controlled trials. Palliat Med 2023; 37: 1365–1378. doi: 10.1177/02692163231194838 [DOI] [PubMed] [Google Scholar]
- 60.Polosa R, Simidchiev A, Walters EH. Nebulised morphine for severe interstitial lung disease. Cochrane Database Syst Rev 2002; 3: CD002872. doi: 10.1002/14651858.CD002872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jennings AL, Davies AN, Higgins JPT, et al. . A systematic review of the use of opioids in the management of dyspnoea. Thorax 2002; 57: 939–944. doi: 10.1136/thorax.57.11.939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Currow D, Watts GJ, Johnson M, et al. . A pragmatic, phase III, multisite, double-blind, placebo-controlled, parallel-arm, dose increment randomised trial of regular, low-dose extended-release morphine for chronic breathlessness: Breathlessness, Exertion And Morphine Sulfate (BEAMS) study protocol. BMJ Open 2017; 7: e018100. doi: 10.1136/bmjopen-2017-018100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Altawalbeh SM, Almomani BA, Alefan Q, et al. . The influence of adverse drug effects on health-related quality of life in chronic obstructive pulmonary disease patients. Int J Pharm Pract 2022; 30: 457–465. doi: 10.1093/ijpp/riac052 [DOI] [PubMed] [Google Scholar]
- 64.de Mol M, Visser S, den Oudsten BL, et al. . Frequency of low-grade adverse events and quality of life during chemotherapy determine patients’ judgement about treatment in advanced-stage thoracic cancer. Support Care Cancer 2019; 27: 3563–3572. doi: 10.1007/s00520-019-4659-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vozoris NT, Wang X, Fischer HD, et al. . Incident opioid drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J 2016; 48: 683–693. doi: 10.1183/13993003.01967-2015 [DOI] [PubMed] [Google Scholar]
- 66.Ekström MP, Bornefalk-Hermansson A, Abernethy AP, et al. . Safety of benzodiazepines and opioids in very severe respiratory disease: national prospective study. BMJ 2014; 348: g445. doi: 10.1136/bmj.g445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verberkt CA, van den Beuken-van Everdingen MHJ, Wouters EFM, et al. . Attitudes of patients with chronic breathlessness towards treatment with opioids. Eur Respir J 2020; 55: 1901752. doi: 10.1183/13993003.01752-2019 [DOI] [PubMed] [Google Scholar]
- 68.Ferreira D, Kochovska S, Honson A, et al. . Patients’ and their caregivers’ experiences with regular, low-dose, sustained-release morphine for chronic breathlessness associated with COPD: a qualitative study. BMJ Open Respir Res 2022; 9: e001210. doi: 10.1136/bmjresp-2022-001210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Antoniu SA. Descriptors of dyspnea in obstructive lung diseases. Multidiscip Respir Med 2010; 5: 216–219. doi: 10.1186/2049-6958-5-3-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.World Health Organization . A Conceptual Framework for Action on the Social Determinants of Health. 2010. www.who.int/publications/i/item/9789241500852
- 71.Smallwood N, Mann J, Guo H, et al. . Patients with fibrotic interstitial lung disease receive supportive and palliative care just prior to death. Am J Hosp Palliat Care 2021; 38: 154–160. doi: 10.1177/1049909120938629 [DOI] [PubMed] [Google Scholar]
- 72.Smallwood N, Ross L, Taverner J, et al. . A palliative approach is adopted for many patients dying in hospital with chronic obstructive pulmonary disease. COPD 2018; 15: 503–511. doi: 10.1080/15412555.2018.1549210 [DOI] [PubMed] [Google Scholar]
- 73.Maher TM, Avram C, Bortey E, et al. . Nalbuphine tablets for cough in patients with idiopathic pulmonary fibrosis. NEJM Evid 2023; 2: EVIDoa2300083. doi: 10.1056/EVIDoa2300083 [DOI] [PubMed] [Google Scholar]
- 74.Wu Z, Banya W, Chaudhuri N, et al. . Pacify Cough – a multicentre, double-blind, placebo-controlled, crossover trial of morphine sulphate for the treatment of pulmonary fibrosis cough. Trials 2022; 23: 184. doi: 10.1186/s13063-022-06068-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Protocol and full search strategy ERR-0265-2023.SUPPLEMENT (302.7KB, pdf)
Supplementary figures and tables ERR-0265-2023.SUPPLEMENT2 (516.3KB, pdf)