Abstract
Bronchiectasis is a chronic respiratory disease characterised by permanent enlargement of the airways associated with cough, sputum production and a history of pulmonary exacerbations. In the past few years, incidence and prevalence of bronchiectasis have increased worldwide, possibly due to advances in imaging techniques and disease awareness, leading to increased socioeconomic burden and healthcare costs. Consistently, a mortality increase in bronchiectasis patient cohorts has been demonstrated in certain areas of the globe, with mortality rates of 16–24.8% over 4–5 years of follow-up. However, heterogeneity in epidemiological data is consistent, as reported prevalence in the general population ranges from 52.3 to more than 1000 per 100 000. Methodological flaws in the designs of available studies are likely to underestimate the proportion of people suffering from this condition worldwide and comparisons between different areas of the globe might be unreliable due to different assessment methods or local implementation of the same method in different contexts. Differences in disease severity associated with diverse geographical distribution of aetiologies, comorbidities and microbiology might explain an additional quota of heterogeneity. Finally, limited access to care in certain geographical areas is associated with both underestimation of the disease and increased severity and mortality. The aim of this review is to provide a snapshot of available real-world epidemiological data describing incidence and prevalence of bronchiectasis in the general population. Furthermore, data on mortality, healthcare burden and high-risk populations are provided. Finally, an analysis of the geographical distribution of determinants contributing to differences in bronchiectasis epidemiology is offered.
Shareable abstract
Despite increasing incidence and prevalence, bronchiectasis is probably underdiagnosed in several areas of the globe. Differences in assessment methods, aetiologies, comorbidities, microbiology and access to care explain epidemiological heterogeneity. https://bit.ly/3WGlEfc
Introduction
Bronchiectasis is a chronic respiratory disease characterised by permanent bronchial dilatation evidenced at chest computed tomography (CT), associated with a clinical syndrome featuring daily cough, daily sputum production and a history of pulmonary exacerbations [1]. In the last few decades, bronchiectasis has rapidly moved from being a rare or orphan disease to a global problem, with a large-scale trend towards increasing incidence and prevalence [2]. Contextually, the scientific community has demonstrated growing interest in this condition, as confirmed by the publication of international guidelines and the foundation of several registries worldwide [3–7] Furthermore, numerous translational and clinical research initiatives have been developed in the last few years, aimed at identifying new therapeutics for people suffering from bronchiectasis [8].
Available data on bronchiectasis prevalence are quite heterogeneous, with reported prevalence roughly ranging from 50 to 1000 cases per 100 000 individuals. However, these data are likely to underestimate bronchiectasis epidemiology for several reasons. First, the definition of bronchiectasis as a clinically and radiologically significant disease requires a chest CT scan to be performed and we can speculate that a proportion of affected patients might not undergo such a radiological test for a variety of reasons [9]. On the other hand, some patients might have radiological evidence of bronchiectasis but no associated signs and symptoms. Secondly, administrative databases may not capture the totality of patients due to an imprecision of assessment methods in the general population, as a recent study identified the poor sensitivity of the International Classification Disease codes ICD-9/10 [10]. Thirdly, patients usually suffer a relevant diagnostic delay, receiving wrong disease labels, such as COPD and/or asthma, and inappropriate treatments for years [9]. Finally, the epidemiology of bronchiectasis can be influenced by external factors with different geographical distributions, such as disease aetiology, comorbidities, microbiology and access to care. All these challenges should be considered when interpreting the results of epidemiological papers on bronchiectasis.
The aim of this narrative review is to provide an overview on bronchiectasis epidemiology through real-world data describing prevalence, incidence, mortality, healthcare burden and high-risk populations associated with this condition.
Search strategy
We conducted a narrative, PubMed-based review of articles mentioning the keyword “bronchiectasis” in combination with the following items: “epidemiology”, “incidence”, “prevalence”, “mortality”, “healthcare burden”, “comorbidities”, “aetiologies”, “microbiology” and “access to care”. Articles focusing exclusively on patients with cystic fibrosis or without radiological confirmation of bronchiectasis were excluded. With the exception of studies on high-risk populations, which occasionally included paediatric patient data, only studies conducted on adults were considered.
Prevalence and incidence
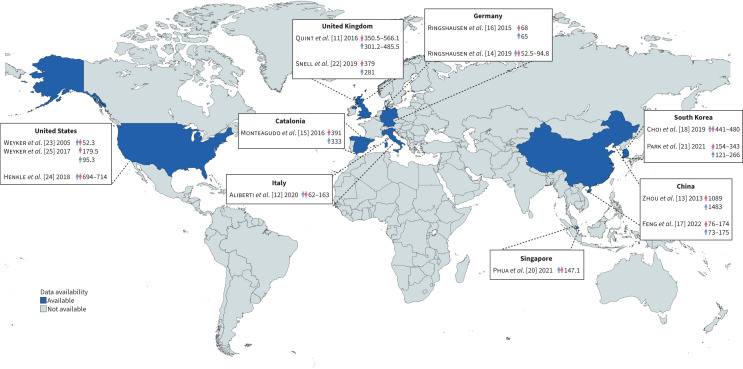
Any examination of bronchiectasis epidemiology should be preceded by the recognition of a number of challenges. Firstly, prevalence data in the general population is only available for a small number of countries, accounting for less than a quarter of the global population, with no information from entire regions of the globe, such as Africa, South America or the Middle East, as displayed in figure 1. Secondly, studies conducted within the same country or in countries expected to be similar, such as Spain and Italy, show consistent differences in prevalence and incidence, as shown in table 1. This effect is probably linked to different database sources and assessment methods. Insurance-based databases are likely to introduce a selection bias in the study population, as included people might undergo medical examinations more frequently and have fewer risk factors than people without stipulated medical insurance. Therefore, this approach may detect patients with radiological evidence of bronchiectasis in the absence of daily signs, symptoms or exacerbations, indicating the likelihood of nonclinically relevant bronchiectasis. Thirdly, some studies conducted using the same database materials, such as the general practitioner (GP)-based studies by Quint et al. [11] in the UK and Aliberti et al. [12] in Italy, show discrepant results. This could reflect differences in standard operating procedures in primary care settings, hinting at difficulties in the homogenisation of data obtained through these sources. Fourthly, small sample size studies, such as the one conducted by Zhou et al. [13], may lack precision and have limited generalisability. Fifthly, real differences in study populations at a global level in terms of genetics, environmental factors, aetiologies, comorbidities, microbiology and access to care might explain the remaining part of epidemiological diversity.
FIGURE 1.
Prevalence of bronchiectasis in the general population according to available data. Countries have been coloured in blue if they had at least one available study describing prevalence in the general population. For these countries, available studies are mentioned in the boxes. Numbers are expressed as number of people affected by the disease per 100 000 individuals. The blue and pink indicator represent males and females, respectively; when they are separated, they indicate gender-related prevalences, when together they indicate overall prevalence. Studies including evaluations at more than one timepoint have the minimum and maximum prevalence indicated. Reference numbers follow the same order as the text.
TABLE 1.
Incidence and prevalence of bronchiectasis
| Country | Study, year | Source | Sample size (n) | Age group | Year | Prevalence (per 100 000) | Incidence (per 100 000 person-years) |
|---|---|---|---|---|---|---|---|
| Europe | |||||||
| UK | Quint [11] 2016 | Primary care database (Clinical Practice Research Datalink database) |
5.4 million | All | 2004 | Female: 350.5 Male: 301.2 |
Female: 21.2 Male: 18.2 |
| 2013 | Female: 566.1 Male: 485.5 |
Female: 35.2 Male: 26.9 |
|||||
| Snell [22] 2019 | Primary care database (The Health Improvement Network records) |
5% of UK population (total ∼52 million) | All | 2004 | Overall: 20 | ||
| 2012 | Female: 379 Male: 281 |
Overall: 33 | |||||
| Catalonia | Monteagudo [15] 2016 | Primary care database (Information System for the Development of Research in Primary Care) |
5.8 million (80% of the total population) |
All | 2012 | Overall: 362 Female: 391 Male: 333 |
Overall: 48.1 Female: 49.3 Male: 46.9 |
| Germany | Ringshausen [16] 2015 | Federal Insurance Authority | 3 895 272 | All | 2013 | Overall: 67 Female: 68 Male: 65 |
|
| Diel [52] 2019 | Health Risk Institute research database from more than 80 German statutory health insurance companies | 3 988 648 | All | 2011 | Overall: 16.77 | ||
| 2012 | Overall: 16.05 | ||||||
| 2013 | Overall: 21.23 | ||||||
| 2013 | Overall: 20.88 | ||||||
| Ringshausen [14] 2019 | Externally validated InGef research database claims data from public health scheme | 4 million | All | 2009 | Overall: 52.5 | ||
| 2017 | Overall: 94.8 | ||||||
| Italy | Aliberti [12] 2020 | Primary care database | 1 054 376 | ≥15 years | 2005 | Overall: 62 | |
| 2015 | Overall: 163 Female: 178 Male: 147 |
Overall: 16.3 Female: 18.2 Male: 14.1 |
|||||
| Poland | Niewiadomska [31] 2016 | National Health Found and Mz/Szp-11 reports | 4 635 882 | ≥19 years | 2006 | Overall: 19.9 Female: 21.4 Male: 18.7 |
|
| 2007 | Overall: 25.1 Female: 27.3 Male:23.4 |
||||||
| 2008 | Overall: 22.2 Female: 23.0 Male: 22.3 |
||||||
| 2009 | Overall: 23.7 Female: 26.0 Male: 21.7 |
||||||
| 2010 | Overall: 21.1 Female: 22.2 Male: 20.6 |
||||||
| America | |||||||
| USA | Weycker [23] 2005 | Healthcare claims | 5.6 million | ≥18 years | 1999–2001 | Overall: 52.3 | |
| Seitz [26] 2012 | 5% sample of the Medicare outpatient claims database | >2 million | ≥65 years | 2000–2007 (total 8-year period) |
Overall: 1106 Female: 414.8 Male: 245.7 |
||
| 2000 | Female: 322 Male: 223 |
||||||
| 2007 | Female: 553 Male: 388 |
||||||
| Weycker [25] 2017 | Truven Health Analytics MarketScan Commercial Claims and Encounters and Medicare Supplemental and Coordination of Benefits databases | Prevalence: 33.2 million Incidence: 23.7 million |
≥18 years | 2013 | Overall: 138.6 Female: 179.5 Male: 95.3 |
Overall: 28.5 Female: 33.6 Male: 23.0 |
|
| Henkle [24] 2018 | 40% of Medicare enrolees with prescription drug plans | NA | ≥65 years | 2006–2014 | Overall: 701 Female: 802 Male: 617 |
||
| 2012 | Overall: 694 | ||||||
| 2013 | Overall: 694 | ||||||
| 2014 | Overall: 714 | ||||||
| Asia | |||||||
| China | Zhou [13] 2013 | Urban population–based cross sectional survey of bronchiectasis | 10 811 | ≥40 years | 2002–2004 | Overall: 1248.7 (135/10 811) Female: 1088.8 (70/6429) Male: 1483.3 (65/4382) |
|
| Feng [17] 2022 | Urban Employee Basic Medical Insurance and Urban Resident Basic Medical Insurance in China | >380 million | ≥18 years | 2013 | Overall: 75.48 Female: 76.12 Male: 73.42 |
||
| 2014 | Overall: 112.61 Female: 115.45 Male: 109.71 |
||||||
| 2015 | Overall: 120.41 Female: 119.19 Male: 122.03 |
||||||
| 2016 | Overall: 131.97 Female: 133.13 Male: 131.20 |
||||||
| 2017 | Overall: 174.45 Female: 173.60 Male: 175.03 |
||||||
| Singapore | Phua [20] 2021 | Ministry of Health-hosted administrative database | NA | All | 2007 | Overall: 13.9 | |
| 2008 | Overall: 12.5 | ||||||
| 2009 | Overall: 12.4 | ||||||
| 2010 | Overall: 11.4 | ||||||
| 2011 | Overall: 12.4 | ||||||
| 2012 | Overall: 11.0 | ||||||
| 2013 | Overall: 9.6 | ||||||
| 2014 | Overall: 9.4 | ||||||
| 2015 | Overall: 9.5 | ||||||
| 2016 | Overall: 10.6 | ||||||
| 2017 | Overall: 147.1 | Overall: 10.6 | |||||
| South Korea | Choi [18] 2019 | Health Insurance Review and Assessment Service, National Patient Sample | 1.4 million | ≥20 years | 2012–2017 | Overall: 464 | |
| 2012 | Overall: 464 | ||||||
| 2013 | Overall: 441 | ||||||
| 2014 | Overall: 455 | ||||||
| 2015 | Overall: 474 | ||||||
| 2016 | Overall: 468 | ||||||
| 2017 | Overall: 480 | ||||||
| Park [21] 2021 | National Health Insurance Service–National Sample Cohort data | 1 025 340 | All | 2007 | Female: 154.3 Male: 120.9 |
||
| 2009 | Female: 183 Male: 174.5 |
||||||
| 2015 | Female: 343.1 Male: 266.4 |
Female: 150.3 Male: 126.6 |
|||||
Studies are grouped according to the geographical area in which they have been conducted (i.e. continents and countries). NA: not available/applicable.
Considering these caveats, existing data seem to demonstrate that the prevalence of bronchiectasis might be lower in continental Europe (53–362 per 100 000 individuals) [12, 14–16] compared to Asia (76–1249 per 100 000) [17–21]. Interestingly, the UK shows higher prevalence (350–566 per 100 000 women and 281–486 per 100 000 men), with a reported annual increase of 8–20% [11, 22]. In the US, the prevalence increased from 52.3 to 714.0 per 100 000 people between 1999 and 2001 and 2014 [23–25]. An 8-year study detected an overall prevalence of 1106 per 100 000 people between 2000 and 2007 [26]. The global prevalence has consistently increased over time [11, 21, 24]. This effect may be at least in part due to increasing disease awareness and advances in imaging techniques. Overall, bronchiectasis prevalence is higher in women, with reported proportion ranging between 51.6 and 68.0%. Furthermore, prevalence generally increases with age, moving from 4.2–43.4 per 100 000 people aged 18–34 years to 153–1365 per 100 000 individuals older than 65–75 years [11, 15, 18, 23]. Interestingly, some studies have highlighted a higher prevalence in men compared to women in the eldest age brackets [12, 14–16]. The mean age of bronchiectasis patients has increased over time moving from 61 to 68 years in the US and from 64.2 to 67.6 years in Germany [14, 23, 25]. Bronchiectasis seems to be more common in individuals with higher socioeconomic status [11]; however, when an association with COPD exists, it is more likely to occur in people with a lower socioeconomic status [24]. A single study highlighted a significantly higher prevalence of bronchiectasis in Asian patients compared to people of other ethnicities [26]. Finally, three studies have measured the prevalence of bronchiectasis in people undergoing CT scans in the context of a lung cancer screening programme [27–29]. Reported prevalence was 9.1% in Korea, 11.7% in Spain and 23% in the US. In these studies, patients with bronchiectasis were generally older and had a more extensive smoking history compared to other people, but no information on the clinical significance of bronchiectasis in these patients was provided [28, 29].
Incidence seems to be distributed with a narrower range of variability across geographic areas, with most regions ranging between 9.4 and 48.1 new cases per 100 000 person-years [11, 12, 15, 20, 22, 25, 30, 31]. In contrast, in 2009, South Korea showed an unexpectedly high incidence of 183 and 175 new cases per 100 000 women and men, respectively [21]. Change of incidence over time was not uniform, but most studies conducted in the UK and Europe found a trend towards growing incidence [11, 22, 30, 31]. This was not the case of some Asian countries, namely Singapore and South Korea, in which incidence seems to have decreased [20, 21]. Similar to prevalence trends, incidence is higher in women at all time-points and increases with age until 79 years [11, 12, 15, 20, 21, 25, 30, 31]. Interestingly, an incidence decline can be noted in people older than 80 years in the UK and Germany and in those older than 85 years in Italy [11, 12, 30].
Mortality
Bronchiectasis patients have a higher mortality rate than the general population [32]. However, studies investigating mortality are heterogeneous in terms of inclusion criteria, assessment methods, associated conditions and follow-up periods, making overall results essentially incomparable. Furthermore, mortality is influenced by the above-mentioned prevalence increase, as a UK population study demonstrated a 3% increase per year in the annual mortality rates between 2001 and 2007 [22, 33].
Published mortality rates are reported in table 2. Different continental European cohorts showed mortality rates ranging from 16% to 24.8%, with follow-up periods of 4.0–5.18 years [34–37]. In the UK, the reported mortality rate in the general population was 1.68 per 100 000 individuals, accounting for 0.18–0.3% of all deaths [22, 33]. In cohorts with longer follow-up periods (4.0–18.8 years), the reported mortality rates were 10.2–29.7% [38–40]. Greater heterogeneity was highlighted in Asia and Australia, with mortality rates ranging between 2.3% and 21% with follow-up periods of 1–10 years [32, 41–47]. In Brazil, a retrospective cohort study reported an anomalously high mortality rate of 38.6% [48]; however, a successive prospective study enrolling 120 bronchiectasis patients demonstrated a probably more reliable 10.8% mortality rate over a 3-year follow-up [49].
TABLE 2.
Mortality of bronchiectasis
| Country | Study, year | Study design | Follow-up (years) | Sample size (n) | Study period | Mortality estimate | Measure of effect | Main causes of deaths | |
|---|---|---|---|---|---|---|---|---|---|
| Europe | |||||||||
| UK | Snell [22] 2019 | Retrospective (GP data) |
1 | ∼52 million (5% of UK population) |
2012 | 0.3% | Deaths in 2012, % | NA | |
| Roberts [33] 2010 | Retrospective (Office of National Statistics data for England and Wales) |
1 | 54 071 900 | 2007 | 1.68% | Deaths per 100 000 | NA | ||
| 0.18% | Deaths in 2007, % | ||||||||
| Loebinger [38] 2009 | Retrospective | 13 | 91 | 1994–2007 | 29.7% | Deaths of cohort, % | Respiratory related (70.4%); renal failure and colon cancer (7.4%); haemoptysis, heart failure, cerebrovascular accident, liver metastasis and pulmonary embolism (3.7%) | ||
| Ellis [39] 2016 | Retrospective | 18.8 | 74 | 1994–2013 | 36% | Deaths of cohort, % | Respiratory related (69.2%) | ||
| Chalmers [40] 2014 | Prospective (BSI derivation cohort) |
4 | 608 | 2008–2012 | 10.2% | Deaths of cohort, % | Respiratory related (51.5%); myocardial infarction (19.3%); malignancy (12.9%); heart failure, stroke and sepsis (3.2%); pulmonary embolism, trauma, alcoholic liver disease and post-operative complications (1.6%) | ||
| Belgium | Goeminne [34] 2014 | Prospective | 5.18 | 245 | 2006–2012 | 20.4% | Deaths of cohort, % | Respiratory related (58%); cardiovascular (16%); unclear (12%); neurological (4%); gastrointestinal, nephrological, haematological, euthanasia and intoxication (2%) | |
| Poland | Nowiński [35] 2021 | Prospective | 5 | 93 | 2015–2019 | 16% | Deaths of cohort, % | NA | |
| Spain | Martínez-García [36] 2014 | Retrospective Seven Spanish hospitals (FACED derivation cohort) |
5 | 397 | Before 2005 | 24.8% | Deaths of cohort, % | Respiratory related (42.9%); malignancy (9.1%); cardiovascular disorders (9.1%) | |
| Turkey | Onen [37] 2007 | Prospective | 4 | 98 | 2000–2005 | 16.3% | Deaths of cohort, % | Pulmonary arrest or cardiopulmonary arrest related to bronchiectasis (100%) | |
| Asia Pacific | |||||||||
| Australia | Darwin: urban | Gibbs [47] 2024 | Retrospective (Hospital medical records) |
11.7 | 23 722 | 7.5% | Mean annual mortality | NA | |
| Darwin: rural | 4.5% | Mean annual mortality | |||||||
| East Arnhem | 3.2% | Mean annual mortality | |||||||
| Katherine | 4.9% | Mean annual mortality | |||||||
| Australia | Rees [46] 2023 | Retrospective | 4 | 145 | 2015–2020 | 21% | Deaths of cohort, % | NA | |
| China | Tang [41] 2017 | Retrospective | 5 | 89 | 2003–2008 | 13.5% | Deaths of cohort, % | NA | |
| China | Wang [42] 2021 | Prospective | 1.3 | 1234 | 2013–2019 | 15.2% | Deaths of cohort, % | NA | |
| India | Dhar [44] 2023 | Prospective | At least 1 year (cumulative observation time of 15 479 months) | 1018 | 2015-ongoing | 2.3% Ages: 18–40 years 0.5% 41–60 years 3.5% 61–80 years 8.1% >80 years 23.5% |
Deaths of cohort, % | NA | |
| Singapore | Young [45] 2021 | Prospective | 2.4 | 168 | 2015–2020 | 10.7% | Deaths of cohort, % | Pneumonia (22.2%); bronchiectasis (5.6%); colorectal cancer (5.6%); unknown (66.7%) | |
| South Korea | Lee [43] 2021 | Retrospective (National Health Insurance Service–Health Screening Cohort) |
10 | 2769 | 2004–2016 | 14.8% | Deaths of cohort, % | Chronic lower respiratory disease (13.9%); other malignancies (17.1%); lung cancer (13.0%); cardiovascular disease (10.0%); cerebrovascular disease (5.9%); pneumonia (5.6%); tuberculosis (2.2%); diabetes mellitus (2.2%); hypertension (0.7%); other (28.6%) | |
| South Korea | Choi [32] 2021 | Retrospective (National Health Insurance Service–National Sample Cohort) |
10 | 14 823 | 2005–2015 | 15.2% 19.6% Male 11.0% Female |
Deaths of cohort, % | Malignancy (29.7%); respiratory related (19.8%); cardiovascular diseases (17.8%); injury, poisoning and external causes (overall 7.3%) | |
| 2505 3362 Male 1759 Female |
Deaths in 100 000 patients/year | ||||||||
| South America | |||||||||
| Brazil | Machado [48] 2018 | Retrospective | 5.5±2.3 | 70 | 2008–2016 | 38.6% | Deaths of cohort, % | Acute infectious exacerbation (60.7%) | |
| Brazil | Mateus [49] 2022 | Prospective | 3 | 120 | 2017–2020 | 10.8% | Deaths of cohort, % | Circulatory system related (30.8%); infectious and parasitic diseases (23.1%); malignancy (15.4%); digestive system diseases (15.4%); respiratory system diseases (7.7%); external morbidities and mortality (7.7%) | |
Studies are grouped according to the geographical area in which they have been conducted (i.e. continents and countries). BSI: bronchiectasis severity index; FACED: forced expiratory volume in 1 s, age, chronic colonisation by Pseudomonas aeruginosa, radiological extension and dyspnoea; GP: general practitioner; NA: not available/applicable.
Healthcare costs and utilisation
Bronchiectasis represents a relevant burden on healthcare systems [50]. In the US between 1999 and 2001, annual health-related costs of bronchiectasis patients were $630 million higher than those without the disease [23]. A similar trend was observed in Europe. In Italy (2016–2018), the mean annual expenditure was €3539 for bronchiectasis patients in the first year after diagnosis, 1.9 times higher than asthmatic patients, but 29% lower than those with COPD. Similarly, in Germany (2012–2015), the total direct expenditure per patient was nearly one third higher in bronchiectasis patients compared to matched controls [51, 52].
Hospital admission costs represent a significant contributor to total medical expenditures, ranging from 20% to 55.8–81.2% of reported costs [20, 53]. Notably, hospitalisation-at-home seems to further increase costs [54]. Outpatient costs are mainly linked to drug prescriptions, especially antibiotics, and account for up to 41% of total expenditures [18, 52]. Indirect costs seem to be relevant as well. A German study estimated an average of 40.5 of sick-leave days during a 3-year follow-up period, equating to indirect costs of €4230.49 [52]. These findings were partially confirmed by a Spanish study enrolling hospitalised bronchiectasis patients that reported an average of 13.4±9 sick-leave days for 7.2% of patients and 6.2±4.9 days for their caregivers, accounting for additional expenditures of €776.9±520.6 and €356.5±286.6, respectively [54].
Overall, bronchiectasis-related costs seem to have increased over time. A Chinese study highlighted a 2.18-fold increase of total annual pro capita costs and a 1.83-fold increase in inpatient-related costs between 2013 and 2017 [17]. Similarly, in Singapore (2007–2017), the annual inpatient costs rose by 5% annually [20]. In the UK, bronchiectasis-related intensive care unit (ICU) admissions increased by 8% annually between 2009 and 2013, with an estimated increase of annual costs from £189 144 to £298 967 [55]. However, against the trend, in Spain overall costs decreased between 2004 and 2013 [56].
Populations at high risk for developing bronchiectasis
Some populations living in specific areas of the globe have higher risk of developing the disease, with interesting mixed outcomes.
In Australia, Indigenous communities living in the Northern Territory have a higher risk of developing bronchiectasis, representing the 79.3–97.0% of cases in this area [57–59]. A possible association with human T-cell lymphotropic virus seropositivity, far higher in Indigenous people compared to non-Indigenous Australians, was highlighted in some studies [60, 61]. Estimated prevalence in these communities reached 1030–1940 per 100 000 individuals, was higher in women and reached its peak at 50–59 years of age. Furthermore, the age-adjusted prevalence was significantly higher in the urban Darwin region compared to rural districts (1800–3600 versus 500 per 100 000) [47, 61]. Limited access to healthcare and higher rates of acute respiratory infections, possibly linked to overcrowded accommodation, were the main determinants of the lower life expectancy of Indigenous people compared to non-Indigenous Australians (men 66.6 versus 78.1 years, women 69.9 versus 82.7 years) [57]. Interestingly, these patients had lower FACED (forced expiratory volume in 1 s (FEV1), age, chronic colonisation by Pseudomonas (P.) aeruginosa, radiological extension and dyspnoea) scores despite having poorer lung function, more exacerbations and poorer prognosis [62, 63]. Mortality rates of Indigenous people with bronchiectasis ranged between 34.2% and 42.5%, with an estimated annual mortality of 4.5–7.5% (see table 2) [47, 60, 61]. The mean age of death was 16–20 years lower in Indigenous compared to non-Indigenous people, and was lower in rural areas compared to the urban Darwin region (60.3 versus 67.8 years) [47, 62, 63].
Māori and Pacific Islanders (PIs) represent two distinct high-risk populations, as cohort studies demonstrated that they represent 14.4–27% and 22.9–41% of bronchiectasis patients in New Zealand, despite representing a lower proportion of the local population according to census data (15% and 17%, respectively) [64, 65]. Māori and PI bronchiectasis patients had higher socioeconomic deprivation scores and worse lung function compared to the general population [64, 65]. When compared to Indigenous Australians, Māori and PI people had better lung function and fewer exacerbations, but the overall respiratory-related mortality was similar [62].
In Alaska, the prevalence of bronchiectasis patients was higher in people living in the Yukon Kuskokwim Delta area, despite a consistent reduction trend in newer generations (18.0–20.5 versus 6.7 per 1000 people born in 1960–1969 and in 1980–1989, respectively) [66–68]. The vast majority of these patients developed bronchiectasis following a lower respiratory tract infection in childhood (91–100%) [68].
In Canada, some case series of Inuit people suffering from primary ciliary dyskinesia (PCD) from the Qikiqtaaluk region have been described [69, 70]. In these patients, bronchiectasis was usually associated with neonatal respiratory distress, meconium aspiration, situs inversus totalis, chronic atelectasis, aspiration pneumonia, gastro-oesophageal reflux and chronic otitis and rhinitis [69, 70]. Based on these series, the estimated prevalence in Inuit people ranges between 70 and 202 per 100 000 children [69, 70].
Determinants of real epidemiological differences
The huge heterogeneity of epidemiological data on bronchiectasis could be the result of numerous factors, even beyond the methodological study limitations already mentioned so far. In this section, we aim to explore these potential determinants.
Aetiologies and associated conditions
Recently, the European bronchiectasis registry (European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC)) described the massive aetiological variation in European bronchiectasis patients, with over 15 different potential causes of the disease, the most common being idiopathic (38%) or post-infective (21%) [5]. Detection of underlying aetiologies is crucial for the related consequences in terms of clinical management and prognosis. Unfortunately, the local ability to perform a complete aetiological investigation can largely influence the aetiological classification of bronchiectasis and particularly the rate of idiopathic disease [71].
While the rarest causes of bronchiectasis show a similar prevalence rate across different European countries, most of the variability is linked to most common aetiologies. The rate of post-infective bronchiectasis is related to local healthcare access and it is often an investigator's assumption due to the absence of a previous negative CT scan [72]. In a similar way, the reported rates of bronchiectasis associated with COPD and asthma have also been extremely variable in the past, possibly due to differences in cohorts features and criteria used to define these associations [73]. Only recently has a consensus definition been published to define the association of COPD and bronchiectasis through the ROSE (radiology, obstruction, symptoms and exposure) criteria [74]. These criteria have identified a proper diagnosis of COPD as either an aetiology or a comorbidity in 19.6% of EMBARC patients, with a huge rate of misdiagnosis (>44%) [75]. Additionally, the prevalence of COPD varies enormously across countries, ranging between almost 60% in Macedonia and 5% in Sweden, likely according to local smoking habits. Unfortunately, the bad prognosis associated with comorbid COPD [34, 76–79] is likely to influence the distribution of the overall disease severity worldwide, defined through the bronchiectasis severity index (BSI). A similar study has described a 31% prevalence rate of asthma in EMBARC registry, but the lack of a standard definition and the poor application of asthma-specific tests (such as IgE and bronchodilation) makes this report a rough estimation that will need to be confirmed in the future, especially considering the potential therapeutic consequences [80]. Similarly, chronic rhinosinusitis has been classically described in association with bronchiectasis in 34–75% of cases and it is considered to contribute to disease activity as potential source of airways inflammation and infection [73, 81]. Furthermore, this association seems to be based on an eosinophilic inflammatory pathway and could represent a differential phenotype of bronchiectasis [82].
Additionally, many comorbidities have been described in association with bronchiectasis, with variable rates depending on cohorts’ demographics and healthcare systems, the most common being cardiovascular diseases (32.5% in EMBARC), anxiety and depression (14% each), osteoporosis (13%), and cancer (11%) [5]. Many of these can considerably contribute to the overall frailty of patients, severity scores and to the mortality risk [83]. Furthermore, beyond the possible causal relationship highlighted for some of them (such as rheumatic disorders or COPD [79]), most comorbidities are likely linked to bronchiectasis through shared inflammatory mediators [84]. For instance, the association between bronchiectasis and inflammatory bowel diseases influences the prognosis, as patients suffering from both conditions have a doubled mortality risk [83–85]. Finally, exposure to air pollution seems to play a role, as a Belgian cohort study identified a significant effect of living close to a major road on bronchiectasis patients’ mortality [86].
Microbiology
Tuberculosis (TB) has been widely described as a possible aetiology of bronchiectasis. A systematic review recently highlighted that a significant proportion of patients previously treated for TB (35.0–86.0%) develops bronchiectasis at chest CT scan [87]. On the other hand, TB is recognised as the underlying aetiology of bronchiectasis in a variable proportion of patients according to the geographic area, ranging between the very low prevalence detected in Australia (1.8%) and the peaks detected in South Korea (20.1%) and India (35.5%) [7, 88, 89]. The estimated prevalence of post-TB bronchiectasis in the US and Taiwan are 4.0 and 12.4%, respectively [6, 90]. In Europe the overall prevalence is 4.9%, but geographical heterogeneity is consistent, as Moldova, Portugal and Turkey reach 20.2, 19.8 and 18.9%, respectively [5]. A description of post-TB bronchiectasis as a distinct phenotype has been proposed by some authors. Fong et al. [91] recently reported on a Singaporean cohort of bronchiectasis patients, in which patients with post-TB bronchiectasis had lower body mass index (BMI) and FEV1, and a higher proportion of non-tuberculous mycobacteria (NTM) infections and haemoptysis during exacerbations. Furthermore, these patients showed an increased severity expressed through the FACED score and a shortened time to first exacerbation when compared to patients with bronchiectasis not associated with TB. These findings are partially confirmed by the Korean cohort described by Choi et al. [92], in which patients suffering from post-TB bronchiectasis had a lower BMI, a more frequent association with COPD, an increased FACED severity and a higher rate of drug prescriptions, especially long-acting beta-agonists/long-acting muscarinic antagonists and mucolytics.
Infection from NTM is common in people suffering from bronchiectasis. Recent meta-analyses estimated a prevalence of NTM infection of about 10% in bronchiectasis patients, with consistent heterogeneity across studies [19, 93]. Although clinical and microbiological procedures may account for a part of this variability, a geographic effect is likely, as the proportion of people with at least one NTM isolation in North American (63%) and South Korean (25–44.5%) cohorts appears far higher than the ones reported in Italy (12.2–26.1%), Israel (8.6%), Spain (8.3%), Netherlands (5%), France (3.6%), Taiwan (3.6%) and Greece (0.9%) [6, 90, 94–102.] Regardless of whether NTM infection should be considered an aetiology or a consequence of bronchiectasis, a proportion of infected patients develops NTM pulmonary disease (NTM-PD) [103]. The “Lady Windermere” phenotype has been proposed in the past to describe elder, underweight women suffering from NTM-PD possibly related to sputum retention; however, similar features can be recognised in men [104, 105]. These patients tend to have lower BMI, inferior BSI and fewer exacerbations when compared to those with P. aeruginosa [96, 97]. On the other hand, healthcare costs associated with NTM management are remarkable [106]. Furthermore, NTM-PD seems to have an impact on mortality, as a Taiwanese matched-cohort study recently demonstrated that both single and multiple NTM isolates predict mortality after adjustment for multiple confounding factors [107].
Bronchiectasis and Aspergillus are partners in a complex relationship. From one side, exposure to environmental Aspergillus fumigatus can cause allergic bronchopulmonary aspergillosis (ABPA), another recognised aetiology for bronchiectasis. The prevalence of ABPA in the general population is estimated to be close to 4.8 million people worldwide, with a heavier burden in the US, China and India [5, 108]. In Europe, ABPA is far more common in Northern and Western countries when compared to Southern or Eastern countries [5]. Aspergillus species are ubiquitous moulds found in air and soil, but the reasons for such variability remain unclear and could possibly be explained through differences in either climate or the immunological properties of people living in different areas of the globe. On the other hand, Aspergillus can infect people with pre-existing lung conditions, including bronchiectasis, and lead to the development of chronic pulmonary aspergillosis (CPA) [109]. Despite being often unrecognised or considered a rare disease, CPA affects more than 6 million people worldwide, with a higher prevalence in low- and middle-income countries. Furthermore, CPA has a considerable prognostic impact, yielding a 20% mortality in the year following the diagnosis and a 50% mortality in a 5-year period [110]. Finally, people suffering from CPA, and especially aspergilloma, have a high of experiencing haemoptysis and undergo hospitalisation.
P. aeruginosa is the most frequently isolated bacterial species in people suffering from bronchiectasis (25.1%, EMBARC) but with huge differences across countries, being markedly more frequent in Southern European countries compared to Northern-Western and Central-Eastern European countries [5]. Similarly, P. aeruginosa was detected in 13.7, 19.5 and 33% of patients enrolled in India, China and the US, respectively [6, 7, 42]. The prevalence of P. aeruginosa has direct consequences on the epidemiology of disease severity, since it is associated with increased mortality, exacerbations, economic burden and worsening lung function and quality of life [111–115]. However, in India Enterobacterales showed even a stronger association with mortality compared with P. aeruginosa (12.8% versus 6.8%) [44]. Consistently, other studies conducted in Europe and China did not find increased mortality in Pseudomonas-infected people after multivariate regression, suggesting that it might serve more as a disease severity marker than a prognostic factor [42, 116].
There is less data available on bacteria other than Pseudomonas. Haemophilus (H.) influenzae is the most frequently detected bacterium in most Northern European countries, including UK, while in other areas of the world seems to be rarer, with detection rates ranging between 0.5 and 9.2% [5–7, 90, 117]. Patients with H. influenzae chronic infection have a higher disease severity (BSI), a more extended radiological involvement and more exacerbations associated with an increase in outpatient morbidity [118–120] However, the impact on hospital admissions, pulmonary function and mortality seems lower than Pseudomonas [119, 120]. Scarce information is available on chronic infection from Staphylococcus (S.) aureus, but data from the Spanish registry RIBRON suggest that it may more frequently affect younger, low-BMI people. Furthermore, chronic S. aureus infection seems to be associated with more exacerbations and faster functional decline when compared to people without chronic infections [121].
Access to care
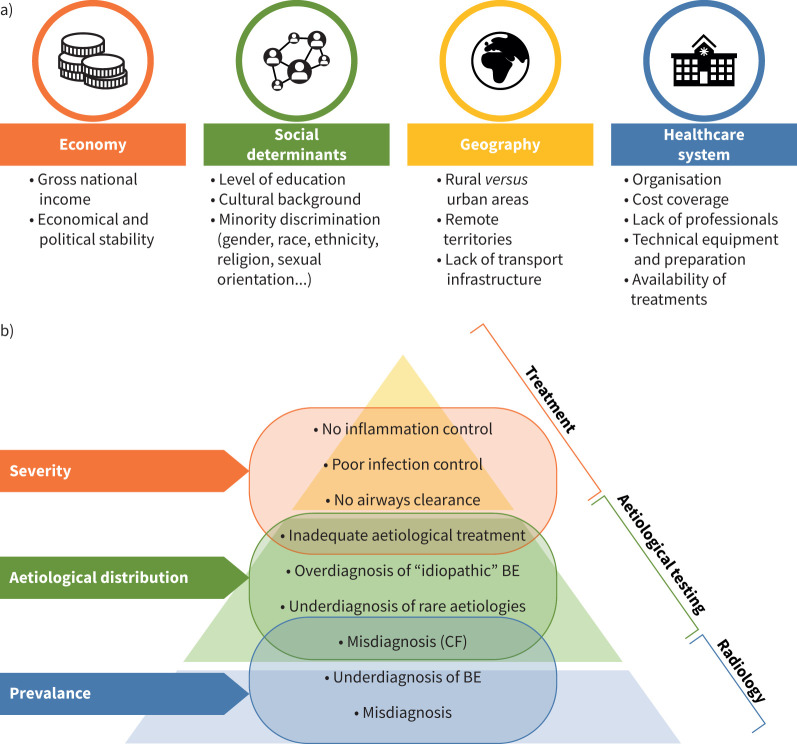
Differences in access to care have a considerable impact on the epidemiology of bronchiectasis. Literature on this topic is very limited, especially from some areas of the globe (i.e. South America, Africa and Asia). Healthcare accessibility depends on several factors, including geographical, infrastructural, cultural, political and economic features of the explored region [122, 123]. Differences in access to care are particularly evident for low- and middle-income countries, especially between rural and urban areas [124], but can be manifest even in high-income countries, particularly concerning remote regions of large nations and minority groups [125, 126]. Figure 2 displays socioeconomic and healthcare-related factors potentially affecting epidemiology.
FIGURE 2.
Causes and effects of unequal distribution to access to care in bronchiectasis. The first panel a) displays the main factors affecting patient's access to healthcare facilities. The second panel b) depicts the effects on bronchiectasis epidemiology of unequal access to management, diagnostic resources and treatments. Access to lower tiers of the pyramid is mandatory to reach the higher ones. BE: bronchiectasis; CF: cystic fibrosis.
To begin with, the diagnosis of bronchiectasis requires high-resolution chest CT scan to be performed [1]. According to World Health Organization reports, the distribution of CT units varies between and within different regions worldwide: a median of 13.84 units per million (UPM) of inhabitants is reported in Europe, 7.23 in America (excluding USA with 44.56 UPM), 5.82 in Western-Pacific countries (excluding Australia, reporting 66.92 UPM), 3.82 in Eastern-Mediterranean countries, 1.51 in South-East Asia and 0.42 in Africa [127]. Data are unavailable for many areas, such as Central Asia. This unequal distribution can definitely preclude patients from being diagnosed and factitiously reduce bronchiectasis prevalence and incidence in areas with lower resources.
The latest European Respiratory Society (ERS) guidelines suggest performing a minimum bundle of aetiological tests (i.e. full blood cell count, immunoglobulin dosage and ABPA testing) in every bronchiectasis patient [3]. However, the application of this recommendation depends on the availability of laboratory tests in different contexts, with the risk of introducing the above-mentioned epidemiological bias related to “idiopathic” bronchiectasis [71]. Data about access to testing are extremely scarce. In Europe, few national audits investigated the aetiological workout applied in their country: immunoglobulins and ABPA were commonly assessed in Belgium and UK, while in Italy less than a quarter of patients were tested [128–130]. However, these data could not be applied to the present day, as British and Italian audits pre-date ERS guidelines. Another British audit focused on PCD recorded that only 2% of bronchiectasis patients underwent testing [131, 132]. Although international guidelines agree on limiting PCD testing to patients with high clinical suspicion, this could contribute to the underestimation of the disease [131]. In the US and Canada, healthcare and reimbursement issues complicate PCD diagnosis even further, as only 10% of affected individuals seem to receive a diagnosis and management in specialised centres [133]. Hence American Thoracic Society diagnostic guidelines slightly differ from ERS ones, facilitating large-scale diagnosis despite territorial and practical limitations [134, 135]. In Asia, a 2020 survey assessed the application of international guidelines in Japan, South Korea, Singapore and Taiwan, demonstrating overall poor adherence [136]. In India, only 3.1% of patients enrolled in the national registry received a complete aetiological testing [7]. We could not find direct information regarding Australia and New Zealand, but evidence of diagnostic delay in patients with primary antibody deficiencies and bronchiectasis in these contexts might indirectly suggest underuse of immunoglobulin testing [137, 138]. Unfortunately, we found no information on South America, Africa, Middle East and the remaining part of Oceania. Details are available in table S1.
Up to date, pharmacological treatments have not been specifically approved for bronchiectasis in the US nor in Europe and most drugs are currently used off-label. Nevertheless, availability and correct applications of pharmacological treatments can potentially affect disease severity and prognosis. This is the case of inhaled antibiotics, recommended in selected cases by latest ERS guidelines [3]. Information about their accessibility is limited, as national drug regulatory agencies websites are often unavailable in English. Using an alternative approach based on research market reports, a list of countries with an inhaled antibiotic investor market was identified, as follows: three in North America, 10 in Europe, eight in Asia, one in Oceania, five in Africa and the Middle East, and two in South America [139]. Although this list may be incomplete, as it was obtained through unofficial sources, it may suggest that a consistent number of countries worldwide may not have access to inhaled antibiotics. Furthermore, drug availability is not the only limiting factor, since prescription, cost coverage and accessibility to administration devices also represent important challenges in some regions [140, 141]. Available data is reported in table S2. In the EMBARC registry, long-term inhaled antibiotics were prescribed in 7.7% of patients, with consistent geographical variation between different European regions. For instance, 20% of Spanish patients receive this treatment, while in Italy only 1.1% do, despite similar geographical and socioeconomic features [5]. A part of this variability could be explained considering different rates of chronic bacterial infections, but this could also reflect difficulties in access to this treatment. In the US, patients treated with inhaled antibiotics reach 10% of the registry, while in India they only represent 3.6% of patients [7, 142]. Availability of inhaled antibiotics seem to be guaranteed in most centres in Singapore, although their effective use was not measured directly. Japanese, Taiwanese and Korean centres seem to rarely have access to this treatment [136]. Finally, Australian data suggest underuse of inhaled antibiotics, that were prescribed in half of the eligible patients according to guidelines [143].
Despite available evidence supporting pulmonary rehabilitation (PR) and airway clearance techniques (ACTs) and the interest expressed by patients, access to physiotherapy remains challenging due to several reasons [3, 144]. First, referral to PR specialists depends on awareness of the treating physicians [145]. Furthermore, availability of dedicated infrastructures, PR rehabilitation teams, economic sustainability and adaptability to certain cultural backgrounds represent possible limitations to patients’ access in both high- and middle/low-income countries [146–148]. Significant differences in PR programmes for chronic respiratory conditions, including bronchiectasis, exist between Europe and the US [149]. In Europe, team expertise is more diversified, programmes usually include more patients and they are prevalently state-funded, while in the US GPs seem to be more likely to make referrals [149]. Attendance to PR/ACT programmes has also been reported to be very heterogeneous. In EMBARC, 51.5% of patients used ACT as part of their treatment [5]. This rate is significantly lower than the one reported in a UK survey (83%), but consistent with the one reported in the US registry (55.8%) [6, 150]. In the Indian registry, whilst 42% of patients had undergone ACT tuition, most eligible subjects missed out (62%) [7]. No publicly available data informs on the use of PR/ACT in the rest of Asia, but a survey reported that only half of the interviewed physicians from South Korea, Taiwan and Singapore regularly consider this treatment in bronchiectasis patients [136]. In Australia, most patients use ACT, while only 22% attended a PR programme despite 67% being eligible. Nonattendance was often due to an unspecified lack of referral [143]. Data from New Zealand are available only during exacerbations, when most patients (89%) received PR [151]. No data from South America and Africa is available. Implementation of new technologies, such as online videos or meetings, may assist in overcoming the barriers to referral by reaching people all over the world and decreasing the geographical differences limiting access to experienced physiotherapists [152]. However, this approach should not replace formal physiotherapy, nor distract both the medical community and patients from advocating access for each patient in need.
Conclusions
Overall, increasing prevalence and incidence rates of bronchiectasis have been described, potentially due to increasing disease awareness and advances in imaging techniques. Factors associated with increased prevalence seem to be female gender, socioeconomic deprivation and poor access to care. However, the ability to detect the disease seems to increase in presence of COPD or in case of screening protocols for cancer. In addition, a high socioeconomic burden has been broadly recognised worldwide in bronchiectasis, with main costs being related to hospitalisations, ICUs, antibiotics and loss of working days for both patients and caregivers.
Nevertheless, huge disparities in terms of disease epidemiology are still evident across different areas. Numerous are the potential determinants of the observed epidemiological variations in bronchiectasis. Relevant differences in geographical distribution of some pathogens have been described. This is the case for TB, NTM and Aspergillus-related conditions, which severely influence disease severity, treatment burden and healthcare costs across different regions. Among the usual pathogens, P. aeruginosa has also recently shown different prevalence rates and this can surely affect the overall disease severity, costs and outcomes. Both environmental factors and patients’ immunological features could contribute to these variations, but still more research is needed to unravel this puzzle, as highlighted in table 3.
TABLE 3.
Research priorities related to the epidemiology of bronchiectasis
| Research question | Related issues | Research perspectives to solve them |
|---|---|---|
| Which bronchiectasis definition should be used for epidemiological studies? | Several studies do not distinguish between the solitary radiological evidence of bronchiectasis and both clinically and radiologically significant bronchiectasis | A uniform, consensus-based definition of bronchiectasis for epidemiological studies |
| How should epidemiological data on bronchiectasis incidence, prevalence and mortality should be presented and analysed? | Different available data formats are hardly comparable | A uniform, consensus-based identification of the most appropriate way to present epidemiological data on bronchiectasis |
| What is the epidemiology of bronchiectasis in parts of the world not explored by currently available studies? | No data is available from highly populated areas of the globe, such as Africa, South America and the Middle East | More epidemiological, population-based studies in the unexplored areas Development of national and international registries in the aforementioned areas |
| How can reliable data on bronchiectasis-related mortality be obtained worldwide? | The mortality rate in bronchiectasis patients can vary according to aetiology, disease severity and activity, comorbidities, and access to medical care | More epidemiological, longitudinal long-term studies employing large-scale registries and real-world data with standardised diagnostic criteria |
| How does having comorbid asthma and/or COPD affect the epidemiology of bronchiectasis? | Do people with comorbid CADs have higher risk of developing bronchiectasis? The association of CAD with bronchiectasis can be chaotic and difficult to standardise at both national and international levels |
A uniform, consensus-based definition of the criteria for the association between asthma and bronchiectasis Large-scale, population-based studies to explore incidence and prevalence of bronchiectasis, asthma, COPD and their association in the general population |
| How does microbiology affect the epidemiology of bronchiectasis? | Few data are available on the clinical implications and outcomes of patients suffering from chronic infections due to bacteria other than Pseudomonas | Studies describing specific characteristics of post-TB and ABPA-related bronchiectasis More epidemiological studies addressing the outcomes of people affected by chronic infections from bacteria different than Pseudomonas and NTM Large-scale studies to describe the true impact of Pseudomonas on the prognosis of bronchiectasis patients |
| How could differences in access to care be approached? | Disease awareness might be low in specific areas of the globe | Increasing patients and healthcare professionals’ awareness of the disease Powering patient advocacy and requests for additional healthcare resources in specific areas of the globe Development of new drugs for bronchiectasis |
ABPA: allergic bronchopulmonary aspergillosis; CAD: chronic airway disease; NTM: nontuberculous mycobacteria; TB: tuberculosis.
More importantly, socioeconomic deprivation and limited access to care can be considered major determinants of increased prevalence and mortality of bronchiectasis in some regions. This is the case of high-risk populations, such as Indigenous people from Australia and New Zealand or Inuit people from Canada. However, current data on availability of most relevant diagnostic tests or treatments suggest enormous inequalities in access to care all over the world. Additionally, appropriate detection of comorbidities and their management can affect disease severity and mortality risk as recently pointed out by EMBARC data.
Certainly, more data is required from some regions such as South America, Africa and Asia, all underrepresented in the literature, to better understand the epidemiology and treatment gaps in the future.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0091-2024.SUPPLEMENT (430.6KB, pdf)
Footnotes
Number 6 in the Series “World Bronchiectasis Conference 2024” Edited by James D. Chalmers, Felix C. Ringshausen and Pieter C. Goeminne
This article has an editorial commentary: https://doi.org/10.1183/16000617.0124-2024
Provenance: Commissioned article, peer reviewed.
Previous articles in this series: No. 1: Perea L, Faner R, Chalmers JD, et al. Pathophysiology and genomics of bronchiectasis. Eur Respir Rev 2024; 33: 240055. No. 2: Mac Aogáin M, Dicker AJ, Mertsch P, et al. Infection and the microbiome in bronchiectasis. Eur Respir Rev 2024; 33: 240038. No. 3: Van Braeckel E, Bosteels C. Growing from common ground: nontuberculous mycobacteria and bronchiectasis. Eur Respir Rev 2024; 33: 240058. No. 4: De Angelis A, Johnson ED, Sutharsan S, et al. Exacerbations of bronchiectasis. Eur Respir Rev 2024; 33: 240085. No. 5: Choi H, Xu J-F, Chotirmall SH, et al. Bronchiectasis in Asia: a review of current status and challenges. Eur Respir Rev 2024; 33: 240096.
Conflict of interest: M. Nigro, I.F. Laska and E. Simonetta have nothing to disclose. L. Traversi reports support for attending meetings from Chiesi, TEVA, Grifols and Pari. E. Polverino reports grants from Grifols, consultancy fees from Grifols, Insmed, Chiesi, Pari, Electromed and AN2 Therapeutics, payment or honoraria for lectures, presentations, manuscript writing or educational events from Insmed, TEVA, Chiesi and Pari, support for attending meetings from INSMED, and a leadership role with EMBARC (Co-Chair).
References
- 1.Aliberti S, Goeminne PC, O'Donnell AE, et al. Criteria and definitions for the radiological and clinical diagnosis of bronchiectasis in adults for use in clinical trials: international consensus recommendations. Lancet Respir Med 2022; 10: 298–306. doi: 10.1016/S2213-2600(21)00277-0 [DOI] [PubMed] [Google Scholar]
- 2.Chalmers JD. Bronchiectasis from 2012 to 2022. Clin Chest Med 2022; 43: 1–6. doi: 10.1016/j.ccm.2021.12.001 [DOI] [PubMed] [Google Scholar]
- 3.Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017; 50: 1700629. doi: 10.1183/13993003.00629-2017 [DOI] [PubMed] [Google Scholar]
- 4.Hill AT, Sullivan AL, Chalmers JD, et al. British Thoracic Society guideline for bronchiectasis in adults. Thorax 2019; 74: Suppl. 1, 1–69. doi: 10.1136/thoraxjnl-2018-212463 [DOI] [PubMed] [Google Scholar]
- 5.Chalmers JD, Polverino E, Crichton ML, et al. Bronchiectasis in Europe: data on disease characteristics from the European Bronchiectasis registry (EMBARC). Lancet Respir Med 2023; 11: 637–649. doi: 10.1016/S2213-2600(23)00093-0 [DOI] [PubMed] [Google Scholar]
- 6.Aksamit TR, O'Donnell AE, Barker A, et al. Adult patients with bronchiectasis: a first look at the US bronchiectasis research registry. Chest 2017; 151: 982–992. doi: 10.1016/j.chest.2016.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhar R, Singh S, Talwar D, et al. Bronchiectasis in India: results from the European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC) and Respiratory Research Network of India Registry. Lancet Glob Health 2019; 7: e1269–e1279. doi: 10.1016/S2214-109X(19)30327-4 [DOI] [PubMed] [Google Scholar]
- 8.Chalmers JD, Aliberti S, Altenburg J, et al. Transforming clinical research and science in bronchiectasis: EMBARC3, a European Respiratory Society Clinical Research Collaboration. Eur Respir J 2023; 61: 2300769. doi: 10.1183/13993003.00769-2023 [DOI] [PubMed] [Google Scholar]
- 9.Chessari C, Simonetta E, Amati F, et al. Diagnostic delay in bronchiectasis: an Italian perspective. ERJ Open Res 2024; 10: 00713-2023. doi: 10.1183/23120541.00713-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green O, Liautaud S, Knee A, et al. Measuring accuracy of International Classification of Diseases codes in identification of patients with non-cystic fibrosis bronchiectasis. ERJ Open Res 2024; 10: 00715-2023. doi: 10.1183/23120541.00715-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quint JK, Millett ERC, Joshi M, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population-based cohort study. Eur Respir J 2016; 47: 186–193. doi: 10.1183/13993003.01033-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aliberti S, Sotgiu G, Lapi F, et al. Prevalence and incidence of bronchiectasis in Italy. BMC Pulm Med 2020; 20: 15. doi: 10.1186/s12890-020-1050-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Y, Wang C, Yao W, et al. [The prevalence and risk factors of bronchiectasis in residents aged 40 years old and above in seven cities in China]. Zhonghua Nei Ke Za Zhi 2013; 52: 379–382. [PubMed] [Google Scholar]
- 14.Ringshausen FC, Rademacher J, Pink I, et al. Increasing bronchiectasis prevalence in Germany, 2009–2017: a population-based cohort study. Eur Respir J 2019; 54: 1900499. doi: 10.1183/13993003.00499-2019 [DOI] [PubMed] [Google Scholar]
- 15.Monteagudo M, Rodríguez-Blanco T, Barrecheguren M, et al. Prevalence and incidence of bronchiectasis in Catalonia, Spain: a population-based study. Respir Med 2016; 121: 26–31. doi: 10.1016/j.rmed.2016.10.014 [DOI] [PubMed] [Google Scholar]
- 16.Ringshausen FC, de Roux A, Diel R, et al. Bronchiectasis in Germany: a population-based estimation of disease prevalence. Eur Respir J 2015; 46: 1805–1807. doi: 10.1183/13993003.00954-2015 [DOI] [PubMed] [Google Scholar]
- 17.Feng J, Sun L, Sun X, et al. Increasing prevalence and burden of bronchiectasis in urban Chinese adults, 2013–2017: a nationwide population-based cohort study. Respir Res 2022; 23: 111. doi: 10.1186/s12931-022-02023-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi H, Yang B, Nam H, et al. Population-based prevalence of bronchiectasis and associated comorbidities in South Korea. Eur Respir J 2019; 54: 1900194. doi: 10.1183/13993003.00194-2019 [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Mu W, Zhang J, et al. Global prevalence of non-tuberculous mycobacteria in adults with non-cystic fibrosis bronchiectasis 2006–2021: a systematic review and meta-analysis. BMJ Open 2022; 12: e055672. doi: 10.1136/bmjopen-2021-05567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phua HP, Lim W-Y, Ganesan G, et al. Epidemiology and economic burden of bronchiectasis requiring hospitalisation in Singapore. ERJ Open Res 2021; 7: 00334-2021. doi: 10.1183/23120541.00334-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park D-I, Kang S, Choi S. Evaluating the prevalence and incidence of bronchiectasis and nontuberculous mycobacteria in South Korea using the nationwide population data. Int J Environ Res Public Health 2021; 18: 9029. doi: 10.3390/ijerph18179029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snell N, Gibson J, Jarrold I, et al. Epidemiology of bronchiectasis in the UK: findings from the British Lung Foundation's “Respiratory Health of the Nation” project. Respir Med 2019; 158: 21–23. doi: 10.1016/j.rmed.2019.09.012 [DOI] [PubMed] [Google Scholar]
- 23.Weycker D, Edelsberg J, Oster G, et al. Prevalence and economic burden of bronchiectasis. Clin Pulm Med 2005; 12: 205–209. doi: 10.1097/01.cpm.0000171422.98696.ed [DOI] [Google Scholar]
- 24.Henkle E, Chan B, Curtis JR, et al. Characteristics and health-care utilization history of patients with bronchiectasis in US Medicare enrollees with prescription drug plans, 2006 to 2014. Chest 2018; 154: 1311–1320. doi: 10.1016/j.chest.2018.07.014 [DOI] [PubMed] [Google Scholar]
- 25.Weycker D, Hansen GL, Seifer FD. Prevalence and incidence of noncystic fibrosis bronchiectasis among US adults in 2013. Chron Respir Dis 2017; 14: 377–384. doi: 10.1177/1479972317709649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seitz AE, Olivier KN, Adjemian J, et al. Trends in bronchiectasis among Medicare beneficiaries in the United States, 2000 to 2007. Chest 2012; 142: 432–439. doi: 10.1378/chest.11-2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwak HJ, Moon J-Y, Choi YW, et al. High prevalence of bronchiectasis in adults: analysis of CT findings in a health screening program. Tohoku J Exp Med 2010; 222: 237–242. doi: 10.1620/tjem.222.237 [DOI] [PubMed] [Google Scholar]
- 28.Cai Q, Triphuridet N, Zhu Y, et al. Bronchiectasis in low-dose CT screening for lung cancer. Radiology 2022; 304: 437–447. doi: 10.1148/radiol.212547 [DOI] [PubMed] [Google Scholar]
- 29.Sanchez-Carpintero Abad M, Sanchez-Salcedo P, de-Torres JP, et al. Prevalence and burden of bronchiectasis in a lung cancer screening program. PLoS One 2020; 15: e0231204. doi: 10.1371/journal.pone.0231204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diel R, Ewig S, Blaas S, et al. Incidence of patients with noncystic fibrosis bronchiectasis in Germany A healthcare insurance claims data analysis. Respir Med 2019; 151: 121–127. doi: 10.1016/j.rmed.2019.04.007 [DOI] [PubMed] [Google Scholar]
- 31.Niewiadomska E, Kowalska M, Zejda JE. Spatial and temporal variability of bronchiectasis cases in Silesian voivodeship in 2006–2010. Int J Occup Med Environ Health 2016; 29: 699–708. doi: 10.13075/ijomeh.1896.00667 [DOI] [PubMed] [Google Scholar]
- 32.Choi H, Yang B, Kim YJ, et al. Increased mortality in patients with non cystic fibrosis bronchiectasis with respiratory comorbidities. Sci Rep 2021; 11: 7126. doi: 10.1038/s41598-021-86407-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts HJ, Hubbard R. Trends in bronchiectasis mortality in England and Wales. Respir Med 2010; 104: 981–985. doi: 10.1016/j.rmed.2010.02.022 [DOI] [PubMed] [Google Scholar]
- 34.Goeminne PC, Nawrot TS, Ruttens D, et al. Mortality in non-cystic fibrosis bronchiectasis: a prospective cohort analysis. Respir Med 2014; 108: 287–296. doi: 10.1016/j.rmed.2013.12.015 [DOI] [PubMed] [Google Scholar]
- 35.Nowiński A, Stachyra K, Szybińska M, et al. The influence of comorbidities on mortality in bronchiectasis: a prospective, observational study. Adv Clin Exp Med 2021; 30: 1315–1321. doi: 10.17219/acem/144200 [DOI] [PubMed] [Google Scholar]
- 36.Martínez-Garcia MA, de Gracia J, Vendrell Relat M, et al. Multidimensional approach to non-cystic fibrosis bronchiectasis: the FACED score. Eur Respir J 2014; 43: 1357–1367. doi: 10.1183/09031936.00026313 [DOI] [PubMed] [Google Scholar]
- 37.Onen ZP, Eris Gulbay B, Sen E, et al. Analysis of the factors related to mortality in patients with bronchiectasis. Respir Med 2007; 101: 1390–1397. doi: 10.1016/j.rmed.2007.02.002 [DOI] [PubMed] [Google Scholar]
- 38.Loebinger MR, Wells AU, Hansell DM, et al. Mortality in bronchiectasis: a long-term study assessing the factors influencing survival. Eur Respir J 2009; 34: 843–849. doi: 10.1183/09031936.00003709 [DOI] [PubMed] [Google Scholar]
- 39.Ellis HC, Cowman S, Fernandes M, et al. Predicting mortality in bronchiectasis using bronchiectasis severity index and FACED scores: a 19-year cohort study. Eur Respir J 2016; 47: 482–489. doi: 10.1183/13993003.01312-2015 [DOI] [PubMed] [Google Scholar]
- 40.Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med 2014; 189: 576–585. doi: 10.1164/rccm.201309-1575OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang X, Bi J, Yang D, et al. Emphysema is an independent risk factor for 5-year mortality in patients with bronchiectasis. Clin Respir J 2017; 11: 887–894. doi: 10.1111/crj.12432 [DOI] [PubMed] [Google Scholar]
- 42.Wang R, Ding S, Lei C, et al. The contribution of Pseudomonas aeruginosa infection to clinical outcomes in bronchiectasis: a prospective cohort study. Ann Med 2021; 53: 459–469. doi: 10.1080/07853890.2021.1900594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JM, Lee SA, Han CH, et al. Body mass index as a predictor of mortality in bronchiectasis: a nationwide population-based study. Respir Med 2021; 180: 106370. doi: 10.1016/j.rmed.2021.106370 [DOI] [PubMed] [Google Scholar]
- 44.Dhar R, Singh S, Talwar D, et al. Clinical outcomes of bronchiectasis in India: data from the EMBARC/Respiratory Research Network of India registry. Eur Respir J 2023; 61: 2200611. doi: 10.1183/13993003.00611-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young SL, Puan Y, Chew SY, et al. Heterogeneity of non-cystic-fibrosis bronchiectasis in multiethnic Singapore: a prospective cohort study at a tertiary pulmonology centre. Ann Acad Med Singap 2021; 50: 556–565. doi: 10.47102/annals-acadmedsg.202178 [DOI] [PubMed] [Google Scholar]
- 46.Rees M, Liu B, Pascoe A, et al. Improving care for people with bronchiectasis: opportunities and challenges highlighted from service evaluation. Intern Med J 2023; 53: 753–759. doi: 10.1111/imj.15730 [DOI] [PubMed] [Google Scholar]
- 47.Gibbs C, Howarth T, Ticoalu A, et al. Bronchiectasis among Indigenous adults in the Top End of the Northern Territory, 2011–2020: a retrospective cohort study. Med J Aust 2024; 220: 188–195. doi: 10.5694/mja2.52204 [DOI] [PubMed] [Google Scholar]
- 48.Machado BC, Jacques PS, Penteado LP, et al. Prognostic factors in adult patients with non-cystic fibrosis bronchiectasis. Lung 2018; 196: 691–697. doi: 10.1007/s00408-018-0165-z [DOI] [PubMed] [Google Scholar]
- 49.Mateus SP, Ribeiro-Alves M, Salles REB, et al. Mortality and comorbidities in patients with bronchiectasis over a 3-year follow-up. Medicine (Baltimore) 2022; 101: e32537. doi: 10.1097/MD.0000000000032537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roberts JM, Goyal V, Kularatna S, et al. The economic burden of bronchiectasis: a systematic review. Chest 2023; 164: 1396–1421. doi: 10.1016/j.chest.2023.06.040 [DOI] [PubMed] [Google Scholar]
- 51.Ronco R, Franco G, Monzio Compagnoni M, et al. Healthcare costs and resource utilisation in bronchiectasis, asthma and COPD. ERJ Open Res 2023; 9: 00158-2023. doi: 10.1183/23120541.00158-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diel R, Chalmers JD, Rabe KF, et al. Economic burden of bronchiectasis in Germany. Eur Respir J 2019; 53: 1802033. doi: 10.1183/13993003.02033-2018 [DOI] [PubMed] [Google Scholar]
- 53.de la Rosa D, Martínez-Garcia M-A, Olveira C, et al. Annual direct medical costs of bronchiectasis treatment: impact of severity, exacerbations, chronic bronchial colonization and chronic obstructive pulmonary disease coexistence. Chron Respir Dis 2016; 13: 361–371. doi: 10.1177/1479972316643698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de la Rosa Carrillo D, Navarro Rolon A, Girón Moreno RM, et al. Cost of hospitalizations due to exacerbation in patients with non-cystic fibrosis bronchiectasis. Respiration 2018; 96: 406–416. doi: 10.1159/000489935 [DOI] [PubMed] [Google Scholar]
- 55.Navaratnam V, Muirhead CR, Hubbard RB, et al. Critical care admission trends and outcomes in individuals with bronchiectasis in the UK. QJM 2016; 109: 523–526. doi: 10.1093/qjmed/hcv206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sánchez-Muñoz G, López de Andrés A, Jiménez-García R, et al. Time trends in hospital admissions for bronchiectasis: analysis of the Spanish national hospital discharge data (2004 to 2013). PLoS One 2016; 11: e0162282. doi: 10.1371/journal.pone.0162282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davey RX. Health disparities among Australia's remote-dwelling aboriginal people: a report from 2020. J Appl Lab Med 2021; 6: 125–141. doi: 10.1093/jalm/jfaa182 [DOI] [PubMed] [Google Scholar]
- 58.Kruavit A, Fox M, Pearson R, et al. Chronic respiratory disease in the regional and remote population of the Northern Territory Top End: a perspective from the specialist respiratory outreach service. Aust J Rural Health 2017; 25: 275–284. doi: 10.1111/ajr.12349 [DOI] [PubMed] [Google Scholar]
- 59.Steinfort DP, Brady S, Weisinger HS, et al. Bronchiectasis in Central Australia: a young face to an old disease. Respir Med 2008; 102: 574–578. doi: 10.1016/j.rmed.2007.11.007 [DOI] [PubMed] [Google Scholar]
- 60.Einsiedel L, Pham H, Au V, et al. Predictors of non-cystic fibrosis bronchiectasis in Indigenous adult residents of central Australia: results of a case–control study. ERJ Open Res 2019; 5: 00001-2019. doi: 10.1183/23120541.00001-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Einsiedel L, Fernandes L, Spelman T, et al. Bronchiectasis is associated with human T-lymphotropic virus 1 infection in an Indigenous Australian population. Clin Infect Dis 2012; 54: 43–50. doi: 10.1093/cid/cir766 [DOI] [PubMed] [Google Scholar]
- 62.Blackall SR, Hong JB, King P, et al. Bronchiectasis in Indigenous and nonIndigenous residents of Australia and New Zealand. Respirology 2018; 23: 743–749. doi: 10.1111/resp.13280 [DOI] [PubMed] [Google Scholar]
- 63.Mehra S, Chang A, Lam C, et al. Bronchiectasis among Australian Aboriginal and non-Aboriginal patients in the regional and remote population of the Northern Territory of Australia. Rural Remote Health 2021; 21: 6390. doi: 10.22605/RRH6390 [DOI] [PubMed] [Google Scholar]
- 64.de Boer S, Lewis CA, Fergusson W, et al. Ethnicity, socioeconomic status and the severity and course of non-cystic fibrosis bronchiectasis. Intern Med J 2018; 48: 845–850. doi: 10.1111/imj.13739 [DOI] [PubMed] [Google Scholar]
- 65.Roberts ME, Lowndes L, Milne DG, et al. Socioeconomic deprivation, readmissions, mortality and acute exacerbations of bronchiectasis. Intern Med J 2012; 42: e129–e136. DOI: 10.1111/j.1445-5994.2011.02444.x [DOI] [PubMed] [Google Scholar]
- 66.Singleton R, Morris A, Redding G, et al. Bronchiectasis in Alaska Native children: causes and clinical courses. Pediatr Pulmonol 2000; 29: 182–187. doi: [DOI] [PubMed] [Google Scholar]
- 67.Sibanda D, Singleton R, Clark J, et al. Adult outcomes of childhood bronchiectasis. Int J Circumpolar Health 2020; 79: 1731059. doi: 10.1080/22423982.2020.1731059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kinghorn B, Singleton R, McCallum GB, et al. Clinical course of chronic suppurative lung disease and bronchiectasis in Alaska Native children. Pediatr Pulmonol 2018; 53: 1662–1669. doi: 10.1002/ppul.24174 [DOI] [PubMed] [Google Scholar]
- 69.Hunter-Schouela J, Geraghty MT, Hegele RA, et al. First reports of primary ciliary dyskinesia caused by a shared DNAH11 allele in Canadian Inuit. Pediatr Pulmonol 2023; 58: 1942–1949. doi: 10.1002/ppul.26414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Das L, Kovesi TA. Bronchiectasis in children from Qikiqtani (Baffin) Region, Nunavut, Canada. Ann Am Thorac Soc 2015; 12: 96–100. doi: 10.1513/AnnalsATS.201406-257OC [DOI] [PubMed] [Google Scholar]
- 71.Araújo D, Shteinberg M, Aliberti S, et al. Standardised classification of the aetiology of bronchiectasis using an objective algorithm. Eur Respir J 2017; 50: 1701289. doi: 10.1183/13993003.01289-2017 [DOI] [PubMed] [Google Scholar]
- 72.Amati F, Franceschi E, Gramegna A, et al. Investigating the etiology of bronchiectasis: you do not find what you do not look for. Respiration 2017; 93: 228–229. doi: 10.1159/000455880 [DOI] [PubMed] [Google Scholar]
- 73.Polverino E, Dimakou K, Hurst J, et al. The overlap between bronchiectasis and chronic airway diseases: state of the art and future directions. Eur Respir J 2018; 52: 1800328. doi: 10.1183/13993003.00328-2018 [DOI] [PubMed] [Google Scholar]
- 74.Traversi L, Miravitlles M, Martinez-Garcia MA, et al. ROSE: radiology, obstruction, symptoms and exposure – a Delphi consensus definition of the association of COPD and bronchiectasis by the EMBARC Airways Working Group. ERJ Open Res 2021; 7: 00399-2021. doi: 10.1183/23120541.00399-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Polverino E, De Soyza A, Dimakou K, et al. The association between bronchiectasis and chronic obstructive pulmonary disease: data from the European Bronchiectasis Registry (EMBARC). Am J Respir Crit Care Med 2024; 210: 119-127. DOI: 10.1164/rccm.202309-1614OC [DOI] [PubMed] [Google Scholar]
- 76.Martínez-García MÁ, de la Rosa-Carrillo D, Soler-Cataluña JJ, et al. Bronchial infection and temporal evolution of bronchiectasis in patients with chronic obstructive pulmonary disease. Clin Infect Dis 2021; 72: 403–410. doi: 10.1093/cid/ciaa069 [DOI] [PubMed] [Google Scholar]
- 77.Martinez-Garcia MA, Miravitlles M. Bronchiectasis in COPD patients: more than a comorbidity? Int J Chron Obstruct Pulmon Dis 2017; 12: 1401–1411. doi: 10.2147/COPD.S132961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martínez-García M-A, de la Rosa Carrillo D, Soler-Cataluña J-J, et al. Prognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 187: 823–831. doi: 10.1164/rccm.201208-1518OC [DOI] [PubMed] [Google Scholar]
- 79.De Soyza A, McDonnell MJ, Goeminne PC, et al. Bronchiectasis rheumatoid overlap syndrome is an independent risk factor for mortality in patients with bronchiectasis: a multicenter cohort study. Chest 2017; 151: 1247–1254. doi: 10.1016/j.chest.2016.12.024 [DOI] [PubMed] [Google Scholar]
- 80.Polverino E, Dimakou K, Traversi L, et al. Bronchiectasis and asthma: data from the European Bronchiectasis Registry (EMBARC). J Allergy Clin Immunol 2024; 153: 1553–1562. doi: 10.1016/j.jaci.2024.01.027 [DOI] [PubMed] [Google Scholar]
- 81.Sheng H, Yao X, Wang X, et al. Prevalence and clinical implications of bronchiectasis in patients with overlapping asthma and chronic rhinosinusitis: a single-center prospective study. BMC Pulm Med 2021; 21: 211. doi: 10.1186/s12890-021-01575-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shteinberg M, Chalmers JD, Narayana JK, et al. bronchiectasis with chronic rhinosinusitis is associated with eosinophilic airway inflammation and is distinct from asthma. Ann Am Thorac Soc 2024; 21: 748–758. doi: 10.1513/AnnalsATS.202306-551OC [DOI] [PubMed] [Google Scholar]
- 83.McDonnell MJ, Aliberti S, Goeminne PC, et al. Comorbidities and the risk of mortality in patients with bronchiectasis: an international multicentre cohort study. Lancet Respir Med 2016; 4: 969–979. doi: 10.1016/S2213-2600(16)30320-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clofent D, Álvarez A, Traversi L, et al. Comorbidities and mortality risk factors for patients with bronchiectasis. Expert Rev Respir Med 2021; 15: 623–634. doi: 10.1080/17476348.2021.1886084 [DOI] [PubMed] [Google Scholar]
- 85.Cavalli CAM, Gabbiadini R, Dal Buono A, et al. Lung involvement in inflammatory bowel diseases: shared pathways and unwanted connections. J Clin Med 2023; 12: 6419. doi: 10.3390/jcm12196419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goeminne PC, Bijnens E, Nemery B, et al. Impact of traffic related air pollution indicators on non-cystic fibrosis bronchiectasis mortality: a cohort analysis. Respir Res 2014; 15: 108. doi: 10.1186/s12931-014-0108-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meghji J, Simpson H, Squire SB, et al. A systematic review of the prevalence and pattern of imaging defined post-TB lung disease. PLoS One 2016; 11: e0161176. doi: 10.1371/journal.pone.0161176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Visser SK, Bye PTP, Fox GJ, et al. Australian adults with bronchiectasis: the first report from the Australian Bronchiectasis Registry. Respir Med 2019; 155: 97–103. doi: 10.1016/j.rmed.2019.07.016 [DOI] [PubMed] [Google Scholar]
- 89.Lee H, Choi H, Chalmers JD, et al. Characteristics of bronchiectasis in Korea: first data from the Korean Multicentre Bronchiectasis Audit and Research Collaboration registry and comparison with other international registries. Respirology 2021; 26: 619–621. doi: 10.1111/resp.14059 [DOI] [PubMed] [Google Scholar]
- 90.Huang H-Y, Chung F-T, Lo C-Y, et al. Etiology and characteristics of patients with bronchiectasis in Taiwan: a cohort study from 2002 to 2016. BMC Pulm Med 2020; 20: 45. doi: 10.1186/s12890-020-1080-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fong I, Low TB, Yii A. Characterisation of the post-tuberculous phenotype of bronchiectasis: a real-world observational study. Chron Respir Dis 2022; 19: 147997312210987. doi: 10.1177/14799731221098714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Choi H, Lee H, Ra S, et al. Clinical characteristics of patients with post-tuberculosis bronchiectasis: findings from the KMBARC registry. J Clin Med 2021; 10: 4542. doi: 10.3390/jcm10194542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chu H, Zhao L, Xiao H, et al. Systematic review/meta-analysis prevalence of nontuberculous mycobacteria in patients with bronchiectasis: a meta-analysis. Arch Med Sci 2014; 4: 661–668. doi: 10.5114/aoms.2014.44857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sin S, Yun SY, Kim JM, et al. Mortality risk and causes of death in patients with non-cystic fibrosis bronchiectasis. Respir Res 2019; 20: 271. doi: 10.1186/s12931-019-1243-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Park J, Kim S, Lee YJ, et al. Factors associated with radiologic progression of non-cystic fibrosis bronchiectasis during long-term follow-up. Respirology 2016; 21: 1049–1054. doi: 10.1111/resp.12768 [DOI] [PubMed] [Google Scholar]
- 96.Faverio P, Stainer A, Bonaiti G, et al. Characterizing non-tuberculous mycobacteria infection in bronchiectasis. Int J Mol Sci 2016; 17: 1913. doi: 10.3390/ijms17111913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Suska K, Amati F, Sotgiu G, et al. Nontuberculous mycobacteria infection and pulmonary disease in bronchiectasis. ERJ Open Res 2022; 8: 00060-2022. doi: 10.1183/23120541.00060-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Izhakian S, Wasser WG, Fuks L, et al. Lobar distribution in non-cystic fibrosis bronchiectasis predicts bacteriologic pathogen treatment. Eur J Clin Microbiol Infect Dis 2016; 35: 791–796. doi: 10.1007/s10096-016-2599-7 [DOI] [PubMed] [Google Scholar]
- 99.Máiz L, Girón R, Olveira C, et al. Prevalence and factors associated with nontuberculous mycobacteria in non-cystic fibrosis bronchiectasis: a multicenter observational study. BMC Infect Dis 2016; 16: 437. doi: 10.1186/s12879-016-1774-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pieters A, Bakker M, Hoek RAS, et al. Predicting factors for chronic colonization of Pseudomonas aeruginosa in bronchiectasis. Eur J Clin Microbiol Infect Dis 2019; 38: 2299–2304. doi: 10.1007/s10096-019-03675-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Buscot M, Pottier H, Marquette C-H, et al. Phenotyping adults with non-cystic fibrosis bronchiectasis: a 10-year cohort study in a French regional university hospital center. Respiration 2016; 92: 1–8. doi: 10.1159/000446923 [DOI] [PubMed] [Google Scholar]
- 102.Dimakou K, Triantafillidou C, Toumbis M, et al. Non CF-bronchiectasis: aetiologic approach, clinical, radiological, microbiological and functional profile in 277 patients. Respir Med 2016; 116: 1–7. doi: 10.1016/j.rmed.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 103.Reich JM. Non-TB mycobacterial infection–bronchiectasis nexus. Chest 2019; 155: 1301–1302. doi: 10.1016/j.chest.2019.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reich JM, Johnson RE. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern. Chest 1992; 101: 1605–1609. doi: 10.1378/chest.101.6.1605 [DOI] [PubMed] [Google Scholar]
- 105.Ku JH, Ranches G, Siegel SAR, et al. Lady Windermere's counterpart? Pulmonary nontuberculous mycobacteria in men with bronchiectasis. Diagn Microbiol Infect Dis 2020; 96: 114916. doi: 10.1016/j.diagmicrobio.2019.114916 [DOI] [PubMed] [Google Scholar]
- 106.Mirsaeidi M, Allen MB, Ebrahimi G, et al. Hospital costs in the US for pulmonary mycobacterial diseases. Int J Mycobacteriol 2015; 4: 217–221. doi: 10.1016/j.ijmyco.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lin C-Y, Huang H-Y, Hsieh M-H, et al. Impacts of nontuberculous mycobacteria isolates in non-cystic fibrosis bronchiectasis: a 16-year cohort study in Taiwan. Front Microbiol 2022; 13: 868435. doi: 10.3389/fmicb.2022.868435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Denning DW, Pleuvry A, Cole DC. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med Mycol 2013; 51: 361–370. doi: 10.3109/13693786.2012.738312 [DOI] [PubMed] [Google Scholar]
- 109.De Soyza A, Aliberti S. Bronchiectasis and Aspergillus: how are they linked? Med Mycol 2017; 55: 69–81. doi: 10.1093/mmy/myw109 [DOI] [PubMed] [Google Scholar]
- 110.Denning DW. Global incidence and mortality of severe fungal disease. Lancet Infect Dis 2024; 24: e428–e438. DOI: 10.1016/S1473-3099(23)00692-8 [DOI] [PubMed] [Google Scholar]
- 111.Martínez-García MA, Soler-Cataluña J-J, Perpiñá-Tordera M, et al. Factors associated with lung function decline in adult patients with stable non-cystic fibrosis bronchiectasis. Chest 2007; 132: 1565–1572. doi: 10.1378/chest.07-0490 [DOI] [PubMed] [Google Scholar]
- 112.Martinez-García MA, Oscullo G, Posadas T, et al. Pseudomonas aeruginosa and lung function decline in patients with bronchiectasis. Clin Microbiol Infect 2021; 27: 428–434. doi: 10.1016/j.cmi.2020.04.007 [DOI] [PubMed] [Google Scholar]
- 113.Davies G, Wells AU, Doffman S, et al. The effect of Pseudomonas aeruginosa on pulmonary function in patients with bronchiectasis. Eur Respir J 2006; 28: 974–979. doi: 10.1183/09031936.06.00074605 [DOI] [PubMed] [Google Scholar]
- 114.Wang L-L, Lu H-W, Li L-L, et al. Pseudomonas aeruginosa isolation is an important predictor for recurrent hemoptysis after bronchial artery embolization in patients with idiopathic bronchiectasis: a multicenter cohort study. Respir Res 2023; 24: 84. doi: 10.1186/s12931-023-02391-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Finch S, McDonnell MJ, Abo-Leyah H, et al. A comprehensive analysis of the impact of Pseudomonas aeruginosa colonisation on prognosis in adult bronchiectasis. Ann Am Thorac Soc 2015; 12: 1602–1611. doi: 10.1513/AnnalsATS.201506-333OC [DOI] [PubMed] [Google Scholar]
- 116.Araújo D, Shteinberg M, Aliberti S, et al. The independent contribution of Pseudomonas aeruginosa infection to long-term clinical outcomes in bronchiectasis. Eur Respir J 2018; 51: 1701953. doi: 10.1183/13993003.01953-2017 [DOI] [PubMed] [Google Scholar]
- 117.Shafiq I, Wahla AS, Uzbeck MH, et al. Etiology and clinical characteristics of a non-cystic fibrosis bronchiectasis cohort in a middle eastern population. BMC Pulm Med 2023; 23: 250. doi: 10.1186/s12890-023-02543-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chalmers JD, Aliberti S, Filonenko A, et al. Characterization of the “frequent exacerbator phenotype” in bronchiectasis. Am J Respir Crit Care Med 2018; 197: 1410–1420. doi: 10.1164/rccm.201711-2202OC [DOI] [PubMed] [Google Scholar]
- 119.McDonnell MJ, Jary HR, Perry A, et al. Non cystic fibrosis bronchiectasis: a longitudinal retrospective observational cohort study of Pseudomonas persistence and resistance. Respir Med 2015; 109: 716–726. doi: 10.1016/j.rmed.2014.07.021 [DOI] [PubMed] [Google Scholar]
- 120.Yang S-H, Song MJ, Kim YW, et al. Understanding the effects of Haemophilus influenzae colonization on bronchiectasis: a retrospective cohort study. BMC Pulm Med 2024; 24: 7. doi: 10.1186/s12890-023-02823-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.García Clemente M, Olveira C, Girón R, et al. Impact of chronic bronchial infection by Staphylococcus aureus on bronchiectasis. J Clin Med 2022; 11: 3960. doi: 10.3390/jcm11143960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.MacKinnon NJ, Emery V, Waller J, et al. Mapping health disparities in 11 high-income nations. JAMA Netw Open 2023; 6: e2322310. doi: 10.1001/jamanetworkopen.2023.22310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Peters DH, Garg A, Bloom G, et al. Poverty and access to health care in developing countries. Ann N Y Acad Sci 2008; 1136: 161–171. doi: 10.1196/annals.1425.011 [DOI] [PubMed] [Google Scholar]
- 124.Orach CG. Health equity: challenges in low income countries. Afr Health Sci 2009; 9: Suppl. 2, S49–S51. [PMC free article] [PubMed] [Google Scholar]
- 125.Schutz KL, Fancourt N, Chang AB, et al. Transition of pediatric patients with bronchiectasis to adult medical care in the Northern Territory: a retrospective chart audit. Front Pediatr 2023; 11: 1184303. doi: 10.3389/fped.2023.1184303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.LaVeist TA, Pérez-Stable EJ, Richard P, et al. The economic burden of racial, ethnic, and educational health inequities in the US. JAMA 2023; 329: 1682–1692. doi: 10.1001/jama.2023.5965 [DOI] [PubMed] [Google Scholar]
- 127.World Health Organization . Computed tomography units (per million population), total density. Date last accessed: 11 March 2024. Date last updated: 2024. www.who.int/data/gho/data/indicators/indicator-details/GHO/total-density-per-million-population-computed-tomography-units
- 128.Schoovaerts K, Lorent N, Goeminne P, et al. National survey on the management of adult bronchiectasis in Belgium. COPD 2019; 16: 72–74. doi: 10.1080/15412555.2019.1566895 [DOI] [PubMed] [Google Scholar]
- 129.Hill AT, Routh C, Welham S. National BTS bronchiectasis audit 2012: is the quality standard being adhered to in adult secondary care? Thorax 2014; 69: 292–294. doi: 10.1136/thoraxjnl-2013-203739 [DOI] [PubMed] [Google Scholar]
- 130.Aliberti S, Hill AT, Mantero M, et al. Quality standards for the management of bronchiectasis in Italy: a national audit. Eur Respir J 2016; 48: 244–248. doi: 10.1183/13993003.00232-2016 [DOI] [PubMed] [Google Scholar]
- 131.Shoemark A, Griffin H, Wheway G, et al. Genome sequencing reveals underdiagnosis of primary ciliary dyskinesia in bronchiectasis. Eur Respir J 2022; 60: 2200176. doi: 10.1183/13993003.00176-2022 [DOI] [PubMed] [Google Scholar]
- 132.Lucas JS, Barbato A, Collins SA, et al. European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia. Eur Respir J 2017; 49: 1601090. doi: 10.1183/13993003.01090-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.O'Connor MG, Horani A, Shapiro AJ. Progress in diagnosing primary ciliary dyskinesia: the North American perspective. Diagnostics 2021; 11: 1278. doi: 10.3390/diagnostics11071278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shapiro AJ, Davis SD, Polineni D, et al. Diagnosis of primary ciliary dyskinesia. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med 2018; 197: e24–e39. doi: 10.1164/rccm.201805-0819ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shapiro AJ, Zariwala MA, Ferkol T, et al. Diagnosis, monitoring, and treatment of primary ciliary dyskinesia: PCD foundation consensus recommendations based on state of the art review. Pediatr Pulmonol 2016; 51: 115–132. doi: 10.1002/ppul.23304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kim HC, Suzuki M, Lim HF, et al. Survey of the management of patients with bronchiectasis: a pilot investigation in Asian populations. Korean J Intern Med 2021; 36: 1402–1409. doi: 10.3904/kjim.2020.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Slade CA, Bosco JJ, Binh Giang T, et al. Delayed diagnosis and complications of predominantly antibody deficiencies in a cohort of Australian adults. Front Immunol 2018; 9: 694. doi: 10.3389/fimmu.2018.00694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ameratunga R, Jordan A, Cavadino A, et al. Bronchiectasis is associated with delayed diagnosis and adverse outcomes in the New Zealand common variable immunodeficiency disorders cohort study. Clin Exp Immunol 2021; 204: 352–360. doi: 10.1111/cei.13595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Coherent Market Insights . Inhaled Antibiotics Market Analysis. Burlingame, Coherent Market Insights, 2023. [Google Scholar]
- 140.Shteinberg M, Spinou A, Goeminne P, et al. Prescribing preferences and availability of nebulisers and inhalers for inhaled medications in bronchiectasis: results of a specialist survey. ERJ Open Res 2024; 10: 00724-2023. doi: 10.1183/23120541.00724-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Greally P, Whitaker P, Peckham D. Challenges with current inhaled treatments for chronic Pseudomonas aeruginosa infection in patients with cystic fibrosis. Curr Med Res Opin 2012; 28: 1059–1067. doi: 10.1185/03007995.2012.674500 [DOI] [PubMed] [Google Scholar]
- 142.Henkle E, Aksamit TR, Barker AF, et al. Pharmacotherapy for non-cystic fibrosis bronchiectasis. Chest 2017; 152: 1120–1127. doi: 10.1016/j.chest.2017.04.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Visser SK, Bye PTP, Fox GJ, et al. Management of Australian adults with bronchiectasis in tertiary care: evidence-based or access-driven? Lung 2019; 197: 803–810. doi: 10.1007/s00408-019-00280-x [DOI] [PubMed] [Google Scholar]
- 144.Lee AL, Smith R, Burr L, et al. “Teach me how to look after myself”: what people with bronchiectasis want from education in a pulmonary rehabilitation setting. Clin Respir J 2023; 17: 59–69. doi: 10.1111/crj.13563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Johnston KN, Young M, Grimmer KA, et al. Barriers to, and facilitators for, referral to pulmonary rehabilitation in COPD patients from the perspective of Australian general practitioners: a qualitative study. Prim Care Respir J 2013; 22: 319–324. doi: 10.4104/pcrj.2013.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sami R, Salehi K, Hashemi M, et al. Exploring the barriers to pulmonary rehabilitation for patients with chronic obstructive pulmonary disease: a qualitative study. BMC Health Serv Res 2021; 21: 828. doi: 10.1186/s12913-021-06814-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Augustine A, Bhat A, Vaishali K, et al. Barriers to pulmonary rehabilitation – a narrative review and perspectives from a few stakeholders. Lung India 2021; 38: 59–63. doi: 10.4103/lungindia.lungindia_116_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jayamaha AR, Perera CH, Orme MW, et al. Protocol for the cultural adaptation of pulmonary rehabilitation and subsequent testing in a randomised controlled feasibility trial for adults with chronic obstructive pulmonary disease in Sri Lanka. BMJ Open 2020; 10: e041677. doi: 10.1136/bmjopen-2020-041677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Spruit MA, Pitta F, Garvey C, et al. Differences in content and organisational aspects of pulmonary rehabilitation programmes. Eur Respir J 2014; 43: 1326–1337. doi: 10.1183/09031936.00145613 [DOI] [PubMed] [Google Scholar]
- 150.McLeese RH, O'Neill K, O'Neill B, et al. Airway clearance treatments in bronchiectasis: feasibility of linking survey results to registry data and a survey of patients’ and physiotherapists’ practices. ERJ Open Res 2023; 9: 00540-2022. doi: 10.1183/23120541.00540-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Phillips J, Lee A, Pope R, et al. Physiotherapists’ use of airway clearance techniques during an acute exacerbation of bronchiectasis: a survey study. Arch Physiother 2021; 11: 3. doi: 10.1186/s40945-020-00097-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nicolson C, Holland A, Lee A. The bronchiectasis toolbox—a comprehensive website for the management of people with bronchiectasis. Med Sci (Basel) 2017; 5: 13. doi: 10.3390/medsci5020013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0091-2024.SUPPLEMENT (430.6KB, pdf)