Abstract
Prostheses or implantable medical devices (IMDs) are parts made of natural or artificial materials intended to replace a body structure and therefore must be well tolerated by living tissues. The types of IMDs currently available and usable are very varied and capable of replacing almost any human organ. A high but imprecise percentage of Spaniards are carriers of one or more IMDs to which they often owe their quality of life or survival. IMDs are constructed with different types of materials that are often combined in the same prosthesis. These materials must combine harmlessness to human tissues with high wear resistance. Their durability depends on many factors both on the host and the type of prosthesis, but the vast majority last for more than 10-15 years or remain in function for the lifetime of the patient. The most frequently implanted IMDs are placed in the heart or great vessels, joints, dental arches or breast and their most frequent complications are classified as non-infectious, particularly loosening or intolerance, and infectious. Complications, when they occur, lead to a significant increase in morbidity, their repair or replacement multiplies the health care cost and, on occasions, can cause the death of the patient. The fight against IMD complications is currently focused on the design of new materials that are more resistant to wear and infection and the use of antimicrobial substances that are released from these materials. Their production requires multidisciplinary technical teams, but also a willingness on the part of industry and health authorities that is not often found in Spain or in most European nations. Scientific production on prostheses and IMD in Spain is estimated to be less than 2% of the world total, and probably below what corresponds to our level of socio-economic development. The future of IMDs involves, among other factors, examining the potential role of Artificial Intelligence in their design, knowledge of tissue regeneration, greater efficiency in preventing infections and taking alternative treatments beyond antimicrobials, such as phage therapy. For these and other reasons, the Ramón Areces Foundation convened a series of experts in different fields related to prostheses and IMDs who answered and discussed a series of questions previously formulated by the Scientific Council. The following lines are the written testimony of these questions and the answers to them.
Keywords: Prostheses, Implantable Medical Devices, arthroplasties, endovascular devices, infection, loosening, endocarditis, materials, biomaterials, cost, scientific production, design, health care expenditure
Abstract
Las prótesis o dispositivos médicos implantables (DMIs) son piezas fabricadas con materiales naturales o artificiales destinadas a sustituir una estructura corporal y por tanto deben ser bien toleradas por los tejidos vivos. Los tipos de DMIs existentes y utilizables en el momento actual son muy variados y capaces de sustituir casi cualquier órgano humano. Un elevado pero impreciso porcentaje de españoles son portadores de uno o más DMIs a los que con frecuencia le deben su calidad de vida o su supervivencia. Los DMIs están construidos con tipos distintos de materiales que con frecuencia se combinan en una misma prótesis. Dichos materiales deben combinar su inocuidad para los tejidos humanos y una gran resistencia al desgaste. Su duración depende de muchos factores tanto del huésped como del tipo de prótesis, pero la gran mayoría duran más de 10-15 años o permanecen en funcionamiento incluso durante toda la vida del paciente. Los DMIs más frecuentemente implantados se ponen en el corazón o grandes vasos, en las articulaciones, en las arcadas dentales o en la mama y sus complicaciones más frecuentes se clasifican en no infecciosas, particularmente el aflojamiento o intolerancia, e infecciosas. Las complicaciones, cuando ocurren, suponen un significativo aumento de la morbilidad, su reparación o sustitución multiplica el coste sanitario y, en ocasiones, pueden causar la muerte del enfermo. La lucha frente a las complicaciones de los DMIs se centra en la actualidad en el diseño de los mismos con nuevos materiales, más resistentes al desgaste y a la infección y en la utilización de sustancias antimicrobianas que se liberan desde dichos materiales. Su producción requiere equipos multidisciplinares técnicos, pero también una disposición por parte de la industria y de las autoridades sanitarias que no se dan frecuentemente en nuestra nación ni en la mayoría de las naciones europeas. La producción científica sobre prótesis y DMIs en España se estima por debajo del 2% de la mundial y verosímilmente por debajo de lo que corresponde a nuestro nivel de desarrollo socioeconómico. El futuro de los DMIs pasa, entre otros factores, por examinar el potencial papel de la Inteligencia Artificial en su diseño, del conocimiento de la regeneración tisular, de una mayor eficiencia en prevenir mejor las infecciones y de llevar más allá de los antimicrobianos los tratamientos alternativos como es el caso de la fagoterapia. Por estas y otras razones, la Fundación Ramón Areces convocó a una serie de expertos en distintas materias relacionadas con las prótesis y DMIs que respondieron y discutieron una serie de preguntas formuladas previamente por el Consejo Científico. Las líneas que siguen son el testimonio escrito de esas preguntas y de las respuestas frente a las mismas.
Palabras clave: Prótesis, Dispositivos Médicos Implantables, artroplastias, dispositivos endovasculares, infección, aflojamiento, endocarditis, materiales, biomateriales, coste, producción científica, diseño, gasto sanitario
INTRODUCTION
Prostheses or implantable medical devices (IMDs) are made from a variety of materials and a high proportion of people over the age of 60 have one or more IMDs that improve their quality of life or enable them to continue living.
The biomaterials industry includes organisations and companies that design, manufacture, and fabricate materials that are used to make prostheses and there have been significant advances in the design of new biomaterials. At the same time, improvements have been made in the surgical techniques for implanting IMDs and in the management of their complications.
Despite all this, most of the scientific information available on IMDs is focused on very specific types of prostheses, such as joint or cardiac prostheses, and the orientation of the publications on them is very much oriented towards either the science of new materials or the field of medical practice.
As far as Spain is concerned, on the other hand, some aspects of the use of some prostheses, the workload they generate for the health system and the expenditure related to their implantation and maintenance are not well known.
For these reasons, the Scientific Council of the Ramón Areces Foundation asked itself a series of questions about the situation of prostheses in Spain, inviting a number of experts from different fields to answer them so that they could give the most global view possible of our situation.
At the meeting held at the Foundation Ramón Areces in Madrid in April 2024, aspects such as the most frequent types of prostheses currently in use in Spain, the materials of which they are composed, research into future new materials, infectious and non-infectious complications, the cost to the system and the evolution of our scientific production on this problem were discussed.
The following lines collect the questions and answers that were produced on the situation of prostheses in humans in Spain.
WHAT DO WE UNDERSTAND BY PROSTHESES AND BIOPROSTHESES? WHAT ARE THE MAIN DEFINITIONS IN THIS FIELD?
According to the Real Academia Española de la Lengua (RAE), prosthesis is: A part or device used to replace an organ or a limb of the body. A piece of animal tissue used to repair or replace a part of the human body, such as a heart valve, is called a bioprosthesis.
All prostheses are made of materials that can be implanted in a living organism, and it is now possible to replace almost all parts of the organism. Physical disability and age are closely linked to the need for prostheses. If during the first 10 years of life the need to replace damaged parts of the human body is very low, by the age of 60 the percentage can reach very high levels.
Prostheses must necessarily be biologically compatible with the human body. They are used to repair or replace damaged natural tissue, such as bones, heart valves, teeth or skin, and in the near future, organ tissues such as liver or kidneys. The goal of using biomaterials is to save people, improve their quality of life, reduce suffering and help them reach the end of life in a better condition. The science that deals with the design of prostheses and the study of the materials of which they are made is biomedical engineering.
Implanted prostheses can be temporary or definitive, but in any case, they must fulfil a specific function without causing any damage to the organism. Provisional prostheses, such as vascular catheters, have different construction requirements to those of definitive prostheses, such as hip prostheses, which must remain in perfect condition indefinitely.
In any case, prostheses must be biocompatible or biologically acceptable and must maintain their performance for the required periods of time, short in the case of provisional pros-theses and very long in the case of definitive prostheses.
The greatest advances in the field of biomaterials have been made in developed countries as a result of the need to treat a large number of patients clinically. The increase in life expectancy and the obligation to ensure a high quality of life for citizens have been key factors in the design and construction of prostheses.
The search for possible solutions to tissue problems has led to a high demand for materials to replace or repair them artificially. On the other hand, the improvement of surgical techniques has led to an accelerated growth in the demand for prostheses, implants and medical systems and devices that must work in contact with body tissues [1-6].
HOW MANY SPANIARDS LIVE WITH ONE OR MORE PROSTHESES? HOW MANY ARE IMPLANTED PER YEAR?. WHAT TYPE OF PROSTHESES ARE WE TALKING ABOUT? DO WE HAVE NATIONAL REGISTRIES IN SPAIN?
It is safe to say that millions of Spaniards today live with one or more implantable medical devices (IMDs) and that their health depends to a large extent on their proper functioning.
The distribution of the use of IMDs varies significantly between countries due to factors such as the availability of medical care, healthcare infrastructure and differences in rates of diseases and medical conditions. Countries with more advanced health systems tend to have a higher rate of IMDs [7].
To give just a few figures, according to the Spanish Society of Plastic, Reconstructive and Aesthetic Surgery (SECPRE), around 25,000 breast augmentation surgeries are performed in Spain every year. This number includes both cosmetic and reconstructive breast implants [8].
As for joint replacements, between 30-35,000 are implanted each year in our country and, at European level, it is estimated that the incidence rate is between 50 and 1,140 joint replacement procedures per 100,000 inhabitants/year [9].
In the cardiovascular field, we know that more than 17,000 valve prostheses are implanted annually by cardiac surgery or transfemoral surgery [10,11]. And if we look at cardiac electrostimulation devices, 8,000 permanent automatic defibrillators and more than 40,000 pacemakers are implanted in Spain every year, which means an implantation rate in this field of around 900 units/million inhabitants/year [12,13].
In Spain there are registers of implants in almost all the Autonomous Communities, but there is a lack of centralised registers as in other countries, which would make it possible to extract a multitude of useful data in a very short period of time, with little effort and at a low cost. By way of example, the Spanish registry of breast prostheses (SREIM) has been operating normally for several years now, and for cardiac pros-theses, electrostimulation devices, circulatory and respiratory assistance devices we also have national registries [14].
In contrast, the national registry of joint replacements (RENAPRO), which was launched in 2019, was not as successful. It should be clarified that these registers are voluntary, usually supplemented by the doctors who perform the implants, so they may differ slightly from the data provided by each manufacturer or even the data provided by the various supply departments of each hospital belonging to a health system in a given Autonomous Community.
Internationally, it is estimated that 8-10% of the American population and 5-6% of the inhabitants of industrialised countries have one or more IMDs to improve body function or for aesthetic reasons. In the case of cardiac implants, it is estimated that at least 400,000 Americans receive a cardiac electrostimulation implant (CIED) each year [15-17].
The most commonly used prostheses in our environment are valvular heart and vascular prostheses, cardiac electrostimulation devices, osteo-articular, mammary, genito-urinary, dental, ocular, auditory, nasal prostheses and also prostheses for the airways and digestive tract (mainly oesophagus) (Table 1).
Table 1.
Summary of surgically implanted prostheses covered by the national health system. Adapted from Ministerio de Sanidad [18].
| CARDIAC IMPLANTS |
|
CA 0 IMPLANTS FOR CARDIAC STIMULATION Include: Single-chamber pacemakers with/without remote monitoring, Single-chamber SSIR pacemakers (rate responsive), dual-chamber pacemakers with or without remote monitoring, pacemakers with cardiac resynchronisation therapy (rate responsive), implantable automatic defibrillators (ICDs) (single or dual-chamber), subcutaneous defibrillator and electrodes for cardiostimulation. CA 1 CARDIOLOGICAL IMPLANTS Include: Directly implanted or self-expandable mechanical or biological valves, transcatheter aortic valves (TAVI), valvuloplasty rings, valved or non-valved conduits, synthetic or biological pericardial substitutes (xenologues), cardiac and vascular occluder devices, VSD closure systems, ventricular assist devices, ..... |
| GASTROENTEROLOGICAL IMPLANTS |
| They include: Oesophageal stents (valved or not), duodenal, colorectal, bilio-pancreatic, rectal anal, artificial anal sphincters and percutaneous portosystemic shunts (TIPS), adjustable gastric bands,... |
| GENITOURINARY IMDs |
| They include: Ureteral endoprostheses (mono or double J), prostatic endoprostheses, anti-incontinence prostheses, penile prostheses for erectile dysfunction, testicular, pelvic organ prolapse implants, tubal obstruction implants via hysteroscopy, ... |
| NERVOUS SYSTEM IMPLANTS |
| They include: Shunt systems and reservoirs, single or multi-channel neurostimulators for both brain and spinal or peripheral nerve channels, electrodes |
| OPHTHALMOLOGIC IMPLANTS |
| Including: anterior and posterior chamber intraocular lenses, capsular tension rings, glaucoma surgery devices, enucleation and evisceration prostheses, paepebral implants, tear duct implants, ….. |
| ENT IMPLANTS |
| They include: middle ear prostheses, transtympanic drainage tubes, hearing implants, phonatory prostheses, laryngeal prostheses, ... |
| IMPLANTABLE DRUG DELIVERY DEVICES |
| These include: implantable infusion pumps, subcutaneous reservoirs, implantable catheters, … |
| RESPIRATORY SYSTEM IMPLANTS |
| They include: Tracheal and bronchial prostheses, endobronchial valves, lung volume reduction devices, ... |
| RESTORATIVE IMPLANTS |
| They include: Breast prostheses, custom-made silicone prostheses, for thoracic defects secondary to congenital malformations, trauma or disease, which cannot be repaired with autologous tissue, prostheses with polyurethane surface, skin expanders, implants for cranio-facial surgery, dental implants, nasal implants, auricular pinna, temporomandibular joint prostheses, prostheses for reconstruction of mastoid cavities, cranial plasties, meshes for containment of eventrations and hernias, ... |
| OSTEOARTICULAR IMPLANTS |
| They include: Hip, knee, shoulder, elbow, wrist, wrist, hand, other joint prostheses, spacers, vertebral body prostheses, intervertebral prostheses, fixators, intramedullary nails, .... |
| VASCULAR IMPLANTS |
| They include: Vascular substitutes, coronary stents, vena cava filters,.... |
| DIAGNOSTIC CARDIAC IMPLANTS |
| They include: Implantable Holters with/without remote monitoring, for the evaluation of patients with cardiac rhythm disorders, ..... |
An example of a very commonly used prosthesis is dental implants which have become an increasingly popular treatment option to replace missing teeth. Between 1999 to 2016 in the US there has been a large increase in the prevalence of dental implants, from 0.7% in 1999 to 2000 to 5.7% in 2015 to 2016. The largest absolute increase in prevalence (12.9%) was among those aged 65-74 years, while the largest relative increase was ~1,000% among those aged 55-64 years. There was a covariate-adjusted mean increase in dental implant prevalence of 14% per year.
The projected prevalence of dental implants until 2026 ranged from 5.7% in the most conservative scenario to 23% in the least conservative scenario [19].
The Italian Society of Otorhinolaryngology, for example, has recently reviewed the discussed situation of cochlear implants, their indications and limitations [20-22].
In addition, implantable biosensors to detect the presence of specific substances, or to monitor brain activity and systems to deliver drugs to specific points in the body, drug-coated vascular stents or nanoparticles for the treatment of different diseases, in particular cancer, should be considered as prosthetic material [23-25].
WHAT KINDS OF BIOMATERIALS ARE USED TO BUILD PROSTHESES?
Major advances in medicine and surgery since the second half of the last century have led to the development of increasingly complex clinical devices, and in particular to the development of prostheses that can be implanted inside the human body.
The main applications of biomaterials are manifold and have already been mentioned [26] (Table 1) and research in the field of biomaterials has grown enormously over the last fifty years. However, in the field of implantable prostheses, the range of biomaterials used remains very similar to those that were first used in the last century. Two reasons for this have to do with the need for clinical implants to meet all the safety and efficiency requirements demanded by regulatory and accreditation agencies and on the other hand that from an economic and production point of view, the new biomaterial, after passing all the tests required by the regulators, will end up being more competitive than the current material on the market [27,28]. Generally speaking, these first-generation bio-materials that are well described by regulatory agencies meet characteristics such as having passed biocompatibility tests, being sterilisable, being stable in the long term (resistance to corrosion, degradation, wear, etc.), and also that they can be manufactured with common techniques (machining, extrusion, moulding, injection, etc.).
The types of biomaterials used in the manufacture of prostheses include the four major groups of materials: metals, polymers, ceramics and composites [24,26,29,30].
Metals are mainly used in joint prostheses, plates and screws for fixations in traumatology, staples, dental implants, etc. The types of metals most commonly used in prostheses are: stainless steels, always austenitic, as they allow cold working and/or forging, are used in plates and screws for traumatology, vertebral fixations and joint prostheses; cobalt-chromium alloys (Co-Cr) which can be used cast (melted and annealed) or forged, have high rigidity and mechanical strength and are mainly used in joint prostheses and dental prostheses; pure titanium (Ti) grade 4 which is used in dental implants and its alloys (especially Ti-6Al-4V containing 6% aluminium and 4% vanadium) which is used in joint prostheses.
Polymers have a wide range of applications due to their diversity and properties. A distinction is made between synthetic polymers and natural polymers. Among the most commonly used synthetic polymers are: high-density polyethylene (PE) with good wear resistance and biostability used in joint replacements; polymethylmethacrylate (PMMA), a rigid, hard, hydrophobic, bioinert and transparent material used as bone cement, also in contact and intraocular lenses and dentures; polypropylene (PP) with high rigidity, mechanical strength and biostability used in non-biodegradable sutures and structures for heart valves; polyethyletheretherketone (PEEK) which has good mechanical properties, is bioinert and is used in orthopaedic implants and spinal implants; polyethyleneterephthalate (PET) (Dacron) which has good mechanical properties and is used in vascular implants, hernia repair and ligament reconstruction; polyurethane (PU) with elastomeric properties is used in tubing and catheters as well as in blood contact applications; polytetrafluoroethylene (PTFE) is hydrophobic and low strength, used in vascular implants, catheter coatings and heart valves; high mechanical strength polyamides (nylons) are used in haemodialysis membranes and non-resorbable sutures; polyglycolic acid (PGA) is bio-degradable and used in drug delivery systems and resorbable sutures; and finally, polylactic acid (PLA) is biodegradable and used in drug delivery systems.
Natural polymers are mainly proteins, polysaccharides and polynucleotides and have the advantage of resembling biological substances in the body and can be degraded in the body. At the same time, they have major disadvantages in that they can produce immunological, toxic and inflammatory reactions. In addition, there can be variability from batch to batch. Among the most commonly used, often in combination with other materials, are collagen, elastin, alginate, chitosan, hyaluronic acid and silk. Mention should be made of the use of decellularised extracellularised matrices as scaffolds for tissue engineering.
Ceramics and glass are rigid and brittle materials that find different applications depending on whether they are inert or bioactive. In the case of inert ceramics, the most commonly used materials are aluminium oxides (alumina Al2O3) and zirconia (zirconia ZrO2), which are used in joint prostheses, mainly hip prostheses (the sphere of the joint, although in the case of alumina the acetabulum can also be made of this material), and zirconia is also used in dental prosthesis crowns. Both alumina and zirconia have been used as coatings for metal substrates. Bioactive ceramics correspond to different formulations of calcium phosphates, with tricalcium phosphate (TCP) and hydroxyapatite (HA) being the most commonly used. Calcium phosphates, chemically close to the mineral phase of bone, induce biological activity when implanted into a bone substrate and help regenerate bone tissue or become anchored to it. They are low-strength and brittle materials. They are used in the form of porous particles or granules for fillers and in coatings for metal substrates. Bioactive glasses consist of different formulations with silicon oxide contents of over 40% and different contents of phosphate, calcium and sodium ions, among others. These glasses exhibit a great capacity for chemical bonding to bone. Their fragility and low strength also makes it necessary to use these materials in granular or particulate form and they find application in traumatology, fracture repair, spinal fusion, craniofacial and maxillofacial applications. Both calcium phosphates and bioactive glasses are also used as reinforcement and bioactive phase in composite materials.
Composite materials seek to combine two or more materials with the aim that their interaction leads to resulting properties that enhance those of the individual constituents. The scope of applications is very broad, as the different combinations allow for optimisation of important parameters such as mechanical properties, biostability, biodegradation, bioactivity, hydrophobicity, etc. For trauma and spinal fixation applications, bioinert combinations of carbon fibres in matrices of different polymers can be considered, which may include epoxy resins, PMMA or PP among others, as well as biodegradable combinations based on HA granules or PGA or PLA fibres in PLA or even PGA matrices. In the case of bone cements, a PMMA matrix has been reinforced with HA or bioactive glass granules. PEEK has been combined with carbon fibres for the manufacture of hip prosthesis stems. For bone filling and regeneration PLA or PGA have been combined with HA granules. Mixtures of natural polymers with synthetic polymers and/ or bioactive ceramics have also been used but can hardly be qualified as composite materials due to the lack of interaction between the constituent phases.
WHAT CARDIAC PROSTHESES ARE IMPLANTED IN SPAIN AT THE PRESENT TIME? WHAT MATERIALS ARE THEY MADE OF? WHAT IS THEIR AVERAGE LIFE SPAN?
The cardiac prostheses currently implanted in Spain are either mechanical or biological. There are two methods of implantation: by open surgery or by catheterisation, known as TAVI procedure, which stands for Transcatheter Aortic Valve Implantation.
According to data from the Spanish Society of Cardiovascular and Endovascular Surgery, 11,257 cardiac prostheses were surgically implanted in Spain in 2021, 70% of which consisted of biological material [10]. On the other hand, according to the registry of the Spanish Society of Cardiology in 2022, 6,672 TAVI procedures were performed in Spain [11].
In terms of materials, mechanical prostheses usually consist of two parts: the suture ring, which is usually made of Dacron or Teflon, highly resistant polyester fabrics; and the structure of the prosthesis itself, which is usually made of graphite bombarded with carbon atoms at high temperatures. They are also impregnated with tungsten to make them radiopaque.
Biological prostheses are classified as follows:
Xenografts(made of porcine or bovine animal material): they are designed in two parts: a Dacron suture ring and the leaflets themselves, sewn to the ring and composed of bovine pericardium or porcine valves. TAVI and some surgical prostheses may have a cobalt-chromium alloy or nickel-titanium (Nitinol) metal framework in addition to the biological tissue.
Homografts: grafts from a cadaveric donor and cold preserved.
Autografts. Composed of the patient’s own biological material.
The half-life of a prosthesis will depend on the type of prosthesis and other factors such as age, position (aortic, mitral, tricuspid, pulmonary), associated pathology and the statistical method used to evaluate its durability.
Biological prostheses have a limited durability, undergoing a degenerative process called primary structural failure. After 10-15 years, approximately 30% of patients have to be operated on again to replace them. This failure is more rapid in young people, in valves in mitral position and in patients with diseases in which calcium metabolism is altered, such as renal insufficiency or hyperparathyroidism. The statistical method used is important when estimating the durability of a prosthesis. The Kaplan-Meir method, the actuarial method and the current method are used for this estimation. The first two are based on a probabilistic calculation by time intervals and assume that patients would live indefinitely and are removed from the calculation when they suffer the event under study, in this case the structural failure of the prosthesis. The potential pitfall with these two methods is that many patients die during the study and are removed from the analysis without being able to accurately estimate the durability of the prosthesis. This is known as the competing risks of the structural failure event and the death event. To solve this problem, the actuarial method with competing risks is used, which takes into account the two aforementioned events and provides a more realistic estimate of the durability of a prosthesis.
In general terms and depending on the patient’s age and other factors, it is estimated that a biological prosthesis in the aortic position has an average durability of 15-20 years and in the mitral position of 10-15 years.
Mechanical prostheses do not undergo the degenerative process and are designed to last a lifetime. However, they may require replacement due to infection (endocarditis) or thrombosis or perivalvular leaks. They have the disadvantage that patients need to take anticoagulants to prevent thrombus formation.
WHAT IS THE NUMBER AND TYPE OF PACEMAKERS USED IN SPAIN TODAY?
There are three types of pacemakers that are implanted: conventional pacemakers (a generator that is connected to wires placed in the heart), pacemakers without wires (implanted directly in the heart) and resynchronisers/defibrillators that improve the contraction of the heart and give an internal shock if there is an episode of severe arrhythmia.
According to data from the Registry of the Spanish Society of Cardiology in 2022, 41,082 conventional pacemakers (866/million inhabitants) were implanted in Spain. To this must be added 4,604 (34/million inhabitants) resynchronisation devices and 813 pacemakers without leads [13].
WHAT ARE THE MAIN NON-INFECTIOUS COMPLICATIONS OF PROSTHESES?
Complications of prostheses are generally classified as infectious and non-infectious (aseptic). In this section we will refer to non-infectious complications.
In the case of prostheses for use in the locomotor apparatus, the work to be carried out during the patient’s lifetime is extremely intense. It has been estimated that the load supported by the main lower limb prostheses (hip, knee) is 1 million cycles per year in a 70-year-old patient, and up to 5 million cycles per year in an active and sporty 40-50-year-old patient. This can lead to the implant withstanding 10 to 50 million load cycles of between 500 and 1,000 Newtons for a patient weighing 70 kg, and this in 10 years of implant survival. The mechanical fatigue problems faced by loaded joint replacements are therefore enormous. Consider that an automotive wiper system is validated by industrial quality controls for 100,000 operating cycles, and here we are talking about millions of cycles. Together with the aggressiveness of the internal environment (highly oxidising, in an aqueous environment), and exposed to trauma and impact, to injuries and atrophy of periarticular soft parts (ligaments, capsule, musculature), to inflammation of the joint which also deteriorates the bone around the implant, joint prostheses fixed to the bony ends of the joint can be said to be in a hostile environment, which will progressively deteriorate their functioning.
There are different sources for defining the complications associated with joint replacements and, as a basis, we will refer to arthroplasty registries and patient series from large hospitals. Different causes of prosthesis replacement due to short-or long-term complications have been identified, and differences are also observed between the main joint replacements of the lower limb, such as the hip and the knee. Due to the current interest and frequency of the knee prosthesis, which is currently 3 times more frequently implanted than the hip prosthesis, we will focus on non-infectious complications of these prostheses.
Non-infectious causes account for 60-70% of reoperations for knee replacement complications in different registries (Swedish Arthroplasty Registry; National Joint Registry of England, Wales, and Northern Ireland; Australian Orthopaedic Association National Joint Replacement Registry) [31]. Aseptic loosening predominates, followed by instability, extensor apparatus and patellar problems. After infection and aseptic loosening, other causes may combine in mechanical complications [32]. Aseptic loosening is associated with particulate matter from material deterioration and the resulting inflammatory reaction. Therefore, the temporal evolution observed after improvements in materials and designs leads to a decrease in aseptic loosening as the main non-infectious complication, although it is still remarkable [33]. In contrast, other long-term complications such as instability and periprosthetic fracture, which occurs in older patients and carriers of certain implant types, are increasing. Joint stiffness is also increasing as an early complication in younger patients and in arthroplasty for post-traumatic osteoarthritis [34].
Monitoring long-term complications of joint implants is essential to correct them, to drive improvements and innovation, and to obtain the best results for, if possible, the patient’s entire life.
WHAT DOES INFECTION AS A COMPLICATION OF PROSTHESES REPRESENT IN TERMS OF NUMBERS? DOES IT DEPEND ON THE MATERIALS OF WHICH THE PROSTHESIS IS MADE? WHAT ARE THE CONSEQUENCES?
Although biomaterial infections have a generally low incidence (2-7% overall), their importance is paramount. Infection of a device is always a major complication that compromises its subsequent functioning, and sometimes even the life of the person who has received the implant, which may be essential for the normal functioning of an organ, such as cardiac pros-theses, in which infection (prosthetic infective endocarditis) can exceed 30% mortality rate. [35]. And if we look strictly at the costs, they literally skyrocket when there is an infection. The cost of acquiring a joint replacement, for example, is around €6-7,000 in European countries [36]. The infection of this implant will increase the total cost of the process by a factor of 10 (€50,000) [37], and the same is true for electrostimulation device infections, as not only does the infected device have to be replaced, but the resulting hospital stays will necessarily be lengthy [38-43].
In the pathogenesis of infection of a biomedical device, the first step is the adherence of the micro-organism to the device. For many decades, when talking about prosthesis-associated infections, the biomaterial was given a secondary and passive role, with greater importance being given to the micro-organism and the patient’s defence mechanisms. Nowadays, however, there are many data available that highlight the importance of the nature of the biomaterial, since adhesion is also decisively influenced by the surface and characteristics of the biomaterial. In fact, initial adhesion will depend on physicochemical interactions such as electrostatic interactions, van der Waals forces or hydrophobic interactions [44,45]. These interactions will also affect the adhesion of proteins and other tissue or serum components and the resultant of this may lead to a second, no longer reversible step, which is the specific adhesion of microorganisms mediated by both host (fibronectin and other adhesins) and host proteins [44]. From here, the micro-organisms will initiate the large-scale production of a “biofilm” or slime that will protect them from the host’s de-fence mechanisms, as well as from other harmful agents such as antimicrobials, which penetrate these structures poorly and are often inactivated by enzymatic mechanisms. Today we also know that the colonies involved in these biofilms coordinate with each other through a fascinating communication network to work together, which has come to be known as quorum sensing, to control certain functions that will facilitate their survival, including through the selection of more resistant mutants [46]. It is undeniable that the nature of the biomaterial will influence the development of these biofilms to a greater or lesser extent, depending also on the microorganisms attached.
HAS THE COST OF IMPLANTING PROSTHESES IN SPAIN BEEN ESTIMATED?
The cost of the prosthesis itself varies depending on the type and complexity of the device. For example, hip and knee replacements can have significant upfront costs, ranging from thousands to tens of thousands of dollars, depending on factors such as the material, brand and technology used.
In addition to the cost of the device, the expenses associated with the surgery must be considered, including staff fees, anaesthesia, hospitalisation and post-operative rehabilitation. These costs can be considerably high and must be taken into account when assessing the economic impact of prosthetic implantation.
On the other hand, the long-term costs associated with maintenance and possible complications of prostheses must be considered. This may include surgical revisions, component replacements and treatment of complications such as infections or loosening of the prosthesis.
Finally, there is a social cost resulting from prolonged periods of inability to work, which can have a significant impact on the patient’s productivity and income, as well as costs associated with social care and disability insurance.
For many patients, the implantation of a prosthesis can mean a significant improvement in mobility and physical function, allowing them to lead a more active life and participate in daily activities that were previously difficult or impossible.
Well-designed and properly implanted prostheses can reduce or eliminate chronic pain associated with conditions such as osteoarthritis, improving the patient’s overall well-being and quality of life.
Restoration of physical function and reduction of pain can have a positive impact on the patient’s mental health and emotional well-being, improving their self-esteem and ability to cope with the challenges of everyday life.
The studies we have found on all these aspects are, however, scattered and partial [47-53]. As examples we can say that costs range from around $4000 to $6000 for expandable aorto-iliac prostheses [54] to figures of around £10,000 to £30,000 for uncomplicated knee prostheses [55] 18,000 to €20,000 for breast reconstruction after cancer resections in data from Spain [56]. Table 2 provides some guidance on pros-thesis procurement costs.
Table 2.
Some estimates of the cost of acquisition and implantation of various prostheses in different countries.
| Type of prosthesis | Acquisition cost | Implantation cost | References |
|---|---|---|---|
| Hip | 2.500-7.000 $ | 15.000-40.000 $ | [59, 60, 61] |
| Knee | 3.000-9.000 $ | 20.000-50.000 $ | [62, 63, 64] |
| Elbow | 5.000-15.000 $ | 20.000-50.000 $ | [65, 66] |
| Shoulder | 5.000-20.000 $ | 20.000-50.000 $ | [67, 68] |
| Implantable Cardiac Electronic Devices (ICED) | 2.500 $-10.000 $ | 10.000-50.0000 $ | [69, 70] |
| Heart valves | 5.000-15.000 $ | 50.000-150.000$ | [71, 72] |
| Mammary | 1.000-3.000 $ | 5.000-20.000 $ | [73] |
| Penis | 5.000-20.000 $ | 5.000-20.000 $ | [74] |
| Hernia meshes | 50-500 $ | 2.000-10.000 $ | [75, 76] |
In patients with heart valve replacements, the cost-effectiveness of having the valve replaced either by TAVI or implantation after open surgery in patients with severe aortic stenosis has been compared, with cost differences between the procedures ranging from $11,000 to $18,000[57]. In the case of mitral surgery, the episodes in which the natural mitral valve can be repaired versus those in which it has to be replaced represent a cost difference of between 34,000 and 55,000 € in favour of the conservative procedure in each episode [58].
WHAT IS THE CURRENT SITUATION OF PROSTHESES WITH NON-ADHESION MATERIALS AND WHAT IS THEIR FUTURE?
Antiadhesion biomaterials usually refer to materials whose surface has repellent properties that prevent adhesion or embedding of micro-organisms or cells. In general, this involves modifying the surface of biomaterials with coatings or treatments that impart these properties. One can speak in general terms of antifouling properties, but in many cases a specific antimicrobial action is also, or above all, sought.
The service life of implants depends in particular on the rejection reaction they receive inside the human body and the risk of infection. Surface modification processes for biomaterials aim to provide solutions to these problems by altering the physical, chemical and biological properties of their surfaces.
Although there are antiadhesion materials whose primary function is not antiinfective, which will be described below, most of them aim to prevent microbial adhesion and infection. There are two main strategies for this. The first involves surface coatings or treatments that kill microbes as soon as they approach the surface. The second is to prevent the accumulation of microbes through their repellent or antifouling properties.
Nosocomial (hospital-acquired) infections are caused by bacterial colonisation of different surfaces of biomedical devices and systems and can affect 4-10% of hospital admissions (and more than 15% in less developed countries), reaching sixth place among causes of death [77].
Biomaterials with antiinfection properties that have been approved by the FDA have increased in recent years, demonstrating their clinical need. Currently the group of biomaterials with antibacterial properties is far superior to those with anti-fungal properties.
The sequence of biological events that take place in the process of infection by microbial attack is complex and includes adsorption of proteins, adhesion of bacteria, proliferation, formation of biofilms with polysaccharide-based extracellular matrix, reaction with inflammatory cells and subsequent inflammation and infection. All this leads to complications, implant failure and, depending on the degree of infection, even death of the patient [77-79].
Antimicrobial coatings can be based either on the release of various antibacterial agents or on coatings that have antibacterial properties themselves. The former release agents such as antibiotics, silver ions, antiseptics, furanones or nitric oxide. They are applied to the biomaterial by techniques such as physical adsorption, impregnation in a biodegradable polymer matrix, complexation or conjugation. The latter are based either on polymers, which themselves have antibacterial properties, or on photoactive metal oxide nanoparticles. The first category includes cationic polymers with biocidal properties, either of natural origin such as chitosan, or of synthetic origin such as polyethyleneimine (highly cytotoxic), polyurethane or cationic silicones, while the second category includes metal oxides such as TiO2, CuO or ZnO, which generate reactive oxygen species capable of damaging organic biomolecules such as carbohydrates, lipids, proteins or DNA [78,79].
On the other hand, antifouling coatings that either show repellent properties towards micro-organisms or affect the biofilm architecture should be considered. In the first category are hydrophilic polymers, especially in polyethylene glycol (PEG), zwitterionic materials that also provide hydrophilic surfaces [80-82] and superhydrophobic surfaces with low surface energy and nanostructured surface topography. The availability of nanotechnology tools has enabled progress in the production of superhydrophobic surfaces with antibacterial activity [83,84]. Their clinical application does not seem immediate, although the successes achieved in paints and fabrics with superhydrophobic properties allow optimism for the future. In the second category are coatings based on enzymes that can degrade the polysaccharide-based extracellular matrix of the biofilm, or by inhibiting bacterial Quorum Sensing (QS), responsible for the regulation of gene expression and chemical signalling among the cell population, which should prevent biofilm formation [77-79].
As stated above, among the antiadhesion biomaterials, those whose function is not antiinfective but to prevent adhesions between tissues should also be considered. In abdominal surgery, almost 80-90% of patients suffer from post-surgical adhesions and this is a complication that can lead to bowel obstruction, chronic pelvic pain, infertility or the risk of having to operate again, and in the case of the intervertebral disc to paraplegia. The general strategy is to use barrier materials that block or prevent the connection between the surgical site and nearby organs or tissues. Gels, liquid solutions or films are used for this purpose. Anti-attachment strategies aim either to create physical barriers using hydrogels or films, or to create chemical barriers using anti-inflammatory agents, anti-coagulant agents or fibrinolytic agents. Natural polymers such as polysaccharides, gelatine, hyaluronic acid or alginate with short resorption times, or chitosan or carboxy methyl cellulose with longer resorption times, as well as biodegradable synthetic polymers such as polylactic acid, polyvinyl alcohol, polycaprolactone or polyethylene glycol with longer degradation times, modulable and non-toxic, can be used for this purpose [85,86].
The future of non-adherent materials will be strongly linked to the industrial scalability of some of the technologies proposed, as well as the demonstration of their effectiveness and cost-efficiency.
TO WHAT EXTENT CAN LOCAL ANTIBIOTICS ATTACHED TO THE STRUCTURAL MATERIALS OF THE PROSTHESIS BE USED TO PREVENT INFECTIONS?
Work is currently underway to develop biomaterials that prevent the initial adherence of the micro-organisms. One possibility is to coat the material with hydrophilic substances (which repel bacteria) such as polyethylene oxide, but once they undergo adsorption of serum proteins, this effect loses its value, so other strategies have been developed, such as the use of biomaterials coated with antimicrobial substances. One of the oldest and proven effective is polymethylmethacrylate (PMMA) bone cement, which can be added with antimicrobials to achieve high concentrations of these drugs after elution. Acrylic cements were developed a long time ago, in the 60s of the last century, and their function is to ensure the fixation of the implant (usually made of metal) to the bone. On the other hand, they also transmit the loads that the prosthesis has to bear, achieve a mechanical locking in the bone interstices and also compensate for imperfections associated with the surgical technique. These cements were subsequently added with antimicrobials to reduce the risk of infection. This can be done manually, but there are also commercial preparations containing gentamicin, vancomycin, ciprofloxacin, and combinations with other antimicrobials. These preparations have demonstrated efficacy over many years and are cost-effective when used in joint revision procedures (where the risk of infection is much higher) and in the eradication of active periprosthetic infection [87]. However, it should be noted that the addition of antimicrobials to these cements can interfere with the mechanical properties (mainly strength) of the material by almost 25%.
Interference with materials could be largely avoided by constructing natural polymers that are reabsorbed once their mission is accomplished. An example of this is tryptophan polymers containing antimicrobials, which have been used to wrap the generators of electrostimulation devices that are usually implanted under the subcutaneous cellular tissue of the pectoral region. These devices release high concentrations of antimicrobials in situ for several days, while degrading naturally, showing a reduction in the incidence of infection of almost 50%, which would make their use cost-effective in patients at high risk of infection [88]. Another strategy would be to inject antimicrobials in the target area (e.g. a joint with an infected prosthesis or in the ocular vitreous humour) with a controlled release, such as would be obtained with their vehicleisation by means of nanospheres.
However, from a microbiological point of view, the use of these antimicrobial-impregnated materials always involves the risk of resistance or even an increase in the generation of bio-films, so it is necessary to ensure adequate release in optimal quantities. It has been shown, for example, that sub-inhibitory concentrations of certain antimicrobials can activate the ica gene responsible for biofilm formation in S. epidermidis [89]. For this reason, polymer coating strategies are also being developed with substances that have a biocidal action other than antimicrobials. Thus, there are designs with anti-fibronectin antibodies, blocking agents of the messengers involved in the quorum sensing phenomenon or even components active against genes that regulate adhesion phenomena [90,91]. This opens up a hitherto unimaginable field of therapeutic possibilities that will change our old patterns. On the other hand, we should not be overconfident without first reflecting on the fact that bacteria have been on Earth for many millions of years before us and that we will always be surprised by their ability to evolve in the face of threats, so that the fight has only just begun. However, small advances in both prevention and treatment will undoubtedly be cost-effective and will, above all, prevent much suffering.
TO WHAT EXTENT WILL TISSUE REGENERATION BE AN ALTERNATIVE TO CURRENT PROSTHESES?
Tissue regeneration aims to restore, replace and increase the ability of a tissue to reproduce. Different animal species differ markedly in their ability to recover injured tissues. Regeneration requires significant plasticity in terms of changes in cell cycle, proliferation, dedifferentiation and transdifferentiation.
Regeneration occurs by transformation of pre-existing body parts or tissues into new structures, which involves dedifferentiation followed by proliferation, and requires a subsequent stage of differentiation into specialised cells to complete tissue reconstruction.
Regeneration mechanisms occur in the complete recovery of amputated limbs in salamanders, starfish, and other animal species, but do not occur spontaneously in humans.
However, in mammals, therefore, in humans, repair of some tissues such as liver regeneration, and self-regeneration of hair, nails, skin, mucous membranes, endometrium, blood, muscles, and bones does occur, achieving the reproduction of the original structure.
In the absence of injury, human tissues regenerate naturally, replacing aged cells with new cells. The regeneration time is different for each tissue; for example, uninjured skin tissue regenerates in two weeks while a bone takes 10 years to fully regenerate.
When a tissue is injured, the body responds with an emergency reaction that leads to scar tissue formation rather than a regenerative response. The possibility of self-regeneration depends on the size of the injury. In the skin, wounds smaller than 2 mm can regenerate naturally before healing occurs. In contrast, if wounds larger than 3 mm are to be prevented from healing, a bridging material must be inserted to induce regeneration.
In the case of bone, when the injury exceeds a certain dimension, a critical defect, tissue repair becomes more difficult or even fails to occur. In addition, with age, the regenerative response becomes less and less effective.
Regeneration generally describes the process by which lost tissue is restored through the proliferation of specialised cells. The aim of regenerative medicine is to regenerate primarily by supplying cells, in particular stem cells that can stimulate regeneration.
Some major goals in regenerative medicine are: Reversing and preventing paralysis, blindness or hearing loss by regenerating bone marrow, optic nerve, retina, auditory nerve, cardiac regeneration after a heart attack, curing Parkinson’s and Alzheimer’s diseases, minimising the after-effects of a thrombus by neuronal repair, cell therapy for diabetes (only 3,000 pancreas transplants are available for every 35,000 potential patients), access to new cartilage, muscle, tendons, ligaments, intervertebral discs in adulthood, reversing disc degeneration in the spine, kidney regeneration (living without dialysis), universal repair of all bone fractures, spinal fusion through bone regeneration, new teeth.....
If all this is achieved, the panorama will undoubtedly change and the alternative to current prostheses will be spectacular [26,92-94] (Figure 1).
Figure 1.
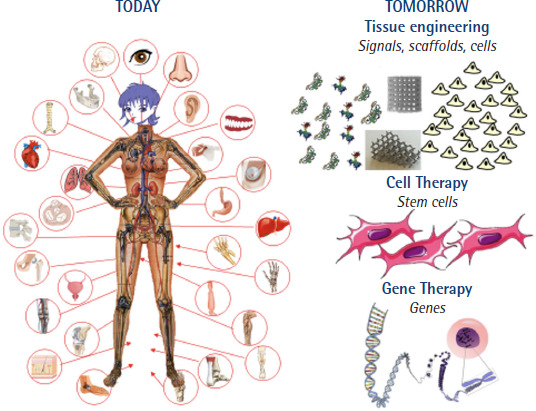
Diagram showing how tissue engineering and cell and gene therapies will change the world of prosthetics today. Figure taken from Vallet-Regí [93]. Copyright © 2022 Vallet-Regí.
WHAT IS THE FUTURE OF PHAGE THERAPY IN THE TREATMENT OF INFECTIONS ON PROSTHESES THAT CANNOT BE REMOVED AND DO NOT RESPOND TO ANTIBIOTICS?
When removal of biofilm and prosthetic material is not technically possible, antibiotics alone often fail to treat prosthetic infections. Bacteriophages are a possible alternative and complement to the use of antibiotics in these circumstances.
Bacteriophages (phages) are viruses, both DNA and RNA, abundant in nature, that have the ability to infect bacteria and can sometimes lyse them. They are harmless to humans and can be administered systemically or locally [95].
For a phage to be able to lyse a bacterium, it must be incorporated into the bacterium after binding to a surface receptor, be replicated by the bacterial machinery and its progeny must have lytic capacity against the bacterium. When the bacterium has been lysed by the phage, the cycle and propagation of the phage ceases. In contrast to lytic phages, temperate phages can remain quiescent as prophages and integrate into the genome.
Phage therapy has some important limitations such as the specificity of phages for certain bacterial species with a very narrow spectrum of action, the need to obtain and maintain phages, and the development of resistance. Fortunately, phage therapy has not been associated with major adverse effects.
Phage therapy was first used in 1917 and has been a therapeutic weapon applied safely and effectively to thousands of patients ever since [96-102], mainly in Eastern European countries where access to antibiotics was not easy. Phage therapy has even been used as monotherapy in urinary tract infections, but it is usually used in association with antibiotics [103].
The current status of phage therapy in the Western world is that of an experimental treatment in need of systematisation and prospective, randomised clinical trials. This treatment has not yet been approved by the FDA.
Phages could be particularly useful in infections on prosthetic material that cannot be removed, but the available studies very often publish only isolated cases or series with very small sample sizes.
The causative microorganisms most frequently treated with phages have been S. aureus, P. aeruginosa, S. epidermidis and others. These were almost always patients who had failed conventional treatments and were not candidates for radical surgery.
Suh et al. [104], in a prospective, open-label, non-randomised study in patients with osteoarticular prosthesis infections who received combined therapy with phage and antibiotics, collected 23 cases that were compared to 22 historical controls who received antibiotics alone. The relapse rate in those treated with antibiotics alone was 8 times higher than in those receiving phages [104].
In another recent paper, Fedorov et al. from Russia publish a non-randomised, prospective, open-label, historically controlled study on the use of combined phage and antibiotic treatment of periprosthetic joint infection (PJI) in 45 adult patients with deep PJI of the hip joint, with a 12-month follow-up after one-stage revision surgery. All 23 patients in the study group were treated with a specific phage preparation and etiotropic antibiotics, while 22 patients in a retrospective comparison group received antibiotics only. The relapse rate of PJI in the phage group was 4.5% and in the control group 36.4% [105].
Experience with phage therapy in patients with infections of prosthetic material other than osteoarthritic material is even more limited and contradictory and does not allow for clear conclusions [106,107]. Phages can reduce bacterial colonisation of surfaces such as catheter tips, endotracheal tubes or urinary catheters [108,109] and could potentially have a prophylactic application but information in this regard is still very limited and partial.
In most cases a local strategy is used with administration of the phages at the surgical site either during surgery or via a catheter left in situ. Intravenous therapy has been used either alone or in combination with local treatment but experience is limited.
Neither ideal dosages nor ideal duration of phage therapy have been established. In published cases the amounts ranged from 1x107 to 1x1011 plaque-forming units and the frequencies of administration varied from daily every 8 hours to once a week. The duration of the process of searching for, selecting and preparing the phages for administration lasted in the work of Suh et al. between 28 and 386 days, which implies that it is a treatment applicable only to patients with chronic diseases, who do not respond to conventional treatment [104].
Before phage therapy can become a tool for common therapeutic use, standardisation of phage preparation processes, systematisation of the study of their spectrum of antibacterial action and the creation of specific banks for therapeutic use are needed [110,111]. At the same time, standard monitoring of phage therapy in both tissues and blood is necessary to optimise doses and duration of treatment [112].
HOW CAN ARTIFICIAL INTELLIGENCE HELP IN THE DESIGN OF PROSTHESES?
Artificial intelligence (AI) is a branch of computer science that aims to create intelligent machines capable of performing tasks that require human intelligence. It is through the use of computer algorithms that AI enables the analysis, understanding and interpretation of complex data sets and from this to learn from experience and make predictions or decisions. AI is completely transforming economic, industrial and cultural sectors. Machine learning (ML) is an AI discipline that uses algorithms to train a machine to identify common patterns in large amounts of data in order to make predictions and decisions. Although from a definitional point of view the differences seem clear, when looking at how AI and ML are applied in the literature related to Biomaterials and Bioengineering in general, the differences are not so clear-cut in terms of the algorithms and computational models used.
As in other sectors, AI and ML are penetrating all areas of the life sciences. In fields such as diagnostic imaging, pharmacology or medicine/healthcare, experience is already well advanced and with tangible successes. In the field of prosthetics, apart from exo-implants, there is an abundance of literature on dental implants and orthopaedic implants [113]. The possibility of carrying out these predictive and design studies is due to the existence of both reliable databases and published works in which both the stress distribution in prostheses and their micro-movements have been calculated by means of finite element models. Therefore, it seems plausible to state that the application of AI can be of great help in finding virtual models of prostheses that can then be transformed into real, clinically usable implants.
In the cardiovascular field, AI also plays an important role in the field of imaging or in the planning of different interventions [114,115]. There are also studies for the development and manufacture of prostheses [116-118], although to a lesser extent than in the dental and orthopaedic fields. Without attempting an exhaustive review, it is also possible to find contributions in other clinical fields such as the design of a bionic eye [119].
The future of the use of AI in the design of prosthetics in general and biomaterials in particular is closely linked to the existence of reliable and accessible databases. There are an increasing number of materials or biological databases that comply with the FAIR (Findable, Accessible, Interoperable, and Reusable) principles of Open Science [120].
The development of new biomaterials that contribute to more effective and efficient implants has come about through trial and error. ML and AI are tools that can enable more holistic views of the material’s working conditions, i.e. take into account the complexity of its environment, and eventually produce a “virtual twin” of the target material. This reduces the time-consuming and costly sequence of tests that could eventually lead to the specification of the desired material. In the field of materials, the US government announced in 2011 its “Materials Genome Initiative” (MGI) programme, which in May 2017 took the form of a workshop on “Advancing and Accelerating Materials Innovation Through the Synergistic Interaction among Computation, Experiment, and Theory: Opening New Frontiers”. It is therefore an initiative that seeks to innovate in Materials Science and Engineering for the development of materials in all industrial fields using all the tools available: modelling and computation, experimental tests and theoretical foundations [121]. It is already at the beginning of the second decade of the present century that seminal works appear that bet on the development of data libraries obtained through the evaluation of cellular interactions with structured surfaces/materials by means of nanotechnological techniques [121-123]. The possibility then arises of asking what genetic responses the materials induce and hence the concept of “Materionomics” appears [124,125], i.e. how biomaterials are involved in the different omics of cellular responses. The application of AI to biomaterials science requires, as mentioned above, databases that comply with the FAIR principles, and in recent years these have become available in the fields of Materials, Biology and different fields of Health Sciences. The concept of “Biomaterialomics” has recently appeared [125] which aims to cover all the above aspects and lay the foundations for the design and development of new biomaterials using AI. In other words, it is about integrating computational tools such as AI or ML, large and different databases, and experimental techniques and tests with the aim of exploring and combining basic elements of materials to discover, design and develop new biomaterials aimed at obtaining clinical products or devices. We are still at the beginning of a new paradigm in innovation in the field of Biomaterials [126,127]. It seems that the more holistic vision generated by the “virtual twin” will allow the properties of the biomaterial to be adjusted, so that it can perform or induce the repair, replacement, integration or regeneration functions for which the biomaterial is used [128,129].
IS THERE A NATIONAL INDUSTRY PRODUCING COMPETITIVE PROSTHESES? IS THE DESIGN OF PROSTHESES ONLY WITHIN THE REACH OF LARGE MULTINATIONALS?
Although there are several national companies producing successful prostheses, the bulk of joint implants come from the USA and other EU countries. The combination of new ideas in joint replacement design, materials that make a difference, and systematic basic and clinical research are the ingredients that encourage innovation in joint replacement. Such a combination of factors, in the highly regulated and competitive environment that surrounds us, does not facilitate the emergence of new products that are commercially successful [130]. New ideas arise from collaboration between surgeons and engineers, scientists and manufacturers, which is not often the case in our country.
The demand for joint replacements continues to grow, due to the success of the technique and the ageing population, which is seeking to prolong its active life. The European market currently accounts for 20-25% of the world market, although its share is decreasing. The main countries in terms of population and deployment capacity are Germany, France, Great Britain, Italy and Spain. However, many of the national and even European companies in the sector are medium-sized, with few large, multinational companies, so competition, both in innovation and in marketing and distribution, may favour multinational companies from outside Europe.
The incentive to bring new solutions to market exists, due to growing demand, but it fades along the way. While most ideas arise from academic and clinical initiative, or from incremental improvements of already marketed products, their development and exploitation is very limited. The regulatory pathway is complex, both for new designs and new materials, and authorisation under the current Medical Device Regulation (MDR) Directive is a challenge [131] requires the demonstration of safety and efficacy of prosthetic implants for large joints (hip, knee, shoulder) through clinical trials, as they are considered Class III devices.
In this context, a solid strategy, based on collaboration between the different agents, is required for our country to position itself in the development of new solutions. Otherwise, the current wave of growing demand will be resolved by resorting to solutions defined in other countries and marketed by foreign multinational companies.
WHAT IS THE EVOLUTION OF SCIENTIFIC PRODUCTION ON PROSTHESIS IN SPAIN AND ABROAD?
A search in Pubmed since its beginnings, with the word “Prosthesis” as the main term MesH lists 416,235 documents published under this heading as of 8 March 2024. Scientific production on this subject has followed a growing trend. The introduction of the word Spain in any field reduces the figure to 7,368 documents, which represents 1.77% of this production.
If the search is done by the term Prosthe* in the title field, PubMed lists 58,514 total documents which when adding the term Spain in any field is reduced to 1,073 representing 1.83%.
In an attempt to compare our scientific production with that of other developed nations, both in the European Union and the United States of America, we have shown in Figure 2 the evolution of the scientific production of some countries of the European Union and the United States of America. It can be seen that the scientific output of the 5 largest nations of the European Union has evolved very much in parallel over the last half century.
Figure 2.
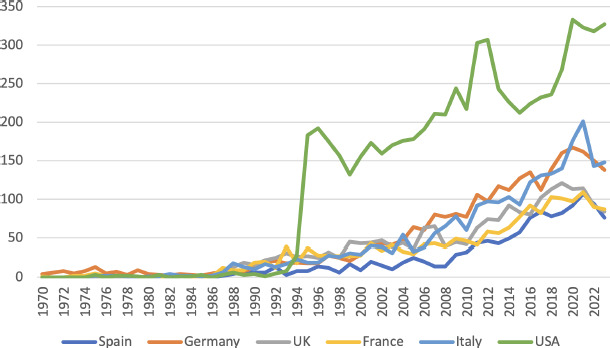
Evolution of scientific production on prosthetics in several developed countries.
FUNDING
None to declare
CONFLICTS OF INTEREST
The author declares no conflicts of interest
References
- 1.Brack R, Amalu EH. A review of technology, materials and R&D challenges of upper limb prosthesis for improved user suitability. J Orthop. 2021;23:88-96. 10.1016/j.jor.2020.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crimì A, Joyce DM, Binitie O, Ruggieri P, Letson GD. The history of resection prosthesis. Int Orthop. 2023;47(3):873-83. 10.1007/s00264-023-05698-w [DOI] [PubMed] [Google Scholar]
- 3.Overmann AL, Forsberg JA. The state of the art of osseointegration for limb prosthesis. Biomed Eng Lett. 2020;10(1):5-16. 10.1007/s13534-019-00133-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandey A, Pragya, Kanoujia J, Parashar P. New Insights into the Applications of 3D-Printed Biomaterial in Wound Healing and Pros-thesis. AAPS PharmSciTech. 2023;24(7):191. 10.1208/s12249-023-02643-3 [DOI] [PubMed] [Google Scholar]
- 5.Sezer HB, Bohu Y, Hardy A, Lefevre N. Knee Prosthesis in the Computer Era. Orthop Surg. 2021;13(2):395-401. 10.1111/os.12762PMC7957434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinke M, Chadwell A, Smit G. State of the art of prosthesis simulators for the upper limb: A narrative review. Ann Phys Rehabil Med. 2022;65(6):101635. 10.1016/j.rehab.2022.101635 [DOI] [PubMed] [Google Scholar]
- 7.Joung YH. Development of implantable medical devices: from an engineering perspective. Int Neurourol J. 2013;17(3):98-106. 10.5213/inj.2013.17.3.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agencia Española de Medicamentos y Productos Sanitarios . Registro de Implantes Mamarios (SREIM). Available at: https://www.aemps.gob.es/productos-sanitarios/protesis-mamarias/?lang=ca
- 9.Merx H, Dreinhöfer K, Schräder P, Stürmer T, Puhl W, Günther KP, et al. International variation in hip replacement rates. Ann Rheum Dis. 2003;62(3):222-6. 10.1136/ard.62.3.222PMC1754456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuerpo Caballero G , Carnero Alcázar M , López Menéndez J, Centella T, Polo López L, García Fuster R , et al. Cirugía cardiovascular en España en el año 2020. Registro de intervenciones de la Sociedad Española de Cirugía Cardiovascular y Endovascular. Cirugía Cardiovascular 2022;29, Issue 4, :207-20. [Google Scholar]
- 11.Jurado-Román A, Freixa X, Cid B, Cruz-González I, Sarnago Cebada F, Baz JA, et al. Spanish cardiac catheterization and coronary intervention registry. 32nd official report of the Interventional Cardiology Association of the Spanish Society of Cardiology (1990-2022). Rev Esp Cardiol (Engl Ed). 2023;76(12):1021-31. 10.1016/j.rec.2023.07.012 [DOI] [PubMed] [Google Scholar]
- 12.Fernández Lozano I, Osca Asensi J, Alzueta Rodríguez J.. Spanish implantable cardioverter-defibrillator registry. 19th official report of Heart Rhythm Association of the Spanish Society of Cardiology (2022). Rev Esp Cardiol (Engl Ed). 2023;76(11):922-35. 10.1016/j.rec.2023.06.015 [DOI] [PubMed] [Google Scholar]
- 13.Pombo Jiménez M, Chimeno García J, Bertomeu González V, Cano Pérez Ó. Spanish pacemaker registry. 20th official report of the Heart Rhythm Association of the Spanish Society of Cardiology (2022). Rev Esp Cardiol (Engl Ed). 2023;76(12):1032-41. 10.1016/j.rec.2023.07.011 [DOI] [PubMed] [Google Scholar]
- 14.Agencia Española de Medicamentos y Productos Sanitarios, Ministerio de Sanidad . Registro Sanitario de Productos Implantables. Gobierno de España. 2023. Availabe at: https://www.aemps.gob.es/productossanitarios_registros_implantables/
- 15.Al-Khatib SM. Cardiac Implantable Electronic Devices. N Engl J Med. 2024;390(5):442-54. 10.1056/NEJMra2308353 [DOI] [PubMed] [Google Scholar]
- 16.Al-Khatib SM, Mi X, Wilkoff BL, Qualls LG, Frazier-Mills C, Setoguchi S, et al. Follow-up of patients with new cardiovascular implantable electronic devices: are experts’ recommendations implemented in routine clinical practice? Circ Arrhythm Electrophysiol. 2013;6(1):108-16. 10.1161/circep.112.974337PMC3640354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kremers MS, Hammill SC, Berul CI, Koutras C, Curtis JS, Wang Y, et al. The National ICD Registry Report: version 2.1 including leads and pediatrics for years 2010 and 2011. Heart Rhythm. 2013;10(4):e59-65. 10.1016/j.hrthm.2013.01.035 [DOI] [PubMed] [Google Scholar]
- 18.Ministerio de Sanidad . Cartera común de implantes quirúrgicos. ANEXO I 2015. Available at: https://www.sanidad.gob.es/profesionales/prestacionesSanitarias/CarteraDeServicios/docs/Anexo_I_Orden_1356_2015_Implantes_EM.pdf
- 19.Elani HW, Starr JR, Da Silva JD, Gallucci GO. Trends in Dental Implant Use in the U.S., 1999-2016, and Projections to 2026. J Dent Res. 2018;97(13):1424-30. 10.1177/0022034518792567PMC6854267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruschini L, Canzi P, Canale A, Covelli E, Laborai A, Monteforte M, et al. Implantable hearing devices in clinical practice. Systematic review and consensus statements. Acta Otorhinolaryngol Ital. 2024;44(1):52-67. 10.14639/0392-100x-n2651PMC10914359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barton GJ, Carlos EC, Lentz AC. Sexual Quality of Life and Satisfaction With Penile Prostheses. Sex Med Rev. 2019;7(1):178-88. 10.1016/j.sxmr.2018.10.003 [DOI] [PubMed] [Google Scholar]
- 22.Reissmann DR, Dard M, Lamprecht R, Struppek J, Heydecke G. Oral health-related quality of life in subjects with implant-supported prostheses: A systematic review. J Dent. 2017;65:22-40. 10.1016/j.jdent.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 23.Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, (Eds). Biomaterials Science, An Introduction to Materials in Medicine: Academic Press; 2013. [Google Scholar]
- 24.Williams D (Ed). Essential Biomaterials Science; . Cambridge: Cambridge University Press; 2014. [Google Scholar]
- 25.U.S. Department of Health & Human Services, Health NIo . Biomaterials; National Institute of Biomedical Imaging and Bioengineering; 2017. Available at: https://www.nibib.nih.gov/science-education/science-topics/biomaterials
- 26.Ratner BD, Hoffman AS, Schoen FJ, Lemons J. Biomaterials Science. An Introduction to Materials in Medicine. 3ª, ed ed: Academic Press. ; 2013. [Google Scholar]
- 27.U.S. Food and Drugs . Medical Device Material Safety Summaries;. Available at: https://www.fda.gov/medical-devices/science-and-re-search-medical-devices/medical-device-material-safety-summaries
- 28.Knothe Tate ML, Detamore M, Capadona JR, Woolley A, Knothe U. Engineering and commercialization of human-device interfaces, from bone to brain. Biomaterials. 2016;95:35-46. 10.1016/j.biomaterials.2016.03.038 [DOI] [PubMed] [Google Scholar]
- 29.Festas AJ, Ramos A, Davim JP. Medical devices biomaterials–A review. Journal of Materials: Design and Applications. 2020;234(1):218-28. 10.1177/1464420719882458 [DOI] [Google Scholar]
- 30.Ralls A, Kumar P, Misra M, Menezes P. Material Design and Surface Engineering for Bio-implants. JOM: The Journal of The Minerals, Metals & Materials Society; . 2019;Available at: 10.1007/s11837-019-03687-2. [DOI] [Google Scholar]
- 31.The Swedish Arthroplasty Registry . Annual Report, 2023. (Accesed 17/03/2024). Available at https://sar.registercentrum.se/about-the-register/annual-reports/p/SJW4-ZGyo
- 32.Dubin JA, Bains SS, Paulson AE, Monarrez R, Hameed D, Nace J, et al. The Current Epidemiology of Revision Total Knee Arthroplasty in the United States From 2016 to 2022. J Arthroplasty. 2024;39(3):760-5. 10.1016/j.arth.2023.09.013 [DOI] [PubMed] [Google Scholar]
- 33.Pietrzak J, Common H, Migaud H, Pasquier G, Girard J, Putman S. Have the frequency of and reasons for revision total knee arthroplasty changed since 2000? Comparison of two cohorts from the same hospital: 255 cases (2013-2016) and 68 cases (1991-1998). Orthop Traumatol Surg Res. 2019;105(4):639-45. 10.1016/j.otsr.2019.01.025 [DOI] [PubMed] [Google Scholar]
- 34.Kerzner B, Kunze KN, O’Sullivan MB, Pandher K, Levine BR. Temporal Trends of Revision Etiologies in Total Knee Arthroplasty at a Single High-Volume Institution: An Epidemiological Analysis. Arthroplast Today. 2021;9:68-72. 10.1016/j.artd.2021.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muñoz P, Kestler M, De Alarcon A, Miro JM, Bermejo J, Rodríguez-Abella H, et al. Current Epidemiology and Outcome of Infective Endocarditis: A Multicenter, Prospective, Cohort Study. Medicine (Baltimore). 2015;94(43):e1816. 10.1097/md.0000000000001816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ziegler A, Heimerl A, Krockenberger K, Hemmelmann C. [Utility of medical devices: approaches to planning and conducting clinical trials]. Z Evid Fortbild Qual Gesundhwes. 2012;106(5):322-31; discussion 32. 10.1016/j.zefq.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 37.Trampuz A, Zimmerli W. Prosthetic joint infections: update in diagnosis and treatment. Swiss Med Wkly. 2005;135(17-18):243-51. 10.4414/smw.2005.10934 [DOI] [PubMed] [Google Scholar]
- 38.Romanek J, Farkowski M, Bukowski H, Gołba K, Wita K, Mitkowski P, et al. The cost of CIED infectious complications treatment in Poland from the perspective of Polish hospitals. Kardiol Pol. 2022;80(9):919-25. 10.33963/KP.a2022.0144 [DOI] [PubMed] [Google Scholar]
- 39.Clémenty N, Carion PL, Léotoing L, Lamarsalle L, Wilquin-Bequet F, Brown B, et al. Infections and associated costs following cardiovascular implantable electronic device implantations: a nationwide cohort study. Europace. 2018;20(12):1974-80. 10.1093/europace/eux387 [DOI] [PubMed] [Google Scholar]
- 40.Ahsan SY, Saberwal B, Lambiase PD, Koo CY, Lee S, Gopalamurugan AB, et al. A simple infection-control protocol to reduce serious cardiac device infections. Europace. 2014;16(10):1482-9. 10.1093/europace/euu126 [DOI] [PubMed] [Google Scholar]
- 41.Brough CEP, Rao A, Haycox AR, Cowie MR, Wright DJ. Real-world costs of transvenous lead extraction: the challenge for reimbursement. Europace. 2019;21(2):290-7. 10.1093/europace/euy291 [DOI] [PubMed] [Google Scholar]
- 42.Eby EL, Bengtson LGS, Johnson MP, Burton ML, Hinnenthal J. Economic impact of cardiac implantable electronic device infections: cost analysis at one year in a large U.S. health insurer. J Med Econ. 2020;23(7):698-705. 10.1080/13696998.2020.1751649 [DOI] [PubMed] [Google Scholar]
- 43.Egea M, García-Urra F, Bellver J A, Alvarez M, Waweru C, A. Q. Economic impact associated with complications of cardiac implantable electronic devices in Spain (Abstract). . Europace 2018;20 (Suppl. 1): i180. [Google Scholar]
- 44.Carniello V, Peterson BW, van der Mei HC, Busscher HJ. Physico-chemistry from initial bacterial adhesion to surface-programmed biofilm growth. Adv Colloid Interface Sci. 2018;261:1-14. 10.1016/j.cis.2018.10.005 [DOI] [PubMed] [Google Scholar]
- 45.Gusnaniar N, van der Mei HC, Qu W, Nuryastuti T, Hooymans JMM, Sjollema J, et al. Physico-chemistry of bacterial transmission versus adhesion. Adv Colloid Interface Sci. 2017;250:15-24. 10.1016/j.cis.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Bian Z, Wang Y. Biofilm formation and inhibition mediated by bacterial quorum sensing. Appl Microbiol Biotechnol. 2022;106(19-20):6365-81. 10.1007/s00253-022-12150-3 [DOI] [PubMed] [Google Scholar]
- 47.Brunelli S, Bonanni C, Foti C, Traballesi M. A Literature Review of the Quality of Life, Health Status and Prosthesis Satisfaction in Older Patients With A Trans-tibial Amputation. Can Prosthet Orthot J. 2020;2(1):33640. 10.33137/cpoj.v3i1.33640PMC10443495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Summers L. Social and quality of life impact using a voice pros-thesis after laryngectomy. Curr Opin Otolaryngol Head Neck Surg. 2017;25(3):188-94. 10.1097/moo.0000000000000361 [DOI] [PubMed] [Google Scholar]
- 49.Samuelsson KA, Töytäri O, Salminen AL, Brandt A. Effects of lower limb prosthesis on activity, participation, and quality of life: a systematic review. Prosthet Orthot Int. 2012;36(2):145-58. 10.1177/0309364611432794 [DOI] [PubMed] [Google Scholar]
- 50.dos Santos DM, Goiato MC, Pesqueira AA, Bannwart LC, Rezende MC, Magro-Filho O, et al. Prosthesis auricular with osseointegrated implants and quality of life. J Craniofac Surg. 2010;21(1):94-6. 10.1097/SCS.0b013e3181c4651a [DOI] [PubMed] [Google Scholar]
- 51.Gallagher P, Desmond D. Measuring quality of life in prosthetic practice: benefits and challenges. Prosthet Orthot Int. 2007;31(2):167-76. 10.1080/03093640600988633 [DOI] [PubMed] [Google Scholar]
- 52.Strassburger C, Kerschbaum T, Heydecke G. Influence of implant and conventional prostheses on satisfaction and quality of life: A literature review. Part 2: Qualitative analysis and evaluation of the studies. Int J Prosthodont. 2006;19(4):339-48. [PubMed] [Google Scholar]
- 53.Strassburger C, Heydecke G, Kerschbaum T. Influence of prosthetic and implant therapy on satisfaction and quality of life: a systematic literature review. Part 1--Characteristics of the studies. Int J Prosthodont. 2004;17(1):83-93. [PubMed] [Google Scholar]
- 54.Warburton TM, Thomas SD, Holden A, Katib N, Varcoe RL. A Cost-Consequence Analysis Comparing Balloon-Expandable Covered Stents for the Management of Aortoiliac Occlusive Disease. J Endovasc Ther. 2024:15266028241234001. 10.1177/15266028241234001 [DOI] [PubMed] [Google Scholar]
- 55.Pennington M, Grieve R, Black N, van der Meulen JH. Cost-Effectiveness of Five Commonly Used Prosthesis Brands for Total Knee Replacement in the UK: A Study Using the NJR Dataset. PLoS One. 2016;11(3):e0150074. 10.1371/journal.pone.0150074PMC4778929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lagares-Borrego A, Gacto-Sanchez P, Infante-Cossio P, Barrera-Pulido F, Sicilia-Castro D, Gomez-Cia T. A comparison of long-term cost and clinical outcomes between the two-stage sequence expander/prosthesis and autologous deep inferior epigastric flap methods for breast reconstruction in a public hospital. J Plast Reconstr Aesthet Surg. 2016;69(2):196-205. 10.1016/j.bjps.2015.11.027 [DOI] [PubMed] [Google Scholar]
- 57.Reynolds MR, Lei Y, Wang K, Chinnakondepalli K, Vilain KA, Magnuson EA, et al. Cost-Effectiveness of Transcatheter Aortic Valve Replacement With a Self-Expanding Prosthesis Versus Surgical Aortic Valve Replacement. J Am Coll Cardiol. 2016;67(1):29-38. 10.1016/j.jacc.2015.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beresniak A, Sabatier B, Achouh P, Menasché P, Fabiani JN. Cost-effectiveness of mitral valve repair versus replacement by biologic or mechanical prosthesis. Ann Thorac Surg. 2013;95(1):98-104. 10.1016/j.athoracsur.2012.08.005 [DOI] [PubMed] [Google Scholar]
- 59.Fidanza A, Schettini I, Palozzi G, Mitrousias V, Logroscino G, Romanini E, et al. What Is the Inpatient Cost of Hip Replacement? A Time-Driven Activity Based Costing Pilot Study in an Italian Public Hospital. J Clin Med. 2022;11(23). 10.3390/jcm11236928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ong CB, Buchan GBJ, Acuña AJ, Hecht CJ, Homma Y, Shah RP, et al. Cost-effectiveness of a novel, fluoroscopy-based robotic-assisted total hip arthroplasty system: A Markov analysis. Int J Med Robot. 2023:e2582. 10.1002/rcs.2582 [DOI] [PubMed] [Google Scholar]
- 61.Agarwal N, To K, Khan W. Cost effectiveness analyses of total hip arthroplasty for hip osteoarthritis: A PRISMA systematic review. Int J Clin Pract. 2021;75(2):e13806. 10.1111/ijcp.13806 [DOI] [PubMed] [Google Scholar]
- 62.Cozzarelli NF, Longenecker AS, Uhr A, Davis DE, Lonner JH. Unicompartmental Knee Arthroplasty Is Cost-Effective in an Outpatient Setting. Cureus. 2023;15(2):e35059. 10.7759/cureus.35059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang JJY, Chen JY, Tay DKJ, Pang HN, Yeo SJ, Liow MHL. Cost-Effectiveness of Robot-Assisted Total Knee Arthroplasty: A Markov Decision Analysis. J Arthroplasty. 2023;38(8):1434-7. 10.1016/j.arth.2023.02.022 [DOI] [PubMed] [Google Scholar]
- 64.Zhang JJY, Chen JY, Tay DK, Pang HN, Yeo SJ, Liow MH. Corrigendum to ‘Cost-Effectiveness of Robot-Assisted Total Knee Arthroplasty: A Markov Decision Analysis’ [Volume 38, Issue 8, August 2023, Pages 1434-1437]. J Arthroplasty. 2024;39(2):568. 10.1016/j.arth.2023.10.001 [DOI] [PubMed] [Google Scholar]
- 65.Wickman JR, Chopra A, Goltz DE, Levin JM, Pereira G, Pidgeon T, et al. Influence of medical comorbidity and surgical indication on total elbow arthroplasty cost of care. J Shoulder Elbow Surg. 2022;31(9):1884-9. 10.1016/j.jse.2022.02.038 [DOI] [PubMed] [Google Scholar]
- 66.Zhou H, Orvets ND, Merlin G, Shaw J, Dines JS, Price MD, et al. Total Elbow Arthroplasty in the United States: Evaluation of Cost, Patient Demographics, and Complication Rates. Orthop Rev (Pavia). 2016;8(1):6113. 10.4081/or.2016.6113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Flinkkilä T, Vähäkuopus M, Sirniö K, Falkenbach P. Cost-effectiveness of shoulder arthroplasty for osteoarthritis and rotator cuff tear arthropathy. An economic analysis using real-world data. Orthop Traumatol Surg Res. 2024:103852. 10.1016/j.otsr.2024.103852 [DOI] [PubMed] [Google Scholar]
- 68.Gowd AK, Agarwalla A, Beck EC, Rosas S, Waterman BR, Romeo AA, et al. Prediction of total healthcare cost following total shoulder arthroplasty utilizing machine learning. J Shoulder Elbow Surg. 2022;31(12):2449-56. 10.1016/j.jse.2022.07.013 [DOI] [PubMed] [Google Scholar]
- 69.Groeneveld PW, Dixit S. Cardiac Pacing and Defibrillation Devices: Cost and Effectiveness. Annu Rev Med. 2017;68:1-13. 10.1146/annurev-med-043015-123540 [DOI] [PubMed] [Google Scholar]
- 70.García-Pérez L, Pinilla-Domínguez P, García-Quintana A, Caballero-Dorta E, García-García FJ, Linertová R, et al. Economic evaluations of implantable cardioverter defibrillators: a systematic review. Eur J Health Econ. 2015;16(8):879-93. 10.1007/s10198-014-0637-x [DOI] [PubMed] [Google Scholar]
- 71.Azari S, Rezapour A, Omidi N, Alipour V, Tajdini M, Sadeghian S, et al. A systematic review of the cost-effectiveness of heart valve replacement with a mechanical versus biological prosthesis in patients with heart valvular disease. Heart Fail Rev. 2020;25(3):495-503. 10.1007/s10741-019-09897-9 [DOI] [PubMed] [Google Scholar]
- 72.Pinar E, García de Lara J, Hurtado J, Robles M, Leithold G, MartíSánchez B, et al. Cost-effectiveness analysis of the SAPIEN 3 transcatheter aortic valve implant in patients with symptomatic severe aortic stenosis. Rev Esp Cardiol (Engl Ed). 2022;75(4):325-33. 10.1016/j.rec.2021.02.013 [DOI] [PubMed] [Google Scholar]
- 73.Palve JS, Luukkaala TH, Kääriäinen MT. Autologous reconstructions are associated with greater overall medium-term care costs than implant-based reconstructions in the Finnish healthcare system: A retrospective interim case-control cohort study. J Plast Reconstr Aesthet Surg. 2022;75(1):85-93. 10.1016/j.bjps.2021.08.020 [DOI] [PubMed] [Google Scholar]
- 74.Chung E, Wang J. State-of-art review of current malleable pe-nile prosthesis devices in the commercial market. Ther Adv Urol. 2023;15:17562872231179008. 10.1177/17562872231179008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aydin M, Fikatas P, Denecke C, Pratschke J, Raakow J. Cost analysis of inguinal hernia repair: the influence of clinical and hernia-specific factors. Hernia. 2021;25(5):1129-35. 10.1007/s10029-021-02372-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Findlay JM, Wood CPJ, Cunningham C. Prophylactic mesh reinforcement of stomas: a cost-effectiveness meta-analysis of randomised controlled trials. Tech Coloproctol. 2018;22(4):265-70. 10.1007/s10151-018-1774-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cloutier M, Mantovani D, Rosei F. Antibacterial Coatings: Challenges, Perspectives, and Opportunities. Trends Biotechnol. 2015;33(11):637-52. 10.1016/j.tibtech.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 78.Hu X, Wang T, Li F, Mao X. Surface modifications of biomaterials in different applied fields. RSC Adv. 2023;13(30):20495-511. 10.1039/d3ra02248j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Francolini I, Vuotto C, Piozzi A, Donelli G. Antifouling and antimicrobial biomaterials: an overview. Apmis. 2017;125(4):392-417. 10.1111/apm.12675 [DOI] [PubMed] [Google Scholar]
- 80.Zhang M, Yu P, Xie J, Li J. Recent advances of zwitterionic-based topological polymers for biomedical applications. J Mater Chem B. 2022;10(14):2338-56. 10.1039/d1tb02323c [DOI] [PubMed] [Google Scholar]
- 81.Chang Y. Designs of zwitterionic polymers. J Polymer Res. 2022; 29: 286. [Google Scholar]
- 82.Pontremoli C, Izquierdo-Barba I, Montalbano G, Vallet-Regí M, Vitale-Brovarone C, Fiorilli S. Strontium-releasing mesoporous bio-active glasses with anti-adhesive zwitterionic surface as advanced biomaterials for bone tissue regeneration. J Colloid Interface Sci. 2020;563:92-103. 10.1016/j.jcis.2019.12.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Falde EJ, Yohe ST, Colson YL, Grinstaff MW. Superhydrophobic materials for biomedical applications. Biomaterials. 2016;104:87-103. 10.1016/j.biomaterials.2016.06.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Modaresifar K, Azizian S, Ganjian M, Fratila-Apachitei LE, Zadpoor AA. Bactericidal effects of nanopatterns: A systematic review. Acta Biomater. 2019;83:29-36. 10.1016/j.actbio.2018.09.059 [DOI] [PubMed] [Google Scholar]
- 85.Chen J, Tang X, Wang Z, Perez A, Yao B, Huang K, et al. Techniques for navigating postsurgical adhesions: Insights into mechanisms and future directions. Bioeng Transl Med. 2023;8(6):e10565. 10.1002/btm2.10565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Park H, Baek S, Kang H, Lee D. Biomaterials to Prevent Post-Operative Adhesion. Materials (Basel). 2020;13(14). 10.3390/ma13143056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dunbar MJ. Antibiotic bone cements: their use in routine primary total joint arthroplasty is justified. Orthopedics. 2009;32(9). 10.3928/01477447-20090728-20 [DOI] [PubMed] [Google Scholar]
- 88.Tarakji KG, Mittal S, Kennergren C, Corey R, Poole JE, Schloss E, et al. Antibacterial Envelope to Prevent Cardiac Implantable Device Infection. N Engl J Med. 2019;380(20):1895-905. 10.1056/NEJMoa1901111 [DOI] [PubMed] [Google Scholar]
- 89.Gomes F, Teixeira P, Cerca N, Ceri H, Oliveira R. Virulence gene expression by Staphylococcus epidermidis biofilm cells exposed to antibiotics. Microb Drug Resist. 2011;17(2):191-6. 10.1089/mdr.2010.0149 [DOI] [PubMed] [Google Scholar]
- 90.Carradori S, Di Giacomo N, Lobefalo M, Luisi G, Campestre C, Sisto F. Biofilm and Quorum Sensing inhibitors: the road so far. Expert Opin Ther Pat. 2020;30(12):917-30. 10.1080/13543776.2020.1830059 [DOI] [PubMed] [Google Scholar]
- 91.Vashistha A, Sharma N, Nanaji Y, Kumar D, Singh G, Barnwal RP, et al. Quorum sensing inhibitors as Therapeutics: Bacterial bio-film inhibition. Bioorg Chem. 2023;136:106551. 10.1016/j.bioorg.2023.106551 [DOI] [PubMed] [Google Scholar]
- 92.Wagner W, Sakiyama-Elbert S, Zhang G, M. Y. Biomaterials Science - An Introduction to Materials in Medicine, . 4th Edition ed: Elsevier, Academic Press; 2020. [Google Scholar]
- 93.Vallet-Regí M. Evolution of Biomaterials. Frontiers in Materials. 2022;March, 01. 10.3389/fmats.2022.864016 [DOI] [Google Scholar]
- 94.Vallet-Regí M, A. S. Mesoporous bioactive glasses for regenerative medicine. MaterToday Bio. 2021; 11, 100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Suh GA, Patel R. Clinical phage microbiology: a narrative summary. Clin Microbiol Infect. 2023;29(6):710-3. 10.1016/j.cmi.2023.02.006 [DOI] [PubMed] [Google Scholar]
- 96.Slopek S, Durlakowa I, Weber-Dabrowska B, Dabrowski M, Kucharewicz-Krukowska A. Results of bacteriophage treatment of suppurative bacterial infections. III. Detailed evaluation of the results obtained in further 150 cases. Arch Immunol Ther Exp (Warsz). 1984;32(3):317-35. [PubMed] [Google Scholar]
- 97.Slopek S, Durlakowa I, Weber-Dabrowska B, Kucharewicz-Krukowska A, Dabrowski M, Bisikiewicz R. Results of bacteriophage treatment of suppurative bacterial infections. II. Detailed evaluation of the results. Arch Immunol Ther Exp (Warsz). 1983;31(3):293-327. [PubMed] [Google Scholar]
- 98.Slopek S, Durlakowa I, Weber-Dabrowska B, Kucharewicz-Krukowska A, Dabrowski M, Bisikiewicz R. Results of bacteriophage treatment of suppurative bacterial infections. I. General evaluation of the results. Arch Immunol Ther Exp (Warsz). 1983;31(3):267-91. [PubMed] [Google Scholar]
- 99.Slopek S, Kucharewicz-Krukowska A, Weber-Dabrowska B, Dabrowski M. Results of bacteriophage treatment of suppurative bacterial infections. VI. Analysis of treatment of suppurative staphylococcal infections. Arch Immunol Ther Exp (Warsz). 1985;33(2):261-73. [PubMed] [Google Scholar]
- 100.Slopek S, Kucharewicz-Krukowska A, Weber-Dabrowska B, Dabrowski M. Results of bacteriophage treatment of suppurative bacterial infections. V. Evaluation of the results obtained in children. Arch Immunol Ther Exp (Warsz). 1985;33(2):241-59. [PubMed] [Google Scholar]
- 101.Slopek S, Kucharewicz-Krukowska A, Weber-Dabrowska B, Dabrowski M. Results of bacteriophage treatment of suppurative bacterial infections. IV. Evaluation of the results obtained in 370 cases. Arch Immunol Ther Exp (Warsz). 1985;33(2):219-40. [PubMed] [Google Scholar]
- 102.Slopek S, Weber-Dabrowska B, Dabrowski M, Kucharewicz-Krukowska A. Results of bacteriophage treatment of suppurative bacterial infections in the years 1981-1986. Arch Immunol Ther Exp (Warsz). 1987;35(5):569-83. [PubMed] [Google Scholar]
- 103.Uyttebroek S, Chen B, Onsea J, Ruythooren F, Debaveye Y, Devolder D, et al. Safety and efficacy of phage therapy in difficult-to-treat infections: a systematic review. Lancet Infect Dis. 2022;22(8):e208-e20. 10.1016/s1473-3099(21)00612-5 [DOI] [PubMed] [Google Scholar]
- 104.Suh GA, Ferry T, Abdel MP. Phage Therapy as a Novel Therapeutic for the Treatment of Bone and Joint Infections. Clin Infect Dis. 2023;77(Supplement_5):S407-s15. 10.1093/cid/ciad533 [DOI] [PubMed] [Google Scholar]
- 105.Fedorov E, Samokhin A, Kozlova Y, Kretien S, Sheraliev T, Morozova V, et al. Short-Term Outcomes of Phage-Antibiotic Combination Treatment in Adult Patients with Periprosthetic Hip Joint Infection. Viruses. 2023;15(2). 10.3390/v15020499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Racenis K, Lacis J, Rezevska D, Mukane L, Vilde A, Putnins I, et al. Successful Bacteriophage-Antibiotic Combination Therapy against Multidrug-Resistant Pseudomonas aeruginosa Left Ventricular Assist Device Driveline Infection. Viruses. 2023;15(5). 10.3390/v150512104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blasco L, López-Hernández I, Rodríguez-Fernández M, Pérez-Florido J, Casimiro-Soriguer CS, Djebara S, et al. Case report: Analysis of phage therapy failure in a patient with a Pseudomonas aeruginosa prosthetic vascular graft infection. Front Med (Lausanne). 2023;10:1199657. 10.3389/fmed.2023.1199657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Amankwah S, Adisu M, Gorems K, Abdella K, Kassa T. Assessment of Phage-Mediated Inhibition and Removal of Multidrug-Resistant Pseudomonas aeruginosa Biofilm on Medical Implants. Infect Drug Resist. 2022;15:2797-811. 10.2147/idr.S367460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sanchez BC, Heckmann ER, Green SI, Clark JR, Kaplan HB, Ramig RF, et al. Development of Phage Cocktails to Treat E. coli Catheter-Associated Urinary Tract Infection and Associated Biofilms. Front Microbiol. 2022;13:796132. 10.3389/fmicb.2022.796132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yerushalmy O, Braunstein R, Alkalay-Oren S, Rimon A, Coppenhagn-Glazer S, Onallah H, et al. Towards Standardization of Phage Susceptibility Testing: The Israeli Phage Therapy Center “Clinical Phage Microbiology”-A Pipeline Proposal. Clin Infect Dis. 2023;77(Supplement_5):S337-s51. 10.1093/cid/ciad514 [DOI] [PubMed] [Google Scholar]
- 111.Yerushalmy O, Khalifa L, Gold N, Rakov C, Alkalay-Oren S, Adler K, et al. The Israeli Phage Bank (IPB). Antibiotics (Basel). 2020;9(5). 10.3390/antibiotics9050269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bosco K, Lynch S, Sandaradura I, Khatami A. Therapeutic Phage Monitoring: A Review. Clin Infect Dis. 2023;77(Supplement_5):S384-s94. 10.1093/cid/ciad497 [DOI] [PubMed] [Google Scholar]
- 113.Revilla-León M, Gómez-Polo M, Vyas S, Barmak BA, Galluci GO, Att W, et al. Artificial intelligence applications in implant dentistry: A systematic review. J Prosthet Dent. 2023;129(2):293-300. 10.1016/j.prosdent.2021.05.008 [DOI] [PubMed] [Google Scholar]
- 114.Sermesant M, Delingette H, Cochet H, Jaïs P, Ayache N. Applications of artificial intelligence in cardiovascular imaging. Nat Rev Cardiol. 2021;18(8):600-9. 10.1038/s41569-021-00527-2 [DOI] [PubMed] [Google Scholar]
- 115.Samant S, Bakhos JJ, Wu W, Zhao S, Kassab GS, Khan B, et al. Artificial Intelligence, Computational Simulations, and Extended Reality in Cardiovascular Interventions. JACC Cardiovasc Interv. 2023;16(20):2479-97. 10.1016/j.jcin.2023.07.022 [DOI] [PubMed] [Google Scholar]
- 116.Karatzia L, Aung N, Aksentijevic D. Artificial intelligence in cardiology: Hope for the future and power for the present. Front Cardiovasc Med. 2022;9:945726. 10.3389/fcvm.2022.945726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zurabov F, Glazunov E, Kochetova T, Uskevich V, Popova V. Bacteriophages with depolymerase activity in the control of antibiotic resistant Klebsiella pneumoniae biofilms. Sci Rep. 2023;13(1):15188. 10.1038/s41598-023-42505-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Solórzano-Requejo W, Aguilar C, Zapata Martínez R, Contreras-Almengor O, Moscol I, Ojeda C, et al. Artificial Intelligence and Numerical Methods Aided Design of Patient-Specific Coronary Stents. Proceedings of the 16th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2023)-Volume 1: BIODEVICES. 2023;SciTePress. 10.5220/0011639000003414P [DOI] [Google Scholar]
- 119.Sarkar P, Dewangan O, Joshi A. A Review on Applications of Artificial Intelligence on Bionic Eye Designing and Functioning. Scandinavian Journal of Information Systems 2023; 35:1119-27 10.5281/SJIS.775137. [DOI] [Google Scholar]
- 120.Gobierno de España, Pública . MplTDylF. Principios FAIR: Buenas prácticas para la gestión y administración de datos científicos | datos.gob.es. Available at: https://datosgobes/es/noticia/principios-fair-buenas-practicas-para-la-gestion-y-administracion-de-datos-cientificos. 2017.
- 121.de Pablo JJ, Nicholas E J, MA; W, Chen L-Q, Moore J E, Morgan D, et al. New frontiers for the materials genome initiative. npj Comput Mater 2019; 41, 5. 10.1038/s41524-019-0173-4 [DOI] [Google Scholar]
- 122.Mei Y, Saha K, Bogatyrev SR, Yang J, Hook AL, Kalcioglu ZI, et al. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nat Mater. 2010;9(9):768-78. 10.1038/nmat2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hook AL, Chang CY, Yang J, Atkinson S, Langer R, Anderson DG, et al. Discovery of novel materials with broad resistance to bacterial attachment using combinatorial polymer microarrays. Adv Mater. 2013;25(18):2542-7. 10.1002/adma.201204936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cranford SW, de Boer J, van Blitterswijk C, Buehler MJ. Materiomics: an-omics approach to biomaterials research. Adv Mater. 2013;25(6):802-24. 10.1002/adma.201202553 [DOI] [PubMed] [Google Scholar]
- 125.Basu B, Gowtham NH, Xiao Y, Kalidindi SR, Leong KW. Biomaterialomics: Data science-driven pathways to develop fourth-generation biomaterials. Acta Biomater. 2022;143:1-25. 10.1016/j.actbio.2022.02.027 [DOI] [PubMed] [Google Scholar]
- 126.Xue K, Wang F, Suwardi A, Han MY, Teo P, Wang P, et al. Biomaterials by design: Harnessing data for future development. Mater Today Bio. 2021;12:100165. 10.1016/j.mtbio.2021.100165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kerner J, Dogan A, von Recum H. Machine learning and big data provide crucial insight for future biomaterials discovery and research. Acta Biomater. 2021;130:54-65. 10.1016/j.actbio.2021.05.053 [DOI] [PubMed] [Google Scholar]
- 128.Al-Kharusi G, Dunne NJ, Little S, Levingstone TJ. The Role of Machine Learning and Design of Experiments in the Advancement of Biomaterial and Tissue Engineering Research. Bioengineering (Basel). 2022;9(10). 10.3390/bioengineering9100561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Suwardi A, Wang F, Xue K, Han MY, Teo P, Wang P, et al. Machine Learning-Driven Biomaterials Evolution. Adv Mater. 2022;34(1):e2102703. 10.1002/adma.202102703 [DOI] [PubMed] [Google Scholar]
- 130.Gómez-Barrena E, N. P-E. Research in Orthopaedics and Trauma. In: Verhaar JAN, Kjærsgaard-Andersen P, Limb D, Günther KP, Karachalios T, editors. The EFORT White Book: “Orthopaedics and Traumatology in Europe”. Lowestoft (UK): Dennis Barber Ltd; 2021.; 2022. (Accessed 17/3/2024). Available at: https://www.ncbi.nlm.nih.gov/books/NBK585952/pdf/Bookshelf_NBK585952.pdf [Google Scholar]
- 131.European Union. EU Regulation 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/ EEC and 93/42/EEC. Official Journal LEU 552017;. 2017;117:1–175. [Google Scholar]


