Abstract
Transcatheter aortic valve replacement (TAVR) has undergone rapid expansion, emerging as a viable therapeutic option for low-risk patients in lieu of surgical aortic valve replacement. This paper aims to provide a review of the scientific evidence concerning TAVR in low-risk patients, encompassing both observational and clinical trial data. Furthermore, a substantial proportion of low-risk patients possesses a bicuspid aortic valve, necessitating careful examination of the pertinent anatomic and clinical considerations to TAVR that is highlighted in this review. Additionally, the review expands upon some of the unique challenges associated with alternate access in low-risk patients evaluated for TAVR. Last, this review outlines the pivotal role of a multidisciplinary heart team approach in the execution of all TAVR procedures and the authors’ vision of ‘minimalist TAVR’ as a new era in low-risk TAVR.
Keywords: Transcatheter aortic valve replacement, transcatheter aortic valve intervention, low risk, systematic review
Transcatheter aortic valve replacement (TAVR) has evolved as a transformative intervention, gaining widespread acceptance and expanding indications to include low-risk patient populations.[1–6] Notably, the annual volume of TAVR procedures in the US surpassed all forms of surgical aortic valve replacement (SAVR) in 2019.[1–3] This paradigm shift is underscored by the approval of SAPIEN 3 (Edwards) and Evolut (Medtronic) valves for low-surgical risk patients, signifying a potential decline in the age threshold for TAVR referrals. As the landscape of TAVR broadens, an increasing number of low-risk patients, including those with bicuspid aortic valves (BAV), will undergo this intervention, presenting unique challenges given their longer life expectancy with bioprosthetic valves. However, the long-term outlook for TAVR outcomes in low-risk patients remains uncertain.
In navigating the complexities of TAVR in low-risk patients, the review explores alternative access strategies and underscores the crucial role of the heart team. Furthermore, the discussion delves into the imperative to establish strategies for lifetime management in young, low-risk patients, emphasising the approach to the selection of the initial procedure. This involves not only optimising durability but also facilitating potential second and third reinterventions, potentially leading to scenarios involving TAVR–TAVR–TAVR.
As we navigate this dynamic landscape, the integration of scientific evidence, individual patient characteristics, and collaborative decision-making within the heart team will be pivotal in defining the trajectory of TAVR in low-risk patients. Additionally, the review anticipates the growing significance of the emerging concept of ‘minimalist TAVR’, projecting its role as the new era of TAVR expansion in the near future.
Scientific Rationale for TAVR in Low-risk Individuals
Over the past decade, evolving research has underscored the efficacy and safety of in comparison to SAVR in low-risk patients. Initial observational studies reported varied outcomes in this patient cohort (Table 1).[7–13] Subsequently, prospective studies and data registries were initiated to further elucidate TAVR outcomes in low-risk patients. In a prospective study of 200 low-risk TAVR patients, favourable 1-year outcomes were observed, with low mortality rates (3%) and a low incidence of stroke (2.1%). Notably, 14% of TAVR recipients displayed hypo-attenuated leaflet thickening at 30 days, correlating with a numerically higher stroke rate (3.8% versus 1.9%; p=0.53), albeit without impacting valve haemodynamics at the 1-year mark.[11] Results from the GARY registry, encompassing 20,549 low-risk patients (6,062 TAVR, 14,487 SAVR) indicated comparable 1-year survival rates between groups. However, in-hospital and 30-day survival rates favoured TAVR over SAVR (98.5% versus 97.3%; p=0.003; 98.1% versus 97.1%; p=0.014, respectively).[8]
Table 1: Observational Studies of Low-risk Transcatheter Aortic Valve Replacement.
| Study | Patients (n) | Population | Major Findings | |
|---|---|---|---|---|
| Waksman et al. 2019[11] | 200 TAVR, 719 SAVR control cohort | Low-risk patients, with | STS-PROM <3% |
|
| Rosato et al. 2016[7] | 3,402 (531 TAVR, 2,871 SAVR) | EuroSCORE II <4% |
|
|
| Bekeredjian et al. 2019[8] | 20,549 (6,062 TAVR, 14,487 SAVR) | Low-risk STS-PROM <4% |
|
|
| Serruys et al. 2018[9] | 254 (131 TAVR, 123 SAVR) | Low-risk based on STS score |
|
|
| Finkelstein et al. 2019[10] | 1,198 low-risk patients who underwent TAVR | Low-risk STS-PROM <4% |
|
PROM = predicted risk of mortality; SAVR = surgical aortic valve replacement; STS = Society of Thoracic Surgeons; TAVR = transcatheter aortic valve replacement.
Serruys et al. analysed 254 patients (131 TAVR, 123 SAVR) and found that the composite endpoint of all-cause mortality or disabling stroke was lower in the TAVR group compared to SAVR among patients with a Society of Thoracic Surgeons (STS) score of < 3% (1.5% versus 6.5%, p=0.04).[9] Conversely, a study involving 3,402 low-risk patients demonstrated that following propensity matching, SAVR exhibited higher 3-year survival rates compared to TAVR (83.4% versus 72.0%, p=0.0015). This analysis also revealed superior freedom from major cardiac and cerebrovascular events with SAVR in comparison to TAVR (80.9% versus 67.3%, p<0.001).[12]
Subsequent to these studies, randomised clinical trials conducted within the low-risk patient population have consistently demonstrated favourable outcomes with TAVR as a therapeutic option (Table 2).[1,2,12,13]
Table 2: Clinical Trials of Low-risk Transcatheter Aortic Valve Replacement.
| Trials | Evolut Low Risk 2019[2]† | NOTION 2019[13] | PARTNER 3 2019[1]‡ | |||
|---|---|---|---|---|---|---|
| TAVR (n=725) | SAVR (n=678) | TAVR (n=139) | SAVR (n=135) | TAVR (n=503) | SAVR (n=497) | |
| Death or disabling stroke at 24 months | 5.3 versus 6.7; -1.4 (-4.9 to 2.1) | |||||
| All-cause mortality, stroke or MI at 5 years | 38 versus 36.3; p=0.86 | |||||
| All-cause death/stroke/rehospitalisation at 12 months | 8.5 versus 15.1; 0.54 [0.37–0.79]§ | |||||
| 30-day Outcomes | ||||||
| Death or disabling stroke | 0.8 versus 2.6; -1.8 (-3.2 to -0.5)§ | 0.4 versus 1.3; 0.30 [0.06–1.51] | ||||
| All-cause mortality, stroke or MI | 6.3 versus 11.9; p=0.10 | |||||
| All-cause death/stroke/rehospitalisation | 4.2 versus 9.3; 0.45 [0.27–0.76]§ | |||||
| All-cause mortality | 0.5 versus 1.3; -0.8 (-1.9 to 0.2) | 2.1 versus 3.7; p=0.43 | 0.4 versus 1.1; 0.37 [0.07–1.88] | |||
| Cardiovascular mortality | 0.5 versus 1.3; -0.8 (-1.9 to 0.2) | 2.1 versus 3.7; p=0.43 | 0.4 versus 0.90; 0.46 [0.08–2.49] | |||
| All stroke | 3.4 versus 3.4; 0.0 (-1.9 to 1.9) | 1.4 versus 3.0; p=0.37 | 0.6 versus 2.4; 0.25 [0.07–0.88]§ | |||
| AF | 7.7 versus 35.4; -27.7 (-31.8 to -23.6)§ | 16.9 versus 57.8; p<0.0001§ | 5.0 versus 39.5; 0.10 [0.06–0.16]§ | |||
| MI | 0.9 versus 1.3; -0.4 (-1.5 to 0.7) | 2.8 versus 6; p=0.20 | 1.0 versus 1.3; 0.76 [0.23–2.50] | |||
| Need for reintervention | 0.4 versus 0.4; 0.0 (-0.8 to 0.7) | 0.0 versus 0.0; p=NA | 0.0 versus 0.0; NA | |||
| Pacemaker placement | 17.4 versus 6.1; 11.3 (8.0–14.7)|| | 34.1 versus 1.6; p<0.0001|| | 6.5 versus 4.0; 1.66 [0.93–2.96] | |||
| Endocarditis | 0.1 versus 0.2; -0.1 (-0.7 to 0.3) | 1.4 versus 0; p=0.17 | 0.0 versus 0.2; 0.00 [NA] | |||
| Coronary obstruction requiring intervention | 0.9 versus 0.4; 0.5 (-0.3 to 1.4) | 0.2 versus 0.7; 0.30 [0.03–2.93] | ||||
| Valve thrombosis | 0.1 versus 0.1; 0.0 (-0.4 to 0.4) | 0.2 versus 0.0; [0] NA | ||||
| Long-term Outcomes | Duration of Follow-up | |||||
| 12 Months | 5 Years | 12 Months | ||||
| All-cause mortality | 2.4 versus 3.0; -0.6 (-2.6 to 1.3) | 27.6 versus 28.9; p=0.75 | 1.0 versus 2.5; 0.41 [0.14–1.17] | |||
| Death from any cause or disabling stroke | 2.9 versus 4.6; -1.8 (-4.0 to 0.4) | 1.0 versus 2.9; 0.34 [0.12–0.97]§ | ||||
| Cardiovascular mortality | 1.7 versus 2.6; -0.9 (-2.7 to 0.7) | 20.8 versus 23; p=0.62 | 0.8 versus 2.0; 0.40 [0.12–1.30] | |||
| All stroke | 4.1 versus 4.3; -0.2 (-2.4 to 1.9) | 9.0 versus 7.4; p=0.65 | 1.2 versus 3.1; 0.38 [0.15–1.00] | |||
| AF | 9.8 versus 38.3; -28.5 (-32.8 to -24.1)§ | 23.4 versus 60.8; p<0.0001§ | ||||
| MI | 1.7 versus 1.6; 0.1 (-1.3 to 1.5) | 7.7 versus 7.4; p=0.96 | 1.2 versus 2.2; 0.54 [0.20–1.49] | |||
| Need for reintervention | 0.7 versus 0.6; 0.0 (-1.0 to 0.9) | 2.1 versus 0.7; p=0.35 | 0.6 versus 0.5; 1.33 [0.22–7.95] | |||
| Pacemaker placement | 19.4 versus 6.7; 12.6 (9.2–16.2)|| | 41.7 versus 7.8; p<0.0001|| | 7.3 versus 5.4; 1.39 [0.83–2.33] | |||
| Endocarditis | 0.2 versus 0.3; -0.1 (-0.9 to 0.5) | 6.2 versus 4.4; p=0.51 | 0.2 versus 0.5; 0.44 [0.04–4.89] | |||
| Coronary obstruction requiring intervention | 0.9 versus 0.4; 0.5 (-0.3 to 1.4) | 0.2 versus 0.7; 0.30 [0.03–2.93] | ||||
| Valve thrombosis | 0.2 versus 0.3; -0.1 (-0.9 to 0.5) | 1.0 versus 0.2; 4.47 [0.52–38.24] | ||||
SAVR = surgical aortic valve replacement; TAVR = transcatheter aortic valve replacement. *All outcomes are represented as percentages. † All event rates are summarised as Bayesian posterior medians with 95% credible intervals. ‡All values are summarised as HR [95% CI]. § Indicates a TAVR-favourable outcome.||Indicates a SAVR-favourable outcome.
In the PARTNER 3 trial encompassing 1,000 patients (503 TAVR with balloon-expandable valves and 497 SAVR), the primary endpoint of death, stroke or rehospitalisation at 1 year significantly favoured TAVR over SAVR, meeting both superiority and non-inferiority margins (8.5% versus 15.1%; p<0.001 for non-inferiority, p=0.001 for superiority).[1]
Similarly, in the Evolut Low Risk trial, involving 1,403 low-risk patients (725 TAVR with self-expanding valves, 678 SAVR), TAVR demonstrated non-inferiority to SAVR in terms of death or disabling stroke rates at 24 months (5.3% in TAVR versus 6.7% in SAVR). Additionally, TAVR displayed lower rates of acute kidney injury, bleeding events, and AF compared to SAVR, although with higher rates of aortic regurgitation and permanent pacemaker implantations.[2] In a recent 4-year report, the authors noted an all-cause mortality or disabling stroke of 10.7% in the TAVR group and 14.1% in the SAVR group (HR 0.74; 95% CI [0.54–1.00]; p=0.05), indicating a 26% relative reduction in the risk for death or disabling stroke with TAVR. Notably, indicators of valve performance (including AV reintervention, valve thrombosis, endocarditis) showed no discernible difference between the two groups.
Within the NOTION trial, encompassing 274 low-risk patients (139 TAVR, 135 SAVR recipients), all-cause mortality at 6 years was comparable between TAVR and SAVR, with similar outcomes persisting at 8 years. Remarkably, this trial represents the lengthiest follow-up data among randomised trials concerning low-risk TAVR outcomes.[12,13] Additionally, investigators documented significantly higher rates of structural valve deterioration (SVD) in SAVR compared to TAVR at 5 years (24.0% versus 4.8%; p<0.001) and at 8 years (13.9% versus 28.3%; p=0.0017).
Long-term Durability: A Closer Look
The long-term analysis of PARTNER 3 raises notable considerations. Beyond the first year, the initially favourable non-hierarchical composite primary endpoint in the TAVR group diminished, revealing a signal in the difference in mortality, primarily driven by non-cardiovascular deaths in the TAVR arm.[14] The 5-year primary endpoint rates for TAVR and surgery were 22.8% and 27.2%, respectively, compared to the 1-year rates (8.5% versus 15.1%; p<0.001) with no significant difference in a win-ratio analysis for a hierarchical composite endpoint. The incidence of stroke at 5 years appeared similar between the two groups, with most strokes being ischaemic, emphasising the continued significance of stroke as a serious complication of aortic-valve replacement. While statistically non-significant, the convergence of mortality curves at this timeframe prompts cautious consideration, especially given the initial assertions of TAVR superiority at 1 year.
In contrast, 4-year data from the Evolut Low Risk trial indicate a persistent benefit with TAVR over time. While all-cause mortality rates were numerically higher with TAVR, the primary endpoint (death or disabling stroke) favoured TAVR, showing a 26% relative reduction in risk.[15] The absolute difference increased from 1.8% at 1 year to 3.4% at 4 years, pointing towards the possible benefits of TAVR in this low-risk population.
These findings underscore the need for continued vigilant monitoring in longer-term follow-up and a nuanced assessment of causes of death to elucidate the evolving landscape of TAVR outcomes in low-risk patients.
Bicuspid TAVR: A Distinct yet Substantial Subset of Low-risk Patients
Traditionally excluded from prior TAVR trials due to safety and outcome uncertainties, patients with BAV have been the focus of recent prospective studies and registries.[1–5,11,13,16] Severe aortic stenosis (AS) in BAV patients, marked by a younger age of onset and unique anatomical challenges, has been addressed in TAVR, with promising short-and intermediate-term success rates. However, this comes with a higher incidence of significant perivalvular regurgitation, a topic of ongoing discussion (Table 3).[6,17–19]
Table 3: Studies of Bicuspid Patients.
| Author | Patients (n) | Population | Major Findings |
|---|---|---|---|
| Forrest et al. 2020[6] | 150 patients | Low-risk bicuspid |
|
| Waksman et al. 2020[17] | 61 bicuspid TAVR | Low-risk patients undergoing TAVR with self-expanding or balloon expandable valves |
|
| Halim et al. 2020[18] | 5,412 bicuspid TAVR procedures | Low-risk TAVR |
|
| Forrest et al. 2020[19] | 932 bicuspid TAVR, 26,154 tricuspid TAVR | Low-risk patients undergoing TAVR with self-expanding valves from TVT registry |
|
TAVR = transcatheter aortic valve replacement; TVT = transcatheter valve therapy.
In a study by Forrest et al., 150 low-risk patients with BAV stenosis undergoing TAVR with self-expanding valves (SEV) showed high device success and a low rate of death or disabling stroke at 30 days, independent of Sievers classification of bicuspid valve type.[6]
Another analysis of 61 low-risk patients with BAV undergoing TAVR reported no mortalities or disabling strokes at 30 days. However, there was a 13.1% rate of new pacemaker implantation and a 10% incidence of hypo-attenuated leaflet thickening at 30 days, unrelated to clinical events.[17]
When comparing bicuspid and tricuspid TAVR patients, an analysis of 932 bicuspid TAVR procedures from the TVT registry revealed comparable all-cause mortality and stroke rates at 30 days and 1 year, albeit with a slightly elevated 30-day risk for stroke in patients with BAV.[19] An analysis by Halim et al. of 5,412 low-risk TAVR procedures in BAV also demonstrated lower adjusted 1-year mortality in bicuspid TAVR compared to tricuspid TAVR, with a slightly higher incidence of residual moderate or severe aortic insufficiency in bicuspid TAVR.[18] The study also noted a higher device success and lower rates of significant aortic insufficiency with current-generation valves compared to older-generation valves.
Despite the promising results, careful patient selection and anatomical assessment are crucial due to the unique anatomical challenges associated with BAV, including asymmetric aortic annulus, eccentric heavy calcification, calcium distribution throughout the aorto-annular complex, raphe resistance to pre-dilatation, and aortic root dilatation.[20] These challenges may impact valve haemodynamics and durability, resulting in elevated transvalvular gradients, paravalvular leak (PVL), device malpositioning, and a higher rate of permanent pacemaker implantations. Due to these anatomical characteristics, valves are often implanted higher and anchored at the narrowest part of the commissural.
Currently, only observational data are available comparing SEV versus balloon-expandable valves (BEV) valves. The BEAT registry compared SAPIEN 3 versus Evolut R/PRO in AS BAV and confirmed favourable procedural results with both platforms. However, the SEV group exhibited a higher rate of moderate-to-severe PVL at 1 year, and BEV were associated with a more frequent occurrence of annular rupture.[21]
Alternate Vascular Access for TAVR
The transfemoral (TF) vascular access route constitutes the primary approach in the majority of TAVR procedures, accounting for approximately 90% of all TAVR interventions, even in cases involving low-risk patients.[1–8] Historically, femoral access has been the standard access employed in randomised clinical trials of TAVR due to its well-established safety profile, consistent outcomes, and the familiarity of operators, with alternative access methods comprising a smaller fraction of these procedures.[1–5,11,13,16] In the Evolut Low Risk trial, the usage of alternative access was approximately 1%, and the absence of TF access served as an exclusion criterion in the PARTNER 3 trial.
It is noteworthy that alternative access approaches have been associated with increased mortality and stroke rates compared to patients with TF access, particularly in cases involving transapical, direct aortic, and transcaval routes.[22–24] While peripheral artery disease and significant vessel tortuosity typically prompt consideration of alternate access TAVR, operators often opt for peripheral vascular interventions to facilitate TF TAVR and avoid the necessity of alternative access procedures.[23] The emergence of intravascular lithotripsy as a novel technology for modifying heavily calcified arteries to accommodate larger-bore access, including TAVR delivery sheaths, is actively under investigation and holds promise, especially in patients with stenotic calcified iliofemoral vessels.[24]
However, given the associated morbidity and mortality of alternative access TAVR, coupled with the exclusion of these patients from low-risk trials, SAVR should remain the preferred choice for low-surgical-risk patients lacking TF access, particularly in centres where routine alternative access procedures are not routinely performed. Furthermore, the most recent American College of Cardiology/American Heart Association (ACC/AHA 2020) valvular heart disease guidelines have recommended SAVR as the preferred treatment if vascular anatomy or other factors preclude TF-TAVR (class I).[25]
Challenges to Low-risk TAVR
Permanent Pacemaker
Presently, the rates of new permanent pacemaker implantation (PPI) after TAVR vary widely, ranging from 2% to 36%. Meta-analyses have indicated an elevated risk of all-cause mortality at 1 year in patients necessitating a new prosthesis–patient mismatch (PPM).[26] Additionally, the requirement for PPI is associated with extended hospital stays and increased healthcare costs. Impingement onto the membranous septum by the TAVR valve is linked to a higher incidence of heart block.[26]
The MInimizing Depth According to the membranous Septum (MIDAS) approach has been shown to significantly reduce the rate of PPI.[27] However, it is imperative to balance between the risk of heart block and the risk of upward migration of the TAVR valve. Consequently, the cusp-overlapping technique has been developed to better assess the true depth of TAVR implantation, which can be misleading when using the traditional co-planar view.[26,27] The cusp overlap view angle can be determined pre-procedurally through CT reconstruction and subsequently confirmed intraoperatively via fluoroscopy, typically employing a right anterior oblique (RAO)-caudal view. By employing this approach, the genuine depth of valve deployment can be accurately gauged during the implantation procedure.[28]
This technique holds particular significance for SEV TAVR for two primary reasons. First, SEV TAVR tend to descend into the ventricular side during implantation, with the degree of descent varying depending on the specific valve used. Second, the gradual implantation process of SEV TAVR allows for more precise adjustments to the depth of implantation. Consequently, the cusp-overlapping technique aids in deploying the TAVR valve at the optimal desired position.[29]
Paravalvular Leak
TAVR has been associated with an increased rate of PVL compared to SAVR, which in turn translates into higher mortality rates.[4,30] Suboptimal device implantation, valve annulus-prosthesis diameter size mismatch, and calcification in the device landing zone have been identified as the primary predictors of PVL.[31,32] Advances in valve technology, including the development of newer generation valves, pre-procedural multidetector computed tomography imaging for precise sizing, and improved sealing mechanisms, have contributed to a reduction in PVL rates.[33,34]
For instance, the PARTNER 3 trial reported similar rates of moderate to severe PVL with the BEV compared to SAVR (0.6% versus 0.5%).[1] In contrast, the low-risk trial involving the Medtronic SEV demonstrated a higher incidence of moderate to severe PVL (3.5% versus 0.55%).[2] This observation aligns with prior studies that consistently show a higher occurrence of moderate to severe PVL with SEV compared to BEV.[3–5,11,16]
In cases involving highly calcified anatomies, including calcification at the annular and left ventricular outflow tract (LVOT) areas, SAVR may be considered a reasonable option for low-risk patients.[34]
Durability/Bioprosthetic Valvular Dysfunction
Long-term durability data for TAVR are limited, especially for patients <65 years of age, due to predominant enrolment of >80 years of age in high-and intermediate-risk trials. This data gap underscores the need for a thorough understanding of TAVR durability across surgical risk levels. Younger patients, expected to live longer, face increased SVD risks, including heightened calcification concerns and microstructural alterations.
A study of 1,128 patients comparing supra-annular SEV TAVR and SAVR in intermediate-and high-risk patients showed a lower 5-year SVD incidence in SEV TAVR (2.57% versus 4.38%), emphasising its significance with a 50% greater risk of all-cause mortality or hospitalisation in both groups.[35]
In the PARTNER 1 trial, 5-year outcomes revealed instances of SVD in the TAVR group, necessitating reoperation, notably with moderate or severe aortic regurgitation.[36] Mortality rates were higher in subgroups with aortic regurgitation. In PARTNER 2, TAVR patients experienced more paravalvular aortic regurgitation, leading to increased hospitalisations and reinterventions, mainly due to aortic regurgitation or progressive stenosis.[5]
In PARTNER 3, haemodynamic valve performance of both TAVR and surgical valves appeared similar at 2 years.[37] The 5-year incidence of bioprosthetic valve failure and reintervention was comparable, with a higher percentage of mild or greater paravalvular aortic regurgitation in TAVR, but without associated higher mortality.[14]
The NOTION trial provided reassuring evidence of long-term durability in low-risk patients comparing TAVR to SAVR, though a substantial percentage in the SAVR arm received later-withdrawn bioprosthetics due to early SVD.[13] The Evolut Low Risk trial suggested SEV TAVR valves may have similar durability, with better valve haemodynamic and lower PPM incidence at 3 years.[2]
Direct comparisons between SAVR-only and TAVR-only studies should be avoided due to varying definitions of SVD used in different studies. The trials mentioned lack sufficient long-term data, preventing definitive conclusions regarding long-term SVD. Ongoing long-term follow-up of low-risk trials is anticipated to provide more reliable data based on standardised definitions and further comparison between balloon-expandable and self-expanding valves.
Lifetime Management
With the increasing prevalence of valve-in-valve (ViV) procedures and the broader application of TAVR in younger, low-risk patients, the imperative to establish comprehensive strategies for lifetime management has grown. Despite the expansion of TAVR indications, data on TAVR in challenging anatomies remain limited. As attention shifts towards the lifetime management of AS in younger patients requiring early interventions, careful consideration of the initial procedure choice becomes paramount. The selection of the first intervention is pivotal, aiming not only for optimal durability but also for facilitating potential second and third reinterventions. This underscores the importance of tailoring strategies based on individual patient characteristics, anatomy, technical considerations, and considering centre and operator experience, as well as patient preferences.
TAVR as First Intervention
TAVR reinterventions involve two primary strategies: TAVR explantation with SAVR and repeat TAVR. Repeat TAVR emerges as a less invasive alternative to TAVR explantation, particularly favoured in high-risk patients. Percy et al. conducted a study comparing TAVR-in-TAVR with TAVR explantation, revealing lower 30-day mortality for TAVR-in-TAVR (6.2% versus 12.3%; p=0.05) and fewer major adverse cardiovascular events (RR for TAV explantation: 2.92; 95% CI [1.88–4.99]; p≤0.001).[38] However, 1-year mortality rates were similar (21.0% versus 20.8%; p=1.000), highlighting the need for further understanding of long-term outcomes.
In another analysis of the international Redo-TAVR registry, encompassing 212 TAVR-in-TAVR patients, both early and late-presenting groups exhibited comparable 30-day and 1-year mortality rates (5.4% versus 1.5%, p=0.427, and 16.4% versus 11.7%, p=0.34, respectively).[39] Periprocedural complications after TAVR-in-TAVR were minimal, with occurrences such as new PPI (9.6%), valve malposition (3.3%), stroke (1.4%), and coronary obstruction (0.9%), and notably, no reported deaths. Stratifying TAVR-in-TAVR outcomes by the type of TAVR (BEV and SEV) revealed no association with procedural safety or mortality, and TAVR-in-TAVR with SEV was associated with a lower residual gradient. The EXPLANT-TAVR registry highlights challenges in TAVR explantation, driven mainly by endocarditis, SVD, PVL, and PPM, resulting in a 30-day mortality of 13% and a 1-year mortality of 28%.[40] Aortic root replacement was required in 13% of cases due to stent endothelialisation. Studies using the Society of Thoracic Surgeons National Database emphasise the complexity of TAVR explantation, with concomitant procedures in 63% of cases and an overall 30-day death rate of 18%.[41] The observed-to-expected mortality ratio for TAVR explant followed by isolated SAVR is 2.2. Despite its increasing prevalence, TAVR explantation remains a high-risk procedure demanding surgical expertise and exhibiting higher in-hospital mortality than standard redo-SAVR.
TAVR-in-TAVR, while a viable option, introduces its own challenges, including a >30% incidence of severe PPM and uncertainties regarding its impact on 1-year survival.[42] With the increasing use of TAVR technology in low-risk, younger patients, strategies to avoid SVD of transcatheter heart valves (THV) become crucial. Additionally, the feasibility of repeat TAVR may be limited in 10–20% of cases.[43,44] This is primarily related to the risk of sinus sequestration and coronary obstruction, particularly for supra-annular THV. Approximately one-quarter of TAVR-in-TAVR patients faces a high probability of coronary obstruction, regardless of the valve type used in the first procedure, while another one-quarter exhibits aortic root anatomy suitable for any combination of THVs during TAVR-in-TAVR.[45]
Currently, the reported incidence of redo-TAVR in TAVR cases is approximately 0.33–0.59%.[39,46] As TAVR becomes more prevalent in younger patients, this incidence is expected to rise. Redo-TAVR is a feasible option for patients experiencing SVD, including THV stenosis and regurgitation. However, it is not recommended for patients with infective endocarditis or PPM.
Coronary Access in TAVR and TAVR-in-TAVR
Coronary ostium obstruction during native valve TAVR is relatively uncommon, occurring in <1% of cases.[47] Coronary obstruction typically arises due to the displacement of native valve leaflets and any accompanying calcium deposits. Patients with coronary ostia positioned <10–11 mm above the lowest point of the associated sinus, effaced sinuses, and a narrow sinus of Valsalva to tubular ascending aorta are at a heightened risk of occlusion. The use of coronary stents to safeguard a coronary artery susceptible to post-TAVR obstruction has demonstrated favourable mid-term survival rates. However, long-term data on this approach remain unavailable.[48]
Despite coronary access being more challenging after the initial TAVR, the challenges after TAVR-in-TAVR, especially with SEV, are projected to be exceedingly difficult in most cases. The displaced leaflets of the first THV, positioned between two stent frames, often extend above the sinotubular junction. This creates a tube graft that holds open the first valve, posing risks to coronary circulation and access.[49] A study of the Redo-TAVR of TAVR-in-TAVR patients showed that 45.5% in the Evolut R/Evolut PRO group and 2.0% in the SAPIEN 3 had high-risk features on sinus sequestration on CT, which included AV commissure level above the sinotubular junction and a close distance between THV and STJ (<2 mm).[49]
Therefore, screening candidates using cardiac CT is crucial for identifying high-risk cases, particularly in younger patients where the need for future procedures might be indicated. This is particularly important because TAVR prosthesis modification is limited and unamenable to fracture, unlike their surgical counterparts. The bioprosthetic or native Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary artery obstruction (BASILICA) technique has exhibited effectiveness in averting coronary obstruction in both native and bioprosthetic valves, thereby providing additional options for patients at risk.[50] It is worth noting that the patients included in this trial were classified as high and intermediate risk, and the procedure was conducted by experienced operators.[28] Other options available are TAVR explantation plus SAVR or the use of new emerging devices (ShortCut [Pi-Cardia]).
SAVR as First Intervention
The decision between redo-SAVR and TAVR within the surgical aortic valve (TAVR-in-SAVR) for individuals with degenerated surgical aortic bioprostheses is a nuanced process influenced by various factors. The considerations extend beyond short-term outcomes, encompassing factors such as age, surgical risk, life expectancy and anatomical considerations.
A 2021 meta-analysis involving a substantial cohort of 8,048 patients undergoing ViV-TAVR and 8,159 patients treated with redo-SAVR presented a comprehensive comparison of outcomes.[51] This analysis demonstrated no significant differences in perioperative rates of stroke, MI, major vascular complications, PVL, PPI, or 30-day readmission. Notably, ViV-TAVR was associated with lower rates of 30-day mortality [OR 0.52; 95% CI [0.39–0.68]; p<0.001), major bleeding and shorter hospital stays. However, a crucial drawback emerged as ViV-TAVR was linked to significantly higher rates of severe post-procedural PPM compared to redo-SAVR. PPM following TAVR-in-SAVR is identified as an independent risk factor for future reinterventions and exhibits inferior long-term survival. The early mortality benefit of TAVR-in-SAVR over redo-SAVR is observed to diminish at 1 year, prompting consideration of the latter for potentially better long-term survival.
An analysis of 717 propensity score-matched pairs from a large French database also showed a lower rate of the composite endpoint (all-cause mortality, stroke, MI, major or life-threatening bleeding) at 30 days following a TAVR.[52] However, no significant differences between the two groups were noted on follow-up. Intriguingly, the incidence curves favouring redo-SAVR over TAVR-in-SAVR became apparent after approximately 1 year, possibly in line with the findings in the meta-analysis reported above.
In contrast, a comprehensive 5-year follow-up study by Hahn et al., part of the PARTNER 2 Aortic ViV registry, reported the outcomes of ViV-TAVR in patients at high surgical risk.[53] The study, encompassing 369 patients who underwent ViV-TAVR, revealed sustained valve performance up to 5 years, with low rates (6.6%) of haemodynamic valve deterioration or bioprosthetic valve failure.
Important factors that have been reported in the literature to correlate with worse outcomes in ViV-TAVR in surgical prosthesis patients include smaller-degenerate valves and suboptimal implantation depth.[54] High implantation during ViV-TAVR was associated with lower gradients in both SEV and BEV. Additionally, in efforts to reduce PPM, proven to independently correlate with mortality, bioprosthetic valve ring fracture has been proposed with promising results.
Collectively, these studies underscore the multifaceted nature of the decision-making process in choosing between redo-SAVR and TAVR-in-SAVR for patients with degenerated surgical aortic bioprostheses. Patient selection, careful evaluation of procedural risks, and an understanding of the long-term implications play pivotal roles in optimising the choice between these interventions.
Challenges and Future Directions
The inclusion of young patients in TAVR discussions portends a potential rise in triple valve interventions, with the possible emergence of TAVR-TAVR-TAVR scenarios. While theoretically feasible, this approach poses considerable limitations, encompassing increased risks of PPM, PVL, need for pacemaker implantation, and significant concerns regarding long-term durability, potential coronary obstruction, restricted future coronary access, and valve thrombosis.
In considering a ‘TAVR first’ strategy, it might be wise to target patients with a large aortic root and favourable coronary anatomy. However, existing drawbacks, including limited current evidence and unknown long-term efficacy in low-risk, young patients, necessitate further investigation. Alternatively, the ‘surgery first’ approach, whether in SAVR-SAVR-TAVR or SAVR-TAVR-TAVR scenarios, remains the gold standard for managing severe AS in low-risk patients below 75 years of age, particularly in the presence of small aortic root or low coronary ostia. This strategy minimises long-term mortality and morbidity risks associated with TAVR but requires attention to procedural characteristics, bioprosthesis choice and consideration of concomitant cardiac diseases (Figure 1).
Figure 1: Special Considerations for Low-risk Transcatheter Aortic Valve Replacement.
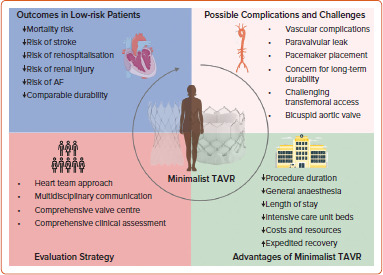
TAVR = transcatheter aortic valve replacement. Figure partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
Heart Team Approach
The heart team approach serves as the fundamental cornerstone in numerous structural heart and coronary interventions, including TAVR. Typically comprised of a structuralist, structural imaging specialist, cardiovascular surgeon, cardiac anaesthesiologist, as well as nursing and ancillary staff involved in the TAVR procedure, this collaborative team plays a pivotal role.[25]
In alignment with the most recent American College of Cardiology/American Heart Association (ACC/AHA) valvular heart disease guidelines, it is recommended that all patients with severe valvular heart disease being considered for intervention undergo evaluation by a multidisciplinary heart valve team (class I). Furthermore, the ACC/AHA guidelines advocate for consultation with or referral to a primary valve centre or a comprehensive valve centre for deliberation on treatment options, particularly in the context of asymptomatic patients with severe valve disease, patients who may benefit from valve repair instead of valve replacement, and those with multiple comorbidities (class IIa).[25]
In the case of younger patients, the heart team’s role is crucial, particularly in the decision-making process for the initial intervention. The focus extends beyond achieving optimal durability to strategically planning for potential second and third reinterventions (Figure 2). The heart team must carefully weigh various factors, including the patient’s age, anatomical considerations and long-term outcomes, to make informed decisions that align with the patient’s individualised needs and maximise the efficacy of subsequent reinterventions. For more complex TAVR procedures, the centre’s experience and procedural volume emerge as important factors influencing optimal outcomes in low-risk patients.[55]
Figure 2: Lifetime Management Strategies in Transcatheter Aortic Valve Replacement.
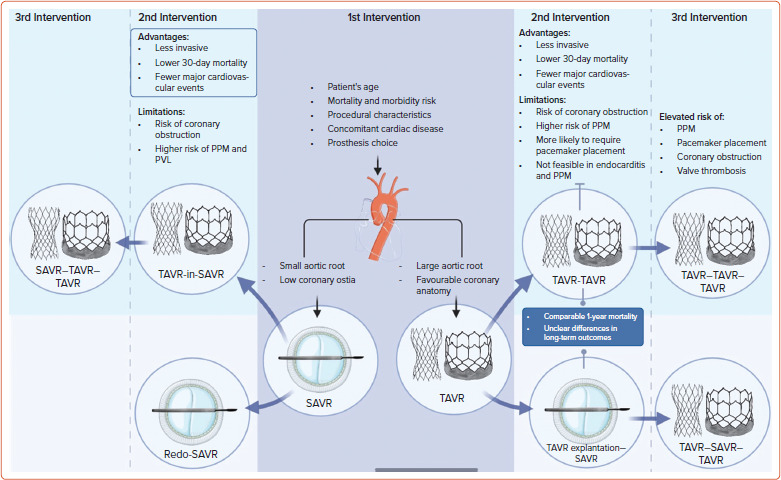
The figure highlights the importance of first intervention and subsequent interventions based on patient and procedural characteristics. PPM = prosthesis–patient mismatch; PVL = paravalvular leak; SAVR = surgical aortic valve replacement; TAVR = transcatheter aortic valve replacement. Figure partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
Minimalist TAVR
Over the past several years, we have witnessed the rapid advancement of TAVR, giving rise to the term ‘minimalist TAVR’, which is increasingly adopted within the structural heart disease community.[56,57] This term characterises TAVR procedures with less invasive peri-procedural approaches, facilitating expedited patient recovery.[57] Such procedures typically involve conscious sedation instead of general anaesthesia, resulting in a standard length of stay of approximately 48 hours. Patients are typically monitored on telemetry floors and often do not require intensive care unit beds.
The 3M TAVR study, conducted collaboratively with 13 North American centres spanning low-, medium-, and high-volume categories, has demonstrated the feasibility of implementing a consistent minimalist TAVR approach across diverse centres. This approach led to safe next-day discharge for 80.1% of participants and discharge within 48 hours for 89.5% of participants.[57] As TAVR increasingly becomes a viable option for lower-risk patients, we anticipate witnessing a greater adoption of the minimalist TAVR approach in clinical practice.
Future Directions
Several ongoing randomised clinical trials are actively investigating outcomes in low-risk patients with asymptomatic severe aortic AS and those with moderate AS and left ventricular dysfunction. These trials, expected to conclude soon, are poised to contribute valuable insights to the prevailing body of research. Table 4 summarises these ongoing trials.
Table 4: Ongoing Randomised Clinical Trials on Outcomes of Transcatheter Aortic Valve Replacement in Low-risk Patients.
| Trial Name | Study Start Date, Estimated Completion Date | Study Population | Primary Outcome |
|---|---|---|---|
| NOTION-2 (NCT02825134) | June 2016, June 2020 | STS-PROM <3% and age <75 years | Composite rate of all-cause mortality, MI and stroke at 1 year |
| DEDICATE (NCT03112980) | May 2017, December 2026 | ‘All-comers’ patient population with low to intermediate risk, age 65–85 years | Freedom of stroke or death at 1 and 5 years |
| TAVR UNLOAD (NCT02661451) | September 2016, December 2021 | Age ≥18 years, LVEF <50% | All-cause death, disabling stroke, hospitalisation due to heart failure or non-disabling stroke, and change in KCCQ relative to baseline at 12 months |
| EARLY TAVR (NCT03042104) | July 2017, December 2021 | Age ≥65 years, asymptomatic severe aortic stenosis undergoing TAVR using SAPIEN 3/SAPIEN 3 Ultra valves | Composite endpoint of all-cause death, all stroke, and unplanned cardiovascular hospitalisation at 2 years |
KCCQ = Kansas City Cardiomyopathy Questionnaire; LVEF = left ventricular ejection fraction; PROM = predicted risk of mortality; STS = Society of Thoracic Surgeons; TAVR = transcatheter aortic valve replacement.
Conclusion
TAVR has undergone rapid evolution in recent years, expanding its scope to include low-risk patients and other previously excluded patient groups. The future of TAVR is poised for further expansion, with a focus on the heart team approach, ongoing enhancements in valve design and durability, and the growing experience of operators. These trends point towards a potential future where minimalist TAVR becomes the standard of care.
References
- 1.Mack MJ, Leon MB, Thourani VH et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 2.Popma JJ, Deeb GM, Yakubov SJ et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706–15. doi: 10.1056/NEJMoa1816885. [DOI] [PubMed] [Google Scholar]
- 3.Carroll JD, Mack MJ, Vemulapalli S et al. STS-ACC TVT registry of transcatheter aortic valve replacement. J Am Coll Cardiol. 2020;76:2492–516. doi: 10.1016/j.jacc.2020.09.595. [DOI] [PubMed] [Google Scholar]
- 4.Smith CR, Leon MB, Mack MJ et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–98. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 5.Leon MB, Smith CR, Mack MJ et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609–20. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 6.Forrest JK, Ramlawi B, Deeb GM et al. Transcatheter aortic valve replacement in low-risk patients with bicuspid aortic valve stenosis. JAMA Cardiol. 2021;6:50–7. doi: 10.1001/jamacardio.2020.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosato S, Santini F, Barbanti M et al. Transcatheter aortic valve implantation compared with surgical aortic valve replacement in low-risk patients. Circ Cardiovasc Interv. 2016;9:e003326. doi: 10.1161/CIRCINTERVENTIONS.115.003326. [DOI] [PubMed] [Google Scholar]
- 8.Bekeredjian R, Szabo G, Balaban Ü et al. Patients at low surgical risk as defined by the Society of Thoracic Surgeons Score undergoing isolated interventional or surgical aortic valve implantation: in-hospital data and 1-year results from the German Aortic Valve Registry (GARY). Eur Heart J. 2019;40:1323–30. doi: 10.1093/eurheartj/ehy699. [DOI] [PubMed] [Google Scholar]
- 9.Serruys PW, Mondolo R, Reardon M et al. One-year outcomes of patients with severe aortic stenosis and an STS PROM of less than three percent in the SURTAVI trial. EuroIntervention. 2018;14:877–83. doi: 10.4244/EIJ-D-18-00460. [DOI] [PubMed] [Google Scholar]
- 10.Finkelstein A, Rozenbaum Z, Halkin A et al. Outcomes of transcatheter aortic valve implantation in patients with low versus intermediate to high surgical risk. Am J Cardiol. 2019;123:644–9. doi: 10.1016/j.amjcard.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Waksman R, Corso PJ, Torguson R et al. TAVR in low-risk patients: 1-year results from the LRT trial. JACC Cardiovasc Interv. 2019;12:901–7. doi: 10.1016/j.jcin.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Jørgensen TH, Thyregod HGH, Ihlemann N et al. Eight-year outcomes for patients with aortic valve stenosis at low surgical risk randomized to transcatheter vs. surgical aortic valve replacement. Eur Heart J. 2021;42:2912–9. doi: 10.1093/eurheartj/ehab375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thyregod HGH, Ihlemann N, Jørgensen TH et al. Five-year clinical and echocardiographic outcomes from the NOTION randomized clinical trial in patients at lower surgical risk. Circulation. 2019;139:2714–23. doi: 10.1161/CIRCULATIONAHA.118.036606. [DOI] [PubMed] [Google Scholar]
- 14.Mack MJ, Leon MB, Thourani VH et al. Transcatheter aortic-valve replacement in low-risk patients at five years. N Engl J Med. 2023;389:1949–60. doi: 10.1056/NEJMoa2307447. [DOI] [PubMed] [Google Scholar]
- 15.Forrest JK, Deeb GM, Yakubov SJ et al. 4-year outcomes of patients with aortic stenosis in the Evolut low risk trial. J Am Coll Cardiol. 2023;82:2163–5. doi: 10.1016/j.jacc.2023.09.813. [DOI] [PubMed] [Google Scholar]
- 16.Leon MB, Smith CR, Mack M et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 17.Waksman R, Craig PE, Torguson R et al. Transcatheter aortic valve replacement in low-risk patients with symptomatic severe bicuspid aortic valve stenosis. JACC Cardiovasc Interv. 2020;13:1019–27. doi: 10.1016/j.jcin.2020.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Halim SA, Edwards FH, Dai D et al. Outcomes of transcatheter aortic valve replacement in patients with bicuspid aortic valve disease: a report from the Society of Thoracic Surgeons/American College of Cardiology transcatheter valve therapy registry. Circulation. 2020;141:1071–9. doi: 10.1161/CIRCULATIONAHA.119.040333. [DOI] [PubMed] [Google Scholar]
- 19.Forrest JK, Kaple RK, Ramlawi B et al. Transcatheter aortic valve replacement in bicuspid versus tricuspid aortic valves from the STS/ACC TVT registry. JACC Cardiovasc Interv. 2020;13:1749–59. doi: 10.1016/j.jcin.2020.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Mylotte D, Lefevre T, Søndergaard L et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol. 2014;64:2330–9. doi: 10.1016/j.jacc.2014.09.039. [DOI] [PubMed] [Google Scholar]
- 21.Mangieri A, Tchetchè D, Kim WK et al. Balloon versus self-expandable valve for the treatment of bicuspid aortic valve stenosis: insights from the BEAT International Collaborative Registrys. Circ Cardiovasc Interv. 2020;13:e008714. doi: 10.1161/CIRCINTERVENTIONS.119.008714. [DOI] [PubMed] [Google Scholar]
- 22.Chandrasekhar J, Hibbert B, Ruel M et al. Transfemoral vs non-transfemoral access for transcatheter aortic valve implantation: a systematic review and meta-analysis. Can J Cardiol. 2015;31:1427–38. doi: 10.1016/j.cjca.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 23.Di Mario C, Goodwin M, Ristalli F et al. A prospective registry of intravascular lithotripsy-enabled vascular access for transfemoral transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2019;12:502–4. doi: 10.1016/j.jcin.2019.01.211. [DOI] [PubMed] [Google Scholar]
- 24.Banks A, Gaca J, Kiefer T. Review of alternative access in transcatheter aortic valve replacement. Cardiovasc Diagn Ther. 2020;10:72–82. doi: 10.21037/cdt.2019.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otto CM, Nishimura RA, Bonow RO et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2021;143:e35–71. doi: 10.1161/CIR.0000000000000932. [DOI] [PubMed] [Google Scholar]
- 26.van Rosendael PJ, Delgado V, Bax JJ. Pacemaker implantation rate after transcatheter aortic valve implantation with early and new-generation devices: a systematic review. Eur Heart J. 2018;39:2003–13. doi: 10.1093/eurheartj/ehx785. [DOI] [PubMed] [Google Scholar]
- 27.Jilaihawi H, Zhao Z, Du R et al. Minimizing permanent pacemaker following repositionable self-expanding transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2019;12:1796–807. doi: 10.1016/j.jcin.2019.05.056. [DOI] [PubMed] [Google Scholar]
- 28.Faroux L, Chen S, Muntané-Carol G et al. Clinical impact of conduction disturbances in transcatheter aortic valve replacement recipients: a systematic review and meta-analysis. Eur Heart J. 2020;41:2771–81. doi: 10.1093/eurheartj/ehz924. [DOI] [PubMed] [Google Scholar]
- 29.Fadahunsi OO, Olowoyeye A, Ukaigwe A et al. Incidence, predictors, and outcomes of permanent pacemaker implantation following transcatheter aortic valve replacement: analysis from the U.S. Society of Thoracic Surgeons/American College of Cardiology TVT registry. JACC Cardiovasc Interv. 2016;9:2189–99. doi: 10.1016/j.jcin.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 30.Adams DH, Popma JJ, Reardon MJ et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790–8. doi: 10.1056/NEJMoa1400590. [DOI] [PubMed] [Google Scholar]
- 31.Athappan G, Patvardhan E, Tuzcu EM et al. Incidence, predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement: meta-analysis and systematic review of literature. J Am Coll Cardiol. 2013;61:1585–95. doi: 10.1016/j.jacc.2013.01.047. [DOI] [PubMed] [Google Scholar]
- 32.Reardon MJ, Van Mieghem NM, Popma JJ et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321–31. doi: 10.1056/NEJMoa1700456. [DOI] [PubMed] [Google Scholar]
- 33.Finkelstein A, Rozenbaum Z, Zhitomirsky S et al. Safety outcomes of new versus old generation transcatheter aortic valves. Catheter Cardiovasc Interv. 2019;94:E44–53. doi: 10.1002/ccd.28021. [DOI] [PubMed] [Google Scholar]
- 34.Seiffert M, Fujita B, Avanesov M et al. Device landing zone calcification and its impact on residual regurgitation after transcatheter aortic valve implantation with different devices. Eur Heart J Cardiovasc Imaging. 2016;17:576–84. doi: 10.1093/ehjci/jev174. [DOI] [PubMed] [Google Scholar]
- 35.Reardon M. Presented at: ACC Annual Scientific Session, ACC22, Washington, DC, US, 4 April 2022: 5-year incidence, outcomes and predictors of structural valve deterioration of transcatheter and surgical aortic bioprostheses: insights from the CoreValve US Pivotal and SURTAVI trials. [Google Scholar]
- 36.Mack MJ, Leon MB, Smith CR et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2477–84. doi: 10.1016/S0140-6736(15)60308-7. [DOI] [PubMed] [Google Scholar]
- 37.Leon MB, Mack MJ, Hahn RT et al. Outcomes 2 years after transcatheter aortic valve replacement in patients at low surgical risk. J Am Coll Cardiol. 2021;77:1149–61. doi: 10.1016/j.jacc.2020.12.052. [DOI] [PubMed] [Google Scholar]
- 38.Percy ED, Harloff MT, Hirji S et al. Nationally representative repeat transcatheter aortic valve replacement outcomes: report from the Centers for Medicare and Medicaid Services. JACC Cardiovasc Interv. 2021;14:1717–26. doi: 10.1016/j.jcin.2021.06.011. [DOI] [PubMed] [Google Scholar]
- 39.Landes U, Webb JG, De Backer O et al. Repeat transcatheter aortic valve replacement for transcatheter prosthesis dysfunction. J Am Coll Cardiol. 2020;75:1882–93. doi: 10.1016/j.jacc.2020.02.051. [DOI] [PubMed] [Google Scholar]
- 40.Bapat VN, Zaid S, Fukuhara S et al. Surgical explantation after TAVR failure: mid-term outcomes from the EXPLANT-TAVR international registry. JACC Cardiovasc Interv. 2021;14:1978–91. doi: 10.1016/j.jcin.2021.07.015. [DOI] [PubMed] [Google Scholar]
- 41.Fukuhara S, Nguyen CTN, Yang B et al. Surgical explantation of transcatheter aortic bioprostheses: balloon vs self-expandable devices. Ann Thorac Surg. 2022;113:138–45. doi: 10.1016/j.athoracsur.2021.01.041. [DOI] [PubMed] [Google Scholar]
- 42.Dvir D, Webb JG, Bleiziffer S et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA. 2014;312:162–70. doi: 10.1001/jama.2014.7246. [DOI] [PubMed] [Google Scholar]
- 43.Forrestal BJ, Case BC, Yerasi C et al. Risk of coronary obstruction and feasibility of coronary access after repeat transcatheter aortic valve replacement with the self-expanding Evolut valve: a computed tomography simulation study. Circ Cardiovasc Interv. 2020;13:e009496. doi: 10.1161/CIRCINTERVENTIONS.120.009496. [DOI] [PubMed] [Google Scholar]
- 44.Tang GHL, Zaid S, Gupta E et al. Feasibility of repeat TAVR after SAPIEN 3 TAVR: A novel classification scheme and pilot angiographic study. JACC Cardiovasc Interv. 2019;12:1290–2. doi: 10.1016/j.jcin.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 45.Russo G, Tang GHL, Sangiorgi G et al. Lifetime management of aortic stenosis: transcatheter versus surgical treatment for young and low-risk patients. Circ Cardiovasc Interv. 2022;15:915–27. doi: 10.1161/CIRCINTERVENTIONS.122.012388. [DOI] [PubMed] [Google Scholar]
- 46.Tang GHL, Zaid S, Kleiman NS et al. Explant vs redo-TAVR after transcatheter valve failure: mid-term outcomes from the EXPLANTORREDO-TAVR international registry. JACC CardioVasc Interv. 2023;16:927–41. doi: 10.1016/j.jcin.2023.01.376. [DOI] [PubMed] [Google Scholar]
- 47.Ribeiro HB, Nombela-Franco L, Urena M et al. Coronary obstruction following transcatheter aortic valve implantation: a systematic review. JACC Cardiovasc Interv. 2013;6:452–61. doi: 10.1016/j.jcin.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 48.Palmerini T, Chakravarty T, Saia F et al. Coronary protection to prevent coronary obstruction during TAVR: a multicenter international registry. JACC Cardiovasc Interv. 2020;13:739–47. doi: 10.1016/j.jcin.2019.11.024. [DOI] [PubMed] [Google Scholar]
- 49.Ochiai T, Oakley L, Sekhon N et al. Risk of coronary obstruction due to sinus sequestration in redo transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2020;13:2617–27. doi: 10.1016/j.jcin.2020.09.022. [DOI] [PubMed] [Google Scholar]
- 50.Khan JM, Greenbaum AB, Babaliaros VC et al. BASILICA trial: one-year outcomes of transcatheter electrosurgical leaflet laceration to prevent TAVR coronary obstruction. Circ Cardiovasc Interv. 2021;14:e010238. doi: 10.1161/CIRCINTERVENTIONS.120.010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sá MPBO, Van den Eynde J, Simonato M et al. Valve-in-valve transcatheter aortic valve replacement versus redo surgical aortic valve replacement: an updated meta-analysis. JACC Cardiovasc Interv. 2021;14:211–20. doi: 10.1016/j.jcin.2020.10.020. [DOI] [PubMed] [Google Scholar]
- 52.Woitek FJ, Stachel G, Kiefer P et al. Treatment of failed aortic bioprostheses: an evaluation of conventional redo surgery and transfemoral transcatheter aortic valve-in-valve implantation. Int J Cardiol. 2020;300:80–6. doi: 10.1016/j.ijcard.2019.09.039. [DOI] [PubMed] [Google Scholar]
- 53.Hahn RT, Webb J, Pibarot P et al. 5-year follow-up from the PARTNER 2 aortic valve-in-valve registry for degenerated aortic surgical bioprostheses. JACC Cardiovasc Interv. 2022;15:698–708. doi: 10.1016/j.jcin.2022.02.014. [DOI] [PubMed] [Google Scholar]
- 54.Bleiziffer S, Simonato M, Webb JG et al. Long-term outcomes after transcatheter aortic valve implantation in failed bioprosthetic valves. Eur Heart J. 2020;41:2731–42. doi: 10.1093/eurheartj/ehaa544. [DOI] [PubMed] [Google Scholar]
- 55.Vemulapalli S, Carroll JD, Mack MJ et al. Procedural volume and outcomes for transcatheter aortic-valve replacement. N Engl J Med. 2019;380:2541–50. doi: 10.1056/NEJMsa1901109. [DOI] [PubMed] [Google Scholar]
- 56.Lauck SB, Sathananthan J, Park J et al. Post-procedure protocol to facilitate next-day discharge: results of the multidisciplinary, multimodality but minimalist TAVR study. Catheter Cardiovasc Interv. 2020;96:450–8. doi: 10.1002/ccd.28617. [DOI] [PubMed] [Google Scholar]
- 57.Wood DA, Lauck SB, Cairns JA et al. The Vancouver 3M (multidisciplinary, multimodality, but minimalist) clinical pathway facilitates safe next-day discharge home at low-, medium-, and high-volume transfemoral transcatheter aortic valve replacement centers: the 3M TAVR study. JACC Cardiovasc Interv. 2019;12:459–69. doi: 10.1016/j.jcin.2018.12.020. [DOI] [PubMed] [Google Scholar]


