Abstract
OBJECTIVE
To explore how Qingfei Zhisou oral liquid (清肺止嗽口服液, QFZS) adjusts body temperature bias and the interaction of inflammatory factors levels and metabolomic differences.
METHODS
Dry yeast was subcutaneously injected at 10 mL/kg to establish the pyrexia model. We randomly divided 60 Sprague-Dawley rats into five groups: control, model, positive, low dose of QFZS and high dose of QFZS. Inflammatory proteins were evaluated by Western blotting and immunohistochemistry. For the examination of the endogenous metabolites, enzyme linked immunosorbent assay and ultra-high-performance liquid chromatography high-resolution mass spectrometry were employed.
RESULTS
QFZS significantly reduced rats' body temperature within 6 h after dry yeast injection and reduced the secretion of the arginine vasopressin, cyclic adenosine monophosphate, prostaglandin E-2, tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β in serum. Meanwhile, we identified 41 metabolites between the model and QFZS groups, including arachidonic acid and lysophospholipids. QFZS restored normal arachidonic acid levels. Based on the differential metabolite enrichment analysis, QFZS's anti-inflammatory and anti-pyrexia effects might be related to the inflammatory pathway regulated by transient receptor potential. Additionally, QFZS treatment reduced transient receptor potential melastatin 2 ion channel expression and affected TNF-α, heat shock protein 70, and cyclooxygenase-2 expression in the hypothalamus.
CONCLUSION
QFZS exerts its regulatory effects on fever by regulating the metabolism of lysophospholipids and arachidonic acid and the regulation of inflammation via transient receptor potential ion channels channels.
Keywords: inflammation, fever, metabolomics, transient receptor potential ion channels, Qingfei Zhisou oral liquid
1. INTRODUCTION
Mammals maintain their body temperature within a narrow range of around 37 ℃. Fever is a condition in which the body's core temperature rises beyond that range by the hypothalamus due to heat generation exceeding heat loss, as well as organ-specific, systemic, and death-causing effects. A high body temperature activates the immune system to modulate inflammation.1 The preoptic anterior hypothalamus is the body's temperature control center. The enhancement of the firing rate upon warming in the warm-sensitive neurons (WSNs) might be significantly involved in the transduction of the information related to temperature to peripheral organs and the promotion of heat loss.2,⇓-4 The transient receptor potential ion channels (TRP) ion channel is an important sensor for heat in several neurons in the preoptic area of the hypothalamus. The transient receptor potential melastatin 2 (TRPM2) channel is a hypothalamic heat sensor involved in temperature regulation.5,6 Activated by temperatures above 35 ℃, TRPM2 is modulated by intracellular adenosine diphosphate-ribose, Ca2+, cyclic adenosine diphosphate-ribose, and reactive oxygen species (ROS).7,⇓-9 Small molecule metabolites are closely connected to the regulation of inflammation and fever. The inhibition of WSNs in the hypothalamus induced by prostaglandin E-2 (PGE-2) could effectively mediate any temperature increase. Arachidonic acid (AA) metabolism is a classic signaling pathway that regulates inflammation.10 Temperature-related metabolites in the blood of yeast-induced rats are significantly affected by warm- or cold-stimulating herbs.11
Non-steroidal drugs associated with anti-inflammation, like cyclooxygenase-2 (COX-2) inhibitors, and aspirin were commonly used in the treatment of inflammation.12 Qingfei Zhisou (清肺止嗽口服液, QFZS) oral liquid is a Chinese compound with five herbal medicines, including Qingdai (Indigo Naturalis), Jinguolan (Radix Tinosporae), Dahuang (Radix Et Rhizoma Rhei Palmati), Bingpian (Borneolum Syntheticum), Gancao (Radix Glycyrrhizae) and has rapidly reduced fever in the clinic. A large number of clinical studies have found that it can reduce infection.13,⇓-15 Several monomers in this formula have presented excellent anti-inflammatory activity. QFZS composition testing is provided in supplementary Figure 1. Indirubin alleviates lipopolysaccharide-caused oxidative stress and inflammation by reducing the expression of malondialdehyde abundance and interleukin-1β and tumor necrosis factor-α.16 Rhein attenuates inflammatory responses by mediating macrophage phenotype polarization, reducing nuclear factor kappa-B p65 phosphorylation, decreasing inducible nitric oxide synthase expression, and inhibiting the expression of COX-2 and proinflammatory cytokines at the protein level.17-18 Emodin compromises lipopolysaccharide-induced inflammation through the peroxisome proliferators-activated receptor γ-dependent inactivation of nuclear factor kappa-B.19 In the present study, we explored how QFZS adjusts body temperature bias and the interaction of inflammatory factors levels and metabolomic differences.
2. MATERIALS AND REAGENTS
2.1. Experimental animals
Sixty male Sprague-Dawley rats (Specific Pathogen Free grade), two-month-old, weighing [(120 ± 20) g] were purchased from the Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China, No. 1103241911033018). Rats were fed with standard food and water, with a 12/12 h light/dark cycle. The committee of the Xiyuan Hospital approved the experimental animal procedures (ethical registration No. 2020XLC007). All efforts were made to minimize animal suffering and the number of animals used.
2.2. Yeast-induced fever model and experimental protocols
By using the random number method, sixty Sprague-Dawley rats were divided into five groups: control, pyrexia model, positive, low-dose QFZS treatment, and high-dose QFZS treatment. The temperature detected in the rectum at a depth of 3 cm before the construction of the models was recorded as the original temperature. Then, after the dissolution of yeast in saline (15%), 10 mL/kg of the solutions were injected into the backs of the rats.20,21 Control rats were only treated with an equal volume of saline. Aspirin (100 mg/kg), low-dose QFZS (2.82 g/kg), and high-dose QFZS (5.64 g/kg) were administered to the treatment groups after modeling, whereas an equal volume of 0.9% saline was administered to the rats in the control and model groups. Considering that the daily dose for a human is 60 mL, the daily dose for rats was calculated according to the body surface area conversion ratio. The low dose was the clinically equivalent dose, and the high dose was twice the clinical dose. Six hours after drug treatment, we detected and recorded the temperatures of rats in the rectum at 1-hour intervals. We strictly complied with The National Guidelines for Animal Protection, including experimental procedures, animal feeding, care, and housing.
2.3. Serum cytokine assay by enzyme linked immunosorbent assay (ELISA)
Serum arginine vasopressin (AVP), cyclic adenosine monophosphate (cAMP), prostaglandin E-2, interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), were detected by indicated ELISA kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer’s instructions.
2.4. Immunohistochemical detection
After fixation using 4% paraformaldehyde, embedding with paraffin, the hypothalamus tissues were sectioned. Then, the tissues were incubated with primary antibodies against transient receptor potential ion channels M2 (DF7533, Affinity, Liyang, China), heat shock protein 70 (HSP70) (66183-1-AP, Proteintech, Wuhan, China), and TNF-α (BS-0078R, Bioss, Beijing, China) 1∶100 dilution for incubation overnight at 4 ℃. After washing using PBS and 1 h incubation with secondary antibodies, the stained sections were observed, and the images were captured using Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, USA).
2.5. Western blotting analysis
As previously described, proteins were extracted from the hypothalamus and analyzed by Western blotting22 using TRPM2 (DF7533, Affinity, 1∶1000 dilution), COX-2 (663511-Ig, Proteintech, 1∶1000 dilution), Hsp70 (66183-1-AP, Proteintech, 1∶10000 dilution) and TNF-α (BS-0078R, Bioss, 1∶1000 dilution). Protein levels were normalized to GAPDH (5174, CST, 1∶1000 dilution). Signals were visualized with an enhanced chemiluminescence detection reagent (Thermo Fisher Scientific, Waltham, MA, USA).
2.6. Quality control (QC) assessment of metabolomics samples
In the 6th hour after modeling, rats were anesthetized with 2% isoflurane and fixed in the prone pose. Blood samples were collected from the abdominal aorta of rats, and the serum was obtained by centrifugation at 4 ℃/300 g for 15 min. Samples (100 μL) were added to L-2-chlorophenyl alanine (0.3 mg/mL) dissolved in methanol as an internal standard. Subsequently, the samples were added to an ice-cold mixture of methanol and acetonitrile (2/1, vol/vol) and ultrasonically extracted for 10 min. After 10-min centrifugation at 1300 × g/4 ℃, 300 μL of supernatant was concentrated. Equal volumes of methanol and water (1/4, vol/vol) were added, and samples were ultrasonically extracted in an ice-water bath followed by a 2 h incubation at −20 ℃. After 10 min centrifugation at 1300 g/4 ℃, 150 μL of supernatants were collected from each tube. Next, after filtering with 0.22 μm microfilters, the collected supernatants were transferred to vials. Vials were stored at −80 ℃ until Liquid Chromatography Mass Spectrometry analysis. A pooled sample created by mixing all samples was used as the quality control sample.
2.7. Ultra HPLC-quadrupole time-of-flight-mass spectrometry (UPLC-Q/TOF-MS)-based metabolomics assay
To analyze the profile of metabolites in both electrospray ionization (ESI)-negative and positive ion modes, we used a Dionex Ultimate 3000RS ultra-high performance liquid chromatograph with a Q-Exactive plus quadrupole-Orbitrap mass spectrometer equipped with a heated ESI source (Thermo Fisher Scientific, Waltham, MA, USA). We also used an Acquity Ultra Performance Liquid Chromatography High Strength Silica T3 column (1.8 μm, 2.1 mm × 100 mm). The binary gradient elution system comprised: (A) formic acid (0.1%, v/v)-contained water; (B) formic acid (0.1%, v/v)-contained acetonitrile. The separation gradient was: 0-2-4-8-10-14-15-15.1-16 min, 5-5-25-50-80-100-100-5-5% B. The column temperature was 45 ℃, and the flow rate was 0.35 mL/min. Samples were maintained at 4 ℃. The injection volume was 2 μL, and the mass/charge ratio (m/z) ranged from 100 to 1000. Samples were randomly analyzed in a single batch with QC spectral acquisition after every six samples. We used the Xcalibur 2.2 SP1.48 software (Thermo Fisher Scientific, Waltham, MA, USA) to operate the LC-MS system and data collection and analysis.
The original LC-MS data were processed by Progenesis QI v2.3 (Nonlinear) for normalization, peak alignment, retention time correction, integral, peak identification, and baseline filtering. The precise mass-to-charge ratio (m/z) identified the compound by qualitative analysis using the Protein Model Database, Electron Microscopy Data Bank, Metlin, Lipidmaps (v2.3), Human Metabolome Database, and self-built databases. If the resulting scores were less than 36 points, we considered the analysis inaccurate, and the compounds were removed. Differential metabolites were selected with P < 0.05. After combining the data matrix from the negative and positive ion data, the matrix was imported into R to conduct principal component analysis (PCA) to verify the stability of the entire analysis and the overall distribution among samples. Partial least-squares-discriminant analysis (PLS-DA) was used to distinguish the metabolites.
2.8. Statistical analysis
All results are presented as mean ± standard deviation (SD). The statistical comparisons were analyzed using Student’s t-test for two groups or one-way analysis of variance followed by Tukey’s post hoc tests for multiple groups (GraphPad Prism 8, San Diego, CA, USA). P < 0.05 was considered significantly different.
3. RESULTS
3.1. Antipyretic therapeutic effects of QFZS and inhibition of inflammatory factors secretion in yeast-induced fever rats
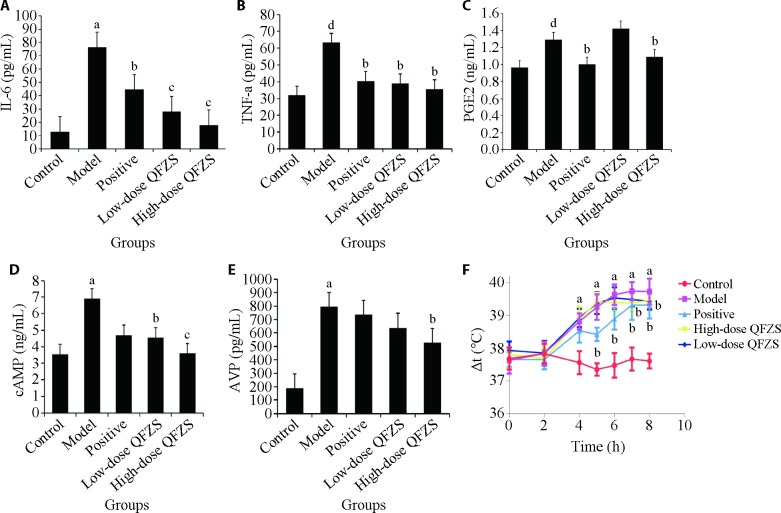
Due to the importance of body temperature in survival, its precise regulation by the nervous system is critical. Thus, to investigate the effects of QFZS on temperature regulation, dynamic temperatures after dry yeast injection were tested from 2 to 8 h. The effects of dry yeast in causing fever were observed after 4 h (Figure 1). The antipyretic effect of the positive drug aspirin appeared at 5 h. The inhibitory effects of QFZS at 5.64 and 2.82 g/kg were observed after 6 h. At 8 h, the rats' body temperature significantly decreased in the positive and QFZS groups. These results indicated the significant antifebrile efficacy of QFZS on yeast-induced fever in rats. However, its antipyretic effects were milder compared to aspirin.
Figure 1. Differences in inflammatory cytokines and hypothalamic-releasing factors.
A: IL-6 level; B: TNF-α level; C: PGE-2 level; D: cAMP level; E: AVP; F: rectal temperature of the rats in each group. Control group: sham operated; Model group: Yeast-induced fever model without treatment; Positive group: Yeast-induced fever model with Aspirin (100 mg/kg); Lose-dose QFZS group: Yeast-induced fever model with low-dose QFZS (2.82 g/kg); High-dose QFZS group: Yeast-induced fever model with high-dose QFZS (5.64 g/kg). IL-6: interleukin-6; TNF-α: tumor necrosis factor-α; AVP: arginine vasopressin; cAMP: cyclic adenosine monophosphate; PGE-2: prostaglandin E-2; QFZS: Qingfei Zhisou oral liquid. Data represent the mean ± standard deviation using one-way analysis of variance (n = 4). Compared with the control group, aP < 0.01, dP < 0.05; compared with the model group, bP < 0.05, cP < 0.01.
The ELISA results (Figure 1) showed that model rats exhibited elevated secretion of TNF-α, IL-6, and PGE-2 compared to control rats. A markedly downstream effect was detected for pyrexia rats treated with QFZS. During fever, cAMP is an important positive regulator for central heating. Endogenous pyrogens, including arginine vasopressin, α-melanocyte-stimulating hormone, β-endorphin, neurotensin, adrenocorticotropic hormone, and bombesin,23,24 are negative regulators of body temperature and will suppress any excessive rise in body temperature. Significantly elevated cAMP and AVP production were also observed in model rats compared to control rats. Correspondingly, QFZS and aspirin treatment groups downregulated the expression levels of these factors in serum.
3.2. QFZS inhibits inflammation by reducing levels of TRP ion channel proteins in yeast-induced febrile rats
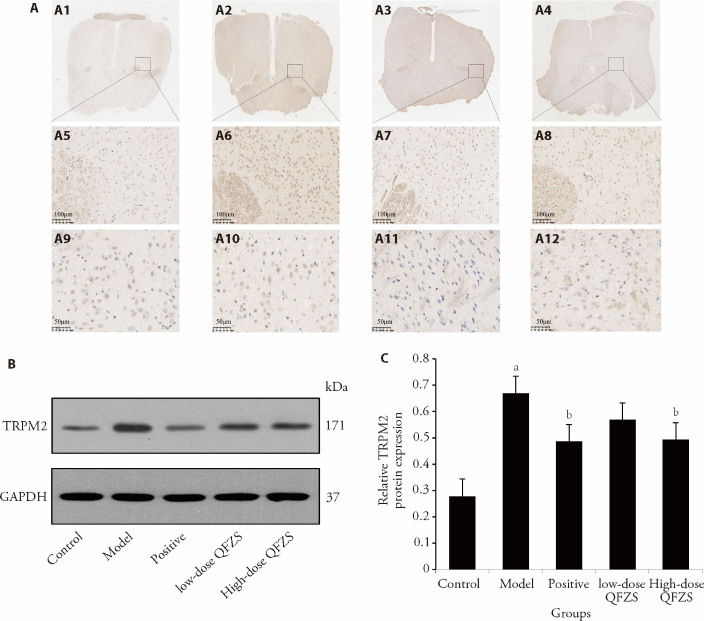
To validate the pathway enrichment results, we determined the levels of TRPM2 in hypothalamus tissue via immunohistochemical staining and Western blot (Figure 2). As expected, the yeast-treated group showed a significant increase in the levels of TRP ion channel, and TRPM2 expression significantly decreased in hypothalamus tissue after treatment. These findings indicated that QFZS treatment regulated hypothalamic temperature-controlled ion channels. The expression results of TNF-α and Hsp70 are shown in supplementary Figures 2, 3.
Figure 2. Effects of QFZS on TRPM2 expression.
A: the immunohistochemistry staining of TRPM2, A1-A4: images of control (A1), model (A2), Lose-dose QFZS (A3), High-dose QFZS(A4) (× 1); A5-A8: images of control (A5), model (A6), Lose-dose QFZS (A7), High-dose QFZS(A8) (× 200, bar = 100 μm); A9-A12: images of control (A9), model (A10), Lose-dose QFZS (A11), High-dose QFZS(A12) (× 400, bar = 50 μm). B: Western blotting representative images of TRPM2 respective quantification in the hypothalamus; C: protein expression levels of TRPM2. Control group: sham operated; Model group: Yeast-induced fever model without treatment; Positive group: Yeast-induced fever model with Aspirin (100 mg/kg); Lose-dose QFZS group: Yeast-induced fever model with low-dose QFZS (2.82 g/kg); High-dose QFZS group: Yeast-induced fever model with high-dose QFZS (5.64 g/kg). TRPM2: transient receptor potential melastatin 2; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; QFZS: Qingfei Zhisou oral liquid. Data represent the mean ± standard deviation using one-way analysis of variance (n = 5). Compared with the sham group, aP < 0.05; compared with the model group, bP < 0.05.
3.3. QC assessment of metabolomics samples and UHPLC/MS data analysis
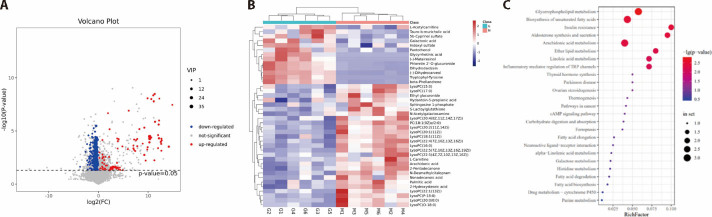
Using UHPLC/MS, total ion chromatography data was collected under the ESI negative and positive ion scan modes (Figures 3A, 3B). We separated 12 QCs and clustered them in the PCA score plots under the ESI positive ion scan mode using the HILIC and C18 columns. Clustered QC samples demonstrated the satisfactory repeatability of the separation system, and the results indicated that these collected data were reliable and could be used for further analysis. Figure 3 shows the PLS-DA and PCA score plots of the serum samples. The parameters of the PLS-DA model were as follows: R2X = 0.652, R2Y = 0.993, and Q2Y = 0.884, indicating satisfactory stability and predictability. The control and model achieved separation. Satisfactory separation was also detected between QFZS treatment and model groups. A tendency to recover to the normal group was observed in the treatment group.
Figure 3. Screening, pathway analysis, and enrichment of differential metabolites.
A: summary analysis of differential metabolites volcano plot, B; heat map of metabolisms, C: pathway enrichment of differential metabolites.
3.4. Identification and evaluation of differential metabolites
The metabolites that significantly contributed to the clustering and discrimination in pattern recognition were selected with P < 0.05 and FC ≥ 2. According to the accurate m/z of ion feature metabolites, we identified 41 fever-related metabolites in rat serum, 31 differential metabolites between low-dose QFZS and the model, and 30 differential metabolites between high-dose QFZS and the model (supplementary Tables 1, 2). Feature metabolites between the control and model were mostly lysophospholipids. Eighteen feature metabolites were found in low-dose QFZS treatment; in the high-dose QFZS group, 30 feature metabolites included 14 kinds of lysophospholipids. Twenty commonly regulated meta-bolites were detected in the QFZS treatment groups: Palmitic acid, Glycyrrhetinic acid, N-Desmethylcitalopram, 2-Hydroxystearic acid, L-Acetylcarnitine, LysoPC [14Z)], LysoPC [19Z)], LysoPC [20∶1 (11Z)], LysoPC [16Z)], LysoPC (15∶0), LysoPC (P-18∶0), LysoPC [16Z)]), LysoPC (20∶0/0∶0), LysoPC (17∶0), PC [18∶1(9Z)e/2∶0], LysoPC [22∶1 (13Z)], LysoPC (O-18∶0), LysoPC [17Z)], LysoPC (16∶0) and LysoPC [18∶1 (11Z)]. Details are presented in supplementary Tables 1 and 2.
3.5. Pathway analysis and validation
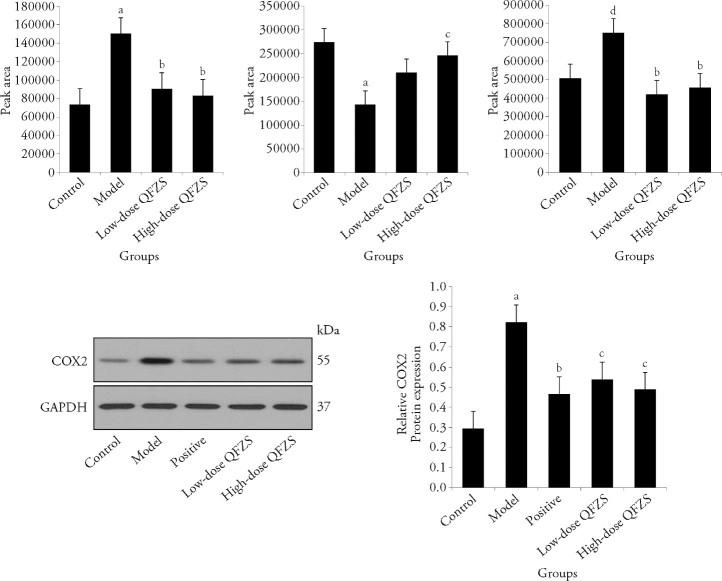
The volcano map showed the outline of differential metabolites, and the heatmap showed that samples in the same group had good parallelism, and the groups could be distinguished. KEGG IDs were applied for each feature metabolite in the QFZS pathway analysis (Figure 3). We found that glycerophospholipid metabolism, biosynthesis of unsaturated fatty acids, insulin resistance, aldosterone synthesis and secretion, inflammatory mediator regulation of TRP channels, linoleic acid metabolism, ether lipid metabolism, and arachidonic acid metabolism were regulated by QFZS. Metabolite level analysis showed that model samples contained a higher level of arachidonic acid, while the QFZS group presented reduced arachidonic acid content. Additionally, QFZS affected the metabolism of arachidonic acid and COX-2 protein levels in the hypothalamus (Figure 4).
Figure 4. Relative levels of common metabolites.
A: arachidonic acid; B: galactonic acid; C: N-desmethylcitalopram; D: Western blotting representative images of COX-2; E: protein expression levels of COX-2. Control group: sham operated; model group: Yeast-induced fever model without treatment; positive group: Yeast-induced fever model with Aspirin (100 mg/kg); Lose-dose QFZS group: Yeast-induced fever model with low-dose QFZS (2.82 g/kg); High-dose QFZS group: Yeast-induced fever model with high-dose QFZS (5.64 g/kg). COX-2: cyclooxygenase-2; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; QFZS: Qingfei Zhisou oral liquid. Data represent the mean ± standard deviation using one-way analysis of variance (n = 5). Compared with the control, aP < 0.01, dP < 0.001; compared with the model, bP < 0.001, cP < 0.01.
4. DISCUSSION
Inflammation can be activated by various compounds, such as adhesion molecules, inflammatory mediators, AA-derived eicosanoids, and inflammatory cytokines, and occurs in several chronic and acute human diseases. Reducing inflammatory factors is a key step to controlling inflammation levels. This study found that QFZS significantly reduced the levels of serum inflammatory factors IL-6 and TNF-α, and reduced the expression of inflammation-related proteins TNF-α and HSP70 in hypothalamic tissues, while the expression of temperature sensing ion channel protein TRPM2 decreased. These results showed that QFZS regulated inflammatory response and body temperature bias in rat pyrexia mode. Through serum metabolomics difference analysis, we found the interaction of inflammatory factors levels and some differential metabolites associated with this progress. It has been reported that an increased concentration of palmitic acid triggers the production of sphingosine 1-phosphate, and causes the recruitment of macrophages to form crown-like structures and produces proinflammatory cytokines, such as TNF-α, interleukin-1β, and IL-6.25 Additionally, palmitic acid can suppress the translocator activity of mitochondrial adenine nucleotide and induce ATP accumulation and ROS production.26 Palmitic acid also induces the activation and dimerization of the toll-like receptor family, followed by recruitment to lipid rafts and phosphate oxidase activation to increase interleukin-1β, TNF-α, and COX-2 expression in macrophages and monocytes.25 Hydroxystearic acid (5-PAHSA) is an endogenous fatty acid that can reduce inflammatory cytokine production and inhibit inflammation by decreasing IL-6 secretion.27 Dexpanthenol also inhibits the release of IL-6 and TNF-α.28 Herein, we observed changes in serum inflammatory factors and metabolite levels, but the specific regulatory mechanism of differential metabolites on inflammation-related proteins still needs further study.
Most differentially produced metabolites between QFZS treatment and model groups were lipid components. Due to the many stimuli and enzymes involved, lipid metabolism is closely regulated and significantly complex. As a common lipid class, glycerophospholipids significantly participate in the regulatory process of cellular membrane integrity and have potential relevance in acute inflammation. Many receptors associated with signal-transducing can be stimulated by oxidized phospholipids, such as nuclear ligand-activated transcription factors, G protein-coupled receptors, receptors coupled to endocytosis, receptor tyrosine kinases, and Toll-like receptors.29 The concentrations of nonadecanoic (C19:0) acids are associated with decreased lipoperoxidation.30 Sphingolipids have many bioactive functions and participate in almost all major aspects of cell biology, including inflammation, immune responses, metabolism, and responses to stress stimuli. There is also a significant association between the cPLA2-COX-2 pathway and bioactive sphingolipids. Studies have shown the regulatory effect of sphingosine 1-phosphate on COX-2 expression, but the underlying mechanisms remain unknown.31 Since the specific biological functions of many lipids are still unknown, more in-depth exploration is needed in this area.
In the present study, we found that QFZS affected the arachidonic acid signaling pathway, reduced arachidonic acid content in serum and tissue COX-2 expression. Both inhibitory and promotive effects of AA-derived mediators on inflammation have been reported.32 Some differential metabolites screened by metabolomics can exert anti-inflammatory effects by affecting COX-2 protease activity. Indoxyl sulfate induced ROS production and increased COX-2 expression in an intestinal inflammation model.33 Additionally, indoxyl sulfate could significantly increase inducible nitric oxide synthase, COX-2, and TNF-α expression and nitrotyrosine formation.34 Eicosapentaenoic acid is a fatty acid rich in omega 3. Studies have observed that docosahexaenoic, eicosapentaenoic acids and dietary n-3 polyunsaturated fatty acids are significantly involved in regulating immune and inflammatory responses. The recent discovery of eicosapentaenoic acid-derived novel autoxidation has generated much interest in the inflammation field.35 Arachidonic acid metabolite 20-hydroxyeicosatetraenoic acid has emerged as a potential endogenous activator of TRPV1. For example, endogenously generated 20-hydroxyeicosatetraenoic acid activates TRPV1.36 We also observed changes in 20-hydroxyeicosatetraenoic acid in the QFZS group. At the same time, the expression of TRP ion channels decreased as inflammatory factors and body temperature decreased.
Thermosensitive neurons are found throughout the central nervous system, but those located in the preoptic anterior hypothalamus are considered the most important for triggering physiologic thermos-effector responses.37 Some peripheral thermoreceptors use TRP channels as thermosensitive elements. TRPM2 can be activated upon exposure to temperatures above 35 ℃.38 The TRPM2 functions as a metabolic and oxidative stress sensor and translates this information into ion fluxes that can affect Ca2+ signaling and the membrane potential.39 By promoting Ca2+ influx via TRPM2, hydrogen peroxide can activate Ca2+-dependent tyrosine kinase signaling. The signaling cascade induced by ROS and associated with chemokine production is controlled by TRPM2 Ca2+ influx, aggravating inflammation. Thus, TRPM2 suppression at the functional level might be a novel strategy for treating inflammatory diseases.40,41 Many TRP channels can be activated or blocked by various compounds found in plants.42 QFZS downregulated TRPM2 expression in the hypothalamus. Our current findings demonstrated that the inflammatory response and body temperature bias triggered TRPM2 increasing in the preoptic area area of model rats. After QFZS intervention, the expression of TRPM2 and inflammatory factors decreased, regulating the inflammatory signaling pathway mediated by TRP channels, and specific active component(s) of QFZS need to be further studied.
In summary, we identified 41 fever-related differential metabolites in rat serum using ultra-high-performance liquid chromatography high-resolution mass spectrometry in a yeast-induced fever rat model. QFZS decreased the concentration of arachidonic acid and inflammatory cytokines in serum. QFZS exerts its regulatory effects on fever by regulating the metabolism of lysophospholipids and arachidonic acid and the regulation of inflammation via TRP channels.
5. ACKNOWLEDGMENTS
The authors would like to thank our team members Prof. LI Lei, Dr. XIN Gaojie, LIU Zixin, ZHANG Huiyu, GUO Fan, CUI Xiaoshan, XU Shujuan, CAO Ce from Xiyuan Hospital of China Academy of Chinese Medical Sciences for helpful discussions on topics related to this work and also thank OE Biotech Co., Ltd. (Shanghai, China) to provide the analysis of metabolomics.
6. SUPPORTING INFORMATION
Supporting data to this article can be found online at http://journaltcm.cn.
Funding Statement
Supported by Beijing Traditional Chinese Medicine Foundation for Science and Technology (JJ-2020-78); Research on the Protection of Intellectual Property Rights of Traditional Chinese Medicine; Scientific and Technological Innovation Project of China Academy of Chinese Medical Sciences (CI2021A00912); Basic and Translational Research on the Application of Traditional Chinese Medicine; the Research Foundation of Major New Drug Creation, from the Ministry of Science and Technology of China (2018ZX09721003-009-022); Research on Key Technologies and Industrialization of Traditional Chinese Medicine Preparations for Children; and National Natural Science Foundation of China (82074060); Study on the Effect of Tanyu Tongzhi Fang in Maintaining Vascular Homeostasis in the Treatment of Atherosclerosis
Contributor Information
Hao GUO, Email: g0502g@163.com.
Jianhua FU, Email: jianhuaffcn@263.net.
<BOLD>REFERENCES </BOLD>
- 1. Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol 2015; 15: 335-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Caterina MJ. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regul Integr Comp Physiol 2007; 292: 64-76. [DOI] [PubMed] [Google Scholar]
- 3. Morrison SF. Central neural control of thermoregulation and brown adipose tissue. Auton. Neurosci 2016; 196: 14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heller HC. The thermostat of vertebrate animals. Sci Am 1978; 239: 102-12. [DOI] [PubMed] [Google Scholar]
- 5. Song K, Wang H, Kamm GB, et al. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science 2016; 353: 1393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tan CH. The TRPM2 ion channel is required for sensitivity to warmth. Nature 2016; 536: 460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beck A, Kolisek M, Bagley LA, et al. Nicotinic acid adenine dinucleotide phosphate and cyclic ADP-ribose regulate TRPM2 channels in T lymphocytes. FASEB J 2006; 20: 962-4. [DOI] [PubMed] [Google Scholar]
- 8. Heiner I, Eisfeld J, Warnstedt M, et al. Endogenous ADP-ribose enables calcium-regulated cation currents through TRPM2 channels in neutrophil granulocytes. Biochem J 2006; 398: 225-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kolisek M, Beck A, Fleig A, et al. Cyclic ADP-ribose and hydrogen peroxide synergize with ADP-ribose in the activation of TRPM2 channels. Mol Cell 2005; 18: 61-9. [DOI] [PubMed] [Google Scholar]
- 10. Wang B, Wu L, Chen J, et al. Metabolism pathways of arachidonic acids: mechanisms and potential therapeutic targets. Signal Transduct Target Ther 2021; 6: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang Y, Yao P, Leung KW, et al. The Chinese medicinal herbs of spleen-meridian property regulate body temperature in yeast-induced fever rats. Phytomedicine 2020; 74: 152815. [DOI] [PubMed] [Google Scholar]
- 12. Sharma V, Bhatia P, Alam O, et al. Recent advancement in the discovery and development of COX-2 inhibitors: insight into biological activities and SAR studies (2008-2019). Bioorg Chem 2019; 89: 103007. [DOI] [PubMed] [Google Scholar]
- 13. Zhu M. 284 cases of lung fever and cough treated with clear lungs and anti-cough decoction. Yao Wu Zi Xun 2011; 24: 3795. [Google Scholar]
- 14. Yang D. 87 cases of cough after treatment of infection. Zhong Yi Yao Yan Jiu Qian Yan 2010; 16: 76. [Google Scholar]
- 15. Qiu YZ. Efficacy of lung cleansing and anti-drinking in the treatment of acute onset of chronic bronchitis. Zhong Yi Yao Xue Kan 2006; 24: 160-1. [Google Scholar]
- 16. Qi T, Li H, Li S, et al. Indirubin improves antioxidant and anti-inflammatory functions in lipopolysaccharide-challenged mice. Oncotarget 2017; 8: 36658-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou Y, Gao C, Vong CT, et al. Rhein regulates redox-mediated activation of NLRP3 inflammasomes in intestinal inflammation through macrophage-activated crosstalk. Br J Pharmacol 2022; 179: 1978-97. [DOI] [PubMed] [Google Scholar]
- 18. Ge H, Tang H, Liang Y, et al. Rhein attenuates inflammation through inhibition of NF-κB and NALP3 inflammasome in vivo and in vitro. Drug Des Devel Ther 2017; 6: 1663-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu T, Zhang W, Feng SJ, et al. Emodin suppresses LPS-induced inflammation in RAW264.7 cells through a PPARγ-dependent pathway. Int Immunopharmacol 2016; 34: 16-24. [DOI] [PubMed] [Google Scholar]
- 20. Gao X, Huang C, Geng T, et al. Serum and urine metabolomics based on UPLC-Q-TOF/MS reveals the antipyretic mechanism of Reduning injection in a rat model. J Ethnopharmacol 2020; 25: 112429. [DOI] [PubMed] [Google Scholar]
- 21. Zhang X, Wang Y, Li S, et al. The potential antipyretic mechanism of gardeniae fructus and its heat-processed products with plasma metabolomics using rats with yeast-induced fever. Front Pharmacol 2019; 9: 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao A, Ma B, Xu L, et al. Jiedu Tongluo granules ameliorates post-stroke depression rat model via regulating NMDAR/BDNF signaling pathway. Front Pharmacol 2021; 20: 662003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lu B, Zhao J, Xu L, et al. Identification of molecular target proteins in Berberine-treated cervix adenocarcinoma HeLa cells by proteomic and bioinformatic analyses. Phytother Res 2012; 26: 646-56. [DOI] [PubMed] [Google Scholar]
- 24. Wang X, Wang R, Xing D, et al. Kinetic difference of berberine between hippocampus and plasma in rat after intravenous administration of Coptidis rhizoma extract. Life Sci 2005; 77: 3058-67. [DOI] [PubMed] [Google Scholar]
- 25. Korbecki J, Bajdak-Rusinek K. The effect of palmitic acid on inflammatory response in macrophages: an overview of molecular mechanisms. Inflamm Res 2019; 68: 915-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ciapaite J, Bakker SJ, Diamant M, et al. Metabolic control of mitochondrial properties by adenine nucleotide translocator determines palmitoyl-CoA effects. Implications for a mechanism linking obesity and type 2 diabetes. FEBS J 2006; 273: 5288-302. [DOI] [PubMed] [Google Scholar]
- 27. Wang YM, Liu HX, Fang NY, et al. High glucose concentration impairs 5-PAHSA activity by inhibiting AMP-activated protein kinase activation and promoting nuclear factor-kappa-B-mediated inflammation. Front Pharmacol 2019; 7: 1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wan LM, Tan Jie, Wan SH, et al. Anti-inflammatory and anti-oxidative effects of dexpanthenol on lipopolysaccharide induced acute lung injury in mice. Inflammation 2016; 39: 1757-63. [DOI] [PubMed] [Google Scholar]
- 29. Bochkov VN, Oskolkova OV, Birukov KG, et al. Generation and biological activities of oxidized phospholipids. Antioxid Redox Signal 2010; 12: 1009-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Handl J, Meloun M, Mužáková V, et al. Inflammatory markers in dependence on the plasma concentration of 37 fatty acids after the coronary stent implantation. J Pharm Biomed Anal 2018; 5: 96-105. [DOI] [PubMed] [Google Scholar]
- 31. Hannun YA, Obeid LM. Sphingolipids and their metabolism in physiology and disease. Nat Rev Mol Cell Biol 2018; 19: 175-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tilley SL, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest 2001; 108: 15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tanaka S, Watanabe H, Nakano T, et al. Indoxyl sulfate contributes to adipose tissue inflammation through the activation of NADPH oxidase. Toxins (Basel) 2020; 12: 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rapa SF, Prisco F, Popolo A, et al. Pro-inflammatory effects of indoxyl sulfate in mice: impairment of intestinal homeostasis and immune response. Int J Mol Sci 2021; 22: 1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yin H, Brooks JD, Gao L, et al. Identification of novel autoxidation products of the omega-3 fatty acid eicosapentaenoic acid in vitro and in vivo. J Biol Chem 2007; 282: 29890-901. [DOI] [PubMed] [Google Scholar]
- 36. Hamers A, Primus CP, Whitear C, et al. 20-hydroxyeicosatetraenoic acid (20-HETE) is a pivotal endogenous ligand for TRPV1-mediated neurogenic inflammation in the skin. Br J Pharmacol 2022; 179: 1450-69. [DOI] [PubMed] [Google Scholar]
- 37. Romanovsky AA. The thermoregulation system and how it works. Handb Clin Neurol 2018; 156: 43. [DOI] [PubMed] [Google Scholar]
- 38. Togashi K, Hara Y, Tominaga T, et al. TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J 2006; 25: 1804-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Knowles H, Li Y, Perraud AL. The TRPM2 ion channel, an oxidative stress and metabolic sensor regulating innate immunity and inflammation. Immunol Res 2013; 55: 241-8. [DOI] [PubMed] [Google Scholar]
- 40. Yamamoto S, Shimizu S, Kiyonaka S, et al. TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med 2008; 14: 738-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kashio M, Tominaga M. The TRPM2 channel: a thermo-sensitive metabolic sensor. Channels (Austin) 2017; 11: 426-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang Y, Sreekrishna K, Lin Y, et al. Modulation of transient receptor potential (TRP) channels by Chinese herbal extracts. Phytother Res 2011; 25: 1666-70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting data to this article can be found online at http://journaltcm.cn.