Abstract
OBJECTIVE
To investigate the effects of acupuncture on learning and memory impairment, oxidative stress and autophagy induced by sleep depriv ation in rats, and to analyze the related mechanism.
METHODS
Thirty Wistar rats were randomly divided into a normal group, sleep deprivation group and acupuncture group. The rat model of sleep deprivation was established by a modified multiplatform sleep deprivation method. The Baihui (GV20), Shenmen (HT7) and Sanyinjiao (SP6) acupoints of rats were located to give electroacupuncture (density wave, frequency 20 Hz, intensity 1 mA) to maintain the needle feeling, and to keep the needle for 15 min and continuous acupuncture for 7 d. The spatial learning and memory abilities of the rats were detected by the water maze test. The content of malondialdehyde (MDA) and the activities of superoxide dismutase (SOD) and glutathione peroxidase (GPX) in the brain were detected by an assay kit, and the autophagy related proteins light chain 3 alpha (LC3A), light chain 3 beta (LC3B) and Beclin 1 and the activation of the protein kinase B (PKB/AKT) and mechanistic target of rapamycin (mTOR) signaling pathway in the rat’s brain were detected by Western blotting.
RESULTS
Compared with the normal group, the time spent in the target quadrant (P < 0.05) and the number of times entering the target quadrant (P < 0.05) in the rats of sleep deprivation group were significantly reduced, and the content of MDA was significantly increased (P < 0.01), while the activities of SOD and GPX (P < 0.01) in the brain were significantly decreased, and LC3A Ⅱ/Ⅰ, LC3B Ⅱ/Ⅰ and Beclin 1 increased significantly (P < 0.01), while p-AKT (ser473)/AKT, p-mTOR (ser2448)/mTOR and p-p70s6K (thr389)/p70S6 decreased significantly (P < 0.01). Compared with the sleep deprivation group, the time spent in the target quadrant and the times of entering the target quadrant (P < 0.05) in the rats of acupuncture group after 7 d of treatment were significantly increased, Additionally, the content of MDA was significantly decreased (P < 0.05), while the activities of SOD and GPX (P < 0.05) in the brain were significantly increased. Moreover, the levels of LC3A Ⅱ/Ⅰ, LC3BⅡ/Ⅰ and Beclin 1 decreased significantly (P < 0.05), and that of p-AKT (ser473)/AKT, p-mTOR (ser2448)/mTOR and p-p70s6K (thr389)/p70s6k increased significantly (P < 0.05).
CONCLUSION
Acupuncture can significantly improve the learning and memory damage caused by sleep deprivation and inhibit oxidative stress and autophagy, and its effect is related to the activation of AKT/mTOR signaling.
Keywords: acupuncture, sleep deprivation, memory and learning tests, oxidative stress, autophagySupporting information
1. INTRODUCTION
Sleep deprivation (SD) refers to the inability to achieve adequate undisturbed nocturnal sleep due to environmental influences or individual reasons. Sleep allows for oxidative clearance,1 while sleep deprivation alters energy metabolism in the brain, leading to the accumulation of reactive oxygen species (ROS).2 When antioxidant enzymes and nonenzymatic antioxidants in the internal environment are relatively deficient and fail to eliminate excessive ROS produced during cellular metabolism,3 they cause an increase in oxidative stress and ultimately lead to a range of adverse effects in the body, such as cognitive and immune abnormalities, as well as neurological, cardiovascular, and gastrointestinal diseases.4,5 Neuroimaging has also demonstrated that sleep deprivation in humans can lead to impaired molecular clearance in the limbic system, including the hippocampus.6
Autophagy is a protective mechanism that protects cells from damage by degrading dysfunctional organelles and misfolded or aggregated proteins. As a self-defense strategy, autophagy promotes cell survival by preventing apoptosis, necrosis, and pyroptosis.7,8 Studies have confirmed that oxidative stress can cause autophagy because reactive oxygen species can act as signaling molecules and oxidatively regulate a variety of signaling pathway proteins for cell proliferation and survival, thereby promoting the formation of autophagosomes.9 It has been confirmed that protein kinase B (AKT) is an important factor in the regulation of survival signaling pathways in cells stimulated by a variety of factors, which can phosphorylate and activate the relevant substrates of rapamycin complex 1 (mTORC1). In addition, the resulting active mTORC1 can control the activity of eukaryotic promoters and elongation factors by phosphorylating p70s6K kinase (p70s6K). This constitutes the AKT-mTOR-p70s6K signaling pathway,10 while the related signals of oxidative stress can inhibit the activation of the AKT-mTOR-p70s6K signaling pathway through phosphatidylinositol 3-kinase (PI3K) and then regulate the level of autophagy.11
The ventral hippocampus is an important brain region involved in cognitive deficits after sleep deprivation.12 This cognitive deficit can be improved by acupuncture to regulate proteins associated with hippocampal synaptic function.13,14 Acupuncture at the Sanyinjiao (SP6) point on the distal limb of the human body can induce extensive activation of the endogenous autonomic nerve circuit and improve sleep deprivation.15 Several clinical reports have shown that acupuncture treatment can effectively improve the sleep status of patients, regulate the physiological discomfort caused by sleep disorders and related emotional and cognitive disorders, and has the potential to inhibit the oxidative stress response.16,17 Our previous study found that acupuncture can play a sleep-improving role by regulating brain-derived nerve growth factor (BDNF) and synaptic vesicle membrane protein (SYP) in the hippocampus of sleep-deprived rats.18 Moreover, we also found that acupuncture could ameliorate depression-like behavior and spatial memory retention deficits, reduce proliferation and differentiation of hippocampal neuronal progenitors by improving impaired field excitatory postsynaptic potential (fEPSP) slope, and elevate BDNF/TrkB protein expression.19 Recent studies have also confirmed that electro-acupuncture promotes hippocampal neurogenesis and synaptic plasticity by activating BDNF/TrkB/Erk signals, thus alleviating SD-induced spatial memory impairment.20 However, the mechanisms involved in hippocampal oxidative stress and the autophagic response remain elusive. In this study, we investigated the effect and mechanism of acupuncture on oxidative stress induced by sleep deprivation in a sleep-deprived rat model.
2. MATERIALS AND METHODS
2.1. Experimental animals and groups
Thirty healthy and clean Wistar rats aged 11-12 months and weighing 150-170 g were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. [Production License No. SCXK (Hu) 2017-0005)]. The rats were housed in the laboratory animal center with an ambient temperature controlled at (25.0 ± 2.0) ℃, a constant dark/light cycle of 12 h/12 h, and free access to water. Thirty rats were randomly divided into normal group, sleep deprivation group and acupuncture group, with 10 rats in each group. All animal experimental procedures were approved by the animal ethics committee of Changchun University of Chinese Medicine and strictly followed the Guiding Opinions on the Treatment of Laboratory Animals issued by the Ministry of Science and Technology of the People's Republic of China in 2006. This study obtained ethical review and approval from the Animal Experimental Center of Changchun University of Chinese Medicine.
2.2. Main reagents and instruments
Blot-related detection reagents (Biyuntian Biote-chnology Co., Ltd. Shanghai, China); Bicinchoninic Acid protein quantifi cation kit (Thermo Fisher, Waltham, MA, USA): p70s6K antibody (9202), phospho-p70s6K (Thr389) antibody (9234), mTOR antibody (2983), phospho-mTOR (Ser2448) antibody (5536), AKT antibody (4691), phospho-AKT (Ser473) antibody (4060), light chain 3 alpha (LC3A) antibody (4599), glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (5174) were purchased from Cell Signaling Technology (Danvers, MA, USA); Beclin 1 antibody (ab207612), light chain 3 beta (LC3B) (ab192890), and corresponding horseradish peroxidase-labeled secondary antibodies were purchased from Abcam (Cambridge, UK). The total superoxide dismutase (SOD) activity, malondialdehyde (MDA) and total glutathione peroxidase (GPX) assay kits were purchased from Shanghai Biyuntian Biotechnology Co., Ltd (Shanghai, China).
Huatuo brand acupuncture needle (Suzhou Acupuncture and Moxibustion Products Factory, Suzhou, China), HANS-100 A electroacupuncture instrument (Nanjing Jisheng Medical Technology Co., Ltd., Nanjing, China), Olympus electron fluorescence microscope (MIS 2000, Olympus, Tokyo, Japan), water maze system (Shanghai Yuyan Scientific Instruments Co., Ltd., Shanghai, China), and chemiluminescence imaging analyzer (ImageQuant LAS 4000, General Motors, New York, NY, USA) were used.
2.3. Sleep deprivation model
A sleep deprivation model was established using a modified multiplatform platform method (MMPM),21 as we previously reported.18 Rat boxes were 110 cm × 60 cm × 40 cm, had 6 platforms with a diameter of 6.5 cm and a height of 8.0 cm, with the platform separated by 15 cm and were filled with water around the platform. The water temperature was maintained at 22 ℃, the water surface was approximately 1.0 cm away from the platform surface, and the rats could feed and drink water by themselves and move on the platform. When the rats enter rapid-eye-movement (REM) sleep, they are awakened by water touch due to reduced muscle tone throughout the body, resulting in physical imbalance, ensuring that rats cannot enter REM sleep. The room temperature was controlled at 22.0-24.0 ℃ and 40 w fluorescent lights were illuminated from 08:30 to 20:30.
2.4. Electroacupuncture treatment
Acupuncture methods: Acupoints were selected according to the location of acupoints provided by Experimental Acupuncture and Moxibustion and Development of Acupoint Atlas in rats, and comparative anatomical methods were used to determine the localization of Baihui (GV20), Shenmen (HT7) and Sanyinjiao (SP6) acupoints in rats. The acupoint location was determined according to our previous reports.18,19 Electroacupuncture (dense wave, frequency 20 Hz, intensity 1 mA) was added after acupuncture to maintain the needle sensation through a medical electronic stimulator (Qingdao Xinsheng Instrument, Qingdao, China), the needle was retained for 15 min, and continuous acupuncture was performed for 7 d. The needles (0.25 mm × 25 mm) were purchased from Suzhou Hualun Medical Appliance Co., Ltd. (Suzhou, China). Shenmen (HT 7) and Sanyinjiao (SP 6) on the same side jointly formed a pair of stimulation electrodes, Baihui (GV20) and the back side of the 0.5 cm open skin jointly formed a pair of stimulation electrodes. Special attention should be given to binding 20 min before electroacupuncture stimulation to reduce stress.
2.5. Morris water maze test
The water maze test was used to evaluate the spatial learning and exploration abilities and long-term memory in rats. It was performed according to the experimental methods described in the literature. The water maze is a circular open pool (approximately 1.2 m in diameter) divided into four quadrants: northeast, northwest, southeast, and southwest. The underwater platform was located in the center of the northwest quadrant (target quadrant), 0.8 cm below the water surface. Four pictures of different shapes were pasted on the pool walls of the four quadrants of the water maze as maze tips. Each rat underwent four experiments per day, and in each water entry experiment, the rat was fed water in a manner facing the pool wall, swam to find the underwater platform, remained on the platform for 15 s and then was removed and placed in the cage; if the rat failed to find the underwater platform within 60 s, it was guided to find the platform and stayed there for 15 s before returning to the cage. Escape latency (time to reach the platform) and swimming speed were recorded for each rat. On the fifth day, the underwater platform was removed, the rat was placed in the southeast quadrant to begin exploration for a maximum of 60 s, and the time spent and number of entries by the rat in the target quadrant were recorded.
2.6. Enzyme-linked immunosorbent assay (ELISA)
The hippocampal samples were weighed, added to tissue cracking solution, homogenized and centrifuged at 12000 r/min for 10 min (4 ℃). The supernatant was taken for testing, and 100 µL of diluted cracking solution was added to the ELISA plate and incubated overnight at 4 ℃. The next day, after washing the plates, the sealing solution was added at 200 µL/well and incubated at 37 ℃ for 1 h. The tissue samples were diluted with washing solution, and then 100 µl/well diluted standard solution was added and incubated at 37 ℃ for 30 min. Freshly prepared enzymatic antibody was added at 50 µL/well and incubated at 37 ℃ for 1 h. After the plate was washed again, TMB substrate liquid was added at 150 µL/well for color development, and 50 µL termination liquid was added after 20 min of reaction at room temperature and away from light. OD values at 450 nm were collected by Tecan Sunrise (Tecan Group Ltd., Männedorf, Switzerland).
2.7. Western blot
At the end of behavioral tests, rats in each group were euthanized by injection of thiopental sodium (1 unit/10 g) to separate the cerebral cortex of rats, and the brain homogenizer on one side was lysed in lysis buffer, cryopreserved at 4 ℃, and centrifuged at 8000 rpm for 10 min to collect the supernatant. The protein concentration was estimated using the BCA method. Each sample (50 μg) was used for sodium dodecyl sulfate polyacrylamide gel electrophoresis (Shanghai Biyuntian Biotechnology Co., Ltd., Shanghai, China), transferred to polyvinylidene fluoride (PVDF) membrane (Shanghai Biyuntian Biotechnology Co., Ltd., Shanghai, China) after protein electrophoresis separation and blocked in Tris-Buffered Saline with Tween (Shanghai Biyuntian Biotechnology Co., Ltd., Shanghai, China) solution containing 5% skimmed milk for 1 h. The corresponding primary antibody working solution was added, and the primary antibody was slowly shaken overnight at a low temperature. The concentrations of the primary antibodies were as follows: p70s6K antibody (9202, 1∶500), phospho-p70s6K (Thr389) antibody (9234, 1∶400), mTOR antibody (2983, 1∶1000), phospho-mTOR (Ser2448) antibody (5536, 1∶500), AKT antibody (4691, 1∶500), phospho-AKT (Ser473) antibody (4060, 1∶400), LC3A antibody (4599, 1∶500), GAPDH antibody (5174), Beclin 1 antibody (ab207612, 1∶800), and LC3B antibody (ab192890, 1∶500). The next day, the primary antibody was removed, the correspondinghorseradish peroxidase-labeled secondary antibody was added, and the PVDF membrane was detected using a chemi-luminescence imaging analyzer after color development by chromogen.
2.8. Data analysis
GraphPad Prism 7.0 (GraphPad Software, San Diego, CA, USA) was used for statistical analysis of experimental data. Enumeration data were expressed as the mean ± standard deviation (). Independent sample t test was used for comparison between two groups. One-way analysis of variance was used for comparisons between multiple groups. If the overall difference was statistically significant, the least significant difference t-test was used for further pairwise comparisons. P < 0.05 indicates a significant difference.
3. RESULTS
3.1. Effect of acupuncture on learning and memory ability in sleep deprived rats
The results of the water maze test showed that the time spent in the target quadrant and times to enter the target quadrant position in the sleep deprivation group were significantly lower than those in the normal group (P < 0.05), indicating that the spatial learning and memory ability of the sleep deprivation group were impaired. However, the time spent in the target quadrant and times to enter the target quadrant position were significantly increased in the acupuncture group compared with sleep deprivation group (P < 0.05), indicating that acupuncture treatment improved the spatial learning and memory ability of rats (Figure 1).
Figure 1. Comparison of spatial learning and memory abilities among groups.
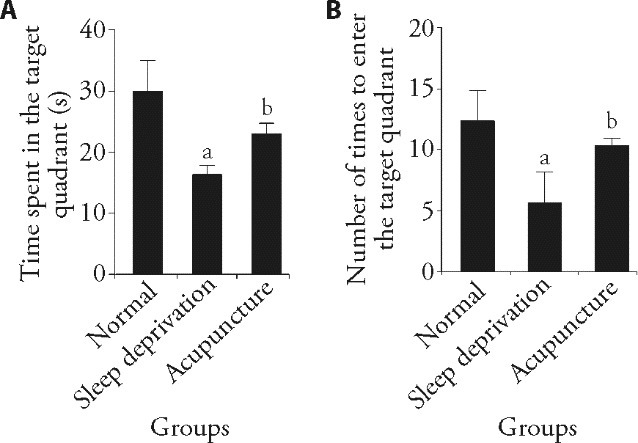
A: time spent in the target quadrant in each group (n = 3); electroacupuncture (20 Hz, 1 mA) was applied 15 min/d for 7 d. B: number of times each group entered the target quadrant position (n = 3). One-way analysis of variance was used for comparisons between multiple groups. If the overall difference was statistically significant, the least significant difference t-test was used for further pairwise comparisons. The data are expressed as mean ± standard deviation. Compared with the normal group, aP < 0.05; compared with the sleep deprivation group, bP < 0.05.
3.2. Effect of acupuncture on oxidative stress in the hippocampus of sleep-deprived rats
The results of oxidative stress analysis showed that the MDA content in the hippocampus of sleep-deprived rats was significantly increased (P < 0.01), while the activities of SOD and GPX were significantly decreased (P < 0.01) compared with those of normal rats, and the differences were statistically significant. Compared with sleep-deprived rats, the MDA content in the brain of acupuncture rats was significantly decreased (P < 0.05), while the activities of SOD and GPX were significantly enhanced (P < 0.05), and the difference was statistically significant (Figure 2).
Figure 2. Comparison of oxidative stress levels in the brains of rats in each group.
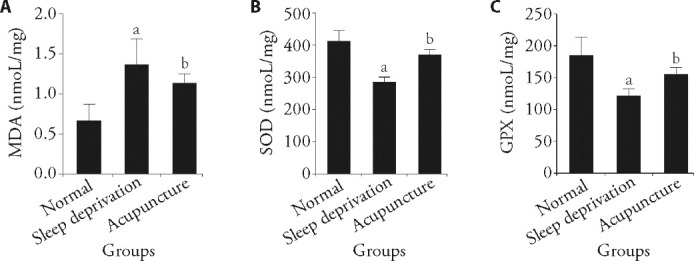
A: MDA content in the brain tissue of rats in each group (n = 3), electroacupuncture (20 Hz, 1 mA) was applied 15 min/d for 7 d; B: SOD content in the brain tissue of rats in each group; C: GPX content in the brain tissue of rats in each group. MDA: malondialdehyde; SOD: superoxide dismutase; GPX: glutathione peroxidase. One-way analysis of variance was used for comparisons between multiple groups. If the overall difference was statistically significant, the least significant difference t-test was used for further pairwise comparisons. The data are expressed as mean ± standard deviation. Compared with the normal group, aP < 0.01; compared with sleep deprivation group, bP < 0.05.
3.3. Effect of acupuncture on autophagy in the hippocampus of sleep-deprived rats
Western blot analysis showed that the LC3A II/I ratio, LC3B II/I ratio and relative expression of Beclin 1 in the hippocampus of sleep-deprived rats were significantly increased compared with those of normal rats (P < 0.01), and the differences were statistically significant. Compared with sleep-deprived rats, the LC3A II/I ratio, LC3B II/I ratio, and relative expression of Beclin 1 were significantly decreased in the acupuncture group (P < 0.05), and the differences were statistically significant (Figure 3).
Figure 3. Comparison of autophagy levels in the brains of rats in each group.
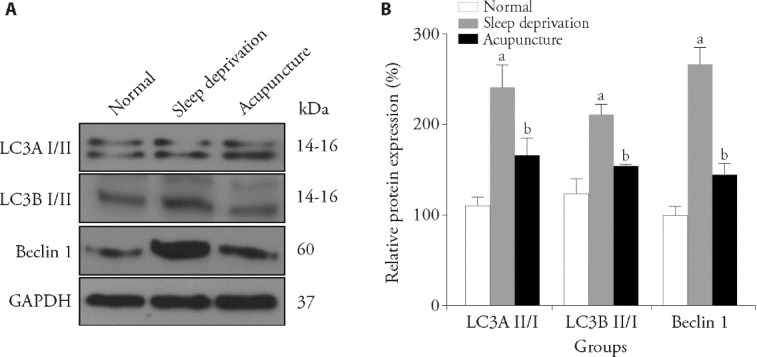
A: representative images of LC3A Ⅱ/Ⅰ ratio, LC3B Ⅱ/Ⅰ ratio and relative expression of Beclin 1 in the normal, sleep deprivation and acupuncture groups (n = 3). Electroacupuncture (20 Hz, 1 mA) was applied 15 min/d for 7 d. B: LC3A Ⅱ/Ⅰ ratio, LC3B Ⅱ/Ⅰ ratio and relative expression of Beclin 1 in the hippocampal tissues of rats in each group. LC3A: Light chain 3 alpha; LC3B: Light chain 3 beta. One-way analysis of variance was used for comparisons between multiple groups. If the overall difference was statistically significant, the least significant difference t-test was used for further pairwise comparisons. Data were presented as mean ± standard deviation (n = 3). Compared with the normal group, aP < 0.01; compared with sleep deprivation group, bP < 0.05.
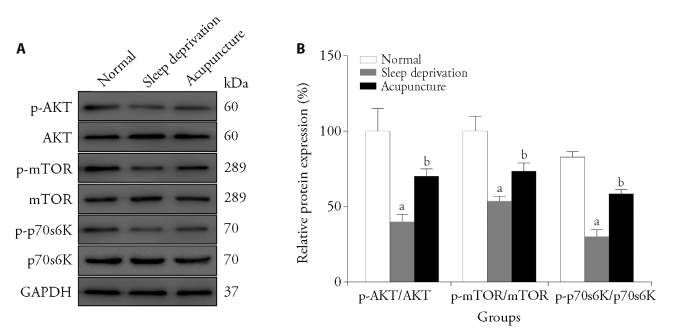
3.4. Effect of acupuncture on hippocampus signaling pathways in sleep deprived rats
Western blot analysis showed that the p-AKT (Ser473)/AKT, p-mTOR (Ser2448)/mTOR, and p-p70s6K (Thr389)/p70s6K ratios were significantly decreased in the brains of sleep-deprived rats compared with normal rats (P < 0.05), and the differences were statistically significant. Compared with sleep-deprived rats, the p-AKT (Ser473)/AKT, p-mTOR (Ser2448)/ mTOR, and p-p70s6K (Thr389)/p70s6K ratios were significantly enhanced in the acupuncture group (P < 0.05), and the differences were statistically significant (Figure 4).
Figure 4. Phosphorylation level of the AKT/mTOR/p70S6K signaling pathway in rat hippocampal tissue.
A: Western blotting was performed to analyze the expression of AKT, mTOR and p70S6K in the hippocampal tissues (n = 3). Electroacupuncture (20 Hz, 1 mA) was applied 15 min/d for 7 d. B: quantitative analysis of AKT, mTOR and p70S6K protein. AKT: protein kinase B; mTOR: mechanistic target of rapamycin. One-way analysis of variance was used for comparisons between multiple groups. If the overall difference was statistically significant, the least significant difference t-test was used for further pairwise comparisons. Data were presented as mean ± standard deviation (n = 3). Compared with the normal group, aP < 0.01; compared with the sleep deprivation group, bP < 0.05.
4. DISCUSSION
Sleep deprivation can cause brain and psychological fatigue, which further accelerate physical fatigue and bring a series of adverse consequences, such as visual hallucinations, illusions, microsomia and behavioral disorders, resulting in deficits in cognitive functions such as learning and memory.22 In clinical studies, neuroimaging techniques were used to find that acupuncture at Shenmen (HT7) and Sanyinjiao (SP6) points had significant effects on extensive brain networks and reversed specific functional connections between networks in acute sleep deprivation subjects.23,24 Our previous prospective clinical studies have demonstrated the efficacy and safety of electroacupuncture in improving negative emotions in sleep-deprived patients.16 Therefore, to explore the effect of acupuncture treatment on cognitive impairment associated with sleep deprivation, we established a sleep deprivation model in rats by a modified multiplatform sleep deprivation method and observed that the time spent in the target quadrant of the water maze and the number of entries into the target quadrant position (P < 0.05) were significantly increased in sleep-deprived rats after acupuncture treatment. The indicated that acupuncture treatment significantly improved learning and memory impairment induced by sleep deprivation in rats. This is consistent with other reports that electroacupuncture promotes hippocampal neuron survival and synaptic plasticity and improves spatial memory impairment caused by sleep deprivation.20
An important function of sleep is to reduce the production of large amounts of reactive oxygen species that cause oxidative stress in the nervous system during prolonged wakefulness. Oxidative stress induced by sleep deprivation in the nervous system has been well-established, and decreases in glycogen synthase kinase (GSK) levels and increases in ROS levels have been observed in the brain in animal models of sleep deprivation.25 Several studies have confirmed the close relationship between sleep deprivation and oxidative stress; for example, Valvassori et al 26 demonstrated that paradoxical sleep deprivation (PSD) induces mania-like behavior in mice by increasing lipid peroxidation and oxidative damage to DNA while destroying antioxidant enzymes in the frontal cortex, hippocampus, and serum. However, oxidative stress in the brain also triggers self-protective mechanisms, resulting in increased activity of antioxidant enzymes such as SOD and GPX, which act as reactive oxygen species scavengers produced by cells and remove excessive reactive oxygen species in a timely manner to avoid neuronal damage. However, decreased levels of SOD and GPX may be the result of enzyme inactivation or lipid peroxidation leading to excessive levels of reactive oxygen species.27 In the present study, MDA levels in the hippocampus were significantly increased and SOD and GPX activities were inhibited in sleep-deprived rats, indicating that sleep deprivation may promote peroxide accumulation and induce oxidative changes in lipids, ultimately leading to membrane function changes in neurons and inhibition of intracellular antioxidant defense systems. These results are consistent with changes in biochemical parameters that have been reported.27 However, after acupuncture treatment, a significant improvement in the level of oxidative stress can be observed, and acupuncture treatment has different degrees of inhibitory effect on oxidative stress caused by other pathological factors. For example, electroacupuncture at Shenmen (HT7) alleviates sleep disruption following acute caffeine exposure by regulating BDNF-mediated endoplasmic reticulum stress,28 which is related to the neuroprotective effect of acupuncture. Acupuncture not only activates the intrinsic antioxidant enzyme system but also inhibits the excessive generation of ROS by regulating a series of molecular signaling pathways involved in redox regulation.
It has been demonstrated that oxidative stress can trigger autophagy and thereby improve cell survival. Sleep deprivation induces increased levels of autophagy in hippocampal neurons.10 LC3 Ⅱ protein is an autophagic marker protein located on the autophagosome membrane, and the proportion of LC3A Ⅱ/Ⅰ and LC3B Ⅱ/Ⅰ is increased, indicating that the conversion of LC3 I to LC3 Ⅱ is increased, which is required for autophagosome formation, while Beclin I protein is involved in initiating autophagy and is used as a marker to monitor the onset of autophagy.29 Our results showed that the relative expression of LC3A Ⅱ/Ⅰ, LC3B Ⅱ/Ⅰ and Beclin 1 was significantly increased in the hippocampus of sleep-deprived rats, indicating that their autophagy levels were increased. However, after acupuncture treatment, the relative expression of LC3A Ⅱ/Ⅰ, LC3B Ⅱ/Ⅰ and Beclin 1 in the brain of sleep-deprived rats was significantly decreased, indicating that acupuncture could inhibit the autophagy level of neurons. At present, there are few reports on the inhibition of autophagy by acupuncture treatment; for example, acupuncture can reduce oxidative stress products by regulating the Nrf2/HO-1 signaling pathway in the hippocampus, thereby preventing apoptosis of hippocampal neurons and playing a role in improving cognitive dysfunction.30
The mTOR signaling pathway plays an important role in inhibiting autophagy initiation and regulating autophagosome maturation after sleep deprivation, and it can integrate some autophagy signals, including oxidative stress.31 In addition, p70S6K is a major downstream target of the mTOR pathway and is essential for regulating cell proliferation and survival.10 The AKT/mTOR pathway may contribute to autophagy-mediated antioxidant protection.32 In the present study, we investigated the major proteins of the AKT/mTOR signaling pathway. The levels of p-AKT (Ser473)/AKT, p-mTOR (Ser2448)/mTOR, and p-p70 S6 (Thr389)/p70S6K were significantly decreased in the hippocampal neurons of sleep-deprived rats, indicating that the AKT/mTOR/p70S6K signaling pathway is inhibited by sleep deprivation. However, p-AKT (Ser473)/AKT, p-mTOR (Ser2448)/mTOR and p-p70s6k (Thr389)/p70s6k were significantly enhanced after acupuncture treatment. AKT/mTOR has been one of the key pathways activated by acupuncture in the study of mechanism of action of acupuncture treatment. AKT/mTOR has been one of the key pathways activated by acupuncture in the study of the mechanism of action of acupuncture treatment and is a target for acupuncture treatment to exert therapeutic effects in sleep deprivation models. Combined with our results, it could be confirmed that increased autophagy caused by sleep deprivation is mediated by inhibition of the AKT/mTOR signaling pathway, whereas inhibition of autophagy by acupuncture treatment may be achieved by activation of the AKT/mTOR signaling pathway, providing an experimental basis for improving the effectiveness of sleep deprivation symptoms and acupoint compatibility theory.
Our study explains the therapeutic effect of acupuncture on sleep deprivation in rats and its related mechanism of regulating AKT/mTOR. However, there are still some observations at the cellular level, which require more precise pharmacological and optogenetic techniques to verify the target cells and internal molecular targets of acupuncture. Its application in clinical practice still needs to be confirmed by further in depth clinical studies in the future.
5. ACKNOWLEDGMENTS
We would like to acknowledge the staff in the Laboratory Animal Centre of Changchun University of Chinese Medicine for their invaluable assistance in the performance of the animals’ work.
Funding Statement
Supported by the Natural Science Foundation of Jilin province: Investigation of the Regulatory Mechanism of Cognitive Function in Sleep-Deprived Rats Treated with Acupuncture based on the Rat Sarcoma Virus Oncogene/Rapidly Accelerated Fibrosarcoma/Mitogen-Activated Protein Kinase/Extracellular Signal-Regulated Kinase Kinase/Extracellular Signal-Regulated Kinase pathway (No. 20210101222JC)
REFERENCES
- 1. Iranzo A. An overview on sleep medicine. Adv Exp Med Biol 2022; 1384: 3-15. [DOI] [PubMed] [Google Scholar]
- 2. Melhuish Beaupre LM, Brown GM, Braganza NA, Kennedy JL, Gonçalves VF. Mitochondria's role in sleep: novel insights from sleep deprivation and restriction studies. World J Biol Psychiatry 2022; 23: 1-13. [DOI] [PubMed] [Google Scholar]
- 3. Luo F, Han S, Xia M, Chen Z, Liu P, Lin J. Study on the mechanism of hydrolyzed seawater pearl tablet in treating chronic sleep deprivation mice model. Endocr Metab Immune Disord Drug Targets 2023; 23: 927-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alrousan G, Hassan A, Pillai AA, Atrooz F, Salim S. Early life sleep deprivation and brain development: insights from human and animal studies. Front Neurosci 2022; 16: 833786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tobaldini E, Costantino G, Solbiati M, et al. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci Biobehav Rev 2017; 74: 321-9. [DOI] [PubMed] [Google Scholar]
- 6. Eide PK, Vinje V, Pripp AH, Mardal KA, Ringstad G. Sleep deprivation impairs molecular clearance from the human brain. Brain 2021; 144: 863-74. [DOI] [PubMed] [Google Scholar]
- 7. Bedont JL, Toda H, Shi M, et al. Short and long sleeping mutants reveal links between sleep and macroautophagy. Elife 2021; 10: e64140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xie Y, Ba L, Wang M, et al. Chronic sleep fragmentation shares similar pathogenesis with neurodegenerative diseases: Endosome-autophagosome-lysosome pathway dysfunction and microglia-mediated neuroinflammation. CNS Neurosci Ther 2020; 26: 215-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yun HR, Jo YH, Kim J, Shin Y, Kim SS, Choi TG. Roles of autophagy in oxidative stress. Int J Mol Sci 2020; 21: 3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cao Y, Li Q, Liu L, et al. Modafinil protects hippocampal neurons by suppressing excessive autophagy and apoptosis in mice with sleep deprivation. Br J Pharmacol 2019; 176: 1282-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frolinger T, Smith C, Cobo CF, et al. Dietary polyphenols promote resilience against sleep deprivation-induced cognitive impairment by activating protein translation. FASEB J 2018; 32: 5390-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sun W, Li J, Cui S, et al. Sleep deprivation disrupts acquisition of contextual fear extinction by affecting circadian oscillation of hippocampal-Infralimbic proBDNF. eNeuro 2019; 6: ENEURO.0165-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hao L, Wu Y, Xie J, Chen X. Electroacupuncture enhances cognitive deficits in a rat model of rapid eye movement sleep deprivation via targeting MiR-132. Evid Based Complement Alternat Med 2022; 2022: 7044208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen D, Zhang Y, Wang C, et al. Modulation of hippocampal dopamine and synapse-related proteins by electroacupuncture improves memory deficit caused by sleep deprivation. Acupunct Med 2020; 38: 343-51. [DOI] [PubMed] [Google Scholar]
- 15. Gao L, Zhang M, Gong H, et al. Differential activation patterns of FMRI in sleep-deprived brain: restoring effects of acupuncture. Evid Based Complement Alternat Med 2014; 2014: 465760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yan B, Wang FC, Ma TS, et al. Efficacy and safety of electroacupuncture treatment in the prevention of negative moods in healthy young men after 30 h of total sleep deprivation: study protocol for a single-center, single-blind, parallel-arm, randomized clinical trial. Trials 2021; 22: 761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yeung WF, Yu BY, Yuen JW, et al. Semi-individualized acupuncture for insomnia disorder and oxidative stress: a randomized, double-blind, sham-controlled trial. Nat Sci Sleep 2021; 13: 1195-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zheng P, Xu X, Zhao H, Lyu T, Song B, Wang F. Tranquilizing and allaying excitement needling method affects BDNF and SYP expression in hippocampus. Evid Based Complement Alternat Med 2017; 2017: 8215949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu X, Zheng P, Zhao H, Song B, Wang F. Effect of electroacupuncture at GV20 on sleep deprivation-induced depression-like behavior in mice. Evid Based Complement Alternat Med 2020; 2020: 7481813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pei W, Meng F, Deng Q, et al. Electroacupuncture promotes the survival and synaptic plasticity of hippocampal neurons and improvement of sleep deprivation-induced spatial memory impairment. CNS Neurosci Ther 2021; 27: 1472-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Machado RB, Suchecki D, Tufik S. Comparison of the sleep pattern throughout a protocol of chronic sleep restriction induced by two methods of paradoxical sleep deprivation. Brain Res Bull 2006; 70: 213-20. [DOI] [PubMed] [Google Scholar]
- 22. Hudson AN, Van Dongen HPA, Honn KA. Sleep deprivation, vigilant attention, and brain function: a review. Neuropsychopharmacology 2020; 45: 21-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ning Y, Zheng S, Feng S, et al. The altered intrinsic functional connectivity after acupuncture at shenmen (HT7) in acute sleep deprivation. Front Neurol 2022; 13: 947379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dai XJ, Min YJ, Gong HH, et al. Evaluation of the post-effect of acupuncture at Sanyinjiao (SP6) under sleep deprivation by resting-state amplitude of low-frequency fluctuation: a fMRI study. Zhong Guo Zhen Jiu 2012; 32: 47-52. [PubMed] [Google Scholar]
- 25. Xue R, Wan Y, Sun X, Zhang X, Gao W, Wu W. Nicotinic mitigation of neuroinflammation and oxidative stress after chronic sleep deprivation. Front Immunol 2019; 10: 2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Valvassori SS, Resende WR, Dal-Pont G, et al. Lithium ameliorates sleep deprivation-induced mania-like behavior, hypothalamic-pituitary-adrenal (HPA) axis alterations, oxidative stress and elevations of cytokine concentrations in the brain and serum of mice. Bipolar Disord 2017; 19: 246-58. [DOI] [PubMed] [Google Scholar]
- 27. Nabaee E, Kesmati M, Shahriari A, Khajehpour L, Torabi M. Cognitive and hippocampus biochemical changes following sleep deprivation in the adult male rat. Biomed Pharmacother 2018; 104: 69-76. [DOI] [PubMed] [Google Scholar]
- 28. Seo SY, Ryu Y. Electroacupuncture stimulation of HT7 alleviates sleep disruption following acute caffeine exposure by regulating BDNF-mediated endoplasmic reticulum stress in the rat medial septum. Biomed Pharmacother 2022; 155: 113724. [DOI] [PubMed] [Google Scholar]
- 29. D'Arcy MS. Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int 2019; 43: 582-92. [DOI] [PubMed] [Google Scholar]
- 30. Cheng WJ, Li P, Huang WY, et al. Acupuncture relieves stress-induced depressive behavior by reducing oxidative stress and neuroapoptosis in rats. Front Behav Neurosci 2022; 15: 783056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Havekes R, Abel T. The tired hippocampus: the molecular impact of sleep deprivation on hippocampal function. Curr Opin Neurobiol 2017; 44: 13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cao Y, Li Q, Zhou A, et al. Notoginsenoside R1 reverses abnormal autophagy in hippocampal neurons of mice with sleep deprivation through melatonin receptor 1A. Front Pharmacol 2021; 12: 719313. [DOI] [PMC free article] [PubMed] [Google Scholar]