Abstract
Antiretroviral therapy (ART) has improved the survival of people living with HIV (PLHIV) but this success has been accompanied by an increase in noncommunicable diseases. We conducted a prospective cohort study of 4000 adult PLHIV who were initiating ART in Dar es Salaam, Tanzania, to assess weight gain during the first year of treatment and associated sociodemographic and clinical factors. Anthropometric data were collected at ART initiation and monthly follow-up visits. The mean weight gain during the first year of treatment was 2.6 ± 0.3 kg, and the prevalence of overweight or obesity increased from 26.3% at baseline to 40.7%. Female sex, greater household wealth, lower CD4-T-cell counts, higher WHO HIV disease stage, and pulmonary tuberculosis were associated with a greater increase in body mass index (P < .05). Weight gain following ART initiation was common but was greater among females and PLHIV with advanced HIV or comorbidities.
Keywords: HIV, nutritional status, weight gain, highly active antiretroviral therapy, Tanzania
Introduction
Antiretroviral therapy (ART) has significantly improved the survival of people living with HIV (PLHIV) and reduced the risk of HIV-associated wasting. However, chronic conditions, including noncommunicable diseases (NCDs), have now become a concern globally for PLHIV.1,2 Epidemiologic research has confirmed weight gain, overweight, and obesity as risk factors for NCDs, including cardiovascular disease, stroke, chronic kidney disease, cancers, and musculoskeletal diseases.3,4 Importantly, obesity is also rapidly increasing in sub-Saharan Africa (SSA) in the general population. 5 It has also been noted that PLHIV experience a combination of general population and HIV-specific risk factors for high body mass index (BMI) and NCDs. 6 Information on weight gain after ART initiation as well as subpopulations that may have greater risk of incident overweight and obesity is currently needed to inform interventions and prevention policies for PLHIV in SSA.
There has been a growing focus on ART regimens and weight gain among PLHIV; however, there are many other potential sociodemographic and clinical risk factors that can influence weight gain. 7 Several studies in SSA have estimated the prevalence of overweight and obesity among PLHIV,8–11 but few have evaluated weight gain following ART initiation. The prevalence of overweight and obesity among PLHIV has ranged between 17% and 61% in research studies.8–13 Generally, studies have documented that patients with low CD4T-cell counts, 14 older age, 15 women, 16 and those with lower baseline weight17–19 may experience greater weight gain after ART initiation. Relatively fewer studies have longitudinally evaluated weight gain and its predictors, particularly non-ART-related risk factors, in the context of cohorts in SSA.12,13
To address this research gap, we conducted a prospective cohort study of adults living with HIV during the first year of ART in Tanzania to evaluate the relationship of sociodemographic and clinical characteristics with changes in BMI. We also examined the cumulative incidence of incident overweight or obesity as well as their predictors. These results are intended to inform populations that may be targeted or have greater benefit from interventions to prevent unhealthy weight gain following ART initiation.
Methods
We conducted a prospective cohort study among participants enrolled in a randomized, double-blind, placebo-controlled trial of vitamin D3 supplementation conducted among adults living with HIV who were initiating ART in Dar es Salaam, Tanzania (ClinicalTrials.gov identifier NCT01798680). The trial protocol and the efficacy results have been reported elsewhere.20,21
The trial was conducted from February 2014 to March 2018 at 4 large public HIV care and treatment centers. Participants were eligible for trial enrollment if they met the following inclusion criteria: (1) adult men or women aged ≥18 years old, (2) documented to be living with HIV, (3) were initiating ART at the time of randomization, (4) had insufficient serum 25(OH)D concentration <30 ng/mL at the screening visit, (5) intended to stay in Dar es Salaam for at least 1 year after enrollment, and (6) provided written informed consent. The trial exclusion criteria were (1) pregnant women and (2) being enrolled in any other clinical trial. All study participants were provided with HIV care and treatment that adhered to Tanzanian national guidelines. 21 Eligible participants were randomly assigned to the vitamin D3 supplementation or placebo regimen. The vitamin D3 supplementation group received oral supplements containing 50 000 IU vitamin D3 at the randomization visit and once a week for the next 3 weeks followed by daily oral supplements containing 2000 IU vitamin D3 starting at the fourth week until trial discharge at 1-year post-randomization. The placebo group received a matching regimen of placebo supplements.
During the trial, the Dar es Salaam HIV care and treatment program changed the ART eligibility criteria. At the start of trial enrollment in 2014, the ART initiation criteria for adults was a CD4T-cell count <350 cells/mL or WHO HIV stage 3 or 4 disease. In January 2016, the ART initiation criteria changed to a CD4T-cell count <500 cells/mL or WHO HIV stage 3 or 4 disease, and in June 2016 the program switched to a test-and-treat strategy whereby all individuals with HIV were eligible for lifelong ART. Efavirenz–lamivudine–tenofovir (EFV/3TC/TDF) was the only first-line ART regimen used during the trial. Participants diagnosed with pulmonary tuberculosis (TB) received directly observed therapy for 6-month starting with a 2-month intensive phase of daily rifampicin–isoniazid–pyrazinamide–ethambutol followed by a 4-month continuation phase of daily rifampicin and isoniazid. All participants received general dietary counseling as the standard of care for PLHIV. No additional food or nutritional supplements were provided as standard of care. In terms of outcomes, vitamin D3 supplementation did not affect mortality, incidence of TB, weight gain, or any clinical outcome after ART initiation.20,22
Data Collection
The screening visit for the trial was integrated into the HIV testing and ART initiation visit. CD4T-cell count was measured at the ART eligibility visit (FACS-Calibur System, Becton Dickinson). At the randomization visit, study physicians conducted a clinical examination, TB screening, and assessed the WHO HIV disease stage. Study nurses collected information on sociodemographic characteristics using standardized questionnaires. Wealth quintile was constructed with a principal component of household assets (electricity, generator, running water, sofa/couch, television, radio, refrigerator, fan, bicycle, car, and roof type). All participants had height and weight collected at the time of randomization. Participants were followed up at weekly clinic visits for the first month of ART and followed by monthly clinic visits until 12 months post-randomization. A full description of the follow-up procedures is published in the trial protocol. Physicians conducted clinical examinations, diagnosed conditions, and provided treatment for comorbidities at each clinic visit. Study nurses assessed participant-reported morbidities and measured weight at each monthly visit.
Statistical Analysis
We first described the population and characteristics of interest for the study population at ART initiation. Height and weight were used to calculate BMI in kg/m2, and standard cutoffs were used to define underweight (<18.5 kg/m2), normal (18.5-24.9 kg/m2), overweight (25.0-29.9 kg/m2), and obesity (≥30.0 kg/m2). First, generalized linear models were used to describe the relationship between sociodemographic and clinical characteristics with continuous BMI at ART initiation. To visualize longitudinal BMI assessed at monthly visits during the first year of ART, we also plotted estimates from linear mixed-effects models that used a random intercept, a compound symmetric covariance structure, and robust standard errors.
We then conducted the primary analysis to examine the association of sociodemographic and clinical characteristics with change in BMI from 1 year of ART as compared to ART initiation (calculated as BMI at 1 year minus BMI at ART initiation). Univariable and multivariable generalized linear models were used to evaluate the relationship between characteristics of interest with change in BMI over 1 year of ART. Predictors of BMI at ART initiation included sex (male or female), age (19-29.9, 30.0-39.9, 40.0-49.9, or 50+ years), education (no formal education, primary, or secondary/advanced), wealth quintile, self-reported current smoking status (yes or no), self-reported current alcohol drinking status (yes or no), CD4T-cell count at ART initiation (<200, 200-349, 350-499, and ≥500 cells/mL), WHO HIV disease stage (I/II, III, or IV), and pulmonary TB status (Yes or No). Analyses of change in BMI evaluated the same characteristics with the addition of baseline BMI categories as a predictor. We also evaluated the relationship of these characteristics with incident overweight or obesity (BMI ≥25.0 kg/m2) among individuals with a normal or underweight BMI at ART initiation (BMI <25.0 kg/m2) with log-binomial to produce relative risk estimates. All multivariable models also included a covariate for randomized trial regimen (vitamin D or placebo). The missing indicator method was used to retain data for those missing covariates in multivariable models. All P values were 2-sided, and P < .05 was considered statistically significant. Statistical analyses were conducted with SAS version 9.3.
Ethical Approval and Informed Consent
The study was approved by the Tanzania's National Health Research Ethics Committee (reference: HQ/R.8a/Vol. IX/1658), the Tanzania Medicines and Medical Devices Authority (reference: 13/CTR/0005/3), and the Harvard TH Chan School of Public Health's Institutional Review Board (reference: IRB13-0231). Written informed consent was obtained from all participants.
Results
A total of 4000 participants were enrolled in the trial and were included in the analysis. Characteristics of the study population at ART initiation are included in Table 1. Briefly, most study participants were female (68.4%), and a total of 181 (4.6%) participants reported current smoking, and 497 (12.7%) reported current alcohol drinking. The majority of participants had a CD4 T-cell count of less than 200 cells/mL (42.8%) and had WHO HIV stage III disease (57.6%). The mean ± standard deviation of BMI at ART initiation was 22.6 ± 5.1 kg/m2 and 21.1% of participants were classified as underweight, 52.6% had a normal BMI, 16.9% were overweight, and 9.4% were obese.
Table 1.
Baseline characteristics of the cohort of adult men and nonpregnant women living with HIV at ART initiation (n = 4000).
| Characteristic | n (%) |
|---|---|
| Sex | |
| Female | 2735 (68.4%) |
| Male | 1265 (31.6%) |
| Age, years | |
| 18-29.9 | 756 (18.9%) |
| 30-39.9 | 1550 (38.8%) |
| 40-49.9 | 1187 (29.7%) |
| 50+ | 507 (12.7%) |
| Education | |
| No formal education | 635 (15.9%) |
| Primary | 2588 (64.7%) |
| Secondary/ advanced | 775 (19.4%) |
| BMI, kg/m2 | |
| Underweight <18.5 | 844 (21.1%) |
| Normal 18.5-24.9 | 2102 (52.6%) |
| Overweight 25.0-29.9 | 677 (16.9%) |
| Obese ≥ 30.0 | 375 (9.4%) |
| Current smoker | 184 (4.6%) |
| Current alcohol drinker | 497 (12.4%) |
| CD4 T-cell count at ART initiation, cells per μL | |
| <200 | 1711 (42.8%) |
| 200-349 | 906 (22.7%) |
| 350-499 | 633 (15.8%) |
| ≥500 | 562 (14.1%) |
| Missing | 188 (4.7%) |
| WHO HIV disease stage at ART initiation | |
| I / II | 1504 (37.6%) |
| III | 2304 (57.6%) |
| IV | 192 (4.8%) |
| Pulmonary tuberculosis co-infection at ART initiation | 364 (9.1%) |
| ART regimen | |
| Efavirenz/lamivudine/tenofovir | 3883 (97.1%) |
| Other ART regimen | 117 (2.9%) |
| Randomized regimen | |
| Vitamin D | 2001 (50.0%) |
| Placebo | 1999 (50.0%) |
The relationship of sociodemographic and clinical characteristics with BMI at ART initiation is presented in Supplemental Table 1. In multivariable analysis, females had 2.2 kg/m2 (95% CI: 1.9-2.6) greater BMI at ART initiation as compared to males. Older age, greater education, greater wealth, and alcohol drinking were independently associated with higher BMI at ART initiation, while current smoking, lower CD4T-cell count, advanced WHO HIV disease stage, and PTB coinfection were associated with a lower BMI (P < .05).
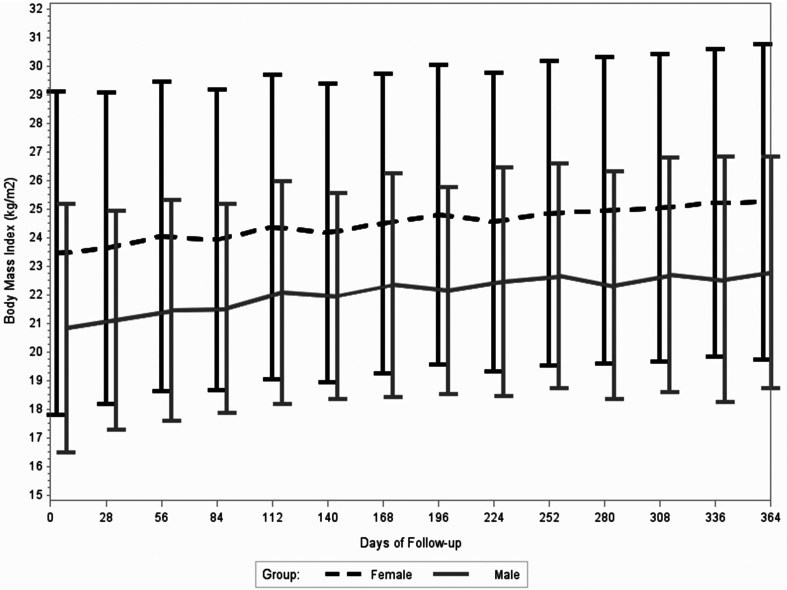
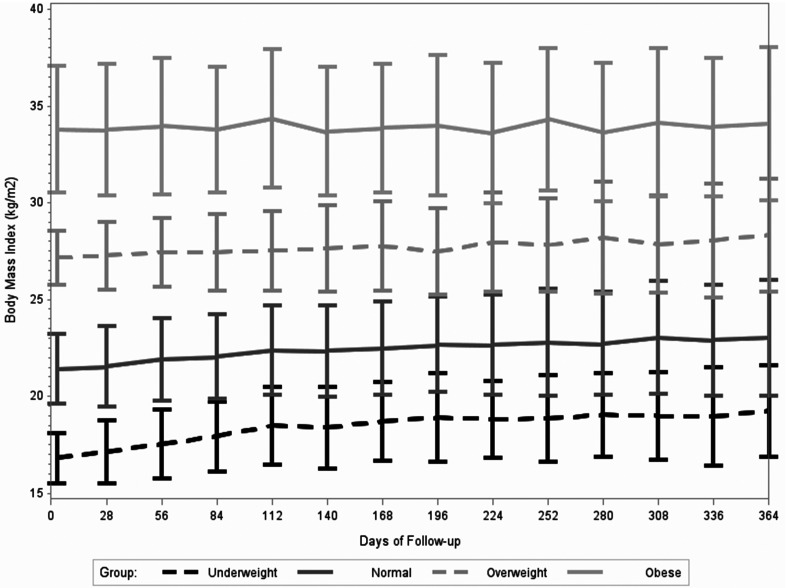
During the first year of ART, participants’ weight increased by a mean of 2.6 ± 0.3 kg, which corresponded to a mean increase in BMI of 1.5 ± 2.6 kg/m2. At one year of ART, 9.6% of participants were classified as underweight, 49.8% had a normal BMI, 25.7% were overweight, and 15.0% were obese. Figures 1 and 2 visualize the mean BMI at monthly visits stratified by sex and baseline BMI category, respectively. Both males and females tended to consistently gain weight during the first year of ART (Figure 1). In terms of BMI groups at ART initiation, underweight individuals had notable weight gain during the first months of treatment (Figure 2). The mean ± SD increase in BMI during the first year of treatment was 2.3 ± 2.3 kg/m2 for underweight participants, 1.6 ± 2.6 kg/m2 for participants with a normal BMI, 1.1 ± 2.8 kg/m2 for participants that were overweight, and 0.3 ± 2.4 kg/m2 for participants that were obese at ART initiation.
Figure 1.
Mean and standard deviation in body mass index (kg/m2) from ART initiation to one year of ART stratified by gender.
Figure 2.
Mean and standard deviation of body mass index (kg/m2) at monthly clinic visits from ART initiation to one year of ART stratified by baseline body mass index (underweight <18.5, normal 18.5-24.9, overweight 25.0-29.9, and obese ≥30.0 kg/m2).
Across all sociodemographic and clinical characteristics, mean BMI increased from ART initiation to one year of ART (Table 2). In univariable models, there was no association of sex with change in BMI. However, in multivariable models, which included adjustment for clinical characteristics, females had +0.29 kg/m2 (95% CI: +0.07, 0.50) greater increase in BMI over the first year of treatment as compared to males. In terms of sociodemographic characteristics, in multivariable models, participants over 50 years of age had a significantly lower change in BMI as compared to those aged 30 to 39 years (mean difference: −0.48 kg/m2; 95% CI: −0.76, −0.19) and participants with greater wealth had a greater increase in BMI (P value for trend in wealth quintiles: <.001). There was no difference in change in BMI by education status, smoking status, and alcohol drinking in multivariable models. In terms of clinical characteristics, lower CD4-T cell counts, advanced WHO HIV disease stage, and PTB infection were associated with greater increases in BMI. Participants with a baseline CD4T-cell count <200 cells/mL at ART initiation had a + 1.61 kg/m2 (95% CI: 1.33-1.88) greater increase in BMI as compared to participants with ≥500 cells/mL over the first year of ART. Participants with stage IV HIV disease had +1.01 kg/m2 (95% CI: 0.50-1.52) greater increase in BMI as compared to participants with stage I/II disease, while participants diagnosed with PTB coinfection had +0.74 kg/m2 (95% CI: 0.39-1.09) greater increase in BMI as compared to those without PTB.
Table 2.
Mean change in body mass index (BMI) in kg/m2 from initiation to 1 year of ART by baseline characteristics.
| Mean ± SD Change in BMI (kg/m2) from ART initiation to 1-year | Univariable Mean difference in change in BMI (kg/m2) (95% CI) | P value | Multivariable* mean difference in change in BMI (kg/m2) (95% CI) | P value | |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 1.46 ± 2.76 | −0.12 (−0.33, 0.09) | .27 | 0.29 (0.07, 0.50) | .009 |
| Male | 1.58 ± 2.21 | Ref. | Ref. | ||
| Age, years | |||||
| 18-29.9 years | 1.44 ± 2.62 | −0.08 (−0.37, 0.20) | .56 | −0.07 (−0.34, 0.20) | .61 |
| 30-39.9 years | 1.52 ± 2.54 | Ref. | Ref. | ||
| 40-49.9 years | 1.66 ± 2.70 | 0.14 (−0.09, 0.37) | .23 | 0.05 (−0.17, 0.26) | .67 |
| 50+ years | 1.08 ± 2.58 | −0.43 (−0.74, −0.13) | .005 | −0.48 (−0.76, −0.19) | .001 |
| Education | |||||
| No formal education | 1.58 ± 2.76 | 0.17 (−0.09, 0.44) | .20 | 0.14 (−0.11, 0.39) | .28 |
| Primary | 1.41 ± 2.55 | Ref. | Ref. | ||
| Secondary/ advanced | 1.71 ± 2.69 | 0.30 (0.05, 0.55) | .02 | 0.21 (−0.03, 0.45) | .09 |
| Wealth quintile | |||||
| Q1 – Poorest | 1.39 ± 2.60 | Ref. | Ref. | ||
| Q2 | 1.44 ± 2.47 | 0.05 (−0.26, 0.35) | 0.01 (−0.27, 0.29) | ||
| Q3 – Middle | 1.35 ± 2.55 | −0.04 (−0.35, 0.27) | 0.13 (−0.16, 0.42) | ||
| Q4 | 1.55 ± 2.67 | 0.16 (−0.14, 0.45) | 0.22 (−0.06, 0.50) | ||
| Q5 – Richest | 1.72 ± 2.74 | 0.32 (0.02, 0.62) | .03** | 0.46 (0.17, 0.75) | <.001** |
| Current smoker | |||||
| No | 1.51 ± 2.63 | Ref. | Ref. | ||
| Yes | 1.17 ± 2.21 | −0.34 (−0.80, 0.11) | .14 | −0.44 (−0.88, 0.01) | .05 |
| Current alcohol drinker | |||||
| No | 1.52 ± 2.65 | Ref. | Ref. | ||
| Yes | 1.28 ± 2.39 | −0.24 (0.53, 0.04) | .10 | −0.04 (−0.31, 0.23) | .14 |
| CD4T-cell count (cells per μL) | |||||
| <200 | 2.33 ± 2.72 | 1.86 (1.58, 2.14) | <.001 | 1.61 (1.33, 1.88) | <.001 |
| 200-349 | 1.15 ± 2.39 | 0.69 (0.39, 0.99) | <.001 | 0.71 (0.41, 1.00) | <.001 |
| 350-499 | 0.79 ± 2.29 | 0.33 (0.00, 0.66) | .05 | 0.35 (0.03, 0.67) | .03 |
| ≥500 | 0.46 ± 2.32 | Ref. | Ref. | ||
| WHO HIV disease stage | |||||
| I/II | 0.77 ± 2.30 | Ref. | Ref. | ||
| III | 1.96 ± 2.69 | 1.20 (1.00, 1.39) | <.001 | 0.89 (0.70, 1.09) | <.001 |
| IV | 2.31 ± 2.92 | 1.54 (1.02, 2.07) | <.001 | 1.01 (0.50, 1.52) | <.001 |
| Pulmonary tuberculosis co-infection | |||||
| No | 2.38 ± 2.58 | Ref. | Ref. | ||
| Yes | 2.88 ± 2.68 | 1.50 (1.14, 1.86) | <.001 | 0.74 (0.39, 1.09) | <.001 |
| BMI at ART initiation (kg/m2) | |||||
| Underweight <18.5 | 2.34 ± 2.33 | 0.74 (0.48, 0.99) | <.001 | 0.39 (0.14, 0.64) | .002 |
| Normal 18.5-24.9 | 1.60 ± 2.58 | Ref. | Ref. | ||
| Overweight 25.0-29.9 | 1.06 ± 2.77 | −0.54 (−0.79, −0.29) | <.001 | −0.23 (−0.48, 0.01) | .06 |
| Obese ≥30.0 | 0.32 ± 2.40 | −1.28 (−1.60, −0.97) | <.001 | −0.81 (−1.12, −0.50) | <.001 |
*Multivariable model included all variables in the table and randomized regimen (vitamin D or placebo).
**P value for trend.
Table 3 presents analyses of predictors of incident overweight/obesity (BMI > 25.0 kg/m2) among participants who had a normal or underweight BMI at ART initiation. The cumulative incidence of overweight/obesity in the cohort was 17.8%. Individuals who were underweight at ART initiation were much less likely to become overweight/obese as compared to those with a normal BMI at ART initiation (RR: 0.06; 95% CI: 0.03-0.10). In multivariable models, females had about twice the risk of incident overweight/obesity as compared to males (RR: 1.85; 95% CI: 1.49-2.27). Individuals with a CD4T-cell count <200 cells/mL had 1.40 times (95% CI: 1.05-1.88) the risk of incident overweight/obesity as compared to those that started ART with a CD4T-cell count >500 cells/mL.
Table 3.
Risk factors for incident overweight or obesity (BMI ≥25.0 kg/m2) among participants with a normal or underweight BMI at ART initiation.
| Percentage of participants with incident overweight or obesity n / N (%) | Univariable Relative Risk (95% CI) | P value | Multivariable* Relative Risk (95% CI) | P value | |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 395 / 1831 (21.6%) | 1.89 (1.56-2.27) | <.001 | 1.85 (1.49-2.27) | <.001 |
| Male | 128 / 1115 (11.5%) | Ref. | Ref. | ||
| Age, years | |||||
| 18-29.9 years | 95 / 595 (16.0%) | 0.90 (0.72-1.12) | .33 | 0.85 (0.66-1.09) | .38 |
| 30-39.9 years | 201 / 1128 (17.8%) | Ref. | Ref. | ||
| 40-49.9 years | 164 / 854 (19.2%) | 1.08 (0.89-1.30) | .43 | 1.19 (0.97-1.47) | .10 |
| 50+ years | 63 / 369 (17.1%) | 0.96 (0.74-1.24) | .74 | 1.14 (0.85-1.51) | .38 |
| Education | |||||
| No formal education | 86 / 486 (17.7%) | 1.01 (0.82-1.25) | .92 | 1.07 (0.84-1.36) | .58 |
| Primary | 334 / 1908 (17.5%) | Ref. | Ref. | ||
| Secondary/advanced | 103 / 550 (18.7%) | 1.07 (0.88-1.31) | .51 | 1.11 (0.88-1.39) | .38 |
| Wealth | |||||
| Q1 – Poorest | 108 / 642 (16.8%) | Ref. | Ref. | ||
| Q2 | 103 / 613 (16.8%) | 1.00 (0.78-1.28) | 1.04 (0.80-1.37) | ||
| Q3 – Middle | 95 / 542 (17.5%) | 1.04 (0.81-1.34) | 0.96 (0.73-1.26) | ||
| Q4 | 110 / 663 (17.4%) | 1.03 (0.81-1.32) | 0.94 (0.71-1.24) | ||
| Q5 – Richest | 107 / 516 (20.7%) | 1.23 (0.97-1.57) | .11** | 1.04 (0.80-1.37) | .11** |
| BMI at ART initiation (kg/m2) | |||||
| Underweight <18.5 | 12 / 844 (1.4%) | 0.06 (0.03-0.10) | <.001 | 0.06 (0.03-0.10) | <.001 |
| Normal 18.5-24.9 | 511 / 2102 (24.3%) | Ref. | Ref. | ||
| Current smoker | |||||
| No | 510 / 2788 (18.3%) | Ref. | Ref. | ||
| Yes | 13 / 158 (8.2%) | 0.45 (0.27-0.76) | .003 | 0.69 (0.39-1.22) | .20 |
| Current alcohol drinker | |||||
| No | 467 / 2631 (17.8%) | Ref. | Ref. | ||
| Yes | 56 / 259 (17.8%) | 1.00 (0.78-1.29) | .99 | 1.06 (0.80-1.41) | .69 |
| CD4T-cell count at ART initiation (cells per μL) | |||||
| <200 | 262 / 1399 (18.7%) | 1.20 (0.92-1.57) | .17 | 1.40 (1.05-1.88) | .02 |
| 200-349 | 121 / 644 (18.8%) | 1.21 (0.90-1.61) | .20 | 1.23 (0.89-1.69) | .21 |
| 350-499 | 68 / 399 (17.0%) | 1.10 (0.79-1.51) | .58 | 1.09 (0.76-1.55) | .65 |
| ≥500 | 56 / 360 (15.6%) | Ref. | Ref. | ||
| WHO HIV disease stage | |||||
| I/II | 179 / 904 (19.8%) | Ref. | Ref. | ||
| III | 321 / 1871 (17.2%) | 0.87 (0.73-1.02) | .09 | 0.98 (0.81-1.18) | .82 |
| IV | 23 / 171 (13.5%) | 0.68 (0.45-1.02) | .06 | 1.10 (0.70-1.71) | .69 |
| Pulmonary tuberculosis coinfection | |||||
| No | 472 / 2609 (18.1%) | Ref. | Ref. | ||
| Yes | 51 / 337 (15.1%) | 0.84 (0.64-1.09) | .19 | 1.23 (0.91-1.67) | .17 |
Discussion
In this prospective cohort study conducted in an urban setting in Tanzania, we found one in 4 adults living with HIV were either overweight or obese at the time of ART initiation. Female sex, older age, higher education, and greater household wealth were associated with higher BMI at ART initiation. The mean weight gain was 2.6 ± 0.3 kg during the first year of treatment and the overall prevalence of overweight/obesity in the cohort increased to 40.7% at 1 year. Sociodemographic risk factors for increased weight gain during the first year of ART included female sex and greater household wealth: while in terms of clinical factors, lower CD4-T-cell counts, advanced WHO HIV disease stage, and PTB infection were associated with greater weight gain. The cumulative incidence of overweight/obesity during the first year of treatment among PLHIV was 17.8% with greater risk for females and those with a CD4-T-cell count <200 cells/mL at ART initiation.
The high prevalence of PLHIV starting ART with overweight or obesity in our study is generally in line with other studies in SSA.12,23–25 In a study by Kintu et al conducted among PLHIV in Dar es Salaam between 2004 and 2014, ∼20% of adults that initiated ART were overweight or obese and the prevalence was found to increase over time during this period. 26 Our study was conducted during 2014 to 2018 in the same setting, and we found greater than 25% prevalence of overweight or obesity. Therefore, similar to the general population of adults in Tanzania, BMI is increasing over time among PLHIV. 27 Further, in a study in Tanzania in the rural Kilombero and Ulanga districts, 17% of PLHIV initiating ART were categorized as overweight and obese, 12 which is slightly lower than the prevalence in our cohort and highlights overweight/obesity being an issue in both urban and rural Tanzania. We found female sex, older individuals, and those with greater wealth initiated ART with a higher BMI and these same groups are at greater risk for overweight and obesity in the general population.28–30 This points to the need for interventions to address overweight/obesity in the general population to reduce the proportion of PLHIV initiating ART with a high BMI, particularly in the context of a test-and-treat program.
In line with other cohort studies in SSA and high-income settings, weight gain following ART initiation was common but differed by sociodemographic, clinical, and HIV-related factors.7,19,31 In our study, females had significantly higher BMI at ART initiation, experienced greater weight gain during the first year of treatment, and had almost twice the risk of incident overweight and obesity as compared to males. These results confirm findings from previous research in SSA and high-income settings.9,16,32–34 Several theories exist that explain the excessive weight gain among women in SSA. Firstly, women are more likely to be diagnosed with HIV at an early stage than men as they are routinely screened during pregnancy.35,36 On the other hand, men living with HIV in SSA may be more likely to be tested for HIV when they have advanced disease or have comorbidities.37,38 This is supported by our study since the sex difference in the change in BMI during the first year of ART was only seen with multivariable adjustment for clinical variables, since men had significantly lower CD4T-cell counts and higher WHO HIV disease stage at ART initiation which were independently associated with greater weight gain. Secondly, larger body size may be considered to be reflective of being “healthy” among females in SSA, especially women living with HIV.34,39 In a qualitative study among women on their perception of weight gain in Uganda, Alhasain and colleagues found that women living with HIV considered gaining a moderate to high amounts of weight as ideal, mostly to appeal to others that they are healthy. 40 As a result, these findings underscore the importance of interventions that take into account the cultural and HIV-related perceptions of weight to address overweight/obesity among women living with HIV in Tanzania.
We also found that adults that were underweight, had low CD4T-cell counts, and those with TB coinfection at the initiation of treatment had more pronounced increases in body weight during the first year of treatment. This finding echoes many previous reports and has been attributed to the “return to health” whereby the inflammation-induced metabolic demand in PLHIV is reversed with virological control following ART initiation. 7 In a study by Bourgi from Kenya, PLHIV with low CD4T-cell count, underweight, as well as those with TB coinfection had a higher risk of gaining >10% of their baseline body weight after ART initiation. 32 The association between TB coinfection and rebound of weight following ART initiation has been previously reported by others in SSA,32,41 and our findings add that this association is independent of factors such as gender, CD4T-cell count, WHO HIV stage, and baseline nutrition status in our population. These findings highlight the complexity of weight gain among PLHIV; some degree of weight gain should be expected for individuals that are underweight, severely immunocompromised, or have comorbidities at the start of ART. However, more research is needed to determine in these groups the amount of weight gain that places these individuals at higher risk for NCDs and other longer-term health outcomes.
Our results have several implications for monitoring and development of interventions to mitigate excess weight gain experienced by PLHIV on ART, especially during the initial months of ART initiation when weight gain is often greatest. Although data are still limited on the long-term effects of excess weight gain among PLHIV on ART, current evidence indicates excess weight will likely be a mediator to other cardiovascular disease risk factors including hypertension, type 2 diabetes, and dyslipidemia. In a context like Tanzania, where PLHIV were once characterized by HIV-associated wasting, education will be needed for both PLHIV and healthcare workers on the need to prevent and manage excess weight gain. Nevertheless, there remains a “double burden” of undernutrition and overnutrition among PLHIV and continued efforts to address food insecurity and patients that become underweight or do not maintain a healthy weight after ART initiation are also needed. Findings of this study will also serve as baseline measures for comparing incident overweight and obesity as well as change in weight gain over time for efavirenz-based regimens as compared with newer regimens including the dolutegravir-based ART regimens.
Our study has several limitations. First, these data were collected before the introduction of dolutegravir-based first-line ART in Tanzania, which is of particular concern for weight gain. Nevertheless, our findings give important information on other non-ART-related sociodemographic and clinical risk factors that are likely to remain associated with weight gain even with new ART regimens. Second, as this is an observational study there may be residual or unmeasured confounding that may have contributed to the differences in weight gain among the study subgroups. Third, our study was conducted in urban Dar es Salaam among adults that were initiating ART. As a result, our findings may not be directly generalizable to other populations within Tanzania, including rural populations, and more broadly in East Africa.
In conclusion, our study confirms that there is an urgent need to address high BMI at ART initiation and weight gain during the initial year of treatment among PLHIV in Dar es Salaam, Tanzania. Given the resources to address HIV and NCDs may be limited in settings like Tanzania, we have provided valuable insights into sociodemographic and clinical factors that might identify subpopulations that may benefit the most from interventions that promote healthy weight, including adult women, that may reduce the risk of NCDs. However, it also remains important to note that underweight remains an issue in this population, and some degree of weight gain may be indicative of a “return to health” for some patients. Overall, our study findings support the development and evaluation of culturally tailored interventions to promote healthy weight among PLHIV in hopes of reducing the risk of NCDs and ultimately enhancing the long-term survival benefits of ART programs in Tanzania.
Supplemental Material
Supplemental material, sj-docx-1-jia-10.1177_23259582241281010 for Sociodemographic and Clinical Predictors of Weight Gain During the First Year of Antiretroviral Therapy among Adults Living With HIV in Urban Tanzania by Pilly Chillo, Alfa Muhihi, Goodarz Danaei, Muhammad Bakari, Gideon Kwesigabo, Marina Njelekela, Nzovu Ulenga, Wafaie W. Fawzi, Ferdinand Mugusi and Christopher R. Sudfeld in Journal of the International Association of Providers of AIDS Care (JIAPAC)
Footnotes
Data Availability Statement: The datasets generated during and/or analyzed during the current study are not publicly available but may be made available from the corresponding author on reasonable request and approval of relevant regulatory bodies.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The parent trial was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R01DK098075; the current study was partially supported by the Fogarty International Center of the National Institutes of Health under Award Number D43TW009775. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ORCID iD: Pilly Chillo https://orcid.org/0000-0001-7073-1122
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Egede LE, Walker RJ, Monroe P, Williams JS, Campbell JA, Dawson AZ. HIV and cardiovascular disease in sub-Saharan Africa: demographic and health survey data for 4 countries. BMC Public Health. 2021;21(1):1122. doi: 10.1186/s12889-021-11218-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah ASV, Stelzle D, Lee KK, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV: systematic review and meta-analysis. Circulation. 2018;138(11):1100–1112. doi: 10.1161/CIRCULATIONAHA.117.033369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh GM, Danaei G, Farzadfar F, et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS One. 2013;8(7):e65174. doi: 10.1371/journal.pone.0065174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emerging Risk Factors C, Wormser D, Kaptoge S, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377(9771):1085–1095. doi: 10.1016/S0140-6736(11)60105-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collaboration NCDRF. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–2642. doi: 10.1016/S0140-6736(17)32129-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakal DR, Coelho LE, Luz PM, et al. Obesity following ART initiation is common and influenced by both traditional and HIV-/ART-specific risk factors. J Antimicrob Chemother. 2018;73(8):2177–2185. doi: 10.1093/jac/dky145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandiwana NC, Siedner MJ, Marconi VC, et al. Weight gain after HIV therapy initiation: pathophysiology and implications. J Clin Endocrinol Metab. 2024;109(2):e478–e487. doi: 10.1210/clinem/dgad411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kagaruki GB, Mayige MT, Ngadaya ES, et al. Magnitude and risk factors of non-communicable diseases among people living with HIV in Tanzania: a cross sectional study from mbeya and Dar es Salaam regions. BMC Public Health. 2014;14:904. doi: 10.1186/1471-2458-14-904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ottaru TA, Kwesigabo GP, Butt Z, et al. Ideal cardiovascular health: distribution, determinants and relationship with health status among people living with HIV in urban Tanzania. Glob Heart. 2022;17(1):74. doi: 10.5334/gh.1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enriquez R, Ssekubugu R, Ndyanabo A, et al. Prevalence of cardiovascular risk factors by HIV status in a population-based cohort in south central Uganda: a cross-sectional survey. J Int AIDS Soc. 2022;25(4):e25901. doi: 10.1002/jia2.25901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Njelekela M, Muhihi A, Aveika A, et al. Prevalence of hypertension and its associated risk factors among 34,111 HAART naive HIV-infected adults in Dar es Salaam, Tanzania. Int J Hypertens. 2016;2016:5958382. doi: 10.1155/2016/5958382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalinjuma AV, Hussey H, Mollel GJ, et al. Body mass index trends and its impact of under and overweight on outcome among PLHIV on antiretroviral treatment in rural Tanzania: a prospective cohort study. PLoS One. 2023;18(8):e0290445. doi: 10.1371/journal.pone.0290445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez-Arboli E, Mwamelo K, Kalinjuma AV, et al. Incidence and risk factors for hypertension among HIV patients in rural Tanzania—A prospective cohort study. PLoS One. 2017;12(3):e0172089. doi: 10.1371/journal.pone.0172089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor BS, Liang Y, Garduno LS, et al. High risk of obesity and weight gain for HIV-infected uninsured minorities. J Acquir Immune Defic Syndr. 2014;65(2):e33–e40. doi: 10.1097/QAI.0000000000000010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taramasso L, Ricci E, Menzaghi B, et al. Weight gain: a possible Side effect of all antiretrovirals. Open Forum Infect Dis. 2017;4(4):ofx239. doi: 10.1093/ofid/ofx239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bares SH, Smeaton LM, Xu A, Godfrey C, McComsey GA. HIV-infected women gain more weight than HIV-infected men following the initiation of antiretroviral therapy. J Womens Health (Larchmt). 2018;27(9):1162–1169. doi: 10.1089/jwh.2017.6717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diggins CE, Russo SC, Lo J. Metabolic consequences of antiretroviral therapy. Curr HIV/AIDS Rep. 2022;19(2):141–153. doi: 10.1007/s11904-022-00600-6 [DOI] [PubMed] [Google Scholar]
- 18.Hasse B, Iff M, Ledergerber B, et al. Obesity trends and body mass index changes after starting antiretroviral treatment: the Swiss HIV cohort study. Open Forum Infect Dis. 2014;1(2):ofu040. doi: 10.1093/ofid/ofu040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis. 2020;71(6):1379–1389. doi: 10.1093/cid/ciz999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sudfeld CR, Mugusi F, Muhihi A, et al. Efficacy of vitamin D3 supplementation for the prevention of pulmonary tuberculosis and mortality in HIV: a randomised, double-blind, placebo-controlled trial. Lancet HIV. 2020;7(7):e463–e471. doi: 10.1016/S2352-3018(20)30108-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sudfeld CR, Mugusi F, Aboud S, Nagu TJ, Wang M, Fawzi WW. Efficacy of vitamin D3 supplementation in reducing incidence of pulmonary tuberculosis and mortality among HIV-infected Tanzanian adults initiating antiretroviral therapy: study protocol for a randomized controlled trial. Trials. 2017;18(1):66. doi: 10.1186/s13063-017-1819-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muhihi A, Fawzi WW, Aboud S, et al. Cholecalciferol supplementation does not affect the risk of HIV progression, viral suppression, comorbidities, weight loss, and depression among Tanzanian adults initiating antiretroviral therapy: secondary outcomes of randomized trial. J Nutr. 2022;152(8):1983–1990. doi: 10.1093/jn/nxac096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Achwoka D, Mutave R, Oyugi JO, Achia T. Tackling an emerging epidemic: the burden of non-communicable diseases among people living with HIV/AIDS in sub-Saharan Africa. Pan Afr Med J. 2020;36:271. doi: 10.11604/pamj.2020.36.271.22810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musinguzi N, Stanford FC, Boatin AA, et al. Association between obesity and combination antiretroviral therapy (cART) adherence among persons with early-stage HIV infection initiating cART. Int J Obes (Lond). 2021;45(8):1855–1859. doi: 10.1038/s41366-021-00837-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adeboye B, Bermano G, Rolland C. Obesity and its health impact in Africa: a systematic review. Cardiovasc J Afr. 2012;23(9):512–521. doi: 10.5830/CVJA-2012-040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kintu A, Liu E, Hertzmark E, et al. Incidence and risk factors for overweight and obesity after initiation of antiretroviral therapy in Dar es Salaam, Tanzania. J Int Assoc Provid AIDS Care. 2018;17:2325958218759759. doi: 10.1177/2325958218759759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chong B, Jayabaskaran J, Kong G, et al. Trends and predictions of malnutrition and obesity in 204 countries and territories: an analysis of the global burden of disease study 2019. EClinicalMedicine. 2023;57:101850. doi: 10.1016/j.eclinm.2023.101850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muhihi AJ, Njelekela MA, Mpembeni R, Mwiru RS, Mligiliche N, Mtabaji J. Obesity, overweight, and perceptions about body weight among middle-aged adults in Dar es Salaam, Tanzania. ISRN Obes. 2012;2012:368520. doi: 10.5402/2012/368520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keding GB, Msuya JM, Maass BL, Krawinkel MB. Obesity as a public health problem among adult women in rural Tanzania. Glob Health Sci Pract. 2013;1(3):359–371. doi: 10.9745/GHSP-D-13-00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pallangyo P, Mkojera ZS, Hemed NR, et al. Obesity epidemic in urban Tanzania: a public health calamity in an already overwhelmed and fragmented health system. BMC Endocr Disord. 2020;20(1):147. doi: 10.1186/s12902-020-00631-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alebel A, Demant D, Petrucka PM, Sibbritt D. Weight change after antiretroviral therapy initiation among adults living with HIV in northwest Ethiopia: a longitudinal data analysis. BMJ Open. 2022;12(2):e055266. doi: 10.1136/bmjopen-2021-055266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bourgi K, Ofner S, Musick B, et al. Weight gain among treatment-naive persons with HIV receiving dolutegravir in Kenya. J Acquir Immune Defic Syndr. 2022;91(5):490–496. doi: 10.1097/QAI.0000000000003087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brennan AT, Berry KM, Rosen S, et al. Growth curve modelling to determine distinct BMI trajectory groups in HIV-positive adults on antiretroviral therapy in South Africa. AIDS. 2019;33(13):2049–2059. doi: 10.1097/qad.0000000000002302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCormick CL, Francis AM, Iliffe K, et al. Increasing obesity in treated female HIV patients from sub-Saharan Africa: potential causes and possible targets for intervention. Front Immunol. 2014;5:507. doi: 10.3389/fimmu.2014.00507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drammeh B, Medley A, Dale H, et al. Sex differences in HIV testing—20 PEPFAR-supported sub-Saharan African countries, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(48):1801–1806. doi: 10.15585/mmwr.mm6948a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beia T, Kielmann K, Diaconu K. Changing men or changing health systems? A scoping review of interventions, services and programmes targeting men's health in sub-Saharan Africa. Int J Equity Health. 2021;20(1):87. doi: 10.1186/s12939-021-01428-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hlongwa M, Mashamba-Thompson T, Makhunga S, Hlongwana K. Barriers to HIV testing uptake among men in sub-Saharan Africa: a scoping review. Afr J AIDS Res. 2020;19(1):13–23. doi: 10.2989/16085906.2020.1725071 [DOI] [PubMed] [Google Scholar]
- 38.UNAIDS. Blind Spot: Reaching Out to Men and Boys. Addressing a Blind Spot in the Response to HIV. UNAIDS Geneva; 2017. [Google Scholar]
- 39.Puoane T, Tsolekile L, Steyn N. Perceptions about body image and sizes among black African girls living in Cape Town. Ethn Dis. 2010;20(1):29–34. [PubMed] [Google Scholar]
- 40.Alhassan Y, Twimukye A, Malaba T, et al. “It's only fatness, it doesn't kill”: a qualitative study on perceptions of weight gain from use of dolutegravir-based regimens in women living with HIV in Uganda. BMC Womens Health. 2022;22(1):246. doi: 10.1186/s12905-022-01814-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munseri P, Jassely L, Tumaini B, Hertzmark E. Body mass index, proteinuria and total lymphocyte counts in predicting treatment responses among ART naïve individuals with HIV initiated on antiretroviral treatment in Dar es Salaam, Tanzania, 2019: a cohort study. BMJ Open. 2022;12(6):e059193. doi: 10.1136/bmjopen-2021-059193 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jia-10.1177_23259582241281010 for Sociodemographic and Clinical Predictors of Weight Gain During the First Year of Antiretroviral Therapy among Adults Living With HIV in Urban Tanzania by Pilly Chillo, Alfa Muhihi, Goodarz Danaei, Muhammad Bakari, Gideon Kwesigabo, Marina Njelekela, Nzovu Ulenga, Wafaie W. Fawzi, Ferdinand Mugusi and Christopher R. Sudfeld in Journal of the International Association of Providers of AIDS Care (JIAPAC)