Abstract
Background
Antipsychotic drugs may have adverse effects on the components of metabolic syndrome. Previous studies have shown that changes in the intestinal microbiome are associated with metabolic disturbances in patients with schizophrenia. The objective of this study was to determine the effects of synbiotics on the components of metabolic syndrome as primary outcomes in patients with schizophrenia. Secondary outcomes were HbA1c, insulin resistance, LDL-c, and anthropometric measurements.
Methods
In this double-blind, placebo-controlled trial, seventy patients with schizophrenia receiving antipsychotic drugs who had at least two criteria of metabolic syndrome were randomly divided into two groups to receive either two capsules of a synbiotic supplement or a placebo daily for 8 weeks. Anthropometric indices and biochemical parameters were measured at baseline and after the intervention.
Results
Fifty-five patients completed the study. The synbiotic supplement significantly decreased waist circumference and HbA1C compared to placebo (-2.66 ± 4.20 vs. 3.03 ± 4.50 and − 0.26 ± 0.54 vs. 0.20 ± 0.75, respectively). Although BMI did not change significantly in the synbiotic + antipsychotic group, it increased in the placebo + antipsychotic group (-0.37 ± 1.00 vs. 0.61 ± 1.09 P < 0.5). LDL-c and triglyceride (TG) levels decreased significantly in the synbiotic + antipsychotic group, but the change was not significantly different from that of the placebo + antipsychotic group. FBS, HDL-c, systolic and diastolic blood pressure, insulin resistance, and total cholesterol were not significantly different between the two groups after intervention.
Conclusion
Synbiotic supplement may decrease waist circumference, HbA1c, LDL and TG and prevent BMI increase in patients receiving antipsychotic drugs.
Trial registration
Iranian Registry of Clinical Trials (IRCT Number: IRCT20090901002394N45), Date: 26-12-2019.
Keywords: Antipsychotic drugs, Hypertriglyceridemia, Intestinal microbiome, Lactobacillus, Waist circumference
Introduction
Metabolic syndrome (MS) characterized by central obesity, hypertriglyceridemia, low levels of high-density lipoprotein (HDL) cholesterol, hyperglycemia and increased blood pressure is very common in patients with schizophrenia [1]. The risk of metabolic syndrome increases with the duration of the disease [2]. Antipsychotic drugs used for the management of clinical symptoms of schizophrenia may increase the risk of metabolic syndrome [2–4]. In addition, even without the consumption of antipsychotic drugs, glucose homeostasis [5], waist circumference and visceral fat are affected in these patients [6]. Although lifestyle modification to reduce body weight is the primary approach to this syndrome, in some patients treatment options need to extend beyond lifestyle modification [7]. It has been shown that metformin is effective in treating antipsychotic-induced weight gain in patients with schizophrenia [8], but it may induce gastrointestinal complications, pancreatitis, hepatitis, vitamin B12 and coagulation abnormalities and reactive hypoglycemia [9].
According to gut microbiota-brain axis, a bidirectional communication exists between central nervous system and gut microbiota [10]. A systematic review suggests that changes in the intestinal microbiome are associated with inflammation, oxidative stress and metabolic disturbances in patients with schizophrenia [11].Probiotics are “live microorganisms that, when administered in sufficient amounts, confer a health benefit on the host” [12], whereas a prebiotic is “a substrate that is selectively utilized by host microorganisms conferring a health benefit” [13]. “Synbiotics are a synergistic combination of both probiotics and prebiotics in the same food or supplement.” [14].
Previous studies have suggested that the consumption of probiotics or synbiotics could improve cardiometabolic outcomes by reducing inflammation and oxidative stress [15, 16]. Moreover, systematic reviews have shown that pre/pro/synbiotics may have favorable effects on the lipid profile, fasting blood sugar, blood pressure, insulin sensitivity, waist circumference, body weight and HbA1c in adults including people with obesity, as well as patients with non-alcoholic fatty liver disease or type 2 diabetes [17–23]. In addition, in two previous studies, probiotics or fiber supplement or their combination had favorable effects on weight and some other cardiometabolic parameters in patients with schizophrenia or bipolar mood disorder [24, 25]. In contrast, in another trial in drug-naïve patients with episode schizophrenia, no significant differences in weight gain were observed between probiotic + olanzapine and olanzapine group [25].
As probiotic bacteria survival during the passage through the upper intestinal tract and implantation in the colon is better in synbiotic preparations [26], we hypothesized that synbiotic supplements may control metabolic syndrome in patients with schizophrenia. The strains and dose of probiotics were based on a previous study which had reported favorable effects of probiotic on insulin resistance [27]. To our knowledge, no previous study has investigated the effect of /synbiotics on the components of metabolic syndrome in patients with schizophrenia. Thus, the present study was conducted to determine the effects of synbiotic supplements on the components of metabolic syndrome in patients with schizophrenia.
Methods
Patients
In this double-blinded placebo-controlled trial, seventy patients with schizophrenia were enrolled from March 2020 to August 2021. Subjects were recruited from the Iran Psychiatric Hospital Clinic, Iran University of Medical Science, Tehran, Iran. Inclusion criteria were as follows; age between 18 and 65 years, having schizophrenia based on DSM-V criteria for at least one year and taking antipsychotic drugs, having a BMI ≥ 23 and at least two criteria of metabolic syndrome. The diagnostic criteria of metabolic syndrome were based on ATP III [28], but he cut-off point for waist circumference was 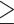 90 cm based on national data [29]. Non-inclusion criteria were having systematic diseases, such as endocrine, renal and gastrointestinal disorders and immune deficiency, a history of weight loss surgery, being pregnant or lactating, change in dose or type of medication for at least two months before enrollment, taking pre/pro/synbiotic supplements, antihypertensive, lipid-lowering, or weight-loss drugs, corticosteroids, metformin, or antibiotics one month before enrollment in the study. Exclusion criteria were the occurrence of any serious adverse event that would seem to be related to the study, the acquisition of any of the criteria for non-inclusion, or hospitalization of the patient during the study.
90 cm based on national data [29]. Non-inclusion criteria were having systematic diseases, such as endocrine, renal and gastrointestinal disorders and immune deficiency, a history of weight loss surgery, being pregnant or lactating, change in dose or type of medication for at least two months before enrollment, taking pre/pro/synbiotic supplements, antihypertensive, lipid-lowering, or weight-loss drugs, corticosteroids, metformin, or antibiotics one month before enrollment in the study. Exclusion criteria were the occurrence of any serious adverse event that would seem to be related to the study, the acquisition of any of the criteria for non-inclusion, or hospitalization of the patient during the study.
Study design
The protocol of the study was described for eligible patients and written informed consent was signed by the patient or his/her legal guardian in case he/she was unable to comprehend research consent form. The patients were randomly divided into two groups to receive either two capsules of a synbiotic supplement or a placebo daily for eight weeks. Each synbiotic capsule contained 109 CFU L. rhamnosus, L.casei, L. acidophilus, L. bulgaricus, L. plantarum, L. gasseri, L. helveticus, B. lactis, B. breve, B. longum, B. bifidum, S. thermophilus, and 21 mg fructooligosaccharide. The placebo capsule contained maltodextrin and was identical in appearance to the supplements. Synbiotic capsules and placebos, having been purchased from Zist Takhmir, Tehran, Iran, were taken after lunch and dinner by the patients. Participants were asked to keep the supplements in the refrigerator door (2–8 ° C). The researcher communicated with participants and their families by telephone during the study to ensure compliance.
Dietary intake and physical activity, as potential confounders, were assessed by 24-hour dietary recall and International Physical Activity Questionnaires [30], respectively. In addition, anthropometric indices and biochemical parameters were measured at baseline and after the intervention.
The study protocol was approved by the Ethics Committee, Iran University of Medical Sciences and registered at Iranian Registry of Clinical Trials (IRCT Number: IRCT20090901002394N45), Date: 26-12-2019. Our manuscript adheres to CONSORT guidelines for reporting clinical trials.
Anthropometric measurements
The weight, height, waist circumference (WC) and blood pressure of the subjects were measured and BMI was calculated at baseline and the end of the intervention. Height was measured barefoot to the nearest 0.5 cm using a tape measure attached to the wall. Weight was measured with a Seca digital scale with a precision of 100 g. Additionally, waist circumference was measured to the nearest 0.5 cm approximately between the lower margin of the last rib and the top of the iliac crest at the level of the navel with a tape measure. BMI was calculated by dividing weight (kg) by the square of height (m2). Blood pressure was measured twice with a 10-minute interval by a vital sign monitor while the patient was in a sitting position after a 15-minute rest.
Measurement of biochemical parameters
Blood samples were taken after a 12-18-hour fasting period. Serum hemoglobin A1c (HbA1c) was measured in whole blood using an automated high-performance liquid chromatography analyzer with commercially available kits (Bio-Rad D-10 Laboratories, Schiltigheim, France).
Serum samples were frozen and stored at − 80 °C for later TG, TC, HDL-c, and insulin assays. All biochemical assessments were performed in the same laboratory using standard methods. Serum triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c) and fasting blood sugar (FBS) concentrations were measured by the photometric enzymatic method using Pars Azmoun kits, Tehran, Iran). Insulin levels were measured using an ELISA kit (Monobind, California, USA) and insulin resistance (HOMA-IR) and the quantitative insulin sensitivity check index (QUICKI) were calculated according to suggested formulas [31]. Low-density lipoprotein cholesterol (LDL-C) concentration was calculated using the Fried–Wald formula [32].
Statistical analysis
Kolmogorov–Smirnov test was used to test normal distribution of variables. For skewed variables, normally transformed data were used, but if they could not be transformed to normal distribution, non-parametric analysis was performed. Two-way analysis of variance with repeated measures (time as within subject and group as between subject factor) was used to compare outcomes before and after 8 weeks of treatment across treatment groups. Furthermore, analysis of covariance adjusted for baseline values and potential confounders was performed to compare outcome variables after intervention between groups and paired T test was used to compare outcomes within groups. The tests were two-sided and P-value less than 0.05 was considered significant.
Sample size
Based on each of the five components of metabolic syndrome, the sample size was calculated. The largest sample size was for TG. Given a power = 80% and alpha = 5%, the sample size was calculated to be at least twenty-seven patients in each group according to Shakeri’s study [33].
Results
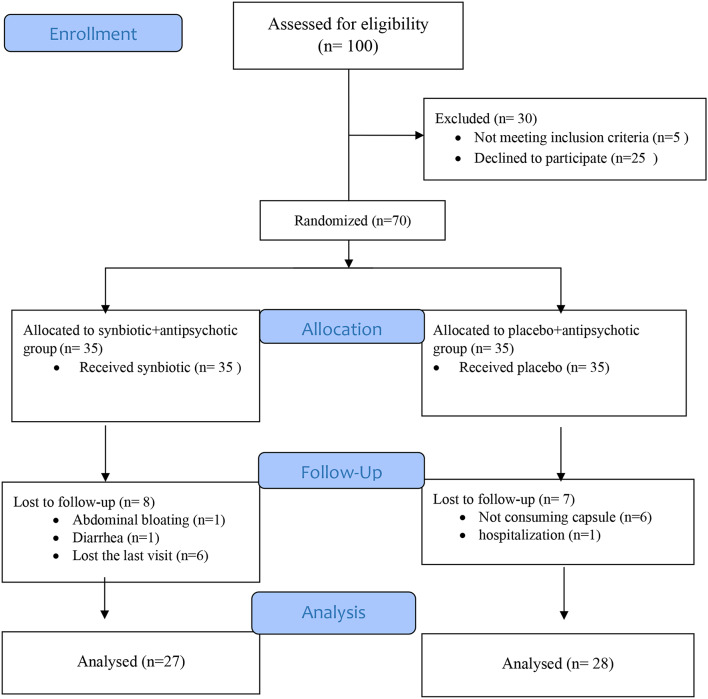
As Fig. 1 shows, eight patients in the synbiotic + antipsychotic group and seven patients in the placebo + antipsychotic group were lost to follow-up. More than 95% of the capsules were consumed throughout the study in both groups. Regarding baseline characteristics, there was no statistically significant difference between the two groups except for smoking status and the level of education (Table 1). At baseline, there were no significant differences regarding the intake of energy and nutrients and the level of physical activity between the two groups, but energy and carbohydrate intakes were significantly different between the two groups at the end of the intervention (Table 2). As there was a correlation between the intake of energy and carbohydrates, only energy intake, in addition to smoking status and the level of education was adjusted in the analysis.
Fig. 1.
Flow diagram of the study
Table 1.
Baseline characteristics of patients in the two groups
| Characteristics | Synbiotic + antipsychotic group(n = 27) | placebo + antipsychotic group (n = 28) | P a | |
|---|---|---|---|---|
| Age (years) | 42.19 ± 11.45 | 44.54 ± 9.47 | 0.410 | |
| Gender | Men (%) | 14 (51.58) | 19 (67.85) | 0.226 |
| Women (%) | 13(48.14) | 9 (32.14) | ||
|
Marital status |
Single (%) | 11 (40.7) | 13 (46.42) | 0.671 |
| Married (%) | 16 (59.3) | 15 (53.57) | ||
| Family history | Yes (%) | 9 (33.33) | 12 (42.85) | 0.467 |
| No (%) | 18 (66.66) | 16 (57.14) | ||
| Smoking | Yes (%) | 4 (14.81) | 11 (39.28) | 0.042 |
| No (%) | 23 (85.18) | 17 (60.17) | ||
| Education | Less than a diploma | 17(62/96) | 25(89.28) | 0.022 |
| Diploma and higher | 10(37.04) | 3(10.71) | ||
| Medications | Typical Antipsychotic (%) | 26(96.3) | 23(82.1) | 0.611 |
| Atypical antipsychotic (%) | 1(3.7) | 5(17.9) | ||
Data are reported as average (standard deviation) for age and as number (percentage) for other variables
Pa: Obtained from Independent Sample t-Test for age and chi-square for other variables
Table 2.
Dietary intake in the two groups
| Factor | Synbiotic + antipsychotic group (n = 27) | placebo + antipsychotic group(n = 28) | P |
|---|---|---|---|
| Energy (Kcal/d) | |||
| Week 0 | 1941.48 ± 642.81 | 1930.96 ± 539.10 | 0.948a |
| Week 8 | 1716.50 ± 600.73 | 2113.01 ± 828.71 | 0.043b |
| Pb | 0.115 | 0.301 | |
| Protein (gr/d) | |||
| Week 0 | 63.27 ± 27.56 | 66.79 ± 25.51 | 0.624 a |
| Week 8 | 69.65 ± 26.97 | 73.17 ± 28.95 | 0.762 b |
| Pb | 0.291 | 0.280 | |
| Carbohydrate (gr/d) | |||
| Week 0 | 308.82 ± 118.20 | 270.26 ± 100.05 | 0.197 a |
| Week 8 | 250.83 ± 94.87 | 332.08 ± 147.47 | 0.007 b |
| Pb | 0.026 | 0.045 | |
| Total fat (gr/d) | |||
| Week 0 | 49.50 ± 24.31 | 56.49 ± 24.82 | 0.066 a |
| Week 8 | 52.55 ± 23.61 | 67.71 ± 31.33 | 0.292 b |
| Pb | 0.625 | 0.180 | |
| Saturated fat (gr/d) | |||
| Week 0 | 12.37 ± 7.54 | 16.93 ± 10.70 | 0.074 a |
| Week 8 | 13.83 ± 7.94 | 16.00 ± 7.61 | 0.267 b |
| Pb | 0.479 | 0.918 | |
| Fiber (gr/d) | |||
| Week 0 | 11.74 ± 6.36 | 15.85 ± 9.11 | 0.674 a |
| Week 8 | 14.23 ± 8.70 | 15.62 ± 10.72 | 0.086 b |
| Pb | 0.293 | 0.868 | |
| EPA(gr/d) | |||
| Week 0 | 0(0–0) | 0(0–0) | 0.151 a |
| Week 8 | 0(0–0) | 0(0–0) | 0.146 b |
| Pb | 0.276 | 0.317 | |
| DHA(gr/d) | |||
| Week 0 | 0.004(0.001 - 0.02) | 0.004(0.001–0.01) | 1.000 a |
| Week 8 | 0.004(0 - 0.01) | 0.003(0–0.007) | 0.317 b |
| Pb | 0.682 | 0.328 | |
| Vitamin B12(µg /d) | |||
| Week 0 | 1.53(0.68 - 2.52) | 1.59(1.03 - 2.34) | 0.729 a |
| Week 8 | 1.72(1.02 - 3.00) | 2.18(1.21 - 3.63) | 0.602 b |
| Pb | 0.970 | 0.295 | |
| Vitamin B9(mg/d) | |||
| Week 0 | 262.67 ± 358.65 | 272.23 ± 264.54 | 0.589 a |
| Week 8 | 199.35 ± 106.86 | 223.14 ± 163.18 | 0.525 b |
| Pb | 0.402 | 0.417 | |
| Vitamin C (mg/d) | |||
| Week 0 | 54.75(18.64 - 104.40) | 52.24(21.39 - 147.40) | 0.506 a |
| Week 8 | 69.70(19.66 - 109.60) | 79.11(15.55 - 119.35) | 0.920 b |
| Pb | 0.564 | 0.820 | |
| Zinc(mg/d) | |||
| Week 0 | 5.69 ± 3.62 | 7.06 ± 3.75 | 0.075 a |
| Week 8 | 6.83 ± 3.70 | 7.28 ± 4.07 | 0.801 b |
| Pb | 0.325 | 0.946 |
Data are reported as mean ± SD for normally distributed variables and median(interquartile range) for other variables
MUFA: monounsaturated fatty acid; PUFA: polyunsaturated fatty acid; SAFA: saturated fatty acid
a: Obtained from the independent t-test for normally distributed variables /Mann-Whitney test for other variables
bAnalysis of covariance adjusted for baseline values for normally distributed variables/ Mann-Whitney test for changes in dietary intake of non-normally distributed variables
Pb: Obtained from the paired sample t-test for normally distributed variables and Wilcoxon test for other variables
As shown in Tables 3, 4 and 5, synbiotic supplements significantly decreased waist circumference and HbA1c compared to placebo (-2.66 ± 4.20 vs. 3.03 ± 4.50 and − 0.26 ± 0.54 vs. 0.20 ± 0.75, respectively). Although BMI did not change significantly in the synbiotic + antipsychotic group, it increased in the placebo + antipsychotic group (-0.37 ± 1.00 vs. 0.61 ± 1.09, between-group P value = 0.01). LDL and TG decreased significantly in the synbiotic + antipsychotic group, but the change was not significantly different from that of the placebo + antipsychotic group. FBS, HDL-c, diastolic blood pressure (DBP), insulin, HOMA-IR, QUIKI, and TC did not change significantly after the intervention (p > 0.05). SBP significantly decreased in both groups, but the change was not significantly different between the two groups.
Table 3.
Anthropometric measurement in the two groups before and after 8 weeks
| Factor | Synbiotic + antipsychotic group (n = 27) |
Placebo + antipsychotic group group(n = 28) |
P | Time and group interaction | |
|---|---|---|---|---|---|
| F | P-value c | ||||
| Bodyweight(Kg) | |||||
| Week 0 | 85.33 ± 11.19 | 82.10 ± 14.24 | 0.356a | 7.982 | 0.007 |
| Week 8 | 84.20 ± 11.38 | 83.85 ± 14.33 | 0.010b | ||
| Pb | 0.058 | 0.006 | |||
| BMI(Kg/m2) | |||||
| Week 0 | 31.14 ± 4.12 | 29.56 ± 5.70 | 0.155a | 7.627 | 0.008 |
| Week 8 | 30.90 ± 4.58 | 30.13 ± 5.77 | 0.008b | ||
| Pb | 0.064 | 0.006 | |||
Data are reported as mean ± SD
a: Obtained from the independent t-test
b:Analysis of covariance adjusted for baseline values, smoking, education, and the change in energy intake
Pb: Obtained from the paired sample t-test
P-value c Obtained from repeated measures analysis of variance adjusted for smoking, education and the change in energy intake
Table 4.
Components of metabolic syndrome in the two groups at baseline and after 8 weeks
| Factor | Synbiotic + antipsychotic group (n = 27) |
Placebo + antipsychotic group group(n = 28) |
P a | Time and group interaction | |
|---|---|---|---|---|---|
| F | P-value c | ||||
| WC(cm( | |||||
| week 0 | 101.2  8.83 8.83 |
101.28 ± 11.69 | 0.903a | ||
| week 8 | 98.96 ± 7.63 | 104.32 ± 12.14 | 0.000b | 19.785 | 0.000 |
| Pb | 0.003 | 0.001 | |||
| HDL(mg/dl)* § | |||||
| Week 0 | 66(51–79) | 57(48–67) | 0.535a | ||
| Week 8 | 60(49–77) | 60(49–71) | 0.129b | 3.050 | 0.087 |
| Pb | 0.395 | 0.354 | |||
| TG(mg/dl) § | |||||
| Week 0 | 191(134–244) | 148(99–176) | 0.030a | ||
| Week 8 | 145(114–186) | 151(99–191) | 0.029b | 0.079 | 0.780 |
| Pb | 0.026 | 0.684 | |||
| FBS(mg/dl) § | |||||
| Week 0 | 100(87–109) | 96.50(90.25–104) | 0.926a | ||
| Week 8 | 95(89–103) | 97.50(89.50 -103.75) | 0.768b | 0.768 | 0.503 |
| Pb | 0.394 | 0.580 | |||
| SBP(mmHg) | |||||
| Week 0 | 134.73 ± 12.99 | 137.29 ± 14.69 | 0.498a | ||
| Week 8 | 127.85 ± 12.56 | 131.82 ± 13.01 | 0.251b | 0.232 | 0.632 |
| Pb | 0.000 | 0.045 | |||
| **DBP(mmHg) | |||||
| Week 0 | 84.64± 9.99 | 83.73 ± 14.07 | 0.160a | ||
| Week 8 | 82.92 ± 12.69 | 85.15± 11.00 | 0.140b | 3.223 | 0.079 |
| Pb | 0.559 | 0.616 |
Data are reported as mean ± SD for normally distributed variables and interquartile range for non-normal distribution
FBS: fasting blood sugar, TG: triglyceride, TC: total cholesterol, HDL-c: high-density lipoprotein cholesterol, SBP: systolic blood pressure, DBP: diastolic blood pressure WC: waist circumference
a: Obtained from the independent t-test for normally distributed variables /Mann-Whitney test for other variables
b:analysis of covariance adjusted for baseline values, smoking, education, and the change in energy intake for normally distributed variables and logHDL/ Mann-Whitney test for changes in serum levels of other variables
Pb: Obtained from the paired sample t-test for normally distributed variables and Wilcoxon test for non-normal variables
P-value c Obtained from repeated measures analysis of variance adjusted for smoking, education and the change in energy intake
* In the placebo group, the data of one patient whose baseline HDL-c was more than 150 mg/dL was excluded from the analysis
** Data of 4 patients were excluded from diastolic blood analysis because they were outliers
§ non-normally distributed variables
Table 5.
Biochemical parameters in the two groups at baseline and after 8 weeks
| Factor | Synbiotic + antipsychotic group (n = 27) |
Placebo + antipsychotic group group(n = 28) |
P a | Time and group interaction | |
|---|---|---|---|---|---|
| F | P-value c | ||||
| TC(mg/dl) | |||||
| Week 0 | 187.66 ± 35.20 | 162.92 ± 26.65 | 0.005a | ||
| Week 8 | 182.25 ± 45.55 | 163.71 ± 32.79 | 0.741b | 0.557 | 0.459 |
| Pb | 0.164 | 0.903 | |||
| LDL(mg/dl) | |||||
| Week 0 | 115.03 ± 24.01 | 98.32 ± 29.35 | 0.007a | ||
| Week 8 | 107.96 ± 27.94 | 104.96 ± 29.22 | 0.194b | 2.906 | 0.094 |
| Pb | 0.011 | 0.152 | |||
| HbA1C (%) | |||||
| Week 0 | 5.2(4.5–5.6) | 4.9(4.5–5.6) | 0.867a | ||
| Week 8 | 4.8(4.3–5.2) | 5(4.6–5.6) | 0.023b | 8.674 | 0.005 |
| Pb | 0.024 | 0.174 | |||
| Insulin(µU/mL) | |||||
| Week 0 | 14.6(11.4–19.6) | 9.3(3.9–17.2) | 0.061a | ||
| Week 8 | 12.3(8.1–21.1) | 9.1(4.9–16.3) | 0.846b | 0.303 | 0.584 |
| Pb | 0.479 | 0.336 | |||
| HOMA-IR | |||||
| Week 0 | 3.4(2.34-5) | 2.2(0.7–4.3) | 0.089a | ||
| Week 8 | 2.7(1.7–4.6) | 1.9(1-4.1) | 0.794b | 1.078 | 0.304 |
| Pb | 0.217 | 0.128 | |||
| QUICKI | |||||
| Week 0 | 0.53(0.4–0.6) | 0.56(0.5–0.7) | 0.235a | ||
| Week 8 | 0.56(0.5–0.6) | 0.6(0.5–0.7) | 0.711b | 0.089 | 0.767 |
| Pb | 0.870 | 0.447 | |||
Data are reported as mean ± SD for normally distributed variables and interquartile range for non-normally distributed variables
a: Obtained from the independent t-test for normally distributed variables /Mann-Whitney test for other variables
b:analysis of covariance adjusted for baseline values, smoking, education, and the change in energy intake for normally distributed variables/ Mann-Whitney test for changes in serum levels of non-normally distributed variables
Pb: Obtained from the paired sample t-test for normally distributed variables and Wilcoxon test for non-normally distributed variables
P-value c Obtained from repeated measures analysis of variance adjusted for smoking, education and the change in energy intake
Discussion
The results of the present study showed that synbiotic supplements decreased waist circumference, HbA1C, LDL and TG and prevented BMI increase in patients receiving antipsychotic drugs probably by reducing inflammation and oxidative stress [15, 16]. To our knowledge, no previous study has investigated the effect of synbiotics on the components of metabolic syndrome in patients with schizophrenia. However, in an experimental study, probiotics significantly reduced weight, total cholesterol, and TG and increased HDL-c in rats with olanzapine-induced metabolic syndrome [34]. In addition, Liu et al. investigated effects of probiotics and fiber, separately, and in combination on metabolic syndrome parameters in patients with antipsychotic-induced metabolic side effects. They found that although probiotics or dietary fiber prevented further weight gain, their combination was superior for weight, BMI, and total cholesterol reduction after 12 weeks [24]. Another paper reported results of two RCTs in drug-naïve patients with first-episode schizophrenia. In Study 1, no significant differences in weight gain were observed between probiotic + olanzapine and olanzapine group. In study 2 adjuvant therapy with probiotics plus dietary fiber supplements attenuated olanzapine-induced weight gain compared to olanzapine monotherapy after 12 weeks [25].
Furthermore, effects of pro/pre/synbiotics on components of metablic syndrome have been investigated in patients with diabetes, obesity, or nonalcoholic fatty liver disease in several trials. Although the results of the trials have been inconsistent, systematic reviews and meta-analyses have suggested that probiotics/synbiotic administration could exert some beneficial effects on components of metablic syndrome. For example John et al., in their systematic review concluded that the intake of probiotics could decrease BMI, body weight, and fat mass in overweight or obese adults [17]. and another systematic review showed that probiotics or synbiotics had beneficial effects on lipid profiles, anthropometric indices and blood pressure in individuals with type 2 diabetes [35]. In addition Li et al. conducted a systematic review and meta-analysis and reported that probiotics decreased the levels of HbA1c, insulin resistance, TC and TG in patients with prediabetes [36]. Another systematic review of trials conducted in patients with non-alcoholic fatty liver disease showed that gut microbiota interventions improved markers of inflammation, glycemia, insulin resistance, dyslipidemia, obesity and liver injury [37]. Moreover, the results of an RCT conducted by Bernie et al. showed that probiotic consumption for 45 days significantly decreased weight and BMI in patients with metabolic syndrome [38]. Finally, based on the results of a recent umbrella review, probiotic supplementation can reduce total cholesterol (TC), triglycerides (TG) and low-density lipoprotein cholesterol, but may have no significant effect on high-density lipoprotein cholesterol [39]. However, some trials have reported no effects of pro/pre/synbiotics on components of metabolic syndrome which may be explained by using different bacterial or probiotic composition and short length of intervention. For example, a recent study reported that L. acidophilus La5 and B. animalis subsp. lactis Bb12 did not improve blood pressure and serum lipid concentrations after 6 weeks in overweight men and women over 55 y with low baseline cholesterol and the authors suggested that the hypocholesterolemic benefits of probiotics may be limited to populations with borderline or high total cholesterol levels [40].In the present study, the synbiotic supplement did not significantly affect FBS, TC, blood pressure or insulin resistance. This may be explained by the duration of the study. As these variables favorably, but insignificantly changed after intervention, it is conceivable that if the duration of intervention had been longer, synbiotics might have favorable effects on the mentioned variables. Huang et al. repotted that after 12 weeks probiotics plus dietary fibers significantly reduced FBS, TC and insulin resistance in patients with severe mental disorders gaining weight more than 10% predrug treatment weight [24].
As a study in rats showed that probiotics modified satiety hormones production [41], significant effects of synbiotics on carbohydrate consumption in this study may be due to effects of this supplement on appetite, however appetite was not assessed in this trial.
Our research had several strengths. To our knowledge, this was the first randomized controlled trial investigating the effects of synbiotics on components of metabolic syndrome in patients with schizophrenia. In addition, important confounding factors including diet and physical activity were assessed and adjusted in the analysis. It should also be noted that the present study had some limitations. First, gut micrbiotia analysis was not performed. Second, due to COVID pandemic, approximately 20% of the patients were lost to follow-up.
Conclusion
Daily consumption of synbiotic supplements for eight weeks reduced waist circumference and HbA1C and prevented BMI increase in patients with schizophrenia receiving antipsychotic drugs. Moreover, LDL-c, and TG decreased significantly in the synbiotic + antipsychotic group, but the changes were not significantly different from those of the placebo + antipsychotic group. HDL, SBP, DBP, FBS, insulin, HOMA_IR, QUIKI, and TC were not significantly different between the two groups after intervention.
Acknowledgements
This work was supported by the Vice-Chancellor for Research, Iran University of Medical Sciences, Tehran, Iran (grant number 14664(. We sincerely thank Dr. Ahmad Mousavi, Ms Mojgan Maghrour and the staff of Iran Psychiatric Hospital for their support. Thanks are also extended to Zist Takhmir Company for providing synbiotic capsules and placebos.
Author contributions
Study conception and design: Basafa-Roodi, Jazayeri, Hadi, Khosravi-darani, Jamali Paghaleh, Malakouti Acquisition of data: Basafa-Roodi, Jazayeri, Hadi, Jamali Paghaleh Analysis and interpretation of data: Basafa-Roodi, JazayeriDrafting of manuscript: Basafa-Roodi, JazayeriAll authors have approved the submitted version of the manuscript.
Funding
This work was supported by the Vice-Chancellor for Research, Iran University of Medical Sciences, Tehran, Iran (grant number 14664).
Data availability
The data are available from the corresponding author, upon reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of Iran University of Medical Sciences (Ethical approval code: IR.IUMS.REC.1398.650) and registered at Iran Registry of Clinical Trials (IRCT Number: 20090901002394N45), Date: 26-12-2019. The protocol of the study was described for eligible patients and written informed consent was signed by the patient or his/her legal guardian in case he/she was unable to comprehend research consent form.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liu J, Fu L. Metabolic syndrome in patients with schizophrenia: why should we care. Med (Baltim). 2022;101(32):e29775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Hert M, et al. Prevalence of diabetes, metabolic syndrome and metabolic abnormalities in schizophrenia over the course of the illness: a cross-sectional study. Clin Pract Epidemiol Ment Health. 2006;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Correll CU, et al. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: baseline results from the RAISE-ETP study. JAMA Psychiatry. 2014;71(12):1350–63. [DOI] [PubMed] [Google Scholar]
- 4.Foley DL, Morley KI. Systematic review of early cardiometabolic outcomes of the first treated episode of psychosis. Arch Gen Psychiatry. 2011;68(6):609–16. [DOI] [PubMed] [Google Scholar]
- 5.Pillinger T, et al. Impaired glucose homeostasis in First-Episode Schizophrenia: a systematic review and Meta-analysis. JAMA Psychiatry. 2017;74(3):261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emul M, Kalelioglu T. Etiology of cardiovascular disease in patients with schizophrenia: current perspectives. Neuropsychiatr Dis Treat. 2015;11:2493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jameson JL. Harrison’s Principles of Internal Medicine. Twenty-First ed; 2022.
- 8.de Silva VA, et al. Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis. BMC Psychiatry. 2016;16(1):341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shurrab NT, Arafa E-SA. Metformin: a review of its therapeutic efficacy and adverse effects. Obes Med. 2020;17:100186. [Google Scholar]
- 10.Kim YK, Shin C. The Microbiota-Gut-Brain Axis in Neuropsychiatric disorders: pathophysiological mechanisms and novel treatments. Curr Neuropharmacol. 2018;16(5):559–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen TT, et al. Overview and systematic review of studies of microbiome in schizophrenia and bipolar disorder. J Psychiatr Res. 2018;99:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill C, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–14. [DOI] [PubMed] [Google Scholar]
- 13.Gibson GR, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502. [DOI] [PubMed] [Google Scholar]
- 14.Swanson KS, et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. 2020;17(11):687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naseri K, et al. The effects of probiotic and synbiotic supplementation on inflammation, oxidative stress, and circulating adiponectin and leptin concentration in subjects with prediabetes and type 2 diabetes mellitus: a GRADE-assessed systematic review, meta-analysis, and meta-regression of randomized clinical trials. Eur J Nutr. 2023;62(2):543–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flaig B et al. Treatment of Dyslipidemia through targeted therapy of gut microbiota. Nutrients, 2023. 15(1). [DOI] [PMC free article] [PubMed]
- 17.John GK et al. Dietary alteration of the gut microbiome and its impact on Weight and Fat Mass: a systematic review and Meta-analysis. Genes (Basel), 2018. 9(3). [DOI] [PMC free article] [PubMed]
- 18.Tabrizi R, et al. The effects of Synbiotic supplementation on glucose metabolism and lipid profiles in patients with diabetes: a systematic review and Meta-analysis of Randomized controlled trials. Probiotics Antimicrob Proteins. 2018;10(2):329–42. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y, et al. Effect of probiotic Lactobacillus on lipid profile: a systematic review and meta-analysis of randomized, controlled trials. PLoS ONE. 2017;12(6):e0178868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikbakht E, et al. Effect of probiotics and synbiotics on blood glucose: a systematic review and meta-analysis of controlled trials. Eur J Nutr. 2018;57(1):95–106. [DOI] [PubMed] [Google Scholar]
- 21.Sáez-Lara MJ et al. Effects of Probiotics and Synbiotics on obesity, insulin resistance syndrome, type 2 diabetes and non-alcoholic fatty liver disease: a review of human clinical trials. Int J Mol Sci, 2016. 17(6). [DOI] [PMC free article] [PubMed]
- 22.Soltani S, et al. Effects of probiotic/synbiotic supplementation on body weight in patients with diabetes: a systematic review and meta-analyses of randomized-controlled trials. BMC Endocr Disord. 2023;23(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saadati S et al. Beneficial effects of the probiotics and synbiotics supplementation on anthropometric indices and body composition in adults: a systematic review and meta-analysis. Obes Rev, 2024 Mar;25(3):e13667. [DOI] [PubMed]
- 24.Huang J, et al. The effects of probiotics plus dietary fiber on antipsychotic-induced weight gain: a randomized clinical trial. Transl Psychiatry. 2022;12(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang J, et al. Probiotics Plus Dietary Fiber supplements Attenuate Olanzapine-Induced Weight Gain in Drug-Naïve First-Episode Schizophrenia patients: two randomized clinical trials. Schizophr Bull. 2022;48(4):850–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandey KR, Naik SR, Vakil BV. Probiotics, prebiotics and synbiotics- a review. J Food Sci Technol. 2015;52(12):7577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sepideh A, et al. Effects of Multistrain Probiotic supplementation on glycemic and inflammatory indices in patients with nonalcoholic fatty liver disease: a double-blind Randomized Clinical Trial. J Am Coll Nutr. 2016;35(6):500–5. [DOI] [PubMed] [Google Scholar]
- 28.Grundy SM et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation, 2004. 109(3): pp. 433-8. [DOI] [PubMed]
- 29.Hadaegh F, Azizi F. Effect of Weight Change on Incident of metabolic syndrome and its components according to Iranian Waist circumference and NHLBI: TLGS. Iran J Endocrinol Metabolism. 2010;12(2):116–30. [Google Scholar]
- 30.Hagströmer M, Oja P, Sjöström M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9(6):755–62. [DOI] [PubMed] [Google Scholar]
- 31.Gutch M, et al. Assessment of insulin sensitivity/resistance. Indian J Endocrinol Metab. 2015;19(1):160–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eblen-Zajjur A, Eblen-Zajjur M. [Estimation of low density lipoprotein cholesterol concentration: regression analysis versus Friedewald’s formula]. Rev Med Chil, 2001. 129(11): pp. 1263-70. [PubMed]
- 33.Shakeri H, et al. Consumption of synbiotic bread decreases triacylglycerol and VLDL levels while increasing HDL levels in serum from patients with type-2 diabetes. Lipids. 2014;49(7):695–701. [DOI] [PubMed] [Google Scholar]
- 34.Syed M, Nayak V. Effect of probiotics on olanzapine-induced metabolic syndrome in Wistar albino rats. 2021.
- 35.Naseri K, et al. Beneficial effects of probiotic and synbiotic supplementation on some cardiovascular risk factors among individuals with prediabetes and type 2 diabetes mellitus: a grade-assessed systematic review, meta-analysis, and meta-regression of randomized clinical trials. Pharmacol Res. 2022;182:106288. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, et al. The effects of probiotic administration on patients with prediabetes: a meta-analysis and systematic review. J Transl Med. 2022;20(1):498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carpi RZ et al. The effects of Probiotics, Prebiotics and Synbiotics in non-alcoholic Fat Liver Disease (NAFLD) and Non-alcoholic Steatohepatitis (NASH): a systematic review. Int J Mol Sci, 2022. 23(15). [DOI] [PMC free article] [PubMed]
- 38.Bernini LJ, et al. Beneficial effects of Bifidobacterium lactis on lipid profile and cytokines in patients with metabolic syndrome: a randomized trial. Effects of probiotics on metabolic syndrome. Nutrition. 2016;32(6):716–9. [DOI] [PubMed] [Google Scholar]
- 39.Zarezadeh M, et al. Probiotics act as a potent intervention in improving lipid profile: an umbrella systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2022;63(2):145–58. [DOI] [PubMed] [Google Scholar]
- 40.Ivey KL, et al. The effect of yoghurt and its probiotics on blood pressure and serum lipid profile; a randomised controlled trial. Nutr Metabolism Cardiovasc Dis. 2015;25(1):46–51. [DOI] [PubMed] [Google Scholar]
- 41.Forssten SD, et al. Changes in satiety hormone concentrations and feed intake in rats in response to lactic acid bacteria. Appetite. 2013;71:16–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from the corresponding author, upon reasonable request.