Abstract
Background
Sarcopenia is common in end-stage liver disease and negatively impacts patients awaiting or undergoing liver transplantation (LT). Magnetic resonance imaging (MRI) may be used to measure body composition and sarcopenia. We aimed to evaluate the feasibility of MRI-based LT body composition profiling, describe waitlist body composition, and assess the natural rate of change in body composition while on the waitlist and post-LT.
Methods
This prospective pilot study recruited adults listed for LT at an urban, tertiary care facility. Eighteen participants were scanned at time of waitlisting and 15 had follow-up MRIs (waitlist and/or post-LT). An 8-min MRI was used to measure body composition (AMRA® Researcher) including thigh fat-free muscle volume (FFMV) and fat infiltration (MFI), visceral (VAT) and abdominal subcutaneous (ASAT) adipose tissue volumes, and liver fat. A sex- and BMI invariant FFMV z-score (z-FFMV) was calculated, and muscle composition (MC) phenotypes were defined using the muscle assessment score (consisting of the FFMV z-score and sex-adjusted MFI). Rate of body composition change was calculated using mixed-effect modelling and is presented as rate per 30 days.
Results
At time of waitlisting, 73% of the 18 participants had high MFI and 39% had the adverse MC (low FFMV z-score and high MFI) phenotype. Seven participants received an LT. Post-LT serial MRIs, at a median of 147 days apart within the first 200 days post-LT, demonstrated increased z-FFMV 0.22 SDs/(30 days) (p = 0.002), VAT 0.23 (p < 0.001), and ASAT 0.52 (p = 0.001) L/(30 days), but no change in MFI (p = 0.200) nor liver fat (p = 0.232).
Conclusion
MRI-based body composition profiling is feasible in LT patients and shortly after LT. This can be amended to routine clinical scans and may help in early identification of patients who may benefit from interventions to improve body composition. In addition, body composition changes significantly over time after LT.
Keywords: Liver transplantation, Body composition, Sarcopenia, Magnetic resonance imaging, Obesity, Sarcopenic obesity, Adverse muscle composition
Background
Sarcopenia, characterized by the progressive loss of muscle volume and function, is a common and major complication in cirrhosis, which negatively impacts outcomes in patients awaiting or undergoing liver transplantation (LT) [1, 2]. While several methods exist to evaluate body composition, imaging techniques, such as magnetic resonance imaging (MRI), enable us to capitalize on images taken as part of standard practice and to append clinical examinations with rapid sequences tailored for quantifying body composition [3]. These techniques have been instrumental in furthering our understanding of body composition in human health, in general, and in sarcopenia, specifically.
Recent practice guidance for patients with liver cirrhosis states that objective measures of muscle loss should be considered to assess the risk for poor outcomes in patients with cirrhosis [2]. Currently, ad hoc analysis of single abdominal CT-images is a common source of such measurements. However, CT is not recommended for the sole purpose of assessing sarcopenia due to exposure to ionizing radiation [2]. In addition, CT-based muscle measurements are typically limited to a two-dimensional analysis of a single or small set of axial slices to minimize ionizing radiation. Furthermore, since the analysis is based on labor intense manual segmentation, only a few slices (of many potential slices) in a 3D volume are feasible to analyze. This means that the area measured is a proxy for the volume. Since the exact location of the slices in relation to internal organs cannot be determined a priori and inevitably differ between scans, the precision of muscle measurement by CT is limited [3].
A standardized MRI-based measurement technique for volumetric neck-to-knee body composition analysis has been developed for research and clinical use. The body composition analysis is based on a rapid (typically 6–10 min) non-contrast set of sequences without surface coils that is comparable across common MR-scanner manufacturers and clinically used field strengths [4, 5]. Based on these measurements, the Muscle Assessment Score (MAsS) was developed to describe muscle health invariant to sex and BMI. The MAsS consists of thigh muscle volume z-score and muscle fat infiltration. Large population studies have shown that MAsS is better than the individual components in predicting physical function and hospitalization, and MAsS has been suggested to enable objective sarcopenia detection [6, 7]. MAsS can be used to detect muscle composition (MC) phenotypes, of which the adverse MC (high muscle fat and low muscle volume z-score) has been shown to independently predict all-cause mortality [8, 9].
Currently, the natural rate of change in body composition measurements, including MAsS, on the LT waiting list and post-LT is unknown. Our aim was to: (i) assess the feasibility of performing a rapid MRI for the assessment of body composition in a LT setting, (ii) describe body composition, including MC phenotypes, for a cohort awaiting LT, and (iii) assess the natural rate of change in body composition in LT waitlist candidates and during the first 6 months after LT. As exploratory analyses, we sought to assess the association between the Karnofsky Performance status as well as long-term survival with MC phenotypes.
Methods
This prospective pilot study recruited 28 adults listed for LT between 2017 and 2018 with long-term follow-up on mortality through 5/2021 at an urban, tertiary care facility. All participants provided informed consent before being enrolled into this study in conformance to the 1964 Declaration of Helsinki and its later amendments. No organs from executed prisoners were used.
Eighteen participants underwent baseline MRI exam while on the LT waitlist and 15 of those had one or two additional MRI examinations; either on the waitlist or post-LT. Eight participants had one additional MRI examination on the waitlist (these participants did not undergo a LT). Seven participants had one post-LT MRI examination, and 5 of those 7 had a second post-LT MRI examination. Details of the MRI examinations and body composition measurements are presented in a sub-section below. Reasons for study dropout included transplantation before the baseline MRI (n = 3), waitlist removal (n = 2), critical illness (n = 2), or patient time constraints (n = 3).
Functional dependency was assessed by trained study personnel at each visit and categorized as ‘independent’, ‘partially dependent’, and ‘fully dependent’ as defined by The Karnofsky Performance Status scale [10].
Body composition
A rapid (8-minute) non-contrast dual-echo Dixon MRI protocol [5], which provides a water- and fat-separated volumetric data set with neck to knee coverage, was used to analyze body composition including: thigh fat-free muscle volume (FFMV) and muscle fat infiltration (MFI), visceral (VAT) and abdominal subcutaneous adipose tissue (ASAT) volumes, and liver proton density fat-fraction (PDFF) using AMRA® Researcher (AMRA Medical AB, Sweden) [5]. The MR examination is separated into six stations with ‘breath-hold’ acquisition over the abdomen and free breathing from the pelvic area and below. The methodology and MR protocol used in this study has been published extensively. Briefly, the analysis consists of the following steps (based on the MR images): (1) automatic image calibration, (2) automatic labeling and registration of fat and muscle regions to the acquired image volumes, (3) quality control of anatomical regions and MR-data performed by trained personnel at AMRA Medical AB, and (4) quantification of fat and muscle volumes based on the calibration images [5, 11–14]. These body composition measurements have been reported to be comparable across commonly used clinical MRI scanners and field strengths [4]. An example of these fat and water separated neck-to-knee images used for the body composition analysis is shown in Fig. 1 for a LT recipient.
Fig. 1.
Example images of a male liver transplant (LT) recipient at baseline (panels a & d; 85 days pre-LT) and post-LT (panels b & e 42 days post-LT, and panels c & f 197 days post-LT). Panels a-c shows the water channel with thigh muscles in false colors, these regions were used to measure muscle volume and muscle fat infiltration. Panels d-f shows the fat channel with the visceral fat (red) and abdominal subcutaneous fat (blue) compartments. The participant improved in the muscles by going from − 2.4 SDs to + 0.1 SDs in muscle volume z-score with a loss of 2 pp in muscle fat. The abdominal fat however increased; visceral fat increased from 3.3 L to 4.9 L and subcutaneous fat increased from 11.0 L to 15.7 L
For each participant, MAsS was also calculated consisting of a sex- and BMI invariant FFMV z-score (z-FFMV) and MFI adjusted for sex-differences using the median MFI in a sex-specific reference population. The MC phenotype adverse MC was defined as high MFI (> 75th percentile in a sex-specific reference population) and low FFMV z-score (< 25th percentile in a reference population). High and only high fat, low and only low volume, as well as normal MC phenotypes were also defined using the same cut-points. The reference population consisted of 40,178 participants aged 44 to 76 from the UK Biobank imaging sub-study [6–8]. This research was conducted using the UK Biobank resource, project ID 6569. The study was approved by the North West Multicenter Research Ethics Committee, UK. Written informed consent was obtained prior to study entry.
Statistics
Rate of change was assessed using mixed-effect modeling (R package lme4; RStudio v1.4). In the modeling, the patient id was used as the random effect and the linear time-dependent change in body composition was calculated. The results are presented graphically and as rate of change per 30 days. P-values were calculated for the rate of change. This method takes into account the individual variability of rate of change over time.
Results
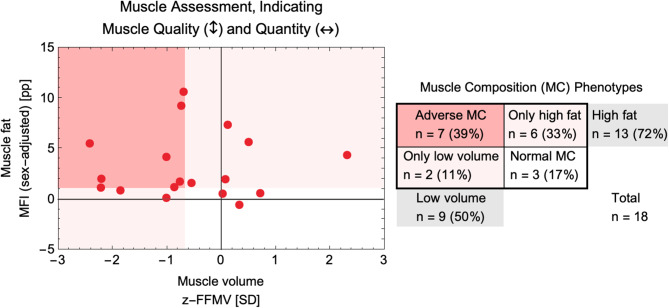
Liver transplant waitlist (baseline) characteristics and MC phenotypes of the 18 participants are presented in Table 1; Fig. 2. The following MC phenotype proportions were found: high fat MC was found in 72% of all patients and low volume in 50%; only 17% presented with normal MC. The adverse MC phenotype (low muscle volume and high muscle fat content) was present in 39% of all patients at baseline.
Table 1.
Participant characteristics at baseline visit (liver transplant waitlist)
| All (n = 18) | |
|---|---|
| Sex (Women) [n (%)] | 5 (28%) |
| Self-Reported Race [n (%)] | |
| Black | 1 (6%) |
| White | 16 (89%) |
| Declined | 1 (6%) |
| Age [years] | 58.0 ± 9.4 |
| Height (m) | 1.75 ± 0.11 |
| Weight [kg] | 94.7 ± 15.4 |
| BMI [kg/m2] | 31.0 ± 5.6 |
| BMI category | |
| 18.5–24.9 kg/m2 | 3 (17%) |
| 25–29.9 kg/m2 | 5 (28%) |
| ≥30 kg/m2 | 10 (56%) |
| Liver-related history | |
| Ascites | 13 (72%) |
| Spontaneous bacterial peritonitis | 2 (11%) |
| Hepatic encephalopathy | 8 (44%) |
| Varices | 14 (78%) |
| Laboratory findings | |
| Albumin [g/dL] | 3.3 ± 0.5 |
| Bilirubin [mg/dL] | 2.9 ± 2.0 |
| ALT [U/L] | 30 ± 19 |
| AST [U/L] | 48 ± 19 |
| Creatinine [mg/dL] | 1.0 ± 0.8 |
| Sodium [mmol/L] | 138 ± 2 |
| INR | 1.3 ± 0.3 |
| Child-Turcotte-Pugh points | 7.5 ± 2.3 |
| Child-Turcotte-Pugh class | |
| A | 7 (39%) |
| B | 8 (44%) |
| C | 3 (17%) |
| MELD-Na | 15.3 ± 5.0 |
| Primary indication for LT | |
| Hepatocellular carcinoma | 8 (44%) |
| Hepatitis C | 7 (39%) |
| Alcohol-associated cirrhosis | 6 (33%) |
| Nonalcoholic steatohepatitis | 4 (22%) |
| Human immunodeficiency virus | 1 (6%) |
| MRI-based body composition measurements | |
| Visceral Adipose Tissue (VAT) volume [L] | 4.70 ± 2.07 |
| Abdominal Subcutaneous Adipose Tissue (ASAT) volume [L] | 9.63 ± 4.38 |
| Liver Proton Density Fat-fraction (PDFF) [%] | 4.26 ± 1.50 |
| Mean Anterior Thigh Muscle Fat Infiltration (MFI) [%] | 10.23 ± 3.60 |
| Total Thigh Fat-free Muscle Volume (FFMV) [L] | 11.44 ± 3.26 |
| MRI-based Muscle Assessment Score (MAsS) | |
| z-FFMV [SDs] | -0.56 ± 1.19 |
| MFI sex-adjusted [pp] | 3.33 ± 3.28 |
| Muscle Composition (MC) Phenotypes | |
| Adverse MC [n (%)] | 7 (39%) |
| Only high fat [n (%)] | 6 (33%) |
| Only low volume [n (%)] | 2 (11%) |
| Normal MC [n (%)] | 3 (17%) |
Numbers are presented as mean ± one standard deviation or count (per cent of cohort). ALT, alanine aminotransferase; AST, aspartate aminotransferase; INR, international normalized ratio; MELD, model for end-stage liver disease; MRI, magnetic resonance imaging; SDs, standard deviations; pp, per centage points
Fig. 2.
Muscle assessment score (MAsS) for 18 patients on LT waitlist with muscle composition (MC) phenotypes. The y-axis shows the muscle fat, indicating the quality of muscle, and on the x-axis shows the muscle quantity as a z-score – negative number indicates smaller muscles than expected given the patients’ sex and BMI. The MAsS-based muscle composition (MC) phenotypes are shown in red/pink/white background according to the legend on the right. On the right legend the number of patients and MC phenotype proportions are also shown
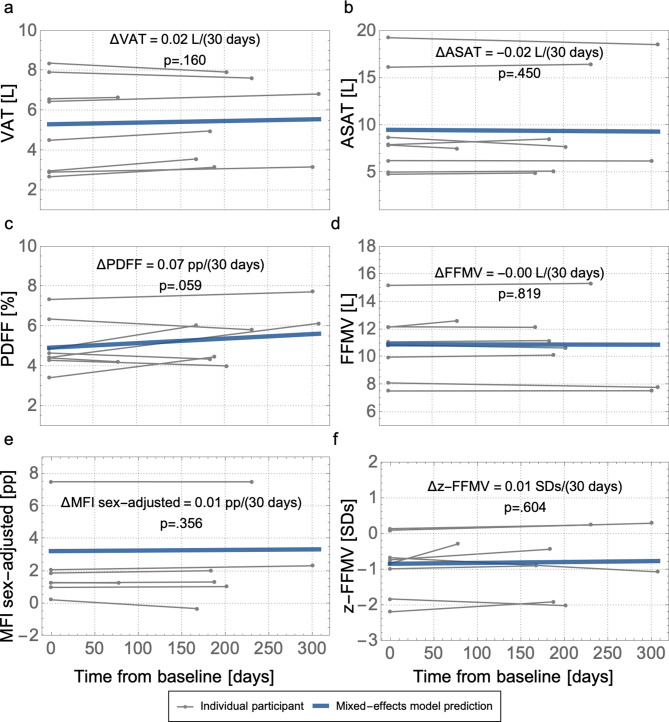
Eleven participants did not receive LT, of whom 8 had a follow-up MRI while on the waitlist. The median waitlist time between baseline and the second MRI was 195 (range, 78–308) days. There was no significant change in body composition for those 8 patients in-between the two MRI examinations, although liver PDFF was borderline insignificantly increased (p = 0.059) (Fig. 3).
Fig. 3.
Longitudinal change on waitlist (n = 8) for body composition measurements; (a) visceral adipose tissue volume (VAT), (b) abdominal subcutaneous adipose tissue volume (ASAT), (c) liver proton density fat-fraction (PDFF), (d) thigh fat-free muscle volume (FFMV), and the Muscle Assessment Score; (e) thigh muscle fat infiltration (MFI) adjusted for sex-differences, and (f) FFMV z-score (z-FFMV). The rate of change is presented per 30 days in each panel based on the mixed-effects model prediction including p-value
Seven participants received LT, all of whom underwent at least one post-LT MRI and 5 had a second post-LT MRI. The median time between the two post-LT MRIs in the 5 patients who had two post-LT MRIs was 147 (range, 76–155) days. The latest repeated MRI took place 198 days post-LT. In-between the post-LT MRIs there was a significant increase in FFMV z-score 0.22 SDs/(30 days) (p = 0.002), FFMV 0.33 (p = 0.002), VAT 0.23 (p < 0.001) L/(30 days), and ASAT 0.52 (p = 0.001) L/(30 days). There was no significant change in MFI nor liver PDFF in-between post-LT MRIs (Fig. 4).
Fig. 4.
Longitudinal change after liver transplant (n = 5) for body composition measurements; (a) visceral adipose tissue volume (VAT), (b) abdominal subcutaneous adipose tissue volume (ASAT), (c) liver proton density fat-fraction (PDFF), (d) thigh fat-free muscle volume (FFMV), and the Muscle Assessment Score; (e) thigh muscle fat infiltration (MFI) adjusted for sex-differences, and (f) FFMV z-score (z-FFMV). The rate of change is presented per 30 days in each panel based on the mixed-effects model prediction including p-value
In terms of functional status, two LT recipients were ‘partially dependent’ at the waitlist and the first post-LT MRI, and the adverse MC phenotype was observed on each MRI exam. Furthermore, in one LT recipient with adverse MC phenotype at waitlist MRI and ‘partial dependency’, the second post-LT MRI showed resolution of adverse MC coupled with functional ‘independency’.
In long-term follow-up there were no deaths among the LT recipients and three deaths in the non-LT group. Among the three deaths in the non-LT group, all had high MFI and two concurrent low muscle volume (i.e., the adverse MC phenotype) at the waitlist MRI.
Discussion
This prospective pilot study demonstrates the feasibility of MRI-based body composition profiling before and after LT and reports body composition measurements and MC phenotype proportions for a cohort of LT waitlist patients. By appending standard practice clinical MRI examinations with rapid sequences (8 min) tailored for measuring body composition, it may be possible to enhance the value of the clinical examinations with quantitative data on muscle health. Such data may aid in risk stratification and potentially identify patients in need of targeted sarcopenia intervention.
Further, the data presented herein demonstrate baseline body composition measurements at time of listing for LT as well as the natural rate of change in body composition before and after LT. These data may be useful for sample size estimations where the aim is to assess body composition changes over time in end-stage liver disease and LT.
Although limited by small sample size, there were significant increases in both muscle and adipose tissue volumes shortly after LT. The muscle volume z-score is effectively BMI and sex-invariant [8] and thus an increase in muscle volume z-score following LT indicates that muscle volume gain is higher than what can be explained by overall weight gain. Whether physical activity interventions early after LT to further increase muscle volume may improve clinical outcomes in LT recipients requires further study. At baseline the adverse MC phenotype was found to be highly prevalent (39%) and close to 4x higher than in a general population (10.5%) and 3x higher than in persons with metabolic dysfunction associated steatotic liver disease (MASLD, formally termed NAFLD) (14%) of similar age [6, 9]. The adverse MC phenotype is a highly vulnerable phenotype linked to poor function, high prevalence of metabolic comorbidity, hospitalization, and all-cause mortality [6, 9, 15]. Larger cohorts of LT patients are required for a clearer understanding of the prevalence of adverse MC and how it may interact with etiology of the disease and outcomes in LT. We also observed an increase in adiposity after LT, especially in the visceral compartment. Visceral adiposity has previously been associated with higher risk for metabolic syndrome, hepatocellular carcinoma, and overall mortality in LT [16–18]. In a recent paper combining two cohort studies, Dallas Heart Study and UK Biobank, high visceral fat together with liver fat content, as measured in the present study, predicted incident cardiovascular disease [19]. In the present study, the levels of visceral fat post-LT were moving towards the reported high-risk level and further adequately powered research could be of interest to assess if such measurements of body composition could be used to detect LT recipients at elevated risk for cardiovascular disease. This is particularly important in LT recipients since cardiovascular disease is a leading cause of morbidity and mortality after LT [20]. Early identification of LT recipients with rapid increase in high-risk adipose tissue phenotypes via MRI may allow for targeted lifestyle interventions to improve cardiometabolic outcomes [21].
The skeletal muscle index at the third lumbar vertebrae (L3-SMI) has extensively been measured based on CT-images using an ad hoc approach with several research papers published. L3-SMI is a recommended method for measuring muscle loss in recent practice guidance for patients with liver cirrhosis [2]. However, due to the exposure to ionizing radiation, routine use of CT solely for the purpose of detecting sarcopenia is not recommended [2]. In two ongoing prospective studies on patients with compensated and decompensated cirrhosis interim results demonstrated a very strong correlation between L3-SMI and thigh muscle volume [22, 23], meaning that outcomes found by L3-SMI can be equally detected with thigh muscle volume measurements. In addition, repeatability of thigh muscle fat and volume measurements in patients with liver cirrhosis were reported to be in line with what has been reported in healthy volunteers [4, 23] and superior to that of L3-SMI in patients with cirrhosis [23], meaning that smaller changes can be detected by using volumetric measurements of the thighs than by using a single slice measurement of muscles around the abdomen and the spine.
We also observed that among participants with low functional status, all also had the adverse MC muscle phenotype (i.e., low muscle volume and high muscle fat). Furthermore, among those who died, all had high muscle fat and most also had concurrent low muscle volume (i.e., adverse MC phenotype). These data provide support for the hypothesis that adverse MC may be linked to mortality in LT candidates though further study in larger populations is required given the small sample in this pilot study.
The very same body composition profiling method used in this study is available for clinical use (presently in the UK, US, and Canada; AMRA® Profiler 4 MAsS Scan), and can be amended to routine clinical liver MRI examinations for patients with chronic liver disease [24]. Notably, there were cases of ascites and other decompensation events present at the time of the MRI in the present study which did not negatively impact the ability to measure body composition in the acquired images. This suggests that it is feasible to perform such measurements in patients with decompensated liver cirrhosis in a pre-LT setting; this is in agreement with another pilot study [25]. However, further study in larger populations with decompensated liver cirrhosis is required.
Our study has some limitations. The sample size was small. In addition, there is variability in the time in-between the repeated MRI examinations. However, since this a pilot study, we saw no rationale to enforce strict time points as it may pose undue cumbersome burden on this frail patient group. Strict time points may also limit the data yield of both pre- and post-LT MRI assessments since receipt of LT is not a completely predictable event. By using mixed-effects modelling we can calculate reasonable rate of change despite these differences. We also did not stratify for primary indication for LT nor etiology of liver disease. It may be of particular interest to conduct similar studies for patients with metabolic disorders such as MASLD since MASLD is becoming a dominant cause of LT. However, the population included in this pilot reflects the clinical heterogeneity of patients with ESLD. Importantly, the data presented herein aims to provide basis for further study. Larger studies should be designed to further describe, validate, and associate body composition changes before and after LT as well as the association of body composition to outcomes in patients with various etiologies of ESLD.
Conclusion
In conclusion, rapid MRI-derived body composition profiling is feasible in LT candidates and shortly after LT. This profiling can easily be amended to routine clinical scans and may help in early identification of patients who may benefit from pre- or re-habilitation interventions. In addition, the adverse MC phenotype may be highly prevalent in this patient group and body composition changes substantially over time after LT and muscle composition phenotypes may be associated to long term and functional outcomes.
Acknowledgements
N/A.
Abbreviations
- MC
Muscle composition
- ASAT
Abdominal subcutaneous adipose tissue
- AST
Aspartate aminotransferase
- BMI
Body mass index
- FFMV
Fat-free muscle volume
- INR
International normalized ratio
- LT
Liver transplant
- MELD
Model for end-stage liver disease
- MFI
Muscle fat infiltration
- MRI
Magnetic resonance imaging
- PDFF
Proton density fat-fraction
- pp
Per centage points
- SDs
Standard deviations
- VAT
Visceral adipose tissue
- z-FFMV
Fat-free muscle volume z-score
Author contributions
Participated in research design: MFF, MR, ODL, LVW. Participated in the writing of the paper: MFF, LVW. Participated in the critical review of the paper: All. Participated in the performance of the research: SP, CRH, DG, MR, LVW. Participated in the image analysis: MFF, ODL. Participated in the data analysis: MFF, MP, ODL, LVW.
Funding
Research reported here was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number UL1TR001422, and a Northwestern Medicine Transplant Endowment Grant. Dr. VanWagner was supported by the National Heart, Lung and Blood Institute, Grant Number K23HL136891 and Grant Number R56HL155093.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. Additional data from UK Biobank used in this study are publicly available through UK Biobank (Category 149, Abdominal composition).
Declarations
Ethics approval and consent to participate
The studies involving humans were approved by Northwestern University Institutional Review Board, USA. Additional data used in this study was acquired from the UK Biobank resource, project ID 6569. The UK Biobank was approved by the North West Multicenter Research Ethics Committee, UK. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Consent for publication
N/A.
Competing interests
MFF is an employee and shareholder of AMRA Medical AB. MP is an employee and shareholder of AMRA Medical AB. ODL is an employee, shareholder, and board member of AMRA Medical AB. Dr. VanWagner serves as an advisor for Gerson Lehrman Group, AlphaSights, and Noble Insights, receives research support from W.L. Gore & Associates and provides expert witness services outside the submitted work.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.van Vugt JL, Levolger S, de Bruin RW, van Rosmalen J, Metselaar HJ. Systematic review and Meta-analysis of the impact of computed tomography-assessed skeletal muscle Mass on Outcome in patients awaiting or undergoing liver transplantation. Am J Transpl. 2016;16(8):2277–92. [DOI] [PubMed] [Google Scholar]
- 2.Lai JC, Tandon P, Bernal W, Tapper EB, Ekong U, Dasarathy S, et al. Malnutrition, Frailty, and Sarcopenia in patients with cirrhosis: 2021 Practice Guidance by the American Association for the study of Liver diseases. Hepatology. 2021;74(3):1611–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borga M, West J, Bell JD, Harvey NC, Romu T, Heymsfield SB, et al. Advanced body composition assessment: from body mass index to body composition profiling. J Investig Med. 2018;66(5):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borga M, Ahlgren A, Romu T, Widholm P, Dahlqvist Leinhard O, West J. Reproducibility and repeatability of MRI-based body composition analysis. Magn Reson Med. 2020;84(6):3146–56. [DOI] [PubMed] [Google Scholar]
- 5.Linge J, Borga M, West J, Tuthill T, Miller MR, Dumitriu A, et al. Body composition profiling in the UK Biobank Imaging Study. Obes (Silver Spring). 2018;26(11):1785–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linge J, Ekstedt M, Dahlqvist Leinhard O. Adverse muscle composition is linked to poor functional performance and metabolic comorbidities in NAFLD. JHEP Rep. 2021;3(1):100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linge J, Heymsfield SB, Dahlqvist Leinhard O. On the definition of Sarcopenia in the Presence of aging and obesity-initial results from UK Biobank. J Gerontol Biol Sci Med Sci. 2020;75(7):1309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linge J, Nasr P, Sanyal AJ, Dahlqvist Leinhard O, Ekstedt M. Adverse muscle composition is a significant risk factor for all-cause mortality in NAFLD. JHEP Rep. 2023;5(3):100663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linge J, Petersson M, Forsgren MF, Sanyal AJ, Dahlqvist Leinhard O. Adverse muscle composition predicts all-cause mortality in the UK Biobank imaging study. J Cachexia Sarcopenia Muscle. 2021;12(6):1513–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolgin NH, Movahedi B, Anderson FA, Brüggenwirth IM, Martins PN, Bozorgzadeh A. Impact of recipient functional status on 1-year liver transplant outcomes. World J Transplantation. 2019;9(7):145–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.West J, Dahlqvist Leinhard O, Romu T, Collins R, Garratt S, Bell JD, et al. Feasibility of MR-Based body composition analysis in large Scale Population studies. PLoS ONE. 2016;11(9):e0163332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.West J, Romu T, Thorell S, Lindblom H, Berin E, Holm AS, et al. Precision of MRI-based body composition measurements of postmenopausal women. PLoS ONE. 2018;13(2):e0192495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karlsson A, Rosander J, Romu T, Tallberg J, Gronqvist A, Borga M, et al. Automatic and quantitative assessment of regional muscle volume by multi-atlas segmentation using whole-body water-fat MRI. J Magn Reson Imaging. 2015;41(6):1558–69. [DOI] [PubMed] [Google Scholar]
- 14.Borga M, Thomas EL, Romu T, Rosander J, Fitzpatrick J, Dahlqvist Leinhard O, et al. Validation of a fast method for quantification of intra-abdominal and subcutaneous adipose tissue for large-scale human studies. NMR Biomed. 2015;28(12):1747–53. [DOI] [PubMed] [Google Scholar]
- 15.Linge J, Nasr P, Sanyal AJ, Dahlqvist Leinhard O, Ekstedt M. Adverse muscle composition is a significant risk factor for all-cause mortality in NAFLD. Hepatology. 2022;76(S1):S746–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodward AJ, Wallen MP, Ryan J, Hall A, Ward LC, Coombes JS, et al. Is post-transplant metabolic syndrome associated with pre-liver transplant visceral adipose tissue area? Clin Nutr ESPEN. 2020;39:61–6. [DOI] [PubMed] [Google Scholar]
- 17.Montano-Loza AJ, Mazurak VC, Ebadi M, Meza-Junco J, Sawyer MB, Baracos VE, et al. Visceral adiposity increases risk for hepatocellular carcinoma in male patients with cirrhosis and recurrence after liver transplant. Hepatology. 2018;67(3):914–23. [DOI] [PubMed] [Google Scholar]
- 18.Terjimanian MN, Harbaugh CM, Hussain A, Olugbade KO Jr., Waits SA, Wang SC, et al. Abdominal adiposity, body composition and survival after liver transplantation. Clin Transpl. 2016;30(3):289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tejani S, McCoy C, Ayers CR, Powell-Wiley TM, Despres JP, Linge J et al. Cardiometabolic Health Outcomes Associated With Discordant Visceral and Liver Fat Phenotypes: Insights From the Dallas Heart Study and UK Biobank. Mayo Clin Proc. 2022;97(2):225 – 37. [DOI] [PMC free article] [PubMed]
- 20.VanWagner LB, Holl JL, Montag S, Gregory D, Connolly S, Kosirog M, et al. Blood pressure control according to clinical practice guidelines is associated with decreased mortality and cardiovascular events among liver transplant recipients. Am J Transpl. 2020;20(3):797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izzy M, Fortune BE, Serper M, Bhave N, deLemos A, Gallegos-Orozco JF, et al. Management of cardiac diseases in liver transplant recipients: Comprehensive review and multidisciplinary practice-based recommendations. Am J Transpl. 2022;22(12):2740–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forsgren MF, Balkhed W, Nasr P, Holmberg M, Kechagias S, Dahlstrom N, et al. Imaging-based test for physical frailty and sarcopenia – interim results from the prospective cirrhosis cohort study ACCESS-ESLD. Hepatology. 2022;76(S1):S1159–60. [Google Scholar]
- 23.Forsgren MF, Bhati C, Evans MC, Kamal H, Dahlqvist Leinhard O, Siddiqui MS. Imaging-based test for sarcopenia – interim results from a liver transplant waitlist natural history study. Hepatology. 2022;76(S1):S841–482. [Google Scholar]
- 24.Jamil O, Desai N, Harmath C, Charlton M. High prevalence of clinically unrecognized low muscle volume in patients with Liver Disease as assessed by Novel MRI-Based muscle Assessment Technology. Gastroenterology. 2023;164(6):S–1388. [Google Scholar]
- 25.Thuluvath AJ, Forsgren MF, Ladner DP, Duarte-Rojo A. Severity of myosteatosis correlates with Frailty in patients with end-stage liver disease. Hepatology. 2022;76(S1):S1192. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. Additional data from UK Biobank used in this study are publicly available through UK Biobank (Category 149, Abdominal composition).