Abstract
Acute coronary syndromes, such as myocardial infarction (MI), lack effective therapies beyond heart transplantation, which is often hindered by donor scarcity and postoperative complications. Human induced pluripotent stem cells (hiPSCs) offer the possibility of myocardial regeneration by differentiating into cardiomyocytes. However, hiPSC-derived cardiomyocytes (hiPSC-cardiomyocytes) exhibit fetal-like calcium flux and energy metabolism, which inhibits their engraftment. Several strategies have been explored to improve the therapeutic efficacy of hiPSC-cardiomyocytes, such as selectively enhancing energy substrate utilization and improving the transplantation environment. In this review, we have discussed the impact of altered mitochondrial biogenesis and metabolic switching on the maturation of hiPSC-cardiomyocytes. Additionally, we have discussed the limitations inherent in current methodologies for assessing metabolism in hiPSC-cardiomyocytes, and the challenges in achieving sufficient metabolic flexibility akin to that in the healthy adult heart.
Keywords: Acute coronary syndromes, Myocardial infarction, Induced pluripotent stem cells, iPSC-cardiomyocytes, Maturation, Energy metabolism
Background
Myocardial infarction (MI) is the most common type of heart failure [1], and is characterized by cardiomyocyte death following restricted blood flow due to coronary artery blockage [1]. MI-induced sudden death can occur in relatively younger patients. Surgical intervention and drugs that improve blood flow and oxygen supply to the heart can slow disease progression and partly maintain heart function, without regenerating new heart muscle [2]. Heart transplantation is currently the only effective treatment for MI, albeit with multiple limitations including scarcity of donors, surgical complexities, and high risk of complications such as postoperative immune rejection [3].
Clinical studies indicate that the above limitations can be circumvented by augmenting the number of functional cardiomyocytes in the injured heart to improve cardiac function [4, 5]. Induced pluripotent stem cells (iPSCs) reprogrammed from somatic cells, including dermal fibroblasts, peripheral blood T cells, renal tubular cells, and keratinocytes (from hair follicles, fat, and oral mucosa), have shown therapeutic potential in regenerative medicine [6, 7]. In the context of cardiac syndromes, the iPSCs can differentiate into all three types of cardiomyocytes, namely ventricular, atrial, and nodal-like cardiomyocytes [8]. However, iPSC-cardiomyocytes exhibit a more immature fetal phenotype and do not recapitulate the major characteristics of adult cardiomyocytes [9]. The metabolic reprogramming of iPSC-cardiomyocytes is a key factor in cardiac tissue remodeling [10, 11]. Therefore, we have discussed the impact of modulating the metabolism of glucose, fatty acids, ketones, and branched-chain amino acids (BCAAs) on the maturation of human iPSC-cardiomyocyte (hiPSC-cardiomyocytes) with particular focus on the limitations of these metabolic strategies.
Metabolic strategies to promote hiPSC-cardiomyocyte maturation
Major metabolic differences between hiPSC-cardiomyocytes and adult cardiomyocytes
During myocardial maturation, cardiomyocytes undergo changes in cell cycle, morphology, contractility, electrophysiological properties, and metabolism [12]. Adult cardiomyocytes produce approximately 60–70% of the total ATP through fatty acid oxidation, and have the ability to switch to carbohydrates, ketone bodies, and certain amino acids (such as BCAAs) when fatty acids are unavailable or fatty acid oxidation is compromised [13]. On the other hand, glycolysis only contributes to less than 10% of the total ATP production in cardiomyocytes (Fig. 1). In contrast, hiPSC-cardiomyocytes cultured as monolayers in vitro produced more than 50% of the total ATP from glycolysis [14, 15], and showed very little fatty acid uptake [16]. Thus, hiPSC-cardiomyocytes are metabolically similar to day 1 neonatal hearts that mainly rely on glycolysis and lactate oxidation to produce ATP [17].
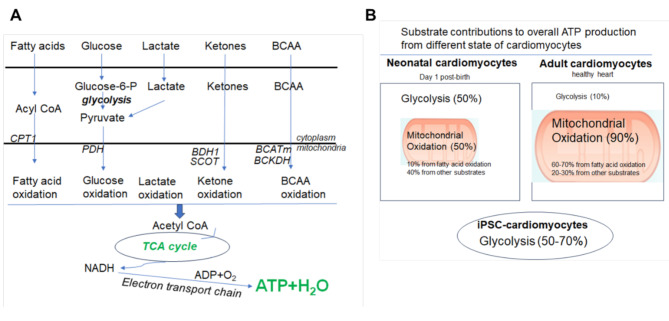
Fig. 1.
A: Energy metabolism in the adult heart: The main fuels of the adult heart are fatty acids, lactate, glucose, ketones, and branched-chain amino acids (BCAA), which must be acquired continuously from the circulation due to a low storage capacity in the heart. Fatty acids are transported into the mitochondria by carnitine-palmitoyl-transferase (CPT1). The major enzymes that control glucose oxidation, ketone oxidation and BCAA oxidation are pyruvate dehydrogenase (PDH), β-hydroxybutyrate dehydrogenase (BDH1), succinyl-CoA:3-ketoacid CoA transferase (SCOT), and mitochondrial branched-chain amino transminase (BCATm) and branched chain ketone dehydrogenase (BCKDH) respectively. The energy substrates are converted to acetyl CoA by the respective key enzymes, which is then fed into the mitochondrial electron transport chain to produce ATP. B: Energy metabolism changes during the development of the heart. Under normal physiological conditions, the contribution of glycolysis to overall ATP is around 50% in the neonatal cardiomyocytes on day 1 post-birth, which is sharply reduced to 10% in the adult cardiomyocytes. Fatty acid oxidation contributes to less than 10% overall ATP in the neonatal hearts, which is gradually increased to 60–70% in the adult heart. In contrast, glycolysis contributes to 50–74% of overall ATP in human iPSC-cardiomyocytes under monolayer culture conditions, indictive of an immature phenotype akin to that of neonatal cardiomyocytes
Why are mature hiPSC-cardiomyocytes required in regenerative therapies? Two major aspects warrant using mature hiPSC-cardiomyocytes: the usefulness of cardiotoxicity tests and the clinical outcomes post-transplantation. Cardiotoxicity tests measure defects in the structure (e.g., cell death), function (e.g., electrophysiology or contractility), and vasculature (e.g., blood flow) of cardiac tissues [18]. Mature hiPSC-cardiomyocytes can display ultrastructural and functional features of native cardiac tissue, which potentially overrides species-related differences in animal models [19, 20], ethical issues [21], and the limitations of clinical trials, such as small sample sizes and the lack of genetic and phenotypic variability [22]. An immature phenotype of hiPSC-cardiomyocytes can affect the above metrics and alter the responses to cardiotoxicity tests, resulting in less accurate estimation of biosafety [23]. Yang et al. recently demonstrated the feasibility of hiPSC-based screening for the identification of mitophagy-targeting drugs using the coral-derived protein mt-Keima, a pH-dependent fluorescent probe attached to the mitochondrial matrix [24].
Preclinical studies have assessed the outcomes of transplanting hiPSC-cardiomyocytes in small animal models [25, 26] and large animals like pigs [27] and monkeys [28]. Ventricular tachyarrhythmia has been observed post-transplantation [29], indicating that hiPSC-cardiomyocytes have limited engraftment potential [30]. At the structural level, the proarrhythmic properties of hiPSC-cardiomyocytes are associated with inadequate electrical cooperation between the implanted cells and the host myocardium [31, 32]. Mechanically, the energy produced by the immature hiPSC-cardiomyocytes is not sufficient to support the formation of electrical junctions, which is possibly the most important barrier to clinical translation.
Dhahri et al. were the first to confirm that transplantation of more mature hiPSC-cardiomyocytes yielded better structural and functional outcomes in vivo [33]. They demonstrated that hiPSC-cardiomyocytes matured through culturing with polydimethylsiloxane efficiently engrafted in infarcted guinea pig hearts, resulting in improved electromechanical integration and left ventricular contractile function, and reduced incidence of ventricular tachycardia [33]. Other studies have also indicated that mature hiPSC-cardiomyocytes have better survival rates following engraftment in the infarcted myocardium [26].
Metabolic strategies and the impact on the maturation of hiPSC-cardiomyocytes
Multiple interventions have been explored to promote the maturation of hiPSC-cardiomyocytes, such as prolonging the duration of culture [34, 35], culturing the cells in 2D [36] or 3D platforms [15, 37], exposing immature cardiomyocytes to physical [38], electrical [39], and mechanical stimuli [40–42], and including neurohormonal factors [43] and micro-RNAs (miRNAs) [44] in the culture media. The heart is an electrically and mechanically specialized organ. While 70% of the cardiac energy generated in the form of ATP supports contractile function, 30% of the ATP ensures the maintenance of ion channels and transporters [45]. Studies increasingly show that improving cellular energy production is advantageous in producing functional hiPSC-cardiomyocytes for tissue regeneration [46].
Glycolysis allows ATP generation even in the absence of oxygen [47]. Therefore, hiPSC-cardiomyocytes can be beneficial during low-flow ischemia [48] and protect the infarcted heart against post-ischemic injury [49]. On the contrary, increased glycolysis allows the shuttling of glycolytic intermediates to other biosynthesis pathways. The hexosamine biosynthesis pathway [50] is of particular interest since it introduces the O-GlcNAcylation modification in protein sequences (Fig. 2A). This post-translational modification regulates mitochondrial function and cell survival [51]. In the cardiovascular system [26, 52], increased O-GlcNAc levels have been associated with pressure-overload-induced hypertrophy [53, 54], heart failure [54], and myocardial remodeling [55], which contribute to mortality during ischemia [52, 56]. Since only 2 mol ATP is generated per mol of substrate consumed through glycolysis compared to 104 mol ATP from palmitate oxidation and 29 mol ATP from glucose oxidation [57], promoting mitochondrial oxidation over glycolysis can improve the function of the infarcted heart. Various metabolic approaches have been investigated to promote the maturation of hiPSC-cardiomyocytes, including fatty acid supplementation [36, 58–64], modulation of transcription factors that control glycolysis and fatty acid oxidation, such as hypoxia-inducible factor-1 alpha (HIF-1α) [44, 60, 65] and peroxisome proliferator-activated receptor alpha (PPARα) [60], and regulating key genes through specific miRNAs [44]. Wang et al. recently showed that the NAD+-dependent mitochondrial deacetylase sirtuin-3 (SIRT3) protein is upregulated during maturation of hiPSC-cardiomyocytes [66], and pharmacological activation of SIRT3 promoted their maturation by improving mitochondrial ultrastructure, and increasing oxidative phosphorylation by deacetylating the mitochondrial inner membrane GTPase optic atrophy 1 (OPA1) [66].
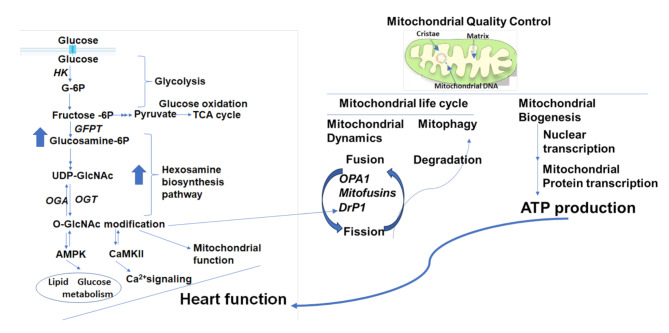
Fig. 2.
A: Hexosamine biosynthesis pathway and cardiac function. In cardiomyocytes, glucose is first converted to glucose-6-phosphate (G-6P) by hexokinase (HK). The glycolytic intermediates go through glycolysis to produce pyruvate and are fed into the mitochondrial tricarboxylic acid (TCA) cycle for glucose oxidation. In addition, glycolytic intermediates can also enter various biosynthesis pathways, such as the hexosamine synthesis pathway (HBP). The end product of this is pathway is uridine diphosphate-GlcNAc (UDP-GlcNAc), which is produced by the activity of L-glutamine-D-fructose-6-phosphate aminotransferase (GFAT). UDP-GlcNAc integrates multiple metabolic pathways to provide a feedback on overall cellular energy levels and the metabolism of fatty acids, glucose, nitrogen, and nucleotides. In addition, UDP-GlcNAc can also be used as a substrate by O-GlcNAc transferase (OGT) to add the sugar moiety into proteins. The sugar moieties are removed by O-GlcNAcase (OGA). Different stresses can increase or decrease O-GlcNAc levels, thereby affecting cardiomyocyte function. Mechanistically, AMP-activated protein kinase (AMPK) and calmodulin-dependent protein kinase II (CaMKII) are known to regulate OGT, and both in turn are regulated by OGT. Moreover, AMPK directly regulates glucose and fatty acid metabolism, and CaMKII is involved in cellular Ca2+ signaling. Therefore, O-GlcNAcylation regulates cardiac function by modulating transcription, metabolism, mitochondrial function, protein quality control, and calcium handling. B: Mechanisms of mitochondria quality control. Mitochondria are double-membraned organelles with cristae formation. Mitochondrial quality control is modulated through biogenesis, and mitochondrial dynamics through fusion, which forms elongated mitochondrial networks, and fission, which creates fragmented mitochondria. Optic atrophy 1 (OPA1) is a mitochondrial fusion protein connecting the inner mitochondrial membranes with a special role in cristae shaping/integrity. The dynamin-related protein-1 (Drp1) executes outer membrane division. Mitochondria can accumulate oxidative damage due to various stresses, resulting in fragmentation. The fission process enables the fragmentation of damaged mitochondria to be degraded via mitophagy. Interestingly, Drp1 can be modified by O-GlcNAc in cardiomyocytes, linking the HBP pathway to mitochondrial quality control
Metabolic alterations involve changes in the expression of genes regulating mitochondrial biogenesis [15, 36], fatty acid oxidation [36, 58, 59, 63, 64], and glycolysis [15, 59, 67]. For instance, upregulated mitochondrial proteins and increased fatty acid utilization are indicative of a successful metabolic shift towards a more adult-like phenotype during maturation protocols [16, 35]. Additionally, glucose oxidation rates in hiPSC-cardiomyocytes were enhanced after 3D culture [14, 15] and fatty acid supplementation [15, 62], and the latter also increased the activity of pyruvate dehydrogenase, the key enzyme of glucose oxidation [58, 68]. Discernible effects are also observed concerning the oxygen consumption rates (OCR) [59–61, 64, 69, 70], and the respiratory rates [14, 15, 68] (Table 1).
Table 1.
Metabolic changes during iPSC-cardiomyocyte maturation by different strategies
| Mitochondrial | Assessed | Maturation strategy | |||
|---|---|---|---|---|---|
| Parameters | level | Prolonged culture | 2D,3D-culture | Fatty acid supplementation | Extracellular matrices |
| Function | Gene | 35 | 15;36;37 | 36;58–64 | 42 |
| Gene | N/A | 15 | N/A | N/A | |
| Glucose uptake | Enzyme activity | N/A | N/A | N/A | N/A |
| Flux | 36 | 14;15 | 64;65 | 94 | |
| Fatty acid uptake | Gene | 35 | 15;60;61 | 42;59 | N/A |
| Flux | 36 | 14;15 | N/A | 42 | |
| Glycolysis | Gene | 15 | 14;15 | 59;67 | N/A |
| Enzyme activity | 15;35;36 | 14;37;65 | 58;64;65 | N/A | |
| OCR | 15;35;36 | 14;37;68;95 | 35;37;59; 67;69;95;96 | N/A | |
| Flux | N/A | N/A | N/A | N/A | |
| Glucose oxidation | Gene | 15 | 14;15 | N/A | N/A |
| OCR | N/A | 14 | N/A | N/A | |
| Flux | 15 | 15 | 15;58;62;68 | N/A | |
| Fatty acid oxidation | Gene | 35 | 14;15 | 36;58;59;63;64 | N/A |
| OCR | 15 | 14;15;35;37;64 | 37; 59–61;64;69;70 | N/A | |
| Flux | 15 | 14;15;62;68 | 15;68 | N/A | |
| ATP production | Gene | 15 | 14;15;36 | 44;60;95 | N/A |
| OCR | 101 | 14;36 | 61;70 | N/A | |
| Flux | 15 | 14;15;36 | 15;68 | N/A | |
Although polydimethylsiloxane-matured hiPSC-cardiomyocytes exhibit an increase in mitochondrial density and a switch from glycolysis to oxidative phosphorylation, their transcriptional profile is more comparable to fetal than adult cardiomyocytes even at day 40 [33]. The high levels of glucose in the culture media and the lack of fatty acid supplementation [33] may favor greater contractility and electrophysiological maturation compared to metabolic maturation, and prevent the hiPSC-cardiomyocytes from attaining a fully adult-like state [33]. The polydimethylsiloxane-matured hiPSC-cardiomyocytes also expressed high levels of fatty acid binding protein 3 (FABP3) [33], a regulator of cardiac fatty acid utilization [71]. However, it is unclear whether the upregulation of FABP3 in these cells led to increased fatty acid oxidation. Thus, supplementing the culture system of hiPSC-cardiomyocytes with fatty acids and polydimethylsiloxane may offer insights on the relationship between increased fatty acid utilization and transplantation outcomes.
Limitations of the maturation strategies from the metabolic aspect
The impact of ketone and BCAA metabolism on the maturation of hiPSC-cardiomyocytes. Recent studies have shown that proteins related to BCAA degradation and ketogenesis are upregulated during the differentiation of hiPSCs to cardiomyocytes, resulting in the accumulation of acetoacetate, one of the metabolic end products of ketone bodies [72]. As ketogenic substrates can be generated from fatty acids and BCAAs like leucine, cardiomyogenic differentiation may be associated with the catabolism of ketones and leucine [72]. In line with this, mitochondrial proteomic analysis has shown that BCAA catabolism is among the top upregulated pathways in the neonatal mouse heart and in hiPSC-cardiomyocytes during differentiation/maturation [73]. It is accompanied by the increased expression of key metabolic enzymes, such as BCAA transaminase, 2-oxoisovalerate dehydrogenase, 2-methylacyl-CoA dehydrogenase, enoyl-CoA hydratase, 3-hydroxy isobutyryl-CoA hydrolase, and 3-hydroxyisobutyrate dehydrogenase [73]. Mice with genetic defects in BCAA metabolic pathways have elevated plasma BCAA levels. In addition, hiPSC-cardiomyocytes incubated with high levels of BCAA showed a significant increase in arrhythmia, and this effect could not be recapitulated by other amino acids such as alanine [74]. In addition, Krüppel-like factor 15, an upstream regulator of BCAA catabolism [75], is significantly activated during the differentiation/maturation of hiPSC-cardiomyocytes [73]. BCAAs, especially leucine, mediate cell growth and proliferation by activating the mTORC1 pathway [76]. Given the role of mTORC1 in mediating cardiac hypertrophy [77], BCAAs may have undefined roles in the maturation of hiPSC-cardiomyocytes.
A ketogenic diet has been shown to protect against cardiac ischemia or reperfusion injury, which may be attributed to the role of ketones in increasing the abundance of cardiac mitochondria [78]. The overexpression of the ketogenic enzyme 3-hydroxy-3-methylglutaryl-CoA synthase 2 improved the cardiac function in a mouse model of MI by increasing the expression of phosphorylated histone H3, a proliferation marker for cardiomyocytes [79]. Given the significant changes observed in the circulating levels of ketones during the postnatal period, and the effect of ketogenic diets on diseased states through metabolic regulation, the involvement of ketones in the maturation protocols of hiPSC-cardiomyocytes and their potential in heart regeneration warrant further investigation.
The association between mitochondrial quality control and the maturation of hiPSC-cardiomyocytes. Due to the high energy requirements of the heart, cardiovascular homeostasis is heavily dependent on mitochondrial quality control (Fig. 2B), which involves mitochondrial fusion, fission, mitophagy, and biogenesis that are regulated by OPA1, mitofusins and dynamic 1-like protein [80]. Compared to mature cardiomyocytes, hiPSC-cardiomyocytes have a higher number of fragmented mitochondria with sparse cristae [73, 81, 82]. Given that mitochondrial cristae are associated with organelle function [83], the preference of iPSC-cardiomyocytes for glycolysis rather than oxidative phosphorylation can be attributed to the defective cristae in their mitochondria. OPA1 maintains mitochondrial quality by regulating fusion, cristae morphology, and oxidative phosphorylation [83–85]. Furthermore, the SIRT3/OPA1 axis promotes maturation of hiPSC-cardiomyocytes by regulating mitochondrial dynamics [66]. However, the impaired mitochondrial respiration and fatty acid oxidation induced by OPA1 inhibition in hiPSC-cardiomyocytes was only partially reversed by a mitochondrial fusion activator [66], indicating that additional pathways may regulate the metabolic changes during maturation of hiPSC-cardiomyocytes. In fact, multiple downstream targets of SIRT3 are involved in mitochondrial dynamics, such as fission regulator dynamin-related protein-1 and mitochondrial fusion protein 1 [86]. Furthermore, SIRT3 also regulates fatty acid oxidation, glycolysis and oxidative stress through long-chain acyl-coenzyme A dehydrogenase [87], HIF-1α and superoxide dismutase 2 [88] respectively. This raises the possibility that SIRT3-mediated deacetylation may play a role in regulating mitochondrial quality control during the maturation of hiPSC-cardiomyocytes. SIRT3-mediated deacetylation stimulated fatty acid oxidation in cardiomyocytes [89, 90], and regulated glycolysis and glucose oxidation in heart tissues [91, 92]. In addition, O-GlcNAcylation of dynamic protein 1in cardiomyocytes has been shown to induce mitochondrial fragmentation [93]. Thus, future studies should explore the impact of mitochondrial quality control on the metabolic changes during maturation of hiPSC-cardiomyocytes, and the possible contributions of post-translational modifications, such as acetylation and O-GlcNAcylation. Mitophagy is a major mitochondrial quality control mechanism [94]. Yang et al. demonstrated that the basal mitophagy rate was higher in differentiating hiPSC-cardiomyocytes throughout an extended period (> 120 days) without causing any overt cellular stress [24]. It remains to be elucidated whether the altered mitophagy is the primary cause or a significant contributor to metabolic reprogramming during the differentiation of hiPSC-cardiomyocytes.
Methodology
The use of distinct sets of readouts to evaluate the maturation profile of hiPSC-cardiomyocytes has limited the direct comparison between different protocols. Glycolysis has been evaluated in hiPSC-cardiomyocytes through the reduction in hexokinase activity [36, 64, 65], lactate secretion [14, 15, 37, 58, 64, 65] and glucose uptake [14, 15, 42]. Although various groups have measured glycolysis rates in terms of extracellular acidification rate (ECAR) by the Seahorse assay, the findings are ambiguous. Some studies report increased glycolysis in the hiPSC-cardiomyocytes [35, 37, 59, 67, 95], while others have reported lower [15, 68] or even unchanged ECAR [69, 96]. Fatty acid metabolism is evaluated by measuring fatty acid uptake [42, 59], fatty acid oxidation rates by assessing the OCR via the Seahorse assay [59–61, 64, 69, 70], or fatty acid flux using 13C-stable isotope [15, 68] or 14C radioactive isotope [14, 62]. Likewise, upregulation of the tricarboxylic acid cycle, along with a reduction in fatty acid synthesis [14, 15] are indicative of fatty acid metabolism in hiPSC-cardiomyocytes cultured with fatty acids [58] or in the 3D format [14, 15].
The Seahorse assay is the most common method used for assessing metabolic changes. However, it does not provide information regarding the substrate preference [97], nor the negative correlation between fatty acid oxidation and glucose metabolism via the Randel cycle [98]. The flux of radioactive or stable isotopes through specific metabolic pathways is more informative, and can be measured directly by tracing 14CO2-capture [14, 62, 69] or calculating the 13C-metabolic flux [68]. Due to the poor solubility of fatty acids in aqueous solutions, and the fact that albumin is the major fatty acid-binding protein, 3–4% albumin-bound fatty acids should be employed in the metabolic assay system to mimic the circulating levels of albumin (0.55 mmol/L in adults and 0.3 mmol/L in infants). However, only a limited number of studies have used the correct amount of albumin with fatty acids in the assay system [63], while others have either not used albumin-bound fatty acids [65] or have not indicated the amount [68, 99]. Thus, the cocktail of albumin-bound fatty acids should be standardized at physiological stoichiometry [63]. Increased overall ATP production has been reported during hiPSC-cardiomyocyte maturation [14, 15, 35, 37, 58–61, 64, 96, 100, 101]. However, whether or to what extent the increased ATP level is a promising readout of iPSC-cardiomyocyte maturation remains to be ascertained.
The mt-Keima fluorescent probe is a valuable tool for detecting mitophagy in hiPSC-cardiomyocytes [24]. Combining the mt-Keima probe with a deactivated Cas9 nuclease-fused system to block transcription of targeted genes will be provide an additional platform for investigating mitophagy-related gene function in hiPSC-cardiomyocytes. For instance, deactivated Cas9 fused to the Krüppel-associated box repression domain can be used for the temporal control of loss-of-function phenotypes in hiPSC-derived cells [102].
Parameters hitherto uninvestigated during iPSC-cardiomyocytes maturation
Besides metabolism, mitochondria are the central signaling hubs for inflammation and oxidative stress, which significantly affect cell fate. Increased mitochondrial oxidative metabolism during postnatal development is accompanied by the production of intracellular reactive oxygen species (ROS), which play a positive role in cardiomyocyte differentiation [103]. Uncoupling adenosine diphosphate phosphorylation and substrate oxidation results in a mitochondrial proton leak, which may indicate oxidative stress in hiPSC-cardiomyocytes undergoing maturation or a physiological response to the maturation strategies [35, 37, 60, 104]. However, the impact of different ambient oxygen conditions on the metabolic changes during maturation of hiPSC-cardiomyocytes has not been investigated [105]. It also remains to be ascertained whether a proton leak is a reliable indicator of hiPSC-cardiomyocyte maturation. Post-MI inflammation significantly affects graft survival and graft-host integration following cell transplantation. A recent study has shown that suppressing inflammation and fibrosis in the infarcted mouse heart increased the survival of the transplanted hiPSC-cardiomyocytes [106]. Thus, preventing inflammasome‐triggered cell death may enhance graft survival.
Several studies have reported sex-related differences in the susceptibility to heart failure, arrhythmias, and other pathological conditions [107]. Huo et al. demonstrated that male- and female-derived hiPSC-cardiomyocytes have differential sensitivity to several cardiac ion channel blockers [108]. Furthermore, Huynh et al. demonstrated a strong effect of sex on the interactions between O-GlcNAcylation, mitochondrial function, and mitochondrial quality control in mouse brains with age-dependent neurodegenerative pathogenesis [109]. Erickson et al. showed that acute elevation of extracellular glucose concentration induced O-GlcNAcylation of calmodulin-dependent protein kinase II in the cardiomyocytes, resulting in an increased propensity of arrhythmias due to the release of Ca2 + from sarcoplasmic reticulum [110, 111]. These findings warrant pre-clinical and clinical studies to investigate the effects of O-GlcNAcylation on hiPSC-cardiomyocytes. Adult cardiomyocytes are characterized by the presence of M-bands, a hallmark of mature sarcomeres, and T-tubules, the key structures of the excitation-contraction coupling mechanism [112]. However, these features have not been assessed in mature iPSC-cardiomyocytes as indicators of normal Ca2+ flux. In addition, the signaling pathway downstream of β-adrenergic receptor (β-AR) is the primary regulator of heart function in human adults [113], and plays a critical role in cardiac remodeling [114]. Sex differences exist in β-adrenergic contractile responsiveness [115], although little is known regarding the activation of this pathway during the maturation of iPSC-cardiomyocytes. Jung et al. have demonstrated that β1-AR signaling in iPSC-cardiomyocytes cultured up to 90 days did not fully recapitulate the pattern seen in adult human ventricles, and chronic stimulation of the β1-AR pathway-induced cardiotoxicity [116]. The authors have suggested that β-AR signaling is a critical readout for the functional maturity of human iPSC-cardiomyocytes in combination with other measurements [116]. Collectively, hiPSC-cardiomyocytes derived from individual male and female patients need to be compared in order to understand the influence of sex hormones on metabolic changes, expression of cardiac ion channel genes, and drug-induced proarrhythmia [108, 117] with potential parameters (Table 2).
Table 2.
Parameters are hitherto untested during iPSC-cardiomyocyte maturation
| Category | Parameters to check | References |
|---|---|---|
| Excitation-contraction coupling | M-bands, upon maturation strategies | 111 |
| T-tubes, upon maturation strategies | 111 | |
| Contractility | β -adrenergic receptors | 112;113;115 |
| Oxygen supply | Ambient oxygen conditions | 104 |
| Energy substrate supplementation | Ketone, on energy metabolism | 72 |
| BCAA, on energy metabolism | 72–75 | |
| Oxidative stress | ROS production | 35;37;60;64 |
| Inflammation | Effect on cell survival | 105 |
| Effect of sex | Effect on energy metabolism | 107; 116 |
Notable challenges and conclusions
The major challenges for the clinical translation of hiPSC-cardiomyocytes are optimization of cell survival in the infarcted heart, scaling up the production of iPSC-cardiomyocytes, and personalized drug screening.
Delivery and survival of hiPSC-cardiomyocytes in the infarcted heart
Both preclinical and clinical studies have shown that hiPSC-cardiomyocytes delivered to the infarcted heart through intracoronary infusion [118], and intravenous [119], transendomyocardial [120], or transepicardial [121] injections have poor retention [122]. The delivery of hiPSC-cardiomyocytes can be improved by using engineered tissues [118, 123], co-transplantation with other cells [124], or encapsulation in biomaterial matrices [125] or extracellular vesicles (EVs) [126]. Tominage et al. have demonstrated that EV-encapsulated hiPSC-cardiomyocytes survive longer in the infarcted rat myocardium and sustainably improve cardiac function for up to four weeks [126]. The short observation time is a limitation for the use of the tissue patch [118] or EVs [126]. Furthermore, Peinkofer et al. demonstrated that hiPSC-cardiomyocytes from the 14th day of differentiation persisted the longest in the infarcted myocardium and showed high functional integration [122], which suggests that the developmental stage is a determinant of the survival of transplanted cells [127]. Thus, future studies need to identify the exact maturation stage of hiPSC-cardiomyocytes for optimal transplantation efficiency and functional improvement. In this regard, the endpoint of maturation needs to be standardized.
Scalability of mature hiPSC-cardiomyocyte production
Regenerative treatments require billions of cells for individual patients, which is time-consuming, technically challenging, and affects the cost and reproducibility of the operation. Dhahri et al. have developed a practical method for the large-scale manufacturing of mature hiPSC-cardiomyocytes that can be cryopreserved and later thawed at high viability through 2D-culture on polydimethylsiloxan-lined roller bottles [33]. Although further improvement is required for clinical translation, as these cells do not achieve a fully adult-like phenotype and the responses to hormonal, metabolic, or pharmacological cues are unclear, a greater host-graft electromechanical integration and reduced proarrhythmic behavior were evident in the infarcted guinea pig heart following transplantation of these cells [33].
Potential for personalized drug screening
Due to the different responses of individual patients to specific drugs, personalized medicine is increasingly being explored for treating MI. The hiPSC-cardiomyocytes derived from individual patients might be effective tools for screening candidate drugs and identifying cardiotoxic drugs. Nevertheless, it is crucial to elucidate the mechanisms underlying hiPSC-cardiomyocyte maturation and to optimize the maturation status.
Conclusions
Several technical hurdles, such as maturation readouts of metabolism, must be optimized for the clinical application of hiPSC-cardiomyocytes. Interventions that simultaneously target multiple metabolic pathways might be necessary to accelerate the maturation process. Furthermore, it is crucial to establish the optimal maturation status of the hiPSC-cardiomyocytes to ensure optimal engrafting and functional improvements. Finally, patient-specific changes in hiPSC-cardiomyocytes need to be further investigated, which will greatly facilitate the development of personalized therapeutic options.
Acknowledgements
None. The authors declare that they have not used Artificial Intelligence in this study.
Abbreviations
- MI
myocardial infarction
- iPSCs
Induced pluripotent stem cells
- hiPSC-cardiomyocytes
human iPSC-derived cardiomyocytes
- BCAAs
branched-chain amino acids
- HIF-1α
hypoxia-inducible factor-1 alpha
- ECAR
extracellular acidification rate
- EVs
extracellular vesicles
- OPA1
optic atrophy 1
- OCR
oxygen consumption rates
- SIRT3
sirtuin-3
- β-AR
β-adrenergic receptor
Author contributions
Conceptualization: XJ. Formal analysis: JZ and KY. Funding acquisition: HM. Investigation: HL. Writing - original draft: YC, LP, XL and KW. Writing - review & editing: YC and LP. All authors read and approved the final manuscript.
Funding
This work was supported by the Natural Science Foundation of Jilin Province (YDZJ202401235ZYTS, YDZJ202401171ZYTS, YDZJ202401412ZYTS) and National Natural Science Foundation (no.82102306), Shenzhen Science and Technology Program (JCYJ20220531090814032). The funders had no role in study design, data collection and analysis, or preparation of the manuscript.
Data availability
No applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rubart M, Field LJ. Cardiac regeneration: repopulating the heart. Annu Rev Physiol. 2006;68:29–49. [DOI] [PubMed] [Google Scholar]
- 2.Hashimoto H, Olson EN, Bassel-Duby R. Therapeutic approaches for cardiac regeneration and repair. Nat Rev Cardiol. 2018;15:585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson CS, Durandt C, van Janse I, Praloran V, Brunet de la Grange P, Pepper MS. Targeting the aryl hydrocarbon receptor nuclear translocator complex with DMOG and Stemregenin 1 improves primitive hematopoietic stem cell expansion. Stem Cell Res. 2017;21:124–31. [DOI] [PubMed] [Google Scholar]
- 4.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–32. [DOI] [PubMed] [Google Scholar]
- 5.Takeda M, Miyagawa S, Fukushima S, Saito A, Ito E, Harada A, et al. Development of in Vitro Drug-Induced Cardiotoxicity Assay by using three-Dimensional Cardiac tissues Derived from Human Induced Pluripotent Stem cells. Tissue Eng Part C Methods. 2018;24:56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. [DOI] [PubMed] [Google Scholar]
- 8.Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat Protoc. 2013;8:162–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X, Pabon L, Murry CE. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Res. 2014;114:511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batho CAP, Mills RJ, Hudson JE. Metabolic regulation of human pluripotent stem cell-derived cardiomyocyte maturation. Curr Cardiol Rep. 2020;22:73. [DOI] [PubMed]
- 11.Ulmer BM, Eschenhagen T. Human pluripotent stem cell-derived cardiomyocytes for studying energy metabolism. Biochim Biophys Acta Mol Cell Res. 2020;1867:118471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karbassi E, Fenix A, Marchiano S, Muraoka N, Nakamura K, Yang X, et al. Cardiomyocyte maturation: advances in knowledge and implications for regenerative medicine. Nat Rev Cardiol. 2020;17:341–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O LH. Metabolism of the heart in health and disease. II. Am Heart J. 1969;77:100–22. contd. [DOI] [PubMed]
- 14.Ulmer BM, Stoehr A, Schulze ML, Patel S, Gucek M, Mannhardt I, et al. Contractile Work Contributes To Maturation of Energy Metabolism in hiPSC-Derived cardiomyocytes. Stem Cell Rep. 2018;10:834–47. [DOI] [PMC free article] [PubMed]
- 15.Correia C, Koshkin A, Duarte P, Hu D, Carido M, Sebastiao MJ, et al. 3D aggregate culture improves metabolic maturation of human pluripotent stem cell derived cardiomyocytes. Biotechnol Bioeng. 2018;115:630–44. [DOI] [PubMed] [Google Scholar]
- 16.Nose N, Werner RA, Ueda Y, Gunther K, Lapa C, Javadi MS, et al. Metabolic substrate shift in human induced pluripotent stem cells during cardiac differentiation: functional assessment using in vitro radionuclide uptake assay. Int J Cardiol. 2018;269:229–34. [DOI] [PubMed] [Google Scholar]
- 17.Lopaschuk GD, Spafford MA, Marsh DR. Glycolysis is predominant source of myocardial ATP production immediately after birth. Am J Physiol. 1991;261:H1698–705. [DOI] [PubMed] [Google Scholar]
- 18.Gavila J, Segui MA, Calvo L, Lopez T, Alonso JJ, Farto M, et al. Evaluation and management of chemotherapy-induced cardiotoxicity in breast cancer: a Delphi study. Clin Transl Oncol. 2017;19:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hochedlinger K, Jaenisch R. Nuclear reprogramming and pluripotency. Nature. 2006;441:1061–7. [DOI] [PubMed] [Google Scholar]
- 20.Rong Z, Wang M, Hu Z, Stradner M, Zhu S, Kong H, et al. An effective approach to prevent immune rejection of human ESC-derived allografts. Cell Stem Cell. 2014;14:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zwi L, Caspi O, Arbel G, Huber I, Gepstein A, Park IH, et al. Cardiomyocyte differentiation of human induced pluripotent stem cells. Circulation. 2009;120:1513–23. [DOI] [PubMed] [Google Scholar]
- 22.Anson BD, Kolaja KL, Kamp TJ. Opportunities for use of human iPS cells in predictive toxicology. Clin Pharmacol Ther. 2011;89:754–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee TYT, Coles JG, Maynes JT. iPSC-cardiomyocytes in the preclinical prediction of candidate pharmaceutical toxicity. Front Pharmacol. 2024;15:1308217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang M, Fu JD, Zou J, Sridharan D, Zhao MT, Singh H, et al. Assessment of mitophagy in human iPSC-derived cardiomyocytes. Autophagy. 2022;18:2481–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Wang D, Chen M, Yang B, Zhang F, Cao K. Intramyocardial transplantation of undifferentiated rat induced pluripotent stem cells causes tumorigenesis in the heart. PLoS ONE. 2011;6:e19012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed RP, Ashraf M, Buccini S, Shujia J, Haider H. Cardiac tumorigenic potential of induced pluripotent stem cells in an immunocompetent host with myocardial infarction. Regen Med. 2011;6:171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye L, Chang YH, Xiong Q, Zhang P, Zhang L, Somasundaram P, et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. 2014;15:750–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiba Y, Gomibuchi T, Seto T, Wada Y, Ichimura H, Tanaka Y, et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature. 2016;538:388–91. [DOI] [PubMed] [Google Scholar]
- 29.Liu YW, Chen B, Yang X, Fugate JA, Kalucki FA, Futakuchi-Tsuchida A, et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat Biotechnol. 2018;36:597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soma Y, Morita Y, Kishino Y, Kanazawa H, Fukuda K, Tohyama S. The Present State and Future perspectives of Cardiac Regenerative Therapy using human pluripotent stem cells. Front Cardiovasc Med. 2021;8:774389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lundy SD, Zhu WZ, Regnier M, Laflamme MA. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 2013;22:1991–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gherghiceanu M, Barad L, Novak A, Reiter I, Itskovitz-Eldor J, Binah O, et al. Cardiomyocytes derived from human embryonic and induced pluripotent stem cells: comparative ultrastructure. J Cell Mol Med. 2011;15:2539–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhahri W, Sadikov Valdman T, Wilkinson D, Pereira E, Ceylan E, Andharia N, et al. In Vitro Matured Human Pluripotent Stem Cell-Derived cardiomyocytes form grafts with enhanced structure and function in injured hearts. Circulation. 2022;145:1412–26. [DOI] [PubMed] [Google Scholar]
- 34.Kamakura T, Makiyama T, Sasaki K, Yoshida Y, Wuriyanghai Y, Chen J, et al. Ultrastructural maturation of human-induced pluripotent stem cell-derived cardiomyocytes in a long-term culture. Circ J. 2013;77:1307–14. [DOI] [PubMed] [Google Scholar]
- 35.Bekhite MM, Gonzalez Delgado A, Menz F, Kretzschmar T, Wu JMF, Bekfani T, et al. Longitudinal metabolic profiling of cardiomyocytes derived from human-induced pluripotent stem cells. Basic Res Cardiol. 2020;115:37. [DOI] [PubMed] [Google Scholar]
- 36.Ramachandra CJA, Mehta A, Wong P, Ja K, Fritsche-Danielson R, Bhat RV, et al. Fatty acid metabolism driven mitochondrial bioenergetics promotes advanced developmental phenotypes in human induced pluripotent stem cell derived cardiomyocytes. Int J Cardiol. 2018;272:288–97. [DOI] [PubMed] [Google Scholar]
- 37.Giacomelli E, Meraviglia V, Campostrini G, Cochrane A, Cao X, van Helden RWJ, et al. Human-iPSC-Derived cardiac stromal cells enhance maturation in 3D Cardiac microtissues and reveal non-cardiomyocyte contributions to Heart Disease. Cell Stem Cell. 2020;26:862–79. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herron TJ, Rocha AM, Campbell KF, Ponce-Balbuena D, Willis BC, Guerrero-Serna G, et al. Extracellular matrix-mediated maturation of human pluripotent stem cell-derived cardiac monolayer structure and electrophysiological function. Circ Arrhythm Electrophysiol. 2016;9:e003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruan JL, Tulloch NL, Razumova MV, Saiget M, Muskheli V, Pabon L, et al. Mechanical stress conditioning and electrical stimulation promote Contractility and Force Maturation of Induced Pluripotent Stem Cell-Derived Human Cardiac tissue. Circulation. 2016;134:1557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nunes SS, Miklas JW, Liu J, Aschar-Sobbi R, Xiao Y, Zhang B, et al. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods. 2013;10:781–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Y, Rafatian N, Feric NT, Cox BJ, Aschar-Sobbi R, Wang EY, et al. A platform for generation of Chamber-Specific Cardiac tissues and Disease modeling. Cell. 2019;176:913–27. e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mesquita FCP, Morrissey J, Monnerat G, Domont GB, Nogueira FCS, Hochman-Mendez C. Decellularized Extracellular Matrix Powder accelerates metabolic maturation at early stages of Cardiac differentiation in Human Induced Pluripotent Stem Cell-Derived cardiomyocytes. Cells Tissues Organs. 2023;212:32–44. [DOI] [PubMed] [Google Scholar]
- 43.Parikh SS, Blackwell DJ, Gomez-Hurtado N, Frisk M, Wang L, Kim K, et al. Thyroid and glucocorticoid hormones promote functional T-Tubule development in Human-Induced pluripotent stem cell-derived cardiomyocytes. Circ Res. 2017;121:1323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu JD, Rushing SN, Lieu DK, Chan CW, Kong CW, Geng L, et al. Distinct roles of microRNA-1 and – 499 in ventricular specification and functional maturation of human embryonic stem cell-derived cardiomyocytes. PLoS ONE. 2011;6:e27417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris DA, Das AM. Control of mitochondrial ATP synthesis in the heart. Biochem J. 1991;280(Pt 3):561–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiong Q, Ye L, Zhang P, Lepley M, Swingen C, Zhang L, et al. Bioenergetic and functional consequences of cellular therapy: activation of endogenous cardiovascular progenitor cells. Circ Res. 2012;111:455–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pfeiffer T, Schuster S, Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science. 2001;292:504–7. [DOI] [PubMed] [Google Scholar]
- 48.McNulty PH, Sinusas AJ, Shi CQ, Dione D, Young LH, Cline GC, et al. Glucose metabolism distal to a critical coronary stenosis in a canine model of low-flow myocardial ischemia. J Clin Invest. 1996;98:62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sabia PJ, Powers ER, Ragosta M, Sarembock IJ, Burwell LR, Kaul S. An association between collateral blood flow and myocardial viability in patients with recent myocardial infarction. N Engl J Med. 1992;327:1825–31. [DOI] [PubMed] [Google Scholar]
- 50.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–64. [DOI] [PubMed] [Google Scholar]
- 51.Chatham JC, Zhang J, Wende AR. Role of O-Linked N-Acetylglucosamine protein modification in Cellular (patho)physiology. Physiol Rev. 2021;101:427–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marsh SA, Collins HE, Chatham JC. Protein O-GlcNAcylation and cardiovascular (patho)physiology. J Biol Chem. 2014;289:34449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Facundo HT, Brainard RE, Watson LJ, Ngoh GA, Hamid T, Prabhu SD, et al. O-GlcNAc signaling is essential for NFAT-mediated transcriptional reprogramming during cardiomyocyte hypertrophy. Am J Physiol Heart Circ Physiol. 2012;302:H2122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lunde IG, Aronsen JM, Kvaloy H, Qvigstad E, Sjaastad I, Tonnessen T, et al. Cardiac O-GlcNAc signaling is increased in hypertrophy and heart failure. Physiol Genomics. 2012;44:162–72. [DOI] [PubMed] [Google Scholar]
- 55.Ritterhoff J, Tian R. Metabolism in cardiomyopathy: every substrate matters. Cardiovasc Res. 2017;113:411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–72. [DOI] [PubMed] [Google Scholar]
- 57.Fillmore N, Wagg CS, Zhang L, Fukushima A, Lopaschuk GD. Cardiac branched-chain amino acid oxidation is reduced during insulin resistance in the heart. Am J Physiol Endocrinol Metab. 2018;315:E1046–52. [DOI] [PubMed] [Google Scholar]
- 58.Nishita M, Park SY, Nishio T, Kamizaki K, Wang Z, Tamada K, et al. Ror2 signaling regulates Golgi structure and transport through IFT20 for tumor invasiveness. Sci Rep. 2017;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feyen DAM, McKeithan WL, Bruyneel AAN, Spiering S, Hormann L, Ulmer B, et al. Metabolic maturation media improve physiological function of human iPSC-Derived cardiomyocytes. Cell Rep. 2020;32:107925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gentillon C, Li D, Duan M, Yu WM, Preininger MK, Jha R, et al. Targeting HIF-1alpha in combination with PPARalpha activation and postnatal factors promotes the metabolic maturation of human induced pluripotent stem cell-derived cardiomyocytes. J Mol Cell Cardiol. 2019;132:120–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Horikoshi Y, Yan Y, Terashvili M, Wells C, Horikoshi H, Fujita S et al. Fatty acid-treated Induced Pluripotent Stem Cell-Derived Human cardiomyocytes exhibit Adult Cardiomyocyte-Like Energy Metabolism Phenotypes. Cells. 2019 Sep 17;8(9):1095. [DOI] [PMC free article] [PubMed]
- 62.Storkanova H, Oreska S, Spiritovic M, Hermankova B, Bubova K, Komarc M, et al. Plasma Hsp90 levels in patients with systemic sclerosis and relation to lung and skin involvement: a cross-sectional and longitudinal study. Sci Rep. 2021;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang X, Rodriguez ML, Leonard A, Sun L, Fischer KA, Wang Y, et al. Fatty acids enhance the maturation of Cardiomyocytes Derived from Human pluripotent stem cells. Stem Cell Rep. 2019;13:657–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ye L, Zhang X, Zhou Q, Tan B, Xu H, Yi Q, et al. Activation of AMPK promotes maturation of Cardiomyocytes Derived from Human Induced Pluripotent Stem cells. Front Cell Dev Biol. 2021;9:644667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu D, Linders A, Yamak A, Correia C, Kijlstra JD, Garakani A, et al. Metabolic maturation of human pluripotent stem cell-derived cardiomyocytes by inhibition of HIF1alpha and LDHA. Circ Res. 2018;123:1066–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang R, Xu H, Tan B, Yi Q, Sun Y, Xiang H, et al. SIRT3 promotes metabolic maturation of human iPSC-derived cardiomyocytes via OPA1-controlled mitochondrial dynamics. Free Radic Biol Med. 2023;195:270–82. [DOI] [PubMed] [Google Scholar]
- 67.Garbern JC, Helman A, Sereda R, Sarikhani M, Ahmed A, Escalante GO, et al. Inhibition of mTOR Signaling enhances maturation of Cardiomyocytes Derived from Human-Induced pluripotent stem cells via p53-Induced Quiescence. Circulation. 2020;141:285–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Correia C, Koshkin A, Duarte P, Hu D, Teixeira A, Domian I, et al. Distinct carbon sources affect structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Sci Rep. 2017;7:8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Emanuelli G, Zoccarato A, Reumiller CM, Papadopoulos A, Chong M, Rebs S, et al. A roadmap for the characterization of energy metabolism in human cardiomyocytes derived from induced pluripotent stem cells. J Mol Cell Cardiol. 2022;164:136–47. [DOI] [PubMed] [Google Scholar]
- 70.Drawnel FM, Boccardo S, Prummer M, Delobel F, Graff A, Weber M, et al. Disease modeling and phenotypic drug screening for diabetic cardiomyopathy using human induced pluripotent stem cells. Cell Rep. 2014;9:810–21. [DOI] [PubMed] [Google Scholar]
- 71.Binas B, Danneberg H, McWhir J, Mullins L, Clark AJ. Requirement for the heart-type fatty acid binding protein in cardiac fatty acid utilization. FASEB J. 1999;13:805–12. [DOI] [PubMed] [Google Scholar]
- 72.Kim S, Jeon JM, Kwon OK, Choe MS, Yeo HC, Peng X, et al. Comparative proteomic analysis reveals the Upregulation of Ketogenesis in Cardiomyocytes differentiated from Induced Pluripotent Stem cells. Proteomics. 2019;19:e1800284. [DOI] [PubMed] [Google Scholar]
- 73.Venkatesh S, Baljinnyam E, Tong M, Kashihara T, Yan L, Liu T, et al. Proteomic analysis of mitochondrial biogenesis in cardiomyocytes differentiated from human induced pluripotent stem cells. Am J Physiol Regul Integr Comp Physiol. 2021;320:R547–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Portero V, Nicol T, Podliesna S, Marchal GA, Baartscheer A, Casini S, et al. Chronically elevated branched chain amino acid levels are pro-arrhythmic. Cardiovasc Res. 2022;118:1742–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fan L, Hsieh PN, Sweet DR, Jain MK. Kruppel-like factor 15: Regulator of BCAA metabolism and circadian protein rhythmicity. Pharmacol Res. 2018;130:123–6. [DOI] [PubMed] [Google Scholar]
- 76.Vellai T. How the amino acid leucine activates the key cell-growth regulator mTOR. Nature. 2021;596:192–4. [DOI] [PubMed] [Google Scholar]
- 77.Davogustto GE, Salazar RL, Vasquez HG, Karlstaedt A, Dillon WP, Guthrie PH, et al. Metabolic remodeling precedes mTORC1-mediated cardiac hypertrophy. J Mol Cell Cardiol. 2021;158:115–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Snorek M, Hodyc D, Sedivy V, Durisova J, Skoumalova A, Wilhelm J, et al. Short-term fasting reduces the extent of myocardial infarction and incidence of reperfusion arrhythmias in rats. Physiol Res. 2012;61:567–74. [DOI] [PubMed] [Google Scholar]
- 79.Cheng YY, Gregorich Z, Prajnamitra RP, Lundy DJ, Ma TY, Huang YH, et al. Metabolic changes Associated with Cardiomyocyte dedifferentiation enable adult mammalian Cardiac Regeneration. Circulation. 2022;146:1950–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Picca A, Mankowski RT, Burman JL, Donisi L, Kim JS, Marzetti E, et al. Mitochondrial quality control mechanisms as molecular targets in cardiac ageing. Nat Rev Cardiol. 2018;15:543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garbern JC, Lee RT. Mitochondria and metabolic transitions in cardiomyocytes: lessons from development for stem cell-derived cardiomyocytes. Stem Cell Res Ther. 2021;12:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dai DF, Danoviz ME, Wiczer B, Laflamme MA, Tian R. Mitochondrial maturation in human pluripotent stem cell derived cardiomyocytes. Stem Cells Int. 2017;2017:5153625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patten DA, Wong J, Khacho M, Soubannier V, Mailloux RJ, Pilon-Larose K, et al. OPA1-dependent cristae modulation is essential for cellular adaptation to metabolic demand. EMBO J. 2014;33:2676–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li A, Gao M, Jiang W, Qin Y, Gong G. Mitochondrial dynamics in Adult cardiomyocytes and Heart diseases. Front Cell Dev Biol. 2020;8:584800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tahrir FG, Langford D, Amini S, Mohseni Ahooyi T, Khalili K. Mitochondrial quality control in cardiac cells: mechanisms and role in cardiac cell injury and disease. J Cell Physiol. 2019;234:8122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Song M, Dorn GW 2. Mitoconfusion: noncanonical functioning of dynamism factors in static mitochondria of the heart. Cell Metab. 2015;21:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yun UJ, Yang DK. Sinapic acid inhibits Cardiac Hypertrophy via activation of mitochondrial Sirt3/SOD2 signaling in neonatal rat cardiomyocytes. Antioxid (Basel). 2020 Nov 21;9(11):1163. [DOI] [PMC free article] [PubMed]
- 89.Parodi-Rullan RM, Chapa-Dubocq XR, Javadov S. Acetylation of mitochondrial proteins in the heart: the role of SIRT3. Front Physiol. 2018;9:1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jayakumari NR, Rajendran RS, Sivasailam A, Parambil ST, Reghuvaran AC, Sreelatha HV, et al. Honokiol regulates mitochondrial substrate utilization and cellular fatty acid metabolism in diabetic mice heart. Eur J Pharmacol. 2021;896:173918. [DOI] [PubMed] [Google Scholar]
- 91.Li L, Zeng H, He X, Chen JX. Sirtuin 3 alleviates Diabetic Cardiomyopathy by regulating TIGAR and Cardiomyocyte Metabolism. J Am Heart Assoc. 2021;10:e018913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang X, Ji R, Liao X, Castillero E, Kennel PJ, Brunjes DL, et al. MicroRNA-195 regulates metabolism in failing Myocardium Via alterations in Sirtuin 3 expression and mitochondrial protein acetylation. Circulation. 2018;137:2052–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gawlowski T, Suarez J, Scott B, Torres-Gonzalez M, Wang H, Schwappacher R, et al. Modulation of dynamin-related protein 1 (DRP1) function by increased O-linked-beta-N-acetylglucosamine modification (O-GlcNAc) in cardiac myocytes. J Biol Chem. 2012;287:30024–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 2018;556:239–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoshida S, Miyagawa S, Fukushima S, Kawamura T, Kashiyama N, Ohashi F, et al. Maturation of Human Induced Pluripotent Stem Cell-Derived cardiomyocytes by Soluble factors from human mesenchymal stem cells. Mol Ther. 2018;26:2681–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rakita A, Nikolic N, Mildner M, Matiasek J, Elbe-Burger A. Re-epithelialization and immune cell behaviour in an ex vivo human skin model. Sci Rep. 2020;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–9. [DOI] [PubMed] [Google Scholar]
- 99.Paige SL, Galdos FX, Lee S, Chin ET, Ranjbarvaziri S, Feyen DAM, et al. Patient-specific Induced pluripotent stem cells implicate intrinsic impaired contractility in hypoplastic Left Heart Syndrome. Circulation. 2020;142:1605–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moore GWK, Howell SEL, Brady M, Xu X, McNeil K. Anomalous collapses of Nares Strait ice arches leads to enhanced export of Arctic Sea Ice. Nat Commun. 2021;12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ding Q, Liu X, Qi Y, Yao X, Tsang SY. TRPA1 promotes the maturation of embryonic stem cell-derived cardiomyocytes by regulating mitochondrial biogenesis and dynamics. Stem Cell Res Ther. 2023;14:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mandegar MA, Huebsch N, Frolov EB, Shin E, Truong A, Olvera MP, et al. CRISPR Interference efficiently induces specific and reversible gene silencing in human iPSCs. Cell Stem Cell. 2016;18:541–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wei H, Cong X. The effect of reactive oxygen species on cardiomyocyte differentiation of pluripotent stem cells. Free Radic Res. 2018;52:150–8. [DOI] [PubMed] [Google Scholar]
- 104.Jastroch M, Divakaruni AS, Mookerjee S, Treberg JR, Brand MD. Mitochondrial proton and electron leaks. Essays Biochem. 2010;47:53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brodarac A, Saric T, Oberwallner B, Mahmoodzadeh S, Neef K, Albrecht J, et al. Susceptibility of murine induced pluripotent stem cell-derived cardiomyocytes to hypoxia and nutrient deprivation. Stem Cell Res Ther. 2015;6:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sirish P, Thai PN, Lee JH, Yang J, Zhang XD, Ren L, et al. Suppression of inflammation and fibrosis using soluble epoxide hydrolase inhibitors enhances cardiac stem cell-based therapy. Stem Cells Transl Med. 2020;9:1570–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hayward CS, Kelly RP, Collins P. The roles of gender, the menopause and hormone replacement on cardiovascular function. Cardiovasc Res. 2000;46:28–49. [DOI] [PubMed] [Google Scholar]
- 108.Huo J, Wei F, Cai C, Lyn-Cook B, Pang L. Sex-related differences in Drug-Induced QT Prolongation and Torsades De Pointes: a New Model System with Human iPSC-CMs. Toxicol Sci. 2019;167:360–74. [DOI] [PubMed] [Google Scholar]
- 109.Huynh VN, Benavides GA, Johnson MS, Ouyang X, Chacko BK, Osuma E, et al. Acute inhibition of OGA sex-dependently alters the networks associated with bioenergetics, autophagy, and neurodegeneration. Mol Brain. 2022;15:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, et al. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature. 2013;502:372–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lu S, Liao Z, Lu X, Katschinski DM, Mercola M, Chen J, et al. Hyperglycemia acutely increases cytosolic reactive oxygen species via O-linked GlcNAcylation and CaMKII activation in mouse ventricular myocytes. Circ Res. 2020;126:e80–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ziman AP, Gomez-Viquez NL, Bloch RJ, Lederer WJ. Excitation-contraction coupling changes during postnatal cardiac development. J Mol Cell Cardiol. 2010;48:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res. 2003;93:896–906. [DOI] [PubMed] [Google Scholar]
- 114.Communal C, Singh K, Pimentel DR, Colucci WS. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the beta-adrenergic pathway. Circulation. 1998;98:1329–34. [DOI] [PubMed] [Google Scholar]
- 115.McIntosh VJ, Chandrasekera PC, Lasley RD. Sex differences and the effects of ovariectomy on the beta-adrenergic contractile response. Am J Physiol Heart Circ Physiol. 2011;301:H1127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jung G, Fajardo G, Ribeiro AJ, Kooiker KB, Coronado M, Zhao M, et al. Time-dependent evolution of functional vs. remodeling signaling in induced pluripotent stem cell-derived cardiomyocytes and induced maturation with biomechanical stimulation. FASEB J. 2016;30:1464–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Akdis D, Saguner AM, Shah K, Wei C, Medeiros-Domingo A, von Eckardstein A, et al. Sex hormones affect outcome in arrhythmogenic right ventricular cardiomyopathy/dysplasia: from a stem cell derived cardiomyocyte-based model to clinical biomarkers of disease outcome. Eur Heart J. 2017;38:1498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gao L, Gregorich ZR, Zhu W, Mattapally S, Oduk Y, Lou X, et al. Large cardiac muscle patches Engineered from Human Induced-Pluripotent Stem Cell-Derived Cardiac cells improve recovery from myocardial infarction in Swine. Circulation. 2018;137:1712–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Halkos ME, Zhao ZQ, Kerendi F, Wang NP, Jiang R, Schmarkey LS, et al. Intravenous infusion of mesenchymal stem cells enhances regional perfusion and improves ventricular function in a porcine model of myocardial infarction. Basic Res Cardiol. 2008;103:525–36. [DOI] [PubMed] [Google Scholar]
- 120.Gyongyosi M, Dib N. Diagnostic and prognostic value of 3D NOGA mapping in ischemic heart disease. Nat Rev Cardiol. 2011;8:393–404. [DOI] [PubMed] [Google Scholar]
- 121.Karantalis V, Hare JM. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res. 2015;116:1413–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Peinkofer G, Maass M, Pfannkuche K, Sachinidis A, Baldus S, Hescheler J, et al. Persistence of intramyocardially transplanted murine induced pluripotent stem cell-derived cardiomyocytes from different developmental stages. Stem Cell Res Ther. 2021;12:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wendel JS, Ye L, Tao R, Zhang J, Zhang J, Kamp TJ, et al. Functional effects of a tissue-Engineered Cardiac Patch from Human Induced Pluripotent Stem Cell-Derived cardiomyocytes in a rat infarct model. Stem Cells Transl Med. 2015;4:1324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sahito RGA, Sheng X, Maass M, Mikhael N, Hamad S, Heras-Bautista CO, et al. In Vitro grown micro-tissues for Cardiac cell replacement therapy in vivo. Cell Physiol Biochem. 2019;52:1309–24. [DOI] [PubMed] [Google Scholar]
- 125.Mayfield AE, Tilokee EL, Latham N, McNeill B, Lam BK, Ruel M, et al. The effect of encapsulation of cardiac stem cells within matrix-enriched hydrogel capsules on cell survival, post-ischemic cell retention and cardiac function. Biomaterials. 2014;35:133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tominaga Y, Kawamura T, Ito E, Takeda M, Harada A, Torigata K et al. Pleiotropic effects of extracellular vesicles from induced pluripotent stem cell-derived cardiomyocytes on ischemic cardiomyopathy: a preclinical study. J Heart Lung Transpl. 2024 Jan;43(1):85–99. [DOI] [PubMed]
- 127.Halbach M, Baumgartner S, Sahito RG, Krausgrill B, Maass M, Peinkofer G, et al. Cell persistence and electrical integration of transplanted fetal cardiomyocytes from different developmental stages. Int J Cardiol. 2014;171:e122–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No applicable.