Abstract
Certain classes of antibiotics, including tetracyclines and macrolides, are known to exert immune suppressive effects in other species but the immune modulatory effects of these antibiotics have not been previously studied in cattle. To address this question, we investigated the effects of oxytetracycline, gamithromycin, and tulathromycin on T cell and macrophage responses to activation, using in vitro assays. In addition, we assessed the impact of these antibiotics on T cell responses in vivo following treatment of healthy cattle with currently recommended doses of each of the three antibiotics. We found that all 3 antibiotics markedly suppressed T cell proliferation in vitro at relevant therapeutic drug concentrations and significantly suppressed macrophage activation responses to LPS. In cattle treated with a single dose of each antibiotic, we observed significant suppression of T cell proliferation and cytokine production beginning as early as 6 h after administration, with increasing immune suppression observed at 48 h. Taken together, these results indicate that commonly used antibiotics in cattle exert significant immune modulatory activity, in addition to their antimicrobial activity. These off-target effects should be considered when using antibiotics for prophylaxis or metaphylaxis in high-risk dairy or beef cattle (192 words).
Keywords: Lymphocytes, Macrophages, Cytokines, Metaphylaxis
Introduction
Stresses imposed on cattle by weaning, shipping and co-mingling of cattle greatly increases their susceptibility to bovine respiratory disease complex (BRDC) (Krehbiel [13]). Accordingly, cattle are frequently treated with antibiotics in these settings (antimicrobial metaphylaxis) to reduce clinical manifestations of infection (Ives and Richeson [11]). While the potential impact of antibiotic therapy on antimicrobial drug-resistance is well known in the dairy and beef cattle industries, much less is known about the potential off-target effects of antibiotic administration on immune responses in cattle. Studies in other species (rodents, humans, porcine) indicate that some commonly used antibiotics (e.g., macrolides and tetracyclines) are capable of suppressing adaptive and innate immune responses, including immune responses to vaccines (Tauber and Nau [32]) (Goscinski et al. [8]) (Wyns et al. [20]) (Parnham [21]). In rodent models, antibiotics can suppress inflammation and innate immune defenses, including defenses against viral and bacterial infections (Zhang et al. [5]) (Feola et al. [7]). In vitro studies have demonstrated similar phenomena with antibiotics and modulation of human immune responses (Lin et al. [15]) (Ratzinger et al. [28]). For example, macrolide antibiotics such as azithromycin can drive macrophage polarization towards an anti-inflammatory state (Zhang et al. [5]). Additionally, in vitro treatment with azithromycin has been shown to reduce the expression of the activation markers CD80 and CD86 on dendritic cells (DCs) (Lin et al. [15]) and to suppress CD4 T cell activation (Ratzinger et al. [28]). Importantly, recent studies in swine demonstrated that antibiotics administered at the time of vaccination significantly suppressed vaccine responses and reduced vaccine titers (Pomorska-Mol et al. [25]) (Pomorska-Mol et al. [26]) (Pomorska-Mol et al. [27]).
Thus, there is strong evidence to suggest that antibiotics may exert similar off-target, immune modulatory effects in cattle (Watts et al. [35]) (Coppin et al. [4]) (Martinez-Cortes et al. [16]). Accordingly, in the present study we investigated, using both in vitro and in vivo studies, the immune modulatory properties of 3 commonly used antibiotics (oxytetracycline, tulathromycin and gamithromycin) in cattle. We found that all 3 of these drugs significantly suppressed T cell and macrophage functions in vitro. Moreover, in vivo we found that a single injection of each of these antibiotics in healthy adult cattle rapidly and significantly suppressed T cell and innate immune responses for at least 48 h. Collectively, these findings indicate that administration of certain commonly used antibiotics to cattle as metaphylaxis may paradoxically increase susceptibility to infection and interfere with vaccine immunity and suggest further reason for caution with antibiotic use in such settings.
Materials and methods
Biochemical reagents and antibiotics
Three different antibiotics were selected for evaluation in this study based on their widespread use in the cattle industry, including oxytetracycline (Millipore-Sigma, St. Louis, MO) (trade name: Bio-Mycin), tulathromycin (Millipore-Sigma, St. Louis, MO) (trade name: Draxxin) and gamithromycin (Millipore-Sigma, St. Louis, MO) (trade name: Zactran). The concentrations of stock antibiotic solutions were prepared as follows: Oxytetracycline stocks were prepared in DMSO at 3 mg/ml and diluted in cell growth medium to either 1, 0.1–0.01 µg/ml immediatlely prior to use. Tulathromycin was prepared as 0.5 mg/ml stock solution in 50% EtOH: PBS and diluted similarly to desirable assay concentrations in complete cellular growth medium immediately before use. Tulathromycin is not stable in pure aqueous solution. Gamithromycin stocks were prepared in DMSO at 5 mg/ml. And diluted in cell growth medium similar to the other antibiotics.
Cell culture medium
Peripheral blood mononuclear cells (PBMC) were prepared from EDTA anti-coagulated blood. obtained from healthy cattle, by Ficoll density gradient purification, as described previously (Wheat et al. [37]). Blood for in vitro assays was obtained from adult (1–3 years) female Holstein cattle. Briefly, for separation of PBMC, blood was diluted 1:2 with sterile PBS followed by layering over a Ficoll-Paque PLUS® (GE Healthcare, Chicago, IL) gradient and centrifuged. All studies involving blood collection from healthy animals were approved by the Institutional Animal Care and Use Committee at Colorado State University. After separation, PBMC were counted and placed in complete medium, which consisted of DMEM (ThermoFisher Scientific, Watham, MA) containing 15% fetal bovine serum (FBS) (Avanti, Alabaster, AL) with L-glutamine, essential and non-essential amino acids and penicillin and streptomycin (Gibco and ThermoFisher Scientific, Pittsburgh, PA). Pen/Strep was omitted or removed from cultures being tested for antibiotic-mediated immune modulation by washing the cells 3X in PBS 24 h prior to treatment with the test antibiotics.
Flow cytometry
For analysis of immune cell modulation by antibiotics, either PBMC or monocyte-derived macrophages (MDM) were harvested and resuspended in flow cytometry buffer (PBS, 5%FBS and 0.01% sodium azide) and immunostained using directly conjugated antibodies for analysis by flow cytometry. The following antibodies were used to measure immune activation of bovine PBMC or MDM: the bovine cross-reactive rat anti-human CD3-Pacific Blue (PB) (clone CD3-12); mouse anti-bovine CD25(IL-2Ra)-RPE and mouse-anti-bovine MHC class II-FITC (Bio-Rad, Hercules, CA). Bovine PBMC were gated on live cells based on propidium iodide (PI) exclusion and typically displayed very low forward vs. side scatter. Single cells were gated on linear events displayed on dot plots of forward scatter-area vs. height. Live MDM were gated on live cells based on PI exclusion. After immunostaining, cells were resuspended in flow cytometry buffer (PBS containing, 2% BSA and 2mM sodium azide) and data acquired using a Beckman-Coulter Gallios multicolor flow cytometer. Flow cytometry data were analyzed using FlowJo software (FlowJo, Ashland, OR).
Impact of antibiotic treatment on T cell proliferation
For assessment of the impact of antibiotics on T cell proliferation, antibiotics were each tested at 3 different concentrations (1.0 µg/ml, 0.1 µg/ml and 0.01 µg/ml), which were selected to reflect the representative range of serum antibiotic concentrations achievable with current animal dosing recommendations. The PBMC were resuspended in complete DMEM tissue culture medium containing 15% FBS. T cell proliferative responses were assessed using Click-iT technology (ThermoFisher, Waltham, MA). Briefly, bovine PBMCs were separated by Ficoll plaque solution (GE Healthcare, Chicago, IL) and plated at a density of 4 × 106 cells per well in a 24 well cell culture plate (Corning) and then incubated for 3 h with antibiotics at the indicated concentrations followed by the addition of Concanavalin A (Con A) (Sigma-Aldrich, St. Louis, MO) at 5 µg/ml. EdU (5-ethynyl-2’-deoxyuridine) was added to a concentration of 0.01 mM at 24 h. At 48 and 72 h post-treatment, PBMCs were washed and fixed with 4% paraformaldehyde in PBS (ThermoFisher, Waltham, MA) then permeabilized with 0.1% saponin-based permeabilization buffer and wash reagent (BD Biosciences, San Jose, CA). Click chemistry-based detection was then performed by adding 1mM CuSO4, 50 mM ascorbic acid according to manufacture’s protocol using the Click-iT™ Plus Alexa Fluor Picolyl Azide Toolkit (ThermoFisher). Cells were evaluated for Edu incorporation in a CD3+ cell gate using a Beckman Coulter Gallios flow cytometer (Brea, CA) and data were analyzed using FlowJo Software (Ashland, OR).
Measurement of macrophage functional responses
PBMC were obtained from healthy cattle as described above and plated in triplicate wells per test in 24-well plates at a concentration of 3 × 106 cells/ml for 4 h at 37ºC to allow for monocyte adherence to plastic. Non-adherent cells were then gently removed by decanting and washing wells with warm PBS, and complete DMEM + 15%FBS containing 30 ng/ml of cross-reactive recombinant human macrophage colony stimulating factor (hM-CSF) (PeproTech, Rocky Hill, NJ) was added to the plastic-adherent monocytes in the culture wells. Every 3 days, 50% of the medium was removed and fresh medium containing hM-CSF was added. After a total of 7 days in culture, adherent cells were washed again (3X) with antibiotic-free medium and the resulting MDM were treated with appropriate concentrations of antibiotics for 3 h prior to adding LPS (300 ng/ml) to activate the cells. Cells were allowed to activate for 36 h. Supernatants were subsequently harvested for determination of cytokine secretion by ELISA and nitric oxide (NO) release. Cells were also stained for surface MHCII expression or intracellularly for total cellular reactive oxygen species (ROS) (see below). MDM were harvested from the wells by washing the adherent cells 3X with pre-warmed PBS followed by addition of 100 µl of ice-cold PBS containing 5 mM EDTA. Plates were then transferred on ice for 15–20 min and MDM were detached from the plate with moderate repeated agitation using a P200 Pipetman® (Gilson, Middleton, WI). Detached cells were transferred to a 96-well plate for immunostaining and flow cytometric analysis.
Reactive oxygen and nitrogen assays for assessment of macrophage bactericidal function and antibiotic effects
Quantification of LPS-induced ROS production in vitro was performed by loading MDM with a chemically reduced fluorescein (H2DCFDA, Invitrogen, Carlsbad, CA) according to manufacturer’s protocol. Briefly, MDM were cultured/maintained in 24-well plates and treated with antibiotics for 12–18 h prior to loading with H2DCFDA. Freshly prepared 100 µM H2DCFDA in complete DMEM was added to the cells and allowed to load for 15 min followed by LPS treatment (500 ng/ml) for 30 min to stimulate ROS production. Cells were immediately washed and resuspended with FACS buffer (above) and analyzed by flow cytometric analysis. To assess ROS production, oxidized fluorescein production was assessed by flow cytometry, as the mean fluorescence intensity (MFI) of events in the FL1 fluorescein channel. The Griess reagent assay system (Promega, Madison, WI) was used to measure nitric oxide (NO) release by MDM by assessing formation of nitrate (NO2 -) in culture supernatants, according to manufacturer’s protocol.
Cytokine assays for assessment of T cell and monocyte functional responses
Conditioned medium was collected from all immune stimulated and antibiotic treated PBMC or MDM and cytokine concentrations (INF-g, TNF-a and IL-6) were determined using commercially available ELISA kits (R & D Systems, Minneapolis, MN) according to manufacturer’s recommendation.
In vivo assessment of antibiotic-induced immune modulation in cattle
Studies in healthy 4–6-month-old weaned Angus and Angus cross cattle were conducted at the Hunter Cattle Company, Wheatland WY. These studies were approved by the Veterinary Research and Consulting Services (VRCS) Institutional Animal Care and Use Committee. Cattle were randomly separated into 4 groups of n = 5 animals per group. Animals in the following groups were treated once with single subcutaneous. injections of the indicated test material: PBS injection (control, Group 1), 2.5 mg/kg dose of tulathromycin (Draxxin Zoetis, Parsippany-Troy Hills, NJ)(2.5 mg/kg); (Group 2), 6 mg/kg gamithromycin (Zactran, Boehringer Ingelheim, Duluth GA) (Group 3), 20 mg/kg oxytetracycline (Bio-Mycin, Boehringer Ingelheim) (Group 4).
Blood for PBMC analysis was collected by jugular venipuncture from all study animals immediately prior to injection and again at 5 h and 48 h after injection. PBMC were prepared from blood samples as described above and were either untreated or treated with 5 µg/ml of ConA to stimulate T cell proliferation and activation for 72 h. Following in vitro activation, PBMC were immunostained for CD3, CD25, and MHCII expression, and quantified by flow cytometry, as noted above. Supernatants from the PBMC cultures were also analyzed for secretion of cytokines (IFN-g, TNF-a, IL-6) by ELISA, as described above.
Data analysis
Statistical analysis
Statistical tests were performed as indicated in the figure legends. Calculations were performed using GraphPad Prism version 9.0 for MacOS (San Diego, CA). Unless specifically indicated One- or Two-Way ANOVA with Tukey’s multiple comparisons test were used throughout this study.
Results
In vitro antibiotic treatment suppresses T cell proliferation
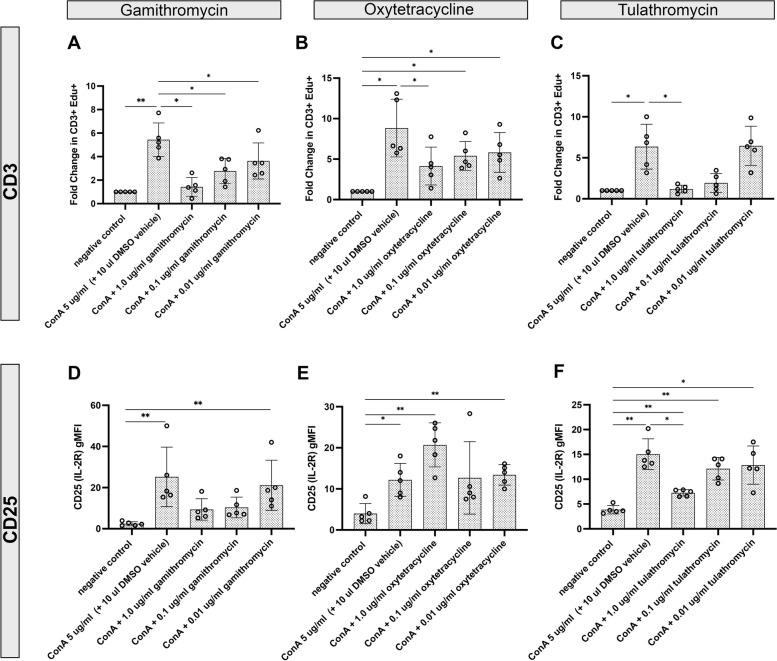
In vitro treatment of PBMC with each of the 3 antibiotics tested each significantly suppressed T cell proliferative responses, and all 3 doses evaluated were found to be suppressive (Fig. 1, panels A, B & C). Notably, the most potent suppression was observed following treatment with gamithromycin and tulathromycin, both of which showed greater than 80% suppression of T cell proliferation compared to untreated PBMC. Gamithromycin treatment significantly (p < 0.05) abrogated ConA-stimulated CD3+ T cell proliferation at all concentrations of the antibiotic evaluated (Fig. 1, panel A). However, only the highest concentration of oxytetracycline (1.0 µg/ml) evaluated significantly reduced CD3+ T cell proliferative responses (Fig. 1, panel B). Tulathromycin treatment resulted in significant reduction in CD3 T cell proliferative responses in the two highest antibiotic concentrations evaluated (Fig. 1, panel C). After 72 h, T cell proliferative responses were significantly reduced with both 1.0 µg/ml and 0.1 µg/ml tulathromycin concentrations (data not shown). To determine whether antibiotic-mediated abrogation of CD3+ T cell proliferative responses was associated with differences in expression of a key T cell activation receptor (high-affinity IL-2 receptor a chain-CD25), flow cytometry was used to assess the impact of treatment on T cell expression of CD25. Only treatment of PBMC with 1.0 µg/ml tulathromycin significantly reduced expression of CD25 on CD3+ T cells after 48 h (Fig. 1, panel F) and at all concentrations (1.0, 0.1 & 0.01 µg/ml) after 72 h of culture (data not shown). Neither gamithromycin nor oxytetracycline treatment significantly reduced CD25 expression on CD3 + cells (Fig. 1, panels D & E).
Fig. 1.
Treatment of blood leukocytes with gamithromycin, oxytetracycline and tulathromycin suppresses T cell proliferation and reduces expression of CD25 in vitro. In vitro treatment of PBMC from healthy cattle with clinically relevant concentrations (indicated on the x-axis) of gamithromycin (A & D), oxytetracycline (B & E), or tulathromycin (C & F) resulted in decreased CD3+ T cell proliferative responses (A, B and C) and expression of CD25 (D, E and F) to mitogenic stimulation with ConA. PBMC cultures were plated in triplicate wells of 24-well plates and were either untreated or treated for 3 h in the absence or presence of the indicated antibiotics followed by treatment with 5 µg/ml ConA and cultured an additional 48 h. After treatment with ConA, EdU was added to each well and cells were analyzed by flow cytometry as described in Methods. The y-axis represents the fold change of cells incorporating EdU or gMFI of cell surface expression of CD25 relative to those treated with ConA in the absence of antibiotics. Negative controls are untreated cells. Each open circle symbol in the bar graphs represent the average of triplicate cultures from each of 5 animals. Significant differences were obtained using an ordinary one-way ANOVA with p values of **, ≤ 0.01 and *, ≤ 0.05
Antibiotic treatment suppresses secretion of IFN-g, TNF-a and IL-6 following T cell activation in vitro
Supernatants were harvested from PBMC that were activated with ConA and treated with antibiotics, as described above and analyzed for INF-g, TNF-a and IL-6 release by cytokine ELISA. Each of the 3 antibiotics evaluated resulted in a significant, dose-dependent reduction in IFN-g secretion. Gamithromycin and oxytetracycline treatment each significantly suppressed secretion of INF-g from ConA stimulated PBMC at all antibiotic concentrations examined after 48 h (Fig. 2, panels A & B). Treatment with tulathromycin, reduced IFN-g only at the 2 highest concentrations (Fig. 2, panel C). Gamithromycin treatment at all concentrations inhibited TNF-a release in 48 h cultures when compared to cells treated with ConA alone (Fig. 2, panel D). Treatment with oxytetracycline significantly abrogated TNF-a release in 48 h cultures (Fig. 2. panel E). Similarly, treatment of PBMC with tulathromycin also significantly reduced TNF-a release (Fig. 2, panel F). Treatment with the two highest dosages of each antibiotic also significantly suppressed IL-6 production (Fig. 2, panel G, H, I). Thus, cytokine secretion was also broadly suppressed by antibiotic treatment, in concordance with the reduction in T cell proliferation noted above.
Fig. 2.
Treatment of Con A stimulated bovine PBMC with either gamithromycin, oxytetracycline or tulathromycin, reduced secretion of IFN-g, TNF-a and IL-6. Supernatants from cultures obtained in Fig. 1 were further analyzed by ELISA for secretion of IFN-g (A, B & C), TNF-a (D, E & F) and IL-6 (G, H & I). Significant differences were obtained using an ordinary one-way ANOVA with p values of ****, ≤ 0.001; ***, ≤ 0.005, **, ≤ 0.01 and *, ≤ 0.05
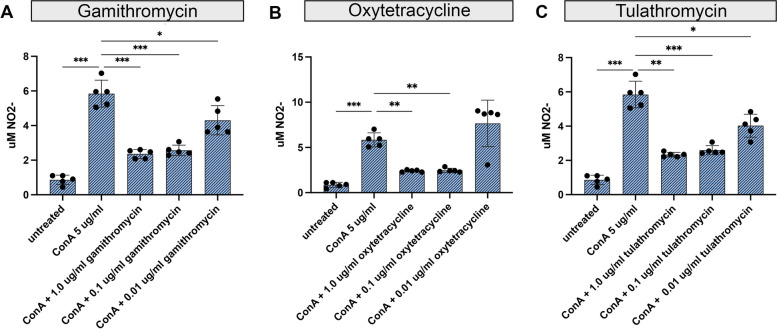
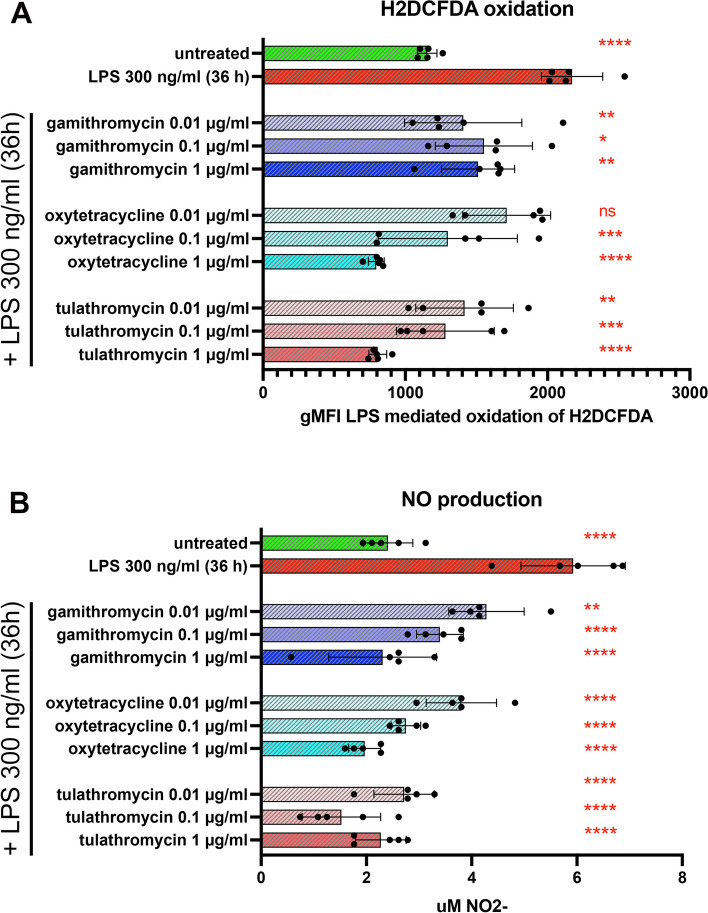
Suppression of nitric oxide (NO) production by antibiotic treatment
Production of NO by macrophages and neutrophils constitutes a key antimicrobial defense mechanism and, as such, the potential impact of antibiotics on this pathway is highly relevant to host bacterial defenses (Wink et al. [38]). Therefore, NO production was examined in supernatants from activated T cell cultures, with or without antibiotic treatment. Treatment with gamithromycin and tulathromycin significantly reduced NO production at all three antibiotic concentrations tested (Fig. 3, Panels A & C). Significant reductions in NO release were observed for oxytetracycline at the highest antibiotic concentrations tested (0.1 and 1.0 µg/ml) (Fig. 3, Panel, B).
Fig. 3.
Treatment of bovine PBMC with gamithromycin, oxytetracycline or tulathromycin, significantly reduced release of nitric oxide. Supernatants from cultures obtained in Fig. 1 were analyzed for nitric oxide (NO) production. Supernatants were analyzed for production of NO2 - release using the Griess Reagent assay. Treatment of bovine PBMC with ConA either alone or pretreated with 3 concentrations of either gamithromycin (A), oxytetracycline (B or tulathromycin (C) is shown. The figure is representative of 3 separate experiments. Significant differences were obtained using an ordinary one-way ANOVA with p values of ****, ≤ 0.001; ***, ≤ 0.005, **, ≤ 0.01 and *, ≤ 0.05
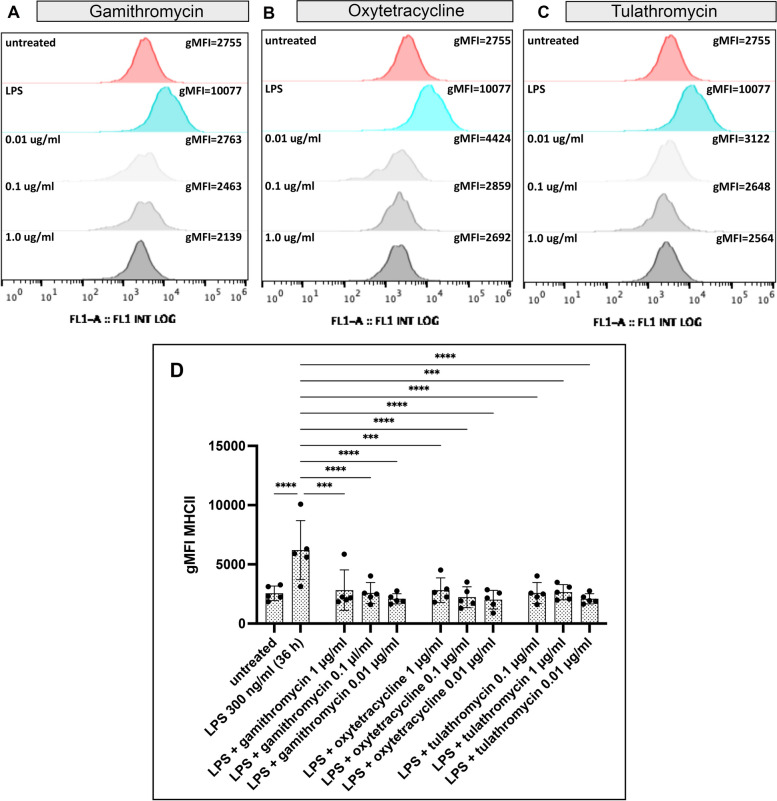
Antibiotic suppression of macrophage MHCII expression
Expression of the MHCII molecule plays a critical role in the ability of macrophages to present antigens to CD4+ T cells. Therefore, we examined the impact of antibiotic exposure on MHCII expression as an indirect measure of how antibiotics might affect the generation of antibody responses. To address the question, MDM cultures were generated from 5 healthy animals, as described in Methods. On day 7, MDM were cultured with gamithromycin, oxtetracycline or tulathromycin for 24 h, and then activated with LPS to stimulate upregulation of MHCII expression for 24 h. The MDM were subsequently detached and MHCII expression was quantitated by flow cytometry, as described in Methods (Fig. 4, Panels A, B, C & D). We observed that all three concentrations of each of the 3 antibiotics each significantly suppressed LPS-mediated upregulation of MHCII expression, reducing the expression to levels expressed by untreated MDM. Therefore, one might expect that this antibiotic-induced block to MHCII expression could negatively impact generation of T cell and antibody responses to vaccines and viral and bacterial infections.
Fig. 4.
Treatment of MDM with gamithromycin oxytetracycline or tulathromycin suppresses LPS-mediated upregulation of MHCII. Cultures of MDC were generated from 5 healthy animals as noted in Methods, and were pre-treated with gamithromycin, oxytetracycline or tulathromycin at the indicated concentrations, for 3 h prior to activation with LPS. Control or antibiotic-treated MDM were incubated for 36 h with 300 ng/ml LPS followed by cell harvesting and immunostaining with FITC-conjugated mouse anti-bovine MHCII antibody. Comparative differences in MHCII expression were determined by flow cytometry. Raw flow cytometric data showing histograms representing levels of the geometric mean fluorescent intensity (gMFI) of antibody bound to cells indicating the expression of MHCII are shown to compared expression levels of MHCII in MDM treated with either nothing, LPS, or LPS-stimulated cells pre-treated with the indicated concentrations of gamithromycin (A), oxytetracycline (B) or tulathromycin (C). A summary of comparisons of groups consisting of 5 animals each were made based on differential flow cytometric quantitative FITC gMFI observed on MDM from each animal (D). The figure is representative of 3 independent experiments. Differences between experimental samples and those treated with LPS alone were determine using an ordinary one-way ANOVA with: *, P ≤ 0.05; **, P ≤ 0.01 and ****, P ≤ 0.001
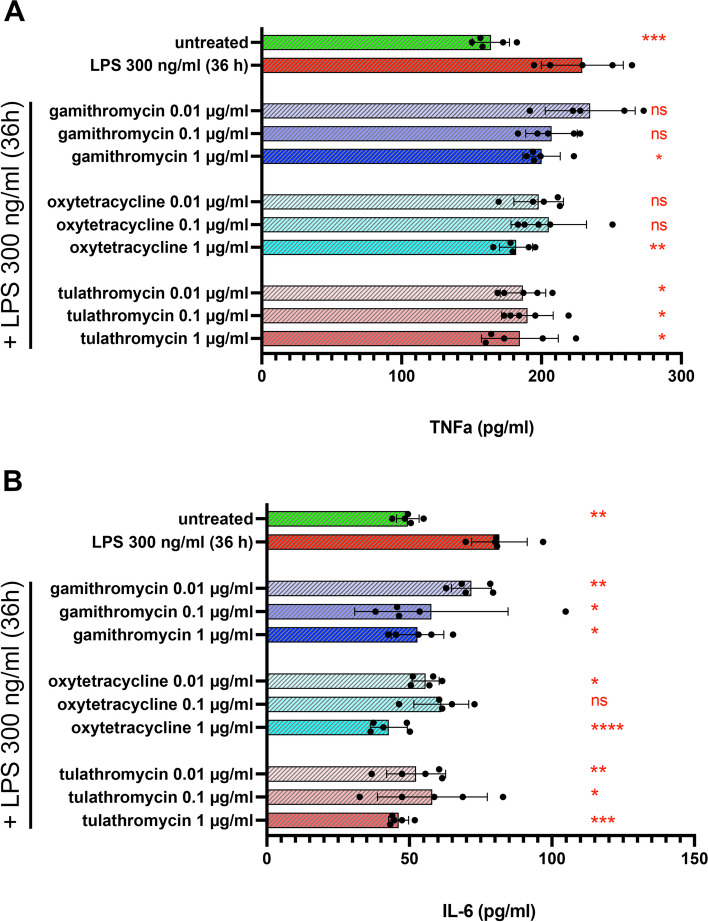
Antibiotic treatment suppresses macrophage production of TNF-a and IL-6
To determine whether antibiotic treatment altered macrophage secretion of two important innate immunity mediators, TNF-a and IL-6, supernatants from LPS-activated MDM cultures were measured to assess the antibiotic impact on another aspect of macrophage activation. At the highest concentrations (1 µg/ml) evaluated, all 3 antibiotics significantly suppressed LPS-induced secretion of TNF-a, while tulathromycin was also active at the two lower concentrations tested (Fig. 5, Panel A). All 3 antibiotics significantly suppressed IL-6 secretion from MDM, at all 3 concentrations evaluated (Fig. 5, Panel B). Thus, these findings provide further evidence that antibiotics exerted substantial in vitro inhibition of key macrophage inflammatory cytokines required to activate macrophages and neutrophils for bactericidal activity.
Fig. 5.
Treatment of MDM with gamithromycin, oxytetracycline or tulathromycin suppresses LPS-mediated upregulation of TNF-a and IL-6. MDM cultures were treated with gamithromycin, oxytetracycline or tulathromycin for 3 h prior to activation with LPS. Control or antibiotic-pre-treated MDM were incubated for 36 h with 300 ng/ml LPS followed by harvesting culture supernatants and analysis for bovine TNF-a (A) and IL-6 (B) by ELISA. Levels of cytokines were compared in MDM treated with either nothing, LPS or LPS in cells pre-treated with the indicated concentrations of either gamithromycin, oxytetracycline or tulathromycin. Comparisons for cytokine release for each animal and differences between control and experimental samples vs. LPS-treated cells were determine using an ordinary one-way ANOVA with: *, P ≤ 0.05; **, P ≤ 0.005, ***, P ≤ 0.0005 and ****, P ≤ 0.0001
Impact of antibiotics on macrophage production of reactive oxygen species (ROS) and nitric oxide (NO) in vitro
As noted previously, both NO and ROS play key roles in intracellular and extracellular killing of bacteria by macrophages, monocytes, and neutrophils. Therefore, we next evaluated the impact of the 3 antibiotics on LPS-stimulated ROS and NO production by macrophages, using the MDM cells as described above. All 3 concentrations evaluated of gamithromycin and tulathromycin resulted in significant reduction of ROS production by MDM, while only the two highest concentrations of oxytetracycline were significantly ROS suppressive (Fig. 6, Panel A). All 3 antibiotics potently and significantly inhibited NO production by macrophages, at all 3 concentrations evaluated (Fig. 6, Panel B). Thus, these data indicate that at reasonably achievable concentrations in live animals, all 3 antibiotics were capable of potent suppression of critical microbial killing pathways.
Fig. 6.
Treatment of MDM with gamithromycin, oxytetracyclin, tulathromycin suppresses LPS-mediated upregulation of ROS and NO. Cultures of MDM were treated with gamithromycin, oxytetracycline or tulathromycin for 3 h prior to activation with LPS. Control or antibiotic-treated MDM were incubated for 36 h with 300 ng/ml LPS followed by cell harvesting and flow cytometric analyses for ROS production and release of NO in culture supernatants. Comparisons for NO and ROS release were made for each animal and differences between experimental samples and LPS-treated cells were determine using an ordinary one-way ANOVA with: *, P ≤ 0.05; **, P ≤ 0.005, ***,P ≤ 0.0005 and ****, P ≤ 0.0001
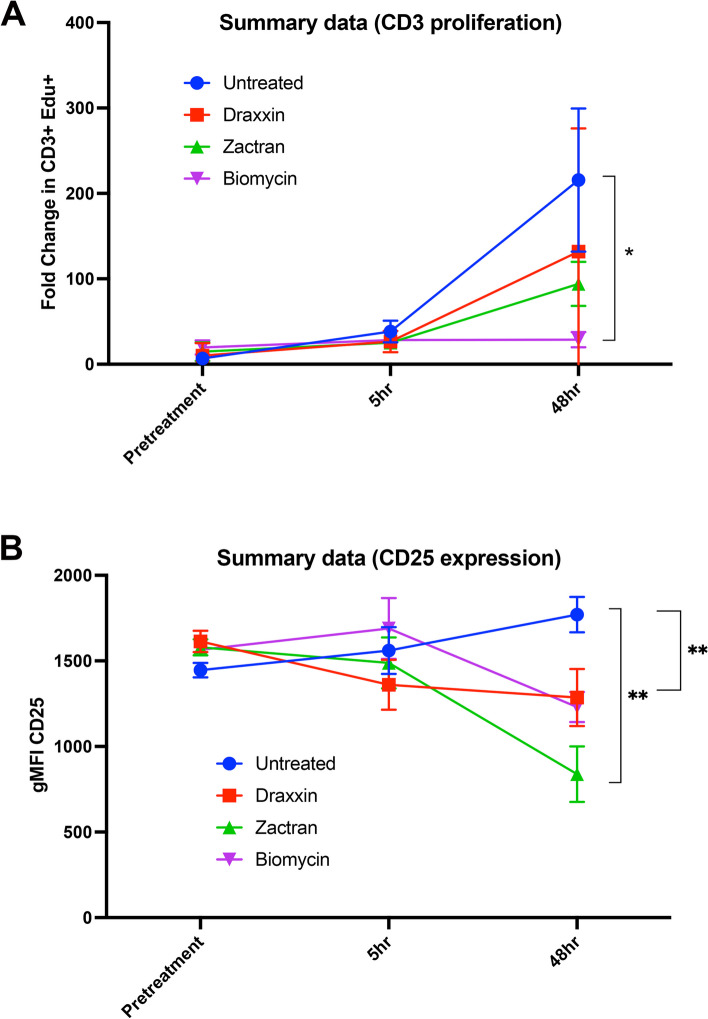
Single antibiotic treatment of cattle significantly suppresses T cell and overall leukocyte immune responses
To validate the in vitro findings described above and to determine the degree to which antibiotic treatment was capable of also suppressing immune responses in vivo, healthy 6–8 month old cattle (n = 5 per group) were treated with a single dose of 3 antibiotics, at standard recommended doses, and immune responses were assessed using blood leukocytes collected at 5 h and 48 h after treatment described in Methods. Four groups of n = 5 animals each were treated once with a single IM injection of tulathromycin, gamithromycin, oxytetracycline or saline (control group). At the indicated time points, PBMC were isolated from blood samples and then stimulated with ConA to induce T cell activation. The impact of antibiotic treatment on T cell proliferation was determined by incorporation of the nucleic acid precursor EdU, while cytokine secretion was measured by ELISA in the cell culture supernatants, as described in Methods.
We observed that T cells from each of the three antibiotic treated groups of animals exhibited a significant reduction in T cell proliferative responses to ConA activation, whereas the responses were not affected in the saline treated animals. For example, at 48 h post-antibiotic injection, proliferative responses in the tulathromycin treated group (Fig. 7, panel A (red line]) were reduced by nearly 50% compared to untreated animals. In cattle treated with gamithromycin and oxytetracycline (Fig. 7, panel A (green and purple lines respectively)), T cell proliferative responses were reduced on average by over 65% and 90%, respectively. The reduction in T cell proliferation was statistically significant only for the oxytetracycline treated groups, but clear statistical trends were apparent for both the tulathromycin and gamithromycin treated groups, particularly considering the relatively small numbers of animals in each group. In addition, when compared to untreated animals, PBMC from cattle injected with each of the three different antibiotics demonstrated reduced up-regulation of MHCII expression (data not shown) and CD25 expression (Fig. 7, panel B) following T cell activation.
Fig. 7.
Treatment of beef cattle with tulathromycin (Draxxin®), gamithromycin (Zactran®), or oxytetracycline (Bio-Mycin®) significantly reduced T cell proliferative responses and expression of CD25.Healthy young cattle (n = 5 per group) were randomly assigned to 4 treatment groups: Saline (control); tulathromycin, gamithromycin, and oxytetracycline, at doses noted in Methods. Cattle were bled prior to antibiotic treatment (pretreatment) and subsequently bled 5 h and 48 h after injection. Cultures of PBMC were isolated from blood samples and activated with ConA and T cell proliferation assessed by EdU incorporation (A). CD25 expression was determined by flow cytometry. (B). Significance of differences in fold change in proliferative responses of T cell events and expression of CD25 in PBMC we determined by a one-way ANOVA analysis of the areas under curve (AUC) of the timeline with *, P ≤ 0.05 and ** ≤ 0.01
Antibiotic treatment in vivo suppresses cytokine production by activated PBMC cultures
Supernatants from ConA-activated PBMC samples from Fig. 7 were also evaluated for secretion of IFN-g, TNF-a and IL-6 by specific ELISA. When compared to untreated animals, activated PBMC from tulathromycin-treated animals exhibited significantly suppressed secretion of TNF-a and IL-6 at 48 h, whereas secretion of IFN-g was unaffected (Fig. 8, compare panel A with B & C (compare blue to red lines). In gamithromycin treated animals, INF-g, TNF-a and IL-6 secretion were all significantly suppressed 48 h post treatment (Fig. 8, panel A, B & C (compare blue to green lines)). Similarly, secretion of all 3 cytokines was significantly suppressed at the 48 h post-treatment time point in oxytetracycline-treated animals (Fig. 8, panel A, B &C (compare blue to purple lines)). Taken together, these findings provide strong evidence that the immune suppressive effects of antibiotics observed in vitro were also recapitulated in vivo, at least with respect to T cell activation and cytokine suppression.
Fig. 8.
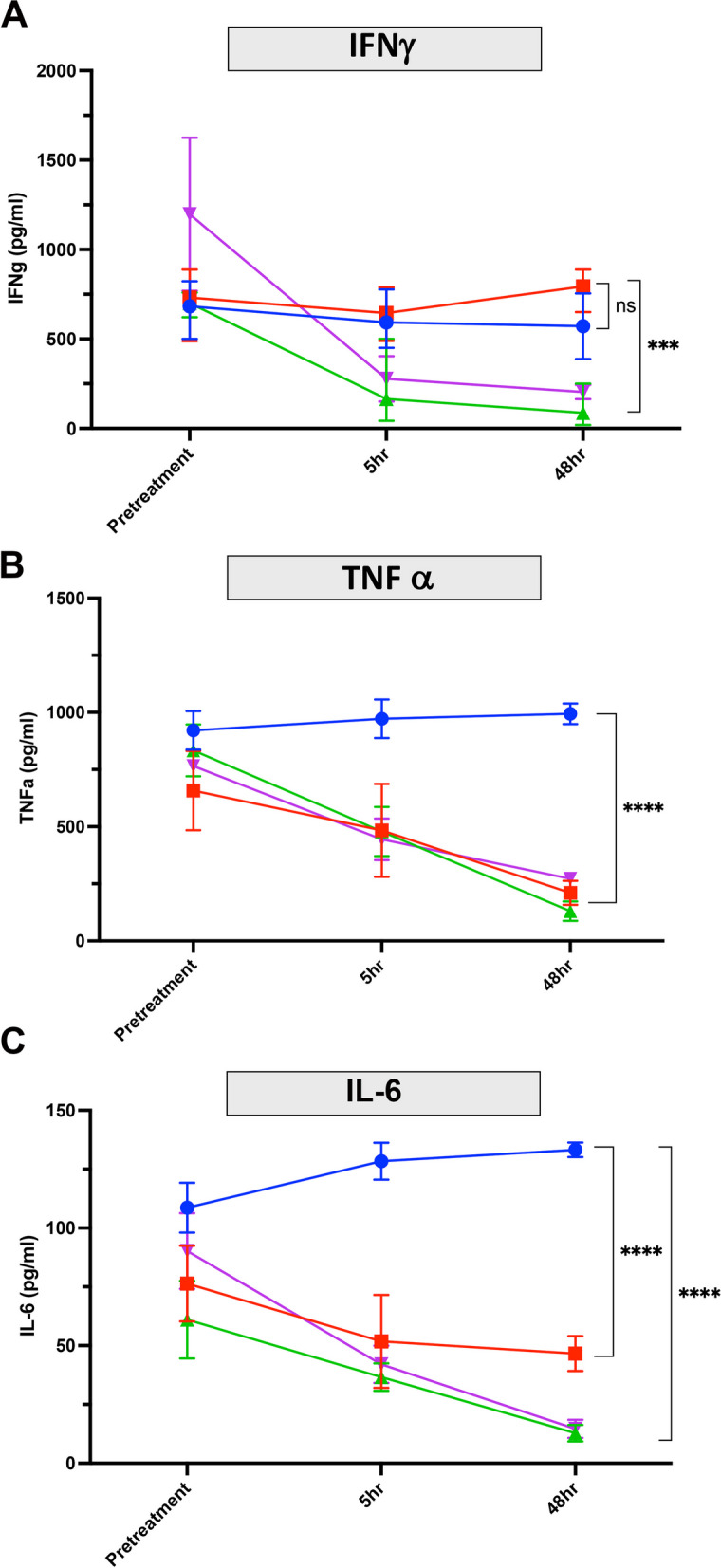
Impact of antibiotic treatment on cytokine production by activated PBMC from in vivo treated animals. Blood was collected from animals before antibiotic injections and after injections at 5 h and 48 h post treatment, as noted above (Fig. 7) and in Methods. Supernatants from activated T cell cultures from study animals in Groups 1–4 were collected and the concentrations of IFN-g, TNF-a, and IL-6 measured by ELISA. INF-g secretion is shown in panel A. TNF-a secretion is shown in panel B and IL-6 secretion is shown in panel C. Statistical comparisons of differences in cytokine release was analyzed by a 2-way ANOVA with multiple comparisons with *, P ≤ 0.05, **, P ≤ 0.01, ***, P ≤ 0.005 and ****, P ≤ 0.001
Discussion
Antibiotics have been shown previously to potentially interfere with host immunity due to off-target effects on mammalian cellular processes, especially those antibiotics that interfere with protein synthesis, including tetracyclines, macrolides, and aminoglycoside antibiotics (Miller and Singer [19]). However, this is the first study to our knowledge where the direct impact of tetracyclines and macrolides on immune responses has been examined in detail in cattle. Our findings indicate that indeed tetracycline and macrolide antibiotics are broadly immune suppressive in cattle, both in vitro and in vivo. The immune processes that were inhibited were quite broad and included T cell proliferation and cytokine production, expression of co-stimulatory molecules by both T cells and macrophages, macrophage cytokine production and production of key antimicrobial pathways (NO and ROS). The immune suppressive effects occurred rapidly (within 5 h of injection for in vivo studies) and at concentrations readily achievable by currently recommended dosing protocols. Importantly, the antibiotic-induced immune suppression was observed in healthy animals with normal immunity. In an animal with pre-existing immune suppression (e.g., after weaning or following shipping long distances), the overall impact of immune suppression exerted by these two classes of antibiotics would likely be even more pronounced since there would potentially be a reduction in T cell number (Murata et al. [20]), as well as a decrease in cytokine production in response to antigens (Murata et al. [20]) (Blecha et al. [3]) (Dixit et al. [5]).
In vitro studies revealed that all 3 antibiotics significantly suppressed T cell proliferation, as well as inducing downregulation of CD25 expression by T cells and MHCII expression by macrophages, indicative of broadly active and potent immune cell suppressive mechanisms. Secretion of IFN-g, TNF-a, and IL-6 were also significantly suppressed by antibiotic treatment of activated PBMC. Key antimicrobial effector pathways involving NO and ROS production were also suppressed in macrophages. These findings suggest that suppression of NO and ROS production by antibiotics could exert an important, negative impact on host immunity to bacterial infections, given the key role played by these processes. These findings therefore speak to the breadth of immune suppression generated by single dose antibiotic exposure.
The immune suppressive effects of tetracyclines and macrolides observed in cattle likely represent off-target effects on host immune cells, involving pathways related to protein synthesis inhibition via interference with 30 S and 50 S ribosomal subunit function, as mammalian cell functional disruption is a common feature associated with tetracyclines, macrolides, aminoglycosides, and oxazolidinones (McCoy et al. [17]) (Barrenechea et al. [2]) (Sutcliffe [31]) (Keating et al. [12]). Other pathways may also be interrupted by antibiotics, in addition to immune function. For example, we and others have reported recently that aminoglycoside antibiotics are potently cytotoxic to normal joint tissues and cells in both horses and dogs (Egerbacher et al. [6]) (Pezzanite et al. [23]) (Pezzanite et al. [22]) (Pezzanite et al., [24]).
Studies in other species have illustrated impacts on multiple immune pathways exerted by tetracyclines and macrolides. For example, tetracyclines suppress protein synthesis in mammalian cells (Kroon et al., [14]) (Riesbeck et al. [29]) (van den Bogert and Kroon [33]) (van den Bogert et al. [34]) (McKee et al. [18]). Additionally, tetracyclines (including oxytetracycline, doxycycline, minocycline) have been shown to suppress human lymphocyte proliferation in vitro (Hauser and Remington [9]). Tetracyclines also inhibit phagocyte chemotaxis and granuloma formation (Webster et al. [36]) while inhibiting production of IL-1b and TNF-a by human macrophages (Shapira et al. [30]), and inhibition of murine macrophage NO and IFN-g responses in vitro (Amin et al. [1]). Recent work has demonstrated that antibiotic treatment can also inhibit immune function by inhibiting the respiratory burst activity (Yang et al. [3]). Finally, macrolide antibiotics have additionally been shown to directly inhibit production of NO, TNF-a, IL-1b and IL-6 in mouse macrophages (Ianaro et al., [10]). Thus, our results in cattle agree with prior work describing a wide range of targets for immune suppression by tetracyclines and macrolide antibiotics.
For example, weanling animals, animals shipped long distances, and animals in feedlots all experience significant stress-related immune suppression and are much more susceptible to development of BRDC. In these animals, an antibiotic that interferes with host immunity, either adaptive or innate immunity, has the potential to further impair host immunity to the viral and bacterial pathogens associated with BRDC (Hauser and Remington [9]). Even though these immune suppressive effects may not be apparent at the level of the individual animal, the net effect on large herds becomes more difficult to ignore. In some cases, it may be preferable to either use different, non-immune suppressive antibiotics, or to avoid administration altogether.
The administration of antibiotics at the time of vaccination is another important consideration since impairment of critical antigen presenting pathways such as the MHCII pathway have the potential to significantly reduce vaccine immunity (Pomorska-Mol et al. [25]). Since antibiotics and routine vaccines may be often administered together, it may be judicious to either separate administration of each by 1–2 weeks, or to consider use of different classes of antibiotics (e.g., beta-lactams) that have not been previously associated with significant immune suppression and interfence with vaccine immunity. Further studies are needed to determine how long the antibiotic immune suppression lasts and to what benefit delaying antibiotic treatment may have on clinically sick animals. Additionally, future research geared toward development of agents stimulating innate immunity may be beneficial to stressed animals as well.
In summary, these studies demonstrate broad immune suppression by 3 commonly used antibiotics in cattle, affecting both innate and adaptive immune responses. Immune suppression was found to occur rapidly following antibiotic administration in vivo and persisted at least several days. These off-target immune suppressive effects of tetracyclines and macrolides in cattle warrant additional study in larger groups of animals, and especially in conditions in the field where co-morbidities such as stress from weaning and co-mingling may further suppress host immune responses.
Acknowledgements
The authors wish to acknowledge the assistance of Ms. Jade Kurihara in cytokine assays.
Authors' contributions
Wheat W, Hunter R, Herman J, Dow, S. study conception and design. Wheat W, Chow L, Dow S data collection and interpretation of results, draft manuscript preparation. Still-Brooks, K and Moore-Foster, R design and implementation of the research, sample collection from cattle. All authors discussed the results and contributed to the final manuscript.
Funding
These studies were supported by a grant from the CSU College of Veterinary Medicine and Biomedical Sciences College Research Council.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
The study did not include human subjects or client owned animals. All cattle used in the study are property of Hunter Cattle Company, Wheatland WY. These studies were approved by the Veterinary Research and Consulting Services (VRCS) Institutional Animal Care and Use Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
W. Wheat, Email: William.Wheat@colostate.edu
S. Dow, Email: sdow@colostate.edu
References
- 1.Amin AR, Attur MG, Thakker GD, Patel PD, Vyas PR, Patel RN, Patel IR, Abramson SB. A novel mechanism of action of tetracyclines: effects on nitric oxide synthases. Proc Natl Acad Sci U S A. 1996;93(24):14014–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrenechea V, Vargas-Reyes M, Quiliano M, Milon P. A complementary mechanism of bacterial mRNA translation inhibition by tetracyclines. Front Microbiol. 2021;12:682682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blecha F, Boyles SL, Riley JG. Shipping suppresses lymphocyte blastogenic responses in Angus and Brahman X Angus feeder calves. J Anim Sci. 1984;59(3):576 – 83. 10.2527/jas1984.593576x. PMID: 6490547. [DOI] [PubMed]
- 4.Coppin CM, Smock TM, Helmuth CL, Manahan JL, Long NS, Hoffman AA, Carroll JA, Broadway PR, Burdick Sanchez NC, Wells JE, Fernando SC, Hales KE. The effects of administering different metaphylactic antimicrobials on growth performance and health outcomes of high-risk, newly received feedlot steers. Transl Anim Sci. 2022;6(4):txac140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixit VD, Marahrens M, Parvizi N. Transport stress modulates adrenocorticotropin secretion from peripheral bovine lymphocytes. J Anim Sci. 2001;79(3):729 – 34. 10.2527/2001.793729x. PMID: 11263834. [DOI] [PubMed]
- 6.Egerbacher M, Edinger J, Tschulenk W. Effects of enrofloxacin and ciprofloxacin hydrochloride on canine and equine chondrocytes in culture. Am J Vet Res. 2001;62(5):704–8. [DOI] [PubMed] [Google Scholar]
- 7.Feola DJ, Garvy BA, Cory TJ, Birket SE, Hoy H, Hayes D Jr., Murphy BS. Azithromycin alters macrophage phenotype and pulmonary compartmentalization during lung infection with Pseudomonas. Antimicrob Agents Chemother. 2010;54(6):2437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goscinski G, Lipcsey M, Eriksson M, Larsson A, Tano E, Sjolin J. Endotoxin neutralization and anti-inflammatory effects of tobramycin and ceftazidime in porcine endotoxin shock. Crit Care. 2004;8(1):R35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauser WE Jr., Remington JS. Effect of antibiotics on the immune response. Am J Med. 1982;72(5):711–6. [DOI] [PubMed] [Google Scholar]
- 10.Ianaro A, Ialenti A, Maffia P, Sautebin L, Rombola L, Carnuccio R, Iuvone T, D’Acquisto F. and M. Di Rosa. 2000. Anti-inflammatory activity of macrolide antibiotics. J Pharmacol Exp Ther 292(1):156–63. [PubMed]
- 11.Ives SE, Richeson JT. Use of Antimicrobial Metaphylaxis for the control of bovine respiratory disease in high-risk cattle. Vet Clin North Am Food Anim Pract. 2015;31(3):341–50. [DOI] [PubMed] [Google Scholar]
- 12.Keating TA, Lister T, Verheijen JC. New antibacterial agents: patent applications published in 2011. Pharm Pat Anal. 2014;3(1):87–112. [DOI] [PubMed] [Google Scholar]
- 13.Krehbiel CR. Bovine respiratory Disease influences on Nutrition and Nutrient Metabolism. Vet Clin North Am Food Anim Pract. 2020;36(2):361–73. [DOI] [PubMed] [Google Scholar]
- 14.Kroon AM, Dontje BH, Holtrop M. and C. Van Den Bogert. 1984. The mitochondrial genetic system as a target for chemotherapy: tetracyclines as cytostatics. Cancer Lett 25(1):33–40. [DOI] [PubMed]
- 15.Lin SJ, Kuo ML, Hsiao HS, Lee PT. Azithromycin modulates immune response of human monocyte-derived dendritic cells and CD4(+) T cells. Int Immunopharmacol. 2016;40:318–26. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Cortes I, Acevedo-Dominguez NA, Olguin-Alor R, Cortes-Hernandez A, Alvarez-Jimenez V, Campillo-Navarro M, Sumano-Lopez HS, Gutierrez-Olvera L, Martinez-Gomez D, Maravillas-Montero JL, Loor JJ, Garcia-Zepeda EA, Soldevila G. Tilmicosin modulates the innate immune response and preserves casein production in bovine mammary alveolar cells during Staphylococcus aureus infection. J Anim Sci. 2019;97(2):644–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCoy LS, Xie Y, Tor Y. Antibiotics that target protein synthesis. Wiley Interdiscip Rev RNA. 2011;2(2):209–32. [DOI] [PubMed] [Google Scholar]
- 18.McKee EE, Ferguson M, Bentley AT, Marks TA. Inhibition of mammalian mitochondrial protein synthesis by oxazolidinones. Antimicrob Agents Chemother. 2006;50(6):2042–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller M, Singer M. Do antibiotics cause mitochondrial and immune cell dysfunction? A literature review. J Antimicrob Chemother. 2022;77(5):1218–27. [DOI] [PubMed] [Google Scholar]
- 20.Murata H, Takahashi H, Matsumoto H. The effects of road transportation on peripheral blood lymphocyte subpopulations, lymphocyte blastogenesis and neutrophil function in calves. Br Vet J. 1987 Mar-Apr;143(2):166 – 74. 10.1016/0007-1935(87)90008-X. PMID: 3495314. [DOI] [PubMed]
- 21.Parnham MJ. Immunomodulatory effects of antimicrobials in the therapy of respiratory tract infections. Curr Opin Infect Dis. 2005;18(2):125–31. [DOI] [PubMed] [Google Scholar]
- 22.Pezzanite L, Chow L, Soontararak S, Phillips J, Goodrich L, Dow S. Amikacin induces rapid dose-dependent apoptotic cell death in equine chondrocytes and synovial cells in vitro. Equine Vet J. 2020;52(5):715–24. [DOI] [PubMed] [Google Scholar]
- 23.Pezzanite L, Chow L, Piquini G, Griffenhagen G, Ramirez D, Dow S, Goodrich L. Use of in vitro assays to identify antibiotics that are cytotoxic to normal equine chondrocytes and synovial cells. Equine Vet J. 2021b;53(3):579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pezzanite L, Chow L, Hendrickson D, Gustafson DL, Russell Moore A, Stoneback J, Griffenhagen GM, Piquini G, Phillips J, Lunghofer P, Dow S. and L. R. Goodrich. Evaluation of Intra-articular Amikacin Administration in an equine non-inflammatory joint model to identify effective bactericidal concentrations while minimizing cytotoxicity. Front Vet Sci. 2021a;8:676774. [DOI] [PMC free article] [PubMed]
- 25.Pomorska-Mol M, Kwit K, Markowska-Daniel I, Pejsak Z. The effect of doxycycline treatment on the postvaccinal immune response in pigs. Toxicol Appl Pharmacol. 2014;278(1):31–8. [DOI] [PubMed] [Google Scholar]
- 26.Pomorska-Mol M, Czyzewska-Dors E, Kwit K, Rachubik J, Lipowski A, Pejsak Z. Immune response in pigs treated with therapeutic doses of enrofloxacin at the time of vaccination against Aujeszky’s disease. Res Vet Sci. 2015;100:68–74. [DOI] [PubMed] [Google Scholar]
- 27.Pomorska-Mol M, Kwit K, Wierzchoslawski K, Dors A, Pejsak Z. Effects of Amoxicillin, ceftiofur, doxycycline, tiamulin and tulathromycin on pig humoral immune responses induced by erysipelas vaccination. Vet Rec. 2016;178(22):559. [DOI] [PubMed] [Google Scholar]
- 28.Ratzinger F, Haslacher H, Poeppl W, Hoermann G, Kovarik JJ, Jutz S, Steinberger P, Burgmann H, Pickl WF, Schmetterer KG. Azithromycin suppresses CD4(+) T-cell activation by direct modulation of mTOR activity. Sci Rep. 2014;4:7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riesbeck K, Bredberg A, Forsgren A. Ciprofloxacin does not inhibit mitochondrial functions but other antibiotics do. Antimicrob Agents Chemother. 1990;34(1):167–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shapira L, Soskolne WA, Houri Y, Barak V, Halabi A, Stabholz A. Protection against endotoxic shock and lipopolysaccharide-induced local inflammation by tetracycline: correlation with inhibition of cytokine secretion. Infect Immun. 1996;64(3):825–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutcliffe JA. Improving on nature: antibiotics that target the ribosome. Curr Opin Microbiol. 2005;8(5):534–42. [DOI] [PubMed] [Google Scholar]
- 32.Tauber SC, Nau R. Immunomodulatory properties of antibiotics. Curr Mol Pharmacol. 2008;1(1):68–79. [PubMed] [Google Scholar]
- 33.van den Bogert C, Kroon AM. Tissue distribution and effects on mitochondrial protein synthesis of tetracyclines after prolonged continuous intravenous administration to rats. Biochem Pharmacol. 1981;30(12):1706–9. [DOI] [PubMed] [Google Scholar]
- 34.van den Bogert C, Dontje BH, Holtrop M, Melis TE, Romijn JC, van Dongen JW, Kroon AM. Arrest of the proliferation of renal and prostate carcinomas of human origin by inhibition of mitochondrial protein synthesis. Cancer Res. 1986;46(7):3283–9. [PubMed] [Google Scholar]
- 35.Watts KM, Lahiri P, Arrazuria R, De Buck J, Knight CG, Orsel K, Barkema HW, Cobo ER. Oxytetracycline reduces inflammation and treponeme burden whereas vitamin D3 promotes beta-defensin expression in bovine infectious digital dermatitis. Cell Tissue Res. 2020;379(2):337–48. [DOI] [PubMed] [Google Scholar]
- 36.Webster GF, Toso SM, Hegemann L. Inhibition of a model of in vitro granuloma formation by tetracyclines and ciprofloxacin. Involvement of protein kinase C. Arch Dermatol. 1994;130(6):748–52. [PubMed] [Google Scholar]
- 37.Wheat W, Chow L, Rozo V, Herman J, Still Brooks K, Colbath A, Hunter R, Dow S. Non-specific protection from respiratory tract infections in cattle generated by intranasal administration of an innate immune stimulant. PLoS ONE. 2020;15(6):e0235422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wink DA, Hines HB, Cheng RY, Switzer CH, Flores-Santana W, Vitek MP, Ridnour LA, Colton CA. Nitric oxide and redox mechanisms in the immune response. J Leukoc Biol. 2011;89(6):873–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.