Abstract
Low temperature is one of the environmental factors that restrict the growth and geographical distribution of Brassica. To investigate the effects of exogenous calcium and calcium inhibitor on the ability of winter turnip rapeseed (Brassica rapa L.) to withstand low temperatures (4℃), we used a strong cold-resistant variety Longyou 7 (L7) and a weak cold-resistant variety Longyou 99 (L99) as the materials. The seedlings were treated with CaCl2 (20 mmol·L-1) and calcium inhibitor LaCl3 (10 mmol·L-1) at 0 h (CK), 6 h, 12 h, 24 h and 48 h after 4℃ treatments. Physiological characteristics, Ca2+ flux and Ca2+ concentration in roots after treatments were analyzed. Results illustrated that under 4℃ treatment, activities of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) increased by both CK and exogenous CaCl2 treatments. Contents of soluble protein (SP) and proline (Pro) increased, while contents of malondialdehyde (MDA) decreased, resulting in reduced membrane lipid peroxidation. But enzyme activity decreased and MDA content increased following treatment with exogenous LaCl3. The rate of Ca2+ flow showed a higher uptake in L7 roots compared with L99. L99 showed Ca2+ efflux with a rate of 30.21 pmol‧cm-2‧s-1, whereas L7 showed short efflux then returned to influx. Calcium ion content in roots decreased in both cultivars after CaCl2 treatment. Results of RNA-seq revealed that genes were differentially expressed in response to low temperatures, hormones, photosystem II, chloroplasts, DNA replication, ribosomal RNA processing, and translation. This study found significant expression genes related to cellular signal transduction (MAPK signaling pathway) and material metabolism (nitrogen metabolism, glycerol ester metabolism).It was also analyzed by WGCNA that two modules had the strongest correlation with physiological indicators. Eight candidate genes were identified among MAPK signaling pathway and the two modules.
Keywords: Winter turnip rapeseed, Low temperature, Calcium chloride, Lanthanum chloride, Antioxidant enzyme activity
Background
Winter rapeseed is an important oil-seed crop globally. In China, it contributes 5.2 million tons of high-quality edible oil per year, which represents over 50% of the country’s total oil crop production [1]. Nevertheless, China is still lacking in the production and edible oil of rapeseed. It has always been important for us to produce more rapeseed. Winter rapeseed is the main cultivation type of rapeseed in China, and it is cultivated in both north and south [2]. But in north, freezing temperature in winter and chilling injury in spring have negatively impacts on the yield, growth, and quality of edible oil in China [3]. Therefore, it is crucial to dig out hub genes related to strong cold-resistance of winter rapeseed and enhance its cold resistance to ensure the safe overwintering in north China. This is of great significance for the development of the rapeseed industry and the protection of China grain and oil security. The application of exogenous substances is one of the simplest and most effective methods.
Calcium chloride (CaCl2), an inorganic salt, is used to help plants withstand environmental stress [4]. Previous studies have shown that calcium ions regulate enzyme activity in plants including synthesis, secretion, and promotion of metabolic reactions which helps maintain normal plant metabolism, growth, and development, as well as aiding in resistance to environmental stresses such as drought, salinity, and low temperatures [5–9]. The use of exogenous Ca2+ to treat rapeseed seedlings under low-temperature stress conditions is practically significant for studying rapeseed resistance [10]. Lanthanum chloride (LaCl3) is a calcium channel inhibitor that binds to calcium channels and blocks the entry of calcium ions, reducing the intracellular calcium ion concentration and inhibiting calcium signaling [11]. Numerous studies have shown that lanthanum chloride can enhance the antioxidant enzyme system and osmoregulatory substances in plants at low concentrations while inhibiting them at high concentrations [12–14]. The impact of lanthanum chloride on plants is influenced by both treatment time and concentration [14].
Winter turnip rapeseed (Brassica rapa L., AA) is a kind of oil crop that can survive in Northwest China where the extreme low temperature is about − 32℃ [15]. Under low-temperature stress, winter turnip rapeseed undergoes morphological changes such as wilting and yellowing of leaves [3, 16]. Throughout winter, parts of the ground of winter turnip rapeseed dry out, leaving only roots in soil [15, 16]. At the physiological level, low temperature triggers the production of a large amount of reactive oxygen species (ROS) in the plant body [3]. This affects the activity of antioxidant enzyme systems, leads to the accumulation of osmoregulatory substances and metabolic abnormalities, and destroys the integrity of cell membranes. Simultaneously, in vivo, there is rapid and extensive regulation at both physiological and molecular levels, involving multiple stages of low-temperature signal perception, conduction, and regulation [17]. This results in a reduction of various physiological activities, which affects the normal growth and development of winter turnip rapeseed [18]. However, at present some studies have not been clear, e.g. what are the hub genes affecting winter turnip rapeseed strong cold resistance, and what are the mechanisms.
In this study we analyzed changes in osmoregulatory substances and antioxidant enzymes using exogenous CaCl2 and LaCl3 after 4℃ treatment. The objective was to research the mechanism of the effects of these two treatments on winter turnip rapeseed seedlings under low temperature stress. Then transcriptome sequencing was used to study the differences in expression patterns of genes in two varieties with varying cold-resistance under low-temperature conditions and different exogenous substance treatments. This is to mine some hub genes involved in low-temperature regulation and calcium signaling. These results will provide a theoretical basis and genetic resources for further study of the cold-resistance mechanism and the selection and breeding of new varieties with strong cold-resistance in winter rapeseed.
Materials and methods
Plant material and treatment
In present study we used winter turnip rapeseed variety Longyou 7 (L7), a strong cold-resistant variety, and Longyou 99 (L99), a weak cold-resistant variety bred by Gansu Agricultural University [15]. Uniform-sized rape seeds were sterilized by immersing them in a 5% sodium hypochlorite solution for a duration of 10 min, subsequently washed 5 times with distilled water for one minute each time. The seeds were placed in petri dishes with a diameter of 15 cm, covered with filter paper to facilitate germination. After germination, seedlings were transferred to pots with a diameter of 15 cm and a height of 18 cm, which were filled with a nutrient-rich substrate (Hoagland) with no Ca ion in it. A total of 54 pots were used to plant each variety, with 3 seedlings kept in each pot.
Seedlings were subjected to stress treatment at five-leaf stage [19]. Seedlings were set in low temperature incubators with 16 h light and 8 h darkness after exogenous spraying of distilled water, 20 mmol·L-1 CaCl2, and 10 mmol·L-1 LaCl3 on leaves. Concentrations of CaCl2 and LaCl3 were chosen by the preliminary test. 20 mmol·L-1 CaCl2, and 10 mmol·L-1 LaCl3 had obvious alleviating and inhibiting effects on seedlings at low temperature. We sprayed treatment reagent on the surface and underside of leaves with a spray bottle until it began to drip. And poured 20 ml fluid into the root of each plant (divided into small amounts along the root system), then immediately place them in incubator. Samples were collected at different time points (0 h, 6 h, 12 h, 24 h, and 48 h) following treatments with distilled water (CK), 20 mmol·L-1 CaCl2, and 10 mmol·L-1 LaCl3, all at a temperature of 4 °C. The leaves and roots were collected individually and preserved by quick-freezing by using liquid nitrogen. Subsequently, the collected samples were stored at -80 °C for further analysis [20].
Analysis of physiological index
A fresh leaf sample weighing 0.5 g was finely crushed in a phosphate buffer solution while being kept in a container filled with ice to maintain a low temperature, while roots were crushed quickly in liquid nitrogen. The crude enzyme solution was prepared through the process of freezing and centrifugation. The activity of superoxide dismutase (SOD) was determined using the nitrogen blue tetrazolium (NBT) photoreduction technique [21]. The activity of peroxidase (POD) was determined using the guaiacol method [22]. Catalase (CAT) activity was determined using the UV absorption method [23]. The level of contents of malondialdehyde (MDA) was determined using the thiobarbituric acid (TBA) method [24]. Soluble protein (SP) content was measured using the Coomassie brilliant blue method [25]. The acid ninhydrin mixing method was used to determine the proline content (Pro) [26]. The non-invasive microtomography (NMT) technique was used to determine the flow of calcium ions at root surface site located 300 μm away from the apex of the root tip [27]. Calcium ion content was determined using inductively coupled plasma emission spectroscopy [28].
RNA isolation and transcriptomics analysis
Total RNA was isolated using a SteadyPure Plant RNA Extraction Kit (Accurate Biotechnology, AG21019, Hunan, China) following the manufacturer’s instructions and removing genomic DNA contamination. After the samples were qualified, the library was constructed [3]. The effective concentration of the library (> 2nM) was quantified accurately by Q-PCR to ensure the quality of the library. After the library was qualified, high-throughput sequencing platform illumina NovaSeq6000 was used for pattern sequencing. A total of 289.78 G clean reads were obtained by filtering the original data. The transcriptome sequencer was provided by Shanghai Ouyi Biomedical Technology Company (https://cloud.oebiotech.com/task/).
The reads from each gene alignment were analyzed using HTSeqv0.6.1, and the FPKM values of the expressed genes were calculated. Gene expression levels were standardized based on the FPKM values. DESeq v1.10.1 was employed to compare groups for differential expression analysis. Genes with a log2 fold change value greater than 2 or less than − 2 and FDR value below 0.01 between two groups were considered to exhibit differential expression.
Differential expressed genes were found by comparing gene expression levels among different samples. After differential expressed genes were obtained, GO function analysis and KEGG enrichment analysis (KEGG, https://www.genome.jp/kegg) were carried out by relying on the online platform provided by Shanghai Ouyi Biomedical Technology Co., LTD. All groups were named for ease of analysis in Table 1 (Table 1).
Table 1.
Description of the sample groups and codes for the experimental treatment
| Sample group | code |
|---|---|
| L7-CK | A |
| L7-12 h | B |
| L7-12 h-Ca | C |
| L7-12 h-La | D |
| L7-24 h | E |
| L7-24 h-Ca | F |
| L7-24 h-La | G |
| L99-CK | H |
| L99-12 h | I |
| L99-12 h-Ca | J |
| L99-12 h-La | K |
| L99-24 h | L |
| L99-24 h-Ca | M |
| L99-24 h-La | N |
Weighted gene co-expression network analysis (WGCNA)
Transcriptome data were combined with physiological indicators and calcium ion content, and core genes were screened in strict compliance with the requirements of ensuring sample size ≥ 15 using WGCNA and gene data filtering and normalization of low-expression genes [29, 30].
A total of 42 winter turnip rape transcriptomes were identified, and 40,578 genes were identified. Genes with low expression fluctuation (standard deviation ≤ 0.5) were filtered and left 11,118 genes for WGCNA network construction. Then, the selected gene expression values were imported into WGCNA software package, and co-expression modules were constructed with the built-in automatic network construction function under the default parameter setting.
Primers and analysis method of qRT-PCR
We used L7 and L99 treated at 4℃ after 12 h and 24 h as PCR experimental materials. Quantitative primers for candidate genes were designed using PrimerQuest™ Tool (Table 2), and the reference gene was Actin. Gene expression levels were tested by two-step qRT-PCR. TaKaRa fluorescent quantitative reagent TB Green kit was used to perform the test according to the instructions. The reaction procedure was: predenaturation at 95 ℃ for 30 s, denaturation at 95 ℃ for 3 s, annealing at 60 ℃ for 30 s, extending at 72 ℃ for 30 s, 35 cycles. Ct values were calculated using the mean value of 3 biological repeats, and the relative gene expression was calculated by 2-ΔΔCt method.
Table 2.
Primers for qRT-PCR
| Gene ID | Upstream primer(5’-3’) | Downstream primer(5’-3’) |
|---|---|---|
| LOC103858143 | AAGTCGTCAGAACAGCCTTCACC | ATCTTCCGACAGCCTCTCCACTC |
| LOC103863198 | CGCAACAACAGGAGCCTAACAAC | CTCGTTCGCTTTCTCGTCGGAAG |
| LOC103866369 | TCAAGCGATGCACATACCTCACAC | ACGGACAGAGACAACCTCAGAG |
| LOC103830167 | TGGAGTGGCGAGGCTGATGAG | ATTGGGATGGCGAAGTGATCTGTG |
| LOC103857966 | GAAGGAGCTTGGCACCGTGATG | TCCGTTACCATCTGCGTCAACTTC |
| LOC103873775 | ACACTCTGCTCGATGGTAGTCCTG | AGGTGCAATGTAAGCTGGAGTTCC |
| LOC103837288 | GACGGGTTGATGAGGGATGTGTTC | GCAGCCAAACCAGCCGACTC |
| WRKY22 | ACCACAGCCACCGTCTTCTCC | GAGGCGTTTGATTCTTGCGTGAAC |
Statistical analysis
The graphs were analyzed using Microsoft Excel 2010, and SPSS 22.0 software was used for ANOVA (one-way).
Results
Effects of exogenous calcium and calcium inhibitor on SOD, POD, and CAT activities in leaves and roots under low-temperature stress
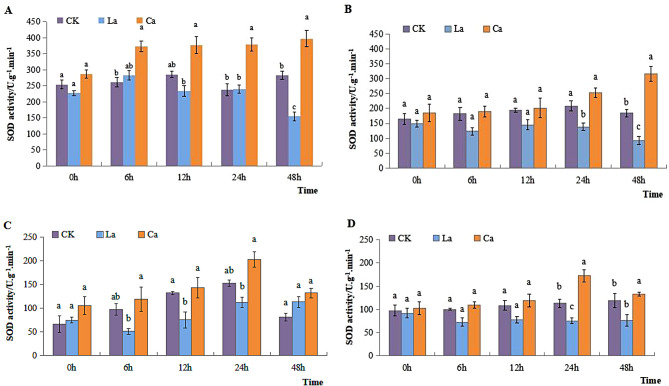
SOD activity in leaves of L7 and L99 increased gradually over time at 4 °C treatment in CK. Following the application of CaCl2 treatment, there was a substantial rise of SOD activity in both cultivars compared to CK. When LaCl3 was applied, it gradually decreased over time. The findings illustrate that SOD activity in leaves of L99 increased by 71.53% compared to CK after CaCl2 treatment. However, it decreased by 49.83% compared to CK after LaCl3 treatment (Fig. 1A and B). SOD activity in roots of two varieties increased gradually over time in CK. It reached its peak at 24 h and subsequently decreased at 48 h. Throughout all treatments, it was consistently lower in LaCl3 treatment compared to CK and CaCl2 treatment (Fig. 1C and D). In addition, SOD activity in leaves of L7 was generally stronger than that of L99. After adding CaCl2, it was significantly different after 6 h in L7, while that of L99 appeared at 48 h.
Fig. 1.
Effects of low temperature on SOD activity of winter rape leaves and roots. Note: (A) L7 leaves; (B) L99 leaves; (C) L7 roots; (D) L99 roots. CK was distilled water treatment under 4 ℃. La means lanthanum chloride treatment under 4 ℃, and Ca was calcium chloride treatment group under 4 ℃. Different lowercase letters indicated that the differences between different treatments at the same time were significant (p < 0.05), the same below
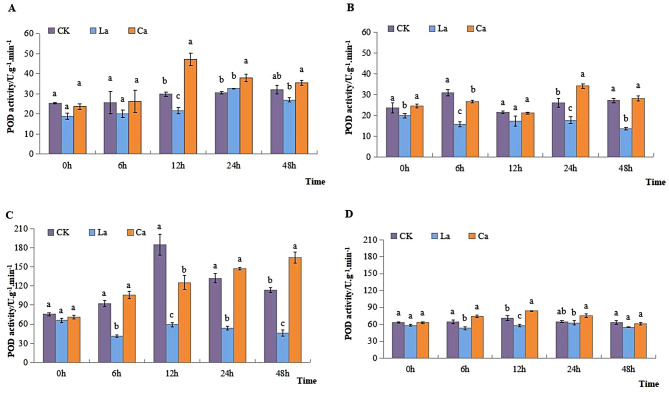
POD activity in leaves of L7 showed a progressive increase over time in CK. The application of CaCl2 resulted in an initial rise on it, which was subsequently followed by a decline as the treatment duration increased. When LaCl3 was applied, it caused a progressive decrease compared to CK over time. POD activity in leaves of L99 initially declined and then increased in response to CK and CaCl2 treatment. However, after the application of CaCl2, it was dramatically increased compared to CK after 24 h. Over time, the application of LaCl3 resulted in a decrease in POD activity relative to CK (Fig. 2A and B). POD activity in roots of L7 increased gradually over time in CaCl2 treatments. However, it was suppressed by LaCl3. POD activity in roots of L99 increased over time both in CK and CaCl2, with a peak at 12 h (Fig. 2C and D).
Fig. 2.
Effects of low temperature on POD activity of winter rapeseed leaves and roots
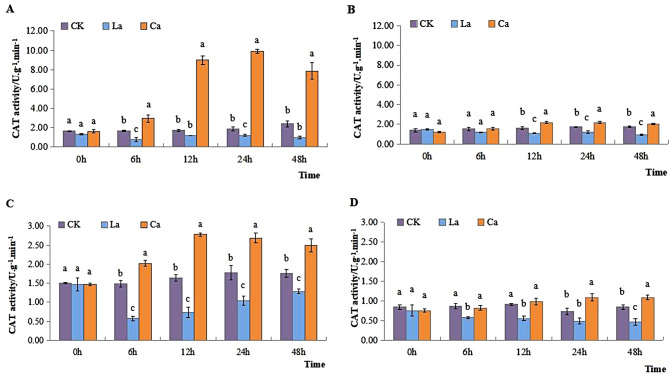
CAT activity in leaves of L7 and L99 increased gradually over time in CK. After applying CaCl2, there was a significant increase in both cultivars compared to CK. Moreover, the duration of treatment showed a direct correlation with the rise in CAT activity. Upon the application of LaCl3, it exhibited a progressive decline over time in comparison to CK. The results demonstrated that CAT activity in leaves of L7 and L99 showed a significant increase of 79.53% and 50.36% respectively, compared to CK after CaCl2 treatment. Nevertheless, it experienced a reduction of 30.83% in comparison to CK with LaCl3 treatment (Fig. 3A and B).
Fig. 3.
Effects of low temperature on CAT activity of winter rapeseed leaves and roots
CAT activity in roots of L7 and L99 increased over time in CK. It reached its maximum level at 24 h. CAT activity was consistently lower than that of CK after LaCl3 treatment. These findings indicate that the application of LaCl3 worsened the oxidative damage to the root system induced by low temperature compared to that of the CK. During all treatment periods, the level of SOD activity was consistently lower in the LaCl3 treatment group compared to both the CK and CaCl2 treatments. The greatest significant alteration was observed after 24 h period (Fig. 3C and D).
Effects of exogenous calcium and calcium inhibitor on MDA, SP, and Pro contents in B. rapa leaves and roots under low-temperature stress
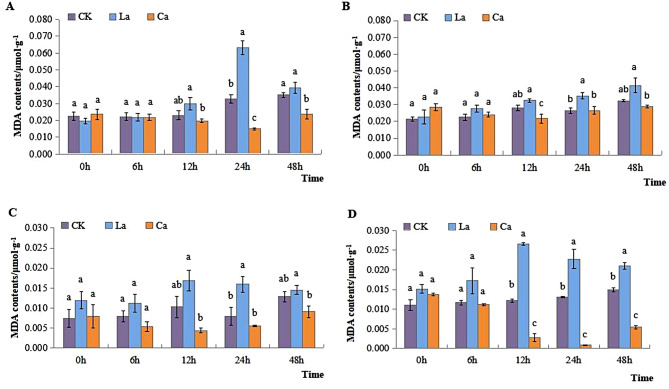
It exhibited an increasing trend of MDA content in their leaves after LaCl3 treatment in the two varieties. However, treatment with CaCl2 resulted in a decrease in MDA content compared to CK at the same treatment time. In L7 leaves it showed a significant increase of 93.68% after LaCl3 treatment at 24 h. On the other hand, after being treated with CaCl2, MDA content initially decreased and then increased over time in both cultivars’ leaves (Fig. 4A and B). The roots of both cultivars displayed an increasing trend, L99 showing more pronounced changes following 12 h treatment with LaCl3 as compared to the CK. The utilization of CaCl2 led to significant decreases in MDA content at 12 h, 24 h, and 48 h as compared to CK in both cultivars( Fig. 4C and D).
Fig. 4.
Effects of low temperature on MDA content of leaves and roots of winter rapeseed
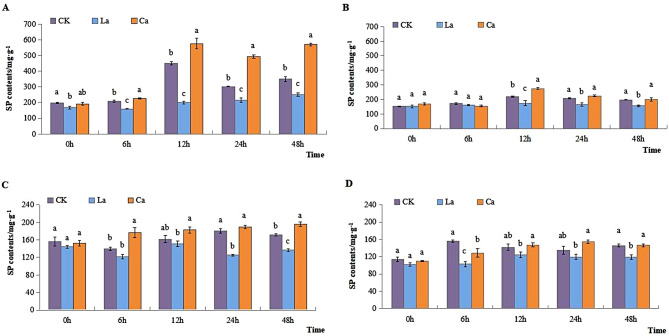
SP content in L7 leaves increased substantially following CaCl2 and LaCl3 treatment at 12 h, 24 h, and 48 h, whereas it remained lower than CK in the case of LaCl3 treatment. While no significant difference was observed between CK and CaCl2 and LaCl3 treatment in L99 leaves (Fig. 5A and B). The root exhibited an upward trend when subjected to CaCl2 in both cultivars. It increased significantly after 6 h of CaCl2 treatment compared to CK, while following the application of LaCl3, it exhibited a decreasing trend compared to CK (Fig. 5C and D). Additionally, it increased significantly in both cultivars after CaCl2 treatment compared to LaCl3 treatment at different times of 6 h, 12 h, 24 h, and 48 h. More precisely, in L99 CaCl2 treatment did not show any significant changes at any time compared to CK. However, the findings illustrated that LaCl3 treatment resulted in a significant reduction of SP content in leaves and roots of both cultivars.
Fig. 5.
Effects of low temperature on SP content of winter rape leaves and roots
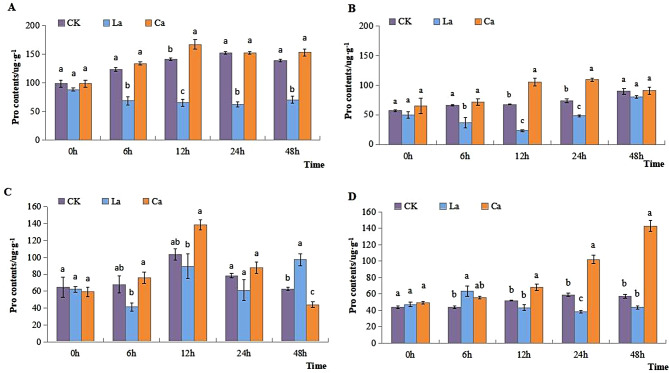
The content of Pro in leaves in CK reached its peak at 24 h, while it reached it maximum at 12 h after CaCl2 application. Conversely, it decreased following LaCl3 treatment in two varieties. In leaves of L99 Pro was significantly higher after CaCl2 at 12 h and 24 h compared to CK. While after the treatment of LaCl3 it dropped down significantly at 12 h and 24 h compared to CaCl2 and CK (Fig. 6A and B). There was a similar trend in L7. Pro in roots of L7 increased both in CK, and CaCl2 treatment, at 12 h and then dropped significantly. There was no significant difference between LaCl3 treatment and CK while Pro in L7 roots was 57.88% higher than CK at 48 h. In L99 Pro content of roots exhibited an increasing trend after CaCl2 treatment, reaching its maximum at 48 h, which was significantly increased by 149.19% compared to CK (Fig. 6C and D).
Fig. 6.
Effects of low temperature on the Pro content of winter rapeseed leaves and roots
Effects of exogenous calcium and calcium inhibitor on Ca2+ content of rapeseed roots under low-temperature stress
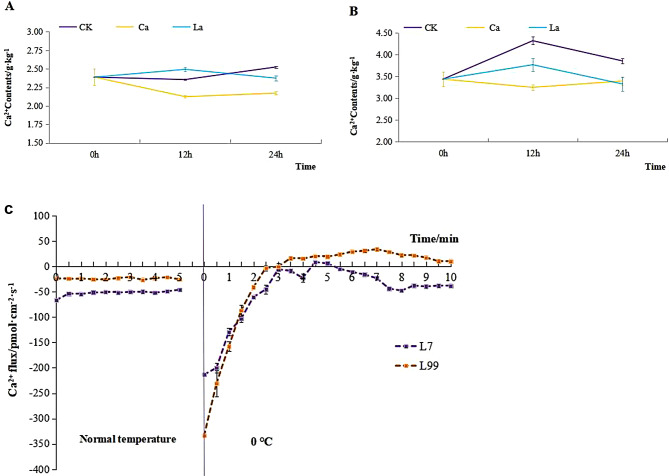
Ca2+ contents in roots of L7 maintained at lower level relative to L99, and there was no significant change at 12 h compared to 0 h but increased at 24 h. However after CaCl2 treatment it dropped significantly at 12 h compared to CK, and after application of LaCl3 it increased significantly at 12 h and then dropped at 24 h compared to CK (Fig. 7A). In case of L99, Ca2+ contents in roots maintained at higher level with a slight drop at 24 h compared to 12 h. After the application of CaCl2 and LaCl3, Ca2+ contents dropped significantly compared to CK. The two varieties showed obvious different trend in CK while similar trend after the application of CaCl2 and LaCl3 (Fig. 7A and B).
Fig. 7.
Comparison of calcium ion content in root under different treatments Note: (A) L7, (B) 99, (C) Comparison of Ca2+ flow rate in rapeseed root before and after 0 ℃ treatment
Analysis of changes in Ca2+ flow rate at low-temperature
Both L7 and L99 roots showed Ca2+ influx at normal temperature, while the influx rate was higher in L7 than in L99 (Fig. 7C). After low-temperature treatment, L99 showed Ca2+ efflux with a rate of 30.21 pmol‧cm-2‧s-1, whereas L7 briefly showed efflux then returned to influx.
Transcriptome analyses
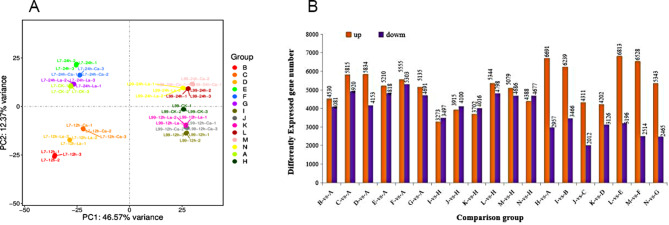
A total of 289.78G of clean data was obtained from sequencing the referenced transcriptomes of 42 samples, with a range of 5.7G to 7.08G per sample. The distribution of Q30 bases ranged from 95.61 to 97.41%, with an average GC content of 46.61%. The percentage of reads that successfully matched the reference genome varied between 88.65% and 90.10%. The matching percentage to the unique position of the reference genome was 86.19–87.55%, and the matching percentage to multiple positions of the reference genome was 2.45–2.92%. A total of 42 samples were subjected to principal component analysis (Fig. 8A and B). The samples exhibited notable disparities, and the replication within each group was satisfactory.
Fig. 8.
Principal component analysis of all samples (left). Analysis of the number of differential expressed genes in each comparison group (right). Note: Each point represents one sample (one biological replicate) and each color represents one treatment
GO and KEGG enrichment analysis of DEGs
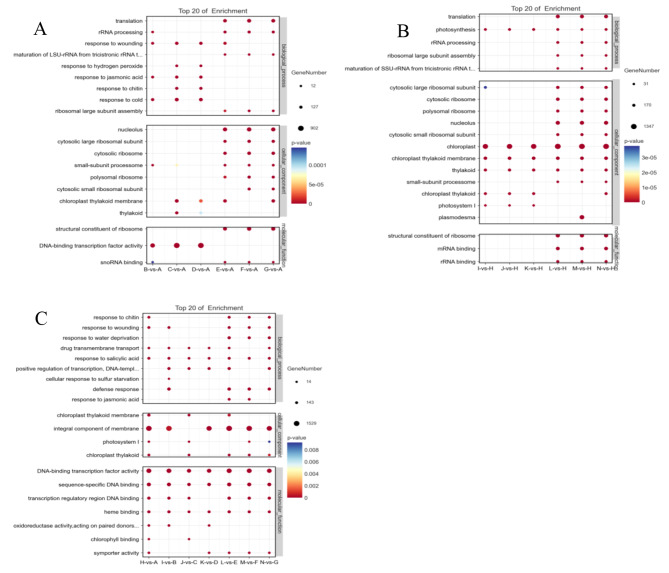
GO functional enrichment analysis was performed on the differential expressed genes of L7 (Fig. 9A). At 12 h treatment time, the biological processes mainly annotated for the response to hydrogen peroxide, low temperature, salicylic acid, Jasmonic acid, and injury. In the cellular component category, annotations were made for photosystem II, chloroplast-like vesicle members, vesicle-like vesicles, chloroplasts, and others. The molecular function category showed significant enrichment in DNA-binding transcription factor activity, calmodulin binding, and phosphatase activity. DNA-binding transcription factor activity was the most enriched among the DEGs in all three group comparisons. In the three group comparisons after 24 h, the biological processes were annotated to ribosomal RNA processing, translation, and DNA replication initiation. Cellular component category showed enrichment mainly in the nucleus and cytoplasmic ribosomes. Nucleus was the most enriched in the three group comparisons. Molecular function category showed significant enrichment on ribosomal structural components, photosystem II binding, and DNA replication initiation binding.
Fig. 9.
Analysis of GO function enrichment results of differential expressed genes (A) Analysis of GO function enrichment results of differential expressed genes of L7; (B) Analysis of GO function enrichment results of differential expressed genes of L99; (C) Analysis of GO function enrichment results of differential expressed genes comparing between two varieties. Note: The horizontal axis Enrichment Score in the figure is the enrichment score, the bigger the bubble the more the number of differential protein-coding genes contained in the entry, the color of the bubble changes from blue-white-yellow-red, and the smaller the enrichment p-value value, the bigger the degree of significance, the bigger the bubble, the more the number of genes. Same as below
GO functional enrichment analysis of DEGs of L99 revealed significant enrichment in biological processes related to light intensity response, photosynthesis, low-temperature response, salicylic acid response, and response to Jasmonic acid in all three treatment groups at 12 h (Fig. 9B). Additionally, cellular components such as chloroplast-like vesicle members, chloroplasts, vesicles, and photosystems I and II were enriched. Molecular function categories were mainly annotated with DNA-binding transcription factor activity and sequence-specific DNA binding. Under 24-hour treatment, biological processes such as translation, rRNA processing, and photosynthesis were significantly enriched. The cellular components were enriched include the nucleus, cytoplasmic ribosomes, cytosolic vesicles, and chloroplast-like vesicles. Molecular function categories that were enriched include ribosomal structural components, chlorophyll-binding, and mRNA binding.
The results illustrated that DEGs were significantly enriched in DNA-binding transcription factor activity, membrane constituents (Fig. 9C), and sequence-specific DNA binding in CK comparison (H-vs-A) of the two varieties. At treatment time of 12 h, the three comparisons (I vs. B, J vs. C, K vs. D) showed enrichment in the following biological process categories: DNA binding transcription factor activity, positive transcriptional regulation, and DNA templates. A large number of genes were differentially expressed and enriched in response to chitin, wounding, water deficit, salicylic acid.
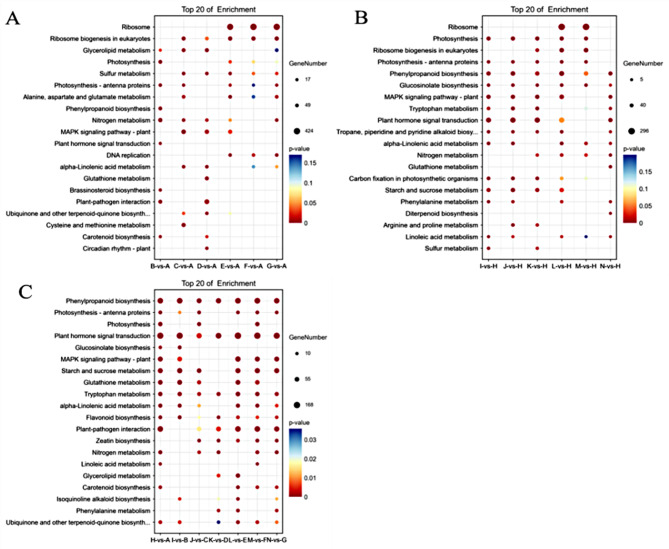
In L7 variety, MAPK signaling pathway, glycerol ester metabolism, nitrogen metabolism, photosynthesis antennae proteins, and phytopathogen interactions were significantly enriched in B-vs-A, C-vs-A, and D-vs-A groups (Fig. 10A). And MAPK signaling pathway, eukaryotic ribosome biogenesis, ribosomes, and photosynthesis were significantly enriched in E-vs-A, F-vs-A and G-vs-A groups. Meanwhile, In L99 variety (Fig. 10B), MAPK signaling pathway, phytohormone signaling, photosynthesis, and photosynthesis antenna proteins were significantly enriched in different groups (I-vs-H, J-vs-H, and K-vs-H). MAPK signaling pathway, ribosomes, eukaryotic ribosome biogenesis, photosynthesis, and photosynthesis antenna proteins were significantly enriched in L-vs-H, M-vs-H, and N-vs-H.
Fig. 10.
Bubble diagram of KEGG functional enrichment of differential expressed genes (A) analysis of KEGG functional enrichment of differential expressed genes of L7; (B) Analysis of KEGG functional enrichment of differential expressed genes of L99; (C) analysis of KEGG functional enrichment of differential expressed genes for the comparison of the two varieties
In comparison group of both varieties, significant enrichments in phytohormone signaling, tryptophan metabolism, starch, and sucrose metabolism, photosynthesis, MAPK signaling pathway, plant-pathogen interactions, and phenylpropanoid biosynthesis were observed (Fig. 10C). Furthermore, transcriptome analysis revealed 220 genes that were differentially expressed and enriched in MAPK signaling pathway in which we identified 6 candidate genes (LOC103873775, WRKY22, LOC103857966, LOC103866369, LOC103830167, LOC103837288) which were relevant to plant growth and development, as well as signal transduction.
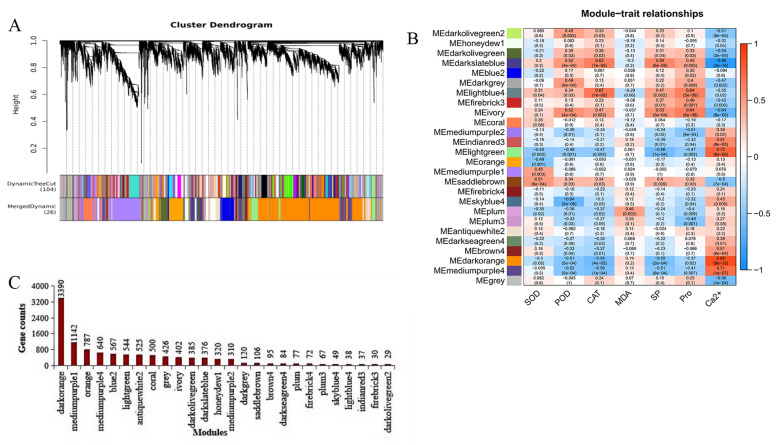
Weighted gene co-expression network analysis and identification of hub genes
A total of 42 samples were taken from winter turnip rapeseed root transcriptome, identifying 40,578 genes. Genes with low fluctuation of expression changes (standard deviation ≤ 0.5) were filtered, leaving 11,118 genes for WGCNA analysis.The power value used in this analysis is 30. Based on pairwise correlations analysis of gene expression 26 merged co-expression modules marked with different colors are shown in Fig. 11A. The analysis of module-trait relationships for the 42 samples revealed that darkslateblue and lightblue4 were significantly positively correlated with POD, CAT, SP, and Pro, and negatively correlated with MDA, and significantly negatively correlated with Ca2+ content (Fig. 11B). The darkorange module contained the most number of genes (3390) and the darkolivegreen2 module contained the least number of genes (29) (Fig. 11C).
Fig. 11.
(A) Gene clustering tree and distribution of modules; (B) Heat map of trait module associations; (C) Number of genes in each module. Note: The figure shows the gene clustering tree (top) and the distribution of genes in each module (bottom). The same color indicates the same module and the Dynamic Tree Cut color indicates the module obtained by using the Dynamic Tree Cut method to identify the modules. Certain modules were merged based on their correlation. The final module obtained is Merged Dynamic in the lower part. These modules were used for subsequent analysis. The correlation between the corresponding module and the trait is indicated by the number (r-value) in each cell. The number in the parentheses stands for the correlation (P-value), and the strength of the correlation is represented by the color of the cell
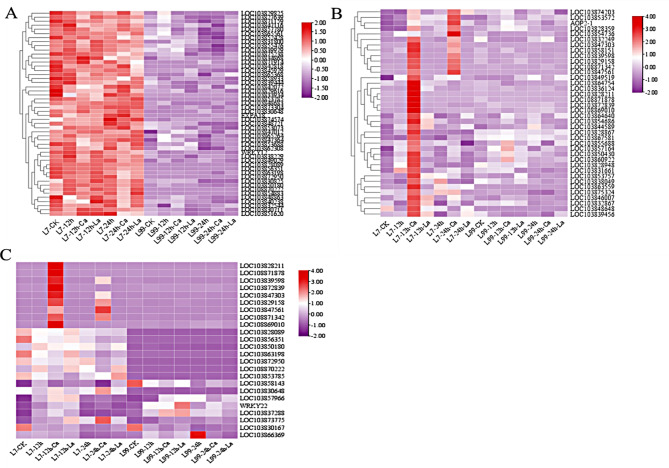
Based on the values of WGCNA edge weight and node scores, the top 18 genes were identified in darkslateblue and lightblue4 module. We choose the top nine genes with the highest connectivity to make functional predictions. In the darkslateblue module, it was observed that 50 genes exhibited up-regulation in L7, however the opposite trend was observed in L99 (Fig. 12A). The lightblue4 module contained a total of 38 genes. The majority of genes exhibited up-regulation following 12 h of CaCl2 treatment in L7, whereas the majority of genes displayed down-regulation following 24 h of CaCl2 treatment. Only a small number of genes showed up-regulation (Fig. 12B). These selected 18 genes from the two modules were subjected to functional annotation and expression analysis, while an additional 6 potential genes were screened within the MAPK signaling pathway (Fig. 12C and Table 3). Furthermore, the expression patterns of these 24 candidate genes were analyzed and whether the changes were significant, and then 8 candidate genes with special expression patterns were screened (Figs. 12C and 13).
Fig. 12.
(A) Darkslateblue module gene expression (B) Lightblue4 module gene expression (C) Heatmap of 24 candidate genes expression
Table 3.
Functional annotation of all candidate genes
| Hub gene ID | Gene abbreviation | Functional annotation |
|---|---|---|
| LOC103828211 | uncharacterized | |
| LOC108871878 | uncharacterized protein At1g43920, Chloroplastic-like | |
| LOC103839598 | uncharacterized | |
| LOC103872839 | 4CLL2 | 4-coumarate–CoA ligase-like 2 |
| LOC103847303 | SPH9 | pumilio homolog 15 |
| LOC103829158 | Os03g0733400 |
zinc finger BED domain-containing protein RICESLEEPER 2-like |
| LOC108871342 | uncharacterized LOC108871342 | |
| LOC103847561 | ZFP2 | zinc finger protein 8-like |
| LOC108869010 | At3g58270 |
MATH domain and coiled-coil domain-containing protein At3g58270-like |
| LOC103828089 | At3g50520 | phosphoglycerate mutase-like protein 4 |
| LOC103856351 | LTA2 | uncharacterized LOC103856351 |
| LOC103850180 | uncharacterized LOC103850180 | |
| LOC103863198 | ATHB-15 | homeobox-leucine zipper protein ATHB-15-like |
| LOC103872950 | DPMS1 | probable dolichol-phosphate mannosyltransferase |
| LOC108870222 | uncharacterized LOC108870222 | |
| LOC103853785 | BI-1 | Bax inhibitor 1 |
| LOC103858143 | WRKY12 | probable WRKY transcription factor 12 |
| LOC103830648 | FIB1 | probable mediator of RNA polymerase II transcription subunit 36b |
| LOC103857966 | CAM5 | calmodulin-5 |
| WRKY22 | WRKY22 | WRKY transcription factor 22 |
| LOC103837288 | CP1 | calmodulin |
| LOC103873775 | SRK2H | serine/threonine-protein kinase SRK2H |
| LOC103830167 | SRK2A | serine/threonine-protein kinase SRK2A |
| LOC103866369 | ERS1 | ethylene response sensor 1 |
Fig. 13.
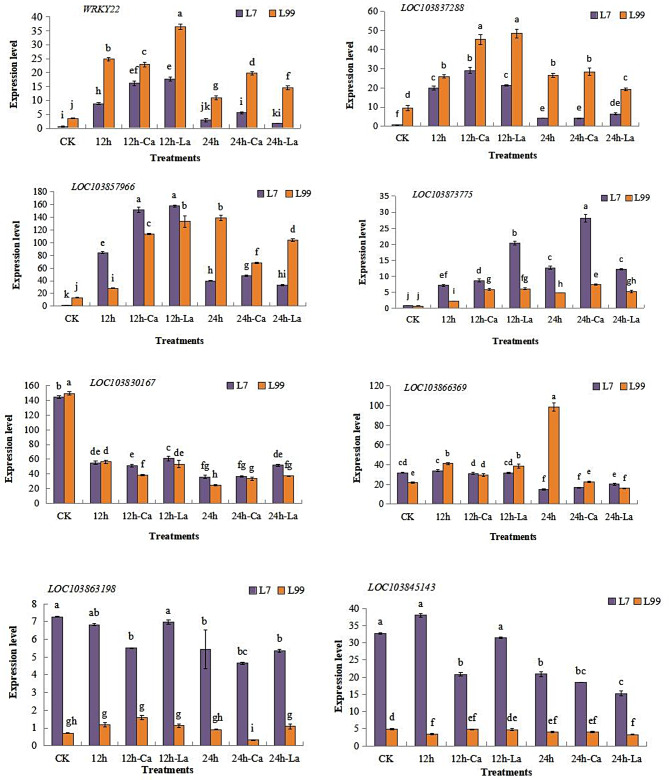
Expression analysis of 8 candidate genes. Note: CK is room temperature control. 12 h is distilled water treatment for 12 h. 12 h-Ca is CaCl2 treatment for 12 h. 12 h-La is LaCl3 treatment for 12 h. 24 h is distilled water treatment for 24 h. 24 h-Ca is CaCl2 treatment for 24 h. 24 h-La is LaCl3 treatment for 24 h
Expression analysis of candidate genes
The expression patterns of 8 candidate genes were further analyzed (Fig. 13). The transcriptional expression of WRKY22 and LOC103837288 in CK were very low, but the expression increased significantly when it was treated with CaCl2 and LaCl3 for 12 h (12 h-Ca, 12 h-La), and decreased significantly at 24 h (24 h-Ca, 24 h-La). LOC103873775 gene expression was significantly higher in L7 than in L99, and it was increased by CaCl2 treatment (12 h-Ca, 24 h-Ca, ). The expression of LOC103857966 in L7was higher than that of L99 after treatment for 12 h (12 h, 12 H-CA, 12 H-LA). It was higher in L99 than that of L7 at 24 h (24 h, 24 H-CA, 24 H-LA), and the expression level of L7 was significantly decreased compared with that of 12 h. The expression of LOC103830167 was the highest in CK, but it was significantly lower than that of CK after adding exogenous substances at low temperature. The expression level of LOC103866369 in L99 (CK, 12 h, 24 h) was significantly increased with time, and the expression level of 24 h was the highest, which was 358.91% higher than CK and 140.49% higher than that of 12 h. LOC103863198 and LOC103858143 were highly expressed in L7 and low in L99, and the expression levels of LOC103863198 and LOC103858143 genes showed a decreasing trend with time in each treatment.
qRT-PCR of candidate genes
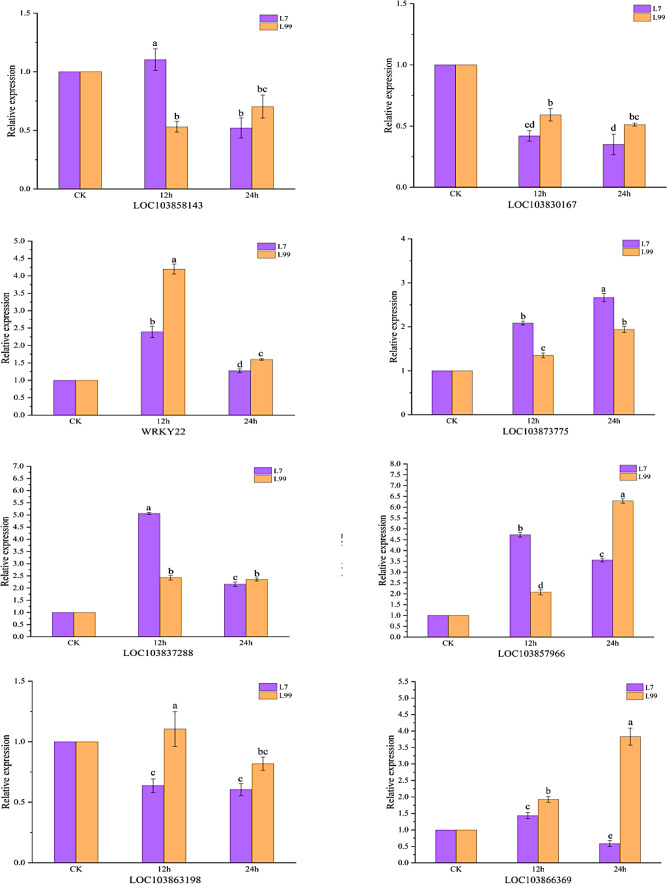
In order to further explore the expression of candidate genes, we analyzed the expression of 8 genes treated at 4℃ by qRT-PCR (Fig. 14). The expression of most genes were consistent with the expression of FPKM value in the transcriptome (Fig. 13).
Fig. 14.
Relative expression of 8 candidate genes. Note: CK is room temperature control. 12 h and 24 h are under 4℃
Discussion
Low temperatures may result in the excessive accumulation of reactive oxygen species, leading to lipid peroxidation and damage to cell membranes, which can ultimately affect seedling growth [31]. The increase in antioxidant enzyme activity is commonly regarded as a crucial mechanism for inducing resistance to oxidative stress in plants [32, 33]. Pu [34] discovered that cold-tolerant rapeseed varieties maintained higher levels of SOD and CAT activity, as well as higher SP content while exhibiting lower MDA content when subjected to freezing treatment. When plants are exposed to low-temperature stress, changes occur not only in antioxidant enzyme activities but also in osmoregulatory substances in the body, which slow down the damage sustained [35]. Li [36] concluded, through the study of cotton seedlings, that the increase in soluble protein content was positively correlated with the cold resistance of varieties under different low-temperature conditions. This study showed that at low temperature, the activities of SOD, CAT and POD increased, as did the SP and Pro. Enzyme activities in leaves and roots in strong cold-resistant variety increased more than weak cold-resistant variety. These findings are consistent with the results of previous studies by Tahmasebi [37] and Wei [38]. Some calcium ions can keep cell membranes stable, reduce MDA levels, keep cell membranes functioning normally, and reduce membrane lipid POD [39]. Exogenous Ca2+ slows down adversity stress, but the required concentration varies among different plants [40]. Zhou [41] found that the addition of 20 mmol·L− 1 CaCl2 significantly increased the activities of antioxidant enzymes (SOD, APX, CAT) in tobacco leaves. In this study, CaCl2 treatment decreased MDA content and increased antioxidant enzyme activities, soluble protein, and Pro content, what reduced chilling injury to leaves and roots of rapeseed. These results agree with those of Pu [34].
It found that the optimal concentration of lanthanum chloride can increase the height and root growth of mauve bean seedlings, as well as root vigor and dry matter accumulation [42, 43]. In their study of Cymbidium macrorrhizum, Wang [44] found that lanthanum chloride had a dual effect on plant growth: at low concentrations, it increased the activity of antioxidant enzymes, the content of physiological regulators and plant resistance, but at high concentrations, it inhibited plant growth. In this study, it was found that after LaCl3 treatment, the oxidase activities of leaves and roots were both reduced and the contents of osmoregulatory substances were significantly increased, which was similar to the results of Wang [44]. It is speculated that the high concentration of LaCl3 in this experiment intensified the peroxidation level of rape membrane, and thus the inhibitory effect appeared.
Calcium plays a direct role in increasing plant resistance to various stresses [45–49]. Researches shows that the higher the calcium ion uptake peak, the higher its cold hardiness [50]. Contrary to the results of the present study, this study found that calcium ion uptake peak does not solely indicate the strength of rapeseed’s cold resistance, calcium ion uptake peak of weak cold resistance was higher than that strong cold resistance. The amount of increase in calcium ions influx and efflux during low-temperature stress plays a key role to a certain extent.
Upon analyzing genes expressed differentially, it was discovered that calcium chloride exhibited the highest number of such genes among the three treatment groups. GO enrichment analysis showed that genes related to low-temperature response, hormone response, photosystem II, chloroplasts, DNA replication, ribosomal RNA processing, and translation were significantly enriched in different exogenous substance treatments under low-temperature conditions. All of these processes are fundamental to cell’s life activities and are essential for its proper functioning [51, 52]. Calmodulin is an important intracellular signaling protein that binds to calcium ions in response to cellular stimuli, thereby regulating cellular biological responses [53]. Changes in the expression levels of these genes can have significant effects on environmental stress [54]. It is important to maintain objectivity and avoid subjective assessments of the response of winter rapeseed to the low-temperature environment.
MAPK signaling pathway is a key pathway in plants that responds to abiotic stress [55–57]. A large number of significant expressed genes enriched on MAPK signaling pathway in L7 and L99. Calcium ions can affect the cell signal transduction process by regulating the activity of MAPK, thus affecting the cold tolerance of winter turnip rapeseed. However, further studies are required to confirm the mechanism of calcium ions regulation of MAPK signaling pathway in rapeseed.
Acknowledgements
We thank Yahong Zhang, Qixian Chen and Feng Li for identification of the cold tolerance of materials. Reviewers are acknowledged for their contribution to the improvement of the manuscript in the revision process.
Abbreviations
- B.
Brassica
- CAT
Catalase
- SOD
Superoxide Dismutase
- POD
Peroxidase
- SP
Soluble Protein
- Pro
Proline
- MDA
Malondialdehyde
- CaCl2
Calcium Chloride
- LaCl3
Lanthanum Chloride
- ROS
Reactive Oxygen Species
- NBT
nitrogen blue tetrazolium
- TBA
Thiobarbituric Acid
- NMT
Non-Invasive Microtomography
- WGCNA
Weighted Gene Co-Expression Network Analysis
- PCA
Principal Component Analysis
Author contributions
J. W. conceived and designed the study. Q. P. and L. Z. analyzed the data. F. A. M. revised and polished the paper. H. G. did the qRT-PCR analysis. L. L., G. Y., W. W., Y. P. and Y. F. contributed materials and analysis tools. J. W. wrote the paper. L. M. and W. S. revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the Gansu Agricultural University youth mentor support fund project (GAU-QDFC-2021-13), Gansu Province college industry support plan project (2023CYZC-51), the China Agriculture Research System of MOF and MARA (CARS-12-09).
Data availability
Data and materials will be made available on request. The datasets generated and/or analysed during the current study are available in the China National Center for Bi-oinformation repository, and the project number is PRJCA028197.
Declarations
Ethics approval and consent to participate
The authors declared that experimental research works on the plants described in this paper comply with institutional, national and international guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhu J, Dai W, Chen B, Cai G, Wu X, Yan G. (2023). Research progress on the effect of nitrogen on rapeseed between seed yield and oil content and its regulation mechanism. Int J Mol Sci 24. [DOI] [PMC free article] [PubMed]
- 2.Li X, Jin J, Ma L, Wu J, Chen Q, Zeng R, Zeng X, Cui X, Sun W. Relationship between height of growth point and cold resistance in strong winter rape (Brassica napus L.) in northern China. Chin J Oil Crop Sci. 2022;44(4):739–50. [Google Scholar]
- 3.Qi W, Wang F, Ma L, Qi Z, Liu S, Chen C, Wu J, Wang P, Yang C, Wu Y, Sun W. Physiological and biochemical mechanisms and cytology of cold tolerance in Brassica napus. Front Plant Sci. 2020;11:1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng Y, Lv JH, Peng MQ, Li J, Wu Y, Gao M, Wu XY, Wang Y, Wu T, Zhang XZ, Xu XF, Han ZH. Genome-wide identification and characterization of the IPT family members in nine Rosaceae species and a functional analysis of MdIPT5b in cold resistance. Hortic Plant J. 2023b;9:616–30. [Google Scholar]
- 5.Zhang X, Ma M, Ye B, Liu L, Ji S. Calcium ion improves cold resistance of green peppers (Capsicum annuum L.) by regulating the activity of protective enzymes and membrane lipid composition. Scientia Hortic. 2021;277:109789. [Google Scholar]
- 6.Ayyaz A, Fang R, Ma J, Hannan F, Huang Q, Athar H, Sun Y, Javed M, Ali S, Zhou W, Farooq MA. Calcium nanoparticles (Ca-NPs) improve drought stress tolerance in Brassica napus by modulating the photosystem II, nutrient acquisition and antioxidant performance. NanoImpact. 2022;28:100423. [DOI] [PubMed] [Google Scholar]
- 7.Huang S, Chen M, Zhao Y, Wen X, Guo Z, Lu S. CBL4-CIPK5 pathway confers salt but not drought and chilling tolerance by regulating ion homeostasis. Environ Exp Bot. 2020;179:104230. [Google Scholar]
- 8.Liu C, Liao W. Potassium signaling in plant abiotic responses: crosstalk with calcium and reactive oxygen species/reactive nitrogen species. Plant Physiol Biochem. 2022;173:110–21. [DOI] [PubMed] [Google Scholar]
- 9.Mazhar MW, Ishtiaq M, Maqbool M, Akram R. Seed priming with calcium oxide nanoparticles improves germination, biomass, antioxidant defence and yield traits of canola plants under drought stress. South Afr J Bot. 2022;151:889–99. [Google Scholar]
- 10.Gupta S, Kaur N, Kant K, Jindal P, Ali A, Naeem M. Calcium: a master regulator of stress tolerance in plants. South Afr J Bot. 2023;163:580–94. [Google Scholar]
- 11.Liu D, Wang X, Chen X, Lin Y, Chen Z, Xu H. Effects of Lanthanum on the change of Calcium Level in the Root cells of Rice. Commun Soil Sci Plant Anal. 2012;43:1994–2003. [Google Scholar]
- 12.Cui WW, Kamran M, Song QH, Zuo BY, Jia ZK, Han QF. Lanthanum chloride improves maize grain yield by promoting photosynthetic characteristics, antioxidants enzymes and endogenous hormone at reproductive stages. J Rare Earths. 2019;37:781–90. [Google Scholar]
- 13.Liu D, Zheng S, Wang X. Lanthanum regulates the reactive oxygen species in the roots of rice seedlings. Sci Rep. 2016;6:31860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Zhang J. (2022). Lanthanum promotes Bahiagrass (Paspalum notatum) roots growth by improving root activity, photosynthesis and respiration. Plants (Basel), 11. [DOI] [PMC free article] [PubMed]
- 15.Niu Z. Molecular mechanism of the height of growth cones respond to low temperature stress in northern winter rapeseed (Brassica rapa L). Doctor Degree, Gansu Agricultural University; 2023.
- 16.Pu Y, Liu L, Wu J, Zhao Y, Bai J, Ma L, Yue J, Jin J, Niu Z, Fang Y, Sun W. (2019). Transcriptome profile analysis of winter rapeseed (Brassica napus L.) in response to freezing stress, reveal potentially connected events to freezing stress. Int J Mol Sci, 20. [DOI] [PMC free article] [PubMed]
- 17.Chao Q, Gao Z, Wang Y, Li Z, Huang X, Wang Y, Mei Y, Zhao B, Li L, Jiang Y, Wang B. The proteome and phosphoproteome of maize pollen uncover fertility candidate proteins. Plant Mol Biol. 2016;91:287–304. [DOI] [PubMed] [Google Scholar]
- 18.Qi J, Luo Y, Huang H, Lu S, Zhao F, Deng Z, Qiu Y. (2023). Molecular mechanism of response and adaptation of antioxidant enzyme system to salt stress in leaves of Gymnocarpos przewalskii. Plants (Basel), 12. [DOI] [PMC free article] [PubMed]
- 19.Ding L, Liu R, Li T, Li M, Liu X, Wang W, Yu Y, Cao J, Tan X. (2022). Physiological and comparative transcriptome analyses reveal the mechanisms underlying waterlogging tolerance in a rapeseed anthocyanin-more mutant. Biotechnology for Biofuels and Bioproducts, 15. [DOI] [PMC free article] [PubMed]
- 20.Zhang y. Study on cold tolerance and cold tolerance mechanism of eight ornamental plants. Master Degree, Shanxi Agricultural University; 2020.
- 21.Wang L, Li S. Thermotolerance and related antioxidant enzyme activities induced by heat acclimation and salicylic acid in grape (Vitis vinifera L.) leaves. Plant Growth Regul. 2006;48:137–44. [Google Scholar]
- 22.Kouakou T, Dué E, Kouadio N, Niamké S, Kouadio Y, Mérillon J. Purification and characterization of cell suspensions peroxidase from cotton (Gossypium hirsutum L). Appl Biochem Biotechnol. 2009;157:575–92. [DOI] [PubMed] [Google Scholar]
- 23.Savaci G. Variations in biochemical compounds of fresh leaves of Castanea sativa in relation to elevation and stand age. J Soil Sci Plant Nutr. 2022;22:2534–44. [Google Scholar]
- 24.Dhindsa R, Plumb-Dhindsa P, Thorpe T. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot. 1981;32:93–101. [Google Scholar]
- 25.Dacheng J, Shan G, Hailun G, Nianwei Q. The details of protein content determination by coomassie brilliant blue staining. Exp Sci Technol. 2018;16:143–7. [Google Scholar]
- 26.Bates L, Waldren R, Teare I. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–7. [Google Scholar]
- 27.Ren Q, Xu Z, Xue Y, Yang R, Ma X, Sun J, Wang J, Lin S, Wang W, Yang L, Sun Z. Mechanism of calcium signal response to cadmium stress in duckweed. Volume 17. Plant Signaling & Behavior; 2022. p. 2119340. [DOI] [PMC free article] [PubMed]
- 28.Fassel V, Kniseley R. Inductively coupled plasma. Optical emission spectroscopy. Anal Chem. 1974;46:A1110–20. [Google Scholar]
- 29.Shannon P, Markiel A, Ozier O, Baliga N, Wang J, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu G, Wang L, Han Y, He Q. ClusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan J, Zhang X, Shang H, Li P, Wang Z, Liao X, Xu X, Yang S, Gong J, Wu J. ORYZA SATIVA SPOTTED-LEAF 41 (OsSPL41) negatively regulates plant immunity in rice. Rice Sci. 2023;30:426–36. [Google Scholar]
- 32.Kong W, Xu X, Li Z, Wang Y, Wu X. Combining ectomycorrhizal fungi and plant growth-promoting rhizobacteria to enhance salt tolerance of metasequoia glyptostroboides. J Forestry Res. 2023;34:1603–14. [Google Scholar]
- 33.Wang M, Ma P, Lin J. (2023). Nanoplatform-based cellular reactive oxygen species regulation for enhanced oncotherapy and tumor resistance alleviation. Chin Chem Lett, 34.
- 34.Pu Y, Zhang L, Miao L, Yang F. Effects of different calcium concentrations on the growth and physiological characteristics of Dalbergia odorifera under low temperatures. Plant Sci J. 2019;37:251–9. [Google Scholar]
- 35.Gao Y, Liu S, Liu H, Ge H, Zhang M, Zhao C, Gong Y, Zhang X, Wang C, Sun X, Wu Z. Application of oxygen vacancy defects in enhanced anti-cancer nanomedicine. Sci China Chem. 2023;66:2492–512. [Google Scholar]
- 36.Li S, Li R, Fen W, Fan Y. Studies on the relationship between cold resistance and increase in non-protein nitrogen content in cotton seedling. China Cotton. 2004;10:14–6. [Google Scholar]
- 37.Tahmasebi A, Pakniyat H. Tahmasebi and Pakniyat comparative analysis of some biochemical responses of winter and spring wheat cultivars under low temperature. Int J Agron Agricultural Res (IJAAR) Tahmasebi Pakniyat. 2015;7:14–22. [Google Scholar]
- 38.Wei J, Zheng G, Yu X, Liu S, Dong X, Cao X, Fang X, Li H, Jin J, Mi W, Liu Z. (2021). Comparative transcriptomics and proteomics analyses of leaves reveals a freezing stress responsive molecular network in winter rapeseed (Brassica rapa L). Front Plant Sci, 12. [DOI] [PMC free article] [PubMed]
- 39.Campo S, Baldrich P, Messeguer J, Lalanne E, Coca M, San Segundo B. Overexpression of a calcium-dependent protein kinase confers salt and drought tolerance in rice by preventing membrane lipid peroxidation. Plant Physiol. 2014;165:688–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi Y, Ke X, Yang X, Liu Y, Hou X. Plants response to light stress. J Genet Genomics. 2022;49:735–47. [DOI] [PubMed] [Google Scholar]
- 41.Zhou S, Wang W, Wang L, Li X. Effects of different calcium effectors on leaf anatomical structure and physiology of banana under salt stress. Genomics Appl Biology. 2017;36:3882–7. [Google Scholar]
- 42.Nikolova V, Kircheva N, Dobrev S, Angelova S, Dudev T. (2023). Lanthanides as Calcium mimetic species in calcium signaling/buffering proteins: the effect of Lanthanide type on the Ca2+/Ln3+ competition. Int J Mol Sci, 24. [DOI] [PMC free article] [PubMed]
- 43.Chen Q, Lu X, Zhang Y, Huang A. Effect of Foliar spraying with different concentrations of Lanthanum Chloride on the growth of Hairy Bean seedlings. Forest by-Product and Speciality in China; 2006. pp. 8–9.
- 44.Wang L, Duan X, Tang M, Sun W. Comprehensive evaluation of physiological indices of cold resistance in cymbidium under Lanthanum treatment. Plant Physiol J. 2014;58:1177–83. [Google Scholar]
- 45.Jones R, Lunt O. The function of calcium in plants. Bot Rev. 1967;33:407–26. [Google Scholar]
- 46.Yasar F, Uzal O. (2020). Effect of calcium applications on ion accumulation in different organs of pepper plant under salt stress. BIO Web Conf, 17.
- 47.Ganapati R, Naveed S, Zafar S, Wensheng W, Jianlong XU. Saline-alkali tolerance in Rice: physiological response, molecular mechanism, and QTL identification and application to breeding. Rice Sci. 2022;29:412–34. [Google Scholar]
- 48.Li XX, Zhang XH, Zhao QS, Liao H. Genetic improvement of legume roots for adaption to acid soils. Crop J. 2023;11:1022–33. [Google Scholar]
- 49.Feng D, Wang X, Gao J, Zhang C, Liu H, Liu P, Sun X. (2023). Exogenous calcium: its mechanisms and research advances involved in plant stress tolerance. Front Plant Sci, 14. [DOI] [PMC free article] [PubMed]
- 50.Xu J, Guo Z, Jiang X, Ahammed G, Zhou Y. Light regulation of horticultural crop nutrient uptake and utilization. Hortic Plant J. 2021;7:367–79. [Google Scholar]
- 51.Feng J, Wang J, Zhang S. Leaf physiological and anatomical responses of two sympatric Paphiopedilum species to temperature. Plant Divers. 2022;44:101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang C, Luo M, Zhang S, Jia G, Tang S, Jia Y, Zhi H, Diao X. Variations in chlorophyll content, stomatal conductance, and photosynthesis in Setaria EMS mutants. J Integr Agric. 2023;22:1618–30. [Google Scholar]
- 53.Du H, Fang C, Li Y, Kong F, Liu B. Understandings and future challenges in soybean functional genomics and molecular breeding. J Integr Plant Biol. 2023;65:468–95. [DOI] [PubMed] [Google Scholar]
- 54.Liu X, Zhang P, Zhao Q, Huang A. Making small molecules in plants: a chassis for synthetic biology-based production of plant natural products. J Integr Plant Biol. 2023;65:417–43. [DOI] [PubMed] [Google Scholar]
- 55.Lu S, Chen J, Wang J, Wu D, Bian H, Jiang H, Sheng L, He C. Toxicological effects and transcriptome mechanisms of rice (Oryza sativa L.) under stress of quinclorac and polystyrene nanoplastics. Ecotoxicology and Environmental Safety; 2023. p. 249. [DOI] [PubMed]
- 56.Yao S, Wang J, Wang J, Liang W. Molecular mechanisms of rice grain size regulation related to plant hormone signaling pathways. Biotechnol Bull. 2023;39:80–90. [Google Scholar]
- 57.Chen X, Hu Y, Chen T, Dai W, Li S, Guo J, Gao S, Wang P, Weng Y, Zheng B, Li J. Progress of chemical regulation on wheat resistance to low temperature stress. J Plant Nutr Fertilizers. 2023;29:1543–55. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and materials will be made available on request. The datasets generated and/or analysed during the current study are available in the China National Center for Bi-oinformation repository, and the project number is PRJCA028197.