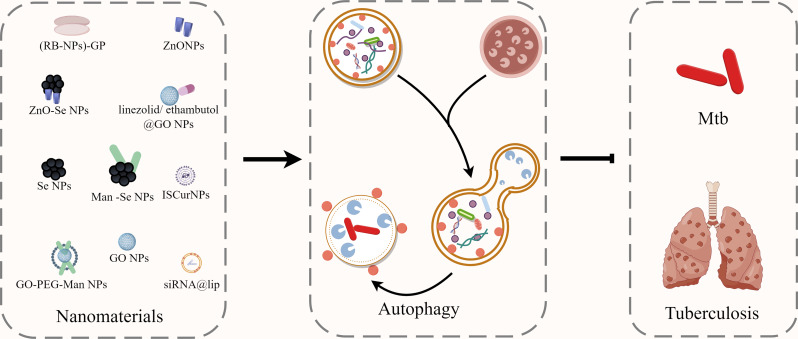
Abstract
Tuberculosis (TB), induced by Mycobacterium tuberculosis (Mtb) infection, remains a major public health issue worldwide. Mtb has developed complicated strategies to inhibit the immunological clearance of host cells, which significantly promote TB epidemic and weaken the anti-TB treatments. Host-directed therapy (HDT) is a novel approach in the field of anti-infection for overcoming antimicrobial resistance by enhancing the antimicrobial activities of phagocytes through phagosomal maturation, autophagy and antimicrobial peptides. Autophagy, a highly conserved cellular event within eukaryotic cells that is effective against a variety of bacterial infections, has been shown to play a protective role in host defense against Mtb. In recent decades, the introduction of nanomaterials into medical fields open up a new scene for novel therapeutics with enhanced efficiency and safety against different diseases. The active modification of nanomaterials not only allows their attractive targeting effects against the host cells, but also introduce the potential to regulate the host anti-TB immunological mechanisms, such as apoptosis, autophagy or macrophage polarization. In this review, we introduced the mechanisms of host cell autophagy for intracellular Mtb clearance, and how functional nanomaterials regulate autophagy for disease treatment. Moreover, we summarized the recent advances of nanomaterials for autophagy regulations as novel HDT strategies for anti-TB treatment, which may benefit the development of more effective anti-TB treatments.
Graphical Abstract
Keywords: Tuberculosis, Nanomaterials, Macrophage, Autophagy, Host directed therapy
Introduction
Tuberculosis (TB), induced by Mycobacterium tuberculosis (Mtb), remains the largest cause of death among infectious diseases after COVID-19 [1]. Drug-resistant TB resulting from Mtb mutations, as well as the emergence of retained bacteria after therapy, have become bottlenecks in TB treatment that must be addressed [2]. Despite extensive efforts in TB control, a more precise understanding of its immunological and pathological mechanisms remains one of the most urgent issues for the development of more effective diagnostics, vaccines and therapeutics [3].
Mtb is an intracellular pathogen primarily transmitted to the lungs through inhalation of droplets containing TB bacteria [4]. Macrophages appear to play critical roles in host-Mtb interactions, utilizing pattern recognition receptors (PRRs) to detect and engulf invading Mtb in phagosomes [5]. In theory, macrophages initiate various host immunological responses, including promoting Mtb-lysosome fusion, apoptosis, autophagy and M1 polarization of infected macrophages to combat Mtb [6, 7]. Additionally, macrophages display antimicrobial activity via reactive oxygen intermediates (ROIs) and reactive nitrogen intermediates (RNIs), along with cytokine-mediated recruitment of immune cells to facilitate local inflammatory responses and antigen presentation to T cells for the development of acquired immunity [8]. Conversely, Mtb employs various strategies to evade the host immune system by counteracting the aforementioned anti-TB mechanisms to ensure intracellular survival [6, 7, 9].
Autophagy, a highly conserved self-degradation mechanism in eukaryotic cells, is crucial for elimination of misfolded proteins, damaged organelles, and pathogenic organisms [10]. Cellular autophagy is categorized into three types based on the manner of intracellular substrate transport to the lysosomal compartment: macroautophagy, microautophagy, and molecular chaperone-mediated autophagy [11]. The most prevalent type of autophagy is macroautophagy, where isolated compartments called phagophores form a lipid bilayer, mature into autophagosomes, and then fuse with lysosomes to form autolysosomes for degradation [12]. During autophagy, nutrients like amino acids and lipids are recycled by the cell, while waste products are eliminated [11]. It has been found that macroautophagy occurs at low levels but can be further induced under stress conditions such as nutritional or energy deprivation. Although macroautophagy is primarily a cytoprotective mechanism for cell metabolism, excessive self-degradation induced by macroautophagy is detrimental [13]. The level of autophagy is closely linked to disease development, suggesting that targeting autophagy regulatory mechanisms could provide new treatment targets for diseases.
Autophagy enables host cells to defend against numerous infections, including Mtb, by preventing pathogens from exploiting host cells for growth and multiplication [14]. Once engulfed by macrophages, a portion of Mtb is sequestered in autophagosome-like compartments, initiating the autophagy process for intracellular clearance of Mtb, confirming the protective role of autophagy in host defense against Mtb [7]. Furthermore, it has been demonstrated that the T helper 1 (Th1) cytokine IFN-γ also promotes macrophage autophagy to eliminate Mtb [15]. However, the inhibitory effects of Mtb on macrophage autophagy dramatically weaken the anti-TB immunity of macrophage for intracellular Mtb clearance, and even allow the survived intracellular Mtb for further infection of other host cells to expand infection [6, 7, 9]. It also has been shown that stimulating macrophage autophagy physiologically can promote the maturation of Mtb phagosomes into phagolysosomes, aiding in intracellular Mtb clearance [16]. Mtb hides and replicates within the human host cells through a variety of methods, and its pathophysiology is heavily reliant on its ability to impair macrophage immune responses [6]. The manipulation of host cell responses to Mtb has been proposed as a potential effective strategy for host-directed therapy. Given the importance of autophagy induction in eliciting protective adaptive immunity, autophagy induction could serve as a novel approach to develop new vaccines or therapeutics [17–19].
In recent decades, the introduction of nanomaterials into medical fields has heralded a new era for novel therapeutics with enhanced efficiency and safety [20–24]. Nanomaterials, characterized by their diameters ranging from 1 to 1000 nm, encompass a diverse array of materials, including metals, lipids, and polymers for potential biomedical uses [25–29]. By deliberate designing and modification, drug-loaded nanomaterials can serve as drug delivery systems to penetrate natural barriers and reach their targets with high efficiency [25, 26]. Despite the promising potential of this drug delivery strategy, several challenges persist, such as unexpected instability, poor bioavailability and unknown in vivo metabolism. However, advancements in nanoparticle engineering now enable the customization of nanoparticle features such as solubility, drug release profile, diffusivity, bioavailability, and immunogenicity, leading to higher treatment efficiency and improved safety [30, 31].
Recently, more and more works are indicating that nanomaterials can also serve as novel agents and drug delivery system for inflammatory and infectious diseases [32, 33], as well as anti-TB treatment [34–36]. The active modification of nanomaterials not only allows the attractive targeting effects of nanosystems against the host cells (such as macrophages), but also introduces the potentials to regulate the host anti-TB immunity, such as apoptosis, autophagy or macrophage polarization [37–39]. In this review, we will summarize the underlying mechanisms of host cell autophagy against the intracellular Mtb, and also introduce the use of functional nanomaterials/ nanomedicines to regulate host cell autophagy for potential anti-TB treatment. Moreover, we will discuss the potentials to develop anti-TB strategies by selectively regulating host cell autophagy and achieving host cell targeting drug delivery by functional nanomaterials, which may contribute to the future development of host cell directed therapies against TB and benefit the control of TB epidemic.
Molecular Mechanisms of Autophagy
Typically, there are three types of autophagy, including macroautophagy, microautophagy, and chaperone-mediated autophagy, with “autophagy” commonly referring to macroautophagy [40]. Macroautophagy differs from the other two types of autophagy as its initial isolation site is located far from the lysosome’s limiting membrane and contains the mechanism of transferring cargo to that cytoplasmic vesicle [13]. Autophagy can be activated by various stimulators or triggers, including hypoxia, amino acid deficiency, stress conditions, as well as some external stimulations [41]. Several structurally and functionally conserved ATG proteins are responsible for the regulations of autophagy process, which can be separated into several successive steps: initiation and nucleation of phagosomes, expansion and closure of phagosomes to produce autophagosomes, fusion with nuclear endosomes and/or lysosomes, and cargo degradation (Fig. 1).
Fig. 1.
The phases of autophagy and its molecular mechanism. (A) Initiation and nucleation of phagosomes. (B) Expansion and closure of phagosomes. (C) Autophagosome-lysosome fusion and degradation
Initiation and Nucleation of Phagosomes
Atg1 homologs (ULK1 or ULK2) from the Unc-51-like kinase family, along with Atg13, RB1CC1/FIP200 and Atg101 are responsible for the induction and development of the initial phagosomal component in mammals [42] (Fig. 1A). The operation of the ULK1/2 complex is regulated by signals from rapamycin complex 1 (MTORC1) and AMP-activated protein kinase (AMPK) [43]. The pre-autophagosomal structure (PAS) is thought to be an essential site for autophagosome development. When cells are stimulated by intracellular or extracellular substances, ATG13 attaches ULK1 to the PAS, followed by the aggregation of additional autophagy-related proteins to the PAS for the initiation of autophagy [44]. The ULK1/2 complex localizes to the PAS to form a scaffolding complex, and the class III phosphatidylinositol 3-kinase (PI3K) complex, will then be recruited to the autophagy formation site [45]. Phagocytes nucleate on endoplasmic reticulum-emitting phosphatidylinositol 3-phosphate (PI3P)-rich membrane structural domains characterized by the presence of the PI3P-binding protein ZFYVE1/DFCP1 [46]. Class III phosphatidylinositol-3-kinase (PI3K) catalyzes the phosphorylation of phosphatidylinositol (PI) to form phosphatidylinositol 3-phosphate (PI3P), which is required for autophagosome elongation and completion because it binds and recruits the membrane-bound protein ATG18 to bilayer membranes [47]. ATG14L begins to engage in phagosome formation by interacting with and binding to Atg13 on the PAS. As a key macrophage central regulator, beclin1 regulates autophagy by interacting with a number of proteins like ATG14L, UVRAG, Rubicon, and Bcl2, thereby regulating autophagy processes [48]. The recruitment of ATG9A-containing cytoplasmic vesicles is also important for autophagosome formation. Phosphorylated ATG9A will be recruited to autophagosome formation sites and is necessary for phagosome expansion and elongation [49].
Expansion and Closure of Phagosomes
Following initiation, two main mechanisms involving ubiquitin-like (UBL) proteins are responsible for phagosome expansion [50] (Fig. 1B). The first system includes the development of the Atg12-Atg5-Atg16 complex. Atg12 forms an irreversible connection with the lysine residue of Atg5, facilitated by the E1 and E2 enzyme activities of Atg7 and Atg10 [51]. Subsequently, Atg12-Atg5 binds noncovalently to Atg16L1 and dimerizes to generate a bigger complex that is required for the creation of the LC3-linked system [52]. The mammalian Atg12-Atg5-Atg16L1 complex binds to phagosomal membranes, and then separates after autophagosome formation, thus could be served as a specific phagosomal marker [53]. The second UBL system involves the Atg8 family, which is linked to phosphatidylethanolamine (PE). PE coupling of Atg8 needs ATG7 (E1) and ATG3 (E2), as well as the ATG12-ATG5-ATG16L1 complex (E3) [54]. Atg8 is first processed at the C-terminus by Atg4, an enzyme involved in ubiquitin processing/decoupling. The E1 enzyme Atg7 then activates Atg8, which is then connected to the amino group of the lipid PE via the E2 enzyme Atg3 [55]. ATG7, the only enzyme shared by both coupling routes, lipolysis ATG8 proteins while also binding ATG12 to ATG5. ATG3, whose major function is to aid in the membrane coupling of Atg8 proteins, accomplishes this through its E2-like activity and direct binding to membranes, allowing the Atg8 proteins to be closer to the membranes. The fundamental function of the ATG12-ATG5-ATG16L1 E3-like complex in ATG8 lipidation is assumed to be membrane recruitment and activation of ATG3 to facilitate the transfer of ATG8 proteins to their PE substrates [54]. Atg4 cleaves Atg8 at the C-terminal peptide link to the glycine residue, which is required for the conjugation process. Furthermore, Atg4 can function as a deconjugating enzyme to cleave the amide link between Atg8 and PE, which would help to liberate the protein from PE in membranes [56]. After processing, lipid-conjugated LC3 (LC3-PE) localized to autophagosomal membranes would contribute to autophagosome formation and elongation [57].
Autophagosome-Lysosome Fusion and Degradation
The ultimate stage in autophagy is the fusion of autophagosomes with lysosomes. Lysosomes tend to occur in the perinuclear area, while autophagosomes always form at random throughout the cytoplasm (Fig. 1C). Thus, once mature autophagosomes are formed, they will be delivered to the perinuclear area where lysosomes located along the microtubules by a dynamin-dependent mechanism [58]. Autophagosome and lysosome formation is mediated by soluble n-ethylmaleimide-sensitive fusion protein attachment protein receptors (SNAREs), membrane tethering proteins, small GTPases, and other fusion-associated proteins [59–61]. Autophagosomal components are digested upon fusion with lysosomes or late endosomes, facilitated by the activation of lysosomal hydrolases, which will destroy the inside proteins and organelles to form lots of amino acids or polypeptides for intracellular recirculation. Autophagic lysosomes eventually degrade to form residues, marking the completion of the autophagy process [62, 63].
Autophagy and Pathogen Infection
Infectious diseases, a major threat to human health and survival, have remained one of the leading causes of mortality and disability worldwide [64]. Autophagy, as an intracellular physiological process, is essential for the host’s defense against foreign pathogens [65]. Traditionally, autophagy has been divided into two types: canonical and non-canonical autophagy. The former is crucial for cell survival and homeostasis, and the latter includes selective autophagy as well as LC3-associated phagocytosis (LAP), which targets a wide range of components, macromolecules, and intracellular microbes [66]. In reaction to foreign pathogen infection, the innate immunological system can eliminate harmful pathogens and their products by the initiation of autophagy, a process known as “xenophagy” [67].
During an infection, host cells use pattern-recognition receptors (PRRs), including pathogen-associated molecular patterns (PAMPs) and pathogen-induced damage-associated molecular patterns (DAMPs) to recognize diverse microorganisms, which further initiate host cell autophagy. Autophagy can selectively catch microorganisms depending on the nature and location of PAMPs and DAMPs within host cells [68, 69]. Recognition of hazardous substances by receptors triggers intracellular signaling cascades, with autophagy thought to be one of the effector mechanisms downstream of these receptors, leading to the activation of antimicrobial effector mechanisms to facilitate the clearance of infection [70].
Invading pathogens are initially ubiquitinated, which may trigger the intracellular xenophagic autophagy signaling pathways. Ubiquitination of cargo necessitates the subsequent action of three enzyme cascades that mediate substrate specificity: ubiquitin-activating (E1), ubiquitin-coupled (E2), and ubiquitin-conjugating (E3) [71]. Following that, the ubiquitinated bacteria are trapped by the ubiquitin-binding protein junction, which binds to the autophagosome membrane containing LC3 [72]. Ubiquitin-binding proteins, or bridging proteins, contain ubiquitin-binding domains and LC3-interacting regions (LIRs) that bind to ubiquitin and ATG8/LC3, thereby linking ubiquitinated substrates to the autophagy machinery [14, 73]. The bridging proteins p62, NDP52, OPTN, and TAX1BP1 have all been implicated in xenophagy [74–77].
Additionally, during LAP, LC3 generates standard phagolysosomes via LC3-associated phagocytosis, which requires autophagic machinery participation, while the bacteria are limited to nascent and possibly intact phagolysosomes [78]. Pathogen attachment to surface receptors of host cells, such as toll-like receptors, Fcgamma receptors, or Dectin-1, can directly induce LC3 binding to the phagosomal membrane during phagocytosis [78, 79]. The activation of Beclin-1/Rubicon complex and NADPH oxidase-2 (NOX2) is required for the above processes. Both PtdIns (3)P and ROS also play important roles in LAP [80]. Related research has demonstrated that macrophages with LAP pathway abnormalities are less effective at controlling bacterial infections such as Legionella pneumophila, Staphylococcus aureus, and Mtb, which indicated the critical roles of LAP in bacterial infection defenses [81]. Another key role of LAP is to aid antigen presentation via MHC class II molecules for adaptive immunological response activation. It has been observed that the absence of LAP impairs the presentation of pathogen antigens on MHC class II molecules, which therefore impairs anti-infection immunity [82].
Viruses, with the exception of bacteria, are also autophagy targets aimed by host cell immunity. The process by which the autophagic machinery coordinates the capture of degraded viral components, viral particles, and even host factors essential for viral reproduction, therefore eliminating RNA and DNA viruses, is known as viral autophagy [83, 84]. Autophagy transports viral particles to lysosomes for destruction; it also binds to cellular antiviral signals, preventing viral reproduction and transmission [65]; To activate innate and adaptive immunity, viral nucleic acids are delivered to Toll-like receptors (TLRs), MHC class I and MHC class II molecules, and endogenous viral antigens are presented to activate adaptive immunity [83]; Notably, reactive oxygen species (ROS), produced by mitochondria, endoplasmic reticulum, and peroxisomes, are acted as signaling molecules involved in the creation and maturation of phagosomes and autophagosomes for virus infection defense [85].
The Confrontation between Mtb and Autophagy
Mtb, an airborne infection, enters the lungs via the respiratory tract and primarily targets alveolar macrophages [86]. Definitively, macrophages are highly capable of recognizing and phagocytosing Mtb, a process that relies on the recognition of different PRR molecules on macrophages with the pattern of microbe-associated molecules on Mtb [87]. During phagocytosis, phagosomes form as a result of membrane invagination, outgrowth, and fusion events. In Mtb-infected macrophages, nitric oxide synthase and antimicrobial peptides exert a direct killing effect on Mtb [4]. In addition, Mtb-infected macrophages up-regulate the expression of various pro-inflammatory cytokines and chemokines, including TNF-α, IL-1β, IL-6, IL-23, and granulocyte-macrophage colony-stimulating factor (GM-CSF). In the presence of these cytokines and chemokines, Mtb was rapidly translocated to lysosomes, where the acidic environments and some anti-bacterial enzymes, such as lysozyme and hydrolase, would execute their pathogen clearance functions.
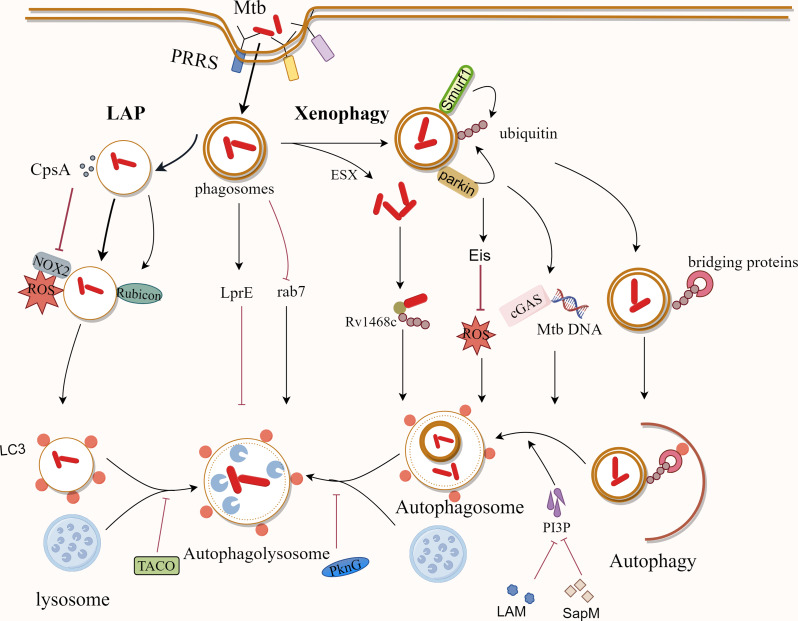
Apparently, autophagy plays a key role in the killing and removal of Mycobacterium. Several studies have suggested possible mechanisms for the ubiquitin-dependent autophagic clearance of Mtb. During xenophagy of Mtb, the ubiquitin ligases Parkin and Smurf1 are involved in the ubiquitination of Mtb, mediating the delivery of Mtb to the autophagosome [88, 89]. The DNA sensor cGAS could identify Mtb DNA in the cytoplasm, which thus triggers autophagy for Mtb clearance [90]. Additionally, ubiquitin can bind directly to the Rv1468c protein on the surface of Mtb, triggering xenophagy [91]. LC3-associated phagocytosis (LAP) also appears to be critical in host resistance to Mtb infection [92]. LAP, characterized by a single-membrane structure, recruits NADPH oxidase to the phagosome, which binds to LC3 and transports the bacteria to the degradative lysosome. At the same time, Rubicon can directly stabilize the NADPH oxidase complex, which leads to the generation of ROS and better bactericidal effects [93] (Fig. 2).
Fig. 2.
The Mechanisms of Mtb clearance by cellular autophagy and inhibition of host cell autophagy by Mtb. Macrophages identify Mtb primarily through PRRs. Nitric oxide synthase and antimicrobial peptides, among other things, destroy Mtb directly after it reaches macrophages via phagocytosis. Mtb can also be removed by ubiquitin-dependent autophagy in macrophages, as well as LAP, which is characterized by a single-membrane structure. Meanwhile, Mtb has developed ways to withstand macrophage destruction and use it as a medium for habitation and dissemination
Conversely, Mtb has evolved to utilize macrophages as habitats and use them as mediators of transmission in the host [94]. Evolved mycobacteria are able to inhibit the antimicrobial activity of reactive oxygen species. For example, the Mycobacterium protein CpsA from Mtb could protect Mtb from NOX2-dependent ROS and LAP-mediated killing [92].
More importantly, Mtb has also evolved some strategies to evade the organism’s autophagic defenses as well as to manipulate host immune functions [95]. Mtb is able to inhibit the acidification of phagosomes, keeping them at a relatively more neutral pH (pH ~ 6.2), thus allowing itself to persist within the phagosome [9]. Macrophages lacking LRG-47 have reduced acidification capacity, impairing their ability to mount immune responses against Mtb [96]. In addition, the protein tyrosine kinase PtkA, a protein tyrosine phosphatase that is closely related to the inhibition of host cell acidification and maturation, also plays an important role in the inhibition of host immunological responses against Mtb [97]. To prevent phagocytosis-mediated bacterial killing, Mtb can modify phagosomes to form pathogen-containing vesicles and avoid the fusion of phagosomes with lysosomes to form the more deadly phagolysosomes [95]. Coronin 1, initially known as a tryptophan aspartate-rich shell protein, is recruited in phagosomes containing active Mtbs, can specifically block lysosomal delivery and death of Mtbs in macrophages and allow Mtb to escape lysosomal bactericidal action [98]. Simultaneously, Mtb can interfere with phagosome-lysosome fusion by blocking transport events dependent on phosphatidylinositol 3-phosphate (PI3P) regulation [99]. Mtb interferes with the activity of the PI3 kinase hVP34 via its cytosolic component, lipoarabinomannan (LAM), thereby preventing phagosome-lysosome fusion [100]. Moreover, SapM, an acid phosphatase released in the cytoplasm of Mtb-infected host cells, hydrolyzes PI3P on the phagosome membrane, thereby preventing its accumulation. Also, PknG has the ability to interfere with the acidification and maturation of Mtb-containing phagosomes, which is closely related to its kinase activity [101]. Furthermore, in macrophages, Mtb can also inhibit the recruitment of Rab7 to the phagosome, thereby evading phagosome-lysosome fusion [102]. Notably, Mtb relies on the ESX system to secrete virulence factors, facilitating their translocation from the phagosome to the cytoplasm for replication [103]. In addition, the Mtb lipoprotein LprE inhibits autophagy and histone expression, promoting bacterial replication [104]. The Mtb protein, enhanced intracellular survival (Eis), inhibits macrophage autophagy by suppressing Jun N-terminal kinase (JNK)-reactive oxygen species (ROS) in Mtb-infected macrophages [105].
Mtb infects human cells, especially macrophages, and has co-evolved over time, leading to a number of survival strategies, such as inhibition of the autophagy pathway. A better exploration and understanding of these strategies will facilitate the development of better ways to treat TB.
Disease Treatment Strategies by Nanomaterials Targeting Autophagy
In recent decades, nanomaterials have been widely reported to show promising potentials for illness prevention, diagnosis and therapy, such as cancer and infectious diseases. Diverse levels of autophagy in diseases have different impacts on the progression of various diseases. It is necessary to investigate how different nanomaterials with varied characteristics can regulate autophagy in disease treatment (Fig. 3). Nanomaterials can be classed as autophagy inhibitors, inducers, or drug delivery systems that actively transport autophagy modulators to target areas. Selecting the appropriate autophagy modulators for different tumors and different roles of autophagy in tumors can more accurately regulate autophagy to achieve the desired therapeutic effects. Some well-designed nanomaterials modulate autophagy, providing preventative or therapeutic synergistic effects on a variety of illnesses, including neurological, autoimmune, and infectious disorders.
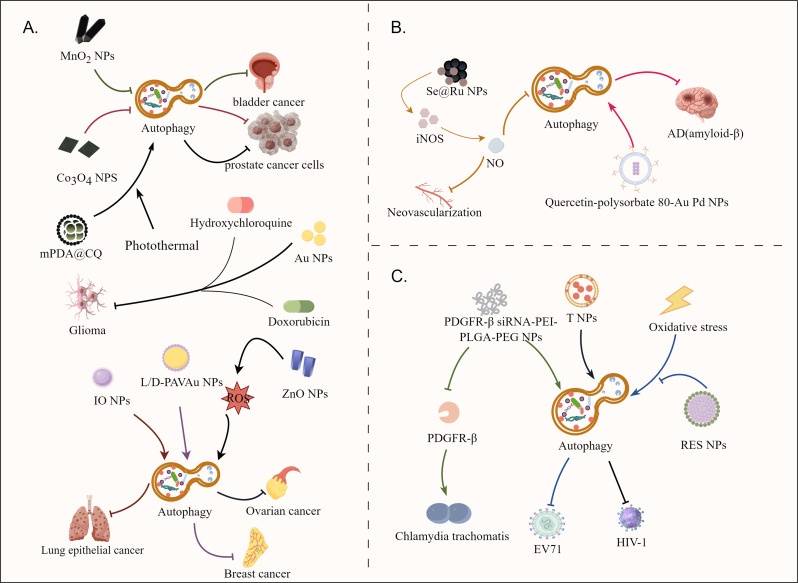
Fig. 3.
The mechanisms of nanomaterial modulation of autophagy. (A) Nanomaterials control autophagy in cancer to limit disease progression. Nanomaterials can be used directly as autophagy inhibitors to limit cancer cell growth, as autophagy inducers to disrupt cancer cell metabolism and inhibit cell growth, and as drug delivery systems to actively deliver autophagy modulators to the tumor site for more effective anti-tumor therapy. (B) Specific nanomaterials show great potential in the treatment of AD and rheumatoid arthritis. (C) Nanomaterials play a role in controlling infectious diseases by modulating autophagy to inhibit pathogens
Cancers
Autophagy plays a dual role in cancer, both preventing and promoting tumor development [106]. This is partially dependent on the microenvironment in which the tumor is placed. The tumor microenvironment is characterized by low oxygen, acidic pH, and a high concentration of reactive oxygen species (ROS). Autophagy is involved in cancer cell development in hypoxic conditions, which acts as a metabolic process to benefit cancer cell growth [107]. Firstly, nanomaterials can directly act as autophagy inhibitors to restrict cancer cell growth. It has been proven that the use of MnO2 based nanomaterials can synergistically alter the aberrant tumor microenvironment and has a role in blocking autophagic flux, successfully sensitizing radiotherapy for bladder cancer treatment [108]. Our previous work also indicated that cobalt oxide nanoparticles (Co3O4 NPs) could also be served as an autophagy inhibitor to enhance the anticancer efficiency of chemotherapeutic drugs [109]. These results suggested that the use of functional nanomaterials can directly block the autophagy processes to promote cancer cell death, thus showing potentials for cancer treatment.
In addition, nanomaterials can also be used directly as autophagy inducers to inhibit the growth of cancer cells by disturbing their normal metabolism. It has been demonstrated that zinc oxide nanoparticles (ZnO NPs), a suitable anticancer agent, can lead to mitochondrial damage via ROS induction and can also induce autophagy in human ovarian cancer cells for anticancer treatment [110]. In a parallel way, iron oxide nanoparticles(IO NPs) also have autophagy-inducing effects [111], which can specifically induce autophagy in human lung epithelial cancer cells (A549) to promote the death of cancer cells. However, these nanoparticles showed no significant cytotoxicity against normal human lung fibroblasts (IMR-90). For breast cancer, the researchers designed monolayers of poly (acryloyl- L, D and racemic valine) chiral molecules anchored on the surface of gold nanoparticles, namely L-PAV-Au NPs, D-PAV-Au NPs and L/D-PAVAu NPs [112]. The results showed that PAV-Au NPs exhibited chiral-dependent cytotoxicity against breast cancer cells through autophagy activation, and this autophagy activation may be attributed to the production of chiral variant ROS, cellular uptake and its continuous autophagy stimulation, especially D-PAV-AuNP. Obviously, the powerful function of nanoparticles that can effectively regulate autophagy and assist in the treatment of diseases was demonstrated.
Moreover, nanomaterials can also act as drug delivery systems to actively deliver autophagy regulators into tumor sites for more effective anti-tumor treatments. Due to the presence of the blood-brain barrier, chemotherapeutic medication administration is particularly problematic, but the use of carefully tailored nanomaterials has made more efficient drug delivery to overcome the blood-brain barrier a reality [113, 114]. A combination therapy strategy based on gold nanoparticles (Au NPs) carrying the medications doxorubicin (DOX) and hydroxychloroquine (HCQ, an autophagy suppresser) and PD-L1 immune checkpoint blockage was reported to improve the efficacy of chemotherapy for glioma [115]. We have also previously fabricated a biomimetic nanoplatform by precisely coating homologous prostate cancer cell membranes (CMs) onto the surface of mesoporous polydopamine nanoparticles (mPDA NPs) encapsulating the autophagy inhibitor chloroquine (CQ) for synergistically manipulating PTT and autophagy for anticancer treatment [116]. The obtained results indicated that combination of an autophagy inhibitor with other anti-cancer effects could synergistically inhibit tumor growth, which therefore led to better anticancer effects. Nanomaterials also can be used to load autophagy-inhibiting microRNAs for downregulating autophagy-associated miRNA targets, enabling robust inhibition of autophagy in breast cancer [117].
Alzheimer Disease
Alzheimer’s disease (AD), a prevalent neurodegenerative disorder, is characterized by the formation of senile plaques composed primarily of amyloid-β (Aβ) peptides [118, 119]. The cleavage of the parental amyloid precursor protein (APP) by β-secretase and γ-secretase results in the formation of amyloid-β (Aβ) peptides [120]. Autophagy dysfunction is thought to be associated with the pathogenesis of AD; decreased levels of autophagy result in abnormally aggregated proteins not being removed in a timely manner, thus accelerating the disease process [121, 122]. As a result, pharmacological activation of autophagy and promotion of autophagic degradation of Aβ proteins offer a therapy option for AD. A quercetin (autophagy inducer)-modified polysorbate 80 (P-80)-coated Au Pd core-shell nanostructure has been developed to activate autophagy of SH-SY5Y cells, promote the fusion of autophagosomes and lysosomes, accelerate the clearance of Aβ, and protect SH-SY5Y cells from Aβ-induced cytotoxicity damage [123]. These results indicated the potentials of nanomaterials for AD treatments by regulating autophagy pathway.
Autoimmune Diseases
Nanomaterials have shown promising potential in treating autoimmune diseases. In rheumatoid arthritis, the main clinical features are proliferative synovitis and angiogenesis [124]. Therefore, using this as a therapeutic target for RA, the researchers carefully prepared a nanomaterial called peptide-composite selenium nanoparticles Se NPs-PEG RGD @Ru (Se @Ru NPs) [125]. The material stimulates NO production by inducing the formation of NO synthase (iNOS) thereby inhibiting the growth of local tissue neovascularization. In addition, NO enhances the phosphorylation of AMPKa and inhibits the phosphorylation of mTOR, which in turn enhances autophagic flux.
Infectious Diseases
Moreover, nanomaterials can also be used for infectious disease treatment by targeting autophagy. Chlamydia trachomatis, a specialist intracellular parasite bacterium, is one of the most prevalent sexually transmitted pathogens [126]. Prolonged infection and the emergence of drug-resistant phenotypes are difficult for antibiotic treatment and may even lead to sequelae caused by severe inflammation, such as pelvic inflammatory disease and infertility [127]. Therefore, there is a need to develop new non-antibiotic therapies to provide new options for clinical treatment. It has been found that the absence or neutralization of platelet-derived growth factor receptor-β (PDGFR-β) reduces the binding of Chlamydia trachomatis to host cells [128]. Based on the previous findings, researchers developed a PDGFR-β siRNA-PEI-PLGA-PEG NP using PDGFR-β, polyethyleneimine (PEI), poly (lactic-hydroxy glycolic acid), and poly (ethylene glycol) as raw materials [129]. The results demonstrated that these nanomaterials can both induce cellular autophagy and reduce the expression of the important Chlamydia trachomatis surface-binding protein PDGFR-β gene, which is an important tool against Chlamydia trachomatis infection.
In addition, nanomaterials are also expected to be used as antiviral therapy by directly modulating host cell autophagy to fight viruses [130]. Resveratrol (Res), a component that can be isolated from natural plants, has shown the ability to fight intestinal viruses, but is restricted by its poor solubility and instability [131, 132]. To this end, Resveratrol nanoparticles (RES NPs) based on monomethoxypoly(ethylene glycol)-b-poly (D, L-propanediol) amphiphilic copolymers were prepared for anti-viral treatment [133]. RES NPs can inhibit viral replication and inflammatory responses in enterovirus 71(EV71)-infected RD cells by inhibiting oxidative stress-mediated ERS/autophagy pathway and suppressing the synthesis of VP1 proteins and cytokine secretions. These results suggest that RES NPs may be a novel strategy for the treatment of EV71 infection by regulation of host cell autophagy. Moreover, nanoengineered CD4 + T cell membrane-coated nanoparticles (T NPs) were designed to neutralize a broad range of HIV-1 strains. Interestingly, T NPs also selectively bound to and induced autophagy in HIV-1-infected CD4 + T cells and macrophages, while having no effect on uninfected cells, which therefore resulted in the inhibited viral release and reduced cell-associated HIV-1 in a dose- and phospholipase D1-dependent manner [134]. These results collectively suggested the emerging potentials of nanomaterials for the development of anti-viral strategy by regulating host cell autophagy.
Thus, there are considerably number of nanomaterials that can be used as autophagy regulators or carriers of autophagy regulators for potential disease treatment. And based on the attractive potentials of nanomaterials in autophagy regulation, more and more researchers are trying to develop novel therapeutics against diseases based on functional nanomaterials, which also introduce new possibilities for more effective anti-TB strategy development (See Table 1).
Table 1.
Currently known about nanomaterials in diseases
| Category | Diseases | Nanomaterials | Function | Reference |
|---|---|---|---|---|
| Cancer | Bladder cancer | MnO2 based nanomaterials | Block autophagic flux | [108] |
| Co3 O4 NPs | Inhibit autophagy | [109] | ||
| Ovarian cancer | ZnO NPs | Lead to mitochondrial damage via ROS and induce autophagy | [110] | |
| Lung epithelial cancer | IO NPs | Induce autophagy | [111] | |
| Breast cancer | Au NPs | Active autophagy | [112] | |
| Glioma | Au NPs | Drug delivery systems | [115] | |
| Prostate cancer | mPDA NPs | [116] | ||
| Breast cancer | NPs loading autophagy-inhibiting microRNAs | [117] | ||
| Neurological diseases | Alzheimer’s disease | A quercetin (autophagy inducer)-modified polysorbate 80 (P-80)-coated AuPd core-shell nanostructure | Promote the fusion of autophagosomes and lysosomes | [123] |
| Autoimmune diseases | Treating autoimmune diseases | Se @Ru NPs by inducing the formation of iNOS | Stimulate NO production | [125] |
| Infectious disease | Chlamydia trachomatis | PDGFR-β siRNA-PEI-PLGA-PEG NP | Induce cellular autophagy | [129] |
| Intestinal viruses | RES NPs | Inhibit viral replication and inflammatory responses | [133] | |
| HIV | T NPs | Induce autophagy | [134] |
Nanomaterial-assisted Anti-TB Therapeutic Strategy by Modulating Autophagy
TB still remains a global disaster that must be carefully addressed. Presently, attaining the desired level of clinical efficacy in anti-TB antibiotics poses challenges, resulting in prolonged treatment duration, adverse effects, and the emergence of drug-resistant strains of Mtb [135]. As a result, the discovery of novel anti-TB medications with improved efficacy is a critical endeavor. The biocompatibility, targeting ability, and efficacy of nanoparticles are superior to existing anti-TB medications, which has sparked worldwide interests [136]. The utilization of nanomaterials has demonstrated significant advancements not only in the management of tumors and autoimmune diseases, but also in the regulation of infectious diseases, such as TB (Fig. 4). Autophagy, a key catabolic mechanism in the body, aids in immunological protection against pathogens such as Mtb [137]. Thus, functional nanomaterials with autophagy promoting effects might be served as potential therapeutics against intracellular Mtb for effective Mtb clearance. Firstly, a wide range of nanomaterials can directly regulate autophagy in their own right and can be utilized as autophagy inducers to inhibit or eliminate intracellular Mtb. It has been reported that ZnO NPs can effectively kill Gram-positive and Gram-negative bacteria (S. aureus and E. coli) with low cytotoxicity and low acute toxicity in mice, which indicates their high biocompatibility for anti-infection uses [138]. Excitingly, ZnO NPs have been reported to directly target and kill Mtb, and also inhibit intracellular Mtb in macrophages by affecting the immune response of the host cells [139, 140]. Bao et al. reported the inhibition effects of ZnO NPs against various strains of Mtb (BCG, H37Rv, and clinically susceptible MDR and XDR strains) and the autophagy promoting effects of low doses of ZnO NPs that for effective intracellular Mtb inhibition [141]. However, high doses of ZnO NPs could induce ferroptosis in Mtb-infected macrophages to impair host anti-TB immunity, while the co-administration of a ferroptosis inhibitor with ZnO NPs could improve the anti-TB activity of ZnO NPs in vivo and alleviated acute lung injury caused by ZnO NPs. However, this ability is also found in normal cells, which indicates the potential cytotoxicity of ZnO NPs. Additionally, M1 macrophages demonstrated decreased expression of polarization markers, mainly pro-inflammatory molecules such as COX-2, iNOS, IL-6, and TNF, in the presence of relatively modest dosages of ZnO NPs, which may be due to their killing effect on bacterial load. Therefore, addressing the effective application of ZnO NPs’ anti-TB activity while mitigating their cytotoxicity-induced side effects remains a challenge.
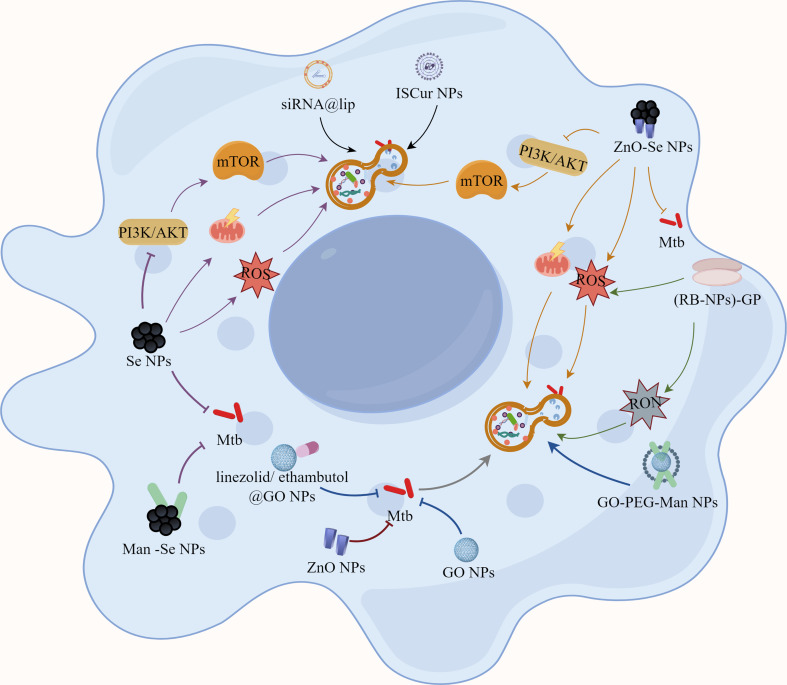
Fig. 4.
The nanomaterials by modulating autophagy-assisted anti-TB therapeutic strategies. Functional nanomaterials with autophagy promoting effects might be served as potential therapeutics against intracellular Mtb for effective Mtb clearance. Some nanomaterials can directly regulate autophagy in their own right and can be utilized as autophagy inducers to inhibit or eliminate intracellular Mtb. Simultaneously, nanoparticles can be employed as drug delivery vehicles to actively transfer pharmacological molecules with autophagy-inducing properties to lesions, resulting in more effective therapeutic effects
Previously, Se nanoparticles (Se NPs) were shown to inhibit the growth and viability of various types of bacteria, with the mode of action involving disruption of energy transduction, increased ROS production, membrane depolarization, and membrane disruption [142]. Meanwhile, the activity they anti-bacterial perform is particle size-dependent and dose-dependent [143]. Furthermore, Se NPs have also previously been proven to be bactericidal by triggering host cell immunological responses [144]. Recently, lots of works have indicated the direct killing effects of Se NPs against Mtb by damaging their cell envelope integrity, which opens new dimensional avenues in the field of Se NPs -induced cell disruption strategies against Mtb [143, 145, 146]. More interestingly, our previous work both indicated the extracellular Mtb killing effects and intracellular Mtb inhibition effects of Man-Se NPs, which were functionalized with mannose on Se NPs for macrophage targeting [144]. Moreover, we further demonstrated that the intracellular Mtb inhibition effects of Se NPs were closely associated with their ability to promote autophagy of Mtb-infected macrophages by altering ROS production, mitochondrial membrane potential and PI3K/Akt/mTOR signaling pathway, while the promoted formation of autolysosomes played a critical role for intracellular Mtb clearance [144].
Combining the anti-TB activity of ZnO NPs and Se NPs, we further fabricated a kind of novel zinc oxide selenium nanoparticles (ZnO-Se NPs) for synergistic anti-TB treatments [39]. These ZnO-Se NPs demonstrated dual functionality: direct extermination of extracellular Mtb and inhibition of intracellular Mtb proliferation by selectively inducing apoptosis and autophagy in Mtb-infected macrophages. ZnO-Se NPs promoted autophagy of Mtb-infected macrophages were closely associated with the increased intracellular ROS, disrupted mitochondrial membrane potential and inhibited PI3K/Akt/mTOR signaling pathway. These results indicated the potentials of ZnO-Se-NPs for anti-TB treatments, suggesting their further exploration as a novel anti-TB adjunct therapy or standalone treatment option.
β-Glucan particles (GP) are polymeric carbohydrates derived from yeast that have been demonstrated to be useful for macrophage-targeted medication delivery [147]. Tarun K. Upadhyay et al. produced glucan particles (GP) with a high payload of Rifabutin (RB) nanoparticles [(RB-NPs)-GP] for anti-mycobacterial application [148]. This multifunctional nanomaterial inherits the beneficial features of GP for macrophage targeting, dramatically improves the Mtb-killing efficacy of RB, and is capable of generating a robust innate immune response within Mtb-infected macrophages. The immune response induced by (RB-NPs)-GP incorporates activation of antimicrobial autophagy and apoptosis in Mtb-infected macrophages as well as induction of reactive oxygen species and reactive nitrogen species, which can lead to a significant decrease in intracellular Mtb survival. Therefore, it was hypothesized from the experimental results that RB- NPs-GP is likely to be an immunotherapeutic tool for activating host immune responses by autophagy regulations and limiting intracellular Mtb growth.
Simultaneously, nanoparticles can be employed as drug delivery vehicles to actively transfer pharmacological molecules with autophagy-inducing properties to lesions, resulting in more effective therapeutic effects. Graphene oxide (GO) nanoparticles have been demonstrated to be useful for the treatment of different kinds of cancers [149]. In cancer, GO regulates apoptosis and autophagy while also increasing the formation of reactive oxygen species, which are used to destroy cancer cells. Surprisingly, GO was also found to block the entry of Mtb into macrophages for the establishment of infection, thus promoting Mtb killings [150]. Furthermore, GO can be employed as a drug delivery system in conjunction with anti-TB pharmaceuticals such as ethambutol and linezolid for the treatment of TB [151, 152]. It not only effectively controlled the drug’s diffusion rate to extend the time to maintain the peak concentration in the body, but it also improved the drug’s activity against Mtb and significantly reduced cytotoxicity. In our previous studies, we developed a GO nanosystem to more effectively target Mtb-infected macrophages, called GO-PEG-MAN, which could significantly enhance intracellular bactericidal efficiency by improving intracellular drug contents and maintaining high drug concentration for intracellular Mtb killings [153]. Although there are still no reports to demonstrate that GO nanosystem can be used to promote autophagy in Mtb-infected macrophages, the native ability of GO to induce autophagy and the ability to act as carriers of autophagy inducer could dramatically contribute to the intracellular Mtb killings in the future researches.
Curcumin (CMN), derived from the rhizome of the perennial herb turmeric, has been demonstrated to reduce Mtb-sensitive and drug-resistant strains’ intracellular survival [154]. Moreover, CMN is widely reported to be an autophagy inducer in different diseases. However, CMN has been criticized for poor bioavailability, which extremely restricts its biomedical uses [155]. The use of nanomaterials as a delivery system to overcome this weakness has made it possible to examine and utilize their biological functions more extensively. For example, to further explore the mechanism of curcumin inhibition in Mtb-sensitive cells, Pramod Kumar Gupta et al. encapsulated CMN in a polylactic acid-glycolic acid (PLGA) shell to obtain polymerized in situ curcumin nanoparticles (ISCur NPs) [156]. Subsequent pharmacokinetic experiments demonstrated that the bioavailability of CMN was significantly improved by ISCur NPs. Meanwhile, ISCur NPs treatment significantly enhanced the autophagy of Mtb-infected macrophages, suggesting that the induction of macrophage autophagy is one of the main mechanisms of ISCur NPs to limit the intracellular survival of Mtb. In addition, ISCur NPs were also found to induce apoptosis in Mtb-infected macrophages and promote phagosome-lysosome fusion for intracellular Mtb clearance. Apparently, the development of more sophisticated ways to treat TB based on ISCur NPs may be one of the options to break through the limitations of existing TB treatment regimens.
Moreover, gene expression regulation is also important for TB progression and TB treatment. siRNA has the property of effectively and selectively inhibiting the expression of homologous genes at the cellular level, encouraging the degradation of homologous mRNAs, and producing specific gene deletion phenotypes [157]. In recent years, the effects of siRNAs on Mtb-infected macrophages have attracted much attention worldwide. For that, liposomal nanoparticles with excellent targeting properties and nucleic acid loading abilities were developed for simultaneous delivery of multiple anti-TB drugs and anti- TGF-β1 siRNAs [158]. Here, chemotherapy, nanotechnology and nucleic acid therapy were then naturally combined for the treatment of intracellular Mtb. The results demonstrated that treatment of Mtb-infected THP-1 cells with a certain concentration of siRNA and drug-loaded liposomes for 24 h resulted in a significant increase in the percentage of autophagic cells, which indicated the potential of siRNA therapy in TB by promoting autophagy of Mtb-infected macrophages. By targeting microRNAs, circRNAs have also been shown to decrease intracellular Mtb growth and induce autophagy in Mtb-infected macrophages [159]. In parallel to siRNAs, circTRAPPC6B has been shown to affect Mtb survival by our previous work. CircTRAPPC6B targets microRNA-874-3p to abrogate its inhibition of ATG16L1, thereby inducing autophagy and inhibiting Mtb growth in macrophages, which also indicates their potentials for TB therapy by regulating autophagy of Mtb-infected macrophages. Both siRNA and circTRAPPC6B have been shown to hold promise as potential therapeutic agents for TB control. And, when combined with nanodelivery systems, such as liposomes, and some anti-TB antibiotics, these anti-TB nuclei acids can function more effectively for synergistic anti-TB treatment.
These results have indicated the roles of functional nanomaterials for anti-TB treatments by directly or indirectly regulating the autophagy of Mtb-infected macrophages, which can be further combined with conventional antimicrobial medications for more effective TB treatment. Although lots of issues remain to be explored for the roles, mechanisms and safety of nanomaterials as novel medication and drug delivery to regulate the autophagy of Mtb-infected macrophages, these recent advances have strongly suggested the potentials of functional nanomaterials for anti-TB therapy targeting the autophagy of Mtb-infected macrophages (See Table 2).
Table 2.
Nanomaterials eliminate Mtb
| Nanomaterials | Function | Reference |
|---|---|---|
| ZnO NPs | Low doses: promote autophagy and the expression of M1 macrophage polarization markers decreases. | [141] |
| High doses: induce ferroptosis | ||
| Se NPs | Energy transduction disruption, increase ROS production, membrane depolarization, and membrane disruption | [142] |
| ZnO-Se NPs | Promoted autophagy, increase intracellular ROS, disrupt mitochondrial membrane potential and inhibit PI3K/Akt/mTOR signaling pathway | [39] |
| (RB-NPs)-GP | Promote antimicrobial autophagy and apoptosis, induce reactive oxygen species and reactive nitrogen species | [148] |
| GO NPs | Regulate apoptosis and autophagy also increase the formation of reactive oxygen species | [151, 152] |
| Drug delivery system | ||
| GO-PEG-MAN NPs | Improve intracellular drug contents and maintain high drug concentration | [153] |
| ISCur NPs | Induce apoptosis and promote phagosome-lysosome fusion | [156] |
| Anti- TGF -β1 siRNAs | Promote autophagy | [158] |
Perspectives and Conclusions
TB is a dangerous infectious respiratory disease that poses a serious health risk globally. Despite the rapid technological advances in modern medicine and the continuous improvement of the hygienic environment, TB remains a common concern for global public health due to the lack of therapeutics to increase the treatment efficiency, shorten the treatment duration, reduce the side effects and avoid the emergence of drug-resistant mutants. In the past decades, biological as well as medical applications of nanomaterials have been continuously investigated with many profound advances. In this review, we summarized the mechanisms of host cell autophagy to inhibit intracellular Mtb, and introduced the potential anti-TB therapeutic effects of nanomaterials/nanomedicines by modulating host cell autophagy.
Autophagy, an important immune response of the organism, eliminates intracellular foreign substances such as foreign pathogens and misfolded proteins and has the ability to cope with unfavorable responses, including starvation and oxidative stress. During the process of Mtb infection, the body makes an attack on Mtb through inducing autophagy to kill intracellular Mtb and inhibit their further spread, which suggests that autophagy is a non-negligible mechanism of anti-TB host immunological processes. Disappointingly, when Mtb enters the host cells (such as macrophages), only a very small portion of the pathogen is eliminated due to the existence of Mtb escaping during the infection process or remaining dormant in old lesions, which keeps the patient in a state of latent TB infection. However, when the host immunity decreases for some unexpected reasons, such as getting very old or getting other diseases that impairing the immunity, when the immunity of the organism decreases, the hidden Mtb in the host will reactivate to infect other cells, and turn the host into activate TB patients. Therefore, in this reality, it is very important to strengthen the host immunity to clear Mtb infection more effectively.
It should be mindful that autophagy frequently plays a dual function in infectious diseases. Autophagy can help prevent or treat infections by killing or eradicating the pathogen that causes the infection via autophagic lysosomes; however, pathogens can secrete components that resist autophagy, and some pathogens convert autophagosomes into their hosts for replication, where autophagosomes provide a membrane-bound, protected environment in which to produce their progeny. As a result, pathogens can evade the organism’s immune defenses, allowing the body to enter a state of latent infection, or they can use the metabolites and energy produced by autophagy for replication during subsequent disease progression, allowing the pathogen to be transmitted during subsequent episodes.
Autophagy activators can moderately upregulate autophagy, preventing a number of bacterial and viral illnesses, as well as inflammatory and autoimmune disorders. On the contrary, excessive autophagy activation in certain disorders may result in self-destruction, and autophagy inhibitors can be employed to down-regulate autophagy in order to avoid the excessive autophagy induced. Here, it is critical not only to actively investigate the beneficial function of autophagy in diseases, particularly infectious diseases, but also to investigate the mechanisms that allow infections to self-purpose autophagy in order to better employ therapeutics based on autophagy control.
Nanomaterials can be well designed to target autophagy pathway, boosting the formation of autophagosomes or accelerating the fusion of autophagosomes and lysosomes in different kinds of cells, which demonstrates the potentials of nanomaterials to destroy the intracellular Mtb in macrophages. In addition, nanomedicines with autophagy-inducing properties can be modified with functional groups or loaded with anti-TB drugs to kill Mtb synergistically and improve therapeutic efficiency. Furthermore, nanomedicines can partially promote the production of reactive oxygen species while inducing autophagy to treat TB or directly interact with the bacterial envelope to cause membrane damage and disrupt the function and growth of bacteria. Combining these anti-TB features, inducing autophagy of Mtb-infected macrophages can be applied to kill intracellular Mtb for better TB treatment, which is now serving as a hot research topic that merits more investigations.
The advantages of pharmaceutical nanomaterials in the treatment, prevention, and diagnosis of diseases are evident to all, providing new ideas to decipher how to effectively treat TB. However, in the era of a big data environment of rapid development and wide application of medical technology, the cross-application of disciplines is a major trend. In order to accelerate the development of nanomedicines through autophagy in sterilization and future clinical applications, there are still many obstacles and problems to be solved. For nanomaterials, more research is needed to determine how to utilize the cytotoxicity of nanomaterials paired with autophagy to destroy intracellular Mtb without inducing toxicity in the patient’s normal tissue cells. The surface charge and morphology of different nanomaterials determine their antimicrobial activity and cytotoxicity, which indicates the critical roles of the complex manufacturing process of nanomaterials. Moreover, how to economically and efficiently produce a large number of nanomaterials with good antimicrobial activity and safety for anti-TB treatment is an urgent issue to be explored. There is also a need to consider how to control the size of the nanomaterials to have a higher diffusion rate as well as bioavailability. The metabolism mechanisms of nanomaterials in vivo also need to be further investigated. It is important to ensure that the metabolites produced during the metabolism of nanomaterials do not cause secondary damage to the organism. Thus, more works should also be aimed at investigating the in vivo short-term and long-term toxicity of nanomaterials that show active anti-TB activities, and clarify their toxicity mechanisms, which can provide the insights about how to apply the nanomaterials for anti-TB treatment while avoiding their toxicity.
Except for the native toxicity of nanomaterials, the innovative design of nanomaterials for host cell targeting also remains a challenge. Although the targeting of macrophages can be achieved by surface modification of nanomaterials, it’s still difficult to selectively target Mtb-infected macrophage while showing no targeting effects on normal macrophages. More works should be focused on developing more selective targeting nanosystems against Mtb-infected macrophages without any toxicity effects on normal macrophages, which would benefit the use of nanomaterials for anti-TB treatment targeting the autophagy of Mtb-infected macrophages.
Moreover, in order to develop nanomaterials for future anti-TB treatment application, we must understand the exact mechanisms of bacterial escape or latency in the host cells or investigate the target point of action of a specific substance in order to design more effective host-cell-targeted therapies regulating autophagy. Then, drug combinations are frequently studied to obtain greater therapeutic effects, and exploring better therapeutic combinations with autophagy regulating by nanomaterials is a subject that has to be further investigated in the future.
Furthermore, the use of new nanomedicines may also address the drug resistance issues in TB, which is frequently created by the low efficiency of traditional medication treatments. Nanomaterial-based nanomedicines are also expected to promote the treatment efficiency of drug-resistant TB by regulating autophagy of the infected host cells, improving the efficacy of therapy and shortening the course of therapy duration. However, up to now, few evidence has indicated the effective application of nanomaterials for drug-resistant TB treatment, which requires more exploration in the future.
In conclusion, we believe that numerous nanomaterials have attractive potential for killing intracellular Mtb to treat TB by utilizing their advanced ability to modulate autophagy directly or indirectly. These autophagy-modulating effects may be in an adjunctive manner to enhance the efficiency of immunotherapy and chemotherapy for more effective anti-TB therapy. With the continuous exploration of nanomaterials, especially anti-TB activity and mechanisms, as well as in vivo metabolism, safety, and degradation modalities, we believe that the exploration of emerging therapeutic modalities based on nanomaterials targeting autophagy regulation would benefit the development of novel therapeutics against TB or drug-resistant TB.
Acknowledgements
Acknowledgments We extend our apologies to those authors whose deserving research was not cited in this manuscript. We also thank Figdraw (https://www.figdraw.com/) for the assistance in creating all figures.
Abbreviations
- TB
Tuberculosis
- Mtb
Mycobacterium tuberculosis
- HDT
Host-directed therapy
- PRRs
Pattern recognition receptors
- ROIs
Reactive oxygen intermediates
- RNIs
Reactive nitrogen intermediates
- Th1 T
Helper 1
- AMPK
AMP-activated protein kinase
- PAS
Pre-autophagosomal structure
- PI3K
Phosphatidylinositol 3-kinase
- PI3P
Phosphatidylinositol 3-phosphate
- PI
Phosphatidylinositol
- UBL
Ubiquitin-like
- PE
Phosphatidylethanolamine
- SNARES
Soluble n-ethylmaleimide-sensitive fusion protein attachment protein receptors
- LAP
LC3-associated phagocytosis
- PAMPs
Pathogen-associated molecular patterns
- Damps
Damage-associated molecular patterns
- LIRs
LC3-interacting regions
- TLRs
Toll-like receptors
- ROS
Reactive oxygen species
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- LAM
Lipoarabinomannan
- JNK
Jun N-terminal kinase
- Co3O4 NPs
Cobalt oxide nanoparticles
- IO NPs
Iron oxide nanoparticles
- ZnO NPs
Zinc oxide nanoparticles
- Au NPs
Gold nanoparticles
- DOX
Doxorubicin
- HCQ
Hydroxychloroquine
- CMS
Cell membranes
- mPDA NPS
Mesoporous polydopamine nanoparticles
- CQ
Chloroquine
- AD
Alzheimer’s disease
- Aβ
Amyloid-β
- APP
Amyloid precursor protein
- Se NPs
Se nanoparticles
- Se @Ru NPs
Peptide-composite selenium nanoparticles
- INOS
Inducible Nitric Oxide Synthase
- PDGFR-β
Platelet-derived growth factor receptor-β
- PEI
Polyethyleneimine
- Res
Resveratrol
- RES NPs
Resveratrol nanoparticles
- EV71
Enterovirus 71
- T NPS CD4 +
T cell membrane-coated nanoparticles
- ZnO-Se NPs
Zinc oxide selenium nanoparticles
- GP
β-Glucan particles
- RB
Rifabutin
- GO
Graphene oxide
- CMN
Curcumin
- PLGA
Polylactic acid-glycolic acid
- ISCur NPs
In situ curcumin nanoparticles
Author contributions
YL and JW drafted this manuscript, JY, JX, JY, DC, YH and FY helped to revise the manuscript, YR, J-FX, and JP helped to revise the manuscript and were responsible for leading this work. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Natural Science Foundation of China (82272348, 82270013 and 82300016), Natural Science Foundation of Guangdong Province (2023A1515030195, 2022A1515011223, 2023A1515140072, 2022A1515010525 and 2023A1515110502), High Talent Project of Guangdong Province (2021QN02Y720), Characteristic Innovation Project of Universities in Guangdong Province (2021KTSCX038), Key Project of Universities in Guangdong Province (2022ZDZX2021), Innovation Team Project of Universities in Guangdong Province (2022KCXTD010), Science and Technology Project of Dongguan (20211800904782, 20211800905542 and 20231800940512), Discipline Construction Project of Guangdong Medical University (4SG23290G, 4SG22259G, 4SG23030G, 4SG24025G and 4SG21229GDGFY01), Special projects of Songshan Lake Medical and Industrial Integration Innovation Center (4SG22309P), Youth Research Projects of Guangdong Medical University (GDMUD2022001) and Construction Project of Nano Technology and Application Engineering Research Center of Guangdong Medical University (4SG24179G).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors in the paper agree to be published.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yilin Liu and Jiajun Wang contributed equally to this work.
Contributor Information
Yongdui Ruan, Email: 13829202566@139.com.
Jun-Fa Xu, Email: yangfen@gdmu.edu.cn.
Jiang Pi, Email: jiangpi@gdmu.edu.cn.
References
- 1.Bagcchi S. WHO’s Global Tuberculosis Report 2022. Lancet Microbe. 2023;4:e20. [DOI] [PubMed] [Google Scholar]
- 2.Bi K, Cao D, Ding C, Lu S, Lu H, Zhang G, Zhang W, Li L, Xu K, Li L, Zhang Y. The past, present and future of tuberculosis treatment. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2022;51:657–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pai M, Behr MA, Dowdy D, Dheda K, Divangahi M, Boehme CC, Ginsberg A, Swaminathan S, Spigelman M, Getahun H. Et al: tuberculosis. Nat Rev Dis Primers. 2016;2:16076. [DOI] [PubMed] [Google Scholar]
- 4.Chai Q, Lu Z, Liu CH. Host defense mechanisms against Mycobacterium tuberculosis. Cell Mol Life Sci. 2020;77:1859–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss G, Schaible UE. Macrophage defense mechanisms against intracellular bacteria. Immunol Rev. 2015;264:182–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hmama Z, Peña-Díaz S, Joseph S, Av-Gay Y. Immunoevasion and immunosuppression of the macrophage by Mycobacterium tuberculosis. Immunol Rev. 2015;264:220–32. [DOI] [PubMed] [Google Scholar]
- 7.Chai Q, Wang L, Liu CH, Ge B. New insights into the evasion of host innate immunity by Mycobacterium tuberculosis. Cell Mol Immunol. 2020;17:901–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flynn JL, Chan J. Immunology of Tuberculosis. Annu Rev Immunol. 2001;19:93–129. [DOI] [PubMed] [Google Scholar]
- 9.Zhai W, Wu F, Zhang Y, Fu Y, Liu Z. The Immune escape mechanisms of Mycobacterium Tuberculosis. Int J Mol Sci 2019;20:340. [DOI] [PMC free article] [PubMed]
- 10.Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fougeray S, Pallet N. Mechanisms and biological functions of autophagy in diseased and ageing kidneys. Nat Rev Nephrol. 2015;11:34–45. [DOI] [PubMed] [Google Scholar]
- 12.Ariosa AR, Lahiri V, Lei Y, Yang Y, Yin Z, Zhang Z, Klionsky DJ. A perspective on the role of autophagy in cancer. Biochim Et Biophys Acta Mol Basis Disease. 2021;1867:166262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014;20:460–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris J, De Haro SA, Master SS, Keane J, Roberts EA, Delgado M, Deretic V. T helper 2 cytokines inhibit Autophagic Control of Intracellular Mycobacterium tuberculosis. Immunity. 2007;27:505–17. [DOI] [PubMed] [Google Scholar]
- 16.Lam A, Prabhu R, Gross CM, Riesenberg LA, Singh V, Aggarwal S. Role of apoptosis and autophagy in tuberculosis. Am J Physiol Lung Cell Mol Physiol. 2017;313:L218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuk J-M, Jo E-K. Host immune responses to mycobacterial antigens and their implications for the development of a vaccine to control tuberculosis. Clin Experimental Vaccine Res 2014, 3. [DOI] [PMC free article] [PubMed]
- 18.Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter RL, Eissa NT. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009;15:267–76. [DOI] [PubMed] [Google Scholar]
- 19.Meerak J, Wanichwecharungruang SP, Palaga T. Enhancement of immune response to a DNA vaccine against Mycobacterium tuberculosis Ag85B by incorporation of an autophagy inducing system. Vaccine. 2013;31:784–90. [DOI] [PubMed] [Google Scholar]
- 20.Jahangirian H, Ghasemian lemraski E, Webster TJ, Rafiee-Moghaddam R, Abdollahi Y. A review of drug delivery systems based on nanotechnology and green chemistry: green nanomedicine. Int J Nanomed. 2017;12:2957–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen M, Sun Y, Liu H. Cell membrane biomimetic nanomedicines for cancer phototherapy. Interdisciplinary Med. 2023;1:e20220012. [Google Scholar]
- 22.Gaytan SL, Beaven E, Gadad SS, Nurunnabi M. Progress and prospect of nanotechnology for cardiac fibrosis treatment. Interdisciplinary Med. 2023;1:e20230018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie F, Wang M, Chen Q, Chi T, Zhu S, Wei P, Yang Y, Zhang L, Li X, Liao Z. Endogenous stimuli-responsive nanoparticles for cancer therapy: from bench to bedside. Pharmacol Res. 2022;186:106522. [DOI] [PubMed] [Google Scholar]
- 24.Wei J, Mu J, Tang Y, Qin D, Duan J, Wu A. Next-generation nanomaterials: advancing ocular anti-inflammatory drug therapy. J Nanobiotechnol. 2023;21:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang EH, Harford JB, Eaton MA, Boisseau PM, Dube A, Hayeshi R, Swai H, Lee DS. Nanomedicine: past, present and future - A global perspective. Biochem Biophys Res Commun. 2015;468:511–7. [DOI] [PubMed] [Google Scholar]
- 26.Lam PL, Wong WY, Bian Z, Chui CH, Gambari R. Recent advances in green nanoparticulate systems for drug delivery: efficient delivery and safety concern. Nanomed (Lond). 2017;12:357–85. [DOI] [PubMed] [Google Scholar]
- 27.Zhou K, Li Z-Z, Cai Z-M, Zhong N-N, Cao L-M, Huo F-Y, Liu B, Wu Q-J, Bu L-L. Nanotheranostics in cancer lymph node metastasis: the long road ahead. Pharmacol Res. 2023;198:106989. [DOI] [PubMed] [Google Scholar]
- 28.Baghban R, Talebnejad MR, Meshksar A, Heydari M, Khalili MR. Recent advancements in nanomaterial-laden contact lenses for diagnosis and treatment of glaucoma, review and update. J Nanobiotechnol. 2023;21:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jatoi I, Fan J. A biomaterials viewpoint for the 2020 SARS-CoV-2 vaccine development. Biomaterials Translational. 2021;2:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018;16:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mabrouk M, Das DB, Salem ZA, Beherei HH. Nanomaterials for Biomedical Applications: production, Characterisations, recent trends and difficulties. Molecules 2021;26:1077. [DOI] [PMC free article] [PubMed]
- 32.Sheng G, Tian N, Duan H, Sun Z, Chu H. Advances in therapeutic nanodrug delivery systems for infectious lung diseases: a review. Acta Materia Med 2022;1:343–36.
- 33.Yi Q, Xu Z, Thakur A, Zhang K, Liang Q, Liu Y, Yan Y. Current understanding of plant-derived exosome-like nanoparticles in regulating the inflammatory response and immune system microenvironment. Pharmacol Res. 2023;190:106733. [DOI] [PubMed] [Google Scholar]
- 34.Xu K, Liang ZC, Ding X, Hu H, Liu S, Nurmik M, Bi S, Hu F, Ji Z, Ren J et al. Nanomaterials in the Prevention, diagnosis, and treatment of Mycobacterium Tuberculosis Infections. Adv Healthc Mater 2021;26:1077. [DOI] [PubMed]
- 35.Sosnik A, Carcaboso ÁM, Glisoni RJ, Moretton MA, Chiappetta DA. New old challenges in tuberculosis: potentially effective nanotechnologies in drug delivery. Adv Drug Deliv Rev. 2010;62:547–59. [DOI] [PubMed] [Google Scholar]
- 36.Griffiths G, Nyström B, Sable SB, Khuller GK. Nanobead-based interventions for the treatment and prevention of tuberculosis. Nat Rev Microbiol. 2010;8:827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maphasa RE, Meyer M, Dube A. The macrophage response to Mycobacterium tuberculosis and opportunities for Autophagy Inducing nanomedicines for Tuberculosis Therapy. Front Cell Infect Microbiol. 2020;10:618414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma A, Vaghasiya K, Gupta P, Gupta UD, Verma RK. Reclaiming hijacked phagosomes: hybrid nano-in-micro encapsulated MIAP peptide ensures host directed therapy by specifically augmenting phagosome-maturation and apoptosis in TB infected macrophage cells. Int J Pharm. 2018;536:50–62. [DOI] [PubMed] [Google Scholar]
- 39.Lin W, Fan S, Liao K, Huang Y, Cong Y, Zhang J, Jin H, Zhao Y, Ruan Y, Lu H et al. Engineering zinc oxide hybrid selenium nanoparticles for synergetic anti-tuberculosis treatment by combining Mycobacterium tuberculosis killings and host cell immunological inhibition. Front Cell Infect Microbiol 2023;12:1074533. [DOI] [PMC free article] [PubMed]
- 40.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–73. [DOI] [PubMed] [Google Scholar]
- 41.Kocaturk NM, Akkoc Y, Kig C, Bayraktar O, Gozuacik D, Kutlu O. Autophagy as a molecular target for cancer treatment. Eur J Pharm Sci. 2019;134:116–37. [DOI] [PubMed] [Google Scholar]
- 42.Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol. 2010;22:132–9. [DOI] [PubMed] [Google Scholar]
- 43.Jung CH, Seo M, Otto NM, Kim DH. ULK1 inhibits the kinase activity of mTORC1 and cell proliferation. Autophagy. 2011;7:1212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kawamata T, Kamada Y, Kabeya Y, Sekito T, Ohsumi Y, Subramani S. Organization of the pre-autophagosomal structure responsible for autophagosome formation. Mol Biol Cell. 2008;19:2039–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, He S, Ma B. Autophagy and autophagy-related proteins in cancer. Mol Cancer 2020;19:12. [DOI] [PMC free article] [PubMed]
- 46.Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, Griffiths G, Ktistakis NT. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol. 2008;182:685–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nascimbeni AC, Codogno P, Morel E. Phosphatidylinositol-3-phosphate in the regulation of autophagy membrane dynamics. FEBS J. 2017;284:1267–78. [DOI] [PubMed] [Google Scholar]
- 48.Wirawan E, Lippens S, Vanden Berghe T, Romagnoli A, Fimia GM, Piacentini M, Vandenabeele P. Beclin1: a role in membrane dynamics and beyond. Autophagy 2012;8:6–17. [DOI] [PubMed]
- 49.Papinski D, Schuschnig M, Reiter W, Wilhelm L, Barnes CA, Maiolica A, Hansmann I, Pfaffenwimmer T, Kijanska M, Stoffel I, et al. Early steps in autophagy depend on direct phosphorylation of Atg9 by the Atg1 kinase. Mol Cell. 2014;53:471–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohsumi Y. Molecular dissection of autophagy: two ubiquitin-like systems. Nat Rev Mol Cell Biol. 2001;2:211–6. [DOI] [PubMed] [Google Scholar]
- 51.Romanov J, Walczak M, Ibiricu I, Schüchner S, Ogris E, Kraft C, Martens S. Mechanism and functions of membrane binding by the Atg5-Atg12/Atg16 complex during autophagosome formation. EMBO J. 2012;31:4304–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walczak M, Martens S. Dissecting the role of the Atg12-Atg5-Atg16 complex during autophagosome formation. Autophagy. 2013;9:424–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weidberg H, Shvets E, Elazar Z. Biogenesis and cargo selectivity of autophagosomes. Annu Rev Biochem. 2011;80:125–56. [DOI] [PubMed] [Google Scholar]
- 54.Lystad AH, Simonsen A. Mechanisms and pathophysiological roles of the ATG8 Conjugation Machinery. Cells 2019;8:973. [DOI] [PMC free article] [PubMed]
- 55.Nakatogawa H. Two ubiquitin-like conjugation systems that mediate membrane formation during autophagy. Essays Biochem. 2013;55:39–50. [DOI] [PubMed] [Google Scholar]
- 56.Yu Z-Q, Ni T, Hong B, Wang H-Y, Jiang F-J, Zou S, Chen Y, Zheng X-L, Klionsky DJ, Liang Y, Xie Z. Dual roles of Atg8-PE deconjugation by Atg4 in autophagy. Autophagy. 2012;8:883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacquet M, Guittaut M, Fraichard A, Despouy G. The functions of Atg8-family proteins in autophagy and cancer: linked or unrelated? Autophagy. 2021;17:599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kimura S, Noda T, Yoshimori T. Dynein-dependent movement of autophagosomes mediates efficient encounters with lysosomes. Cell Struct Funct. 2008;33:109–22. [DOI] [PubMed] [Google Scholar]
- 59.Zhao YG, Codogno P, Zhang H. Machinery, regulation and pathophysiological implications of autophagosome maturation. Nat Rev Mol Cell Biol. 2021;22:733–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang P, Nishimura T, Sakamaki Y, Itakura E, Hatta T, Natsume T, Mizushima N. The HOPS complex mediates autophagosome-lysosome fusion through interaction with syntaxin 17. Mol Biol Cell. 2014;25:1327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tian X, Teng J, Chen J. New insights regarding SNARE proteins in autophagosome-lysosome fusion. Autophagy. 2021;17:2680–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu H, Wang W, Li Y. Molecular mechanism and regulation of Autophagy and its potential role in Epilepsy. Cells 2022;11:2621. [DOI] [PMC free article] [PubMed]
- 63.Zhao YG, Zhang H. Autophagosome maturation: an epic journey from the ER to lysosomes. J Cell Biol. 2019;218:757–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430:242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou A, Zhang W, Dong X, Liu M, Chen H, Tang B. The battle for autophagy between host and influenza a virus. Virulence. 2022;13:46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paik S, Kim JK, Chung C, Jo E-K. Autophagy: a new strategy for host-directed therapy of tuberculosis. Virulence. 2019;10:448–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei S, Xu T, Chen Y, Zhou K. Autophagy, cell death, and cytokines in K. pneumoniae infection: therapeutic perspectives. Emerg Microbes Infections. 2023;12:2140607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yuk JM, Yoshimori T, Jo EK. Autophagy and bacterial infectious diseases. Exp Mol Med 2012;44:99–108. [DOI] [PMC free article] [PubMed]
- 69.Bah A, Vergne I. Macrophage autophagy and bacterial infections. Front Immunol. 2017;8:1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Orvedahl A, Levine B. Eating the enemy within: autophagy in infectious diseases. Cell Death Differ. 2009;16:57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morreale FE, Walden H. Types of Ubiquitin Ligases. Cell 2016;165:248–248. [DOI] [PubMed]
- 72.Hu W, Chan H, Lu L, Wong KT, Wong SH, Li MX, Xiao ZG, Cho CH, Gin T, Chan MTV, et al. Autophagy in intracellular bacterial infection. Semin Cell Dev Biol. 2020;101:41–50. [DOI] [PubMed] [Google Scholar]
- 73.Sil P, Muse G, Martinez J. A ravenous defense: canonical and non-canonical autophagy in immunity. Curr Opin Immunol. 2018;50:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sakowski ET, Koster S, Portal Celhay C, Park HS, Shrestha E, Hetzenecker SE, Maurer K, Cadwell K, Philips JA. Ubiquilin 1 promotes IFN-γ-Induced Xenophagy of Mycobacterium tuberculosis. PLoS Pathog. 2015;11:e1005076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Minowa-Nozawa A, Nozawa T, Okamoto-Furuta K, Kohda H, Nakagawa I. Rab35 GTPase recruits NDP52 to autophagy targets. EMBO J. 2017;36:2790–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Slowicka K, van Loo G. Optineurin functions for optimal immunity. Front Immunol. 2018;9:769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tumbarello DA, Manna PT, Allen M, Bycroft M, Arden SD, Kendrick-Jones J, Buss F. Correction: the Autophagy receptor TAX1BP1 and the Molecular Motor myosin VI are required for clearance of Salmonella Typhimurium by Autophagy. PLoS Pathog. 2016;12:e1005433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sanjuan MA, Dillon CP, Tait SWG, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, Green DR. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–7. [DOI] [PubMed] [Google Scholar]
- 79.Huang J, Canadien V, Lam GY, Steinberg BE, Dinauer MC, Magalhaes MAO, Glogauer M, Grinstein S, Brumell JH. Activation of antibacterial autophagy by NADPH oxidases. Proc Natl Acad Sci USA. 2009;106:6226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martinez J, Malireddi RKS, Lu Q, Cunha LD, Pelletier S, Gingras S, Orchard R, Guan J-L, Tan H, Peng J, et al. Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nat Cell Biol. 2015;17:893–906. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 81.Abnave P, Mottola G, Gimenez G, Boucherit N, Trouplin V, Torre C, Conti F, Ben Amara A, Lepolard C, Djian B, et al. Screening in planarians identifies MORN2 as a key component in LC3-associated phagocytosis and resistance to bacterial infection. Cell Host Microbe. 2014;16:338–50. [DOI] [PubMed] [Google Scholar]
- 82.Romao S, Gasser N, Becker AC, Guhl B, Bajagic M, Vanoaica D, Ziegler U, Roesler J, Dengjel J, Reichenbach J, Münz C. Autophagy proteins stabilize pathogen-containing phagosomes for prolonged MHC II antigen processing. J Cell Biol. 2013;203:757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dong X, Levine B. Autophagy and viruses: adversaries or allies? J Innate Immun. 2013;5:480–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Choi Y, Bowman JW, Jung JU. Autophagy during viral infection - a double-edged sword. Nat Rev Microbiol. 2018;16:341–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pareja MEM, Colombo MI. Autophagic clearance of bacterial pathogens: molecular recognition of intracellular microorganisms. Front Cell Infect Microbiol. 2013;3:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaufmann SH. How can immunology contribute to the control of tuberculosis? Nat Rev Immunol. 2001;1:20–30. [DOI] [PubMed] [Google Scholar]
- 87.Liu CH, Liu H, Ge B. Innate immunity in tuberculosis: host defense vs pathogen evasion. Cell Mol Immunol. 2017;14:963–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Manzanillo PS, Ayres JS, Watson RO, Collins AC, Souza G, Rae CS, Schneider DS, Nakamura K, Shiloh MU, Cox JS. The ubiquitin ligase parkin mediates resistance to intracellular pathogens. Nature. 2013;501:512–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Franco LH, Nair VR, Scharn CR, Xavier RJ, Torrealba JR, Shiloh MU, Levine B. The Ubiquitin Ligase Smurf1 functions in selective autophagy of Mycobacterium tuberculosis and anti-tuberculous host defense. Cell Host Microbe. 2017;21:59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Watson RO, Bell SL, MacDuff DA, Kimmey JM, Diner EJ, Olivas J, Vance RE, Stallings CL, Virgin HW, Cox JS. The Cytosolic Sensor cGAS detects Mycobacterium tuberculosis DNA to induce type I interferons and activate autophagy. Cell Host Microbe. 2015;17:811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chai Q, Wang X, Qiang L, Zhang Y, Ge P, Lu Z, Zhong Y, Li B, Wang J, Zhang L et al. A Mycobacterium tuberculosis surface protein recruits ubiquitin to trigger host xenophagy. Nature Communications 2019, 10:1973. [DOI] [PMC free article] [PubMed]
- 92.Köster S, Upadhyay S, Chandra P, Papavinasasundaram K, Yang G, Hassan A, Grigsby SJ, Mittal E, Park HS, Jones V, et al. Mycobacterium tuberculosis is protected from NADPH oxidase and LC3-associated phagocytosis by the LCP protein CpsA. Proc Natl Acad Sci USA. 2017;114:E8711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sprenkeler EGG, Gresnigt MS, van de Veerdonk FL. LC3-associated phagocytosis: a crucial mechanism for antifungal host defence against Aspergillus Fumigatus. Cell Microbiol. 2016;18:1208–16. [DOI] [PubMed] [Google Scholar]
- 94.Awuh JA, Flo TH. Molecular basis of mycobacterial survival in macrophages. Cell Mol Life Sci. 2017;74:1625–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiao Y, Sun J. Bacterial manipulation of autophagic responses in infection and inflammation. Front Immunol. 2019;10:2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.MacMicking JD, Taylor GA, McKinney JD. Immune control of tuberculosis by IFN-gamma-inducible LRG-47. Sci (New York NY). 2003;302:654–9. [DOI] [PubMed] [Google Scholar]
- 97.Wong D, Li W, Chao JD, Zhou P, Narula G, Tsui C, Ko M, Xie J, Martinez-Frailes C, Av-Gay Y. Protein tyrosine kinase, PtkA, is required for Mycobacterium tuberculosis growth in macrophages. Sci Rep. 2018;8:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ferrari G, Langen H, Naito M, Pieters J. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell. 1999;97:435–47. [DOI] [PubMed] [Google Scholar]
- 99.Fratti RA, Chua J, Vergne I, Deretic V. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. Proc Natl Acad Sci USA. 2003;100:5437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pieters J. Mycobacterium tuberculosis and the macrophage: maintaining a balance. Cell Host Microbe. 2008;3:399–407. [DOI] [PubMed] [Google Scholar]
- 101.Zulauf KE, Sullivan JT, Braunstein M. The SecA2 pathway of Mycobacterium tuberculosis exports effectors that work in concert to arrest phagosome and autophagosome maturation. PLoS Pathog. 2018;14:e1007011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chandra P, Ghanwat S, Matta SK, Yadav SS, Mehta M, Siddiqui Z, Singh A, Kumar D. Mycobacterium tuberculosis inhibits RAB7 recruitment to selectively modulate Autophagy Flux in macrophages. Sci Rep. 2015;5:16320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Houben D, Demangel C, van Ingen J, Perez J, Baldeón L, Abdallah AM, Caleechurn L, Bottai D, van Zon M, de Punder K, et al. ESX-1-mediated translocation to the cytosol controls virulence of mycobacteria. Cell Microbiol. 2012;14:1287–98. [DOI] [PubMed] [Google Scholar]
- 104.Padhi A, Pattnaik K, Biswas M, Jagadeb M, Behera A, Sonawane A. Mycobacterium tuberculosis LprE Suppresses TLR2-Dependent Cathelicidin and Autophagy Expression to Enhance Bacterial Survival in Macrophages. Journal of Immunology (Baltimore, Md: 1950) 2019, 203:2665–2678. [DOI] [PubMed]
- 105.Silwal P, Paik S, Kim JK, Yoshimori T, Jo E-K. Regulatory mechanisms of Autophagy-targeted antimicrobial therapeutics against mycobacterial infection. Front Cell Infect Microbiol. 2021;11:633360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wen X, Klionsky DJ. At a glance: a history of autophagy and cancer. Sem Cancer Biol 2020;66:3–11. [DOI] [PMC free article] [PubMed]
- 107.Paskeh MDA, Entezari M, Clark C, Zabolian A, Ranjbar E, Farahani MV, Saleki H, Sharifzadeh SO, Far FB, Ashrafizadeh M, et al. Targeted regulation of autophagy using nanoparticles: New insight into cancer therapy. Biochim Et Biophys Acta Mol Basis Disease. 2022;1868:166326. [DOI] [PubMed] [Google Scholar]
- 108.Lin T, Zhang Q, Yuan A, Wang B, Zhang F, Ding Y, Cao W, Chen W, Guo H. Synergy of Tumor Microenvironment Remodeling and Autophagy Inhibition to Sensitize Radiation for bladder Cancer Treatment. Theranostics. 2020;10:7683–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang X, Cai H, Zhou H, Li T, Jin H, Evans CE, Cai J, Pi J. Cobalt oxide nanoparticle-synergized protein degradation and phototherapy for enhanced anticancer therapeutics. Acta Biomater. 2021;121:605–20. [DOI] [PubMed] [Google Scholar]
- 110.Bai D-P, Zhang X-F, Zhang G-L, Huang Y-F, Gurunathan S. Zinc oxide nanoparticles induce apoptosis and autophagy in human ovarian cancer cells. Int J Nanomed. 2017;12:6521–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Khan MI, Mohammad A, Patil G, Naqvi SAH, Chauhan LKS, Ahmad I. Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials. 2012;33:1477–88. [DOI] [PubMed] [Google Scholar]
- 112.Yuan L, Zhang F, Qi X, Yang Y, Yan C, Jiang J, Deng J. Chiral polymer modified nanoparticles selectively induce autophagy of cancer cells for tumor ablation. J Nanobiotechnol. 2018;16:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. [DOI] [PubMed] [Google Scholar]
- 114.Gao H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm Sinica B. 2016;6:268–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ruan S, Xie R, Qin L, Yu M, Xiao W, Hu C, Yu W, Qian Z, Ouyang L, He Q, Gao H. Aggregable nanoparticles-enabled chemotherapy and autophagy inhibition combined with Anti-PD-L1 antibody for Improved Glioma Treatment. Nano Lett. 2019;19:8318–32. [DOI] [PubMed] [Google Scholar]
- 116.Huang X, Chen L, Lin Y, Tou KI, Cai H, Jin H, Lin W, Zhang J, Cai J, Zhou H, Pi J. Tumor targeting and penetrating biomimetic mesoporous polydopamine nanoparticles facilitate photothermal killing and autophagy blocking for synergistic tumor ablation. Acta Biomater. 2021;136:456–72. [DOI] [PubMed] [Google Scholar]
- 117.Unal O, Akkoc Y, Kocak M, Nalbat E, Dogan-Ekici AI, Yagci Acar H, Gozuacik D. Treatment of breast cancer with autophagy inhibitory microRNAs carried by AGO2-conjugated nanoparticles. J Nanobiotechnol. 2020;18:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Du Z, Gao N, Wang X, Ren J, Qu X. Near-Infrared Switchable Fullerene-Based Synergy Therapy for Alzheimer’s Disease. Small 2018;14:e1801852. [DOI] [PubMed]
- 119.Chen W, Ouyang J, Yi X, Xu Y, Niu C, Zhang W, Wang L, Sheng J, Deng L, Liu Y-N, Guo S. Black Phosphorus Nanosheets as a Neuroprotective Nanomedicine for Neurodegenerative Disorder Therapy. Advanced Materials (Deerfield Beach, Fla) 2018, 30. [DOI] [PubMed]
- 120.Caruso G, Caraci F, Jolivet RB. Pivotal role of carnosine in the modulation of brain cells activity: Multimodal mechanism of action and therapeutic potential in neurodegenerative disorders. Prog Neurobiol. 2019;175:35–53. [DOI] [PubMed] [Google Scholar]
- 121.Maulik M, Peake K, Chung J, Wang Y, Vance JE, Kar S. APP overexpression in the absence of NPC1 exacerbates metabolism of amyloidogenic proteins of Alzheimer’s disease. Hum Mol Genet. 2015;24:7132–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kerr JS, Adriaanse BA, Greig NH, Mattson MP, Cader MZ, Bohr VA, Fang EF. Mitophagy and Alzheimer’s Disease: Cellular and Molecular mechanisms. Trends Neurosci. 2017;40:151–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu Y, Zhou H, Yin T, Gong Y, Yuan G, Chen L, Liu J. Quercetin-modified gold-palladium nanoparticles as a potential autophagy inducer for the treatment of Alzheimer’s disease. J Colloid Interface Sci. 2019;552:388–400. [DOI] [PubMed] [Google Scholar]
- 124.Serhal L, Lwin MN, Holroyd C, Edwards CJ. Rheumatoid arthritis in the elderly: characteristics and treatment considerations. Autoimmun rev. 2020;19:102528. [DOI] [PubMed] [Google Scholar]
- 125.Liu Y, Ma L, Zhou H, Zhu X, Yu Q, Chen X, Zhao Y, Liu J. Polypeptide nano-Se targeting inflammation and theranostic rheumatoid arthritis by anti-angiogenic and NO activating AMPKα signaling pathway. J Mater Chem B. 2018;6:3497–514. [DOI] [PubMed] [Google Scholar]
- 126.Ammerdorffer A, Stojanov M, Greub G, Baud D. Chlamydia trachomatis and chlamydia-like bacteria: new enemies of human pregnancies. Curr Opin Infect Dis. 2017;30:289–96. [DOI] [PubMed] [Google Scholar]
- 127.Sandoz KM, Rockey DD. Antibiotic resistance in Chlamydiae. Future Microbiol. 2010;5:1427–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Elwell CA, Ceesay A, Kim JH, Kalman D, Engel JN. RNA interference screen identifies Abl kinase and PDGFR signaling in Chlamydia trachomatis entry. PLoS Pathog. 2008;4:e1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yang S, Traore Y, Jimenez C, Ho EA. Autophagy induction and PDGFR-β knockdown by siRNA-encapsulated nanoparticles reduce chlamydia trachomatis infection. Sci Rep. 2019;9:1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Granato M. Nanotechnology frontiers in γ-Herpesviruses treatments. Int J Mol Sci 2021;22:11407. [DOI] [PMC free article] [PubMed]
- 131.Zhang L, Li Y, Gu Z, Wang Y, Shi M, Ji Y, Sun J, Xu X, Zhang L, Jiang J, Shi W. Resveratrol inhibits enterovirus 71 replication and pro-inflammatory cytokine secretion in rhabdosarcoma cells through blocking IKKs/NF-κB signaling pathway. PLoS ONE. 2015;10:e0116879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tian B, Liu J. Resveratrol: a review of plant sources, synthesis, stability, modification and food application. J Sci Food Agric. 2020;100:1392–404. [DOI] [PubMed] [Google Scholar]
- 133.Du N, Li X-H, Bao W-G, Wang B, Xu G, Wang F. Resveratrol–loaded nanoparticles inhibit enterovirus 71 replication through the oxidative stress–mediated ERS/autophagy pathway. Int J Mol Med. 2019;44:737–49. [DOI] [PubMed] [Google Scholar]
- 134.Zhang G, Campbell GR, Zhang Q, Maule E, Hanna J, Gao W, Zhang L, Spector SA. CD4 + T Cell-Mimicking Nanoparticles Broadly Neutralize HIV-1 and Suppress Viral Replication through Autophagy. MBio 2020, 11. [DOI] [PMC free article] [PubMed]
- 135.Seung KJ, Keshavjee S, Rich ML. Multidrug-resistant tuberculosis and extensively drug-resistant tuberculosis. Cold Spring Harbor Perspect Med. 2015;5:a017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Foulkes R, Man E, Thind J, Yeung S, Joy A, Hoskins C. The regulation of nanomaterials and nanomedicines for clinical application: current and future perspectives. Biomaterials Sci. 2020;8:4653–64. [DOI] [PubMed] [Google Scholar]
- 137.Bento CF, Empadinhas N, Mendes V. Autophagy in the fight against tuberculosis. DNA Cell Biol. 2015;34:228–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bai X, Li L, Liu H, Tan L, Liu T, Meng X. Solvothermal synthesis of ZnO nanoparticles and anti-infection application in vivo. ACS Appl Mater Interfaces. 2015;7:1308–17. [DOI] [PubMed] [Google Scholar]
- 139.Behzad F, Sefidgar E, Samadi A, Lin W, Pouladi I, Pi J. An overview of Zinc Oxide nanoparticles produced by plant extracts for anti-tuberculosis treatments. Curr Med Chem. 2022;29:86–98. [DOI] [PubMed] [Google Scholar]
- 140.Taranath TC, Patil BN. Limonia acidissima L. leaf mediated synthesis of zinc oxide nanoparticles: a potent tool against Mycobacterium tuberculosis. Int J Mycobacteriology. 2016;5:197–204. [DOI] [PubMed] [Google Scholar]
- 141.Geng S, Hao P, Wang D, Zhong P, Tian F, Zhang R, Qiao J, Qiu X, Bao P. Zinc oxide nanoparticles have biphasic roles on Mycobacterium-induced inflammation by activating autophagy and ferroptosis mechanisms in infected macrophages. Microb Pathog. 2023;180:106132. [DOI] [PubMed] [Google Scholar]
- 142.Huang T, Holden JA, Heath DE, O’Brien-Simpson NM, O’Connor AJ. Engineering highly effective antimicrobial selenium nanoparticles through control of particle size. Nanoscale. 2019;11:14937–51. [DOI] [PubMed] [Google Scholar]
- 143.Estevez H, Palacios A, Gil D, Anguita J, Vallet-Regi M, González B, Prados-Rosales R, Luque-Garcia JL. Antimycobacterial Effect of Selenium nanoparticles on Mycobacterium tuberculosis. Front Microbiol. 2020;11:800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pi J, Shen L, Yang E, Shen H, Huang D, Wang R, Hu C, Jin H, Cai H, Cai J, et al. Macrophage-targeted isoniazid-selenium nanoparticles promote Antimicrobial Immunity and Synergize Bactericidal Destruction of Tuberculosis Bacilli. Angewandte Chemie (International Ed English). 2020;59:3226–34. [DOI] [PubMed] [Google Scholar]
- 145.Parveen S, Sur T, Sarkar S, Roy R. Antagonist impact of selenium-based nanoparticles against Mycobacterium tuberculosis. Appl Biochem Biotechnol. 2023;195:3606–14. [DOI] [PubMed] [Google Scholar]
- 146.Ifijen IH, Atoe B, Ekun RO, Ighodaro A, Odiachi IJ. Treatments of Mycobacterium tuberculosis and Toxoplasma Gondii with Selenium nanoparticles. BioNanoScience. 2023;13:249–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Upadhyay TK, Fatima N, Sharma D, Saravanakumar V, Sharma R. Preparation and characterization of beta-glucan particles containing a payload of nanoembedded rifabutin for enhanced targeted delivery to macrophages. EXCLI J. 2017;16:210–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Upadhyay TK, Fatima N, Sharma A, Sharma D, Sharma R. Nano-Rifabutin entrapment within glucan microparticles enhances protection against intracellular Mycobacterium tuberculosis. Artif Cells Nanomed Biotechnol. 2019;47:427–35. [DOI] [PubMed] [Google Scholar]
- 149.Taheriazam A, Abad GGY, Hajimazdarany S, Imani MH, Ziaolhagh S, Zandieh MA, Bayanzadeh SD, Mirzaei S, Hamblin MR, Entezari M, et al. Graphene oxide nanoarchitectures in cancer biology: Nano-modulators of autophagy and apoptosis. J Controlled Release: Official J Controlled Release Soc. 2023;354:503–22. [DOI] [PubMed] [Google Scholar]
- 150.De Maio F, Palmieri V, Salustri A, Perini G, Sanguinetti M, De Spirito M, Delogu G, Papi M. Graphene oxide prevents mycobacteria entry into macrophages through extracellular entrapment. Nanoscale Adv. 2019;1:1421–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Saifullah B, Chrzastek A, Maitra A, Naeemullah B, Fakurazi S, Bhakta S, Hussein MZ. Novel anti-tuberculosis Nanodelivery Formulation of Ethambutol with Graphene Oxide. Molecules 2017;22:1560. [DOI] [PMC free article] [PubMed]
- 152.De Maio F, Palmieri V, Santarelli G, Perini G, Salustri A, Palucci I, Sali M, Gervasoni J, Primiano A, Ciasca G et al. Graphene Oxide-Linezolid combination as potential New Anti-tuberculosis Treatment. Nanomaterials (Basel Switzerland) 2020;10:1431. [DOI] [PMC free article] [PubMed]
- 153.Pi J, Shen L, Shen H, Yang E, Wang W, Wang R, Huang D, Lee B-S, Hu C, Chen C, et al. Mannosylated graphene oxide as macrophage-targeted delivery system for enhanced intracellular M.tuberculosis killing efficiency. Mater Sci Eng C Mater Biol Appl. 2019;103:109777. [DOI] [PubMed] [Google Scholar]
- 154.Pramod Kumar Gupta SK, Ramakrishna R. Inhibition of intracellular survival of multi drug resistant clinical isolates of Mycobacterium tuberculosis in macrophages by curcumin. Open Antimicrob Agents J 2018;113:81–90.
- 155.Lopresti AL. The Problem of Curcumin and its bioavailability: could its gastrointestinal influence contribute to its overall Health-Enhancing effects? Adv Nutr (Bethesda Md). 2018;9:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gupta PK, Jahagirdar P, Tripathi D, Devarajan PV, Kulkarni S. Macrophage targeted polymeric curcumin nanoparticles limit intracellular survival of Mycobacterium tuberculosis through induction of autophagy and augment anti-TB activity of isoniazid in RAW 264.7 macrophages. Front Immunol. 2023;14:1233630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Niu N-K, Yin J-J, Yang Y-X, Wang Z-L, Zhou Z-W, He Z-X, Chen X-W, Zhang X, Duan W, Yang T, Zhou S-F. Novel targeting of PEGylated liposomes for codelivery of TGF-β1 siRNA and four antitubercular drugs to human macrophages for the treatment of mycobacterial infection: a quantitative proteomic study. Drug Des Devel Ther. 2015;9:4441–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Luo H-L, Pi J, Zhang J-A, Yang E-Z, Xu H, Luo H, Shen L, Peng Y, Liu G-B, Song C-M, et al. Circular RNA TRAPPC6B inhibits intracellular Mycobacterium tuberculosis growth while inducing autophagy in macrophages by targeting microRNA-874-3p. Clin Translational Immunol. 2021;10:e1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.