Abstract
We sought to determine the relationship between virus-mediated CD4+ T-lymphocyte cytopathicity and viral coreceptor preference among various human immunodeficiency virus type 1 (HIV-1) subtypes in an ex vivo-infected human lymphoid tissue model. Our data show that all R5 HIV-1 infections resulted in mild depletion of CD4+ T lymphocytes, whereas all X4 HIV-1 infections caused severe depletion of CD4+ T lymphocytes regardless of their subtype origin. Thus, at least for the viruses within subtypes A, B, C, and E that were tested, coreceptor specificity is a critical factor that determines the ability of HIV-1 to deplete CD4+ T cells in human lymphoid tissue infected ex vivo.
The ever-increasing magnitude of the human immunodeficiency virus (HIV)-AIDS pandemic has been paralleled by an increasing number of viral subtypes. To date, at least 10 different genetic HIV type 1 (HIV-1) subtypes (A to J) have been identified (12, 16), each with a unique geographical pattern of distribution. For example, subtypes A, C, and E are the predominate subtypes in sub-Saharan Africa and central and southeast Asia, while subtype B has been associated with HIV-1 epidemics in North and South America and Europe (12). Most of our present understanding regarding HIV-AIDS pathogenesis and the underlying mechanisms of HIV-mediated cytopathicity has been derived from studies focused on subtype B viruses. However, investigations of subtype A, C, D, and E viruses, which are associated with the overwhelming majority of HIV-AIDS cases globally, have been comparatively lacking.
In a significant proportion of subtype B infections, viral quasispecies evolution is phenotypically evident in a shift in coreceptor usage usually from CCR5 to CXCR4 (4, 17, 18). This shift in coreceptor preference has been associated with a rapid decline in CD4+ T cells in vivo as well as with an increase in the ability of the virus to deplete CD4+ T lymphocytes ex vivo (4, 6, 11, 18). Although this coreceptor switch has been evident among subtype B infections, a relative lack of CXCR4-utilizing strains among non-B subtypes has been noted, despite aggressive progression of disease (1, 2, 14, 19). Given these observations, we sought to determine whether the observed dichotomy in cytopathicity between CCR5- and CXCR4-utilizing strains, widely reported among subtype B isolates, was consistent across other HIV-1 subtypes. For this purpose, we utilized a well-defined system of ex vivo-infected human lymphoid tissue that supports productive HIV-1 infection without the addition of exogenous cytokines or immunomodulators (5).
Matched blocks of human tonsillar tissue were infected ex vivo as described earlier (4, 10) with a panel of primary X4 and R5 HIV-1 isolates of subtypes A and E; R5 variants of subtype C; and prototypic X4 and R5 variants of subtype B. Primary isolates RW023, RW008-REI, UG92031 and UG92029 (subtype A), THA001, CMU006 and CMU002 (subtype E), and 931N101 and 301905 (subtype C) were obtained through the National Institutes of Health AIDS Reagent Program with their coreceptor specificities as reported by contributors. SF162 and LAV.04 were chosen because their behavior in ex vivo tissue is typical for R5 and X4 isolates, respectively. For infection, each block was inoculated with 7 to 10 μl of viral suspension. Normalization of viral infection was based on p24 measurements of the stocks, since the titration performed on peripheral blood mononuclear cells does not faithfully reflect viral infectivity for ex vivo tissues.
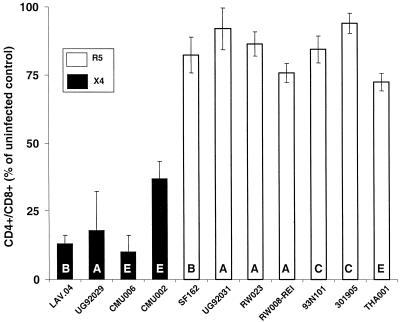
All of the HIV-1 subtypes tested in this report were able to productively infect human lymphoid tissue ex vivo as assessed by monitoring p24 in the culture medium. Infection kinetics were similar to those described for HIV-1 subtype B viruses in this system (5, 9). In ex vivo tissues, viral replication became evident starting approximately on day 6 after infection and continued until the end of the experiment. The maximal level of viral replication in experiments with tissues from different donors was observed between days 9 and 12. The amount of supernatant p24 varied between 11.0 and 56.0 pg per tissue block. Overall, we observed no relationship between the maximal level of viral replication and either coreceptor usage or viral subtype. However, we did observe a strong correlation between viral coreceptor usage and CD4+ T-lymphocyte depletion across all subtypes tested (Fig. 1). We utilized the CD4+/CD8+ T-cell ratio as a measure for CD+ T-cell depletion, since as shown earlier for subtype B viruses, there is no significant difference in the number of CD8+ T cells between infected and control (uninfected) tissue blocks (7). To confirm this for non-B subtypes, we infected histoculture tissue with three different viruses (UG92029, CMU006, and CMU002); the average number of CD8+ T cells was not statistically different from uninfected controls (P = 0.95), whereas CD4+ T cells were significantly depleted (P < 0.05).
FIG. 1.
CD4+ T-cell depletion in tonsillar tissues infected ex vivo with HIV-1 of various subtypes. Cells were mechanically isolated from uninfected and infected tissues (two sets of tissue blocks; nine blocks each from every donor were cultured in 4 ml of medium) and were stained for CD45, CD3, CD4, and CD8 and analyzed with flow cytometry as described earlier (7, 8). The CD4+/CD8+ T-cell ratio, relative to that of matched uninfected control tissue on day 12 postinfection was used as a measure for CD4+ T-cell depletion (for details, see text). The means ± standard error of the means data were obtained from experiments with tissues from three or four donors. In experiments with all the virus isolates except UG92031 and 301905, the mean number of CD4+ T cells was statistically significantly different (99.0% confidence) in comparison to uninfected controls.
Subtype B.
As previously reported (4, 10) and confirmed in the present work LAV.04, a prototypic CXCR4-utilizing isolate, depleted about 90% of CD4+ T cells in ex vivo-infected human lymphoid tissues. In contrast, SF162, a prototypic HIV-1 R5 isolate, depleted about 20% of CD4+ T cells.
Subtype A.
Ex vivo tissue infection with three R5 isolates, RW023, RW008-REI, and UG92031, resulted in depletion of approximately 15, 25 and 10% of CD4+ T cells, respectively. In contrast, X4 isolate UG92029 depleted approximately 80% of CD4+ T cells.
Subtype E.
Ex vivo tissue infection with an R5 isolate, THA001, resulted in approximately 30% depletion of CD4+ T cells. Two X4 isolates, CMU006 and CMU002, caused 90 and 70% of CD4+ T-cell depletion, respectively.
Subtype C.
Ex vivo tissue infection with two R5 isolates, 931N101 and 301905, resulted in depletion of approximately 15 and 5% of CD4+ T cells, respectively. Our data show that all R5 HIV-1 infections resulted in mild depletion of CD4+ T cells, whereas all X4 HIV-1 infections caused severe depletion of CD4+ T cells as measured by the decrease of the CD4+/CD8+ T-cell ratio, irrespective of their subtype origin.
Thus, at least for the viruses within subtypes A, B, C, and E that were tested, coreceptor specificity was a critical factor that determined the ability of HIV-1 to deplete CD4+ T cells in human lymphoid tissue infected ex vivo. The differential pathogenicity of X4 and R5 HIV-1 variants could be explained by the difference in the amount of cognate targets for these viruses in human lymphoid tissue (8). Whereas about 80% of CD4+ T cells in tonsil histocultures express CXCR4, less than 10% express CCR5 (8). However, this difference in the amount of the cognate T-cell targets does not correlate to lower production of R5 viruses, probably because other cellular reservoirs may contribute to viral production. For example, using the tonsillar histoculture system, tissue macrophages were more readily infected by R5 than by X4 viruses (9).
Progression to AIDS in vivo does not necessarily involve a switch in coreceptor usage from CCR5 to CXCR4 (15). Previous studies have reported that even in approximately 50% of subtype B-infected individuals, disease progression is not associated with a coreceptor switch (4, 11). However, our results do not address the various hypotheses that could account for the relative lack of CXCR4-utilizing strains of HIV-1 among the various non-B subtypes and even among subtype B infections that progress to AIDS in the absence of CXCR4-utilizing strains. For instance, it is possible that in those individuals who evidenced no coreceptor switch, increasingly cytopathic R5 variants evolve during disease progression. Second, host factors and the duration of infection may also play significant roles in the emergence of CXCR4-utilizing strains (10, 17). In this regard, individuals infected with HIV-1 subtype C may have a shorter time course to advanced disease, resulting in decreased intrahost evolution of viral quasispecies. Host environmental issues could also play a significant role in determining coreceptor utilization due to persistent immune activation, which could result in an increased CCR5 expression on CD4+ T lymphocytes (3). This would increase the availability and susceptibility of CD4+ T-cell targets in vivo to R5 viruses and thus might provide a selective advantage for R5 strains (10, 13). Further studies of primary isolates obtained from well-characterized, non-B infected cohorts would help in testing these hypotheses.
In conclusion, although a coreceptor switch from CCR5 to CXCR4 may not be necessary for disease progression, particularly in non-B HIV-1 subtype infections (such as subtype C), CXCR4 viruses are nonetheless associated with increased virally mediated CD4+ T-lymphocyte cytopathicity regardless of viral subtype and may help to explain the more rapid disease course in infected individuals harboring these strains.
Acknowledgments
N.M. and C.W. contributed equally to this work.
REFERENCES
- 1.Abebe A, Demissie D, Goudsmit J, Brouwer M, Kuiken C L, Pollakis G, Schuitemaker H, Fontanet A L, Rinke de Wit T F. HIV-1 subtype C syncytium- and non-syncytium-inducing phenotypes and coreceptor usage among Ethiopian patients with AIDS. AIDS. 1999;13:1305–1311. doi: 10.1097/00002030-199907300-00006. [DOI] [PubMed] [Google Scholar]
- 2.Bjorndal A, Sonnerborg A, Tscherning C, Albert J, Fenyo E M. Phenotypic characteristics of human immunodeficiency virus type 1 subtype C isolates of Ethiopian AIDS patients. AIDS Res Hum Retrovir. 1999;15:647–653. doi: 10.1089/088922299310944. [DOI] [PubMed] [Google Scholar]
- 3.Clerici, M., S. Butto, M. Lukwiya, M. Saresella, S. Declich, D. Trabattoni, C. Pastori, S. Piconi, C. Fracasso, M. Fabiani, P. Ferrante, G. Rizzardini, and L. Lopalco. Immune activation in Africa is environmentally driven and is associated with upregulation of CCR5. Italian-Ugandan AIDS Project. AIDS 14:2083–2092. [DOI] [PubMed]
- 4.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1–infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glushakova S, Baibakov B, Margolis L B, Zimmerberg J. Infection of human tonsil histocultures: a model for HIV pathogenesis. Nat Med. 1995;1:1320–1322. doi: 10.1038/nm1295-1320. [DOI] [PubMed] [Google Scholar]
- 6.Glushakova S, Yi Y, Grivel J C, Singh A, Schols D, De Clercq E, Collman R G, Margolis L. Preferential coreceptor utilization and cytopathicity by dual-tropic HIV-1 in human lymphoid tissue ex vivo. J Clin Investig. 1999;104:R7–R11. doi: 10.1172/JCI7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grivel J-C, Malkevitch N, Margolis L. Human immunodeficiency virus type 1 induces apoptosis in CD4+ but not in CD8+ T cells in ex vivo infected human lymphoid tissue. J Virol. 2000;74:8077–8084. doi: 10.1128/jvi.74.17.8077-8084.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grivel J C, Margolis L B. CCR5- and CXCR4-tropic HIV-1 are equally cytopathic for their T-cell targets in human lymphoid tissue. Nat Med. 1999;5:344–346. doi: 10.1038/6565. [DOI] [PubMed] [Google Scholar]
- 9.Grivel J C, Penn M L, Eckstein D A, Schramm B, Speck R F, Abbey N W, Herndier B, Margolis L, Goldsmith M A. Human immunodeficiency virus type 1 coreceptor preferences determine target T-cell depletion and cellular tropism in human lymphoid tissue. J Virol. 2000;74:5347–5351. doi: 10.1128/jvi.74.11.5347-5351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalinkovich A, Weisman Z, Leng Q, Borkow G, Stein M, Greenberg Z, Zlotnikov S, Eitan S, Bentwich Z. Increased CCR5 expression with decreased beta chemokine secretion in Ethiopians: relevance to AIDS in Africa. J Hum Virol. 1999;2:283–289. [PubMed] [Google Scholar]
- 11.Koot M, Keet I P, Vos A H, de Goede R E, Roos M T, Coutinho R A, Miedema F, Schellekens P T, Tersmette M. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann Intern Med. 1993;118:681–688. doi: 10.7326/0003-4819-118-9-199305010-00004. [DOI] [PubMed] [Google Scholar]
- 12.Kuiken C L, Foley B, Hahn B, Korber B, McCutchan F, Marx P A, Mellors J W, Mullins J I, Sodroski J, Wolinsly S, editors. Human retroviruses and AIDS 1999: a compilation and analysis of nucleic acid and amino acid sequences. Los Alamos, N. Mex: Theoretical Biology and Biophysics Group, Los Alamos National Laboratory; 1999. [Google Scholar]
- 13.Messele T, Abdulkadir M, Fontanet A L, Petros B, Hamann D, Koot M, Roos M T, Schellekens P T, Miedema F, Rinke de Wit T F. Reduced naive and increased activated CD4 and CD8 cells in healthy adult Ethiopians compared with their Dutch counterparts. Clin Exp Immunol. 1999;115:443–450. doi: 10.1046/j.1365-2249.1999.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peeters M, Vincent R, Perret J L, Lasky M, Patrel D, Liegeois F, Courgnaud V, Seng R, Matton T, Molinier S, Delaporte E. Evidence for differences in MT2 cell tropism according to genetic subtypes of HIV-1: syncytium-inducing variants seem rare among subtype C HIV-1 viruses. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:115–121. doi: 10.1097/00042560-199902010-00002. [DOI] [PubMed] [Google Scholar]
- 15.Ping L H, Nelson J A, Hoffman I F, Schock J, Lamers S L, Goodman M, Vernazza P, Kazembe P, Maida M, Zimba D, Goodenow M M, Eron J J, Jr, Fiscus S A, Cohen M S, Swanstrom R. Characterization of V3 sequence heterogeneity in subtype C human immunodeficiency virus type 1 isolates from Malawi: underrepresentation of X4 variants. J Virol. 1999;73:6271–6281. doi: 10.1128/jvi.73.8.6271-6281.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robertson D L, Anderson J P, Bradac J A, Carr J K, Foley B, Funkhouser R K, Gao F, Hahn B H, Kalish M L, Kuiken C, Learn G H, Leitner T, McCutchan F, Osmanov S, Peeters M, Pieniazek D, Salminen M, Sharp P M, Wolinsky S, Korber B. HIV-1 nomenclature proposal. Science. 2000;288:55–56. doi: 10.1126/science.288.5463.55d. [DOI] [PubMed] [Google Scholar]
- 17.Scarlatti G, Tresoldi E, Bjorndal A, Fredriksson R, Colognesi C, Deng H K, Malnati M S, Plebani A, Siccardi A G, Littman D R, Fenyo E M, Lusso P. In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine-mediated suppression. Nat Med. 1997;3:1259–1265. doi: 10.1038/nm1197-1259. [DOI] [PubMed] [Google Scholar]
- 18.Schultemaker H, Koot M, Kootstra N A, Dercksen M W, de Goede R E, van Steenwijk R P, Lange J M, Schattenkerk J K, Miedema F, Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tscherning C, Alaeus A, Fredriksson R, Bjorndal A, Deng H, Littman D R, Fenyo E M, Albert J. Differences in chemokine coreceptor usage between genetic subtypes of HIV-1. Virology. 1998;241:181–188. doi: 10.1006/viro.1997.8980. [DOI] [PubMed] [Google Scholar]