Abstract
Background
Temporal heterogeneity in human epidermal growth factor receptor 2 (HER2) status may be associated with the prognosis of breast cancer. We aimed to clarify the relationship of HER2-low transition during neoadjuvant therapy with survival outcomes under the new classification of HER2 status.
Methods
This retrospective study was conducted based on the prospective database of breast cancer patients treated with neoadjuvant therapy from September 2013 to August 2020.
Results
This analysis enrolled 185 patients, including 44 patients with HER2-zero tumours, 93 patients with HER2-low tumours and 48 patients with HER2-positive tumours after neoadjuvant therapy. Nearly, 57.6% of HER2-zero tumours turned into HER2-low tumours after neoadjuvant therapy, while 25.0% of HER2-low patients changed to HER2-zero or HER2-positive tumours. We found that at least once diagnosis as HER2-low breast cancer was related to hormone receptor status (p < .001) and Ki-67 expression (p = .036). Patients ever diagnosed as HER2-low tumours had favourable clinicopathological features (less Ki-67 expression, lower pathological staging, etc.) as well as significantly better locoregional relapse-free survival (LRFS; p = .007) and overall survival (OS; p = .026) compared with those never exhibiting HER2-low expression. Furthermore, the 6-year OS rates were 94.2% (95% confidence interval (CI) 83.1–98.1), 88.7% (74.4–95.2) and 78.1% (65.4–86.6) for patients with stable, once and none HER2-low expression, respectively (adjusted HR, 0.514 [95%CI, 0.294–0.897], p = .019).
Conclusions
Our study first indicated in patients across all expression levels of HER2 that stable or at least once HER2-low status may confer favourable attributes including less malignant biological behaviour and long-term survival benefit for breast cancer receiving neoadjuvant therapy.
Keywords: Neoadjuvant chemotherapy, HER2 transition, HER2-low breast cancer, prognosis, HER2-positive
KEY MESSAGES
Stable or at least once HER2-low status may confer favourable attributes including less malignant biological behaviour and long-term survival benefit for breast cancer receiving neoadjuvant therapy.
HER2-low expression was highly instable during disease evolution from primary lesion to residual tumour and was associated with hormone receptor status, which warrants HER2 re-test in residual lesion, especially for patients with HER2-zero disease at initial diagnosis, so as to give a clear picture of not only prognostic significance but also treatment availability.
Introduction
Breast cancer is a highly heterogeneous disease, especially with the presence of intra-tumour heterogeneity such as temporal heterogeneity, which may associate with the prognosis of breast cancer. Several studies have been exploring whether heterogeneity exists in human epidermal growth factor receptor 2 (HER2) status during neoadjuvant therapy for breast cancer, and whether changes in HER2 status (positive to negative, negative to positive) affect long-term survival of these patients [1–3]. Recently, HER2 immunohistochemistry (IHC) 1+ and IHC 2+/in situ hybridization (ISH) negative breast cancers have been newly classified as HER2-low breast cancer [4], and HER2 status has evolved from binary (positive and negative) to ternary (positive, low and zero). Accordingly, increasing attention has been attracted on whether and how HER2-low status changes from baseline (before neoadjuvant therapy) to post-surgery [5,6].
HER2-low breast cancer accounts for about 45–55% of all breast cancers, and many studies have demonstrated the prognostic value of HER2-low expression in patients undergoing neoadjuvant chemotherapy [7–9]. However, it is yet to be elucidated whether discordances in HER2-low expression during neoadjuvant therapy are associated with survival outcomes of breast cancer patients.
On these premises, we aimed to conduct a retrospective study based on prospective database to further clarify the heterogeneity of HER2 status during neoadjuvant therapy and the relationship of changes in HER2-low expression with long-term prognosis of breast cancer under the newly classification of HER2 status.
Methods
Study population
This retrospective study was performed based on the prospective database of breast cancer patients treated with neoadjuvant therapy from September 2013 to August 2020 at Renji Hospital, School of Medicine, Shanghai Jiao Tong University. The majority of inclusion and exclusion criteria have been published in detail previously [10]. Female patients with histologically confirmed breast cancer (T1 N1–3 M0 or T2–4 N0–3 M0) and available HER2 status were included. In addition, patients were excluded if they achieved pathological complete response after neoadjuvant treatment. All patients received weekly paclitaxel based chemotherapy and underwent surgery as planned after neoadjuvant treatment. In brief, paclitaxel 80 mg/m2 was intravenously given on day 1, 8, 15 and 22, combined with cisplatin 25 mg/m2 on day 1, 8 and 15 every 28 days for four cycles. For HER2-positive patients, trastuzumab based anti-HER2 treatment was recommended concurrently with neoadjuvant chemotherapy. Adjuvant treatment was tailored after surgery according to the guidelines at that time or at the discretion of physicians.
The baseline clinicopathological characteristics, pathological information and follow-up data were prospectively collected from patients’ medical records as described previously [10].
All the biopsy and surgical tissues were confirmed at the Department of Pathology, Renji Hospital. Tumours were considered hormone receptor positive if more than 10% of tumour cells exhibited immunostaining for ER or PR. HER2 positivity was defined as IHC 3+ or IHC 2+ with amplification confirmed by fluorescent in situ hybridization (FISH) based on the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) 2018 guideline [11]. As per recent consensus [4,12], HER2-low status was defined as IHC 1+ or 2+ with a nonamplified FISH assay.
Outcomes
The outcomes in the study were disease-free survival (DFS), relapse-free survival (RFS), locoregional relapse-free survival (LRFS), distant relapse-free survival (DRFS) and overall survival (OS). DFS was defined as the time from surgery until the first occurrence of any relapse, secondary malignancy or death from any cause. RFS was calculated as the time from surgery to first appearance of locoregional, ipsilateral, contralateral, distant relapse or death from any cause. LRFS was estimated from surgery to first occurrence of locoregional relapse, or death, regardless of cause. DRFS referred to the time from surgery to first event of distant relapse or death from any cause. OS denoted the time from surgery to death, irrespective of cause.
Statistical analysis
Correlations between HER2-low status and clinicopathological parameters were evaluated using the chi-square, Yates’s correction or Fisher’s exact test for categorical variables, where appropriate. The continuous variables were analysed using the Wilcoxon or Kruskal–Wallis test, where appropriate.
Associations between HER2-low status transition and clinicopathological variables during neoadjuvant therapy were tested via multivariate logistic regression controlling for hormone receptor status and Ki-67, and then evaluated using odds ratios (ORs) with 95% confidence intervals (CIs). Kaplan–Meier’s curves and log-rank test were estimated for different survival outcomes. Multivariate Cox proportional hazard models were applied to report hazard ratios (HRs) with 95%CIs. Multivariate analyses for survival outcomes were performed by adjusting age, HER2 status, hormone receptor status, Ki-67 expression, clinical staging and pathological staging.
Statistical tests were by default two-sided with a significance level of .05. All statistical analyses were conducted by RStudio v4.1.1 (http://www.R-project.org).
Results
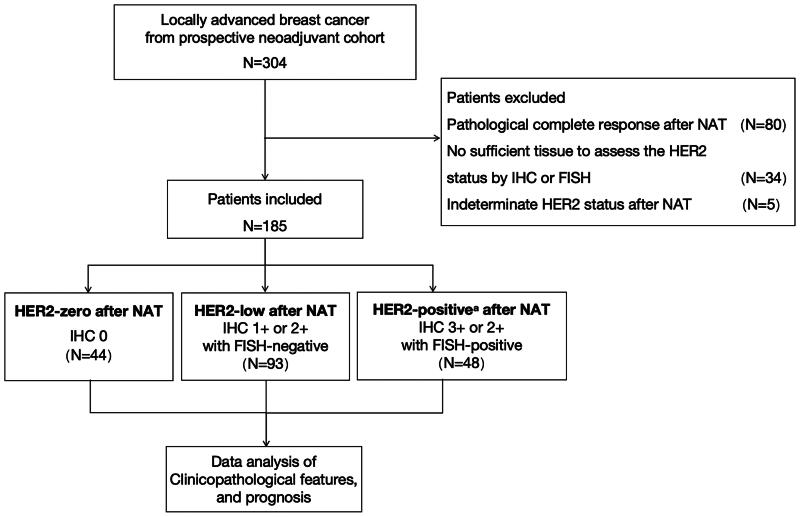
Among 304 patients who were screened between September 2013 and August 2020, 125 patients were excluded due to pathological complete response (n = 80), no sufficient tissue to assess the HER2 status by IHC or FISH (n = 34) and indeterminate HER2 status in residual tumours (n = 5). Finally, 185 breast cancer patients treated with neoadjuvant therapy were included in the analysis (Figure 1), including 44 patients with HER2-zero tumours, 93 patients with HER2-low tumours and 48 patients with HER2-positive tumours after neoadjuvant therapy. The median follow-up interval was 5.01 years.
Figure 1.
Diagram of the study design. IHC: immunohistochemistry; FISH: fluorescent in situ hybridization; HER2: human epidermal growth factor receptor 2; NAT: neoadjuvant therapy. aHER2-positive patients were required to receive anti-HER2 target therapy concurrently.
Changes in HER2 status during neoadjuvant therapy
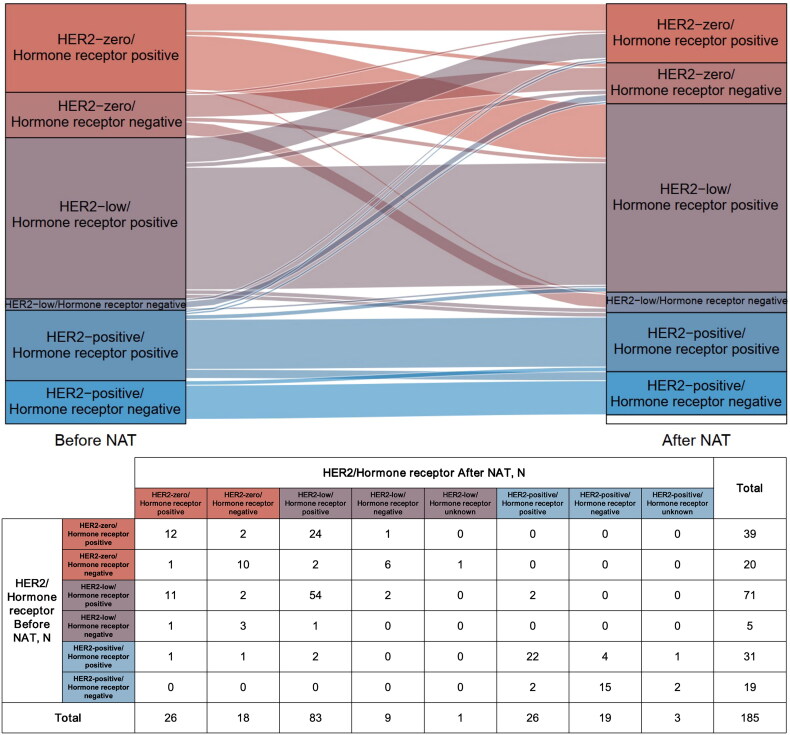
HER2 status may change in breast cancer patients during neoadjuvant therapy (Figure 2). Among 76 patients with HER2-low tumours before neoadjuvant therapy, 25.0% (19/76) changed to HER2-zero (n = 17) or HER2-positive tumours (n = 2) after neoadjuvant therapy, with 21.1% (15/71) in the hormone receptor-positive subgroup and 80.0% (4/5) in the hormone receptor-negative subgroup showing HER2 transition. Nearly, 57.6% (34/59) of HER2-zero breast cancers turned into HER2-low tumours after neoadjuvant therapy, of which 64.1% (25/39) were HER2-zero/hormone receptor-positive patients and 45.0% (9/20) were HER2-zero/hormone receptor-negative patients; however, none of HER2-zero breast cancer converted to HER2-positive breast cancer. HER2-positive tumours rarely changed into HER2-zero or HER2-low ones across neoadjuvant therapy, especially in the hormone receptor-negative/HER2-positive patients.
Figure 2.
Changes in HER2 status from the primary tumour to residual lesion after neoadjuvant therapy. HER2: human epidermal growth factor receptor 2; NAT: neoadjuvant therapy.
Multivariate logistic regression analysis showed that changes in HER2-low status during neoadjuvant therapy might be associated with hormone receptor status (among patients with HER2-low diseases before neoadjuvant therapy: OR, 0.067 [95%CI, 0.007–0.654], p = .020, Table 1A; among patients with HER2-low tumours after neoadjuvant therapy: OR, 0.057 [95%CI, 0.007–0.483], p = .009, Table 1B). Besides, multivariate logistic regression analysis suggested that at least once diagnosis as HER2-low breast cancer (no matter before or after neoadjuvant therapy) was related to hormone receptor status (OR, 4.273 [95%CI, 2.032–8.983], p < .001) and Ki-67 expression (OR, 0.505 [95%CI, 0.267–0.955], p = .036; Table 1C).
Table 1A.
Multivariate logistic regression analysis of HER2 status (change versus not change) among patients with HER2-low tumours before neoadjuvant therapy.
| Characteristics | OR | 95%CI | p Value |
|---|---|---|---|
| Hormone receptor (positive versus negative) | 0.067 | 0.007–0.654 | .020 |
| Ki-67 (>30% versus ≤30%) | 1.705 | 0.546–5.329 | .359 |
Abbreviations: HER2, human epidermal growth factor receptor 2; OR, odds ratio; CI, confidence interval.
Note: Bold values indicate statistical significance.
Table 1B.
Multivariate logistic regression analysis of HER2 status (change versus not change) among patients with HER2-low tumours after neoadjuvant therapy.
| Characteristics | OR | 95%CI | p Value |
|---|---|---|---|
| Hormone receptor (positive versus negative) | 0.057 | 0.007–0.483 | .009 |
| Ki-67 (>30% versus ≤30%) | 1.150 | 0.437–3.028 | .777 |
Abbreviations: HER2, human epidermal growth factor receptor 2; OR, odds ratio; CI, confidence interval.
Note: Bold values indicate statistical significance.
Table 1C.
Multivariate logistic regression analysis of at least once HER2 status among all the enrolled patients.
| Characteristics | OR | 95%CI | p Value |
|---|---|---|---|
| Hormone receptor (positive versus negative) | 4.273 | 2.032–8.983 | <.001 |
| Ki-67 (>30% versus ≤30%) | 0.505 | 0.267–0.955 | .036 |
Abbreviations: HER2, human epidermal growth factor receptor 2; OR, odds ratio; CI, confidence interval.
Note: Bold values indicate statistical significance.
Clinicopathological characteristics between different subgroups by HER2-low status
Patients diagnosed with HER2-low tumours before or after neoadjuvant therapy accounted for 60.5% (n = 112). Of these, 50.9% (n = 57) appeared constant HER2-low status after neoadjuvant therapy, whereas 49.1% (n = 55) had a HER2 transition (low to zero or positive, or vice versa). The clinicopathological differences between patients ever or never diagnosed with HER2-low tumours (zero or positive) were mainly characterized by more hormone receptor-positive status (p < .001), less Ki-67 expression (p < .001), lower histological grade (p = .033), as well as lower pathological T stage (p = .033), pathological N stage (p = .012) and pathological staging (p = .010; Table 2). In addition, statistically significant differences were also observed among patients with concordant HER2-low breast cancer, those with HER2-low expression only once (before or after neoadjuvant therapy), and those who never showed HER2-low status in terms of hormone receptor status (p < .001), Ki-67 expression (p < .001), histological grade (p = .028), pathological N stage (p = .018) and pathological staging (p = .031; Table 3).
Table 2.
Clinicopathological differences between patients ever or never diagnosed with HER2-low breast cancers.
| Never (n = 73) | Ever (n = 112) | p Value | |
|---|---|---|---|
| Age, years | |||
| Mean ± SD | 49.945 ± 10.629 | 52.795 ± 10.07 | .067 |
| ≤50 | 39 (53.4%) | 45 (40.2%) | .077 |
| >50 | 34 (46.6%) | 67 (59.8%) | |
| Hormone receptor status | |||
| Positive | 43 (58.9%) | 98 (87.5%) | <.001 |
| Negative | 30 (41.1%) | 14 (12.5%) | |
| Ki-67 index | |||
| Median (IQR) | 40 (30, 60) | 30 (20, 40) | <.001 |
| ≤30% | 31 (42.5%) | 72 (64.3%) | .004 |
| >30% | 42 (57.5%) | 40 (35.7%) | |
| Histological grade | |||
| 1 | 0 (0%) | 1 (0.9%) | .033 |
| 2 | 37 (52.1%) | 76 (68.5%) | |
| 3 | 34 (47.9%) | 34 (30.6%) | |
| NAa | 2 | 1 | |
| Clinical T stage | |||
| cT1 | 1 (1.4%) | 0 (0%) | .521 |
| cT2 | 16 (21.9%) | 22 (19.6%) | |
| cT3 | 23 (31.5%) | 43 (38.4%) | |
| cT4 | 33 (45.2%) | 47 (42%) | |
| Clinical N stage | |||
| cN0 | 8 (11%) | 22 (19.6%) | .080 |
| cN1 | 53 (72.6%) | 60 (53.6%) | |
| cN2 | 6 (8.2%) | 15 (13.4%) | |
| cN3 | 6 (8.2%) | 15 (13.4%) | |
| Clinical staging | |||
| IIA | 2 (2.7%) | 4 (3.5%) | .615 |
| IIB | 18 (24.7%) | 19 (17%) | |
| IIIA | 19 (26%) | 33 (29.5%) | |
| IIIB | 28 (38.4%) | 41 (36.6%) | |
| IIIC | 6 (8.2%) | 15 (13.4%) | |
| Pathological T stage | |||
| ypT0b | 13 (17.8%) | 8 (7.1%) | .033 |
| ypTis | 1 (1.3%) | 1 (0.9%) | |
| ypT1 | 38 (52.1%) | 73 (65.2%) | |
| ypT2 | 18 (24.7%) | 30 (26.8%) | |
| ypT3 | 3 (4.1%) | 0 (0%) | |
| Pathological N stage | |||
| ypN0 | 37 (50.7%) | 36 (32.1%) | .012 |
| ypN1–3 | 36 (49.3%) | 76 (67.9%) | |
| Pathological staging | |||
| 0–I | 30 (41.1%) | 23 (20.5%) | .010 |
| II | 25 (34.2%) | 55 (49.1%) | |
| III | 18 (24.7%) | 34 (30.4%) |
Abbreviations: HER2, human epidermal growth factor receptor 2; SD, standard deviation; IQR: interquartile range; NA, not applicable.
Note: Bold values indicate statistical significance.
Histological grade could not be assessed in three patients.
Involved lymph nodes were used for pathology examination.
Table 3.
Clinicopathological differences among patients with none, once HER2-low and stable HER2-low status.
| None (n = 73) | Once HER2-low (n = 55) | Stable HER2-low (n = 57) | p Value | |
|---|---|---|---|---|
| Age, years | ||||
| Mean ± SD | 49.945 ± 10.629 | 52.564 ± 10.451 | 53.018 ± 9.777 | .183 |
| ≤50 | 39 (53.4%) | 24 (43.6%) | 21 (36.8%) | .161 |
| >50 | 34 (46.6%) | 31 (56.4%) | 36 (63.2%) | |
| Hormone receptor status | ||||
| Positive | 43 (58.9%) | 42 (76.4%) | 56 (98.2%) | <.001 |
| Negative | 30 (41.1%) | 13 (23.6%) | 1 (1.8%) | |
| Ki-67 index | ||||
| Median (IQR) | 40 (30, 60) | 30 (17.5, 45) | 30 (20, 40) | <.001 |
| ≤30% | 31 (42.5%) | 32 (58.2%) | 40 (70.2%) | .006 |
| >30% | 42 (57.5%) | 23 (41.8%) | 17 (29.8%) | |
| Histological grade | ||||
| 1 | 0 (0%) | 0 (0%) | 1 (1.8%) | .028 |
| 2 | 37 (52.1%) | 34 (63%) | 42 (73.7%) | |
| 3 | 34 (47.9%) | 20 (37%) | 14 (24.5%) | |
| NAa | 2 | 1 | 0 | |
| Clinical T stage | ||||
| cT1 | 1 (1.4%) | 0 (0%) | 0 (0%) | .368 |
| cT2 | 16 (21.9%) | 13 (23.6%) | 9 (15.8%) | |
| cT3 | 23 (31.5%) | 24 (43.7%) | 19 (33.3%) | |
| cT4 | 33 (45.2%) | 18 (32.7%) | 29 (50.9%) | |
| Clinical N stage | ||||
| cN0 | 8 (11%) | 10 (18.2%) | 12 (21.1%) | .239 |
| cN1 | 53 (72.6%) | 28 (50.9%) | 32 (56.1%) | |
| cN2 | 6 (8.2%) | 9 (16.4%) | 6 (10.5%) | |
| cN3 | 6 (8.2%) | 8 (14.5%) | 7 (12.3%) | |
| Clinical staging | ||||
| IIA | 2 (2.7%) | 3 (5.6%) | 1 (1.8%) | .622 |
| IIB | 18 (24.7%) | 8 (14.5%) | 11 (19.3%) | |
| IIIA | 19 (26%) | 19 (34.5%) | 14 (24.5%) | |
| IIIB | 28 (38.4%) | 17 (30.9%) | 24 (42.1%) | |
| IIIC | 6 (8.2%) | 8 (14.5%) | 7 (12.3%) | |
| Pathological T stage | ||||
| ypT0b | 13 (17.8%) | 6 (10.9%) | 2 (3.5%) | .113 |
| ypTis | 1 (1.3%) | 1 (1.8%) | 0 (0%) | |
| ypT1 | 38 (52.1%) | 34 (61.8%) | 39 (68.4%) | |
| ypT2 | 18 (24.7%) | 14 (25.5%) | 16 (28.1%) | |
| ypT3 | 3 (4.1%) | 0 (0%) | 0 (0%) | |
| Pathological N stage | ||||
| ypN0 | 37 (50.7%) | 21 (38.2%) | 15 (26.3%) | .018 |
| ypN1–3 | 36 (49.3%) | 34 (61.8%) | 42 (73.7%) | |
| Pathological staging | ||||
| 0–I | 30 (41.1%) | 14 (25.5%) | 9 (15.8%) | .031 |
| II | 25 (34.2%) | 26 (47.3%) | 29 (50.9%) | |
| III | 18 (24.7%) | 15 (27.2%) | 19 (33.3%) |
Abbreviations: HER2, human epidermal growth factor receptor 2; SD, standard deviation; IQR, interquartile range; NA, not applicable.
Note: Bold values indicate statistical significance.
Histological grade could not be assessed in three patients.
Involved lymph nodes were used for pathology examination.
Association of HER2-low status change with survival outcomes
Survival outcomes between patients with at least once or none HER2-low expression
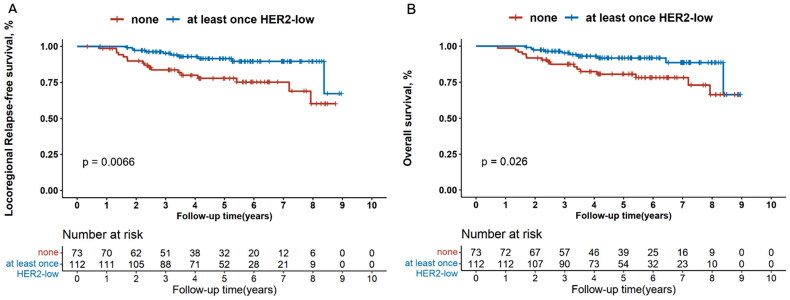
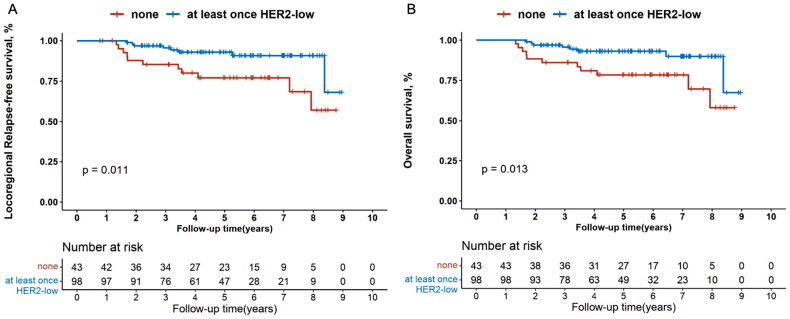
Kaplan–Meier’s curves showed that patients ever diagnosed as HER2-low tumours had significantly better LRFS compared with those never exhibiting HER2-low expression (p = .007, Figure 3(A)), and multivariate Cox regression analysis revealed the same results (HR, 0.371 [95%CI, 0.159–0.869]; p = .022, Figure S1). Furthermore, in the hormone receptor-positive subgroup, LRFS was also superior in patients with at least once HER2-low status for both univariate (p = .011, Figure 4(A)) and multivariate analysis (HR, 0.327 [95%CI, 0.123–0.869]; p = .025, Figure S2). This trend was not observed in the hormone receptor-negative subgroup (data not shown).
Figure 3.
Kaplan–Meier’s survival analysis between patients with none or at least once HER2-low expression for locoregional relapse-free survival (A) and overall survival (B) in all the enrolled patients. HER2: human epidermal growth factor receptor 2.
Figure 4.
Kaplan–Meier’s survival analysis between patients with none or at least once HER2-low expression for locoregional relapse-free survival (A) and overall survival (B) in the hormone receptor-positive patients. HER2: human epidermal growth factor receptor 2.
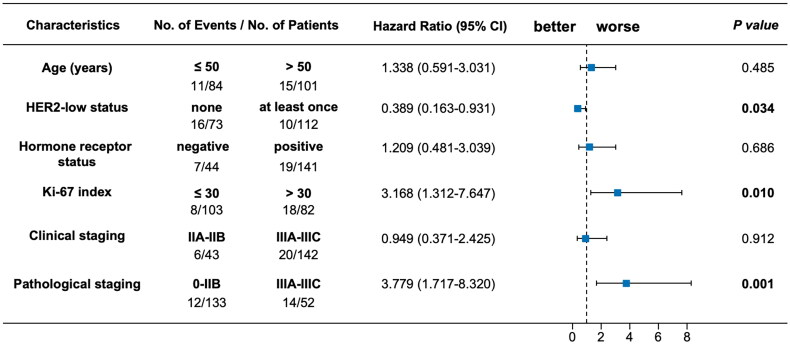
The OS outcome was also remarkably better for patients who had ever been diagnosed with HER2-low tumours compared with those who had never (p = .026, Figure 3(B)). Multivariate Cox regression analysis demonstrated that at least once HER2-low expression was significantly associated with longer OS compared with the others (HR, 0.389 [95%CI, 0.163–0.931]; p = .034; Figure 5). When analysed separately by hormone receptor status, superior OS outcome was observed for patients ever diagnosed with HER2-low breast cancers compared with the others in the hormone receptor-positive subset (univariate analysis: p = .013, Figure 4(B); multivariate analysis: HR, 0.325 [95%CI, 0.123–0.864]; p = .024, Figure S3), rather than in the hormone receptor-negative subset (data not shown).
Figure 5.
Multivariate Cox regression analysis between patients with none or at least once HER2-low expression for overall survival in all the enrolled patients. HER2: human epidermal growth factor receptor 2; CI: confidence interval.
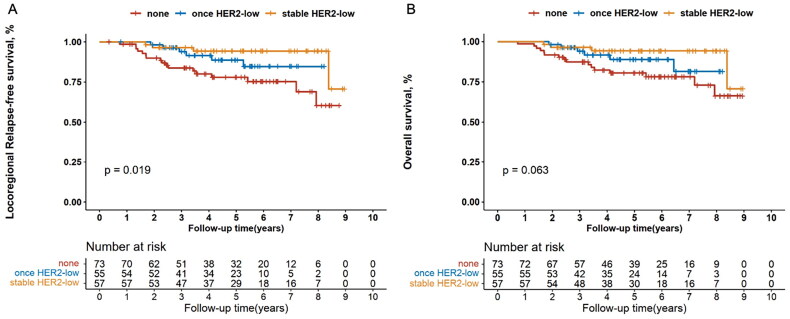
Survival outcomes among patients with stable, once and none HER2-low expression
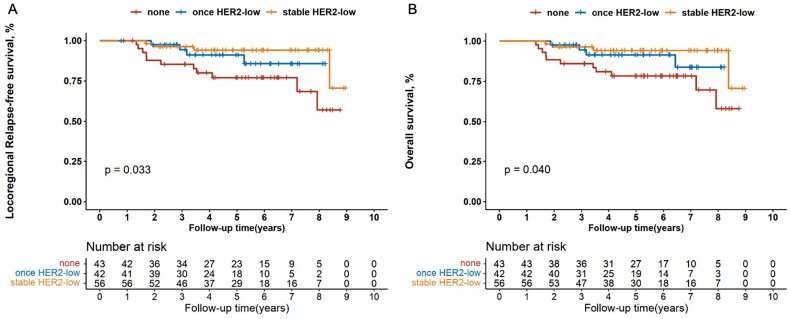
Patients with stable, once and none HER2-low breast cancers exhibited significantly different LRFS (p = .019; Figure 6(A)). The LRFS outcome of patients with once HER2-low expression either before or after neoadjuvant therapy was visually intermediate between the other two subgroups. Multivariate Cox regression analysis supported the above finding (HR, 0.502 [95%CI, 0.289–0.872], p = .014). Similar trend was also observed in the hormone receptor-positive subgroup (univariate analysis: p = .033, Figure 7(A); multivariate analysis: HR, 0.496 [95%CI, 0.273–0.900], p = .021), rather than in the hormone receptor-negative subgroup (data not shown).
Figure 6.
Kaplan–Meier’s survival analysis among patients with stable, once and none HER2-low expression for locoregional relapse-free survival (A) and overall survival (B) in all the enrolled patients. HER2: human epidermal growth factor receptor 2.
Figure 7.
Kaplan–Meier’s survival analysis among patients with stable, once and none HER2-low expression for locoregional relapse-free survival (A) and overall survival (B) in the hormone receptor-positive subgroup. HER2: human epidermal growth factor receptor 2.
On the other hand, 6-year OS rates were 94.2% (95%CI 83.1–98.1) for patients with stable HER2-low expression, 88.7% (74.4–95.2) for those with once HER2-low status and 78.1% (65.4–86.6) for those with none HER2-low diseases, respectively (p = .063, Figure 6(B)). Multivariate Cox regression analysis indicated that HER2-low status was an independent prognostic factor for OS among the three groups (HR, 0.514 [95%CI, 0.294–0.897], p = .019; Table S1A). When analysed in the hormone receptor-positive subgroup, the number of HER2-low status was markedly associated with distinct OS (6-year OS rates were 94.2% [95%CI 82.8–98.1] for stable HER2-low, 91.6% [75.8–97.2] for once HER2-low and 78.3% [62.3–88.1] for none HER2-low; univariate analysis: p = .040, Figure 7(B); multivariate analysis: HR, 0.495 [95%CI, 0.272–0.901], p = .021, Table S1B). However, we did not observe this trend in the hormone receptor-negative subgroup (data not shown).
Furthermore, reverse HER2 transition between HER2-low and HER2-zero expression in patients with once HER2-low breast cancers did not affect any of the survival outcomes (Figure S4).
Discussion
To the best of our knowledge, we investigated for the first time how transition between different HER2 expression from initial diagnosis to the completion of neoadjuvant therapy affected the survival outcomes of breast cancer patients under the trichotomization of HER2 status (HER2-positive, HER2-low and HER2-zero). Our study revealed that the presence of HER2-low expression before or after neoadjuvant therapy was associated with better prognosis, even when HER2-low status was instable.
HER2-low expression was highly instable during disease evolution from primary lesion to residual tumour, indicating the temporal heterogeneity of HER2-low status. Similar to other studies [5,6,13], we found that HER2-low expression was strongly associated with hormone receptor-positive status. Our study first suggested that HER2-low/hormone receptor-positive breast cancers were more likely to maintain stable HER2-low status compared with HER2-low/hormone receptor-negative tumours (hormone receptor-positive subgroup versus hormone receptor-negative subgroup: 78.9% versus 20.0%) after neoadjuvant therapy. In contrast, HER2-zero/hormone receptor-positive breast cancers were prone to conversion to HER2-low expression compared with HER2-zero/hormone receptor-negative tumours (hormone receptor-positive subgroup versus hormone receptor-negative subgroup: 64.1% versus 45.0%). As further suggested by the multivariable logistic regression analysis (hormone receptor-positive versus hormone receptor-negative: OR, 4.273 [95%CI, 2.032–8.983], p < .001), hormone receptor-positive breast cancers might have a susceptibility to HER2-low diseases. In this context, re-testing of HER2 status was essential for residual disease, especially for patients with HER2-zero/hormone receptor-positive breast cancer. Reassessment of HER2 status may increase their chances of HER2-low expression, and then of potential candidates for novel anti-HER2 targeting agents in the subsequent treatment.
Our study revealed that HER2-positive breast cancers exhibited stable HER2 status before and after neoadjuvant therapy, and HER2-positive/hormone receptor-negative tumours rarely experienced loss of strong HER2 expression, as supported by the results of previous studies [3,6,14,15]. Preclinical research found that gene expression was remarkably different between HER2-negative and HER2-positive cell lines. HER2-negative cells mainly showed low expression of cell–cell adhesion genes, high expression of epithelial–mesenchymal transition (EMT) genes and stem-cell-like/less differentiated gene expression patterns [16]. In contrast, the vast majority of differentially expressed genes in HER2-positive breast cancers originated from the 17q12 DNA amplicon, where the HER2 gene is located [17].
Previous studies had confirmed that HER2-low breast cancer diagnosed by biopsy tissue prior to neoadjuvant therapy had lower Ki-67 level and histological grade compared with HER2-zero patients [7,8], but few studies have focused on patients who gained HER2-low expression after neoadjuvant therapy. Our study first showed that patients who appeared HER2-low expression at least once from initial diagnosis to the completion of neoadjuvant therapy had significantly lower Ki-67 level and histological grade than those who never exhibited HER2-low expression, even if HER2-positive status were taken into consideration. If patients could conserve stable HER2-low status from the primary tumour to residual disease, they were at even lower risk of aggressive behaviour. These findings implied that stable or at least once HER2-low status during neoadjuvant chemotherapy may serve as the promising biomarker for less malignant breast cancer.
Additionally, we also found that patients who presented at least once HER2-low expression during neoadjuvant therapy had significantly better LRFS and OS outcomes than patients who never exhibited HER2-low status, which was first reported with HER2-positive breast cancer included. Many previous studies explored the prognostic value of HER2-low status solely among HER2-negative patients, which showed that HER2-low breast cancer had significantly superior survival outcomes compared with HER2-zero breast cancer in different treatment settings (neoadjuvant [7,8], adjuvant [18] and advanced [19] settings), even in systemic treatment-naive node-negative breast cancer [20]. Their work partially supported our conclusion that HER2-low expression might be an indicator of superior prognosis in breast cancer.
Several limitations still existed in our study. First, this was a single-centre retrospective study. However, all patients included in the analysis were collected from prospective databases. They had received similar neoadjuvant chemotherapy regimens, which may reduce the effect of treatment on tumour behaviour. Besides, single-centre pathology testing was more likely to maintain consistency in HER2 interpretation. Second, the sample size of this study is relatively small and we will continue to accumulate the cases for further verification. Third, the median follow-up time in this study was only 5.01 years. However, most patients (96.8%) in our study were diagnosed with locally advanced breast cancer and were prone to early recurrence. Besides, the follow-up is ongoing for further analysis.
In conclusion, our study first indicated in patients across all expression levels of HER2 (HER2-zero, HER2-low and HER2-positive) that stable or at least once HER2-low status may confer favourable attributes including less malignant biological behaviour and long-term survival benefit for breast cancer receiving neoadjuvant therapy. What is more, we also corroborated dynamic change of HER2-low expression during neoadjuvant therapy and its association with hormone receptor status, which warrants HER2 re-test in residual lesion, especially for patients with HER2-zero disease at initial diagnosis, so as to give a clear picture of not only prognostic significance but also treatment availability.
Supplementary Material
Funding Statement
This study was funded by National Natural Science Foundation of China (Nos. 82173115 and 82103695), Science and Technology Commission of Shanghai Municipality (No. 20DZ2201600), Shanghai Rising-Star Program (No. 22QC1400200), Innovative Research Team of High-level Local Universities in Shanghai (No. SHSMU-ZLCX20212601), Shanghai Municipal Health Commission Health Industry Clinical Research Special Project (No. 202340085) and Nurturing Fund of Renji Hospital (Nos. PYIII20-09 and RJPY-LX-002).
Author contributions
Yingying Zhao wrote the original draft of the manuscript. Yingying Zhao and Xinru Chen: data curation, formal analysis, investigation, methodology, software and visualization. Xinru Chen, Yaohui Wang, Xueqing Zhang and Wenjin Yin: data curation and validation. Shuguang Xu, Liheng Zhou, Yanping Lin and Yumei Ye: investigation and project administration. Jingsong Lu and Wenjin Yin: conceptualization. Yaohui Wang, Jingsong Lu and Wenjin Yin: funding acquisition. Jingsong Lu and Wenjin Yin: supervision and writing-review and editing. All authors contributed to the final version of the manuscript.
Ethics statement
Our study was performed under the provisions of the Ethics Committee of Renji Hospital, School of Medicine, Shanghai Jiao Tong University (approval number, LY2022-028-B).
Consent form
The informed consent was waived under the provisions of the Ethics Committee of Renji Hospital.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1.Parinyanitikul N, Lei X, Chavez-MacGregor M, et al. Receptor status change from primary to residual breast cancer after neoadjuvant chemotherapy and analysis of survival outcomes. Clin Breast Cancer. 2015;15(2):153–160. doi: 10.1016/j.clbc.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Tural D, Karaca M, Zirtiloglu A, et al. Receptor discordances after neoadjuvant chemotherapy and their effects on survival. J BUON. 2019;24(1):20–25. [PubMed] [Google Scholar]
- 3.Yoshida A, Hayashi N, Suzuki K, et al. Change in HER2 status after neoadjuvant chemotherapy and the prognostic impact in patients with primary breast cancer. J Surg Oncol. 2017;116(8):1021–1028. doi: 10.1002/jso.24762. [DOI] [PubMed] [Google Scholar]
- 4.Tarantino P, Hamilton E, Tolaney SM, et al. HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol. 2020;38(17):1951–1962. doi: 10.1200/JCO.19.02488. [DOI] [PubMed] [Google Scholar]
- 5.Kang S, Lee SH, Lee HJ, et al. Prognostic implications of HER2 changes after neoadjuvant chemotherapy in patients with HER2-zero and HER2-low breast cancer. Eur J Cancer. 2023;191:112956. doi: 10.1016/j.ejca.2023.112956. [DOI] [PubMed] [Google Scholar]
- 6.Miglietta F, Griguolo G, Bottosso M, et al. HER2-low-positive breast cancer: evolution from primary tumor to residual disease after neoadjuvant treatment. NPJ Breast Cancer. 2022;8(1):66. doi: 10.1038/s41523-022-00434-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denkert C, Seither F, Schneeweiss A, et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021;22(8):1151–1161. doi: 10.1016/S1470-2045(21)00301-6. [DOI] [PubMed] [Google Scholar]
- 8.Kang S, Lee SH, Lee HJ, et al. Pathological complete response, long-term outcomes, and recurrence patterns in HER2-low versus HER2-zero breast cancer after neoadjuvant chemotherapy. Eur J Cancer. 2022;176:30–40. doi: 10.1016/j.ejca.2022.08.031. [DOI] [PubMed] [Google Scholar]
- 9.Shao Y, Yu Y, Luo Z, et al. Clinical, pathological complete response, and prognosis characteristics of HER2-low breast cancer in the neoadjuvant chemotherapy setting: a retrospective analysis. Ann Surg Oncol. 2022;29(13):8026–8034. doi: 10.1245/s10434-022-12369-4. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y, Chen X, Wang Y, et al. The Modified Neo-Bioscore System for staging breast cancer treated with neoadjuvant therapy based on prognostic significance of HER2-low expression. J Clin Med. 2024;13(7):1850. doi: 10.3390/jcm13071850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol. 2018;36(20):2105–2122. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 12.Gradishar WJ, Moran MS, Abraham J, et al. Breast cancer, Version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(6):691–722. doi: 10.6004/jnccn.2022.0030. [DOI] [PubMed] [Google Scholar]
- 13.Ma Y, Zhu M, Zhang J, et al. Prognostic value of the evolution of HER2-low expression after neoadjuvant chemotherapy. Cancer Res Treat. 2023;55(4):1210–1221. doi: 10.4143/crt.2022.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guarneri V, Dieci MV, Barbieri E, et al. Loss of HER2 positivity and prognosis after neoadjuvant therapy in HER2-positive breast cancer patients. Ann Oncol. 2013;24(12):2990–2994. doi: 10.1093/annonc/mdt364. [DOI] [PubMed] [Google Scholar]
- 15.Niikura N, Tomotaki A, Miyata H, et al. Changes in tumor expression of HER2 and hormone receptors status after neoadjuvant chemotherapy in 21,755 patients from the Japanese Breast Cancer Registry. Ann Oncol. 2016;27(3):480–487. doi: 10.1093/annonc/mdv611. [DOI] [PubMed] [Google Scholar]
- 16.Giusti V, Ruzzi F, Landuzzi L, et al. Evolution of HER2-positive mammary carcinoma: HER2 loss reveals claudin-low traits in cancer progression. Oncogenesis. 2021;10(11):77. doi: 10.1038/s41389-021-00360-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prat A, Pineda E, Adamo B, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015;24(Suppl. 2):S26–S35. doi: 10.1016/j.breast.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Mutai R, Barkan T, Moore A, et al. Prognostic impact of HER2-low expression in hormone receptor positive early breast cancer. Breast. 2021;60:62–69. doi: 10.1016/j.breast.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Abudureheiyimu N, Mo H, et al. In real life, low-level HER2 expression may be associated with better outcome in HER2-negative breast cancer: a study of the National Cancer Center, China. Front Oncol. 2021;11:774577. doi: 10.3389/fonc.2021.774577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almstedt K, Heimes A-S, Kappenberg F, et al. Long-term prognostic significance of HER2-low and HER2-zero in node-negative breast cancer. Eur J Cancer. 2022;173:10–19. doi: 10.1016/j.ejca.2022.06.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.