Abstract
Background
Several surgical options for degenerative lumbar spinal stenosis (LSS) are available, but current guidelines do not recommend which one should be prioritized. Although previous network meta-analyses (NMAs) have been performed on this topic, they have major methodological problems and could not provide the convincing evidence and clinical practical information required.
Methods
Randomized controlled trials (RCTs) comparing at least two surgical interventions were included by searching AMED, CINAHL, EMBASE, the Cochrane Library, and MEDLINE (inception to August 2023). A frequentist random-effects NMA was performed for physical function and adverse events due to any reason. For physical function, three follow-up time points were included: short-term (< 6 months post-intervention), mid-term (≥ 6 months but < 12 months), and long-term (≥ 12 months). Laminectomy was the reference comparison intervention.
Results
A total of 43 RCTs involving 5017 participants were included in the systematic review and 28 RCTs encompassing 14 types of surgical interventions were included in the NMA. For improving physical function (scale 0–100), endoscopic-assisted laminotomy (mean difference: − 8.61, 95% confidence interval: − 10.52 to − 6.69; moderate-quality evidence), laminectomy combined with Coflex (− 8.41, − 13.21 to − 3.61; moderate quality evidence), and X-stop (− 6.65, − 8.60 to − 4.71; low-quality evidence) had small effects at short-term follow-up; no statistical difference was observed at mid-term follow-up (very low- to low-quality evidence); at long-term follow-up, endoscopic-assisted laminotomy (− 7.02, − 12.95 to − 1.08; very low-quality evidence) and X-stop (− 10.04, − 18.16 to − 1.93; very low-quality evidence) had a small and moderate effect, respectively. Compared with laminectomy, endoscopic-assisted laminotomy was associated with fewer adverse events due to any reason (odds ratio: 0.27, 0.09 to 0.86; low-quality evidence).
Conclusions
For adults with degenerative LSS, endoscopic-assisted laminotomy may be the safest and most effective intervention in improving physical function. However, the available data were insufficient to indicate whether the effect was sustainable after 6 months.
Trial registration
PROSPERO (CRD42018094180).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-024-03653-z.
Keywords: Lumbar spinal stenosis, Surgery, Systematic review, Network meta-analysis, Musculoskeletal disease, Orthopaedics
Background
Degenerative lumbar spinal stenosis (LSS) is a narrowing of the spinal canal diameter due to age-related changes, with a prevalence of up to 47% in adults aged 60 years and older, and is also a major cause of low back pain and associated disability [1, 2]. Although conservative care (e.g. exercise and physical therapy) is recommended as initial treatment, patients still need surgical interventions if pain persists after receiving conservative care [3]. For example, a recent study which is based on US nationally representative data found that about 165,000 patients who were hospitalized for LSS received surgery in 2019 and the rate increased from 54.2 per 100,000 in 2016 to 64.7 per 100,000 in 2019 [4]. There are a few surgical options (e.g. decompression only, decompression with fusion, and interspinous process spacer device) available for surgeons to manage degenerative LSS; however, current clinical guidelines do not recommend which surgical intervention should be prioritized [5, 6].
Network meta-analysis (NMA) is an extension of traditional pairwise meta-analysis. An advantage of NMA is that it combines direct evidence (referring to data from competing interventions through direct head-to-head comparisons) and indirect evidence (referring to data from competing interventions that have not been compared directly but with the same comparator intervention) to simultaneously compare more than two competing interventions within a single network [7]. In addition, a key advantage of NMA over pairwise meta-analysis is that it increases the precision of effect estimates by combining both direct and indirect evidence [8]. An NMA can also provide a relative ranking of competing interventions, thus, the superiority of different interventions for a given outcome [9].
Although previous NMAs have been performed on this topic, they have important limitations, such as the inclusion of non-randomized controlled trials, misclassification of the surgical intervention, inappropriate selection of the comparison group, and inclusion of a mixed population (e.g. patients with spinal instability) [10–15].
To address these gaps, we performed a systematic review with NMA to investigate the comparative effectiveness and safety of different types of surgical interventions for degenerative LSS.
Methods
Study design
The study followed the PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions (Additional file 1) [16]. The protocol was registered on PROSPERO (registration No CRD42018094180) and published elsewhere [17]. There have been two deviations from the original protocol. Firstly, we have deleted the non-surgical nodes (e.g. exercise) given the high crossover rates involved in these comparisons potentially preventing meaningful analyses of the results [18]. Secondly, we further refined the surgical nodes (e.g. conventional decompression was refined to laminectomy, midline preserving decompression was refined to laminotomy) due to inconsistencies found in the initial node analysis. This refinement resolved the inconsistencies (Additional file 2: Supplementary A. Tables S1-S3) [17, 18].
Data sources
We searched AMED, CINAHL, EMBASE, the Cochrane Library, and MEDLINE from the database inception until August 2023. Search strategies are detailed in Additional file 2: Supplementary B. We also screened the reference lists of all included studies, relevant systematic reviews and meta-analyses, and guidelines for eligible additional studies.
Study selection
We included parallel randomized controlled trials that enrolled participants who were aged 40 years or older with a diagnosis of degenerative LSS. Included studies had to compare one type of surgical intervention with another.
Exclusion criteria
We excluded studies on patients with malignancy, trauma, vertebral fracture, infection, and inflammatory disease. In addition, for studies that included patients with degenerative LSS and associated spondylolisthesis, only those with Meyerding grade I spondylolisthesis were included [17].
Outcome measures
The primary outcome for effectiveness was physical function. Secondary outcomes for effectiveness included the intensity of back pain and leg pain. Considering that different measurement tools could be used for these outcomes, we followed the study by Busse et al. [19] and harmonized the different scoring systems as 0–100 (lower score means less pain and better physical function), with the magnitude of effect estimates being classified as small (5–10), moderate (> 10–20), and large (> 20) [20]. Three follow-up time points were included: (1) short-term follow-up (< 6 months post-intervention), (2) mid-term follow-up (≥ 6 months but < 12 months post-intervention), and (3) long-term follow-up (≥ 12 months post-intervention) [17]. If two or more follow-up assessments occurred within a given time point, we used data that were assessed at the time point closest to the lower limit of the respective category.
The primary outcome for safety was the rate of adverse events due to any reason. Secondary outcomes for safety included reoperation rate and treatment withdrawal due to any reason. Adverse events due to any reason and reoperation rate were defined as the rate of events considering the reporting mainly summarized the number of events rather than the proportion of participants who had events. Given the impact of reoperations on patients and the healthcare system, the outcome of adverse events due to any reason did not include reoperations. Treatment withdrawal due to any reason was defined as the proportion of participants.
Other planned outcomes were not included in the analysis due to insufficient data to perform NMAs or the sparsely connected network from the available data (descriptive results are detailed in Additional file 2: Supplementary C).
Data extraction
Two authors (LC and BG) independently extracted data on study characteristics (e.g. publication year, geographical region, study duration, and funding source) and patient characteristics (e.g. mean age, sex ratio, stenosis level, and stenosis type). Differences were resolved by discussion between the two authors, with a third author (MF) consulted if disagreement persisted.
Risk of bias in individual studies and confidence in the evidence
Two authors (LC and BG) independently assessed the risk of bias through the revised Cochrane risk-of-bias tool for randomized trials (RoB 2)[21]. RoB 2 includes five domains (randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result), with each domain rated, and an overall score provided as either: low risk of bias, some concerns, or high risk of bias. Two authors (LC and BG) independently used the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach to evaluate the quality of evidence through the Confidence in Network Meta-Analysis (CINeMA) web application [8]. CINeMA covers six domains (within-study bias, reporting bias, indirectness, imprecision, heterogeneity, and incoherence) [8]. For each domain, there are three ratings: no concerns, some concerns, and major concerns [8]. The default overall level for each evidence is “high”. With “major concerns” in one domain, the rating is degraded by two levels; with “some concerns” in one domain, the rating is degraded by one level, and with “no concerns” in one domain, the rating is not degraded [8, 22]. The final level for each evidence is judged as “high” (when it is not degraded in total), “moderate” (when it is degraded by one level in total), “low” (when it is degraded by two levels in total), and “very low” (when it is degraded by more than two levels in total) [8, 22].
Treatment node classification
The final NMA included 14 nodes for surgical interventions (detailed in Table 1), which were classified as four nodes for traditional surgical approaches (i.e. laminectomy, laminectomy with fusion, laminectomy with Coflex, and subtotal laminectomy), seven nodes for minimally invasive surgical approaches (i.e. microscopic-assisted subtotal laminectomy, endoscopic-assisted subtotal laminectomy, microscopic-assisted split–spinous process subtotal laminectomy, microscopic-assisted laminotomy, endoscopic-assisted laminotomy, microscopic-assisted split–spinous process laminotomy, and microscopic-assisted spinous process osteotomy laminotomy), and three nodes for interspinous process spacer devices (i.e. Coflex, Superion, and X-stop). Laminectomy was the reference comparison intervention.
Table 1.
Final treatment nodes included in the network meta-analysis
| Treatment node | Description |
|---|---|
| Traditional surgical approaches | |
| Laminectomy | A type of surgery removing osseous elements (vertebrae lamina, vertebral arch, and spinous process) to provide decompression. [23, 24] |
| Laminectomy with fusion | A type of surgery involving the use of spinal implants (e.g. pedicle screws) to stabilize the fused segments after laminectomy. [23] |
| Laminectomy with Coflex | A type of surgery implanting the Coflex after laminectomy |
| Subtotal laminectomy | A type of surgery similar to laminectomy but preserving both facet joints as much as possible while leaving the upper part of the spinous process and lamina. [25] |
| Minimally invasive surgical approaches | |
| Microscopic-assisted subtotal laminectomy | A microscopic-assisted partial laminectomy limiting the extent of bony decompression (compared with laminectomy) with or without resection of the spinous process. [24] |
| Endoscopic-assisted subtotal laminectomy | An endoscopic-assisted partial laminectomy limiting the extent of bony decompression (compared with laminectomy) with or without resection of the spinous process. [24] |
| Microscopic-assisted split–spinous process subtotal laminectomy | A type of surgery involving longitudinally splitting of the spinous process, partial laminectomy, and reconstructing/repositioning the split sections of the spinous process. [26, 27] |
| Microscopic-assisted laminotomy | A type of microscopic-assisted surgery removing unilateral or bilateral vertebrae lamina but leaving the spinous process intact. [23, 24] |
| Endoscopic-assisted laminotomy | A type of endoscopic-assisted surgery removing unilateral or bilateral vertebrae lamina but leaving the spinous process intact. [23, 24] |
| Microscopic-assisted split–spinous process laminotomy | A type of microscopic-assisted surgery involving longitudinally splitting the spinous process into halves with removing vertebrae lamina to provide decompression. [23, 28] |
| Microscopic-assisted spinous process osteotomy laminotomy | A type of microscopic-assisted surgery involving an osteotomy at the base of the spinous process with removing vertebrae lamina to provide decompression. [29] |
| Interspinous process spacer devices | |
| X-stop | A titanium device, consisting of an oval titanium core that is designed to fit within the interspinous ligament, and secured within the ligament by two lateral wings. [30, 31] |
| Superion | A titanium device, consisting of an implant body and two cam lobes that rotate during deployment to encompass the lateral aspects of the superior and inferior spinous processes. [30, 32] |
| Coflex | A titanium implant that fits between the spinous processes of the lumbar spine, consisting of two components: a wing assembly and a spacer assembly. [33, 34] |
Statistical analysis
Basic characteristics of all included studies were summarized (detailed in Additional file 2: Supplementary D. Tables S1-S5)[25, 26, 28, 33, 35–79]. Given the different symptomatology of different types (i.e. central, lateral, or foraminal) of degenerative LSS, we initially planned to analyse them separately. However, a number of studies did not specify the type, so we classified the included studies as non-foraminal and foraminal stenosis. As only four studies were related to foraminal stenosis, quantitative analyses were only performed for non-foraminal stenosis.
The random effects NMA through a frequentist approach was performed using the restricted maximum likelihood method, and the 95% confidence intervals (CI) were estimated using the Hartung-Knapp-Sidik-Jonkman approach [80]. We assumed that the heterogeneity variance across different comparisons within the NMA model was the same [81]. Continuous (e.g. physical function) and binary (e.g. adverse events due to any reason) outcomes were reported mean difference (MD) with 95% CI and odds ratio (OR) with 95% CI, respectively. We estimated the mean rank and relative treatment rankings for each intervention node according to the surface under the cumulative ranking curve (SUCRA) values [82]. We produced rankograms for the primary outcomes at each time point of analysis [82].
Transitivity was assessed by visual inspection of a table containing study characteristics. The global and local inconsistency was assessed through the design-by-treatment interaction model and the Bucher method, respectively [83]. Small-study effects were evaluated through two methods: first, visual inspection of comparison-adjusted funnel plots; second, meta-regression based on the total sample size. To examine the robustness of the results, we performed extensive sensitivity analyses: first, excluding studies that received commercial fundings; second, excluding studies that only recruited patients with multiple level stenosis; third, excluding studies that recruited patients without degenerative spondylolisthesis; fourth, excluding studies that recruited patients with mixed type stenosis; fifth, excluding studies with high risk of bias; sixth, excluding studies that published prior to year 2010; seventh, excluding studies with imputed data; eighth, for adverse events due to any reason, excluding studies where the study endpoints were not long term. The following sensitivity analyses have been conducted to verify the robustness of our conclusions: first, excluding studies with unclear participant description of a history of lumbar spine surgery in the inclusion and exclusion criteria; second, adopting an alternative definition of treatment nodes (i.e. traditional surgical approaches, minimally invasive surgical approaches, interspinous process spacer devices); third, using the change score as the value.
Results
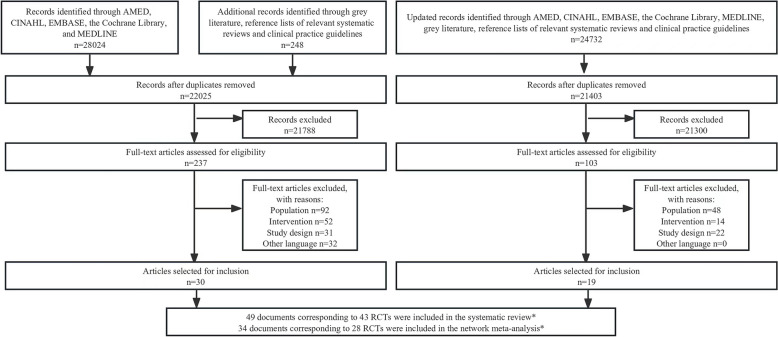
A total of 28,272 records were retrieved through electronic databases (AMED, CINAHL, EMBASE, the Cochrane Library, and MEDLINE) and other sources (grey literature and reference lists of relevant systematic reviews and clinical practice guidelines) from inception to April 2018, and a total of 24,732 records were retrieved through the same sources from May 2018 to August 2023. After screening 43,428 titles and abstracts and 340 full-text articles, 49 documents corresponding to 43 randomized controlled trials (RCTs) were included in the systematic review, and 34 documents corresponding to 28 RCTs were eligible for the network meta-analysis (Fig. 1). Six studies [65–70] had undefined surgical interventions (e.g. decompression only without specific surgical details [e.g. laminectomy]) that could not be classified and were, therefore, excluded. Four studies [71–74] had treatment arms with mixed surgical interventions (e.g. participants in the decompression group received laminectomy or laminotomy, but no detailed proportions of participants who received laminectomy or laminotomy), which could not be assigned exclusively to a single treatment node and were, therefore, excluded. Four studies [75–78] included surgical interventions for foraminal stenosis and could not form a network structure, so the data could not be pooled and these studies were, therefore, excluded. One study [79] had a high risk of bias in the randomization process, so it was excluded with reference to our published protocol paper in BMJ Open [17] (Additional file 2: Supplementary E. Table S1). Figure 2 shows the network plots for the primary outcomes.
Fig. 1.
Study selection flowchart. *One RCT reported data on three documents (results on 2-year follow-up, 3-year follow-up, and 5-year follow-up), one RCT reported data on two documents (results on 6-month follow-up and 3-year follow-up), one RCT reported data on three documents (results on 2-year follow-up, 3-year follow-up, and 5-year follow-up), one RCT reported data on two documents (results on 2-week follow-up and 1-year follow-up). RCT: Randomized controlled trial
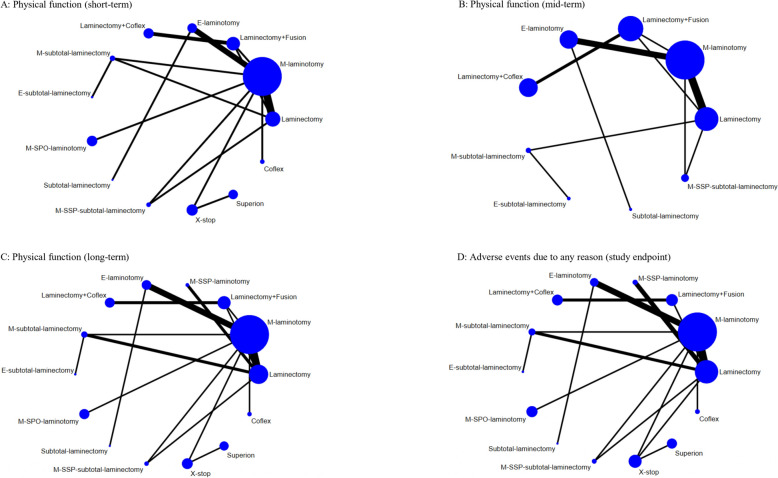
Fig. 2.
Network plots of physical function and adverse events due to any reason. The width of the lines is proportional to the number of trials comparing each pair of interventions. The size of the nodes is proportional to the number of participants. Laminectomy + fusion: Laminectomy with fusion; Laminectomy + Coflex: Laminectomy with Coflex; M-subtotal laminectomy: Microscopic-assisted subtotal laminectomy; E-subtotal laminectomy: Endoscopic-assisted subtotal laminectomy; M-SSP-subtotal laminectomy: Microscopic-assisted split–spinous process subtotal laminectomy; M-laminotomy: Microscopic-assisted laminotomy; E-laminotomy: Endoscopic-assisted laminotomy; M-SSP-laminotomy: Microscopic-assisted split–spinous process laminotomy; M-SPO-laminotomy: Microscopic-assisted spinous process osteotomy laminotomy
Overview of studies
Table 2 presents the general characteristics of the 43 included studies, separated by the primary outcomes. Most studies were published between 2016 and 2023, did not report funding sources, and were two-arm trials. The top three continents were Asia, Europe, and North America. The long-term duration of follow-up was the most frequently assessed time point. Among traditional surgical approaches, laminectomy was the most frequently investigated intervention. Among minimally invasive surgical approaches, microscopic-assisted laminotomy was the most frequently investigated intervention. Among interspinous process spacer devices, X-stop was the most frequently investigated intervention.
Table 2.
General characteristics of included studies in the systematic review
| Characteristics | Primary outcomes | |
|---|---|---|
| Physical function | Adverse events due to any reason | |
| Publication characteristics | ||
| Total number of unique studies included | 42 | 37 |
| Publication year | ||
| 1995–2000 | 0 | 1 |
| 2000–2007 | 4 | 3 |
| 2008–2015 | 14 | 14 |
| 2016–2023 | 24 | 19 |
| Funding | ||
| Commercial | 15 | 14 |
| Non-commercial | 11 | 8 |
| Not reported | 16 | 15 |
| Study design characteristics | ||
| Range of study sample size | 22–437 | 22–437 |
| No of intervention arms included | ||
| 2 arms | 38 | 33 |
| 3 arms | 4 | 4 |
| Studies with durations of follow-upa | ||
| Short-term (< 6 months) | 26 | 1 |
| Mid-term (6–12 months) | 22 | 1 |
| Long-term (≥ 12 months) | 39 | 35 |
| Study setting | ||
| Single centre | 19 | 17 |
| Multicentre | 14 | 12 |
| Not reported | 9 | 8 |
| No. of studies containing the following treatment nodes (only included in the NMA) | ||
| Traditional surgical approaches | ||
| Laminectomy | 13 | 12 |
| Laminectomy with fusion | 4 | 3 |
| Laminectomy with Coflex | 2 | 2 |
| Subtotal laminectomy | 1 | 1 |
| Minimally invasive surgical approaches | ||
| Microscopic-assisted subtotal laminectomy | 4 | 4 |
| Endoscopic-assisted subtotal laminectomy | 1 | 1 |
| Microscopic-assisted split–spinous process subtotal laminectomy | 1 | 1 |
| Microscopic-assisted laminotomy | 18 | 14 |
| Endoscopic-assisted laminotomy | 6 | 4 |
| Microscopic-assisted split–spinous process laminotomy | 3 | 3 |
| Microscopic-assisted spinous process osteotomy laminotomy | 1 | 1 |
| Interspinous process spacer devices | ||
| X-stop | 3 | 3 |
| Superion | 1 | 1 |
| Coflex | 1 | 1 |
| Continent | ||
| Asia | 19 | 15 |
| Europe | 15 | 14 |
| North America | 4 | 4 |
| Oceania | 1 | 1 |
| Africa | 2 | 2 |
| International | 1 | 1 |
| Patient characteristics | ||
| Stenosis level | ||
| Single | 16 | 12 |
| Multiple | 5 | 4 |
| Mixed | 20 | 20 |
| Not reported | 1 | 1 |
| Stenosis type | ||
| Central | 9 | 7 |
| Lateral | 1 | 1 |
| Foraminal | 4 | 3 |
| Mixed | 7 | 6 |
| Not reported | 21 | 20 |
| Degenerative spondylolisthesis | ||
| Low grade spondylolisthesis (Meyerding I grade) | 32 | 28 |
| No spondylolisthesis | 10 | 9 |
| Range of mean age (years); No. of studies | 53.5–73.7; 41 | 53.5–73.7; 36 |
| Range of males (%); No. of studies | 19.7–79.2; 40 | 19.7–63.6; 35 |
| Range of mean body mass index; No. of studies | 23.3–29.7; 19 | 23.3–29.7; 16 |
| Range of smokers (%); No. of studies | 0–51.4; 12 | 0–51.4; 8 |
| Range of mean baseline physical function (0–100)b; No. of studies | 30.4–86; 41 | NA |
aFor physical function, if a unique study involves short-term, mid-term, and long-term outcomes, it will be counted in the corresponding items separately; for adverse events due to any reason, only the study endpoint of a unique study will be counted in the corresponding item
bMean baseline physical function measured by different instruments have been adjusted to a range of 0 to 100, a score closed to 0 means less disability, and a score closed to 100 means more disability
Transitivity
We summarized the study characteristics across direct comparisons within the network for physical function and adverse events due to any reason (Additional file 2: Supplementary F. Tables S1-S2). The mean age was similar across the different comparisons, mostly between 55 and 70 years. The percentage of males was similar across the different comparisons, mostly between 40 and 60%. The included studies provided insufficient data on the percentage of smokers and the mean BMI. Further meta-regression did not suggest mean age, or the proportion of males were effect modifiers (Additional file 2: Supplementary G. Tables S1-S6) [17, 81]. Additionally, meta-regression based on mean baseline levels of physical function did not suggest it was an effect modifier. Overall, we considered the assumption of transitivity to be valid.
Risk of bias
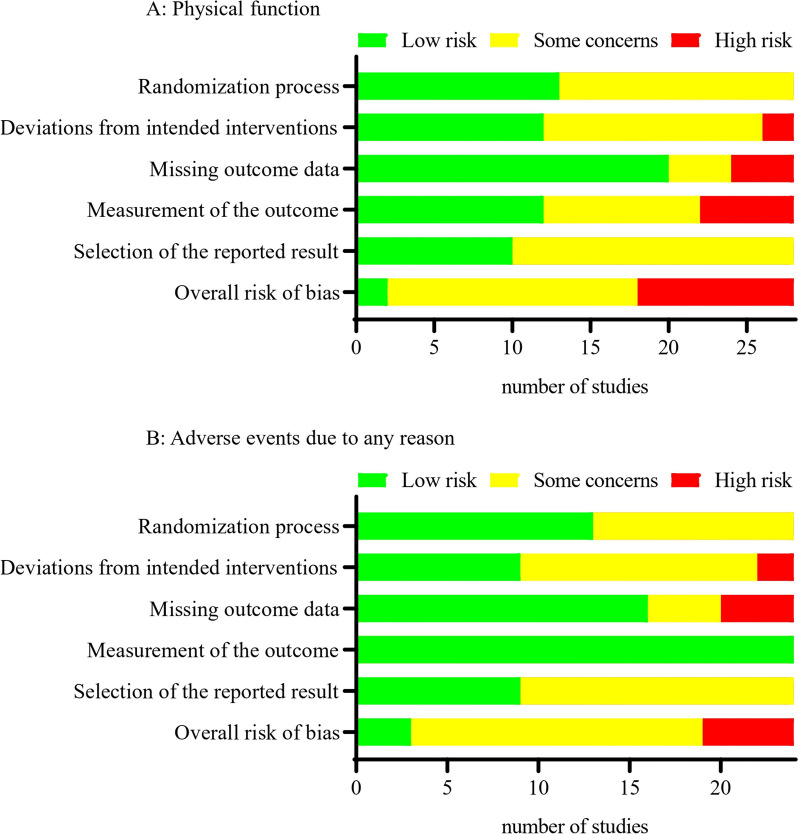
For physical function, of the 28 unique studies included in the NMA, two were judged as having low risk of bias, 16 as having some concerns, and 10 as having high risk of bias (Fig. 3 and Additional file 2: Supplementary H. Tables S1-S2). For adverse events due to any reason, of the 24 unique studies included in the NMA, three were judged as having low risk of bias, 16 as having some concerns, and five as having high risk of bias (Fig. 3 and Additional file 2: Supplementary H. Tables S3-S4). For back pain, of the 18 unique studies included in the NMA, two were judged as having a low risk of bias, ten as having some concerns, and six as having a high risk of bias (Additional file 2: Supplementary H. Tables S5-S6). For leg pain, of the 18 unique studies included in the NMA, two were judged as having a low risk of bias, ten as having some concerns, and six as having a high risk of bias (Additional file 2: Supplementary H. Tables S7-S8). For the reoperation rate, of the 18 unique studies included in the NMA, three were judged as having a low risk of bias, 11 as having some concerns, and four as having a high risk of bias (Additional file 2: Supplementary H. Tables S9-S10). For treatment withdrawal due to any reason, of the 18 unique studies included in the NMA, three were judged as having a low risk of bias, 12 as having some concerns, and three as having a high risk of bias (Additional file 2: Supplementary H. Tables S11-S12). The main concerns related to risk of bias were from deviations from intended interventions, missing outcome data, and measurement of the outcome.
Fig. 3.
Summary plot for primary outcomes showing the number of studies included in the network meta-analysis judged to be low, some, or high risk of bias
Physical function
We did not detect any inconsistency at short-term follow-up (Additional file 2: Supplementary I. Tables S1-S3 and Supplementary J. Tables S1-S3). However, we detected global inconsistency at mid-term and long-term follow-ups. At these time points, local inconsistency was detected. Sensitivity analyses were conducted at these two time points, which resolved the presence of inconsistency by removing one three-arm study [26] (i.e. laminectomy, microscopic-assisted laminotomy, and microscopic-assisted split–spinous process subtotal laminectomy).
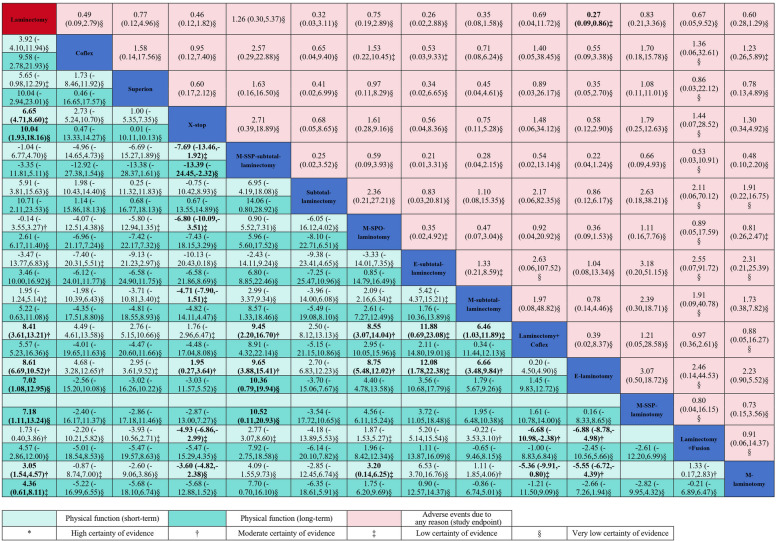
For the short-term follow-up, 20 trials including 2939 patients and 13 interventions were included in the NMA (Fig. 2). Compared with laminectomy, endoscopic-assisted laminotomy (MD − 8.61, 95% CI − 10.52 to − 6.69, 0–100 scale; moderate-quality evidence), laminectomy combined with Coflex (− 8.41, − 13.21 to − 3.61; moderate-quality evidence), and X-stop (− 6.65, − 8.60 to − 4.71; low-quality evidence) had small effects on improving physical function; microscopic-assisted laminotomy had a statistically significant effect (− 3.05, − 4.57 to − 1.54; moderate-quality evidence); no statistical difference was observed for other surgical interventions (Fig. 4 and Additional file 2: Supplementary K. Table S1) [8, 17, 20–22, 83–86]. For physical function at mid-term follow-up, 18 trials including 1649 patients and 9 interventions were included in the NMA (Fig. 2). Compared with laminectomy, no statistical difference was observed for other surgical interventions (very low- to low-quality evidence; Additional file 2: Supplementary K. Table S2 and Supplementary L. Figure S1 and Table S1). For physical function at long-term follow-up, 26 trials including 3251 patients and 14 interventions were included in the NMA (Fig. 2). Compared with laminectomy, X-stop (− 10.04, − 18.16 to − 1.93; very low-quality evidence) had a moderate effect on improving physical function; endoscopic-assisted laminotomy (− 7.02, − 12.95 to − 1.08) and microscopic-assisted split–spinous process laminotomy (− 7.18, − 13.24 to − 1.11) had small effects (very low-quality evidence); microscopic-assisted laminotomy had a statistically significant effect (− 4.36, − 8.11 to − 0.61; low-quality evidence) without clinical importance; no statistical difference was observed for other surgical interventions (Fig. 4 and Additional file 2: Supplementary K. Table S3).
Fig. 4.
Network meta-analyses for physical function and adverse events due to any reason. Laminectomy + fusion: Laminectomy with fusion; Laminectomy + Coflex: Laminectomy with Coflex; M-subtotal laminectomy: Microscopic-assisted subtotal laminectomy; E-subtotal laminectomy: Endoscopic-assisted subtotal laminectomy; M-SSP-subtotal laminectomy: Microscopic-assisted split–spinous process subtotal laminectomy; M-laminotomy: Microscopic-assisted laminotomy; E-laminotomy: Endoscopic-assisted laminotomy; M-SSP-laminotomy: Microscopic-assisted split–spinous process laminotomy; M-SPO-laminotomy: Microscopic-assisted spinous process osteotomy laminotomy. Laminectomy was the reference comparison intervention. Comparisons should be read from left to right. Physical function and adverse events due to any reason estimates are located at the intersection between the column-defining intervention and the row-defining intervention. For physical function, data are in mean difference (95% CI), and data below 0 favour the column-defining intervention. For adverse events due to any reason, data are in odds ratio (95% CI), and data below 1 favour the column-defining intervention. The certainty of the evidence (according to confidence in network meta-analysis [CINeMA]) was also incorporated in this figure. Estimates in bold denoted significance at p < 0.05
Sensitivity analyses showed similar results at short- and mid-term follow-up with unstable estimates at long-term follow-up (Additional file 2: Supplementary M. Tables S1-S30). The comparison-adjusted funnel plots, and meta-regression based on sample size, did not suggest small-study effects (Additional file 2: Supplementary G. Table S1 and Supplementary N. Figures S1-S3).
Based on the ranking results (Additional file 2: Supplementary O. Figures S1-S3), the most highly ranked intervention was endoscopic-assisted laminotomy at short-term follow-up and X-stop at long-term follow-up.
Adverse events due to any reason
We did not detect any inconsistency (Additional file 2: Supplementary I. Table S4 and Supplementary J: Table S4). A total of 24 trials including 2995 patients and 14 interventions were included in the NMA (Fig. 2). Compared with laminectomy, endoscopic-assisted laminotomy was associated with fewer adverse events due to any reason (OR 0.27, 95% CI 0.09 to 0.86; low-quality evidence); no statistical difference was observed for other surgical interventions (Fig. 4 and Additional file 2: Supplementary K. Table S4). Sensitivity analyses showed similar results (Additional file 2: Supplementary M. Tables S31-S39). The comparison-adjusted funnel plots, and meta-regression based on sample size, did not suggest small-study effects (Additional file 2: Supplementary G. Table S2 and Supplementary N. Figure S4). Based on the ranking results (Additional file 2: Supplementary O. Figure S4), the most highly ranked intervention was endoscopic-assisted laminotomy. Further details on specific adverse events in included studies in the NMA are listed in Additional file 2: Supplementary P.
Secondary outcomes
For reducing back pain, microscopic-assisted laminotomy (MD -22.58, 95% CI − 30.53 to − 14.62, 0–100 scale), endoscopic-assisted laminotomy (− 25.70, − 42.37 to − 9.03), microscopic-assisted subtotal laminectomy (− 29.85, − 43.81 to − 15.89), subtotal laminectomy (− 26.67, − 48.76 to − 4.58), X-stop (− 25.67, − 38.73 to − 12.61), Superion (− 23.64, − 42.64 to − 4.64), and Coflex (− 23.55, − 38.62 to − 8.47) had large effects (very low-quality evidence), microscopic-assisted spinous process osteotomy laminotomy (− 16.91, − 30.95 to − 2.87) had moderate effects at short-term follow-up (very low-quality evidence); microscopic-assisted subtotal laminectomy (− 27.60, − 51.10 to − 4.10) and endoscopic-assisted subtotal laminectomy (− 33.85, − 66.83 to − 0.87) had large effects (very low-quality evidence) at mid-term follow-up; microscopic-assisted laminotomy (− 16.83, − 30.54 to − 3.12) had moderate effect (very low-quality evidence) at long-term follow-up (Additional file 2: Supplementary K. Tables S5-S7 and Supplementary L. Figures S2-S4 and Tables S2-S4).
For reducing leg pain, laminectomy was better than microscopic-assisted split-spinous process subtotal laminectomy (− 17.03, − 24.15 to − 9.92) at short-term follow-up (very low-quality evidence); laminectomy was better than microscopic-assisted laminotomy (− 5.31, − 7.29 to − 3.34), laminectomy combined with fusion (− 5.91, − 8.91 to − 2.91), and microscopic-assisted split-spinous process subtotal laminectomy (− 8.64, − 10.50 to − 6.79) at mid-term follow-up (low-quality evidence); laminectomy was better than microscopic-assisted split-spinous process subtotal laminectomy (− 9.38, − 13.06, to − 5.70) at long-term follow-up (low-quality evidence); compared with laminectomy, X-stop had small effect (− 5.13, − 10.10 to − 0.17) at long-term follow-up (very low-quality evidence) (Additional file 2: Supplementary K. Tables S8-S10 and Supplementary L. Figures S5-S7 and Tables S5-S7).
For reoperation rate (very low- to moderate-quality evidence) and treatment withdrawal due to any reason (very low- to low-quality evidence), there was no statistical difference between laminectomy and other surgical interventions (Additional file 2: Supplementary K. Tables S11-S12 and Supplementary L. Figures S8-S9 and Tables S8-S9).
Results of the inconsistency assessment, ranking results, and assessment of small-study effects are detailed in Additional file 2: Supplementary G. Tables S3-S6, Supplementary I. Tables S5-S12, Supplementary J. Tables S5-S12, Supplementary N. Figures S5-S12, and Supplementary O. Figures S5-S12.
Other planned outcomes
Six planned outcomes, including overall pain (referring to pain that is not specific to a part of the body), health-related quality of life, mobility, global impression of recovery, all-cause mortality, and work absenteeism, were not included in the NMA due to insufficient data or a poorly connected network. For the outcome of overall pain, Cho et al. [44] reported that microscopic-assisted split–spinous process laminotomy was better than laminectomy at long-term follow-up (p = 0.001). For the outcome of health-related quality of life, Ghogawala et al. [45] reported that laminectomy with fusion was better than laminectomy at mid-term follow-up (p = 0.02), but the significant difference was not maintained at long-term follow-up. For the global impression of recovery, Haddadi et al. [26] reported that microscopic-assisted laminotomy was better than laminectomy (at short [p < 0.05], mid [p < 0.01], and long-term [p < 0.01] follow-up) or microscopic-assisted split–spinous process subtotal laminectomy (at mid [p < 0.001] and long-term [p < 0.001] follow-up). For the outcomes of mobility, all-cause mortality and work absenteeism, no significant differences were found in any of the comparisons. (Additional file 2: Supplementary C).
Discussion
Principal findings
Our study has identified that for adults with LSS, endoscopic-assisted laminotomy was the most effective surgical intervention to improve physical function at short-term follow-up, compared to laminectomy. For improving physical function at short-term follow-up, other surgical interventions with small effects compared to laminectomy include laminectomy combined with Coflex and X-stop. However, the available data were insufficient to indicate whether the effect was sustainable after 6 months. The available safety data indicated that endoscopic-assisted laminotomy may be the safest surgical intervention.
Comparison with other studies
We searched previous NMAs which included surgical interventions for LSS (Additional file 2: Supplementary Q) [87] and found six relevant studies [10–15]. All six NMAs presented ‘critically low’ methodological quality according to the AMSTAR 2 tool (assessed by LC and BG). The main methodological limitations of these NMAs include inclusion of non-randomized controlled trials [11, 13], inclusion of heterogeneous participants (e.g. degenerative LSS with instable spondylolisthesis [> Meyerding grade I] [10, 11, 13], spinal metastatic disease [10]), inappropriate comparison group (e.g. non-surgery [13, 14], undefined decompression [10, 12, 15]), misclassification of the surgical intervention (e.g. decompression and laminectomy appeared simultaneously in the classification [14]). It is, therefore, not appropriate to directly compare our results with theirs. Our study has addressed the main limitations of previous NMAs by including RCT only, including more homogeneous participants, selecting appropriate comparison groups, and providing more detailed descriptions of surgical interventions. Since the last NMA was published, we have also included five additional trials and two follow-up reports of previous published trials and therefore provide the most up to date and comprehensive evidence to date.
The cost of endoscopic techniques should also be considered. Although there is no comprehensive economic analysis for all available surgical options for LSS, several existing studies have indicated that the endoscopic approach is more expensive than the open approach [88, 89]. For instance, a recent study analysed 633 open and 195 endoscopic decompression lumbar surgical procedures and found that patients who received endoscopic approaches had significantly higher total in-hospital costs compared to those who received open approaches. Clinicians and policy makers should be aware of this point to better guide clinical practice.
Although leg pain is a secondary outcome in our study, it is an important outcome that clinicians are interested in for patients with LSS. Our study showed that laminectomy was superior to several types of minimally invasive surgical approaches for leg pain, which differs from the findings for the primary outcome, physical function. This suggests that clinicians should discuss with patients which outcomes they prioritize and use this information to guide the decision-making process for selecting the most suitable surgical intervention. Further studies are needed, as the quality of almost all the evidence for leg pain ranged from very low to low.
Limitations
Some limitations of this NMA should be mentioned. Firstly, it is important to note that the number of studies included in most comparisons is limited. While it is necessary to restrict the number of studies to obtain less biased estimates, it is also important to acknowledge that this may result in relatively imprecise estimates, which could lead to misunderstandings of the result. To enhance comprehension, we used GRADE to assess the quality of evidence and refrained from overinterpreting our results. Secondly, due to the poor and inconsistent reporting, the influence of several important factors (e.g. stenosis level, and stenosis type) could not be investigated. Future studies could consider individual patient data meta-analyses to explore this topic further, as performing high-quality RCTs, especially in the short term, is challenging [90]. Thirdly, the reporting of safety outcomes is inadequate. The majority of the studies included did not report the timing of adverse events or use a systematic method to gather relevant data. This should be improved in the design of future trials.
Conclusions
For adults with degenerative LSS, endoscopic-assisted laminotomy may be the safest and most effective intervention in improving physical function. However, the available data were insufficient to indicate whether the effect was sustainable after 6 months. The results of this network meta-analysis can enhance the clarity of guideline recommendations on the most effective surgical interventions, helping patients and clinicians to make better-informed treatment decisions.
Supplementary Information
Additional file 1. PRISMA NMA checklist.
Additional file 2: Supplementary A. Deviations from the initial protocol. Table S1. [Initial treatment nodes classification]. Table S2. [Adjustments to pre-specified treatment nodes.]. Table S3. [Revised treatment node classification.]. Supplementary B. Search strategies. Supplementary C. Descriptive results on protocol planned outcomes not included in the NMA. Supplementary D. Individual characteristics of included studies. Table S1. [Individual study design characteristics and publication characteristics of included studies in the NMA]. Table S2. [Individual patient characteristics of included studies in the NMA]. Table S3. [Individual study design characteristics and publication characteristics of included studies in the systematic review but excluded in the NMA]. Table S4. [Individual patient characteristics of included studies in the systematic review but excluded in the NMA.]. Table S5. [Individual description on participant inclusion and exclusion criteria of included studies in the NMA.]. Supplementary E. Studies not included in the NMA. Table S1. [Effect estimates for studies included in the systematic review but excluded in the NMA.]. Supplementary F. Assessment of transitivity. Table S1. [Assessment of transitivity: network of interventions for physical function.]. Table S2. [Assessment of transitivity: network of interventions for adverse events due to any reason.]. Supplementary G. Results of meta-regression. Table S1. [Results of meta-regression for physical function.]. Table S2. [Results of meta-regression for adverse events due to any reason.]. Table S3. [ Results of meta-regression for back pain.]. Table S4. [Results of meta-regression for leg pain.]. Table S5. [Results of meta-regression for reoperation rate.]. Table S6. [Results of meta-regression for treatment withdrawal due to any reason.]. Supplementary H. Risk of bias judgments. Table S1. [Assessing physical function: studies included in the NMA.]. Table S2. [Assessing physical function: studies included in the systematic review but excluded in the NMA.]. Table S3. [Assessing adverse events due to any reason: studies included in the NMA.]. Table S4. [Assessing adverse events due to any reason: studies included in the systematic review but excluded in the NMA.]. Table S5– [Assessing back pain: studies included in the NMA.]. Table S6– [Assessing back pain: studies included in the systematic review but excluded in the NMA.]. Table S7– [Assessing leg pain: studies included in the NMA.]. Table S8– [Assessing leg pain: studies included in the systematic review but excluded in the NMA.]. Table S9– [Assessing reoperation rate: studies included in the NMA.]. Table S10– [Assessing reoperation rate: studies included in the systematic review but excluded in the NMA.]. Table S11– [Assessing treatment withdrawal due to any reason: studies included in the NMA.]. Table S12– [Assessing treatment withdrawal due to any reason: studies included in the systematic review but excluded in the NMA.]. Supplementary I. Assessment of global inconsistency. Table S1– [Physical function at short-term follow-up.]. Table S2a– [Physical function at mid-term follow-up (primary analysis).]. Table S2b– [Physical function at mid-term follow-up (sensitivity analysis conducted to resolve inconsistency).]. Table S3a– [Physical function at long-term follow-up (sensitivity analysis conducted to resolve inconsistency).]. Table S3b– [Physical function at long-term follow-up (sensitivity analysis conducted to resolve inconsistency).]. Table S4– [Adverse events due to any reason at study endpoint.]. Table S5a– [Back pain at short-term follow-up (primary analysis).]. Table S5b– [Back pain at short-term follow-up (sensitivity analysis conducted to resolve inconsistency).]. Table S6a– [Back pain at mid-term follow-up (primary analysis).]. Table S6b– [Back pain at mid-term follow-up (sensitivity analysis conducted to resolve inconsistency).]. Table S7– [Back pain at long-term follow-up.]. Table S8a– [Leg pain at short-term follow-up (primary analysis).]. Table S8b– [Leg pain at short-term follow-up (sensitivity analysis conducted to resolve inconsistency).]. Table S9– [Leg pain at mid-term follow-up.]. Table S10a– [Leg pain at long-term follow-up (primary analysis).]. Table S10b– [Leg pain at long-term follow-up (sensitivity analysis conducted to resolve inconsistency).]. Table S11– [Reoperation rate at study endpoint.]. Table S12– [Treatment withdrawal due to any reason at study endpoint.]. Supplementary J. Assessment of local inconsistency. Tables S1-S12. Table S1– [Physical function at short-term follow-up.]. Table S2a– [Physical function at mid-term follow-up (primary analysis).]. Table S2b– [Physical function at mid-term follow-up (sensitivity analysis conducted to resolve inconsistency).]. Table S3a– [Physical function at long-term follow-up (primary analysis).]. Table S3b– [Physical function at long-term follow-up (sensitivity analysis conducted to resolve inconsistency).]. Table S4– [Adverse events due to any reason at study endpoint.]. Table S5a– [Back pain at short-term follow-up (primary analysis).]. Table S5b– [Back pain at short-term follow-up (sensitivity analysis conducted to resolve inconsistency).]. Table S6a– [Back pain at mid-term follow-up (primary analysis).]. Table S6b– [Back pain at mid-term follow-up (sensitivity analysis conducted to resolve inconsistency).]. Table S7– [Back pain at long-term follow-up.]. Table S8a– [Leg pain at short-term follow-up (primary analysis).]. Table S8b– [Leg pain at short-term follow-up (sensitivity analysis conducted to resolve inconsistency).]. Table S9– [Leg pain at mid-term follow-up.]. Table S10a– [Leg pain at long-term follow-up (primary analysis).]. Table S10b– [Leg pain at long-term follow-up (sensitivity analysis conducted to resolve inconsistency).]. Table S11– [Reoperation rate at study endpoint.]. Table S12– [Treatment withdrawal due to any reason at study endpoint.]. Supplementary K. CINeMA results. Table S1– [Physical function at short-term follow-up.]. Table S2– [Physical function at mid-term follow-up.]. Table S3– [Physical function at long-term follow-up.]. Table S4– [Adverse events due to any reason at study endpoint.]. Table S5– [Back pain at short-term follow-up.]. Table S6– [Back pain at mid-term follow-up.]. Table S7– [Back pain at long-term follow-up.]. Table S8– [Leg pain at short-term follow-up.]. Table S9– [Leg pain at mid-term follow-up.]. Table S10– [Leg pain at long-term follow-up.]. Table S11– [Reoperation rate at study endpoint.]. Table S12– [Treatment withdrawal due to any reason at study endpoint.]. Supplementary L. Results from the NMA. Figure S1– [Network plot for physical function at mid-term follow-up.]. Figure S2– [Network plot for back pain at short-term follow-up.]. Figure S3– [Network plot for back pain at mid-term follow-up.]. Figure S4– [Network plot for back pain at long-term follow-up.]. Figure S5– [Network plot for leg pain at short-term follow-up.]. Figure S6– [Network plot for leg pain at mid-term follow-up.]. Figure S7– [Network plot for leg pain at long-term follow-up.]. Figure S8– [Network plot for reoperation rate at study endpoint.]. Figure S9– [Network plot for treatment withdrawal due to any reason at study endpoint.]. Table S1– [Physical function at mid-term follow-up.]. Table S2– [Back pain at short-term follow-up.]. Table S3– [Back pain at mid-term follow-up.]. Table S4– [Back pain at long-term follow-up.]. Table S5– [Leg pain at short-term follow-up.]. Table S6– [Leg pain at mid-term follow-up.]. Table S7– [Leg pain at long-term follow-up.]. Table S8– [Reoperation rate at study endpoint.]. Tables S9– [Treatment withdrawal due to any reason at study endpoint.]. Supplementary M. Sensitivity analyses. Table S1– [Physical function at short-term follow-up, excluding studies that received commercial fundings.]. Table S2– [Physical function at short-term follow-up, excluding studies that only recruited patients with multiple level stenosis.]. Table S3– [Physical function at short-term follow-up, excluding studies that recruited patients with mixed type stenosis.]. Table S4– [Physical function at short-term follow-up, excluding studies with high risk of bias.]. Table S5a– [Physical function at short-term follow-up, excluding studies published prior to year 2010 (Result 1).]. Table S5b– [Physical function at short-term follow-up, excluding studies published prior to year 2010 (Result 2).]. Table S6– [Physical function at short-term follow-up, excluding studies with data imputed from p value, confidence interval, standard error, or median (interquartile ranges).]. Table S7– [Physical function at mid-term follow-up, excluding studies that received commercial fundings.]. Table S8– [Physical function at mid-term follow-up, excluding studies that only recruited patients with multiple level stenosis.]. Table S9– [Physical function at mid-term follow-up, excluding studies that recruited patients with mixed type stenosis.]. Table S10– [Physical function at mid-term follow-up, excluding studies with high risk of bias.]. Table S11– [Physical function at mid-term follow-up, excluding studies published prior to year 2010.]. Table S12– [Physical function at mid-term follow-up, excluding studies with data imputed from p value, confidence interval, standard error, or median (interquartile ranges).]. Table S13– [Physical function at mid-term follow-up, excluding studies to resolve inconsistency.]. Table S14– [Physical function at long-term follow-up, excluding studies that received commercial fundings.]. Table S15– [Physical function at long-term follow-up, excluding studies that only recruited patients with multiple level stenosis.]. Table S16a– [Physical function at long-term follow-up, excluding studies that recruited patients without degenerative spondylolisthesis (Result 1).]. Table S16b– [Physical function at long-term follow-up, excluding studies that recruited patients without degenerative spondylolisthesis (Result 2).]. Table S17– [Physical function at long-term follow-up, excluding studies that recruited patients with mixed type stenosis.]. Table S18a– [Physical function at long-term follow-up, excluding studies with high risk of bias (Result 1).]. Table S18b– [Physical function at long-term follow-up, excluding studies with high risk of bias (Result 2).]. Table S19– [Physical function at long-term follow-up, excluding studies published prior to year 2010.]. Table S20– [Physical function at long-term follow-up, excluding studies with data imputed from p value, confidence interval, standard error, or median (interquartile ranges).]. Table S21– [Physical function at long-term follow-up, excluding studies to resolve inconsistency.]. Table S22– [Physical function at short-term follow-up, excluding studies with unclear participant description of a history of lumbar spine surgery in the inclusion and exclusion criteria.]. Table S23– [Physical function at mid-term follow-up, excluding studies with unclear participant description of a history of lumbar spine surgery in the inclusion and exclusion criteria.]. Table S24a– [Physical function at long-term follow-up, excluding studies with unclear participant description of a history of lumbar spine surgery in the inclusion and exclusion criteria (Result 1).]. Table S24b– [Physical function at long-term follow-up, excluding studies with unclear participant description of a history of lumbar spine surgery in the inclusion and exclusion criteria (Result 2).]. Table S25– [Physical function at short-term follow-up, adopting an alternative definition of treatment nodes (i.e., traditional surgical approaches, minimally invasive surgical approaches, interspinous process spacer devices).]. Table S26– [Physical function at mid-term follow-up, adopting an alternative definition of treatment nodes (i.e., traditional surgical approaches, minimally invasive surgical approaches, interspinous process spacer devices).]. Table S27– [Physical function at long-term follow-up, adopting an alternative definition of treatment nodes (i.e., traditional surgical approaches, minimally invasive surgical approaches, interspinous process spacer devices).]. Table S28– [Physical function at short-term follow-up, using the change score as the value.]. Table S29– [Physical function at mid-term follow-up, using the change score as the value.]. Table S30– [Physical function at long-term follow-up, using the change score as the value.]. Table S31– [Adverse events due to any reason at study endpoint, excluding studies that received commercial fundings.]. Table S32– [Adverse events due to any reason at study endpoint, excluding studies that only recruited patients with multiple level stenosis.]. Table S33– [Adverse events due to any reason at study endpoint, excluding studies that recruited patients without degenerative spondylolisthesis.]. Table S34– [Adverse events due to any reason at study endpoint, excluding studies that recruited patients with mixed type stenosis.]. Table S35– [Adverse events due to any reason at study endpoint, excluding studies with high risk of bias.]. Table S36– [Adverse events due to any reason at study endpoint, excluding studies published prior to year 2010.]. Table S37– [Adverse events due to any reason at study endpoint, excluding studies where the study endpoints were not long-term.]. Table S38– [Adverse events due to any reason at study endpoint, excluding studies with unclear participant description of a history of lumbar spine surgery in the inclusion and exclusion criteria.]. Table S39– [Adverse events due to any reason at study endpoint, adopting an alternative definition of treatment nodes (i.e., traditional surgical approaches, minimally invasive surgical approaches, interspinous process spacer devices).]. Supplementary N. Comparison-adjusted funnel plots. Figure S1– [Physical function at short-term follow-up.]. Figure S2– [Physical function at mid-term follow-up.]. Figure S3– [Physical function at long-term follow-up.]. Figure S4– [Adverse events due to any reason at study endpoint.]. Figure S5– [Back pain at short-term follow-up.]. Figure S6– [Back pain at mid-term follow-up.]. Figure S7– [Back pain at long-term follow-up.]. Figure S8– [Leg pain at short-term follow-up.]. Figure S9- [Leg pain at mid-term follow-up.]. Figure S10– [Leg pain at long-term follow-up.]. Figure S11– [Reoperation rate at study endpoint.]. Figure S12– [Treatment withdrawal due to any reason at study endpoint.]. Supplementary O. Rank results. Figures S1-S12. Figure S1– [Physical function at short-term follow-up.]. Figure S2– [Physical function at mid-term follow-up.]. Figure S3– [Physical function at long-term follow-up.]. Figure S4– [Adverse events due to any reason at study endpoint.]. Figure S5– [Back pain at short-term follow-up]. Figure S6– [Back pain at mid-term follow-up.]. Figure S7– [Back pain at long-term follow-up.]. Figure S8– [Leg pain at short-term follow-up.]. Figure S9– [Leg pain at mid-term follow-up.]. Figure S10- [Leg pain at long-term follow-up.]. Figure S11– [Reoperation rate at study endpoint.]. Figure S12– [Treatment withdrawal due to any reason at study endpoint.]. Supplementary P. Details on specific adverse events in included studies in the NMA. Supplementary Q. AMSTAR2 rating results on previous NMA.
Acknowledgements
Not applicable.
Abbreviations
- CI
Confidence interval
- CINeMA
The Confidence in Network Meta-Analysis web application
- GRADE
The Grading of Recommendations, Assessment, Development and Evaluation
- LSS
Lumbar spinal stenosis
- MD
Mean difference
- NMA
Network meta-analysis
- OR
Odds ratio
- RCT
Randomized controlled trial
- ROB2
The revised Cochrane risk-of-bias tool for randomized trials
- SUCRA
The surface under the cumulative ranking curve
Authors’ contributions
LC and MLF generated the planning and designed the study. LC and BG developed the study methods. LC and BG did the statistical analysis. LC and BG drafted the manuscript. All authors discussed results, commented on the manuscript, and critically revised the manuscript. Each author contributed important intellectual content during manuscript drafting or revision. SF and HZ are the study guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. All authors read and approved the final manuscript.
Authors’ Twitter handles
MLF: @ProfManuelaF.
Funding
LC is funded by the International Postdoctoral Exchange Fellowship Program (Talent-Introduction Program, YJ20220294) and the China Postdoctoral Science Foundation under Grant Number 2024M751834. MRR is funded by the National Institute for Health and Social Care (NIHR) Manchester Biomedical Research Centre (NIHR203308). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. SF is funded by Taishan Scholars Program of Shandong Province-Pandeng Taishan Scholars (tspd20210320). HZ is funded by funded by Taishan Scholars Program of Shandong Province-Young Taishan Scholars (tsqn201909197) and Cutting Edge Development Fund of Advanced Medical Research Institute (Shandong University). MLF is funded by the National Health and Medical Research Council of Australia Leadership Fellowship.
Availability of data and materials
Requests for data sharing should be sent to the corresponding authors.
Declarations
Ethics approval and consent to participate
Not applicable. This study is a network meta‑analysis, of previously collected data; thus, additional ethical approval was not required.
Consent for publication
All authors agreed to the publication of this manuscript.
Competing interests
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: no support from any organization for the submitted work; MLF provided consulting advice on the scientific advisory board for Novartis, no other relationships or activities that could appear to have influenced the submitted work.
Footnotes
Hengxing Zhou and Shiqing Feng are joint corresponding authors.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lingxiao Chen and Bin Guan contributed equally and are joint first authors.
Contributor Information
Shiqing Feng, Email: shiqingfeng@sdu.edu.cn.
Hengxing Zhou, Email: zhouhengxing@sdu.edu.cn.
References
- 1.Katz JN, Zimmerman ZE, Mass H, Makhni MC. Diagnosis and management of lumbar spinal stenosis: a review. JAMA. 2022;327:1688–99. [DOI] [PubMed] [Google Scholar]
- 2.Tomkins-Lane C, Melloh M, Lurie J, Smuck M, Battié MC, Freeman B, et al. ISSLS Prize Winner: Consensus on the Clinical Diagnosis of Lumbar Spinal Stenosis: Results of an International Delphi Study. Spine (Phila Pa 1976). 2016;41:1239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson DB, Beard DJ, Rannou F, Hunter DJ, Suri P, Chen L, et al. Clinical assessment and management of lumbar spinal stenosis: clinical dilemmas and considerations for surgical referral. Lancet Rheumatol. 2024;6:e727–32. [DOI] [PubMed] [Google Scholar]
- 4.Chen L, Sun Q, Chou R, Anderson DB, Shi B, Chen Y, et al. Low back pain-driven inpatient stays in the United States: a nationwide repeated cross-sectional analysis. Int J Surg.2024;110:1411–9. [DOI] [PMC free article] [PubMed]
- 5.Anderson DB, Luca K, Jensen RK, Eyles JP, Van Gelder JM, Friedly JL, et al. A critical appraisal of clinical practice guidelines for the treatment of lumbar spinal stenosis. Spine J. 2021;21:455–64. [DOI] [PubMed] [Google Scholar]
- 6.Bussières A, Cancelliere C, Ammendolia C, Comer CM, Zoubi FA, Châtillon CE, et al. Non-surgical interventions for lumbar spinal stenosis leading to neurogenic claudication: a clinical practice guideline. J Pain. 2021;22:1015–39. [DOI] [PubMed] [Google Scholar]
- 7.Ho E, Ferreira M, Chen L, Simic M, Ashton-James C, Comachio J, et al. Psychological interventions for chronic non-specific low back pain: protocol of a systematic review with network meta-analysis. BMJ Open. 2020;10:e034996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikolakopoulou A, Higgins JPT, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, et al. CINeMA: An approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17:e1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331:897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan Y, Zhu L. Decompression alone versus fusion and Coflex in the treatment of lumbar degenerative disease: a network meta-analysis. Medicine (Baltimore). 2020;99:e19457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W, Wei H, Zhang R. Different lumbar fusion techniques for lumbar spinal stenosis: a Bayesian network meta-analysis. BMC Surg. 2023;23:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang Z, Xu X, Chen X, Zhuang Y, Wang R, Chen C. Clinical evaluation of surgery for single-segment lumbar spinal stenosis: a systematic review and Bayesian network meta-analysis. Orthop Surg. 2022;14:1281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma H, Hai B, Yan M, Liu X, Zhu B. Evaluation of effectiveness of treatment strategies for degenerative lumbar spinal stenosis: a systematic review and network meta-analysis of clinical studies. World Neurosurg. 2021;152:95–106. [DOI] [PubMed] [Google Scholar]
- 14.Wei FL, Zhou CP, Liu R, Zhu KL, Du MR, Gao HR, et al. Management for lumbar spinal stenosis: a network meta-analysis and systematic review. Int J Surg. 2021;85:19–28. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Lu D, Ji W, He F, Chen AC, Yang H, et al. Which is the most effective treatment for lumbar spinal stenosis: Decompression, fusion, or interspinous process device? A Bayesian network meta-analysis. J Orthop Translat. 2021;26:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–84. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Ferreira PH, Beckenkamp PR, Ferreira ML. Comparative efficacy and safety of surgical and invasive treatments for adults with degenerative lumbar spinal stenosis: protocol for a network meta-analysis and systematic review. BMJ Open. 2019;9:e024752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu C, Ferreira GE, Abdel Shaheed C, Chen Q, Harris IA, Bailey CS, et al. Surgical versus non-surgical treatment for sciatica: systematic review and meta-analysis of randomised controlled trials. BMJ. 2023;381:e070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Busse JW, Wang L, Kamaleldin M, Craigie S, Riva JJ, Montoya L, et al. Opioids for chronic Noncancer pain: a systematic review and meta-analysis. JAMA. 2018;320:2448–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou R, Deyo R, Friedly J, Skelly A, Weimer M, Fu R, et al. Systemic pharmacologic therapies for low back pain: a systematic review for an American college of physicians clinical practice guideline. Ann Intern Med. 2017;166:480–92. [DOI] [PubMed] [Google Scholar]
- 21.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 22.De Crescenzo F, D’Alò GL, Ostinelli EG, Ciabattini M, Di Franco V, Watanabe N, et al. Comparative effects of pharmacological interventions for the acute and long-term management of insomnia disorder in adults: a systematic review and network meta-analysis. Lancet. 2022;400:170–84. [DOI] [PubMed] [Google Scholar]
- 23.Machado GC, Ferreira PH, Yoo RI, Harris IA, Pinheiro MB, Koes BW, et al. Surgical options for lumbar spinal stenosis. Cochrane Database Syst Rev. 2016;11:Cd012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Overdevest GM, Jacobs W, Vleggeert-Lankamp C, Thomé C, Gunzburg R, Peul W. Effectiveness of posterior decompression techniques compared with conventional laminectomy for lumbar stenosis. Cochrane Database Syst Rev. 2015;3:Cd010036. [DOI] [PMC free article] [PubMed]
- 25.Kim JH, Kim YJ, Ryu KS, Kim JS. Comparison of the Clinical and Radiological Outcomes of Full-Endoscopic Laminotomy and Conventional Subtotal Laminectomy for Lumbar Spinal Stenosis: A Randomized Controlled Trial. Global Spine J. 2024;14:1760–70. [DOI] [PMC free article] [PubMed]
- 26.Haddadi K, Ganjeh Qazvini HR. Outcome after surgery of lumbar spinal stenosis: a randomized comparison of bilateral Laminotomy, trumpet laminectomy, and conventional laminectomy. Front Surg. 2016;3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henky J, Yasuda M, Arifin MZ, Takayasu M, Faried A. Trumpet laminectomy microdecompression for lumbal canal stenosis. Asian Spine J. 2014;8:667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Yuan S, Tian Y. Modified unilateral laminotomy for bilateral decompression for lumbar spinal stenosis: technical note. Spine (Phila Pa 1976). 2013;38:E732-737. [DOI] [PubMed] [Google Scholar]
- 29.Hermansen E, Austevoll IM, Hellum C, Storheim K, Myklebust T, Aaen J, et al. Comparison of 3 Different Minimally Invasive Surgical Techniques for Lumbar Spinal Stenosis: A Randomized Clinical Trial. JAMA Netw Open. 2022;5:e224291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel VV, Whang PG, Haley TR, Bradley WD, Nunley PD, Davis RP, et al. Superion interspinous process spacer for intermittent neurogenic claudication secondary to moderate lumbar spinal stenosis: two-year results from a randomized controlled FDA-IDE pivotal trial. Spine (Phila Pa 1976). 2015;40:275–82. [DOI] [PubMed] [Google Scholar]
- 31.Yi X, McPherson B. Application of X STOP device in the treatment of lumbar spinal stenosis. Pain Physician. 2010;13:E327-336. [PubMed] [Google Scholar]
- 32.Loguidice V, Bini W, Shabat S, Miller LE, Block JE. Rationale, design and clinical performance of the Superion® Interspinous Spacer: a minimally invasive implant for treatment of lumbar spinal stenosis. Expert Rev Med Devices. 2011;8:419–26. [DOI] [PubMed] [Google Scholar]
- 33.Moojen WA, Arts MP, Jacobs WC, van Zwet EW, van den Akker-van Marle ME, Koes BW, et al. Interspinous process device versus standard conventional surgical decompression for lumbar spinal stenosis: randomized controlled trial. BMJ. 2013;347:f6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moojen WA, Arts MP, Brand R, Koes BW, Peul WC. The Felix-trial. Double-blind randomization of interspinous implant or bony decompression for treatment of spinal stenosis related intermittent neurogenic claudication. BMC Musculoskelet Disord. 2010;11:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aygun H, Abdulshafi K. Unilateral Biportal Endoscopy Versus Tubular Microendoscopy in Management of Single Level Degenerative Lumbar Canal Stenosis: a prospective study. Clin Spine Surg. 2021;34:E323-e328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bae HW, Davis RJ, Lauryssen C, Leary S, Maislin G, Musacchio MJ Jr. Three-Year Follow-up of the Prospective, Randomized, Controlled Trial of Coflex Interlaminar Stabilization vs Instrumented Fusion in Patients With Lumbar Stenosis. Neurosurgery. 2016;79:169–81. [DOI] [PubMed] [Google Scholar]
- 37.Davis RJ, Errico TJ, Bae H, Auerbach JD. Decompression and Coflex interlaminar stabilization compared with decompression and instrumented spinal fusion for spinal stenosis and low-grade degenerative spondylolisthesis: two-year results from the prospective, randomized, multicenter, Food and Drug Administration Investigational Device Exemption trial. Spine (Phila Pa 1976). 2013;38:1529–39. [DOI] [PubMed] [Google Scholar]
- 38.Musacchio MJ, Lauryssen C, Davis RJ, Bae HW, Peloza JH, Guyer RD, et al. Evaluation of decompression and Interlaminar stabilization compared with decompression and fusion for the treatment of lumbar spinal stenosis: 5-year Follow-up of a Prospective, Randomized. Controlled. Trial Int J Spine Surg. 2016;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borg A, Hill CS, Nurboja B, Critchley G, Choi D. A randomized controlled trial of the X-Stop interspinous distractor device versus laminectomy for lumbar spinal stenosis with 2-year quality-of-life and cost-effectiveness outcomes. J Neurosurg Spine. 2021;34:544. [DOI] [PubMed] [Google Scholar]
- 40.Carrascosa-Granada A, Velazquez W, Wagner R, Saab Mazzei A, Vargas-Jimenez A, Jorquera M, et al. Comparative Study Between Uniportal Full-Endoscopic Interlaminar and Tubular Approach in the Treatment of Lumbar Spinal Stenosis: a Pilot Study. Global spine journal. 2020;10:70S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Süner HI, Castaño JP, Vargas-Jimenez A, Wagner R, Mazzei AS, Velazquez W, et al. Comparison of the Tubular Approach and Uniportal Interlaminar Full-Endoscopic Approach in the Treatment of Lumbar Spinal Stenosis: Our 3-Year Results. World Neurosurg. 2023;173:e148–55. [DOI] [PubMed] [Google Scholar]
- 42.Cavuşoğlu H, Kaya RA, Türkmenoglu ON, Tuncer C, Colak I, Aydin Y. Midterm outcome after unilateral approach for bilateral decompression of lumbar spinal stenosis: 5-year prospective study. Eur Spine J. 2007;16:2133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Celik SE, Celik S, Göksu K, Kara A, Ince I. Microdecompressive laminatomy with a 5-year follow-up period for severe lumbar spinal stenosis. J Spinal Disord Tech. 2010;23:229–35. [DOI] [PubMed] [Google Scholar]
- 44.Cho DY, Lin HL, Lee WY, Lee HC. Split-spinous process laminotomy and discectomy for degenerative lumbar spinal stenosis: a preliminary report. J Neurosurg Spine. 2007;6:229–39. [DOI] [PubMed] [Google Scholar]
- 45.Ghogawala Z, Dziura J, Butler WE, Dai F, Terrin N, Magge SN, et al. Laminectomy plus Fusion versus Laminectomy Alone for Lumbar Spondylolisthesis. N Engl J Med. 2016;374:1424–34. [DOI] [PubMed] [Google Scholar]
- 46.Gurelik M, Bozkina C, Kars Z, Karadag O, Ozum U, Bayrakli F. Unilateral Laminotomy for decompression of lumbar stenosis is effective and safe: a prospective randomized comparative study. J Neurol Sci Turkish. 2012;29:744–53. [Google Scholar]
- 47.Hamawandi SA, Sulaiman, II, Al-Humairi AK. Microdecompression versus Open Laminectomy and Posterior Stabilization for Multilevel Lumbar Spine Stenosis: A Randomized Controlled Trial. Pain Res Manag. 2019;2019:1–6. [DOI] [PMC free article] [PubMed]
- 48.Hermansen E, Austevoll IM, Hellum C, Storheim K, Myklebust TÅ, Aaen J, et al. Comparison of 3 Different Minimally Invasive Surgical Techniques for Lumbar Spinal Stenosis: a randomized clinical trial. JAMA Netw Open. 2022;5:e224291–e224291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kang T, Park S, Young MdP, Kang C, Lee S, Hyuck MdP, et al. Is biportal technique/endoscopic spinal surgery satisfactory for lumbar spinal stenosis patients?: A prospective randomized comparative study. Medicine (Baltimore). 2019;98:e15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ko S, Oh T. Comparison of bilateral decompression via unilateral laminotomy and conventional laminectomy for single-level degenerative lumbar spinal stenosis regarding low back pain, functional outcome, and quality of life - a randomized controlled, prospective trial. J Orthop Surg Res. 2019;14:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Komp M, Hahn P, Oezdemir S, Giannakopoulos A, Heikenfeld R, Kasch R, et al. Bilateral spinal decompression of lumbar central stenosis with the full-endoscopic interlaminar versus microsurgical laminotomy technique: a prospective, randomized, controlled study. Pain Physician. 2015;18:61–70. [PubMed] [Google Scholar]
- 52.Kotheeranurak V, Tangdamrongtham T, Lin GX, Singhatanadgige W, Limthongkul W, Yingsakmongkol W, et al. Comparison of full-endoscopic and tubular-based microscopic decompression in patients with lumbar spinal stenosis: a randomized controlled trial. Eur Spine J. 2023;32:2736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lønne G, Johnsen LG, Rossvoll I, Andresen H, Storheim K, Zwart JA, et al. Minimally invasive decompression versus x-stop in lumbar spinal stenosis: a randomized controlled multicenter study. Spine (Phila Pa 1976). 2015;40:77–85. [DOI] [PubMed] [Google Scholar]
- 54.Mobbs RJ, Li J, Sivabalan P, Raley D, Rao PJ. Outcomes after decompressive laminectomy for lumbar spinal stenosis: comparison between minimally invasive unilateral laminectomy for bilateral decompression and open laminectomy: clinical article. J Neurosurg Spine. 2014;21:179–86. [DOI] [PubMed] [Google Scholar]
- 55.Moojen WA, Arts MP, Jacobs WC, van Zwet EW, van den Akker-van Marle ME, Koes BW, et al. IPD without bony decompression versus conventional surgical decompression for lumbar spinal stenosis: 2-year results of a double-blind randomized controlled trial. Eur Spine J. 2015;24:2295–305. [DOI] [PubMed] [Google Scholar]
- 56.Schenck CD, Terpstra SES, Moojen WA, van Zwet E, Peul W, Arts MP, et al. Interspinous process device versus conventional decompression for lumbar spinal stenosis: 5-year results of a randomized controlled trial. J Neurosurg Spine. 2022;36:909–17. [DOI] [PubMed] [Google Scholar]
- 57.Park SM, Kim GU, Kim HJ, Choi JH, Chang BS, Lee CK, et al. Is the Use of a Unilateral Biportal Endoscopic Approach Associated with Rapid Recovery After Lumbar Decompressive Laminectomy? A Preliminary Analysis of a Prospective Randomized Controlled Trial. World Neurosurg. 2019;128:e709–18. [DOI] [PubMed] [Google Scholar]
- 58.Park S-M, Park J, Jang HS, Heo YW, Han H, Kim H-J, et al. Biportal endoscopic versus microscopic lumbar decompressive laminectomy in patients with spinal stenosis: a randomized controlled trial. Spine Journal. 2020;20:156–65. [DOI] [PubMed] [Google Scholar]
- 59.Patel VV, Whang PG, Haley TR, Bradley WD, Nunley PD, Davis RP, et al. Superion interspinous process spacer for intermittent neurogenic claudication secondary to moderate lumbar spinal stenosis: two-year results from a randomized controlled FDA-IDE pivotal trial. Spine. 2015;40:275–82. [DOI] [PubMed] [Google Scholar]
- 60.Rajasekaran S, Thomas A, Kanna RM, Prasad Shetty A. Lumbar spinous process splitting decompression provides equivalent outcomes to conventional midline decompression in degenerative lumbar canal stenosis: a prospective, randomized controlled study of 51 patients. Spine (Phila Pa 1976). 2013;38:1737–43. [DOI] [PubMed] [Google Scholar]
- 61.Simon RB, Dowe C, Grinberg S, Cammisa FP, Abjornson C. The 2-level experience of interlaminar stabilization: 5-year follow-up of a prospective, randomized clinical experience compared to fusion for the sustainable management of spinal stenosis. Int J Spine Surg. 2018;12:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soliman MAR, Ali A. Decompression of lumbar canal stenosis with a bilateral interlaminar versus classic laminectomy technique: a prospective randomized study. Neurosurg Focus. 2019;46:E3. [DOI] [PubMed] [Google Scholar]
- 63.Thomé C, Zevgaridis D, Leheta O, Bäzner H, Pöckler-Schöniger C, Wöhrle J, et al. Outcome after less-invasive decompression of lumbar spinal stenosis: a randomized comparison of unilateral laminotomy, bilateral laminotomy, and laminectomy. J Neurosurg Spine. 2005;3:129–41. [DOI] [PubMed] [Google Scholar]
- 64.Yagi M, Okada E, Ninomiya K, Kihara M. Postoperative outcome after modified unilateral-approach microendoscopic midline decompression for degenerative spinal stenosis. J Neurosurg Spine. 2009;10:293–9. [DOI] [PubMed] [Google Scholar]
- 65.Azzazi A, Elhawary Y. Dynamic Stabilization Using X-stop Versus Transpedicular Screw Fixation in the Treatment of Lumbar Canal Stenosis; Comparative Study of the Clinical Outcome. Neurosurg Q. 2010;20:165–9. [Google Scholar]
- 66.Cuo AJ, Li ZY, Wang DY, Jia SL, Zhang WH. Clinical efficacy of percutaneous endoscopic bilateral decompression via unilateral interlaminar approach in the treatment of degenerative lumbar spinal stenosis. Indian J Pharm Sci. 2021;83:62. [Google Scholar]
- 67.Grob D, Humke T, Dvorak J. Degenerative lumbar spinal stenosis. Decompression with and without arthrodesis. J Bone Joint Surg Am. 1995;77:1036–41. [DOI] [PubMed] [Google Scholar]
- 68.Hu D, Fei J, Chen G, Yu Y, Lai Z. Treatment for lumbar spinal stenosis in elderly patients using percutaneous endoscopic lumbar discectomy combined with postoperative three-dimensional traction. Expert Rev Med Devices. 2019;16:317. [DOI] [PubMed] [Google Scholar]
- 69.Marsh GD, Mahir S, Leyte A. A prospective randomised controlled trial to assess the efficacy of dynamic stabilisation of the lumbar spine with the Wallis ligament. Eur Spine J. 2014;23:2156–60. [DOI] [PubMed] [Google Scholar]
- 70.Ruetten S, Komp M, Merk H, Godolias G. Surgical treatment for lumbar lateral recess stenosis with the full-endoscopic interlaminar approach versus conventional microsurgical technique: a prospective, randomized, controlled study. J Neurosurg Spine. 2009;10:476–85. [DOI] [PubMed] [Google Scholar]
- 71.Försth P, Ólafsson G, Carlsson T, Frost A, Borgström F, Fritzell P, et al. A Randomized, Controlled Trial of Fusion Surgery for Lumbar Spinal Stenosis. N Engl J Med. 2016;374:1413–23. [DOI] [PubMed] [Google Scholar]
- 72.Meyer B, Baranto A, Schils F, Collignon F, Zoega B, Tan L, et al. Percutaneous Interspinous Spacer vs Decompression in Patients with Neurogenic Claudication: an Alternative in Selected Patients? Neurosurgery. 2018;82:621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmidt S, Franke J, Rauschmann M, Adelt D, Bonsanto MM, Sola S. Prospective, randomized, multicenter study with 2-year follow-up to compare the performance of decompression with and without interlaminar stabilization. J Neurosurg Spine. 2018;28:406. [DOI] [PubMed] [Google Scholar]
- 74.Strömqvist BH, Berg S, Gerdhem P, Johnsson R, Möller A, Sahlstrand T, et al. X-stop versus decompressive surgery for lumbar neurogenic intermittent claudication: randomized controlled trial with 2-year follow-up. Spine (Phila Pa 1976). 2013;38:1436–42. [DOI] [PubMed] [Google Scholar]
- 75.Cheng X, Zhang K, Sun X, Tian H, Zhao C, Zhao J. Unilateral versus bilateral pedicle screw fixation with transforaminal lumbar interbody fusion for treatment of lumbar foraminal stenosis. Spine J. 2022;22:1687–93. [DOI] [PubMed] [Google Scholar]
- 76.Hallett A, Huntley JS, Gibson JN. Foraminal stenosis and single-level degenerative disc disease: a randomized controlled trial comparing decompression with decompression and instrumented fusion. Spine (Phila Pa 1976). 2007;32:1375–80. [DOI] [PubMed] [Google Scholar]
- 77.Truszczyńska A, Rąpała K, Łukawski S, Trzaskoma Z, Tarnowski A, Drzal-Grabiec J, et al. Evaluation of functional outcomes in individuals 10 years after posterior lumbar interbody fusion with corundum implants and decompression: a comparison of 2 surgical techniques. Med Sci Monit. 2014;20:1400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y, Zhu H, Zhou Z, Wu J, Sun Y, Shen X, et al. Comparison between Percutaneous Transforaminal Endoscopic Discectomy and Fenestration in the Treatment of Degenerative Lumbar Spinal Stenosis. Med Sci Monit. 2020;26:e926631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Škoro I, Stančić M, Kovačević M, Đurić KS. Long-Term Results and Efficacy of Laminectomy with Fusion Versus Young Laminoplasty for the Treatment of Degenerative Spinal Stenosis. World Neurosurg. 2016;89:387–92. [DOI] [PubMed] [Google Scholar]
- 80.IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ho EK, Chen L, Simic M, Ashton-James CE, Comachio J, Wang DXM, et al. Psychological interventions for chronic, non-specific low back pain: systematic review with network meta-analysis. BMJ. 2022;376:e067718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salanti G, Nikolakopoulou A, Efthimiou O, Mavridis D, Egger M, White IR. Introducing the Treatment Hierarchy Question in Network Meta-Analysis. Am J Epidemiol. 2022;191:930–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3:98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Noetel M, Sanders T, Gallardo-Gómez D, Taylor P, Del Pozo CB, van den Hoek D, et al. Effect of exercise for depression: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2024;384:e075847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rickers KW, Pedersen PH, Tvedebrink T, Eiskjær SP. Comparison of interventions for lumbar disc herniation: a systematic review with network meta-analysis. Spine J. 2021;21:1750–62. [DOI] [PubMed] [Google Scholar]
- 86.Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. Bmj. 2011;342:d549. [DOI] [PubMed] [Google Scholar]
- 87.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Findlay MC, Hamrick FA, Kim RB, Twitchell S, Mahan MA. Hospital cost differences between open and endoscopic lumbar spine decompression surgery. J Neurosurg Spine. 2024;40:77–83. [DOI] [PubMed] [Google Scholar]
- 89.Gadjradj PS, Broulikova HM, van Dongen JM, Rubinstein SM, Depauw PR, Vleggeert C, et al. Cost-effectiveness of full endoscopic versus open discectomy for sciatica. Br J Sports Med. 2022;56:1018–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010;340:c221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. PRISMA NMA checklist.
Additional file 2: Supplementary A. Deviations from the initial protocol. Table S1. [Initial treatment nodes classification]. Table S2. [Adjustments to pre-specified treatment nodes.]. Table S3. [Revised treatment node classification.]. Supplementary B. Search strategies. Supplementary C. Descriptive results on protocol planned outcomes not included in the NMA. Supplementary D. Individual characteristics of included studies. Table S1. [Individual study design characteristics and publication characteristics of included studies in the NMA]. Table S2. [Individual patient characteristics of included studies in the NMA]. Table S3. [Individual study design characteristics and publication characteristics of included studies in the systematic review but excluded in the NMA]. Table S4. [Individual patient characteristics of included studies in the systematic review but excluded in the NMA.]. Table S5. [Individual description on participant inclusion and exclusion criteria of included studies in the NMA.]. Supplementary E. Studies not included in the NMA. Table S1. [Effect estimates for studies included in the systematic review but excluded in the NMA.]. Supplementary F. Assessment of transitivity. Table S1. [Assessment of transitivity: network of interventions for physical function.]. Table S2. [Assessment of transitivity: network of interventions for adverse events due to any reason.]. Supplementary G. Results of meta-regression. Table S1. [Results of meta-regression for physical function.]. Table S2. [Results of meta-regression for adverse events due to any reason.]. Table S3. [ Results of meta-regression for back pain.]. Table S4. [Results of meta-regression for leg pain.]. Table S5. [Results of meta-regression for reoperation rate.]. Table S6. [Results of meta-regression for treatment withdrawal due to any reason.]. Supplementary H. Risk of bias judgments. Table S1. [Assessing physical function: studies included in the NMA.]. Table S2. [Assessing physical function: studies included in the systematic review but excluded in the NMA.]. Table S3. [Assessing adverse events due to any reason: studies included in the NMA.]. Table S4. [Assessing adverse events due to any reason: studies included in the systematic review but excluded in the NMA.]. Table S5– [Assessing back pain: studies included in the NMA.]. Table S6– [Assessing back pain: studies included in the systematic review but excluded in the NMA.]. Table S7– [Assessing leg pain: studies included in the NMA.]. Table S8– [Assessing leg pain: studies included in the systematic review but excluded in the NMA.]. Table S9– [Assessing reoperation rate: studies included in the NMA.]. Table S10– [Assessing reoperation rate: studies included in the systematic review but excluded in the NMA.]. Table S11– [Assessing treatment withdrawal due to any reason: studies included in the NMA.]. Table S12– [Assessing treatment withdrawal due to any reason: studies included in the systematic review but excluded in the NMA.]. Supplementary I. Assessment of global inconsistency. Table S1– [Physical function at short-term follow-up.]. Table S2a– [Physical function at mid-term follow-up (primary analysis).]. Table S2b– [Physical function at mid-term follow-up (sensitivity analysis conducted to resolve inconsistency).]. Table S3a– [Physical function at long-term follow-up (sensitivity analysis conducted to resolve inconsistency).]. Table S3b– [Physical function at long-term follow-up (sensitivity analysis conducted to resolve inconsistency).]. Table S4– [Adverse events due to any reason at study endpoint.]. Table S5a– [Back pain at short-term follow-up (primary analysis).]. Table S5b– [Back pain at short-term follow-up (sensitivity analysis conducted to resolve inconsistency).]. Table S6a– [Back pain at mid-term follow-up (primary analysis).]. Table S6b– [Back pain at mid-term follow-up (sensitivity analysis conducted to resolve inconsistency).]. Table S7– [Back pain at long-term follow-up.]. Table S8a– [Leg pain at short-term follow-up (primary analysis).]. Table S8b– [Leg pain at short-term follow-up (sensitivity analysis conducted to resolve inconsistency).]. Table S9– [Leg pain at mid-term follow-up.]. Table S10a– [Leg pain at long-term follow-up (primary analysis).]. Table S10b– [Leg pain at long-term follow-up (sensitivity analysis conducted to resolve inconsistency).]. Table S11– [Reoperation rate at study endpoint.]. Table S12– [Treatment withdrawal due to any reason at study endpoint.]. Supplementary J. Assessment of local inconsistency. Tables S1-S12. Table S1– [Physical function at short-term follow-up.]. Table S2a– [Physical function at mid-term follow-up (primary analysis).]. Table S2b– [Physical function at mid-term follow-up (sensitivity analysis conducted to resolve inconsistency).]. Table S3a– [Physical function at long-term follow-up (primary analysis).]. Table S3b– [Physical function at long-term follow-up (sensitivity analysis conducted to resolve inconsistency).]. Table S4– [Adverse events due to any reason at study endpoint.]. Table S5a– [Back pain at short-term follow-up (primary analysis).]. Table S5b– [Back pain at short-term follow-up (sensitivity analysis conducted to resolve inconsistency).]. Table S6a– [Back pain at mid-term follow-up (primary analysis).]. Table S6b– [Back pain at mid-term follow-up (sensitivity analysis conducted to resolve inconsistency).]. Table S7– [Back pain at long-term follow-up.]. Table S8a– [Leg pain at short-term follow-up (primary analysis).]. Table S8b– [Leg pain at short-term follow-up (sensitivity analysis conducted to resolve inconsistency).]. Table S9– [Leg pain at mid-term follow-up.]. Table S10a– [Leg pain at long-term follow-up (primary analysis).]. Table S10b– [Leg pain at long-term follow-up (sensitivity analysis conducted to resolve inconsistency).]. Table S11– [Reoperation rate at study endpoint.]. Table S12– [Treatment withdrawal due to any reason at study endpoint.]. Supplementary K. CINeMA results. Table S1– [Physical function at short-term follow-up.]. Table S2– [Physical function at mid-term follow-up.]. Table S3– [Physical function at long-term follow-up.]. Table S4– [Adverse events due to any reason at study endpoint.]. Table S5– [Back pain at short-term follow-up.]. Table S6– [Back pain at mid-term follow-up.]. Table S7– [Back pain at long-term follow-up.]. Table S8– [Leg pain at short-term follow-up.]. Table S9– [Leg pain at mid-term follow-up.]. Table S10– [Leg pain at long-term follow-up.]. Table S11– [Reoperation rate at study endpoint.]. Table S12– [Treatment withdrawal due to any reason at study endpoint.]. Supplementary L. Results from the NMA. Figure S1– [Network plot for physical function at mid-term follow-up.]. Figure S2– [Network plot for back pain at short-term follow-up.]. Figure S3– [Network plot for back pain at mid-term follow-up.]. Figure S4– [Network plot for back pain at long-term follow-up.]. Figure S5– [Network plot for leg pain at short-term follow-up.]. Figure S6– [Network plot for leg pain at mid-term follow-up.]. Figure S7– [Network plot for leg pain at long-term follow-up.]. Figure S8– [Network plot for reoperation rate at study endpoint.]. Figure S9– [Network plot for treatment withdrawal due to any reason at study endpoint.]. Table S1– [Physical function at mid-term follow-up.]. Table S2– [Back pain at short-term follow-up.]. Table S3– [Back pain at mid-term follow-up.]. Table S4– [Back pain at long-term follow-up.]. Table S5– [Leg pain at short-term follow-up.]. Table S6– [Leg pain at mid-term follow-up.]. Table S7– [Leg pain at long-term follow-up.]. Table S8– [Reoperation rate at study endpoint.]. Tables S9– [Treatment withdrawal due to any reason at study endpoint.]. Supplementary M. Sensitivity analyses. Table S1– [Physical function at short-term follow-up, excluding studies that received commercial fundings.]. Table S2– [Physical function at short-term follow-up, excluding studies that only recruited patients with multiple level stenosis.]. Table S3– [Physical function at short-term follow-up, excluding studies that recruited patients with mixed type stenosis.]. Table S4– [Physical function at short-term follow-up, excluding studies with high risk of bias.]. Table S5a– [Physical function at short-term follow-up, excluding studies published prior to year 2010 (Result 1).]. Table S5b– [Physical function at short-term follow-up, excluding studies published prior to year 2010 (Result 2).]. Table S6– [Physical function at short-term follow-up, excluding studies with data imputed from p value, confidence interval, standard error, or median (interquartile ranges).]. Table S7– [Physical function at mid-term follow-up, excluding studies that received commercial fundings.]. Table S8– [Physical function at mid-term follow-up, excluding studies that only recruited patients with multiple level stenosis.]. Table S9– [Physical function at mid-term follow-up, excluding studies that recruited patients with mixed type stenosis.]. Table S10– [Physical function at mid-term follow-up, excluding studies with high risk of bias.]. Table S11– [Physical function at mid-term follow-up, excluding studies published prior to year 2010.]. Table S12– [Physical function at mid-term follow-up, excluding studies with data imputed from p value, confidence interval, standard error, or median (interquartile ranges).]. Table S13– [Physical function at mid-term follow-up, excluding studies to resolve inconsistency.]. Table S14– [Physical function at long-term follow-up, excluding studies that received commercial fundings.]. Table S15– [Physical function at long-term follow-up, excluding studies that only recruited patients with multiple level stenosis.]. Table S16a– [Physical function at long-term follow-up, excluding studies that recruited patients without degenerative spondylolisthesis (Result 1).]. Table S16b– [Physical function at long-term follow-up, excluding studies that recruited patients without degenerative spondylolisthesis (Result 2).]. Table S17– [Physical function at long-term follow-up, excluding studies that recruited patients with mixed type stenosis.]. Table S18a– [Physical function at long-term follow-up, excluding studies with high risk of bias (Result 1).]. Table S18b– [Physical function at long-term follow-up, excluding studies with high risk of bias (Result 2).]. Table S19– [Physical function at long-term follow-up, excluding studies published prior to year 2010.]. Table S20– [Physical function at long-term follow-up, excluding studies with data imputed from p value, confidence interval, standard error, or median (interquartile ranges).]. Table S21– [Physical function at long-term follow-up, excluding studies to resolve inconsistency.]. Table S22– [Physical function at short-term follow-up, excluding studies with unclear participant description of a history of lumbar spine surgery in the inclusion and exclusion criteria.]. Table S23– [Physical function at mid-term follow-up, excluding studies with unclear participant description of a history of lumbar spine surgery in the inclusion and exclusion criteria.]. Table S24a– [Physical function at long-term follow-up, excluding studies with unclear participant description of a history of lumbar spine surgery in the inclusion and exclusion criteria (Result 1).]. Table S24b– [Physical function at long-term follow-up, excluding studies with unclear participant description of a history of lumbar spine surgery in the inclusion and exclusion criteria (Result 2).]. Table S25– [Physical function at short-term follow-up, adopting an alternative definition of treatment nodes (i.e., traditional surgical approaches, minimally invasive surgical approaches, interspinous process spacer devices).]. Table S26– [Physical function at mid-term follow-up, adopting an alternative definition of treatment nodes (i.e., traditional surgical approaches, minimally invasive surgical approaches, interspinous process spacer devices).]. Table S27– [Physical function at long-term follow-up, adopting an alternative definition of treatment nodes (i.e., traditional surgical approaches, minimally invasive surgical approaches, interspinous process spacer devices).]. Table S28– [Physical function at short-term follow-up, using the change score as the value.]. Table S29– [Physical function at mid-term follow-up, using the change score as the value.]. Table S30– [Physical function at long-term follow-up, using the change score as the value.]. Table S31– [Adverse events due to any reason at study endpoint, excluding studies that received commercial fundings.]. Table S32– [Adverse events due to any reason at study endpoint, excluding studies that only recruited patients with multiple level stenosis.]. Table S33– [Adverse events due to any reason at study endpoint, excluding studies that recruited patients without degenerative spondylolisthesis.]. Table S34– [Adverse events due to any reason at study endpoint, excluding studies that recruited patients with mixed type stenosis.]. Table S35– [Adverse events due to any reason at study endpoint, excluding studies with high risk of bias.]. Table S36– [Adverse events due to any reason at study endpoint, excluding studies published prior to year 2010.]. Table S37– [Adverse events due to any reason at study endpoint, excluding studies where the study endpoints were not long-term.]. Table S38– [Adverse events due to any reason at study endpoint, excluding studies with unclear participant description of a history of lumbar spine surgery in the inclusion and exclusion criteria.]. Table S39– [Adverse events due to any reason at study endpoint, adopting an alternative definition of treatment nodes (i.e., traditional surgical approaches, minimally invasive surgical approaches, interspinous process spacer devices).]. Supplementary N. Comparison-adjusted funnel plots. Figure S1– [Physical function at short-term follow-up.]. Figure S2– [Physical function at mid-term follow-up.]. Figure S3– [Physical function at long-term follow-up.]. Figure S4– [Adverse events due to any reason at study endpoint.]. Figure S5– [Back pain at short-term follow-up.]. Figure S6– [Back pain at mid-term follow-up.]. Figure S7– [Back pain at long-term follow-up.]. Figure S8– [Leg pain at short-term follow-up.]. Figure S9- [Leg pain at mid-term follow-up.]. Figure S10– [Leg pain at long-term follow-up.]. Figure S11– [Reoperation rate at study endpoint.]. Figure S12– [Treatment withdrawal due to any reason at study endpoint.]. Supplementary O. Rank results. Figures S1-S12. Figure S1– [Physical function at short-term follow-up.]. Figure S2– [Physical function at mid-term follow-up.]. Figure S3– [Physical function at long-term follow-up.]. Figure S4– [Adverse events due to any reason at study endpoint.]. Figure S5– [Back pain at short-term follow-up]. Figure S6– [Back pain at mid-term follow-up.]. Figure S7– [Back pain at long-term follow-up.]. Figure S8– [Leg pain at short-term follow-up.]. Figure S9– [Leg pain at mid-term follow-up.]. Figure S10- [Leg pain at long-term follow-up.]. Figure S11– [Reoperation rate at study endpoint.]. Figure S12– [Treatment withdrawal due to any reason at study endpoint.]. Supplementary P. Details on specific adverse events in included studies in the NMA. Supplementary Q. AMSTAR2 rating results on previous NMA.
Data Availability Statement
Requests for data sharing should be sent to the corresponding authors.