Abstract
Background
Patients receiving methadone, buprenorphine, and naltrexone for either chronic pain or substance use disorder (SUD) pose perioperative challenges. Due to their complex pharmacology, perioperative recommendations continue to evolve. Deviations from these recommendations may result in worse perioperative outcomes. A formal preoperative evaluation (POE) and optimization of patients on these medications are recommended to address these concerns.
Methods
A single-center retrospective electronic health record review was performed with adult patients on methadone, buprenorphine, and naltrexone undergoing elective surgery between January 1, 2010 and December 31, 2020. The primary outcome of interest was the percentage of patients referred to the POE clinic for evaluation prior to the scheduled elective surgery. In addition, we assessed differences in variables (perioperative opioid, hospital length of stay, perioperative multimodal analgesics, perioperative complications, inpatient pain service consult, readmission within 30 days, cancellation of surgery, addiction medicine consult) based on POE clinic evaluation. This analysis was performed separately for patients prescribed these medications for SUD versus chronic pain. Continuous outcomes were analyzed using linear regression with generalized estimating equations (GEE) and robust variance estimates.
Results
A total of 714 patients were included in the final analysis, of which 572 (80%) took buprenorphine, methadone, or naltrexone for chronic pain and 142 (20%) took these medications for SUD. Within the chronic pain and SUD subpopulations, 193 (34%) and 35 (25%) patients had formal POE clinic assessments, respectively. Among those taking these medications for chronic pain, POE clinic evaluation was associated with a higher likelihood for receiving non-opioid multimodal analgesics perioperatively (p = 0.016).
Conclusion
Formal preoperative evaluations are currently underutilized in patients who take buprenorphine, methadone, or naltrexone for chronic pain or SUD. These patients may benefit from POE clinic assessment to optimize perioperative outcomes.
Keywords: preoperative evaluation, chronic opioid therapy, substance use disorder, naltrexone, buprenorphine, methadone, perioperative pain management
Introduction
It is estimated that roughly 19.6 million Americans suffer from chronic pain that negatively impacts their quality of life and work.1 Additionally, roughly 2.5 million in the United States have opioid use disorder (OUD) according to the 2020 National Survey on Drug Use and Health, and incident prescription opioid use also continues to remain high.2,3 Buprenorphine, methadone, and naltrexone are approved by the US Food and Drug Administration (FDA) for the treatment of OUD.4 These medications are also used in the management of refractory chronic pain and for the treatment of alcohol use disorder. The various indications of these medications are dependent on dose and frequency of use given differences in pharmacodynamics. Given the unique pharmacology of these three medications along with evolving perioperative recommendations, patients who take these medications for either chronic pain or substance use disorder (SUD) often require special perioperative considerations to ensure adequate management of acute pain and minimize withdrawal and relapse.4–7 It is currently recommended that patients maintain methadone and buprenorphine which are mu-opioid receptor agonists while discontinuing naltrexone, a mu-opioid receptor antagonist, in the perioperative period. More importantly, multimodal analgesia is highly recommended for patients on these medications for adequate pain control and relapse prevention.4,8 Further, a substantial portion of surgeons and anesthesiologists report discomfort or unfamiliarity with caring for patients who take these medications.9–11 It is therefore imperative that these patients are identified and optimized preoperatively to limit adverse perioperative outcomes.
It has been shown that Preoperative Evaluation (POE) Clinics may lead to improved perioperative outcomes by decreasing same-day surgical cancellations, costs associated with unnecessary testing, in-hospital mortality rates, and hospital length of stay.12–14 Despite the well-known advantages of POE clinic assessments, its impact on patients utilizing buprenorphine, methadone, and naltrexone for either chronic pain or SUD have not been studied. A recent comprehensive review highlighted preoperative management of various types of surgical patients but did not address patients on these unique medications.15 At our institution, patients referred to the POE Clinic typically undergo a thorough assessment and are provided with the most UpToDate perioperative recommendations of medications which could have significant perioperative implications. Subsequently, these recommendations influence the care received in the intraoperative and postoperative period by patients on these medications.
The aim of this study was to determine the overall rate of POE clinic referral and utilization in patients on buprenorphine, methadone, and naltrexone for SUD or chronic pain. Additionally, we sought to determine if POE clinic referral improved perioperative analgesic through the use of multimodal analgesia and clinical outcomes for patients on these medications. We hypothesized that patients on buprenorphine, methadone, and naltrexone for SUD or chronic pain evaluated in the POE clinic would have better perioperative analgesic outcomes compared to those who were not evaluated in the POE clinic.
Materials and Methods
Selection Criteria
We performed a single-center retrospective review of electronic health records of adult patients who underwent elective surgery between January 1, 2010 and December 31, 2020, at Mayo Clinic in Rochester, Minnesota. Inclusion criteria included any patient who was scheduled for elective surgery during this time period who took methadone, buprenorphine, or naltrexone as an outpatient before the surgery for an indication of SUD or chronic pain. For the purposes of this study, patients taking these medications for SUD included patients with the diagnoses of opioid use disorder and/or alcohol use disorder. Patients who were admitted prior to surgery and emergent surgical procedures were excluded from this study as well as any patients taking these medications for indications other than SUD or chronic pain. In addition, procedures that occurred within 30 days of the most recent procedure were excluded as a repeat history and physical was not required for the subsequent procedure.
Outcomes and Data Extraction
The Anesthesia Clinical Research Unit collected chart data of patients on these medications who received monitored anesthesia care or general anesthesia. All perioperative outcomes were stratified based on if the patient was evaluated in the POE clinic before elective surgery. Additionally, analyses were performed separately for patients who took these medications for chronic pain versus those who took these medications for SUD.
The primary outcome of interest was the percentage of all included patients who were referred to the POE clinic for evaluation prior to the scheduled elective surgery. Another primary outcome of interest was difference in analgesic and clinical variables based on if the patient was evaluated in the POE clinic. Specific perioperative analgesic and clinical variables that were extracted included: total perioperative opioids (in oral morphine equivalents [OMEs]), hospital length of stay (in days), use of perioperative multimodal analgesics (yes/no), presence of perioperative complications (yes/no), postoperative consultation with the inpatient pain service (yes/no), readmission within 30 days (yes/no), cancellation of surgery (yes/no), and postoperative consultation with addiction medicine (yes/no).
Postoperative complication was defined as at least one of the following: postoperative cerebrovascular accident, postoperative deep vein thrombosis, postoperative pulmonary embolism, postoperative renal replacement therapy, in-hospital mortality, 30-day mortality, 90-day mortality, or admitted to ICU later in hospitalization. Additional, baseline demographic and clinical covariates that were extracted included: age, sex, race, body mass index (BMI), surgery duration (in minutes), Charlson comorbidity index score, use of regional block (yes/no), surgery type (general, neurosurgery, orthopedic, urology, and other), and American Society of Anesthesiologists’ (ASA) type. Due to the low percentage of specific surgery types (eg, thoracic, cardiac, vascular, oral, plastic, trauma, and gynecological), these were combined in category designated as “other”.
Statistical Analysis
Baseline demographic and clinical characteristics of patients were summarized using median (interquartile range [IQR]) for continuous variables and counts and percentages for categorical variables. These characteristics were presented separately for patients who had a POE clinic evaluation and those who did not.
Opioid doses were converted to OMEs (milligrams) and total perioperative opioid consumption was calculated as the sum of total OME (milligrams) in the OR, PACU, and postoperatively. To satisfy distributional assumptions, total preoperative opioids (OME) and hospital length of stay were analyzed using log transformation. Since some patients had zero preoperative opioid consumption, a value of one was added to the original opioid consumption prior to log transformation. Continuous outcomes were analyzed using linear regression with generalized estimating equations (GEE) and robust variance estimates. Binary outcomes were analyzed with logistic regression with GEE. In all cases, the explanatory variable of interest is whether the patient visited the POE clinic. In addition to unadjusted analyses, propensity-adjusted analyses were performed using inverse probability of treatment weighting (IPTW) to account for potential confounding. Logistic regression was used to calculate propensity scores for POE visits. Covariates included in the propensity score model included age, sex, race, BMI, surgery duration, Charlson score, OR regional block (yes/no), surgery type (general, neurosurgery, orthopedic, urology, and other), and ASA type. The propensity score model also factored in patient group (chronic pain vs SUD) with interaction terms. For each of the patient and procedural characteristics, the standardized difference between patients who visited a POE clinic and those who did not was calculated before and after propensity score adjustment to assess whether the IPTW approach was able to adequately control for potential confounding. Due to convergence issues, several variables needed to be combined prior to analysis, including sex, race, and ASA type.
Two-tailed p-values of 0.05 or less were considered statistically significant. Data management and statistical analysis were performed in SAS version 9.4 (SAS Institute Inc, Cary, North Carolina) and R version 4.1.2 (RStudio Team 2021, Boston, Massachusetts).
Results
Baseline Characteristics
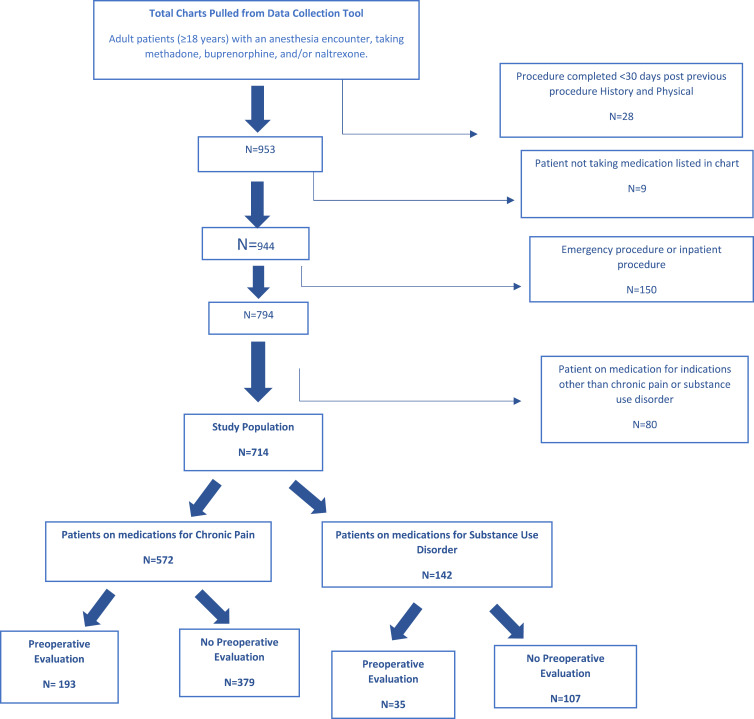
An initial population of 981 subjects were assessed for eligibility with 714 patients included based on inclusion and exclusion criteria (Figure 1). Baseline demographic and clinical characteristics are summarized in Table 1. Based on the magnitude of the unadjusted standardized differences, there was a meaningful imbalance between groups for several characteristics including age, surgery duration, and procedure types with patients evaluated in the POE clinic having older age, longer surgeries, and higher or lower percentage of specific surgery types. These variables were adjusted using IPTW.
Figure 1.
Consort Diagram.
Table 1.
Patient and Procedural Characteristics
| Variable | POE Visit (N=228) |
No POE Visit (N=486) |
Standardized Difference | |
|---|---|---|---|---|
| Unadjusted | IPTW | |||
| Age, median (IQR) | 58.5 (50.0, 66.0) | 51.0 (37.0, 60.0) | 0.545 | 0.064 |
| Sex, n (%) | 0.072 | |||
| Female | 104 (46%) | 252 (52%) | 0.125 | – |
| Male | 123 (54%) | 234 (48%) | 0.116 | – |
| Unknown | 1 (0%) | 0 (0%) | 0.028 | – |
| Race, n (%) | 0.093 | |||
| White | 208 (91%) | 455 (94%) | 0.091 | – |
| American Indian/Alaskan Native | 9 (4%) | 5 (1%) | 0.187 | – |
| Asian | 2 (1%) | 2 (0%) | 0.030 | – |
| Black or African American | 3 (1%) | 6 (1%) | 0.005 | – |
| Other | 6 (3%) | 18 (4%) | 0.061 | – |
| BMI, median (IQR) | 28.3 (24.9, 33.5) | 27.3 (23.3, 32.7) | 0.102 | 0.042 |
| Surgery duration (min) median (IQR) | 127.0 (78.5, 204.5) | 103.5 (46.0, 197.0) | 0.136 | 0.005 |
| Charlson Score median (IQR) | 3.0 (2.0, 5.0) | 3.0 (1.0, 5.0) | 0.124 | 0.056 |
| OR Regional Block, n (%) | 10 (4%) | 21 (4%) | 0.003 | 0.027 |
| Surgery type, n (%) | ||||
| General | 40 (18%) | 125 (26%) | 0.200 | 0.055 |
| Neurologic | 43 (19%) | 31 (6%) | 0.383 | 0.003 |
| Orthopedic | 82 (36%) | 119 (24%) | 0.252 | 0.081 |
| Urology | 32 (14%) | 57 (12%) | 0.069 | 0.010 |
| Other | 57 (25%) | 210 (43%) | 0.391 | 0.003 |
| ASA Type, n (%) | 0.021 | |||
| I | 0 (0%) | 12 (2%) | 0.158 | – |
| II | 109 (48%) | 212 (44%) | 0.084 | – |
| III | 112 (49%) | 226 (47%) | 0.052 | – |
| IV | 6 (3%) | 23 (5%) | 0.112 | – |
| Unknown | 1 (0%) | 13 (3%) | 0.143 | – |
Outcomes of Interest
Of those included in the study, only 228 (32%) patients had a formal POE clinic evaluation while 486 (68%) patients did not. Chronic pain was the most common indication for use of buprenorphine, methadone, or naltrexone, consisting of 572 patients (80.1%) in the total sample.
Of 572 patients taking these medications for chronic pain, 193 (34%) had a formal POE clinic evaluation prior to elective surgery while 379 (66%) did not. Outcome analysis is summarized in Table 2. The median (IQR) OME for perioperative opioids among those who were evaluated in the POE clinic was 76.1 mg (15.1, 156.6) versus 56.0 (0.1, 150.1) for those who were not evaluated. From inverse probability treatment weighting analyses, the estimated multiplicative change in total perioperative opioids was 1.43 (95% CI 0.95, 2.16; p = 0.087). Odds of receiving perioperative multimodal analgesics were higher among patients who were evaluated in the POE clinic (OR = 2.33, 95% CI 1.17, 4.65; p = 0.016) compared to those who were not. The remaining outcomes did not significantly differ between groups, and no surgical procedures were canceled in either group.
Table 2.
Outcomes for Patients on Buprenorphine, Methadone and Naltrexone for Chronic Pain
| POE Visit (N=193) | No POE Visit (N=379) | Unweighted | Weighted | |||||
|---|---|---|---|---|---|---|---|---|
| Estimate* | 95% CI | p-value | Estimate* | 95% CI | p-value | |||
| Total perioperative opioids, median (IQR)ª | 76.1 (15.1, 156.6) | 56.0 (0.1, 150.1) | 1.45 | (1.02, 2.08) | 0.041 | 1.43 | (0.95, 2.16) | 0.087 |
| Hospital length of stay, median (IQR)ª | 2.4 (1.3, 4.4) | 2.3 (1.0, 5.3) | 1.02 | (0.86, 1.22) | 0.816 | 1.01 | (0.81, 1.25) | 0.939 |
| Perioperative multimodal analgesics, n (%) | 179 (93%) | 331 (87%) | 1.86 | (1.01, 3.43) | 0.046 | 2.33 | (1.17, 4.65) | 0.016 |
| Perioperative complications, n (%) | 18 (9%) | 53 (14%) | 0.63 | (0.36, 1.10) | 0.105 | 0.71 | (0.39, 1.29) | 0.261 |
| Inpatient pain service, n (%) | 61 (32%) | 118 (31%) | 1.03 | (0.73, 1.46) | 0.870 | 1.13 | (0.74, 1.71) | 0.568 |
| Readmitted within 30 days, n (%) | 41 (21%) | 84 (22%) | 0.93 | (0.62, 1.42) | 0.751 | 1.19 | (0.75, 1.88) | 0.462 |
| Procedure cancelled, n (%) | 0 (0%) | 0 (0%) | – | – | – | – | – | – |
| Post-surgical addiction medicine consult, n (%) | 0 (0%) | 0 (0%) | – | – | – | – | – | – |
Notes: The reference group is no POE visit. Estimates are the multiplicative change in the outcome for total perioperative opioid consumption, hospital length of stay, and total multimodal perioperative analgesics. Estimates are Odds Ratios for perioperative complications, inpatient pain service, readmitted within 30 days, and withdrawal or relapse. ªData were log-transformed for normality.
Of 142 patients taking these medications solely for SUD (Table 3), only approximately one-quarter (35 patients) underwent a formal POE clinic evaluation prior to elective surgery while 107 (75.3%) did not. The median (IQR) for perioperative opioids among those who were evaluated in the POE clinic was 48.1mg (8.0, 108.1) and 30.1mg (0.1, 126.0) for those who were not evaluated. The estimated multiplicative change in total perioperative opioids was 0.97 (95% CI 0.44, 2.15; p = 0.943), indicating no difference in total perioperative opioids between patients who were evaluated in the POE clinic versus those who were not. Similarly, there were no statistically significant difference based on POE clinic evaluation.
Table 3.
Outcomes for Patients on Buprenorphine, Methadone, and Naltrexone for Substance Use Disorder
| POE Visit (N=35) | No POE Visit (N=107) | Unweighted | Weighted | |||||
|---|---|---|---|---|---|---|---|---|
| Estimate* | 95% CI | p-value | Estimate* | 95% CI | p-value | |||
| Total perioperative opioids, median (IQR)ª | 48.1 (8.0, 108.1) | 30.1 (0.1, 126.0) | 1.79 | (0.88, 3.65) | 0.107 | 0.97 | (0.44, 2.15) | 0.943 |
| Hospital length of stay, median (IQR)ª | 2.2 (1.0, 5.2) | 1.3 (0.7, 3.2) | 1.43 | (0.98, 2.07) | 0.061 | 0.96 | (0.59, 1.57) | 0.882 |
| Perioperative multimodal analgesics, n (%) | 31 (89%) | 103 (96%) | 0.30 | (0.07, 1.21) | 0.090 | 0.43 | (0.09, 2.03) | 0.285 |
| Perioperative complications, n (%) | 2 (6%) | 10 (9%) | 0.75 | (0.18, 3.24) | 0.705 | 0.34 | (0.07, 1.65) | 0.180 |
| Inpatient pain service, n (%) | 13 (37%) | 26 (24%) | 1.85 | (0.83, 4.10) | 0.132 | 1.14 | (0.40, 3.28) | 0.803 |
| Readmitted within 30 days, n (%) | 9 (26%) | 34 (32%) | 1.09 | (0.35, 3.37) | 0.883 | 1.05 | (0.30, 3.64) | 0.942 |
| Procedure cancelled, n (%) | 0 (0%) | 0 (0%) | – | – | – | – | – | – |
| Post-surgical addiction medicine consult, n (%) | 1 (3%) | 7 (7%) | 0.43 | (0.05, 3.60) | 0.439 | 0.35 | (0.04, 3.06) | 0.342 |
Notes: The reference group is no POE visit. Estimates are the multiplicative change in the outcome for total perioperative opioid consumption, hospital length of stay, and total multimodal perioperative analgesics. Estimates are Odds Ratios for perioperative complications, inpatient pain service, readmitted within 30 days, and withdrawal or relapse. ªData were log-transformed for normality.
Discussion
In the present study, we assessed the effects of preoperative assessment through the POE clinic on perioperative outcomes in patients on buprenorphine, methadone, or naltrexone undergoing elective surgery. The patients were separated based on the indication of use (chronic pain versus SUD) as the perioperative needs of these patients will likely differ based on this distinction. Despite the established benefits of preoperative assessments on various clinical indications and diagnoses, to our knowledge, this is the first study to specifically evaluate patients on these unique set of medications.
Regardless of indication, we observed that only a minority of patients were referred to the POE clinic for preoperative evaluation prior to elective surgery. In patients receiving these medications for SUD, only 25% of patients were evaluated in the POE clinic before their elective surgery. In patients receiving these medications primarily for chronic pain (cancer and non-cancer), only about one-third (36%) were seen in the POE clinic before their elective surgeries. This indicates an underutilization of preoperative assessment services in a unique patient population on medications, which a substantial portion of surgeons, anesthesiologists, and clinicians report feeling uncomfortable or unfamiliar with managing.9–11
We observed that among patients taking buprenorphine, methadone, or naltrexone for chronic pain, those who were evaluated in the POE clinic had a higher likelihood of receiving non-opioid multimodal analgesia in the perioperative period versus those who were not evaluated in the POE clinic. This observation suggests a potential link between POE clinic evaluation and the implementation of comprehensive analgesic strategies, which may stem from a deliberate emphasis on prioritizing multimodal analgesic pathways and meticulous preoperative planning aimed at optimizing pain control.16 The adoption of multimodal pathways in the perioperative setting has gained prominence due to compelling evidence endorsing their efficacy in achieving superior outcomes, such as mitigating the onset of chronic post-surgical pain.17 Consequently, the POE clinic appears to confer a crucial advantage by advocating for the incorporation of multimodal analgesia pathways, highly recommended in established guidelines, thereby potentially enhancing perioperative pain management and patient outcomes.4,8,18
The main limitation of our study is its retrospective design. There was also a change in the electronic medical record (EMR) system utilized by our institution during our study’s time frame, which may have underestimated the number of patients who underwent formal preoperative assessment in the POE clinic. The relatively small number of patients, especially those taking these medications for SUD, is another significant limitation. Further, the analysis did not separate patients based on medication type as the number remaining in each group would have been insufficient for meaningful statistical analysis. As previously stated, the doses of these medications exert varying pharmacodynamics with possible major implications on perioperative pain control and overall outcomes. Unfortunately, we were unable to stratify our analysis based on medication dosage given paucity of this information in our EMR. Lastly, this was a single-center study with perioperative referral processes unique to our institution and may not be generalizable to other institutions.
Despite these findings, we still consider a formal preoperative assessment to be highly valuable in patients receiving buprenorphine, methadone, or naltrexone perioperatively. The unique pharmacology of these medications has significant perioperative implications that have been well documented in the literature.7,11,13,19–23 Buprenorphine, a partial mu-opioid receptor agonist with high receptor affinity, has historically been challenging to manage in the perioperative period. These properties were foundational to prior recommendations to discontinue preoperative buprenorphine for acute pain control optimization with full mu-opioid agonists. While the basis for this recommendation was within reason, it had negative consequences for patients on buprenorphine for opioid use disorder due to the increased risk of relapse with abrupt discontinuation.24 Given this risk, recently published consensus guidelines strongly recommend continuing buprenorphine perioperatively to avoid substance use relapse and associated sequelae.4 Similarly, it is highly recommended to maintain methadone, a full mu-opioid receptor agonist, in the perioperative period regardless of indication. On the contrary, it is recommended that patients discontinue naltrexone, a full mu-opioid receptor antagonist, in the perioperative period.5 The duration of discontinuation can vary depending on naltrexone dose and route of administration. Lower doses of oral naltrexone typically used for chronic pain conditions require roughly a 24 hour hold while larger doses used for substance use disorder require longer hold times (48–72 hours) preoperatively. Unfortunately, patients often receive conflicting preoperative recommendations for these medications, especially from providers who do not routinely prescribe or manage them. This further highlights the importance of POE clinics that can give definitive and guideline-based recommendations on the perioperative management of these medications.
Conclusion
In summary, this is the first study to our knowledge that assessed the impact of formal preoperative assessment in patients on buprenorphine, methadone, and naltrexone. We observed that the POE clinic is a heavily underutilized service in this population. Further, the implementation of a formal preoperative assessment may be associated with higher utilization of multimodal analgesia pathways in patients with chronic pain Although we did not not differences in perioperative OME or other analgesic outcomes, we strongly advocate that patients on these medications receive a referral for formal preoperative assessment. These assessments will help streamline and standardize recommendations provided to patients on the perioperative management of these medications, and ultimately minimize the aforementioned risks of relapse while improving overall perioperative outcomes.
Future directions include a prospective observational or randomized multi-center study that can investigate the true effect of formal preoperative assessment on the perioperative outcomes of patients on buprenorphine, methadone, and naltrexone. A future study including other complex pain patients such as those with implanted pain devices (eg, spinal cord stimulator or peripheral nerve stimulator) could provide additional insight on the utility of the preoperative assessment in this population.
Funding Statement
This research was partially funded by a grant from the Mayo Clinic Pain Medicine Divisions research funds.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors certify that there is no conflict of interest with any financial organization regarding the materials discussed in the manuscript.
References
- 1.Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults-United States, 2016. MMWR Surveill Summ. 2018;67(36):1001–1006. doi: 10.15585/mmwr.mm6736a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: results from the 2020 National Survey on Drug Use and Health. (HHS Publication No. PEP21-07-01-003, NSDUH Series H-56). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. Available from: https://www.samhsa.gov/data/. Accessed September 25, 2024.
- 3.D’Souza RS, Nahin RL. Nationally Representative Rates of Incident Prescription Opioid Use Among United States Adults and Selected Subpopulations: Longitudinal Cohort Study From the National Health Interview Survey, 2019 to 2020. J Pain. 2024:104665. doi: 10.1016/j.jpain.2024.104665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohan L, Potru S, Barreveld AM, et al. Buprenorphine management in the perioperative period: educational review and recommendations from a multisociety expert panel. Reg Anesth Pain Med. 2021;46(10):840–859. PMID: 34385292.asdf. doi: 10.1136/rapm-2021-103007 [DOI] [PubMed] [Google Scholar]
- 5.Harrison TK, Kornfeld H, Aggarwal AK, Lembke A. Perioperative considerations for the patient with opioid use disorder on buprenorphine, methadone, or naltrexone maintenance therapy. Anesthesiol Clin. 2018;36(3):345–359. doi: 10.1016/j.anclin.2018.04.002 [DOI] [PubMed] [Google Scholar]
- 6.Oya R, Ogawa S, Oya K, Hirakawa Y, Maeda C, Amaya F. Prevalence of preoperative opioid usage and its impact on postoperative outcomes: a retrospective cohort study. J Anesth. 2023;37(4):532–538. doi: 10.1007/s00540-023-03198-0 [DOI] [PubMed] [Google Scholar]
- 7.Edwards DA, Hedrick TL, Jayaram J, et al. American society for enhanced recovery and perioperative quality initiative joint consensus statement on perioperative management of patients on preoperative opioid therapy. A a Pract. 2019;129(2):553–566. doi: 10.1213/ANE.0000000000004018 [DOI] [PubMed] [Google Scholar]
- 8.Dickerson DM, Mariano ER, Szokol JW, et al. Multiorganizational consensus to define guiding principles for perioperative pain management in patients with chronic pain, preoperative opioid tolerance, or substance use disorder. Reg Anesth Pain Med. 2023;25:rapm–2023–104435. PMID: 37185214. doi: 10.1136/rapm-2023-104435 [DOI] [PubMed] [Google Scholar]
- 9.Burgess JR, Heneghan KC, Barot TG, Stulberg JJ. Surgeons’ knowledge regarding perioperative pain management in patients with opioid use disorder: a survey among 260 members of the American College of Surgeons. Patient Saf Surg. 2024;18(1):9. PMID: 38438902; PMCID: PMC10910809. doi: 10.1186/s13037-024-00392-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cioe K, Biondi BE, Easly R, Simard A, Zheng X, Springer SA. A systematic review of patients’ and providers’ perspectives of medications for treatment of opioid use disorder. J Subst Abuse Treat. 2020;119:108146. PMID: 33138929; PMCID: PMC7609980. doi: 10.1016/j.jsat.2020.108146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sritapan Y, Clifford S, Bautista A. Perioperative management of patients on buprenorphine and methadone: a narrative review. Balkan Med J. 2020;37(5):247–252. doi: 10.4274/balkanmedj.galenos.2020.2020.5.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blitz JD, Kendale SM, Jain SK, Cuff GE, Kim JT, Rosenberg AD. Preoperative evaluation clinic visit is associated with decreased risk of in-hospital postoperative mortality. Anesthesiology. 2016;125:280–294. doi: 10.1097/ALN.0000000000001193 [DOI] [PubMed] [Google Scholar]
- 13.Ferschl MB, Tung A, Sweitzer BJ, Huo D, Glick DB. Preoperative clinic visits reduce operating room cancellations and delays. Anesthesiology. 2005;103:855. doi: 10.1097/00000542-200510000-00025 [DOI] [PubMed] [Google Scholar]
- 14.Burton BN, Arastoo S, Wu S, Liu N, Ong MK, Vazirani S. The association of medical preoperative evaluation using clinical video telehealth with hospital length of stay: descriptive analysis. JMIR Form Res. 2022;6(7):e38054. doi: 10.2196/38054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Rourke MJ, Keshock MC, Boxhorn CE, et al. Preoperative management of opioid and nonopioid analgesics: society for perioperative assessment and quality improvement (SPAQI) consensus statement. Mayo Clin Proc. 2021;96(5):1325–1341. PMID: 33618850. doi: 10.1016/j.mayocp.2020.06.045 [DOI] [PubMed] [Google Scholar]
- 16.D’Souza RS, Langford B, Wilson RE, et al. The state-of-the-art pharmacotherapeutic options for the treatment of chronic non-cancer pain. Expert Opin Pharmacother. 2022;23(7):775–789. PMID: 35354341. doi: 10.1080/14656566.2022.2060741 [DOI] [PubMed] [Google Scholar]
- 17.Hussain N, Brull R, Weber L, et al. The analgesic effectiveness of perioperative lidocaine infusions for acute and chronic persistent postsurgical pain in patients undergoing breast cancer surgery: a systematic review and meta-analysis. Br J Anaesth. 2024;132(3):575–587. PMID: 38199928. doi: 10.1016/j.bja.2023.12.005 [DOI] [PubMed] [Google Scholar]
- 18.Mariano ER, Dickerson DM, Szokol JW, et al. A multisociety organizational consensus process to define guiding principles for acute perioperative pain management. Reg Anesth Pain Med. 2022;47(2):118–127. PMID: 34552003. doi: 10.1136/rapm-2021-103083 [DOI] [PubMed] [Google Scholar]
- 19.Kaye AD, Helander EM, Vadivelu N, et al. Consensus statement for clinical pathway development for perioperative pain management and care transitions. Pain Ther. 2017;6(2):129–141. doi: 10.1007/s40122-017-0079-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coluzzi F, Bifulco F, Cuomo A, et al. The challenge of perioperative pain management in opioid-tolerant patients. Ther Clin Risk Manag. 2017;13:1163–1173. doi: 10.2147/TCRM.S141332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huxtable CA, Roberts LJ, Somogyi AA, MacIntyre PE. Acute pain management in opioid-tolerant patients: a growing challenge. Anaesth Intensive Care. 2011;39(5):804–823. doi: 10.1177/0310057X1103900505 [DOI] [PubMed] [Google Scholar]
- 22.Ward EN, Quaye AN, Wilens TE. Opioid use disorders: perioperative management of a special population. Anesth Analg. 2018;127(2):539–547. doi: 10.1213/ANE.0000000000003477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaye AD, Kandregula S, Kosty J, et al. Chronic pain and substance abuse disorders: preoperative assessment and optimization strategies. Best Pract Res Clin Anaesthesiol. 2020;34:255–267. doi: 10.1016/j.bpa.2020.04.014 [DOI] [PubMed] [Google Scholar]
- 24.Warner NS, Warner MA, Cunningham JL, et al. A practical approach for the management of the mixed opioid agonist-antagonist buprenorphine during acute pain and surgery. Mayo Clin Proc. 2020;95(6):1253–1267. doi: 10.1016/j.mayocp.2019.10.007 [DOI] [PubMed] [Google Scholar]