Abstract
Hybrid viruses between human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus strain mac (SIVMAC) are invaluable to various fields of HIV-1 research. To date, however, no replication-competent HIV-1 strain containing the gag capsid (CA) region of SIVMAC has been reported. To obtain the viable gag gene chimeric virus in an HIV-1 background, seven HIV-1 strains carrying a part of SIVMAC CA or a small deletion in the CA region were constructed and examined for their biological and biochemical characteristics. While all the recombinants and mutants were found to express Gag and to produce progeny virions on transfection, only one chimeric virus, which has 18 bp of SIV gag CA sequence in place of the region encoding the HIV-1 CA cyclophilin A (CyPA)-binding loop, was infectious for human cell lines. Although this chimeric virus was unable to grow in monkey lymphocytic cells like wild-type (wt) HIV-1 did, it grew much better than wt virus in the presence of cyclosporin A in a human cell line which supports HIV-1 replication in a CyPA-dependent manner. These results indicate that the transfer of a small portion of the SIVMAC CA region to HIV-1 could confer the CyPA-independent replication potential of SIVMAC on the virus.
Human immunodeficiency virus type 1 (HIV-1) Gag proteins, like those of the other retroviruses, play roles in many steps of the virus life cycle (for a review, see reference 12). The HIV-1 Gag proteins are initially synthesized as precursor protein p55, and p55 is cleaved by virus protease into mature proteins p17 matrix (MA), p24 capsid (CA), p7 nucleocapsid (NC), and p6 during or shortly after virus budding (for reviews, see references 28 and 32). The Gag proteins are critical not only for the assembly, release of enveloped virions, and maturation of virions but also for the early postentry steps in virus replication. It is generally accepted, therefore, that HIV-1 Gag interacts with numerous viral and cellular factors. One prominent consequence of retroviral Gag-mediated biological functions is the virus host range. For murine leukemia virus, it is well established that Gag CA determines the Fv-1 tropism (for a review, see reference 30). It is also well known for HIV-1 that non-env sequence is critical for the species tropism (25). While simian immunodeficiency virus strain mac (SIVMAC) grows well both in human and simian lymphocytes, HIV-1 does not replicate in the latter cells, and the viral determinant for this restriction is most likely to be the Gag CA of HIV-1 (10, 24, 25, 27, 29). Furthermore, some mutations in the gag gene of HIV-1 affect the cellular tropism of the virus. Mutant viruses with alterations in the HIV-1 gag encoding MA, CA, or NC were able to grow in some human lymphocytic cell lines but not in other (13, 15, 21). Recent studies have raised the idea that the early function of HIV-1 Gag, i.e., that of uncoating and/or reverse transcription, is involved in the restriction of HIV-1 replication mentioned above (18, 27).
To study the molecular basis for the functionality of HIV-1 Gag CA in human and simian cells, replication-competent gag chimeric viruses between HIV-1 and SIVMAC are important tools. Biological and biochemical characterization of such viruses would help us to understand the unique biology of HIV-1 mediated by the Gag CA. So far, however, while a viable chimeric virus that carries the HIV-1 gag CA region in an SIVMAC background has been reported, the construction of replication-competent HIV-1 containing the gag CA region of SIVMAC has been unsuccessful, probably for technical reasons (10). In this study, we have generated seven gag recombinants and mutants of wild-type (wt) HIV-1 NL432 (2) and characterized them biologically and biochemically. We demonstrate here that one HIV-1 clone with a small portion of the SIVMAC gag CA region grows well in human lymphocytic cells but not in monkey cells. We also show that this virus grows in human cells in a cyclophilin A (CyPA)-independent manner, which is an SIVMAC property.
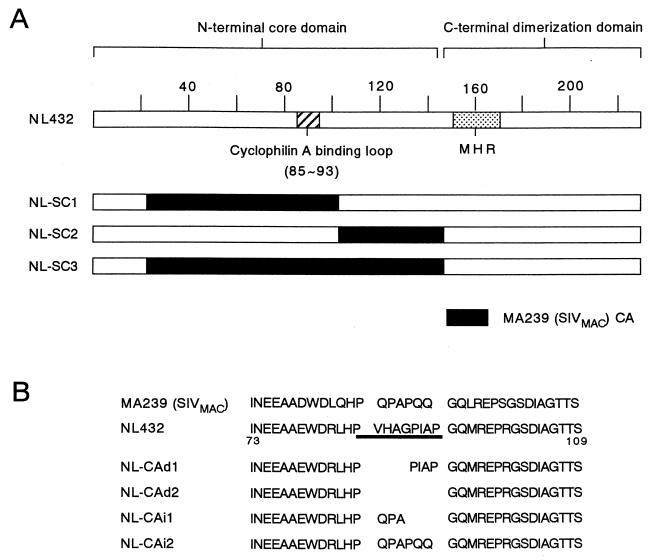
To design the HIV-1/SIVMAC CA recombinants, the amino acid sequences of viruses of HIV-1 and HIV-2/SIVMAC groups with distinct growth properties in monkey peripheral blood mononuclear cells (PBMCs) were compared. Whereas HIV-1 NL432 does not grow in monkey PBMCs, SIVMAC MA239 grows very well in the cells (25). HIV-2 GH123 (26) grows in the monkey cells but more slowly than MA239 (our unpublished observation). The sequence of CA is more highly conserved among the three virus clones than are those of MA, NC, and p6, but a heterologous region (CyPA binding loop [14]) of 6 to 8 amino acids in the N-terminal half of CA was readily noticed (Fig. 1). In addition, because the N-terminal core domain of CA contains sequence important for early postentry steps (12, 16, 18), HIV-1/SIVMAC CA recombinants designated NL-SC1, NL-SC2, and NL-SC3 were constructed, as shown in Fig 1A. Insertion of the whole SIVMAC CA sequence into HIV-1 resulted in a replication-incompetent virus (10). Recombinants NL-SC1, NL-SC2, and NL-SC3 contained translationally silent mutations and were expected to express chimeric CA. To obtain replication-competent virus clones with a high possibility, more HIV-1/SIVMAC CA recombinants with a small portion of the SIVMAC CA region, designated NL-CAi1 and NL-CAi2, were constructed, as shown in Fig. 1B. Deletion mutants designated NL-CAd1 and NL-CAd2 were made as intermediates between wt NL432 and NL-CAi1 and NL-CAi2.
FIG. 1.
Structures of the CA recombinants and mutants used in this study. (A) Construction of CA recombinants NL-SC1, NL-SC2, and NL-SC3. Three restriction enzymes (NsiI, SpeI, and XbaI from left to right in the schema) were used to make these recombinants. Two translationally silent mutations were introduced to generate a NsiI site in MA239 CA and a XbaI site in NL432 CA. The SpeI site is commonly present in authentic NL432 CA and MA239. Structural characteristics are indicated on the upper part. Numbers represent amino acid numbers of NL432 CA. MHR, major homology region in CA (32). (B) Amino acid sequences of deletion and insertion mutants of NL432 CA. The sequences of the mutants were compared to those of MA239 and NL432. The CyPA-binding loop of HIV-1 (14) is underlined. The structures of the CA recombinants and mutants in the figure were confirmed by nucleotide sequencing.
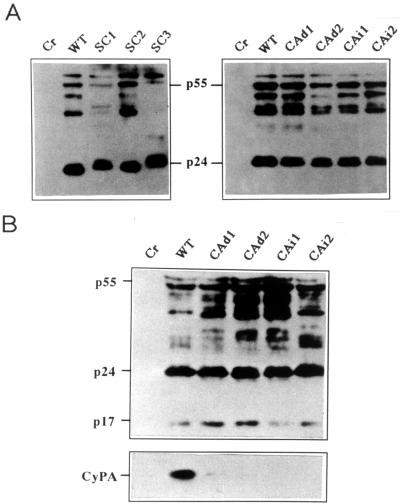
On transfection into 293T cells (19) by calcium phosphate coprecipitation (2), the recombinants NL-SC1, NL-SC2, and NL-SC3 produced progeny virions as judged by reverse transcriptase (RT) activity (31) in the culture supernatants (Table 1). The level of progeny production by recombinant NL-SC1 was significantly lower than that by wt NL432. The infectivity of progeny virions derived from NL-SC1, NL-SC2, and NL-SC3 was also determined by the MAGI assay (17) (Table 1). None of NL-SC1, NL-SC2, and NL-SC3 were infectious for MAGI cells. The four clones NL-CAd1, NL-CAd2, NL-CAi1, and NL-CAi2 were then examined for their progeny production and infectivity, as described above. As shown in Table 1, all four clones generated progeny virions on transfection into 293T cells. In particular, NL-CAi2 produced a similar level of progeny to that of wt NL432. Moreover, only NL-CAi2 was found to be infectious for MAGI cells. We then asked whether the recombinants and mutants in Fig. 1 were infectious for various human and simian lymphocytic cell lines including M8166 (25), A3.01 (11), H9 (20), and HSC-F (5). HSC-F is a CD4+ CXCR4+ CCR5− cynomolgus macaque (Macaca fascicularis) cell line which displays a susceptibility to various HIV and SIV strains similar to that of macaque monkey PBMCs (our unpublished data). To monitor the growth in these lymphocytic cells of various virus clones in Fig. 1, input cell-free virus samples were prepared from transfected 293T cells and inoculated into the cell lines indicated in Table 1. The CA recombinants NL-SC1, NL-SC2, and NL-SC3 did not grow at all in M8166, A3.01, or H9 cells. Similarly, NL-CAd1, NL-CAd2, and NL-CAi1 were not infectious for M8166, A3.01, or H9 cells. In contrast, recombinant NL-CAi2, which has a short stretch of SIVMAC CA sequence (6 amino acids) (Fig. 1), grew in all the cell lines in Table 1 except for HSC-F. While NL-CAi2 grew in A3.01 and H9 cells with somewhat delayed kinetics relative to those of wt NL432, it did well in M8166 cells. To determine the biochemical basis for the replication ability of the recombinants and mutants in Table 1, 293T cells were transfected with various clones and then cell lysates were prepared and monitored for Gag expression by Western blotting. As shown in Fig. 2A, all the clones expressed mature Gag p24, and no major abnormality was observed.
TABLE 1.
Replication property of the CA recombinants and mutants
| Clone | Single-cycle replication (%)a
|
Growth inb:
|
||||
|---|---|---|---|---|---|---|
| Virus production | Infectivity | M8166 | A3.01 | H9 | HSC-F | |
| NL432 (wt) | 100 | 100 | + | + | + | − |
| NL-SC1 | 31 ± 6 | 0 | − | − | − | ND |
| NL-SC2 | 77 ± 1 | 0 | − | − | − | ND |
| NL-SC3 | 70 ± 11 | 0 | − | − | − | ND |
| NL-CAd1 | 57 ± 2 | 0 | − | − | − | ND |
| NL-CAd2 | 58 ± 5 | 0 | − | − | − | ND |
| NL-CAi1 | 59 ± 7 | 0 | − | − | − | ND |
| NL-CAi2 | 97 ± 8 | 122 ± 4 | + | +D | +D | − |
293T cells were transfected with the proviral clones indicated (20 μg of each), and at 48 h posttransfection virus production in the culture supernatants and virus infectivity of the supernatants were monitored by RT (31) and MAGI (17) assays, respectively. Titers relative to that of the wt clone are shown.
The cells were infected with equivalent RT units of cell-free viruses prepared from transfected 293T cells, and virus growth was monitored by RT production in the culture supernatants for 3 weeks. −, no virus growth; +, virus growth; +D, virus growth with delayed kinetics; ND, not done.
FIG. 2.
Analysis of Gag proteins expressed by various recombinants and mutants. (A) Expression of Gag proteins in cells. 293T cells were transfected with the proviral clones indicated (20 μg of each) (2), and at 48 h posttransfection the cells were harvested for Western blot analysis (3, 4, 31) using serum from an individual infected with HIV-1. Because CAs of NL-SC1, NL-SC3, and NL-CAd2 were undetectable or hardly detectable by the Gag p24 enzyme-linked immunosorbent assay (ZeptoMetrix Corp., Buffalo, N.Y.), virus amounts were adjusted by RT activity (31). Cr, pUC19; WT, pNL432. (B) Western blot analysis of the virion proteins. 293T cells were transfected with the proviral clones indicated (20 μg of each) (2), and at 48 h posttransfection the culture supernatants were harvested for virion preparation. Virions were pelleted by ultracentrifugation through a sucrose cushion as previously described (7) and analyzed by Western blotting (3, 4, 31) with a human anti-HIV-1 antiserum (upper panel) and a rabbit anti-CyPA antiserum (BIOMOL Research Labs., Inc., Plymouth Meeting, Pa.) (lower panel). Equal amounts of virions as determined by RT activity were used. Cr, pUC19; WT, pNL432.
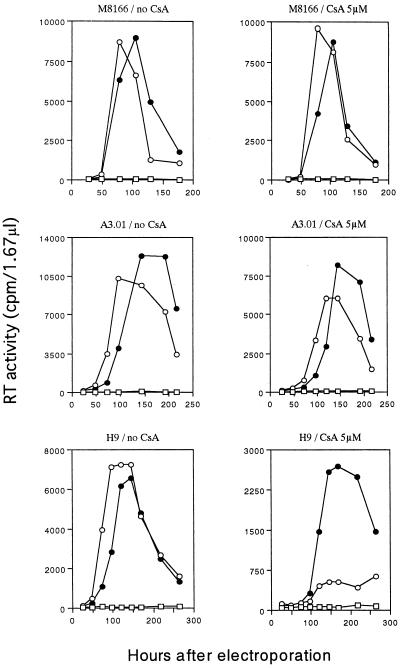
Based on the structure of chimeric CA (Fig. 1), we predicted that NL-CAi2 would grow in the cells in a CyPA-independent manner as reported previously (1, 6, 8). To confirm this, we examined the incorporation of CyPA into virions of various virus clones as previously reported (6–9). 293T cells were transfected with various clones, and the progeny virions produced were prepared by ultracentrifugation through a sucrose cushion (7). Virion lysates were then subjected to Western blot analysis as previously described (3, 4). As shown in Fig. 2B, the protein profiles of virions of wt NL432, NL-CAd1, NL-CAd2, NL-CAi1, and NL-CAi2 with respect to Gag were similar to one another. In contrast, CyPA was detected only in the lysates prepared from wt virions. The growth in lymphocytic cells of NL432 and NL-CAi2 in the presence of cyclosporin A (CsA) (Calbiochem-Novabiochem Corp., La Jolla, Ca.), which binds with high affinity to CyPA, was then monitored (Fig. 3). Infection for this experiment was initiated by electroporation (2) of proviral DNA clones into cells. Virus growth can be recognized more rapidly by electroporation than by the routine infection method, and the effect of cytotoxic drugs on the virus replication could be estimated in a short (our unpublished observation). The presence of CsA at 5 μM in the culture medium did not appear to affect the cells during the observation period. In M8166 cells, unexpectedly, wt NL432 and the recombinant NL-CAi2 grew similarly well irrespective of the presence or absence of CsA (Fig. 3). Both viruses also grew similarly well in A3.01 cells in the presence of CsA. As monitored by Western blot analysis, no significant incorporation of CyPA into NL432 virions was observed when infected M8166 and A3.01 cells were cultured in the presence of CsA (data not shown). In H9 cells, while NL432 grew somewhat better than NL-CAi2 in the absence of CsA, NL-CAi2 grew much better than NL432 in the presence of CsA (Fig. 3).
FIG. 3.
Effect of CsA on the replication of NL-CAi2. The cells indicated were electroporated (2) with 10 μg of pNL432 (○), pNL-CAi2 (●), or pUC19 (□) and cultured in the absence or presence of CsA. Virus replication was monitored by RT (31) production in the culture supernatants.
In this study, we have demonstrated that an HIV-1 strain containing 6 amino acid residues of SIVMAC CA in its CA (Fig. 1) replicates well in human cells in a CyPA-independent manner like SIVMAC (9, 10) (Fig. 3). Although the virus designated NL-CAi2 did not grow in monkey lymphocytic cells (Table 1), this is the first Gag hybrid virus between HIV-1 and SIVMAC in an HIV-1 background that is replication competent (10, 24). Improvements of the Gag structure of NL-CAi2 may result in the generation of hybrid viruses that are infectious for monkey lymphocytic cells. The molecular basis for the replication incompetence of various virus clones constructed in this study (Fig. 1) is not clear. These virus clones generated progeny virions on transfection, albeit relatively inefficiently (Table 1). Furthermore, no drastic abnormality for the expression and processing of Gag proteins was noted in cells and virions (Fig. 2). We noticed, however, that the virions of NL-SC1, NL-SC2, NL-CAd1, NL-CAd2, and NL-CAi1 were unstable relative to those of NL-SC3, NL-CAi2, and wt NL432 as judged by the recovery of RT activity after ultracentrifugation (our unpublished observation). It is possible that some chimeric CAs in this report affected the stability of the virions. Identification of the functional defect in these noninfectious viruses is important, and a systematic functional study on these viruses needs to be carried out.
Another point of this study is the identification of human cell lines which support HIV-1 replication very well in the presence of CsA (Fig. 3). In M8166 and A3.01 cells, HIV-1 clones with or without a functional CyPA-binding site grew well in the presence or absence of CsA. In contrast to these CyPA-independent cell lines, CyPA-dependent H9 cells strongly supported the replication of HIV-1 lacking the CyPA-binding site but not of HIV-1 with the binding site in the presence of CsA (Fig. 3). It has been reported previously that wt HIV-1 replicates in human PBMCs and in CEMx174, Jurkat, and HeLa-CD4+ cells in a CyPA-dependent manner (1, 6, 8–10). The CsA sensitivity of HIV-1 replication in host cells can be modulated by levels of CyPA expression (33). In M8166 and A3.01 cells in this study, wt HIV-1 grew well without significant incorporation of CyPA into virions. It is very likely, therefore, that some cell factor(s) other than CyPA is involved in the replication of HIV-1 in the CyPA-independent cells such as M8166 and A3.01. Identification of a cell factor(s) in human and monkey cells that is responsible for the CyPA-independent replication of HIV/SIV is intriguing and remains to be carried out.
The exact role of CyPA in the early events of HIV-1 replication is not completely understood. It has been reported that CyPA enhances HIV-1 replication at the stages of virus attachment (22, 23) and uncoating (8). Although these functions are not mutually exclusive, more experimental data are required to obtain a clear model for the role of CyPA in HIV-1 replication. The chimeric NL-CAi2 construct in this study is useful for our understanding of the molecular basis of CyPA-independent and -dependent replication of HIV-1.
Acknowledgments
We thank Kazuko Yoshida for editorial assistance.
This work was supported by grants-in-aid for AIDS research from the Ministry of Education, Science, Sports and Culture of Japan and the Ministry of Health and Welfare of Japan.
REFERENCES
- 1.Aberham C, Weber S, Phares W. Spontaneous mutations in the human immunodeficiency virus type 1 gag gene that affect viral replication in the presence of cyclosporins. J Virol. 1996;70:3536–3544. doi: 10.1128/jvi.70.6.3536-3544.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akari H, Arold S, Fukumori T, Okazaki T, Strebel K, Adachi A. Nef-induced major histocompatibility complex class I down-regulation is functionally dissociated from its virion incorporation, enhancement of viral infectivity, and CD4 down-regulation. J Virol. 2000;74:2907–2912. doi: 10.1128/jvi.74.6.2907-2912.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akari H, Fukumori T, Adachi A. Cell-dependent requirement of human immunodeficiency virus type 1 gp41 cytoplasmic tail for Env incorporation into virions. J Virol. 2000;74:4891–4893. doi: 10.1128/jvi.74.10.4891-4893.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akari H, Fukumori T, Iida S, Adachi A. Induction of apoptosis in Herpesvirus saimiri-immortalized T lymphocytes by blocking interaction of CD28 with CD80/CD86. Biochem Biophys Res Commun. 1999;263:352–356. doi: 10.1006/bbrc.1999.1364. [DOI] [PubMed] [Google Scholar]
- 6.Braaten D, Aberham C, Franke E K, Yin L, Phares W, Luban J. Cyclosporin A-resistant human immunodeficiency virus type 1 mutants demonstrate that gag encodes the functional target of cyclophilin A. J Virol. 1996;70:5170–5176. doi: 10.1128/jvi.70.8.5170-5176.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braaten D, Ansari H, Luban J. The hydrophobic pocket of cyclophilin is the binding site for the human immunodeficiency virus type 1 Gag polyprotein. J Virol. 1997;71:2107–2113. doi: 10.1128/jvi.71.3.2107-2113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braaten D, Franke E K, Luban J. Cyclophilin A is required for an early step in the life cycle of human immunodeficiency virus type 1 before the initiation of reverse transcription. J Virol. 1996;70:3551–3560. doi: 10.1128/jvi.70.6.3551-3560.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braaten D, Franke E K, Luban J. Cyclophilin A is required for the replication of group M human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus SIVCPZ GAB but not group O HIV-1 or other primate immunodeficiency viruses. J Virol. 1996;70:4220–4227. doi: 10.1128/jvi.70.7.4220-4227.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorfman T, Gottlinger H G. The human immunodeficiency virus type 1 capsid p2 domain confers sensitivity to the cyclophilin-binding drug SDZ NIM811. J Virol. 1996;70:5751–5757. doi: 10.1128/jvi.70.9.5751-5757.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folks T, Benn S, Rabson A, Theodore T, Hoggan M D, Martin M, Lightfoote M, Sell K. Characterization of a continuous T-cell line susceptible to the cytopathic effects of the acquired immune deficiency syndrome (AIDS)-associated retrovirus. Proc Natl Acad Sci USA. 1985;82:4539–4543. doi: 10.1073/pnas.82.13.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freed E O. HIV-1 Gag proteins: diverse functions in the virus life cycle. Virology. 1998;251:1–15. doi: 10.1006/viro.1998.9398. [DOI] [PubMed] [Google Scholar]
- 13.Furuta R A, Shimano R, Ogasawara T, Inubushi R, Amano K, Akari H, Hatanaka M, Kawamura M, Adachi A. HIV-1 capsid mutants inhibit the replication of wild-type virus at both early and late infection phase. FEBS Lett. 1997;415:231–234. doi: 10.1016/s0014-5793(97)01132-0. [DOI] [PubMed] [Google Scholar]
- 14.Gamble T R, Vajdos F F, Too S, Worthylake D K, Houseweart M, Sundquist W I, Hill P C. Crystal structure of human cyclophilin A bound to the amino-terminal domain of HIV-1 capsid. Cell. 1996;87:1285–1294. doi: 10.1016/s0092-8674(00)81823-1. [DOI] [PubMed] [Google Scholar]
- 15.Kawamura M, Shimano R, Inubushi R, Akari H, Adachi A. Early function of HIV-1 Gag proteins is cell-dependent. Biochem Biophys Res Commun. 1998;248:899–903. doi: 10.1006/bbrc.1998.9065. [DOI] [PubMed] [Google Scholar]
- 16.Kawamura M, Shimano R, Inubushi R, Amano K, Ogasawara T, Akari H, Adachi A. Functional domain mapping of HIV-1 Gag proteins. Biochem Biophys Res Commun. 1997;241:317–320. doi: 10.1006/bbrc.1997.7814. [DOI] [PubMed] [Google Scholar]
- 17.Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koh K-B, Miyaura M, Yoshida A, Sakurai A, Fujita M, Adachi A. Cell-dependent gag mutants of HIV-1 are crucially defective at the stage of uncoating/reverse transcription in non-permissive cells. Microbes Infect. 2000;2:1419–1423. doi: 10.1016/s1286-4579(00)01295-8. [DOI] [PubMed] [Google Scholar]
- 19.Lebkowski J S, Clancy S, Calos M P. Simian virus 40 replication in adeno-virus-transformed human cells antagonizes gene expression. Nature. 1985;317:169–171. doi: 10.1038/317169a0. [DOI] [PubMed] [Google Scholar]
- 20.Mann D L, O'Brien S J, Gilbert D A, Reid Y, Popovic M, Read-Connel E, Gallo R C, Gazdar A F. Origin of the HIV-susceptible human CD4+ cell line H9. AIDS Res Hum Retroviruses. 1989;5:253–255. doi: 10.1089/aid.1989.5.253. [DOI] [PubMed] [Google Scholar]
- 21.Sakuragi J, Sakai H, Kawamura M, Tokunaga K, Ueda S, Adachi A. Generation and characterization of a host cell-dependent gag gene mutant of human immunodeficiency virus type 1. Virology. 1995;212:251–254. doi: 10.1006/viro.1995.1478. [DOI] [PubMed] [Google Scholar]
- 22.Saphire A C S, Bobardt M D, Gallay P A. Host cyclophilin A mediates HIV-1 attachment to target cells via heparans. EMBO J. 1999;18:6771–6785. doi: 10.1093/emboj/18.23.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sherry B, Zybarth G, Alfano M, Dubrovsky L, Mitchell R, Rich D, Ulrich P, Bucala R, Cerami A, Bukrinsky M. Role of cyclophilin A in the uptake of HIV-1 by macrophages and T lymphocytes. Proc Natl Acad Sci USA. 1998;95:1758–1763. doi: 10.1073/pnas.95.4.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibata R, Adachi A. SIV/HIV recombinants and their use in studying biological properties. AIDS Res Hum Retroviruses. 1992;8:403–409. doi: 10.1089/aid.1992.8.403. [DOI] [PubMed] [Google Scholar]
- 25.Shibata R, Kawamura M, Sakai H, Hayami M, Ishimoto A, Adachi A. Generation of a chimeric human and simian immunodeficiency virus infectious to monkey peripheral blood mononuclear cells. J Virol. 1991;65:3514–3520. doi: 10.1128/jvi.65.7.3514-3520.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibata R, Miura T, Hayami M, Ogawa K, Sakai H, Kiyomasu T, Ishimoto A, Adachi A. Mutational analysis of the human immunodeficiency virus type 2 (HIV-2) genome in relation to HIV-1 and simian immunodeficiency virus SIVAGM. J Virol. 1990;64:742–747. doi: 10.1128/jvi.64.2.742-747.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shibata R, Sakai H, Kawamura M, Tokunaga K, Adachi A. Early replication block of human immunodeficiency virus type 1 in monkey cells. J Gen Virol. 1995;76:2723–2730. doi: 10.1099/0022-1317-76-11-2723. [DOI] [PubMed] [Google Scholar]
- 28.Swanstrom R, Wills J W. Synthesis, assembly, and processing of viral proteins. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1997. pp. 263–334. [PubMed] [Google Scholar]
- 29.Uberla K, Stahl-Hennig C, Bottiger D, Matz-Rensing K, Kaup F J, Li J, Haseltine W A, Fleckenstein B, Hunsmann G, Oberg B. Animal model for the therapy of acquired immunodeficiency syndrome with reverse transcriptase inhibitors. Proc Natl Acad Sci USA. 1995;92:8210–8214. doi: 10.1073/pnas.92.18.8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varmus H, Swanstrom R. Replication of retroviruses. In: Weiss R, Teich N, Varmus H, Coffin J, editors. RNA tumor viruses. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1984. pp. 369–512. [Google Scholar]
- 31.Willey R L, Smith D H, Lasky L A, Theodore T S, Earl P L, Moss B, Capon D J, Martin M A. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J Virol. 1988;62:139–147. doi: 10.1128/jvi.62.1.139-147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wills J W, Craven R C. Form, function, and use of retroviral Gag proteins. AIDS. 1991;5:639–654. doi: 10.1097/00002030-199106000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Yin L, Braaten D, Luban J. Human immunodeficiency virus type 1 replication is modulated by host cyclophilin A expression levels. J Virol. 1998;72:6430–6436. doi: 10.1128/jvi.72.8.6430-6436.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]