Summary:
The surgical delay technique can be used effectively in autologous breast reconstruction when there is unfavorable flap vascular anatomy or when the reconstruction necessitates a larger volume of donor tissue to obtain optimal results. The length of time between surgically delaying the flap to pedicle division and inset of the flap often varies based on surgeon preference but is typically approximately a week or longer. The authors present a case in which a 24-hour surgical delay was successfully used to augment deep inferior epigastric perforator flaps for autologous reconstruction. This technique is beneficial as it does not allow time for scarring and adhesions to develop between stages and allows for both stages to be performed in the same hospital admission.
Takeaways
Question: When can a clinically meaningful improvement in perfusion be seen after a surgical delay for autologous breast reconstruction?
Findings: We present a case of a successful deep inferior epigastric perforator reconstruction after a 24-hour surgical delay. This shortened delay period allows for full perforator and pedicle dissection during the initial delay procedure.
Meaning: Though historically performed for a longer interval, it is possible that a clinically significant delay effect can be seen with as short of a delay interval as 24 hours and opens the possibility of performing both the surgical delay and the subsequent reconstruction within the same hospital admission.
INTRODUCTION
The surgical delay phenomenon is a well-described physiologic adaptation in which relative ischemia is used to increase the vascularity of tissue. In the context of breast reconstruction, it has provided an effective means of augmenting flaps in which there is unfavorable vascular anatomy or when the reconstruction necessitates a larger volume of donor tissue to obtain optimal results.1 The authors present a case in which a novel 24-hour surgical delay was performed in autologous bilateral breast reconstruction using deep inferior epigastric perforator (DIEP) and profunda artery perforator (PAP) flaps in a patient with previous abdominal liposuction.
CASE
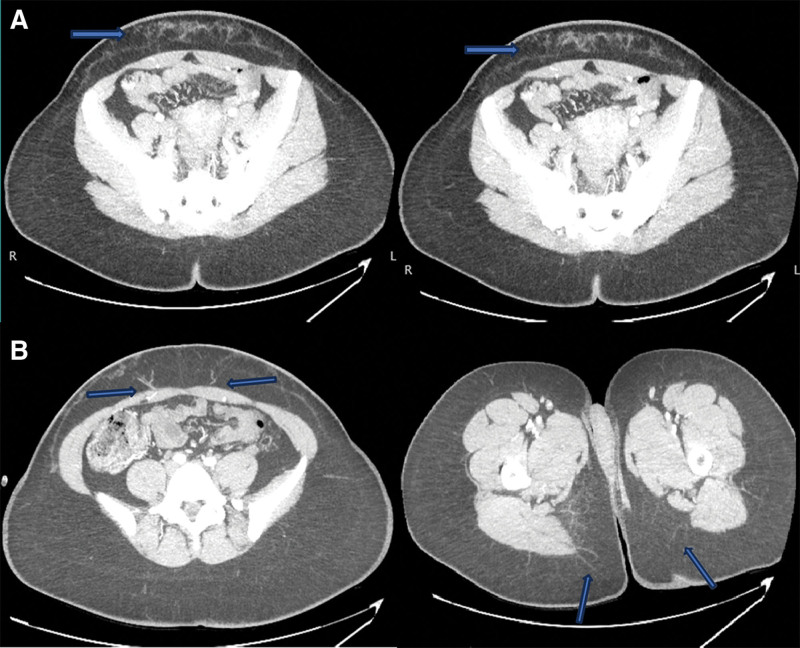
A 35-year-old woman with a history of breast cancer and bilateral mastectomies with tissue expander placement presented for autologous reconstruction consultation. Her surgical history included cosmetic liposuction of the abdomen, leaving the vascularity of the tissue in question. Alternative autologous donor sites were considered; however, the patient lacked adequate volume in the back, groin, and buttocks. A preoperative computed tomography scan with angiography (CTA) was performed which showed significant infraumbilical subcutaneous scarring. However, viable deep inferior epigastric artery (DIEA) and PAPs were also visualized (Fig. 1).
Fig. 1.
Preoperative CTA for surgical planning in a 35-year-old woman with a history of cosmetic liposuction. A, Subcutaneous scarring is designated by the blue arrows. B, Deep inferior epigastric and profunda artery perforators designated by blue arrows.
Based on the history of liposuction and scarring on CTA, we had reasonable concern about the vascularity of the flap, and indocyanine green angiography was considered to assess the vasculature. However, as our index of suspicion for suboptimal perfusion was high, indocyanine green angiography would not have affected management, and we elected not to perform it. Ultimately, the reconstructive plan for the volume required was a stacked DIEP and PAP flap reconstruction with a 24-hour surgical delay of the abdominal flaps to optimize vascularity.
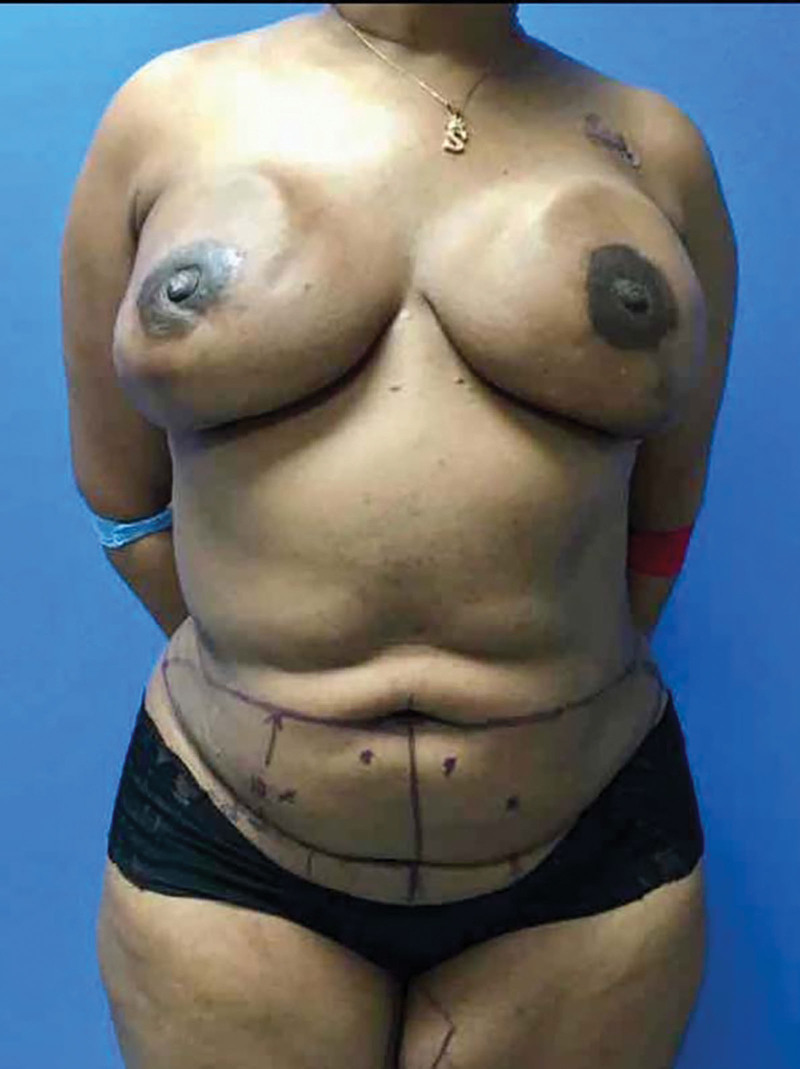
For the delay stage, the DIEA perforators visualized on the CTA scan were identified with handheld Doppler, and the DIEP flaps were marked in typical fashion preoperatively (Fig. 2). [See Video 1 (online), which displays preoperative Doppler ultrasound of the dominant perforator.]
Fig. 2.
Preoperative markings.
Video 1. which displays preoperative Doppler ultrasound of the dominant perforator.
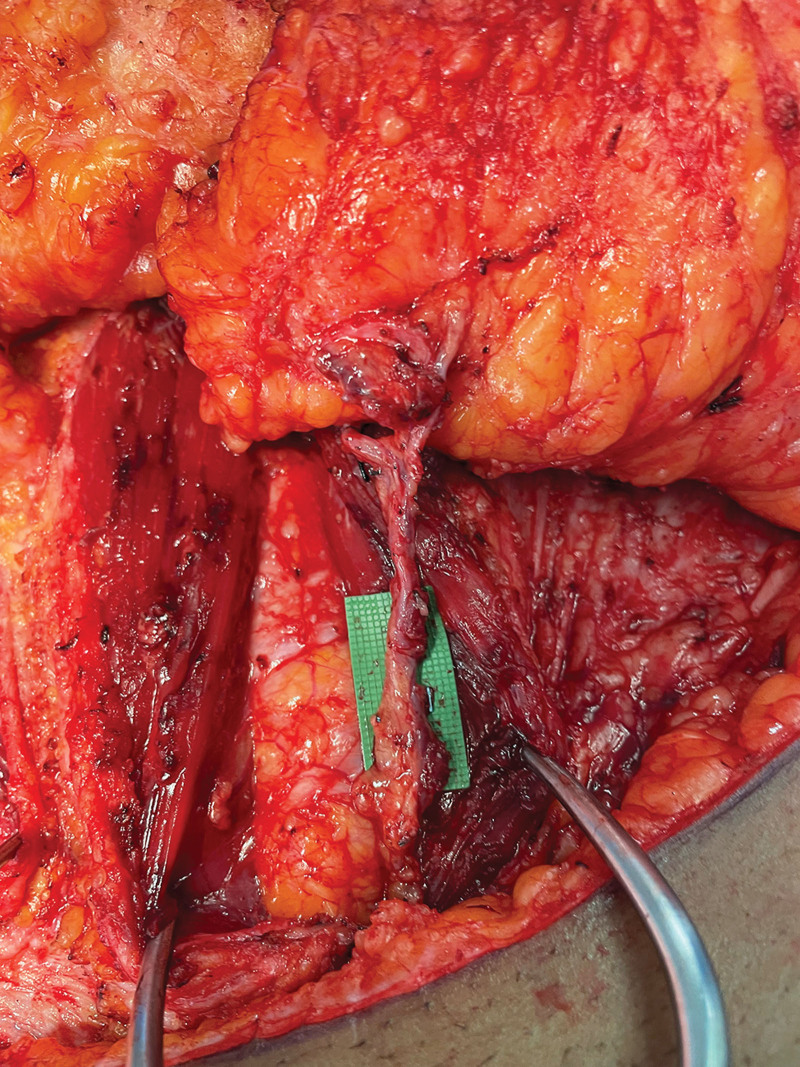
Intraoperatively, the incisions were made while leaving a 4-cm skin bridge laterally to allow supplemental perfusion to zone IV bilaterally and reduce the risk of necrosis to the distal flap tips. The flaps were then elevated based on the dominant perforators only, and the perforator dissection was carried through the rectus fascia. The pedicles were dissected to the origin near the external iliac arteries, whereas all other perforating and intramuscular vessels were divided (Fig. 3). The rectus fascia was then loosely repaired with interrupted suture, and the incisions were repaired in a layered manner.
Fig. 3.
Intraoperative photograph of the completely dissected perforator/pedicle.
The patient returned to the operating room 24 hours later for transfer. [See Video 2 (online), which displays Doppler ultrasound after the 24-hour surgical delay.] The internal mammary vessels were exposed, and the PAP flaps were harvested. The DIEP flaps were then de-epithelialized with clinically reassuring bleeding throughout. Due to the satisfactory bleeding in all four zones of the DIEP flap, no zones were discarded. The DIEP flaps were then harvested with ease given the delay procedure. The ipsilateral PAP and contralateral DIEP flaps were transferred to the corresponding breast with inset and anastomosis performed in the typical fashion.
Video 2. which displays Doppler ultrasound after the 24-hour surgical delay.
Postoperatively, all flaps remained healthy and viable on serial examinations, and the patient was subsequently discharged home with no major complications. On postoperative follow-up, the patient had healed well, with no areas of flap necrosis, and appropriate volume and symmetry was achieved.
DISCUSSION
The surgical delay phenomenon is a powerful tool to optimize flap vasculature; however, the optimal timing between delay and transfer remains uncertain and often varies according to surgeon preference. Historically, Myers and Cherry2 suggested a delay of 8–10 days to obtain optimal effect based on the study of the delay phenomenon in an animal model. More recent clinical studies have shown successful free tissue transfer in breast reconstruction utilizing a delay period of as little as 6 days.3
In physiologic studies performed by Dhar and Taylor,4 it was shown that, after an initial 3-hour period of vasoconstriction, the choke vessels progressively dilate and reach their maximum dilatation rate 48–72 hours after the surgical delay. As such, it is reasonable to question the timing between stages and evaluate when a clinically significant effect can be seen rather than the maximal effect. A shorter period between delay and transfer would allow full perforator and pedicle dissection and, subsequently, a less challenging flap harvest due to minimal adhesions and scarring. Additionally, a shortened delay period opens the possibility of performing both stages during the same hospital admission, potentially reducing healthcare costs.
In the case presented here, the authors considered whether the previous liposuction received by the patient may have influenced flap perfusion, as evidenced by the size of the perforators on the CTA in Figure 1. Although liposuction, in and of itself, may induce a degree of the delay phenomenon by disrupting the blood supply, the authors believe that the net effect of the subscarpal scarring associated with liposuction ultimately worsens flap perfusion. Therefore, it was felt that a surgical delay was the best course of action in this instance to optimize the flap vasculature and mitigate the negative effects of the scarring.
This technique is not without its drawbacks. The downsides to a 24-hour delay are similar to other surgical delay procedures, in that having an additional procedure increases anesthesia time and the risk for infection, bleeding, and other complications inherent to all surgical procedures. The additional procedure also increases the financial burden for patients, but this is somewhat offset in 24-hour delay cases compared with longer surgical delays when a second hospital admission is avoided. In our senior authors’ experience, they have had no issues receiving insurance approval for surgical delay procedures, regardless of the exact timeframe of the delay. Logistically, this technique requires two consecutive days of operating room block time, which may not always be feasible.
CONCLUSIONS
The authors demonstrate a successful autologous breast reconstruction utilizing a 24-hour surgical delay of DIEP flaps in the setting of unfavorable vascularity from previous liposuction. Future research endeavors comparing surgical delay timeframes and their effect on flap vasculature and clinical outcomes would assist in determining the optimal length of time between surgical delay and reconstruction.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Footnotes
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Christiano JG, Rosson GD. Clinical experience with the delay phenomenon in autologous breast reconstruction with the deep inferior epigastric artery perforator flap. Microsurgery. 2010;30:526–531. [DOI] [PubMed] [Google Scholar]
- 2.Myers MB, Cherry G. Augmentation of tissue survival by delay: an experimental study in rabbits. Plast Reconstr Surg. 1967;39:397–401. [DOI] [PubMed] [Google Scholar]
- 3.Hillberg NS, van Mulken TJM, Meesters-Caberg MAJ, et al. Autologous breast reconstruction with a delay procedure of the deep inferior epigastric artery perforator flap because of venous congestion of the flap on pedicle: a case series. Ann Plast Surg. 2019;82:537–540. [DOI] [PubMed] [Google Scholar]
- 4.Dhar SC, Taylor GI. The delay phenomenon: the story unfolds. Plast Reconstr Surg. 1999;104:2079–2091. [DOI] [PubMed] [Google Scholar]