Abstract
Substitution of Y223F disrupts the ability of simian immunodeficiency virus (SIV) Nef to down-modulate major histocompatibility complex (MHC) class I from the cell surface but has no effect on other Nef functions, such as down-regulation of CD4, CD28, and CD3 cell surface expression or stimulation of viral replication and enhancement of virion infectivity. Inoculation of three rhesus macaques with the SIVmac239 Y223F-Nef variant revealed that this point mutation consistently reverts and that Nef activity in MHC class I down-modulation is fully restored within 4 weeks after infection. Our results demonstrate a strong selective pressure for a tyrosine at amino acid position 223 in SIV Nef, and they constitute evidence that Nef-mediated MHC class I down-regulation provides a selective advantage for viral replication in vivo.
The Nef protein is a regulatory factor of human and simian immunodeficiency viruses (HIV and SIV, respectively) that is important for efficient viral replication and persistence in vivo (11, 20, 21). Several in vitro activities of HIV and SIV Nef which may be relevant for viral pathogenicity have been described. Nef down-regulates cell surface expression of CD4 (1, 4, 14, 38), CD28 (39), and major histocompatibility complex class I (MHC-I) molecules (25, 26, 34). It also alters the normal function of the T-cell-receptor (TCR)-CD3 signaling complex in T lymphocytes and modulates cellular activation pathways (3, 5, 18, 33, 35). Furthermore, Nef induces Fas ligand expression and might promote the killing of bystander cells (15, 41). Finally, Nef increases the infectivity of viral particles and enhances viral replication in primary lymphocytes and in human lymphoid tissue ex vivo (8, 12, 16, 24, 30, 36). Recent findings suggest that several conserved in vitro functions of Nef are independently selected in HIV type 1 (HIV-1)-infected individuals and contribute to AIDS pathogenesis (7). However, the exact role of Nef in AIDS pathogenesis still remains unclear.
Several lines of evidence suggest that MHC-I down-regulation might contribute to efficient viral replication and disease induction in vivo. Both HIV-1 and SIV proteins down-regulate steady-state expression of MHC-I complexes assembled with A and B, but not C, heavy chains from the surfaces of T lymphocytes and macrophages (9, 26). Selective down-regulation of HLA-A and HLA-B antigens protects HIV-infected cells from killing by cytotoxic T cells and by natural killer cells in vitro (9, 10). Such protection likely helps HIV-1 to evade the host immune response in vivo (for reviews, see references 13 and 31). Recently, it has been demonstrated that only nef alleles obtained during the asymptomatic phase of HIV-1 infection efficiently down-modulate MHC-I (7). Thus, a selective pressure for this particular Nef function apparently exists only in immunocompetent hosts.
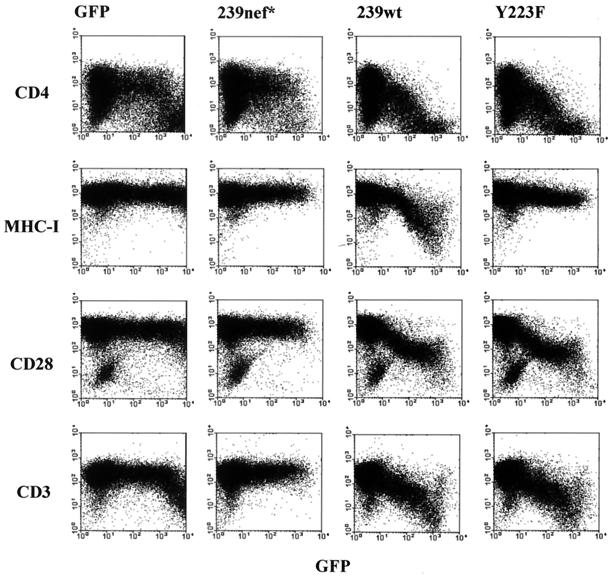
These results are highly suggestive. However, the physiological relevance of MHC-I down-regulation has been called into question, particularly since this effect requires relatively high Nef expression levels (25, 26, 34). To date no direct evidence that MHC-I down-regulation contributes to viral spread in vivo has been provided. To address this point, we analyzed a Nef variant of the pathogenic SIVmac239 clone, containing a Y223F substitution in the unique C-terminal region of Nef (38), both in vitro and in vivo in rhesus macaques. Flow cytometry analysis of Jurkat T cells, transfected with a bicistronic vector coexpressing Nef and green fluorescent protein (GFP) (28), confirmed previous reports (38, 39), showing that this alteration disrupts MHC-I down-regulation but does not affect down-regulation of cell surface expression of CD4, CD28, and CD3 molecules (Fig. 1). Furthermore, the Y223F-Nef increased SIV infectivity and replication, with an efficiency undistinguishable from that of 239wt Nef (reference 38 and data not shown). Thus, the Y223F substitution in SIV Nef selectively disrupts MHC-I down-regulation.
FIG. 1.
Substitution of Y223F in 239-Nef selectively disrupts down-regulation of MHC-I. Jurkat T cells were transiently transfected with a control vector expressing GFP alone or with plasmids coexpressing GFP and the truncated 239nef*, 239wt, or 239Y223F nef genes. CD4, MHC-I, CD28, and CD3 expression was analyzed by two-color flow cytometry.
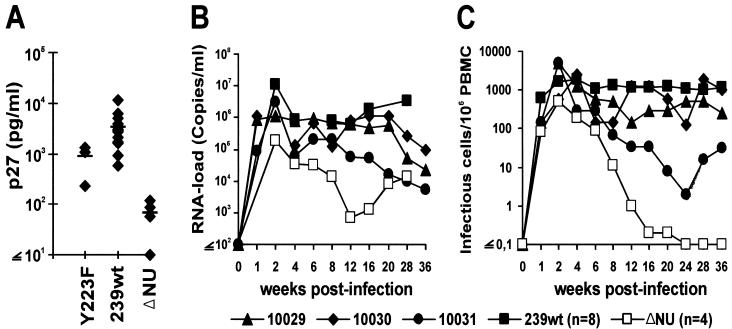
Three juvenile rhesus macaques, Mm10029, Mm10030, and Mm10031, were infected by intravenous inoculation of SIVmac239 Y223F-Nef, containing 5 ng of p27 produced by transfected 293T cells. The animals were healthy and seronegative for SIV, D-type retroviruses, and simian T-lymphotropic virus type 1 at the time of infection. Sera and cells were collected at regular intervals, and serological, virological, and immunological analyses of all samples were performed as described previously (6, 19, 24, 40). The same methods were used to quantitate viral replication in animals infected with the Y223F variant and in historical controls that received similar doses of virus. During acute infection, the levels of p27 gag antigenemia and of viral RNA in cell-free plasma for Mm10030 and Mm10031 were similar to those of some 239wt-infected animals (Fig. 2A and B). In the remaining animal, Mm10029, the amount of detectable p27 (226 pg/ml) was intermediate between SIVmac239wt (3,612 ± 2,741 pg/ml; n = 15) and ΔNU infection (68 ± 45 pg/ml; n = 4) (Fig. 2A). Thus, the Y223F-Nef variant was capable of enhancing SIVmac replication during acute infection.
FIG. 2.
Replication of the SIVmac239 Y223F variant in acutely infected rhesus macaques. (A) Peak levels of p27 plasma antigenemia observed at 2 weeks p.i. For comparison, values obtained from four macaques infected with ΔNU and from 15 animals infected with 239wt are indicated. (B) Viral RNA load. The detection limit for viral RNA is approximately 40 copies/ml (40). (C) Number of infectious cells per 1 million PBMC. For comparison, average values obtained from rhesus macaques infected with SIVmac239 ΔNU (□) or wild-type SIVmac239 (▪) are shown in panels B and C.
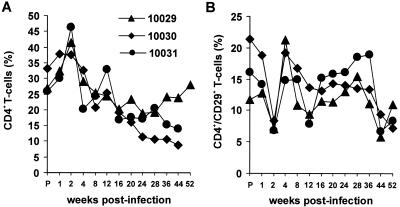
The three macaques that received the Y223F-Nef variant became chronically infected and survived the 1st year of infection. In contrast, about 60 to 70% of 239wt-infected animals die of AIDS within this period (20). Cell-associated viral loads were comparable or only slightly reduced compared to 239wt infection in Mm10029 and Mm10030 (Fig. 2C). Mm10031 showed about 100-fold-reduced frequencies of infectious peripheral blood mononuclear cells (PBMC) by week 20 after infection and very low levels of viral plasma RNA (Fig. 2B and C). The SIVmac239 Y223F-Nef-infected animals remained clinically healthy throughout the 56-week observation period. All three animals exhibited transient anemia to varying degrees from week 4 to 8 postinfection (p.i.). However, blood counts recovered thereafter. Mm10030 developed a moderate persistent lymphadenopathy by week 4 after infection. In Mm10030 and Mm10031 the percentage of CD4+ T cells declined slowly during the investigation period (Fig. 3A). Mm10029 also showed declining CD4+ lymphocyte counts until 28 weeks p.i., followed by partial recovery. The percentage of CD4+ CD29+ memory T cells decreased transiently at 2 weeks p.i. (Fig. 3B), when maximum levels of viral replication were observed (Fig. 2). Overall, the characteristics of infection with the Y223F-Nef variant were more similar to those of 239wt than nef-deleted infection. However, it is remarkable that the three macaques that received the Y223F variant are clinically healthy at 56 weeks p.i., whereas the majority of 239wt-infected macaques develop simian AIDS and die within the 1st year. Our results suggest that the Y223F variant might be more effectively controlled by the antiviral immune response than 239wt.
FIG. 3.
CD4+ T-cell counts in macaques infected with the SIVmac239 Y223F-Nef variant. Shown are percentages of total CD4+ T cells (A) and of CD4+CD29+ memory T cells (B) in peripheral blood of the three animals inoculated with the SIVmac239 Y223F-Nef variant.
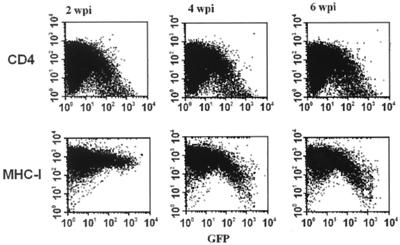
Point mutations in nef that disrupt important functions revert rapidly in infected animals (6, 20). Therefore, we analyzed the stability of the Y223F substitution in vivo. As shown in Fig. 4, Nef activity in MHC-I down-regulation was efficiently restored by 4 weeks p.i. Next, we investigated whether the phenotypic reversions correlated with the selection of revertants. SIV sequences spanning the entire nef gene were amplified from recombinant PBMC DNA or from viral plasma RNA as described previously (19, 24). Sequence analysis revealed that most nef alleles obtained after the acute phase of infection predicted reversion of Y223F→Y (data not shown). Reversions were consistently observed in all three animals, and forms containing a tyrosine at position 223 in Nef predominated throughout the course of infection.
FIG. 4.
Phenotypic reversions of the Y223F-Nef mutant in vivo. Viral RNA was isolated from plasma obtained from Mm10029 at the indicated number of weeks p.i. (wpi), reverse transcribed, and subjected to nested PCR. Amplified PCR fragments were cloned as a pool into a vector coexpressing Nef and GFP from a single bicistronic RNA. Subsequently, the effects of these nef alleles on CD4 and MHC-I expression were determined as described in Materials and Methods. Similar results were obtained with plasma samples obtained from Mm10030 and Mm10031.
Our results demonstrate that a point mutation of Y223F in SIVmac239 Nef reverts efficiently in infected macaques. The Y223F change selectively disrupts MHC-I down-regulation, having no detectable effect on functional Nef activity in down-modulation of CD3, CD28, and CD4 cell surface expression. Furthermore, it did not reduce the ability of Nef to increase the infectivity of viral particles or to stimulate SIV replication in PBMC or in 221 cells. Therefore, our results are evidence that Nef-mediated MHC-I down-regulation is associated with a selective advantage for SIVmac replication in vivo. Our findings are in agreement with those of a recent study suggesting an important role of the C-terminal domain of SIVmac Nef in viral pathogenesis (23).
Mutation of Y223F consistently reverted between 2 and 4 weeks after infection. The efficiency of reversion indicates a strong selective pressure for a tyrosine at position 223 in SIV Nef. However, stop codons in Nef usually revert even faster, within 1 to 2 weeks p.i. (20). The slightly delayed selection of revertants might be explained by the fact that the specific nucleotide change required to restore Y223 simply takes more time to occur than the changes required for reversion of a premature stop codon. However, we have previously observed that even three point mutations in Nef can revert within 2 weeks p.i. (6). Notably, in SIV-infected macaques the virus-specific cytotoxic T-lymphocyte (CTL) response usually peaks after about 2 weeks (22). Thus, the efficient selection of revertants between weeks 2 and 4 p.i. likely reflects the selective pressure to restore the ability of Nef to down-regulate MHC-I due to an efficient antiviral CTL response.
The Y223F mutation that disrupted MHC-I down-regulation predominated only very early after infection. The forms that evolved thereafter expressed wild-type Nef and replicated relatively efficiently compared to SIV with nef deleted. Notably, however, all three animals showed a nonprogressor or slow-progressor phenotype. These findings suggest that the inability of the virus to down-modulate MHC-I during the acute phase of infection might have long-term beneficial effects on the clinical outcome of infection. It has been shown that the levels of HIV-1 replication during acute infection are an important prognostic marker for disease progression and that long-lasting effects can be obtained by short-term antiviral therapy during primary HIV-1 and SIV infection (27, 32, 37). Down-regulation of MHC-I by Nef likely protects infected T cells from recognition and destruction by the host immune system (10). The Y223F mutation should enhance viral propagation and provide a selective advantage for SIV replication only after the onset of the antiviral immune response. It was beyond the scope of this study to quantitate the CTL responses in both Y223F-Nef- and 239wt-infected animals. It is conceivable, however, that impaired MHC-I down-regulation early after infection results in enhanced presentation of viral antigens and enables the infected host to mount stronger CTL responses, resulting in more-efficient immune control than in 239wt infected-macaques.
SIV and HIV-1 Nef proteins use different surfaces to down-regulate MHC-I cell surface expression (2, 17, 25, 29, 38). Therefore, our results with the SIV macaque model cannot be directly applied to HIV-1-infected humans. Nonetheless, we believe that MHC-I down-regulation likely plays a similar role in efficient viral persistence of both HIV-1 and SIVmac infection. First, both HIV-1 and SIV Nef proteins use a similar mechanism to down-regulate MHC-I expression, which requires the conserved tyrosine residue Y320 in class I heavy chains and involves the accelerated endocytosis of the MHC-I complex from the cell surface (38). Second, we have recently demonstrated that nef alleles obtained from asymptomatic HIV-1-infected individuals, but not those derived from AIDS patients, effectively down-modulate MHC-I (7). Concordant with the results of the present study, these findings strongly suggest that MHC-I down-regulation by both SIV and HIV-1 Nef contributes to viral spread in vivo.
Much remains to be done to fully elucidate the relative contributions of the different in vitro Nef activities to viral pathogenesis. However, accumulating evidence suggests that multiple Nef functions, including CD4 down-regulation, contribute to efficient replication in vivo (7, 19). Furthermore, independent Nef functions are modulated during disease progression to optimize viral spread at different clinical stages of HIV-1 infection (7). Thus, HIV-1 and SIV have evolved complex mechanisms for efficient viral persistence in vivo. We utilized the Y223F mutation because it was highly selective for MHC-I down-regulation and because a single homologous substitution minimizes the risk that additional Nef functions or interactions are affected. While this possibility cannot be entirely dismissed, it is highly likely that the reversion of the Y223F substitution indeed reflects a selective pressure for Nef-mediated MHC-I down-regulation in vivo. However, because of the efficient and rapid selection of revertant viruses, our study does not provide information on the consequences of inefficient class I down-modulation for viral persistence and disease progression. Therefore, studies with SIVmac239 mutants containing difficult-to-revert deletions in the C-terminal domain of Nef are required to further clarify the importance of MHC-I down-regulation for SIV replication and AIDS pathogenesis in infected rhesus macaques.
Acknowledgments
We thank Mandy Krumbiegel and Nathaly Finze for excellent technical assistance; Ronald C. Desrosiers, Jacek Skowronski, and John Iafrate for helpful discussions and for sharing unpublished results; and Ingrid Bennett for critical reading of the manuscript. We also thank Bernhard Fleckenstein for constant support and encouragement and Peter Ten Haaft and Jonathan L. Heeney for quantitation of viral RNA loads.
This work was supported by the Wilhelm-Sander Foundation, BMBF grant 01Ki9478, and the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Aiken C, Konner J, Landau N R, Lenburg M E, Trono D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1994;76:853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 2.Akari H, Arold S, Fukumori T, Okazaki T, Strebel K, Adachi A. Nef-induced major histocompatibility complex class I down-regulation is functionally dissociated from its virion incorporation, enhancement of viral infectivity, and CD4 down-regulation. J Virol. 2000;74:2907–2912. doi: 10.1128/jvi.74.6.2907-2912.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander L, Du Z, Rosenzweig M, Jung J U, Desrosiers R C. A role for natural simian immunodeficiency virus and human immunodeficiency virus type 1 nef alleles in lymphocyte activation. J Virol. 1997;71:6094–6099. doi: 10.1128/jvi.71.8.6094-6099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson S, Shugars D C, Swanstrom R, Garcia J V. Nef from primary isolates of human immunodeficiency virus type 1 suppresses surface CD4 expression in human and mouse T cells. J Virol. 1993;67:4923–4931. doi: 10.1128/jvi.67.8.4923-4931.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell I, Ashman C, Maughan J, Hooker E, Cook F, Reinhart T A. Association of simian immunodeficiency virus Nef with the T-cell receptor (TCR) ζ chain leads to TCR down-modulation. J Gen Virol. 1998;79:2717–2727. doi: 10.1099/0022-1317-79-11-2717. [DOI] [PubMed] [Google Scholar]
- 6.Carl S, Iafrate A J, Lang S M, Stahl-Hennig C, Kuhn E M, Fuchs D, Mätz-Rensing K, ten Haaft P, Heeney J L, Skowronski J, Kirchhoff F. The acidic region and conserved putative protein kinase C phosphorylation site in Nef are important for SIV replication in rhesus macaques. Virology. 1999;257:138–155. doi: 10.1006/viro.1999.9645. [DOI] [PubMed] [Google Scholar]
- 7.Carl S, Greenough T C, Krumbiegel M, Greenberg M, Skowronski J, Sullivan J L, Kirchhoff F. Modulation of different human immunodeficiency virus type 1 Nef functions during progression to AIDS. J Virol. 2001;75:3657–3665. doi: 10.1128/JVI.75.8.3657-3665.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chowers M Y, Spina C A, Kwoh T J, Fitch N J, Richman D D, Guatelli J C. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J Virol. 1994;68:2906–2914. doi: 10.1128/jvi.68.5.2906-2914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen G B, Gandhi R T, Davis D M, Mandelboim O, Chen B K, Strominger J L, Baltimore D. The selective dowregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10:661–671. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 10.Collins K L, Chen B K, Kalams S A, Walker B D, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 11.Deacon N J, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker D J, McPhee D A, Greenway A L, Ellett A, Chatfield C. Genomic structure of an attenuated quasispecies of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 12.deRonde A, Klaver B, Keulen W, Smit L, Goudsmit J. Natural HIV-1 NEF accelerates virus replication in primary human lymphocytes. Virology. 1992;188:391–395. doi: 10.1016/0042-6822(92)90772-h. [DOI] [PubMed] [Google Scholar]
- 13.Desrosiers R C. Strategies used by human immunodeficiency virus that allow persistent viral replication. Nat Med. 1999;5:723–725. doi: 10.1038/10439. [DOI] [PubMed] [Google Scholar]
- 14.Garcia J V, Miller A D. Serine phosphorylation-independent down-regulation of cell-surface CD4 by nef. Nature. 1991;350:508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- 15.Geleziunas R, Xu W, Takeda K, Ichijo H, Greene W C. HIV-1 Nef inhibits ASK1-dependent death signalling, providing a potential mechanism for protecting the infected host cell. Nature. 2001;410:834–838. doi: 10.1038/35071111. [DOI] [PubMed] [Google Scholar]
- 16.Glushakova S, Grivel J C, Suryanarayana K, Meylan P, Lifson J D, Desrosiers R C, Margolis L. Nef enhances human immunodeficiency virus replication and responsiveness to interleukin-2 in human lymphoid tissue ex vivo. J Virol. 1999;73:3968–3974. doi: 10.1128/jvi.73.5.3968-3974.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenberg M E, Iafrate A J, Skowronski J. The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J. 1998;17:2777–2789. doi: 10.1093/emboj/17.10.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iafrate A J, Bronson S, Skowronski J. Separable functions of Nef disrupt two aspects of T cell receptor machinery: CD4 expression and CD3 signaling. EMBO J. 1997;16:673–684. doi: 10.1093/emboj/16.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iafrate A J, Carl S, Bronson S, Stahl-Hennig C, Swigut T, Skowronski J, Kirchhoff F. Disrupting surfaces of Nef required for down-regulation of CD4 and for enhancement of virion infectivity attenuates simian immunodeficiency virus replication in vivo. J Virol. 2000;74:9836–9844. doi: 10.1128/jvi.74.21.9836-9844.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kestler H W, Ringler D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 21.Kirchhoff F, Greenough T C, Brettler D B, Sullivan J L, Desrosiers R C. Absence of intact nef sequences in a long-term, nonprogressing survivor of HIV-1 infection. N Engl J Med. 1995;332:228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 22.Kuroda M J, Schmitz J E, Charini W A, Nickerson C E, Lifton M A, Lord C I, Forman M A, Letvin N L. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J Immunol. 1999;162:5127–5133. [PubMed] [Google Scholar]
- 23.Lafont B A, Riviere Y, Gloecker L, Beyer C, Hurtrel B, Kieny M P, Kirn A, Aubertin A M. Implication of the C-terminal domain of Nef in the reversion to pathogenicity of attenuated SIV macBK28–41 in macaques. Virology. 2000;266:286–298. doi: 10.1006/viro.1999.9991. [DOI] [PubMed] [Google Scholar]
- 24.Lang S M, Iafrate A J, Stahl-Hennig C, Kuhn E M, Nißlein T, Haupt M, Hunsmann G, Skowronski J, Kirchhoff F. Association of simian immunodeficiency virus Nef with cellular serine/threonine kinases is dispensable for the development of AIDS in rhesus macaques. Nat Med. 1997;3:860–865. doi: 10.1038/nm0897-860. [DOI] [PubMed] [Google Scholar]
- 25.Le Gall S, Prevost M C, Heard J M, Schwartz O. Human immunodeficiency virus type 1 Nef independently affects virion incorporation of major histocompatibility complex class I molecules and virus infectivity. Virology. 1997;229:295–301. doi: 10.1006/viro.1996.8417. [DOI] [PubMed] [Google Scholar]
- 26.Le Gall S, Erdtmann L, Benichou S, Berlioz-Torrent C, Liu L, Benarous R, Heard J M, Schwarz O. Nef interacts with the mu subunit of clathrin adapter complexes and reveals a cryptic sorting signal in MHC-I molecules. Immunity. 1998;8:483–495. doi: 10.1016/s1074-7613(00)80553-1. [DOI] [PubMed] [Google Scholar]
- 27.Lisziewicz J, Rosenberg E, Lieberman J, Jessen H, Lopalco L, Siliciano R, Walker B, Lori F. HIV control following discontinuation of antiretroviral therapy. N Engl J Med. 1999;340:1683–1684. doi: 10.1056/NEJM199905273402114. [DOI] [PubMed] [Google Scholar]
- 28.Lock M, Greenberg M E, Iafrate A J, Swigut T, Münch J, Kirchhoff F, Shohdy N, Skowronski J. Two elements target SIV Nef to the AP-2 clathrin adaptor complex, but only one is required for the induction of CD4 endocytosis. EMBO J. 1999;18:2722–2733. doi: 10.1093/emboj/18.10.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mangasarian A, Piguet V, Wang J K, Chen Y L, Trono D. Nef-induced CD4 and major histocompatibility complex class I (MHC-I) down-regulation are governed by distinct determinants: N-terminal α helix and proline repeat of Nef selectively regulate MHC-I trafficking. J Virol. 1999;73:1964–1973. doi: 10.1128/jvi.73.3.1964-1973.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller M D, Warmerdam M T, Gaston I, Greene W C, Feinberg M B. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J Exp Med. 1994;179:101–114. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ploegh H L. Viral strategies of immune evasion. Science. 1998;280:248–253. doi: 10.1126/science.280.5361.248. [DOI] [PubMed] [Google Scholar]
- 32.Rosenwirth B, ten Haaft P, Bogers W M J M, Nieuwenhuis I G, Niphuis H, Kuhn E-M, Bischofberger N, Heeney J L, Überla K. Antiretroviral therapy during primary immunodeficiency virus infection can induce persistent suppression of virus load and protection from heterologous challenge in rhesus macaques. J Virol. 2000;74:1704–1711. doi: 10.1128/jvi.74.4.1704-1711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schrager J A, Marsh J W. HIV-1 Nef increases T cell activation in a stimulus-dependent manner. Proc Natl Acad Sci USA. 1999;96:8167–8172. doi: 10.1073/pnas.96.14.8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard J M. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 35.Skowronski J, Parks D, Mariani R. Altered T cell activation and development in transgenic mice expressing the HIV-1 nef gene. EMBO J. 1993;12:703–713. doi: 10.1002/j.1460-2075.1993.tb05704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spina C A, Kwoh T J, Chowers M Y, Guatelli J C, Richman D D. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J Exp Med. 1994;179:115–123. doi: 10.1084/jem.179.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spring M, Stahl-Hennig C, Stolte N, Bischofberger N, Heeney J L, ten Haaft P, Tenner-Racz K, Racz P, Lorenzen D, Hunsmann G, Dittmer U. Enhanced cellular immune response and reduced CD8+ lymphocyte apoptosis in acutely SIV-infected rhesus macaques after short-term antiretroviral treatment. Virology. 2001;279:221–232. doi: 10.1006/viro.2000.0720. [DOI] [PubMed] [Google Scholar]
- 38.Swigut T, Iafrate A J, Münch J, Kirchhoff F, Skowronski J. Simian and human immunodeficiency virus Nef proteins use different surfaces to down-regulate class I major histocompatibility antigen expression. J Virol. 2000;74:5691–5701. doi: 10.1128/jvi.74.12.5691-5701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swigut T, Shody N, Skowronski J. Mechanism for down-regulation of CD28 by Nef. EMBO J. 2001;20:1593–1604. doi: 10.1093/emboj/20.7.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.ten Haaft P, Verstrepen B, Überla K, Rosenwirth B, Heeney J. A pathogenic threshold of virus load defined in simian immunodeficiency virus- or simian-human immunodeficiency virus-infected macaques. J Virol. 1998;72:10281–10285. doi: 10.1128/jvi.72.12.10281-10285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu X N, Laffert B, Screaton G R, Kraft M, Wolf D, Kolanus W, Mongkolsapay J, McMichael A J, Baur A S. Induction of Fas ligand expression by HIV involves the interaction of Nef with the T cell receptor ζ chain. J Exp Med. 1999;189:1489–1496. doi: 10.1084/jem.189.9.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]