Abstract
Short-chain fatty acids (SCFAs) are the main metabolites produced by bacterial fermentation of dietary fiber within gastrointestinal tract. SCFAs produced by gut microbiotas (GMs) are absorbed by host, reach bloodstream, and are distributed to different organs, thus influencing host physiology. However, due to the limited budget or the poor sensitivity of instruments, most studies on GMs have incomplete blood SCFA data, limiting our understanding of the metabolic processes within the host. To address this gap, we developed an innovative multi-task multi-view integrative approach (M2AE, Multi-task Multi-View Attentive Encoders), to impute blood SCFA levels using gut metagenomic sequencing (MGS) data, while taking into account the intricate interplay among the gut microbiome, dietary features, and host characteristics, as well as the nuanced nature of SCFA dynamics within the body. Here, each view represents a distinct type of data input (i.e., gut microbiome compositions, dietary features, or host characteristics). Our method jointly explores both view-specific representations and cross-view correlations for effective predictions of SCFAs. We applied M2AE to two in-house datasets, which both include MGS and blood SCFAs profiles, host characteristics, and dietary features from 964 subjects and 171 subjects, respectively. Results from both of two datasets demonstrated that M2AE outperforms traditional regression-based and neural-network based approaches in imputing blood SCFAs. Furthermore, a series of gut bacterial species (e.g., Bacteroides thetaiotaomicron and Clostridium asparagiforme), host characteristics (e.g., race, gender), as well as dietary features (e.g., intake of fruits, pickles) were shown to contribute greatly to imputation of blood SCFAs. These findings demonstrated that GMs, dietary features and host characteristics might contribute to the complex biological processes involved in blood SCFA productions. These might pave the way for a deeper and more nuanced comprehension of how these factors impact human health.
Keywords: Short-Chain Fatty Acids, Gut Microbiotas, Metagenome, Imputation, Deep learning
Introduction
Short-chain fatty acids (SCFAs) are vital metabolites produced by the bacterial fermentation of dietary fiber in the gastrointestinal tract 1. In a healthy gut, common bacteria such as Prevotella, Bacteroides, Ruminococcaceae, and Lachnospiraceae 2, 3 generate principal SCFAs, including acetate, propionate, and butyrate. These SCFAs are absorbed and distributed to various organs, influencing host physiology by maintaining an intestinal anaerobic environment and regulating energy metabolism 4-7. Given the effects of blood SCFAs on host health, understanding the factors that regulate their production is important for the development of strategies to modulate blood SCFA levels to promote health and prevent diseases 8.
Recent studies have shown that fermentative bacteria species such as Faecalibacterium prausnitzii and Eubacterium rectale, which are abundant in the human gut, could efficiently metabolize complex carbohydrates, particularly resistant starches, into butyrate 9 Wang et al. found that an imbalance of “Good” and “Bad” gut microbiota led to the attenuation of the bacterial metabolite SCFAs 10, 11. These findings demonstrated that gut microbiotas (GMs) are crucial determinants for blood SCFAs. Dietary features, specifically fibers and macronutrients (i.e., fat, protein, and carbohydrate) intake, are pivotal, as they determine the substrate availability for microbial fermentation in the gut, which subsequently impacts the synthesis of SCFAs 12. Meanwhile, host characteristics (e.g., age, race) can significantly modulate blood levels of SCFAs by influencing the synthesis, uptake, and utilization of blood SCFAs within the body, possibly through the direct or indirect regulation of metabolic processes and immune responses 13. For instance, the increase in blood SCFAs may be due to increased uptake of SCFAs in the colon, in part due to increased nutrient intake, a complete bypass of SCFA transporters and increased passive uptake of SCFAs 14. Despite the growing interest in SCFAs research, the majority of existing research does not fully account for the complex interplay among host characteristics, dietary features, and GMs, which are crucial for generating accurate results across a wide range of applications 15.
Due to the limited budget or the poor sensitivity of instruments, most of current studies focusing on the gut microbiome are lacking in complete measurements of blood SCFAs 16, which can limit subsequent analyses and conceivably results in the neglect of pivotal insights into metabolic processes within the host. Gut microbiome as measured by metagenomic sequencing (MGS) data can determine SCFA concentrations, influencing host phenotypes by affecting metabolism, immune responses, and energy homeostasis 17, 18. Integrating SCFA data with MGS data enables multi-omic analyses that reveal broader metabolic impacts and potential links between gut microbiota composition and physiological or disease-related outcomes 19. Hence, developing a model to impute blood SCFA levels using the MGS data is beneficial and essential for advancing our understanding in this field. To the best of our knowledge, the imputation of blood SCFAs in the host using the MGS data remains largely untapped, marking a significant gap in our comprehension of the dynamics and implications of blood SCFAs production. By developing predictive models that integrate gut microbial compositions with host characteristics and dietary features, we can better understand the complex interplay between these variables and their impacts on blood SCFA production, potentially paving the way for personalized interventions to optimize blood SCFA levels and promote overall well-being 20.
In this study, leveraging metagenomic sequencing technology and deep learning methods, we unveiled an innovative approach that captures the intricate interplays among GMs, dietary features, and host characteristics to impute the absolute abundances of human blood SCFAs. Our method addresses the challenge of incomplete SCFA data by imputing it using MGS data, which will further facilitate the integration of SCFAs and MGS. This integration will enable more comprehensive multi-omic analyses, providing deeper insights into the influence of gut microbial composition on SCFA levels and their subsequent impact on host phenotypes in future research. By applying our approach to two in-house generated datasets, we demonstrated our model outperforms traditional regression-based and neural-network based approaches in imputing blood SCFAs. Accurate imputation of incomplete blood SCFA data will enable researchers to conduct more comprehensive studies exploring metabolic processes and their potential implications for health.
Methods
Subject recruitment and sample collection
A total of 964 unrelated males, aged 20-51 years, were recruited for this study as the first dataset (Dataset 1). An additional 171 unrelated subjects, aged 20-85 years, were recruited for this study, forming the second dataset (Dataset 2). All the subjects were living in New Orleans, Louisiana and its surrounding areas. We excluded subjects who had chronic or recent temporary conditions (e.g., gastroenteritis or inter-continental travel in the past 3 months) that may have significantly disturbed gut microbiota compositions, as described previously 21-27. Each subject provided stool and blood samples for metagenomic and SCFA profiling, respectively. We used the OMNIgene•GUT (OMR-200) all-in-one system (DNA GenoTEK, Ottawa, CA) for stool sample collection. Stool samples were frozen at −80°C after sample procurement until DNA extraction. Serum (for 964 subjects) or plasma (for 171 subjects) was extracted from 10 ml of blood samples from each subject according to the protein precipitation protocol 28 developed for metabolomics analysis, aliquoted, and stored at −80°C until used for further analysis. The 964 subjects in the first dataset (Dataset 1) also completed three questionnaires—the Louisiana Osteoporosis Questionnaire, the Metagenomic Study Supplementary Questionnaire, and the Food Frequency Questionnaire—to provide relevant covariate information (e.g., demographic factors, lifestyle factors and dietary features). The 171 subjects in the second dataset (Dataset 2) completed only two questionnaires—the Louisiana Osteoporosis Questionnaire and the Metagenomic Study Supplementary Questionnaire—to provide similar covariate information (e.g., demographic factors, but part of lifestyle factors and dietary features). Each subject signed an informed consent, and the study protocols were approved by the Institutional Review Boards (IRBs) of Tulane University. All data were treated with confidentiality, ensuring the anonymity of the participants.
Metagenome profiling
Metagenomic DNA was extracted from stool samples using the Nucleospin Soil kit (MACHEREY-NAGEL) according to manufacturer’s instructions, as previously described 19, 29-33. After a few washes, DNA was eluted with 50 μl elution buffer and stored at −80°C until used for further sequencing. For Dataset 1, 530 samples were sequenced at LC Sciences (Houston, TX), and 434 samples were sequenced at BGI Americas (Cambridge, MA). For Dataset 2, all the 171 subjects were sequenced at LC Sciences.
For the samples sequenced in LC Sciences (Houston, TX), the DNA library was constructed by TruSeq Nano DNA LT Library Preparation Kit (Illumina Inc.). And then we performed the paired-end 2×150 bp sequencing on an Illumina Hiseq 4000 platform at the LC Sciences following the vendor's recommended protocol.
Raw sequencing reads were processed to obtain valid reads for further analysis. First, sequencing adapters were removed from sequencing reads using cutadapt v1.9 34. Secondly, low quality reads were trimmed by fqtrim v0.94 35 using a sliding-window algorithm. Thirdly, reads were aligned to the host genome using bowtie2 v2.2.0 36 to remove host contamination. Once quality-filtered reads were obtained, they were de novo assembled to construct the metagenome for each sample by IDBA-UD v1.1.1 37. All coding regions (CDS) of metagenomic contigs were predicted by MetaGeneMark v3.26 38. CDS sequences of all samples were clustered by CD-HIT v4.6.1 39 to obtain unigenes. Unigene abundance for a certain sample were estimated by TPM based on the number of aligned reads by bowtie2 v2.2.0 36. The lowest common ancestor taxonomy of unigenes were obtained by aligning them against the NCBI NR database by DIAMOND v 0.9.40.
For samples sequenced in BGI Americas, the sequencing library was generated using MGI Easy Universal DNA Library Prep Set Kit (MGI Inc.). The established library was sequenced on BGI DNBSEQ platform using the 100 bp pair-end sample preparation protocol. Quality control (QC) of raw reads was performed using Fastp 41 to filter low-quality reads. The high-quality reads were aligned to the host genome using bowtie2 36 to remove human reads. The gene profiles were generated by aligning high-quality sequencing reads to the 9.9M integrated gene catalog (IGC) by using the Human Microbiome Project Unified Metabolic Analysis Network (HUMAnN2) 42 .
Serum/Plasma SCFA profiling
Eight SCFAs (acetic acid, propionic acid, isobutyric acid, butyric acid, 2-methylbutyric acid, isovaleric acid, valeric acid and hexanoic acid) in serum/plasma samples were analyzed by Metabolon Inc. (Durham, NC) using LC-MS/MS, as previously described 29-33. The serum/plasma samples were spiked with stable labelled internal standards and were homogenized and subjected to protein precipitation with an organic solvent. After centrifugation, an aliquot of the supernatant was derivatized. The reaction mixture was injected onto an Agilent 1290/AB Sciex QTrap 5500 LC MS/MS system equipped with a C18 reversed phase UHPLC column. The mass spectrometer was operated in negative mode using electrospray ionization (ESI). The peak area of the individual analyte product ions was measured against the peak area of the product ions of the corresponding internal standards. Quantitation was performed using a weighted linear least squares regression analysis generated from fortified calibration standards prepared immediately prior to each run. LC-MS/MS raw data were collected and processed using SCIEX OS-MQ software v1.7. Three levels of QC samples were prepared by diluting with phosphate-buffered saline (PBS) and/or spiking with stock solutions to obtain the appropriate concentrations for each level (low-concentration QC, medium-concentration QC, and high-concentration QC). Sample analysis was carried out in a 96-well plate format containing two calibration curves to determine SCFA concentrations and six QC samples (per plate) to monitor assay performance. Accuracy was evaluated using the corresponding QC replicates in the sample runs. QCs met acceptance criteria at all levels for all analytes. QC acceptance criteria are at least 50% of QC samples at each concentration level per analyte should be within ±20.0% of the corresponding historical mean, and at least 2/3 of all QC samples per analyte should fall within ±20.0% of the corresponding historical mean.
While SCFA levels were measured in Datasets 1 and 2 using serum and plasma samples, respectively, SCFA concentrations tend to be highly consistent between serum and plasma samples 43, 44. This consistency arises because both serum and plasma SCFAs reflect similar metabolic states and distributions in the bloodstream 44. Thus, validating the model on these two datasets is appropriate, as both serum and plasma SCFA data provide reliable and comparable insights into SCFA dynamics.
Data preprocessing
To remove noise and experimental artifacts in the data and better interpret the results, proper preprocessing for each view data is essential. For gut metagenomic sequencing data, we kept only the GM species that exist in all the subjects for data harmonization across the two data sets. For dietary and clinical data, we filtered out variables with missing rate > 20% and kept all the variables that, to our knowledge 45-48, could be pertinent to the study. Missing data for dietary and host characteristics data were imputed using multiple imputation through R package 'mice' 49. Missing values in SCFAs were imputed with the minimum of values of all subjects for each SCFA (missing rate <1%). We randomly selected 75% of the samples as the training set and the remaining 25% of the samples in the dataset as the test set. To avoid potential bias, the training data and testing data have been processed for data normalization separately. We applied log normalization to each type of data, transforming the features to reduce skewness and bring the values into a comparable scale.
Overview of Multi-task Multi-View Attentive Encoders (M2AE) model
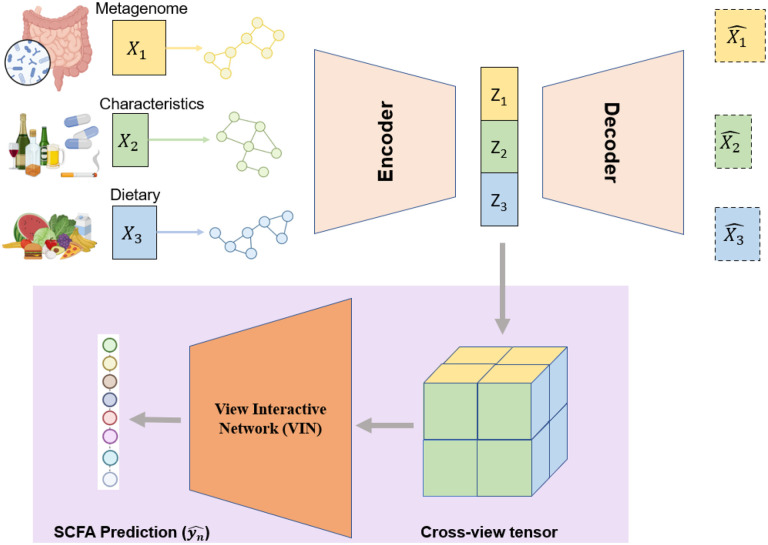
M2AE is a framework for prediction tasks with multi-view data as input. Each view corresponds to a distinct category of data input, i.e., gut microbiome compositions, dietary features, or host characteristics. The workflow of M2AE is shown in Fig. 1 and can be summarized into two components. (1) View-specific representation learning via attentive encoders. For each view, an attentive encoder is designed in a symmetric auto-encoder fashion, where the encoder part is composited with one graph convolutional module and two fully-connected layers for view-specific representation learning. (2) Multi-view integration via the View Interactive Network (VIN). A cross-view interactive tensor is calculated using the latent representations from all the view-specific networks. A VIN is then trained with the cross-view discovery tensor to produce the final predictions. VIN can effectively learn the intra-view and inter-view correlations in the higher-level space for better prediction with multi-view data. M2AE is an end-to-end model, where both view-specific attentive encoders and VIN module are trained jointly. We describe each component in detail in the following sections.
Fig. 1. Overview of M2AE.
M2AE is a framework for prediction tasks with multi-view data as input. The workflow of M2AE can be summarized into two components. (1) View-specific representation learning via attentive encoders. For each view, an attentive encoder was designed in a symmetric auto-encoder fashion, where the encoder part is composed with one graph convolutional module and two fully-connected layers for view-specific representation learning. (2) Multi-view integration via the View Interactive Network (VIN). A cross-view interactive tensor was calculated using the latent representations from all the view-specific networks. A VIN was then trained with the cross-view discovery tensor to produce the final predictions. VIN can effectively learn the intra-view and inter-view correlations in the higher-level space for better prediction with multi-view data. M2AE is an end-to-end model, where both view-specific attentive encoders and VIN module are trained jointly.
Attentive encoders (AEs) for view-specific representation learning
We design each attentive encoder (AE) in an autoencoder manner with one encoder and one symmetric decoder. The encoder contains one graph convolutional module and two fully-connected layers for view-specific representation learning of each type of data input.
The graph convolutional module is implemented to map node features to low-dimensional space and utilizes a simple inner product layer to aggregate the features for feature embedding 50. By viewing each sample as a node, a view-specific graph can be constructed for each type of view by utilizing both the features (relative microbial abundance/dietary features/host characteristics) of each node and the relationships between nodes. Specifically, in each view, the input sample feature matrix contains the features of all samples, where is the number of samples and is the number of features. The input adjacency matrix characterizes the relationships between samples by computing the cosine similarity among pairs of nodes and edges. Thus, the graph convolutional module can be built by stacking multiple convolutional layers with each layer defined as:
| (1) |
where is the input of the layer and is the weight matrix of the -th layer and . denotes a non-linear activation function. For , it states the adjacency between node and node in the graph and is calculated as:
| (2) |
where and are the feature vectors of node and node , respectively. is the cosine similarity between node and node . The threshold is determined given a parameter , which represents the average number of edges per node that are retained including self-connections:
| (3) |
where is the indicator function. The parameter in Eq. (3) is tuned over 51 with the training data, and the same value is adopted across all experiments on the same dataset. Note that for , will turn out to be an identity matrix.
Our graph convolutional module will output a latent feature per view, which is then fed into the subsequent two fully-connected layers generating a further latent feature for each view. Thus, the adjacency matrix in the decoder is calculated as 52, which is sent to the decoder to reconstruct the original input.
For the model training, we aim to minimize the mean absolute error (MAE) between the input feature matrix and the reconstructed matrix for all views:
| (4) |
where represents the mean absolute error function. is the input feature of the sample and feature, is the predicted feature of the sample and feature.
So far, we have learned the view-specific representation and we will introduce to fuse each view for the final prediction task in the following section.
VIN for multi-view integration
Current approaches leveraging multi-view data for biomedical prediction tasks traditionally either concatenate features from disparate views directly or fuse these features within a low-level feature space 53-56. However, properly aligning multiple views remains a consistent challenge, as improper alignment can have detrimental effects. On the other hand, view correlation discovery network (VCDN) 57 can exploit the higher-level cross-view correlations in class label level, as different types of data can provide unique distinctiveness for the production of SCFAs. Inspired by this, we develop VIN, which consolidates three latent features from gut microbiome, dietary features, and host characteristics to learn higher-level intra-view and cross-view correlations, thereby improving SCFA predictions.
For the latent representations of the sample from three types of views , , we construct a cross-view interactive tensor , where each entry of is calculated as:
| (5) |
where denotes the entry of . Then, the obtained tensor is reshaped to a dimensional vector and is forwarded to the final prediction. is designed as a network with one graph convolutional layer and one fully-connected layer with the output dimension of (In this case, we have eight SCFAs as outputs, so we set ). We aim to minimize the mean absolute error between the predicted and ground-truth SCFAs as:
| (6) |
where represents the absolute abundances of eight SCFAs in the sample, represents the sample size.
To this end, could reveal the latent intra-view and cross-view correlations and help to improve the learning performance. By utilizing to integrate latent representations from different types of views, the final prediction made by M2AE is based on both the latent representation from each view and the learned cross-view correlation knowledge.
Overall, we optimize our M2AE by minimizing the attentive encoder loss and view interactive network losses in an iterative manner. During one epoch of the training process, we first fix and update , , for each type of view to minimize the loss function . Then we fix the view-specific AEs and update to minimize . View-specific AEs and VIN are updated alternately until convergence.
Model performance evaluation
To evaluate the model's performance in imputing blood SCFAs, we computed the mean absolute errors (MAE) and root mean squared errors (RMSE) for each subject. The average MAE and RMSE were then calculated by averaging these metrics across all subjects. We evaluated the models on five different randomly generated training and test splits, and the mean and standard deviation of the evaluation metrics across these five experiments were computed.
Identification of influential factors for blood SCFAs
To identify significant factors for SCFAs, we defined a feature contribution score for each SCFA across three different views as:
| (7) |
where denotes the contribution score of the feature to the SCFA in view and denotes the gradient of the SCFA with respect to the input feature in view . Using this approach, we analyzed the contribution of each feature in different types of views on the test set. Features with the largest contribution scores in each view were considered to be the most important ones. Considering the inherent variability during training, we executed five repeated experiments in one dataset and reported the results by summing up the feature contribution scores across these five repeated experiments.
KEGG pathway analyses were conducted to identify significant biological pathways enriched in prominent bacterial species associated with SCFAs, by searching on the website of Kyoto Encyclopedia of Genes and Genomes (https://www.genome.jp/kegg/).
Results
Datasets
To validate the proposed M2AE model, we applied it to two different in-house datasets. We adopted the same data preprocessing pipeline described in the Methods section. For a fair comparison with existing approaches, we used the same methodology to construct the training and testing sets for evaluation.
Dataset 1 consists of data from 964 unrelated males who provided both stool and blood samples for metagenomic and serum SCFAs profiling, along with dietary features, and host characteristics data. The basic characteristics of the samples are shown in Table 1. Features used to predict serum SCFAs include 194 gut bacterial species (relative abundance), 33 dietary features (e.g., intake of fruits, vegetables) and 17 host characteristics (e.g., age, race). The host characteristics and dietary habits used in Dataset 1 are listed in Supplementary Table 1.
Table 1.
Sample Characteristics for Dataset 1 (964 subjects).
| Caucasian | African American | Total | |
|---|---|---|---|
| N (%) | 577 (59.85%) | 387 (40.15%) | 964 (100%) |
| Age: Mean (range) | 35.84 (20-51) | 39.20 (20-51) | 37.19 (20-51) |
| Height (cm): Mean (SD) | 175.27 (6.90) | 174.94 (6.97) | 175.14 (6.93) |
| Weight (kg): Mean (SD) | 82.97 (15.83) | 82.85 (17.46) | 82.92 (16.52) |
| BMI (kg/m2): Mean (SD) | 27.02 (5.07) | 27.04 (5.31) | 27.03 (5.18) |
| Regular exercise: n (%) | 459 (79.55%) | 256 (66.15%) | 715 (74.17%) |
| Smoking: n (%) | 391 (67.76%) | 292 (75.45%) | 683 (70.85%) |
| Alcohol drinking: n (%) | 427 (74.00%) | 210 (54.26%) | 637 (66.08%) |
Dataset 2 includes data from 171 unrelated subjects who provided both stool and blood samples for metagenomic profiling and plasma SCFAs profiling, along with dietary features and host characteristics data. The basic characteristics of these samples are presented in Table 2. Features used to predict plasma SCFAs include 646 gut bacterial species (relative abundance), 3 dietary features (e.g., intake of milk and yogurt), and 11 host characteristics (e.g., age, gender, and race). The host characteristics and dietary habits used in Dataset 2 are listed in Supplementary Table 2.
Table 2.
Sample Characteristics for Dataset 2 (171 subjects).
| Male (58 (33.92%)) | Female (113 (66.08%)) | ||||
|---|---|---|---|---|---|
| Caucasian | African American | Caucasian | African American |
Total | |
| N (%) | 19 (77.19%) | 39 (22.81%) | 77 (78.95%) | 36 (21.05%) | 171 (100%) |
| Age: Mean (range) | 58.16 (44-76) | 58.13 (51-72) | 41.66 (20-85) | 40.00 (21-69) | 46.90 (20-85) |
| Height (cm): Mean (SD) | 173.32 (8.07) | 172.93 (7.57) | 161.80 (6.96) | 165.52 (6.33) | 166.40 (8.63) |
| Weight (kg): Mean (SD) | 85.41 (14.12) | 83.72 (18.85) | 66.10 (14.53) | 77.34 (17.30) | 74.63 (17.97) |
| BMI (kg/m2): Mean (SD) | 28.53 (4.93) | 27.81 (4.90) | 25.24 (5.27) | 28.20 (6.02) | 26.82 (5.47) |
| Regular exercise: n (%) | 29 (83.04%) | 29 (16.96%) | 88 (85.38%) | 25 (14.62%) | 124 (72.51%) |
| Smoking: n (%) | 35 (86.55%) | 23 (13.45%) | 103 (94.15%) | 10 (5.85%) | 75 (43.86%) |
| Alcohol drinking: n (%) | 41 (90.06%) | 17 (9.94%) | 98 (91.23%) | 15 (8.77%) | 110 (64.33%) |
M2AE outperformed existing multi-view integration prediction methods
As shown in Table 3, we compared the prediction performance of M2AE with the following existing regression algorithms for our data: (1) K-nearest neighbor regression (KNN), (2) Random forest regression (RF), (3) Gradient boosting-based regression (XGBoost), (4) Fully-connected neural network (NN) regression and (5) Linear regression. Deep fully-connected NN were also trained with MAE loss. Among the compared methods, KNN, RF, XGBoost, and NN were trained with the direct concatenation of the processed multi-view data as input. All methods were trained with the same processed data. The average MAE and average RMSE across all subjects were computed to compare the performance of different models. The choices for each hyper-parameter are relegated to Supplementary Table 3.
Table 3.
Performance Comparison of different models for Dataset 1 and Dataset 2.
| Dataset 1 | Dataset 2 | |||
|---|---|---|---|---|
| Methods | Mean MAE | Mean RMSE | Total MAE | Total RMSE |
| Linear Regression | 0.641 ± 0.016 | 0.813 ± 0.020 | 0.495 ± 0.016 | 0.620 ± 0.002 |
| RF | 0.481 ± 0.012 | 0.619 ± 0.014 | 0.393 ± 0.018 | 0.494 ± 0.004 |
| NN | 0.951 ± 0.100 | 1.170 ± 0.121 | 2.232 ± 0.160 | 2.673 ± 0.062 |
| KNN | 0.518 ± 0.011 | 0.662 ± 0.014 | 0.423 ± 0.021 | 0.530 ± 0.005 |
| XGBoost | 0.529 ± 0.013 | 0.679 ± 0.013 | 0.447 ± 0.021 | 0.569 ± 0.005 |
| AE(MLP) | 0.458 ± 0.007 | 0.589 ± 0.010 | 0.392 ± 0.021 | 0.502 ± 0.004 |
| AE(GCN) | 0.467 ± 0.006 | 0.601 ± 0.008 | 0.385 ± 0.018 | 0.494 ± 0.004 |
| AE(2GCN+1MLP) | 0.470 ± 0.015 | 0.602 ± 0.015 | 0.388 ± 0.021 | 0.498 ± 0.003 |
| VIN(MLP) | 0.458 ± 0.011 | 0.590 ± 0.015 | 0.384 ± 0.019 | 0.493 ± 0.003 |
| VIN(GCN) | 0.597 ± 0.029 | 0.731 ± 0.032 | 0.384 ± 0.019 | 0.493 ± 0.003 |
| M2AE | 0.449 ± 0.010 | 0.581 ± 0.012 | 0.382 ± 0.017 | 0.489 ± 0.030 |
Note: Bold represents the best performance in different criteria.
We observed that M2AE outperformed the other methods in the prediction tasks by showing the smallest mean MAE and mean RMSE in both Dataset 1 and Dataset 2 (Table 3), indicating the superior learning capability of M2AE. Interestingly, although deep learning-based methods have shown great promises in regression applications, the deep learning-based method NN did not show clear improvements over other approaches. This observation suggested that proper design of deep learning algorithms specific to multi-view integration applications was to some degree required to achieve superior prediction performance.
M2AE outperformed its variations and other methods in SCFA prediction tasks
M2AE integrates view-specific learning via AEs with cross-view interactive fusion via VIN for effective SCFA predictions. To examine the necessity of AEs and VIN for effective SCFA predictions, we performed extensive ablation studies of our proposed method where two additional variations of M2AE were compared. (1) AutoEncoder_VIN: a) Fully-connected NNs with the same number of layers and the same dimensions of hidden layers as the encoder part in M2AE were used for view-specific representation learning; b) GCNs with the same number of layers and the same dimensions of hidden layers as the encoder part in M2AE were used for view-specific representation learning; c) An AE containing two graph convolutional layers and one fully-connected layer with the same dimensions of hidden layers as the encoder part in M2AE were used for view-specific representation learning. The multi-view integration component utilized VIN, which was the same as M2AE. (2) AE_NN/GCN: the view-specific representation component utilized one graph convolutional layer and two fully-connected layers, which was the same as M2AE. a) A fully-connected NN with the same number of layers and the same dimensions of hidden layers as VIN was used for multi-view integration; b) A GCN with the same number of layers and the same dimensions of hidden layers as VIN was used for multi-view integration. Note that Autoencoder_VIN itself is also a novel approach. To the best of our knowledge, there is no existing method that applies AEs to multi-view data integration and imputation problems.
As shown in Table 3, we observed that M2AE outperformed Autoencoder_VIN and AE_NN/GCN in all prediction tasks across both Datasets 1 and 2. The better performance of average MAE and average RMSE in M2AE than AE_NN/GCN indicates that our usage of VIN combines one graph convolutional layer and one fully-connected layer for multi-view integration and prediction tasks makes important contributions to the performance boost of M2AE comparing with existing methods. Compared with traditional NN and GCN that only learn from view information from one pathway, the AE further exploits the graph structural information within the data using a more flexible way. This can be essential to a more comprehensive understanding of the type of view as it captures the connections and correlations among samples. Therefore, AEs were needed for effective view-specific representation learning to fully exploit the advantages of VIN, and these two components could be trained jointly to achieve superior results for multi-view prediction tasks across both datasets.
Performance of M2AE under different types of views
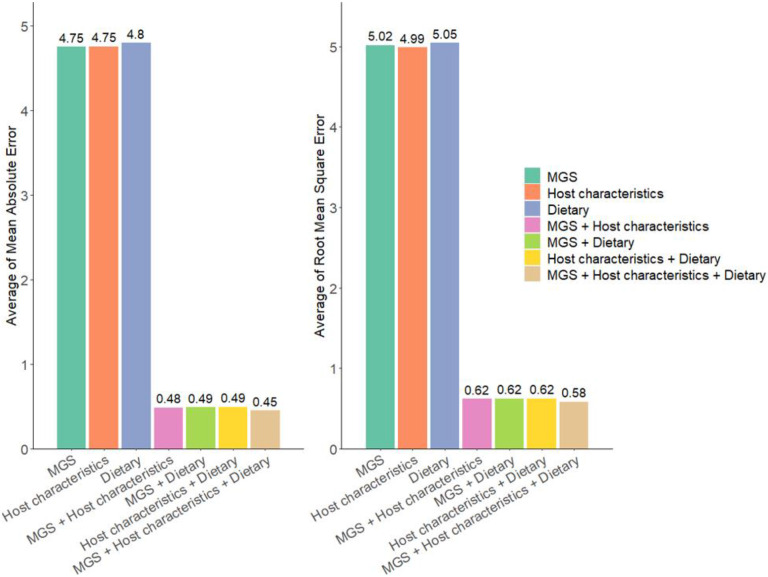
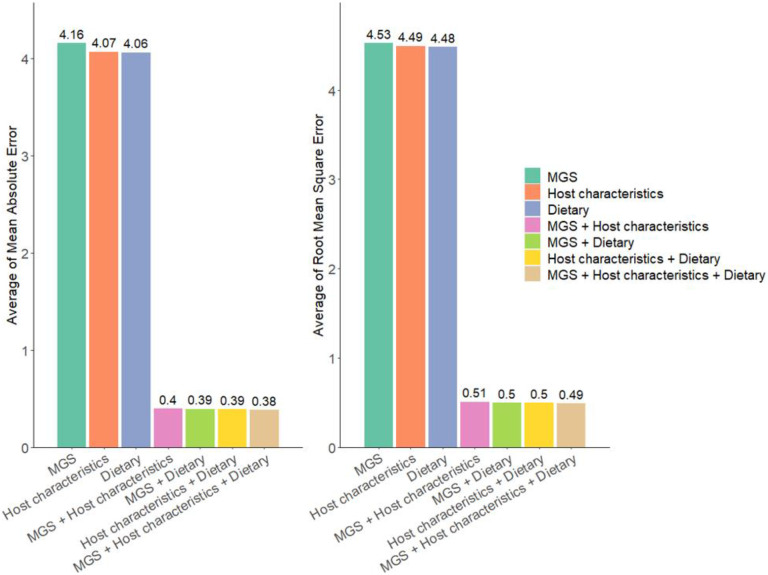
To further demonstrate the necessity of integrating multiple types of data to boost the prediction performance, we compared the prediction performance of M2AE with three types of views (MGS + host characteristics + dietary features), M2AE with two types of views (MGS + host characteristics, MGS + dietary features, and host characteristics + dietary features), and the view-specific AEs trained with single-view data before integration (MGS only, host characteristics only, and dietary features only). Figs. 2 and 3 show that by exploring the cross-view interactive fusion through VIN, the prediction performance was consistently improved by integrating prediction results from multiple views. Specifically, in all the prediction tasks, M2AE models trained with three types of views achieved the best performance compared with M2AE models trained with two types of views. Moreover, the M2AE models trained with two types of views both outperformed the single-view AE models.
Fig. 2. Performance comparison of multi-view data prediction via M2AE and single-view data prediction via Attentive Encoders in Dataset 1 (n = 5 experiments for each model).
Means of evaluation metrics from different experiments are shown in the figure. MGS + Host characteristics + Dietary refers to M2AE with three types of views combining MGS, host characteristics, and dietary features data. MGS + Host characteristics refers to M2AE with two types of views combining gut microbiomes and host characteristics. MGS + Dietary refers to M2AE with two types of views combining gut microbiomes and dietary features. Host characteristics + Dietary refers to M2AE with two types of views combining host characteristics and dietary features. MGS, host characteristics and dietary features refer to the view-specific attentive encoders trained with single-view gut microbiomes, host characteristics, and dietary features.
Fig. 3. Performance comparison of multi-view data prediction via M2AE and single-view data prediction via Attentive Encoders in Dataset 2 (n = 5 experiments for each model).
Means of evaluation metrics from different experiments are shown in the figure. MGS + Host characteristics + Dietary refers to M2AE with three types of views combining MGS, host characteristics, and dietary features data. MGS + Host characteristics refers to M2AE with two types of views combining gut microbiomes and host characteristics. MGS + Dietary refers to M2AE with two types of views combining gut microbiomes and dietary features. Host characteristics + Dietary refers to M2AE with two types of views combining host characteristics and dietary features. MGS, host characteristics and dietary features refer to the view-specific attentive encoders trained with single-view gut microbiomes, host characteristics, and dietary features.
It is well known that gut microbiome is closely influenced by host characteristics and dietary habits, as noted in previous studies 12, 13. This interdependence suggests that these factors, when considered together, may collectively enhance the predictive power of models. The results, therefore, strongly support the necessity of integrating multiple types of data in predictive models. By leveraging cross-view interactions and fusing diverse data types, we captured a more comprehensive understanding of the complex interplay between microbiome, host, and environmental factors, leading to significantly improved prediction accuracy. This multi-view approach is crucial for advancing personalized medicine and for a more profound understanding of complex biological phenomena.
M2AE identified important factors associated with blood SCFAs
In our analysis, we identified key features influencing SCFA production by selecting top-ranked features from two datasets, as detailed in Supplementary Tables 4-11. Among these, Faecalibacterium prausnitzii and Rothia mucilaginosa emerged as significant contributors to various SCFAs production. Several species from the Bacteroides genus were also highlighted for their role in SCFA biosynthesis. For example, Bacteroides thetaiotaomicron and Bacteroides fragilis were major producers of acetic acid, while Bacteroides vulgatus was linked to valeric acid. Bacteroides fragilis was also associated with 2-methylbutyric acid and isobutyric acid, and Bacteroides eggerthii was found to be important for isovaleric acid production. Numerous species within the Clostridium genus were identified as key contributors to specific SCFAs. Clostridium bolteae was particularly relevant for isobutyric acid production, while Clostridium asparagiforme played a significant role in butyric acid synthesis. Streptococcus salivarius was associated with butyric acid production. Additionally, species like Leuconostoc gelidum was noted for its relevance to valeric acid biosynthesis pathways. KEGG pathway analysis further revealed that several of these bacterial species, including Faecalibacterium prausnitzii, Rothia mucilaginosa, Bacteroides thetaiotaomicron, Bacteroides fragilis, Bacteroides vulgatus, Clostridium asparagiforme, and Leuconostoc gelidum, are enriched in SCFA-related biological processes, such as fatty acid biosynthesis, degradation, and metabolism.
Moreover, host characteristics, such as gender, race, age, height, weight, physical activity levels, and the use of probiotics, antibiotics, and gastric acid-lowering medications, were found to correlate with SCFA levels (Supplementary Tables 4-11). Dietary habits, including the consumption of pickles, fruits, cereals, eggs, meat, fats, coffee, and chocolate, also significantly influenced SCFA production. Overall, the factors identified by the M2AE model showed substantial diversities between different SCFAs.
Discussion
Recent development in high-throughput profiling technologies and integrative analysis of multi-view data offered advanced and powerful approaches to dissect complex biological problems. In this study, we pioneered an innovative approach, M2AE, for imputing the abundances of blood SCFAs, and performed multi-view prediction for blood SCFAs data by synthesizing the information of gut microbiome, dietary features and host characteristics. This method jointly explores view-specific representation and cross-view correlation for effective prediction, and demonstrated superior performance compared with other methods. M2AE also effectively identified prominent factors that showed strong associations with blood SCFAs.
Through literature mining, we found interesting evidence supporting the biological connections between these prominent factors and blood SCFAs and interesting relationships among some of these prominent factors.
Gut microbiotas
Our analysis identified several GM species, such as Bacteroides thetaiotaomicron, Bacteroides fragilis, Bacteroides vulgatus, Bacteroides eggerthii, Clostridium asparagiforme, Clostridium bolteae, Faecalibacterium prausnitzii, Rothia mucilaginosa, Streptococcus salivarius and Leuconostoc gelidum, as significant contributors to the production of blood SCFAs. Specifically, Bacteroides thetaiotaomicron and Bacteroides fragilis are prominent contributors to acetic acid production due to their ability to ferment complex carbohydrates into intermediate metabolites, such as lactate and succinate, which can be further converted into SCFAs by other gut bacteria 30, 58, 59. Bacteroides vulgatus exhibited a negative association with blood valeric acid levels 60, possibly due to its negative interactions with other gut bacteria, which affect substrate availability for valeric acid production 61. Bacteroides eggerthii has been identified as a significant contributor to the production of isovaleric acid, primarily via leucine fermentation 62. Moreover, consistent with prior study, Clostridium asparagiforme plays a major role in butyrate production by fermenting glucose into lactate, which is then converted to butyrate by other bacteria 63. Clostridium bolteae can utilize valine through fermentation pathways, leading to the production of isobutyric acid as a metabolic byproduct 64. Faecalibacterium prausnitzii is positively correlated with butyric and valeric acid 59, 65, 66. Rothia mucilaginosa ferments glucose to produce acetate 67.
As above, we identified a series of gut bacterial species that have been proved to play a role in SCFA production. In addition, we identified some novel putative factors that might affect blood SCFAs. For example, Bacteroides fragilis was associated with 2-methylbutyric acid and isobutyric acid, Streptococcus salivarius was associated with butyric acid production, and Leuconostoc gelidum was related to valeric acid. Bacteroides species contribute to amino acid metabolism 68, which might lead to the production of SCFAs, such as isobutyric acid and 2-methylbutyric acid. Streptococcus salivarius, primarily known for its presence in the oral cavity, can also inhabit the gut and metabolize carbohydrates via fermentation 69, potentially contributing to the production of butyric acid. Similarly, Leuconostoc gelidum is a lactic acid bacterium known for fermenting carbohydrates to produce lactic acid 70, which might serve as a substrate for other gut microbes, potentially leading to the production of valeric acid. These findings enhance our understanding of the complex interactions between GM and blood SCFA levels. Meanwhile, these insights could help in validating our model by supporting the observed associations between specific bacterial species and SCFA production, as well as their potential influence on systemic SCFA levels. However, more in-depth studies might be needed to further unravel the underlying mechanisms.
Dietary features
Incorporating dietary features into our study enhanced our understanding in regulating the complex biological regulation of blood SCFAs production. Our findings demonstrated that the intake of various dietary components, such as pickles, fruits, cereals, eggs, meat, fat oil, coffee, and chocolate, influences blood SCFA levels. Diets could shape the microbiome by promoting the growth of bacteria that preferentially use the ingested nutrients 71. For instance, fermented foods like pickles and fiber-rich foods like fruits and cereals promote the growth of beneficial bacteria that ferment sugars and fibers into SCFAs, such as acetic, propionic, and butyric acids 72, 73. High-protein and high-fat diets, including eggs, steak, and fat oil, can promote the growth of Bacteroides species, which are adept at protein degradation and fat metabolism, thereby affecting SCFA production 10, 74-76. Additionally, coffee and dark chocolate were linked to SCFA production due to their bioactive compounds, like caffeine, chlorogenic acid, and polyphenols, which modulate the gut microbiota and fermentation activities 77, 78. These findings emphasize the critical role of diet in regulating SCFA levels and support our model’s effectiveness in identifying dietary determinants of SCFA production.
Host characteristics
Our study identified several factors in host characteristics—such as gender, race, age, height, weight, BMI, physical activity, and the use of probiotics, antibiotics, and gastric acid-lowering medications—were correlated with blood SCFA levels. Race was associated with SCFA production, aligning with previous findings that African Americans have lower fecal acetate levels compared to white participants 45, potentially reflecting similar trends in blood SCFAs 79. Gender and age also affect SCFA production due to differences in gut microbiota diversity and composition across groups 80, 81. Body composition indicators, such as BMI, weight, and height, correlate with SCFA levels, as reduced gut microbiota diversity in overweight or obese individuals often results in increased SCFA production 82-84, which is linked to energy storage and lipid metabolism 85-87. Physical activities such as biking and swimming affect muscle lactate metabolism 88-90, and Veillonella species in the gut can convert lactate into SCFAs like acetic acid 91. Additionally, probiotics increase SCFA production by boosting SCFA-producing bacteria 92, whereas antibiotics and gastric acid-lowering medications reduce microbial diversity and alter gut environments, impacting SCFA levels 84, 93, 94. These identified host characteristics, in turn, demonstrate the effectiveness of our model in imputing SCFA levels by integrating gut microbiome compositions, dietary features, and host characteristics, providing a comprehensive understanding of the determinants influencing SCFA production.
Our current results indicated that the regulation of blood SCFAs could be a complex procedure, the GMs can be important factors. Besides, dietary habits and host characteristics might also influence blood SCFAs directly or through interactions with GMs. However, there are a few limitations in this study. First, all the subjects in our study were Caucasians and African Americans, making it necessary yet to generalize the results to other racial populations. The validation of our model in diverse populations would enhance its applicability. This necessitates further model validation in different cohorts that incorporate metagenomic and blood SCFAs profiling, dietary features, and host characteristics of a similar scope. Second, our study utilized two different datasets for model validation: one with serum SCFA measurements and the other with plasma SCFA measurements. While previous studies suggest that SCFA levels are generally consistent between serum and plasma samples, minor variations might still exist due to the different biological matrices. These variations were not directly analyzed in our study, as the primary goal was to validate the model's performance across different datasets rather than compare serum and plasma SCFA levels. To strengthen the model's accuracy and reliability, future studies should validate our models using additional datasets with more serum and plasma SCFA measurements. Third, another limitation of our study is the sex distribution across the two datasets used for model validation. Dataset 1 included males, while Dataset 2 included both males and females. This imbalance in sex representation between the two datasets could affect the model's ability to generalize across different sexes. However, because Dataset 2 has a relatively small number of male participants, splitting this dataset by sex for separate analyses would result in low statistical power, leading to potentially unreliable results. To maintain robust model validation, we combined the male and female samples in Dataset 2, which helps to preserve adequate sample size for analysis. We adjusted for sex as a covariate in our model to account for potential sex-specific differences in SCFA production. However, future studies with more balanced sex distributions or larger sample sizes for both sexes would provide a more comprehensive understanding and enhance the robustness of the model. Fourth, the integration of data from two different sequencing platforms in Dataset 1 could be considered a limitation. However, deep learning models are particularly capable of finding common patterns across heterogeneous data by learning generalizable features that are not specific to any single sequencing platform. We applied uniform normalization within the model to standardize the data and employed regularization techniques like dropout and mean absolute error loss to prevent overfitting to sequencing platform-specific characteristics. While these strategies allow the model to generalize effectively, future studies could include more data generated from either consistent or varied sequencing platforms to further validate the model's robustness across different technical conditions. Fifth, another limitation of our study is that the features in each view of the two datasets differ, which could result in variability in the important features identified by the model. Although many of the identified important features are well-known factors related to SCFA production, demonstrating the model's effectiveness to some extent, this variability highlights the need for caution in interpreting results. However, it is worth noting that the imputation performance was strong across both datasets, highlighting the model's generalizability despite these differences. It also opens up new opportunities to explore and identify additional relevant features that might be specific to certain datasets or conditions. Future studies should aim to include datasets with consistent feature sets across all views to enhance comparability and validate the model's ability to generalize findings across different contexts.
To summarize, we have blazed a trail with our innovative method that synthesizes information from the gut microbiome, dietary features, and host characteristics to perform multi-view imputation for blood SCFAs data. This can also help us identify key factors or pathways that regulate blood SCFAs in the future study. Our research highlights the utility of integrating information on gut microbiome, dietary features, and host characteristics, providing fresh perspectives on the potential regulatory mechanisms affecting blood SCFAs.
Supplementary Material
Acknowledgement
This work is made possible with partial support by grants from the NIH (U19AG055373, R01AG061917, R01AG068232, P20GM109036 and P20GM103629).
Data Availability
The raw data presented in this study can be found in online repositories. The names of the repositories and accession numbers can be found below: NCBI BioProjects PRJNA1015234 and PRJNA1015228.
Code Availability
The source code of this work can be downloaded from GitHub (https://github.com/Wonderangela123/M2AE).
Reference
- 1.Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16(8):461–78. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 2.Sakamoto M, Takagaki A, Matsumoto K, Kato Y, Goto K, Benno Y. Butyricimonas synergistica gen. nov., sp. nov. and Butyricimonas virosa sp. nov., butyric acid-producing bacteria in the family 'Porphyromonadaceae' isolated from rat faeces. Int J Syst Evol Microbiol. 2009;59(Pt 7):1748–53. Epub 20090619. doi: 10.1099/ijs.0.007674-0. [DOI] [PubMed] [Google Scholar]
- 3.Huo W, Feng Z, Hu S, Cui L, Qiao T, Dai L, Qi P, Zhang L, Liu Y, Li J. Effects of polysaccharides from wild morels on immune response and gut microbiota composition in non-treated and cyclophosphamide-treated mice. Food Funct. 2020;11(5):4291–303. Epub 20200501. doi: 10.1039/d0fo00597e. [DOI] [PubMed] [Google Scholar]
- 4.Berni Canani R, Di Costanzo M, Leone L. The epigenetic effects of butyrate: potential therapeutic implications for clinical practice. Clin Epigenetics. 2012;4(1):4. Epub 20120227. doi: 10.1186/1868-7083-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Yao W, Li B, Qian S, Wei B, Gong S, Wang J, Liu M, Wei M. Nuciferine modulates the gut microbiota and prevents obesity in high-fat diet-fed rats. Exp Mol Med. 2020;52(12):1959–75. Epub 20201201. doi: 10.1038/s12276-020-00534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Overby HB, Ferguson JF. Gut Microbiota-Derived Short-Chain Fatty Acids Facilitate Microbiota:Host Cross talk and Modulate Obesity and Hypertension. Curr Hypertens Rep. 2021;23(2):8. Epub 20210203. doi: 10.1007/s11906-020-01125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54(9):2325–40. Epub 20130702. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rios-Covian D, Ruas-Madiedo P, Margolles A, Gueimonde M, de Los Reyes-Gavilan CG, Salazar N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front Microbiol. 2016;7:185. Epub 20160217. doi: 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294(1):1–8. Epub 20090213. doi: 10.1111/j.1574-6968.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Xiao Y, Li D, Zhang S, Wu Y, Zhang Q, Bai W. New insights into the mechanisms of high-fat diet mediated gut microbiota in chronic diseases. iMeta. 2023;2(1):e69. doi: 10.1002/imt2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang G, Li X, Zhao J, Zhang H, Chen W. Lactobacillus casei CCFM419 attenuates type 2 diabetes via a gut microbiota dependent mechanism. Food Funct. 2017;8(9):3155–64. doi: 10.1039/c7fo00593h. [DOI] [PubMed] [Google Scholar]
- 12.Conlon MA, Bird AR. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients. 2015;7(1):17–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Besten G, Eunen K, Groen AK, Venema K, Reijngoud K, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54(9):2325–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.You H, Tan Y, Yu D, Qiu S, Bai Y, He J, Cao H, Che Q, Guo J, Su Z. The Therapeutic Effect of SCFA-Mediated Regulation of the Intestinal Environment on Obesity. Front Nutr. 2022;9:886902. Epub 20220517. doi: 10.3389/fnut.2022.886902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M, Suez J, Mahdi JA, Matot E, Malka G, Kosower N, Rein M, Zilberman-Schapira G, Dohnalova L, Pevsner-Fischer M, Bikovsky R, Halpern Z, Elinav E, Segal E. Personalized Nutrition by Prediction of Glycemic Responses. Cell. 2015;163(5):1079–94. doi: 10.1016/j.cell.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Gao X, Lv J, Zeng Y, Li Q, Wang L, Zhang Y, Gao W, Wang J. Gut Microbiome Signature Are Correlated With Bone Mineral Density Alterations in the Chinese Elders. Front Cell Infect Microbiol. 2022;12:827575. Epub 20220331. doi: 10.3389/fcimb.2022.827575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramos Meyers G, Samouda H, Bohn T. Short Chain Fatty Acid Metabolism in Relation to Gut Microbiota and Genetic Variability. Nutrients. 2022;14(24). Epub 20221216. doi: 10.3390/nu14245361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.den Besten G, Lange K, Havinga R, van Dijk TH, Gerding A, van Eunen K, Muller M, Groen AK, Hooiveld GJ, Bakker BM, Reijngoud DJ. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am J Physiol Gastrointest Liver Physiol. 2013;305(12):G900–10. Epub 20131017. doi: 10.1152/ajpgi.00265.2013. [DOI] [PubMed] [Google Scholar]
- 19.Lin X, Xiao HM, Liu HM, Lv WQ, Greenbaum J, Gong R, Zhang Q, Chen YC, Peng C, Xu XJ, Pan DY, Chen Z, Li ZF, Zhou R, Wang XF, Lu JM, Ao ZX, Song YQ, Zhang YH, Su KJ, Meng XH, Ge CL, Lv FY, Luo Z, Shi XM, Zhao Q, Guo BY, Yi NJ, Shen H, Papasian CJ, Shen J, Deng HW. Gut microbiota impacts bone via Bacteroides vulgatus-valeric acid-related pathways. Nat Commun. 2023;14(1):6853. Epub 20231027. doi: 10.1038/s41467-023-42005-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zmora N, Suez J, Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16(1):35–56. doi: 10.1038/s41575-018-0061-2. [DOI] [PubMed] [Google Scholar]
- 21.Greenbaum J, Su KJ, Zhang X, Liu Y, Liu A, Zhao LJ, Luo Z, Tian Q, Shen H, Deng HW. A multiethnic whole genome sequencing study to identify novel loci for bone mineral density. Hum Mol Genet. 2022;31(7):1067–81. doi: 10.1093/hmg/ddab305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He H, Liu Y, Tian Q, Papasian CJ, Hu T, Deng HW. Relationship of sarcopenia and body composition with osteoporosis. Osteoporos Int. 2016;27(2):473–82. Epub 2015/08/06. doi: 10.1007/s00198-015-3241-8. [DOI] [PubMed] [Google Scholar]
- 23.Song M, Greenbaum J, Luttrell Jt, Zhou W, Wu C, Luo Z, Qiu C, Zhao LJ, Su KJ, Tian Q, Shen H, Hong H, Gong P, Shi X, Deng HW, Zhang C. An autoencoder-based deep learning method for genotype imputation. Front Artif Intell. 2022;5:1028978. Epub 20221103. doi: 10.3389/frai.2022.1028978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du Y, Xu T, Yin Z, Espinoza S, Xie Y, Gentry C, Tian Q, Zhao LJ, Shen H, Luo Z, Deng HW. Associations of physical activity with sarcopenia and sarcopenic obesity in middle-aged and older adults: the Louisiana osteoporosis study. BMC Public Health. 2022;22(1):896. Epub 2022/05/06. doi: 10.1186/s12889-022-13288-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du Y, Zhao LJ, Xu Q, Wu KH, Deng HW. Socioeconomic status and bone mineral density in adults by race/ethnicity and gender: the Louisiana osteoporosis study. Osteoporos Int. 2017;28(5):1699–709. Epub 2017/02/27. doi: 10.1007/s00198-017-3951-1. [DOI] [PubMed] [Google Scholar]
- 26.Jeng C, Zhao LJ, Wu K, Zhou Y, Chen T, Deng HW. Race and socioeconomic effect on sarcopenia and sarcopenic obesity in the Louisiana Osteoporosis Study (LOS). JCSM Clin Rep. 2018;3(2). Epub 2019/08/30. [PMC free article] [PubMed] [Google Scholar]
- 27.Ning H, Du Y, Zhao LJ, Tian Q, Feng H, Deng HW. The mediating effect of skeletal muscle index on the relationship between menarcheal age and bone mineral density in premenopausal women by race/ethnicity. Menopause. 2021;28(10):1143–9. Epub 2021/07/28. doi: 10.1097/GME.0000000000001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruce SJ, Tavazzi I, Parisod V, Rezzi S, Kochhar S, Guy PA. Investigation of human blood plasma sample preparation for performing metabolomics using ultrahigh performance liquid chromatography/mass spectrometry. Anal Chem. 2009;81(9):3285–96. doi: 10.1021/ac8024569. [DOI] [PubMed] [Google Scholar]
- 29.Lv WQ, Lin X, Shen H, Liu HM, Qiu X, Li BY, Shen WD, Ge CL, Lv FY, Shen J, Xiao HM, Deng HW. Human gut microbiome impacts skeletal muscle mass via gut microbial synthesis of the short-chain fatty acid butyrate among healthy menopausal women. J Cachexia Sarcopenia Muscle. 2021;12(6):1860–70. Epub 20210901. doi: 10.1002/jcsm.12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen WD, Lin X, Liu HM, Li BY, Qiu X, Lv WQ, Zhu XZ, Greenbaum J, Liu RK, Shen J, Xiao HM, Deng HW. Gut microbiota accelerates obesity in peri-/post-menopausal women via Bacteroides fragilis and acetic acid. Int J Obes (Lond). 2022;46(10):1918–24. Epub 20220817. doi: 10.1038/s41366-022-01137-9. [DOI] [PubMed] [Google Scholar]
- 31.Liu HM, Lin X, Meng XH, Zhao Q, Shen J, Xiao HM, Deng HW. Integrated metagenome and metabolome analyses of blood pressure studies in early postmenopausal Chinese women. J Hypertens. 2021;39(9):1800–9. doi: 10.1097/HJH.0000000000002832. [DOI] [PubMed] [Google Scholar]
- 32.Tian B, Xu LL, Jiang LD, Lin X, Shen J, Shen H, Su KJ, Gong R, Qiu C, Luo Z, Yao JH, Wang ZQ, Xiao HM, Zhang LS, Deng HW. Identification of the serum metabolites associated with cow milk consumption in Chinese Peri-/Postmenopausal women. Int J Food Sci Nutr. 2024;75(6):537–49. Epub 20240625. doi: 10.1080/09637486.2024.2366223. [DOI] [PubMed] [Google Scholar]
- 33.Tian B, Yao JH, Lin X, Lv WQ, Jiang LD, Wang ZQ, Shen J, Xiao HM, Xu H, Xu LL, Cheng X, Shen H, Qiu C, Luo Z, Zhao LJ, Yan Q, Deng HW, Zhang LS. Metagenomic study of the gut microbiota associated with cow milk consumption in Chinese peri-/postmenopausal women. Front Microbiol. 2022;13:957885. Epub 20220816. doi: 10.3389/fmicb.2022.957885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. Embnet Journal. 2011;17. [Google Scholar]
- 35.Pertea G. fqtrim: v0.9.4 (Version 0.9.4) 2015. Available from: http://ccb.jhu.edu/software/fqtrim/index.shtml.
- 36.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–9. Epub 20120304. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng Y, Leung HC, Yiu SM, Chin FY. IDBA-UD: a de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics. 2012;28(11):1420–8. Epub 20120411. doi: 10.1093/bioinformatics/bts174. [DOI] [PubMed] [Google Scholar]
- 38.Zhu W, Lomsadze A, Borodovsky M. Ab initio gene identification in metagenomic sequences. Nucleic Acids Res. 2010;38(12):e132. Epub 20100419. doi: 10.1093/nar/gkq275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22(13):1658–9. Epub 20060526. doi: 10.1093/bioinformatics/btl158. [DOI] [PubMed] [Google Scholar]
- 40.Buchfink B, Xie C, Huson DH. Fast and sensitive protein alignment using DIAMOND. Nat Methods. 2015;12(1):59–60. Epub 20141117. doi: 10.1038/nmeth.3176. [DOI] [PubMed] [Google Scholar]
- 41.Chen S, Zhou Y, Chen Y, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34(17):i884–i90. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franzosa EA, McIver LJ, Rahnavard G, Thompson LR, Schirmer M, Weingart G, Lipson KS, Knight R, Caporaso JG, Segata N, Huttenhower C. Species-level functional profiling of metagenomes and metatranscriptomes. Nat Methods. 2018;15(11):962–8. Epub 20181030. doi: 10.1038/s41592-018-0176-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bowen RA, Remaley AT. Interferences from blood collection tube components on clinical chemistry assays. Biochem Med (Zagreb). 2014;24(1):31–44. Epub 20140215. doi: 10.11613/BM.2014.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao HJ, Chen Y, Liu T, McArthur K, Mueller NT. Short-Chain Fatty Acids and Preeclampsia: A Scoping Review. Nutr Rev. 2024. Epub 20240525. doi: 10.1093/nutrit/nuae057. [DOI] [PubMed] [Google Scholar]
- 45.Hester CM, Jala VR, Langille MG, Umar S, Greiner KA, Haribabu B. Fecal microbes, short chain fatty acids, and colorectal cancer across racial/ethnic groups. World J Gastroenterol. 2015;21(9):2759–69. doi: 10.3748/wjg.v21.i9.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jenkins DJ, Kendall CW, Popovich DG, Vidgen E, Mehling CC, Vuksan V, Ransom TP, Rao AV, Rosenberg-Zand R, Tariq N, Corey P, Jones PJ, Raeini M, Story JA, Furumoto EJ, Illingworth DR, Pappu AS, Connelly PW. Effect of a very-high-fiber vegetable, fruit, and nut diet on serum lipids and colonic function. Metabolism. 2001;50(4):494–503. doi: 10.1053/meta.2001.21037. [DOI] [PubMed] [Google Scholar]
- 47.Sowah SA, Riedl L, Damms-Machado A, Johnson TS, Schubel R, Graf M, Kartal E, Zeller G, Schwingshackl L, Stangl GI, Kaaks R, Kuhn T. Effects of Weight-Loss Interventions on Short-Chain Fatty Acid Concentrations in Blood and Feces of Adults: A Systematic Review. Adv Nutr. 2019;10(4):673–84. doi: 10.1093/advances/nmy125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guinan J, Wang S, Hazbun TR, Yadav H, Thangamani S. Antibiotic-induced decreases in the levels of microbial-derived short-chain fatty acids correlate with increased gastrointestinal colonization of Candida albicans. Sci Rep. 2019;9(1):8872. Epub 20190620. doi: 10.1038/s41598-019-45467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software. 2011;45(3):1–67. doi: 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- 50.Li J, Lu G, Wu Z, Ling F. Multi-view representation model based on graph autoencoder. Information Sciences. 2023;632:439–53. [Google Scholar]
- 51.2022 Alzheimer's disease facts and figures. Alzheimers Dement. 2022;18(4):700–89. Epub 2022/03/16. doi: 10.1002/alz.12638. [DOI] [PubMed] [Google Scholar]
- 52.Zhang X, Wang X, Shivashankar GV, Uhler C. Graph-based autoencoder integrates spatial transcriptomics with chromatin images and identifies joint biomarkers for Alzheimer's disease. Nat Commun. 2022;13(1):7480. Epub 20221203. doi: 10.1038/s41467-022-35233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh A, Shannon CP, Gautier B, Rohart F, Vacher M, Tebbutt SJ, Le Cao KA. DIABLO: an integrative approach for identifying key molecular drivers from multi-omics assays. Bioinformatics. 2019;35(17):3055–62. doi: 10.1093/bioinformatics/bty1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Serra A, Fratello M, Fortino V, Raiconi G, Tagliaferri R, Greco D. MVDA: a multi-view genomic data integration methodology. BMC Bioinformatics. 2015;16:261. Epub 20150819. doi: 10.1186/s12859-015-0680-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu X, Suk HI, Zhu Y, Thung KH, Wu G, Shen D. Multi-view Classification for Identification of Alzheimer's Disease. Mach Learn Med Imaging. 2015;9352:255–62. Epub 20151002. doi: 10.1007/978-3-319-24888-2_31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Y, Wu FX, Ngom A. A review on machine learning principles for multi-view biological data integration. Brief Bioinform. 2018;19(2):325–40. doi: 10.1093/bib/bbw113. [DOI] [PubMed] [Google Scholar]
- 57.Wang L. Correlation Discovery for Multi-view and Multi-label Learning: Northeastern University; 2021. [Google Scholar]
- 58.Samuel BS, Gordon JI. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc Natl Acad Sci U S A. 2006;103(26):10011–6. Epub 20060616. doi: 10.1073/pnas.0602187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wrzosek L, Miquel S, Noordine ML, Bouet S, Joncquel Chevalier-Curt M, Robert V, Philippe C, Bridonneau C, Cherbuy C, Robbe-Masselot C, Langella P, Thomas M. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013;11:61. Epub 20130521. doi: 10.1186/1741-7007-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin X, et al. Gut microbiota impacts bone via B.vulgatus-valeric acid-related pathways 2020. doi: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.medrxiv.org/content/10.1101/2020.03.16.20037077v2.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coyte KZ, Rakoff-Nahoum S. Understanding Competition and Cooperation within the Mammalian Gut Microbiome. Curr Biol. 2019;29(11):R538–R44. doi: 10.1016/j.cub.2019.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang H, Xie L, Liu S, Dai A, Chi X, Zhang D. Non-targeted metabolomics and microbial analyses of the impact of oat antimicrobial peptides on rats with dextran sulfate sodium-induced enteritis. Front Nutr. 2022;9:1095483. Epub 20230111. doi: 10.3389/fnut.2022.1095483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thomson P, Medina DA, Ortuzar V, Gotteland M, Garrido D. Anti-inflammatory effect of microbial consortia during the utilization of dietary polysaccharides. Food Res Int. 2018;109:14–23. Epub 20180411. doi: 10.1016/j.foodres.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 64.Watanabe M, Kaku N, Ueki K, Ueki A. Falcatimonas natans gen. nov., sp. nov., a strictly anaerobic, amino-acid-decomposing bacterium isolated from a methanogenic reactor of cattle waste. Int J Syst Evol Microbiol. 2016;66(11):4639–44. Epub 20160808. doi: 10.1099/ijsem.0.001403. [DOI] [PubMed] [Google Scholar]
- 65.Xu D, Feng M, Chu Y, Wang S, Shete V, Tuohy KM, Liu F, Zhou X, Kamil A, Pan D, Liu H, Yang X, Yang C, Zhu B, Lv N, Xiong Q, Wang X, Sun J, Sun G, Yang Y. The Prebiotic Effects of Oats on Blood Lipids, Gut Microbiota, and Short-Chain Fatty Acids in Mildly Hypercholesterolemic Subjects Compared With Rice: A Randomized, Controlled Trial. Front Immunol. 2021;12:787797. Epub 20211209. doi: 10.3389/fimmu.2021.787797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Song CH, Kim N, Nam RH, Choi SI, Jang JY, Kim EH, Choi J, Choi Y, Yoon H, Lee SM, Seok YJ. The Possible Preventative Role of Lactate- and Butyrate-Producing Bacteria in Colorectal Carcinogenesis. Gut Liver. 2024;18(4):654–66. Epub 20231130. doi: 10.5009/gnl230385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao B, Gallagher T, Zhang Y, Elbadawi-Sidhu M, Lai Z, Fiehn O, Whiteson KL. Tracking Polymicrobial Metabolism in Cystic Fibrosis Airways: Pseudomonas aeruginosa Metabolism and Physiology Are Influenced by Rothia mucilaginosa-Derived Metabolites. mSphere. 2018;3(2). Epub 20180425. doi: 10.1128/mSphere.00151-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allison C, Macfarlane GT. Influence of pH, nutrient availability, and growth rate on amine production by Bacteroides fragilis and Clostridium perfringens. Appl Environ Microbiol. 1989;55(11):2894–8. doi: 10.1128/aem.55.11.2894-2898.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abranches J, Zeng L, Kajfasz JK, Palmer SR, Chakraborty B, Wen ZT, Richards VP, Brady LJ, Lemos JA. Biology of Oral Streptococci. Microbiol Spectr. 2018;6(5). doi: 10.1128/microbiolspec.GPP3-0042-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johansson P, Sade E, Hultman J, Auvinen P, Bjorkroth J. Pangenome and genomic taxonomy analyses of Leuconostoc gelidum and Leuconostoc gasicomitatum. BMC Genomics. 2022;23(1):818. Epub 20221209. doi: 10.1186/s12864-022-09032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zeng X, Xing X, Gupta M, Keber FC, Lopez JG, Lee YJ, Roichman A, Wang L, Neinast MD, Donia MS, Wuhr M, Jang C, Rabinowitz JD. Gut bacterial nutrient preferences quantified in vivo. Cell. 2022;185(18):3441–56 e19. doi: 10.1016/j.cell.2022.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Swain MR, Anandharaj M, Ray RC, Parveen Rani R. Fermented fruits and vegetables of Asia: a potential source of probiotics. Biotechnol Res Int. 2014;2014:250424. Epub 20140528. doi: 10.1155/2014/250424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chung WS, Walker AW, Louis P, Parkhill J, Vermeiren J, Bosscher D, Duncan SH, Flint HJ. Modulation of the human gut microbiota by dietary fibres occurs at the species level. BMC Biol. 2016;14:3. Epub 20160111. doi: 10.1186/s12915-015-0224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mendez-Salazar EO, Ortiz-Lopez MG, Granados-Silvestre MLA, Palacios-Gonzalez B, Menjivar M. Altered Gut Microbiota and Compositional Changes in Firmicutes and Proteobacteria in Mexican Undernourished and Obese Children. Front Microbiol. 2018;9:2494. Epub 20181016. doi: 10.3389/fmicb.2018.02494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luo J, Zhang C, Liu R, Gao L, Ou S, Liu L, Peng X. Ganoderma lucidum polysaccharide alleviating colorectal cancer by alteration of special gut bacteria and regulation of gene expression of colonic epithelial cells. Journal of Functional Foods. 2018;47:127–35. [Google Scholar]
- 76.Bisht A, Goh KKT, Matia-Merino L. The fate of mamaku gum in the gut: effect on in vitro gastrointestinal function and colon fermentation by human faecal microbiota. Food Funct. 2023;14(15):7024–39. Epub 20230731. doi: 10.1039/d3fo01665j. [DOI] [PubMed] [Google Scholar]
- 77.Nishitsuji K, Watanabe S, Xiao J, Nagatomo R, Ogawa H, Tsunematsu T, Umemoto H, Morimoto Y, Akatsu H, Inoue K, Tsuneyama K. Effect of coffee or coffee components on gut microbiome and short-chain fatty acids in a mouse model of metabolic syndrome. Sci Rep. 2018;8(1):16173. Epub 20181101. doi: 10.1038/s41598-018-34571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garcia-Cordero J, Martinez A, Blanco-Valverde C, Pino A, Puertas-Martin V, San Roman R, de Pascual-Teresa S. Regular Consumption of Cocoa and Red Berries as a Strategy to Improve Cardiovascular Biomarkers via Modulation of Microbiota Metabolism in Healthy Aging Adults. Nutrients. 2023;15(10). Epub 20230513. doi: 10.3390/nu15102299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamamura R, Nakamura K, Kitada N, Aizawa T, Shimizu Y, Nakamura K, Ayabe T, Kimura T, Tamakoshi A. Associations of gut microbiota, dietary intake, and serum short-chain fatty acids with fecal short-chain fatty acids. Biosci Microbiota Food Health. 2020;39(1):11–7. Epub 20191005. doi: 10.12938/bmfh.19-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mueller S, Saunier K, Hanisch C, Norin E, Alm L, Midtvedt T, Cresci A, Silvi S, Orpianesi C, Verdenelli MC, Clavel T, Koebnick C, Zunft HJ, Dore J, Blaut M. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol. 2006;72(2):1027–33. doi: 10.1128/AEM.72.2.1027-1033.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fransen F, van Beek AA, Borghuis T, Meijer B, Hugenholtz F, van der Gaast-de Jongh C, Savelkoul HF, de Jonge MI, Faas MM, Boekschoten MV, Smidt H, El Aidy S, de Vos P. The Impact of Gut Microbiota on Gender-Specific Differences in Immunity. Front Immunol. 2017;8:754. Epub 20170630. doi: 10.3389/fimmu.2017.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu BN, Liu XT, Liang ZH, Wang JH. Gut microbiota in obesity. World J Gastroenterol. 2021;27(25):3837–50. doi: 10.3748/wjg.v27.i25.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salazar N, Ponce-Alonso M, Garriga M, Sanchez-Carrillo S, Hernandez-Barranco AM, Redruello B, Fernandez M, Botella-Carretero JI, Vega-Pinero B, Galeano J, Zamora J, Ferrer M, de Los Reyes-Gavilan CG, Del Campo R. Fecal Metabolome and Bacterial Composition in Severe Obesity: Impact of Diet and Bariatric Surgery. Gut Microbes. 2022;14(1):2106102. doi: 10.1080/19490976.2022.2106102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lange O, Proczko-Stepaniak M, Mika A. Short-Chain Fatty Acids-A Product of the Microbiome and Its Participation in Two-Way Communication on the Microbiome-Host Mammal Line. Curr Obes Rep. 2023;12(2):108–26. Epub 20230519. doi: 10.1007/s13679-023-00503-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TM, Comelli EM. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes. 2014;4(6):e121. Epub 20140630. doi: 10.1038/nutd.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Heiss CN, Olofsson LE. Gut Microbiota-Dependent Modulation of Energy Metabolism. J Innate Immun. 2018;10(3):163–71. Epub 20171108. doi: 10.1159/000481519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chambers ES, Preston T, Frost G, Morrison DJ. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr Nutr Rep. 2018;7(4):198–206. doi: 10.1007/s13668-018-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Richard NA, Koehle MS. Optimizing recovery to support multi-evening cycling competition performance. Eur J Sport Sci. 2019;19(6):811–23. Epub 20181227. doi: 10.1080/17461391.2018.1560506. [DOI] [PubMed] [Google Scholar]
- 89.Cieminski K, Flis DJ, Dzik KP, Kaczor JJ, Wieckowski MR, Antosiewicz J, Ziolkowski W. Swim Training Affects on Muscle Lactate Metabolism, Nicotinamide Adenine Dinucleotides Concentration, and the Activity of NADH Shuttle Enzymes in a Mouse Model of Amyotrophic Lateral Sclerosis. Int J Mol Sci. 2022;23(19). Epub 20220929. doi: 10.3390/ijms231911504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fasching P, Rinnerhofer S, Wultsch G, Birnbaumer P, Hofmann P. The First Lactate Threshold Is a Limit for Heavy Occupational Work. J Funct Morphol Kinesiol. 2020;5(3). Epub 20200825. doi: 10.3390/jfmk5030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scheiman J, Luber JM, Chavkin TA, MacDonald T, Tung A, Pham LD, Wibowo MC, Wurth RC, Punthambaker S, Tierney BT, Yang Z, Hattab MW, Avila-Pacheco J, Clish CB, Lessard S, Church GM, Kostic AD. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat Med. 2019;25(7):1104–9. Epub 20190624. doi: 10.1038/s41591-019-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Markowiak-Kopec P, Slizewska K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients. 2020;12(4). Epub 20200416. doi: 10.3390/nu12041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Modi SR, Collins JJ, Relman DA. Antibiotics and the gut microbiota. J Clin Invest. 2014;124(10):4212–8. Epub 20141001. doi: 10.1172/JCI72333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6(11):e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data presented in this study can be found in online repositories. The names of the repositories and accession numbers can be found below: NCBI BioProjects PRJNA1015234 and PRJNA1015228.
The source code of this work can be downloaded from GitHub (https://github.com/Wonderangela123/M2AE).