Abstract
Stem-loop B is a 12-nucleotide [nt]-long completely conserved sequence postulated to form a 4-bp stem and a 4-nt internal loop under the kissing-loop hairpin (klh) (nt 248 to 270) of human immunodeficiency virus type 1 (HIV-1) genomic RNA. We investigated its role in viral replication, genomic RNA dimerization, and dimerization of partial HIV-1 RNA transcripts. The putative CUCG246-CGAG277 duplex was replaced by nine alternative complementary sequences, five likely to base pair only in short RNAs and four likely to base pair in long (∼500-nt) RNAs, as assessed by the algorithm mfold. Among the five former sequences, none preserved genome dimerization and all reduced viral replication by 98 to 99.9%. Among the four latter sequences, three (MB6, -9, and -10) preserved genome dimerization, one (MB7) did not significantly inhibit it, and two (MB9 and -10) preserved viral replication. We conclude that duplex formation by stem B nucleotides is necessary for viral infectivity and complete genome dimerization. Deleting the 5′ or 3′ side of loop B or of stem B had little impact on dimerization of partial RNA transcript and no impact on klh folding (and, for loop B mutations, on stem B folding), but each deletion inhibited genome dimerization almost as much as klh destruction. This suggests that loop B is required for complete genome dimerization and that loop B and stem B stimulate dimerization only in very long RNAs and/or in the presence of unidentified viral and cellular factors. Finally, we asked if nine deletions or nucleotide substitutions within nt 200 to 242 and/or nt 282 to 335 could influence genome dimerization. These mutations had intermediate inhibitory impacts consistent with their predicted influence on stem B, loop B, and klh formation. Two exceptions were Δ200–226 and Δ236–242 genomic RNAs, which dimerized relatively poorly despite having neutral or positive influences on stem B, loop B, and klh folding.
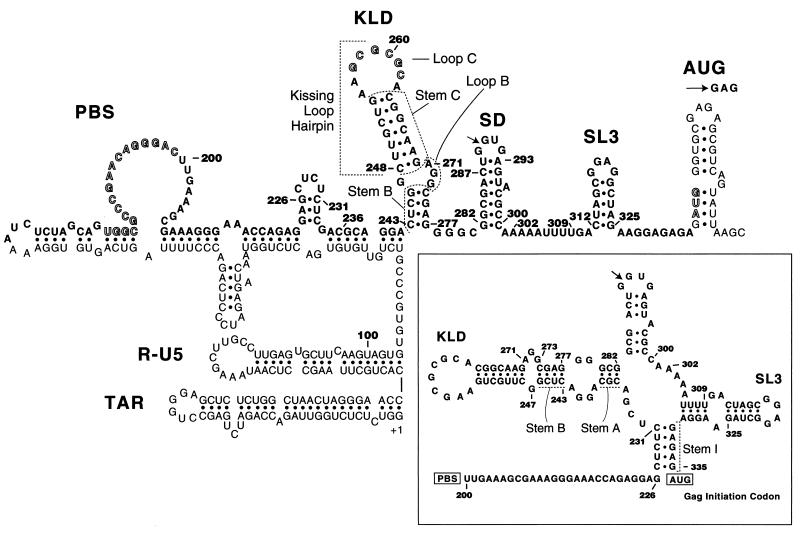
A dimeric genome appears required for human immunodeficiency virus type 1 (HIV-1) replication (20). Stem-loop B (nucleotide [nt] 243 to 247 and nt 271 to 277 in HIV-1Lai and HIV-1Hxb2 genomic RNA) is a completely conserved RNA sequence postulated to form a 4-bp stem and a 4-nt internal loop under the kissing-loop hairpin (klh) (11, 13) (Fig. 1). Together with the klh, it forms the kissing-loop domain (KLD) (13), also called stem-loop 1 (SL1). The klh is crowned by a palindrome called the dimerization initiation site (DIS) (20, 21). The existence of the klh is supported by considerable phylogenetic evidence (11, 13), and much information is available on its structure and function (e.g., see references 2, 4, 5, 7, 11–15, 17, 18, 20, and 21). It is well established that the klh sequence stimulates genomic RNA dimerization (5, 7, 13, 20), genomic RNA encapsidation (2, 5, 13, 14, 17, 18, 20), and proviral DNA synthesis (18, 20). In contrast, little is known on the existence, structure, and function of stem-loop B (4, 5, 14, 20, 22), despite the fact that it is more conserved than the klh (10, 13) and that deleting its 5′ side inhibits viral replication as much as klh destruction (14). Since the existence of stem-loop B is neither proven nor universally accepted (Fig. 1 [see legend]), stem-loop diagrams appear where CUCG246 forms a loop (1), base pairs to an upstream sequence (3, 6, 19), or base pairs to the tip of the AUG hairpin (8) (Fig. 1). The first purpose of this paper is to test the stem-loop B hypothesis by investigating the role of stem B and loop B nucleotides in viral replication, genomic RNA dimerization, and in vitro dimerization of partial RNA transcripts. The second purpose is to ask if nine deletions or nucleotide substitutions near the KLD, namely, within nt 200 to 242 and/or nt 282 to 335 (which encompass residues stimulating reverse transcription, splicing, encapsidation, and translation of viral RNA), could influence genome dimerization.
FIG. 1.
Postulated stem-loop diagram of the 5′ untranslated region of HIV-1Hxb2 and HIV-1Lai genomic RNA (3, 4, 6, 19). The cleavage site within the 5′ major splice donor is marked by an arrow within the SD hairpin. The AUG initiation codon of the gag gene is highlighted and somewhat arbitrarily presented as hydrogen bonded in the interest of a compact figure. The PBS and the GCGCGC262 palindrome are also highlighted. Focusing on the L sequence, the model can be considered proven regarding SL3 and the klh (5, 17, 20). It is rather inconclusive regarding stem-loop B and the PBS hairpin; for example, there is little evidence for the formation of stem-loop B in transcripts starting near the physiological 5′ end (1, 6, 8). A different model of the L sequence is shown in the insert (23). Note that stem-loop B and the klh can be theoretically formed from the sequence of any HIV-1 or simian immunodeficiency virus (strain cpz) (SIVcpz) subtype (10, 13). In contrast, stem I is not found in subtype O and in SIVcpz, stem-loop A is not found in subtypes D and O, the SD hairpin is not found in subtype N, and SL3 is not found in most SIVcpz (10), indicating that these four separate folds are extensively but not as absolutely preserved as the stem-loop B and klh folds.
Production and analysis of mutant viruses.
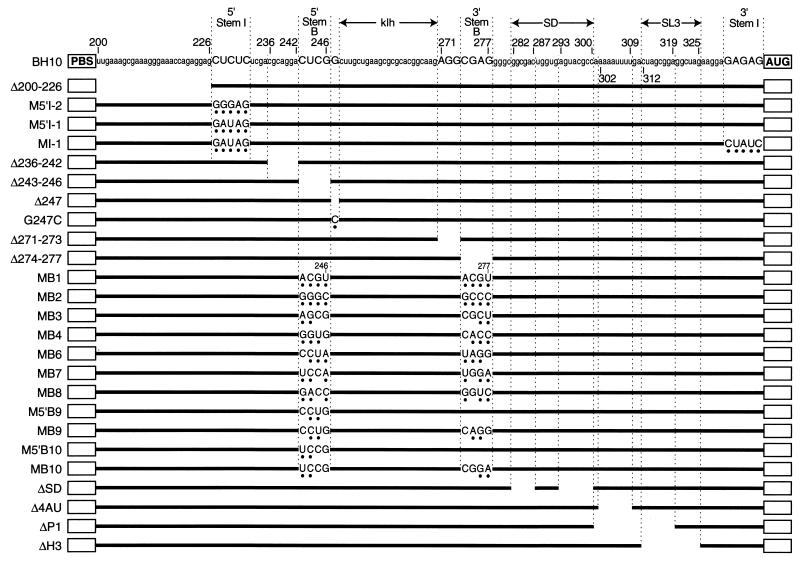
COS-7 cells were transfected in parallel with wild-type plasmid pSVC21.BH10 and derived mutant plasmids, which are defined in Fig. 2. For PCR mutagenesis 5′ of the klh, the PCR fragments synthesized extended from restriction site NarI to site BssHII; for mutagenesis 3′ of the klh, they extended from BssHII to SpeI. Plasmid ΔP1 was altered by cassette mutagenesis (14) to construct C258GΔP1, in which C258 of the DIS was replaced by G and nt 301 to 319 (Fig. 1) were deleted. All cassette and PCR fragments introduced into pSVC21.BH10 and ΔP1 were completely sequenced to verify that the correct and no extraneous mutation had been introduced. Virus titer was determined by estimating the amount of capsid protein (CAp24) in purified viruses or in clarified COS-7 culture supernatant fluid (13, 14). Genomic RNA packaging was derived from densitometry of the Northern blots whenever the titer of the purified viruses was known. To assay infectivity, the number of 50% tissue culture infective doses contained in a mutant and a wild-type clarified COS-7 culture supernatant fluid was measured in two 96-well flat-bottom plates (15).
FIG. 2.
Sequence of most mutations constructed for this study. PBS, tRNALys3 (primer) binding site; AUG, initiation codon of the Pr55gag polyprotein. Numbering is in reference to the genomic RNA cap site. The secondary structure of stem I, stem B, the klh, the SD hairpin, and SL3 is shown in Fig. 1. ΔSD maintains a wild-type-consensus CUGGUGAR splice site sequence.
Impact on viral replication, genome dimerization, and genome packaging of deletions within postulated loop B or postulated stem B.
COS-7 cells were transfected in parallel with equal amounts of plasmid pSVC21.BH10 and mutant plasmids pSVC21Δ243–246, Δ247, Δ271–273, and Δ274–277 (Fig. 2). The infectious titers of the resulting viruses are presented in rows 1 to 4 of Table 1; loop B mutants were equally attenuated (deleting 1 nt of loop B was at least as damaging as inactivating the DIS [20]) and stem B mutants were also equally highly attenuated. This is most consistent with the existence of stem B.
TABLE 1.
Genomic RNA dimerization yield and infectious titers of 26 HIV-1 mutantsa
| Mutation location and sequence | % Dimerization C ± SD | Log BH10/mutant ratio ± SEM |
|---|---|---|
| Within KLD | ||
| Δ247 | 54 ± 2.5 | 1.6 ± 0.45 |
| Δ271–273 | 51.5 ± 3 | 2.0 ± 0.35 |
| Δ243–246 | 48 ± 4.5 | 3.35 ± 0.20 |
| Δ274–277 | 52.5 ± 2 | 3.5 ± 0.20 |
| BH10 | 84 ± 3 | 0.0 |
| G247C | 62.5 ± 3 | 2.5 ± 0.15 |
| MB1 | 38 ± 2.5 | 3.2 ± 0.15 |
| MB2 | 55 ± 1.5 | 2.4 ± 0.1 |
| MB3 | 61 ± 6 | 2.65 ± 0.25 |
| MB4 | 64 ± 2.5 | 2.2 ± 0.25 |
| MB6 | 85 ± 1 | 3.2 ± 0.15 |
| MB7 | 73.5 ± 7.5 | 2.5 ± 0.2 |
| MB8 | 70 ± 5 | 1.6 ± 0.1 |
| MB9 | 85 ± 2 | 0.2 ± 0.25 |
| MB10 | 86.5 ± 2 | 0.3 ± 0.2 |
| M5′B9 | 69 ± 4 | 3.5 ± 0.2 |
| M5′B10 | 63.5 ± 3 | 3.6 ± 0.1 |
| 5′ or 3′ of KLD | ||
| Δ200–226 | 63 ± 3.5 | 3.5 ± 0.65 |
| M5′I-1 | 64 ± 2 | 3.3 ± 0.35 |
| MI-1 | 63 ± 5 | 3.1 ± 0.45 |
| M5′I-2 | 67.5 ± 8.5 | 3.5 ± 0.3 |
| Δ236–242 | 55 ± 3 | >4.2 |
| ΔSD | 76 ± 2 | >4.3 |
| Δ4AU | 73 ± 5.5 | 1.2 ± 0.15 |
| ΔP1 | 56 ± 3.5 | NDb |
| C258GΔP1 | 55.5 ± 2 | ND |
| ΔH3 | 73 ± 2.5 | 1.5 ± 0.15 |
In each infectivity test, the number of 50% tissue culture infective doses per ng of CAp24 was determined in octuplicate (14) by using a fourfold serial dilution against a control BH10 population. The number of independent infectivity tests per mutant was 2 to 4.
ND, not done.
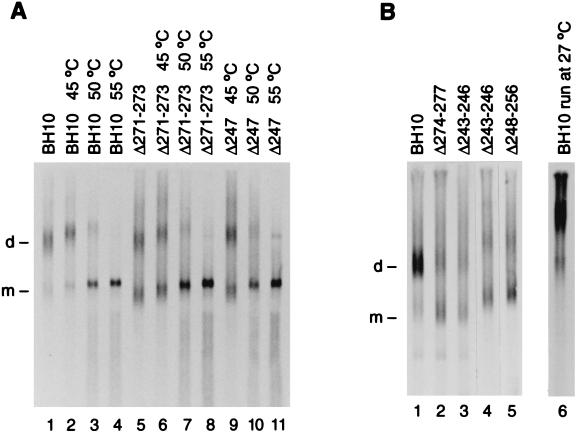
Genomic RNA was extracted from the isolated viruses, electrophoresed on a nondenaturing agarose gel, and visualized by Northern blotting with a 35S-labeled HIV-1 riboprobe specific for unspliced viral RNA (13, 15). Densitometry of Fig. 3, as well as many other gel lanes from independent transfections, reveals that BH10, Δ247, Δ271–273, Δ243–246, and Δ274–277 genomic RNAs were, respectively, 84, 54, 51.5, 48, and 52.5% dimeric (Table 1), and that the dissociation temperatures of BH10, Δ271–273, and Δ247 genomic RNAs were similar (∼49 to 50°C); deleting one side or the other of loop B, or deleting one side or the other of stem B thus inhibited genome dimerization almost as much as klh or KLD destruction (which results in 40 to 45% dimeric genomic RNA [13, 15, 20]; lane 5 of Fig. 3B]). This is most consistent with the existence of stem B at some point in the viral life cycle. Densitometry also indicates that Δ243–246 and Δ274–277 viruses encapsidated genomic RNA 2.5 to 4 times less efficiently than BH10 viruses (Fig. 3 [legend]). This is the first demonstration that mutating stem B inhibits genome encapsidation and the first demonstration that mutating loop B or stem B inhibits genome dimerization.
FIG. 3.
(A) Dimerization level and thermal stability of the viral RNA isolated from BH10, Δ271–273, and Δ247 viruses, respectively, containing 1.5 × 1012, 4.5 × 1012, and 4.5 × 1012 CAp24. Samples were dissolved in 8 μl of buffer S (10 mM Tris [pH 7.5], 100 mM NaCl, 1 mM EDTA, and 1% sodium dodecyl sulfate) and incubated at the temperatures indicated for 10 min (samples in lanes 1 and 5 were left on ice for 10 min). After the incubations, all samples were loaded without delay and with the voltage on. After electrophoresis (70 V; 4 h; 1% agarose in TBE2 [89 mM Tris, 89 mM borate, and 2 mM EDTA] at 4°C), the samples were Northern blotted, hybridized, and autoradiographed for 4 h. (B) Lanes 1 to 5: dimerization level of viral RNA isolated from BH10, Δ274–277, Δ243–246, and Δ248–256 viruses, respectively, containing 3.6 × 1012, 7.1 × 1012, 7.1 × 1012, 8.6 × 1012, and 4 × 1012 CAp24; experimental conditions were unchanged except for 0.8% agarose in lanes 1 to 3, and autoradiographic exposures of, respectively, 1 h (lanes 1 to 3) and 2 h 10 min (lanes 4 to 5). Lane 6: BH10 viral RNA electrophoresed (45 V, 3 h 10 min, 1% agarose in TBE2) in a room maintained at 27°C, i.e., under conditions where only tight dimers of partial HIV-1 RNA transcripts remain dimeric. Each lane represents a different transfection. Densitometry of lanes 1, 5, and 9 of panel A, lanes 1 to 5 of panel B, and equivalent lanes from other Northern blots indicate that Δ271–273, Δ247, Δ274–277, Δ243–246, and Δ248–256 viruses package genomic RNA, respectively, 44% ± 4%, 65% ± 11%, 40%± 6%, 25% ± 4%, and 40% ± 6% as well as BH10. Abbreviations: d, dimeric RNA; m, monomer.
While klh mutations reduce the loose (4, 13, 14, 21) and tight (12, 13) dimerization of partial HIV-1 RNAs by ≥90%, deletions Δ247, Δ271–273, Δ243–246, and Δ274–277 yielded, on average, ∼0% inhibition of tight dimerization and an insignificant ∼20% reduction of loose dimerization of partial transcripts ending at nt 295 or 508 (not shown). This suggests that loop B and stem B fully stimulate dimerization only in RNAs longer than partial transcripts and/or in the presence of unidentified viral and cellular factors.
Base-pair-preserving mutations in postulated stem B preserve genome dimerization; mutations which change base pair number inhibit genome dimerization even when they preserve klh formation.
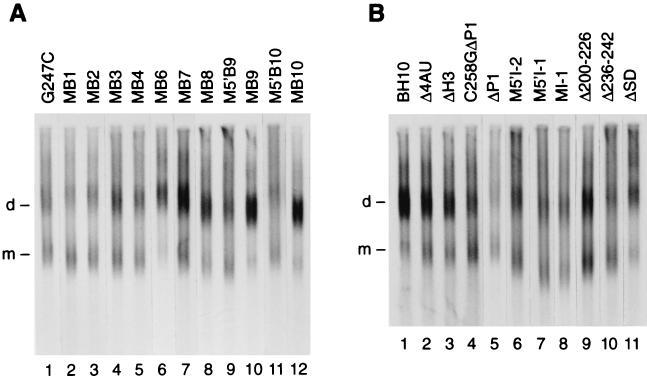
Figure 4A investigates the impact on genome dimerization of nucleotide substitutions in loop B (G247C; Fig. 2) or in stem B (MB1 to MB10, M5′B9, and M5′B10; Fig. 2). Nucleotide substitutions MB1 to MB10 preserve strand complementarity, and G247C makes possible a longer stem B and a smaller loop B. Densitometry of Fig. 4A and other gel lanes from at least two independent transfections reveals a range of dimerization yields; e.g., MB6, -9, and -10 did not affect genome dimerization, while MB1 was as inhibitory as klh or KLD destruction (Table 1).
FIG. 4.
Dimerization level of viral RNA isolated from viruses purified from 10 petri dishes. Experimental conditions as in Fig. 3 except for 5 h electrophoresis. (A) G247C, MB1, MB2, MB3, MB4, MB6, MB7, MB8, M5′B9, MB9, M5′B10 and MB10 genomic RNA. (B) BH10, Δ4AU, ΔH3, C258GΔP1, ΔP1, M5′I-2, M5′I-1, MI-1, Δ200–226, Δ236–242, and ΔSD genomic RNAs.
We used the algorithm mfold (version 3.1) (16, 24) to fold the first 508 nt of BH10 and mutant genomic RNAs. All foldings minimizing ΔG or producing ΔGs within 5% of the predicted minimum—typically 16 per sequence—were accepted as equally probable (not shown). Mutating loop B or deleting the 5′ or 3′ side of stem B left klh folding intact: interference with klh formation is not a plausible explanation for the poor dimerization of Δ243–246, Δ247, G247C, Δ271–273, and Δ274–277 genomic RNAs (54% dimeric on average). MB6, MB7, MB9, and MB10 RNAs (82.5% dimeric on average) were about as likely as BH10 RNA to form a KLD; MB8 and M5′B9 RNAs (69.5% dimeric on average) had a small P (probability of forming a KLD) but a large Pklh (probability of forming a klh); MB2, MB3, MB4, and M5′B10 RNAs (61% dimeric on average) had a small P and a small but nonzero Pklh; and MB1 RNA (38% dimeric) had a P and a Pklh of 0. Thus, it appears that complete genome dimerization requires KLD formation: a klh unsupported by loop B and stem B typically loses its dimerization potential, though it may in some mutants (two out of seven in our sample) keep half of it.
The G247C, Δ247, and Δ271–273 mutations left both klh and stem B folding intact (not shown; G247C stabilized stem B via addition of the C247-G273 base pair). The poor genome dimerization associated with these mutations stresses the importance of loop B in genome dimerization: loop B may orient the klh in a direction optimal for genome dimerization and/or interact with factors favoring dimerization. One such factor could be the nucleocapsid (NC) protein, because it stimulates genome dimerization in isolated viruses (15) and a glutathione-S-transferase–NC fusion protein protects loop B from RNase T1 cleavage (6). Loop B may also favor dimerization by lowering the transition from loose to tight dimer (22). (Tight dimerization of HIV-1 RNAs ending at nt 295 or 508 was stimulated ∼2-fold by deleting the 5′ or 3′ strand of stem B and inhibited ∼3-fold by deleting the 3′ side of loop B; Δ247 had neutral impacts [not shown].)
Two base pair-preserving stem B mutations (MB9 and MB10) preserve viral replication and genome dimerization.
Mutants MB1 to MB8 were, per unit CAp24, 1.6 to 3.2 logarithmic units less active than BH10 (Table 1), indicating that mere strand complementarity did not preserve viral infectivity. But MB9 and MB10 replicated at near-wild-type levels, in contrast with M5′B9 and M5′B10, which preserved neither genome dimerization nor viral replication (Table 1). However, MB6 and MB7 viruses were highly attenuated, suggesting that there may be no mechanistic overlap between how the stem B sequence controls genome dimerization and its other functions, which minimally include genome encapsidation (Fig. 3 [legend]) and reverse transcription (20). This notion is supported by our recent identification of klh mutations which inhibit genome dimerization without affecting genome encapsidation or reverse transcription (20) and of NC mutations which inhibit genome encapsidation without affecting genome dimerization (15). Note also that our four stem B or loop B deletions had identical effects on genome dimerization (Table 1) but variant effects on genome encapsidation (Fig. 3 [legend]).
Specifically inhibiting the dimerization function of the KLD reduces viral replication by 1.3 logarithmic units (20). Since mutations MB1 to MB8, M5′B9, and M5′B10 inhibited viral replication by ≥1.6 logarithmic units (2.7 on average), it follows that each one also inhibited functions other than genome dimerization, such as, we presume, proviral DNA synthesis and/or genome encapsidation.
Out of 255 alternative complementary stem B sequences tested by applying mfold to RNA positions 1 to 508, only 7 (e.g., MB6, -7, and -10) were as or more likely than BH10 to preserve KLD structure, and 210 (e.g., MB1, -2, -3, -4, and -8) had a P of 0 or close to 0 (not shown). This may be an important clue towards understanding why the stem-loop B sequence appears phylogenetically conserved. The simplest explanation for the results with MB1 to MB10 is that the stem B helix exists at some point(s) in the viral cycle, that duplex formation by stem B nucleotides promotes genome dimerization, that few alternative complementary stem B sequences preserve stem B helix formation, and that still fewer preserve helix formation and viral replication.
Impact on genome dimerization and viral replication of mutations 5′ and 3′ of stem B.
The primer binding site (PBS) and the gag gene are separated by 136 nt (the L sequence) which fold into stem B, loop B, the klh, and conceivably five other regions, such as nt 200 to 226, stem I, stem-loop A, the splice donor (SD) hairpin, and AAAAUUUU-SL3 (Fig. 1). We constructed nine mutant viruses bearing various deletions or nucleotide substitutions in each of these five other regions (first five and last four mutants in Fig. 2). (The mfold-derived probability of stem I formation [PI] was 0.31 in MI-1, 0.38 in BH10, and 0 in M5′I-1 and M5′I-2 viruses [not shown]). Genomic RNAs from these mutant viruses are shown in Fig. 4B. Their dimerization yields (from densitometry of Fig. 4B and many other gel lanes from independent transfections) are shown in Table 1. Δ236–242, ΔP1, Δ200–226, MI-1, M5′I-1, and M5′I-2 genomic RNAs had intermediate yields (55 to 56% for ΔP1 and Δ236–242; ∼64% for Δ200–226 and stem I mutants), while ΔSD, ΔH3, and Δ4AU had rather large yields (73 to 76%). Dimerization yields were consistent with the mfold-predicted impact on KLD and klh folding, except that Δ200–226, Δ236–242, and ΔP1 genomic RNAs were less dimeric than predicted. The Δ200–226 and Δ236–242 results invite further studies on the role of the PBS hairpin (Fig. 1) in genome dimerization. ΔP1 destroys SL3 (Fig. 2), a stem-loop as important as the KLD for genome encapsidation (9, 17). But SL3 destruction may only have a weak or indirect impact on genome dimerization, for two reasons: ΔH3 genomic RNA specifically lacks SL3 but is closer to BH10 than to ΔP1 in dimerization yield (Table 1); ΔP1 plus DIS inactivation had no additive effects (C258GΔP1, C258G, and ΔP1 genomic RNAs were, respectively, 55.5, 48, and 56% dimeric [Table 1] [20]). Similarly, though disrupting nt 200 to 226, nt 227 to 231, nt 236 to 242, stem B, loop B, or the klh inhibited genome dimerization by 25 to 50% in each instance, the inhibitions were not additive, because Δ200–256 genomic RNA was indistinguishable from Δ248–256 genomic RNA in dimerization yield (20).
Mutants Δ200–226, MI-1, M5′I-1, M5′I-2, Δ236–242, and ΔSD were, per unit CAp24, at least 3 logarithmic units less active than BH10, while ΔH3 and Δ4AU viruses were ∼1.35 logarithmic units less active than BH10 (Table 1). Thus, nt 200 to 226, nt 227 to 231, or nt 236 to 242 were as crucial for viral replication as stem B (Table 1) or the klh (13, 14) and at least twice as crucial as SL3 and the AU-rich sequence, whose impact was smaller than that of loop B and comparable to that of the central nucleotides of the DIS (14, 20). Since MI-1 viruses were as attenuated as M5′I-1 and M5′I-2 viruses, despite preserving the consensus sequence for protein synthesis initiation and despite a PI close to that of BH10, it follows that a stem I helix need not form during viral replication or it is not easily replaced.
Acknowledgments
This work was supported by a grant (MT-12312) from the Medical Research Council of Canada to M.L.
REFERENCES
- 1.Baudin F, Marquet R, Isel C, Darlix J-L, Ehresmann B, Ehresmann C. Functional sites in the 5′ region of human immunodeficiency virus type 1 RNA form defined structural domains. J Mol Biol. 1993;229:382–397. doi: 10.1006/jmbi.1993.1041. [DOI] [PubMed] [Google Scholar]
- 2.Berkhout B, Van Wamel J L B. Role of the DIS hairpin in replication of human immunodeficiency virus Type 1. J Virol. 1996;70:6723–6732. doi: 10.1128/jvi.70.10.6723-6732.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berkhout B, Van Wamel J L B. The leader of the HIV-1 RNA genome forms a compactly folded tertiary structure. RNA. 2000;6:282–295. doi: 10.1017/s1355838200991684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clever J L, Wong M L, Parslow T G. Requirements for kissing-loop-mediated dimerization of human immunodeficiency virus RNA. J Virol. 1996;70:5902–5908. doi: 10.1128/jvi.70.9.5902-5908.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clever J L, Parslow T G. Mutant human immunodeficiency virus type 1 genomes with defects in RNA dimerization or encapsidation. J Virol. 1997;71:3407–3414. doi: 10.1128/jvi.71.5.3407-3414.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damgaard C K, Dyhr-Mikkelsen H, Kjems J. Mapping the RNA binding sites for human immunodeficiency virus type 1 Gag and NC proteins within the complete HIV-1 and -2 untranslated leader regions. Nucleic Acids Res. 1998;26:3667–3676. doi: 10.1093/nar/26.16.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haddrick M, Lear A L, Cann A J, Heaphy S. Evidence that a kissing loop structure facilitates genomic RNA dimerization in HIV-1. J Mol Biol. 1996;259:58–68. doi: 10.1006/jmbi.1996.0301. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi T, Ueno Y, Okamoto T. Elucidation of a conserved RNA stem-loop structure in the packaging signal of human immunodeficiency virus type 1. FEBS Lett. 1993;327:213–218. doi: 10.1016/0014-5793(93)80172-q. [DOI] [PubMed] [Google Scholar]
- 9.Kim H-J, Lee K, O'Rear J J. A short sequence upstream of the 5′ major splice site is important for encapsidation of HIV-1 genomic RNA. Virology. 1994;198:336–340. doi: 10.1006/viro.1994.1037. [DOI] [PubMed] [Google Scholar]
- 10.Kuiken C, Foley B, Hahn B, Marx P, McCutchan F, Mellors J W, Mullins J, Wolinsky S, Korber B, editors. Human retroviruses and AIDS. Los Alamos, N.Mex: Los Alamos National Laboratory; 1999. [Google Scholar]
- 11.Laughrea M, Jetté L. A nineteen nucleotide sequence upstream of the 5′ major splice donor is part of the dimerization domain of the HIV-1 genome. Biochemistry. 1994;33:13464–13475. doi: 10.1021/bi00249a035. [DOI] [PubMed] [Google Scholar]
- 12.Laughrea M, Jetté L. Kissing-loop model of HIV-1 genome dimerization: HIV-1 RNAs can assume alternative dimeric forms and all sequences upstream or downstream of hairpin 248–271 are dispensable for dimer formation. Biochemistry. 1996;35:1589–1598. doi: 10.1021/bi951838f. [DOI] [PubMed] [Google Scholar]
- 13.Laughrea M, Jetté L, Mak J, Kleiman L, Liang C, Wainberg M A. Mutations in the kissing-loop hairpin of human immunodeficiency virus type 1 reduce viral infectivity as well as genomic RNA packaging and dimerization. J Virol. 1997;71:6003–6010. doi: 10.1128/jvi.71.5.3397-3406.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laughrea M, Shen N, Jetté L, Wainberg M A. Variant effects of non-native kissing-loop hairpin palindromes on HIV replication and HIV RNA dimerization; role of stem-loop B in HIV replication and HIV RNA dimerization. Biochemistry. 1999;38:226–234. doi: 10.1021/bi981728j. [DOI] [PubMed] [Google Scholar]
- 15.Laughrea M, Shen N, Jetté L, Darlix J-L, Kleiman L, Wainberg M A. Role of distal zinc finger of nucleocapsid protein in genomic RNA dimerization of human immunodeficiency virus type 1; no role for the palindrome crowning the R-U5 hairpin. Virology. 2001;281:109–116. doi: 10.1006/viro.2000.0778. [DOI] [PubMed] [Google Scholar]
- 16.Mathews D H, Sabina J, Zuker M, Turner D H. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J Mol Biol. 1999;288:911–940. doi: 10.1006/jmbi.1999.2700. [DOI] [PubMed] [Google Scholar]
- 17.McBride M S, Panganiban A T. Position dependence of functional hairpins important for human immunodeficiency virus type 1 RNA encapsidation in vivo. J Virol. 1997;71:2050–2058. doi: 10.1128/jvi.71.3.2050-2058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paillart J-C, Berthoux L, Ottmann M, Darlix J-L, Marquet R, Ehresmann B, Ehresmann C. Dual role of the putative dimerization initiation site of human immunodeficiency virus type 1 in genomic RNA packaging and proviral DNA synthesis. J Virol. 1996;70:8348–8354. doi: 10.1128/jvi.70.12.8348-8354.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rizvi T A, Panganiban A T. Simian immunodeficiency virus RNA is efficiently encapsidated by human immunodeficiency virus type 1 particles. J Virol. 1993;67:2681–2688. doi: 10.1128/jvi.67.5.2681-2688.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen N, Jetté L, Liang C, Wainberg M A, Laughrea M. Impact of human immunodeficiency virus type 1 RNA dimerization on viral infectivity and of stem-loop B on RNA dimerization and reverse transcription and dissociation of dimerization from packaging. J Virol. 2000;74:5729–5735. doi: 10.1128/jvi.74.12.5729-5735.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skripkin E, Paillart J-C, Marquet R, Ehresmann B, Ehresmann C. Identification of the primary site of the human immunodeficiency virus type 1 RNA dimerization in vitro. Proc Natl Acad Sci USA. 1994;91:4945–4949. doi: 10.1073/pnas.91.11.4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi K I, Baba S, Chattopadhyay P, Koyanagi Y, Yamamoto N, Takaku H, Kawai G. Structural requirement for the two-step dimerization of human immunodeficiency virus type 1 genome. RNA. 2000;6:96–102. doi: 10.1017/s1355838200991635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeffman A, Hassard S, Varani G, Lever A. The major HIV-1 packaging signal is an extended bulged stem loop whose structure is altered on interaction with the Gag polyprotein. J Mol Biol. 2000;297:877–893. doi: 10.1006/jmbi.2000.3611. [DOI] [PubMed] [Google Scholar]
- 24.Zuker M, Matthews D H, Turner D H. Algorithms and thermodynamics for RNA secondary structure prediction: a practical guide. In: Barciszewski J, Clark B F C, editors. RNA biochemistry and bio/technology. NATO ASI Series. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1999. pp. 11–43. [Google Scholar]