Abstract
Thirteen components were identified in the methanol extract of Moricandia sinaica leaves (MSLE) through analysis utilizing HPLC-ESI-MS/MS., including flavonoids, anthocyanins, phenolic acids, and fatty acids. The methanol extract of M. sinaica leaves contained total phenolics and flavonoids (59.37 ± 2.19 mg GAE/g and 38.94 ± 2.72 mg QE/g), respectively. Furthermore, it revealed in vitro antioxidant properties as determined by the DPPH and FRAP assays, with respective IC50 values of 10.22 ± 0.64 and 20.89 ± 1.25 μg/mL. The extract exhibited a notable hepatoprotective effect in rats who experienced paracetamol-induced hepatotoxicity. When a dose of 250 mg/kg was given, there was a 52% reduction in alanine transaminase and a 30% reduction in aspartate transaminase compared to the group with the disease. Furthermore, it demonstrated a 3.4-fold, 2.2-fold, and 2.6-fold increase in superoxide dismutase, non-protein sulfhydryl, and glutathione peroxidase, respectively. In addition, it demonstrated a 68% decrease in lipid peroxide levels compared to the group with paracetamol-induced condition. The verification was conducted using a histological study, which identified improved liver histology with a small number of distended hepatocytes. Moreover, in silico studies focused on the enzymes NADPH oxidase, butyrylcholinesterase, and tyrosinase as the targets for the major compounds. In conclusion, MSLE showed promising hepatoprotective and antioxidant activities due to its richness in antioxidant metabolites.
1. Introduction
The liver is a vital organ that performs several functions, including the storage of vitamins, iron, and glycogen, as well as the metabolism of carbohydrates, lipids, and proteins. Moreover, the liver has a vital function in eliminating many external compounds from the body by facilitating their removal through processes like reduction, hydrolysis, oxidation, and conjugation. Moreover, it is accountable for the synthesis of 90% of the plasma proteins and the production of bile [1–3].
Chronic liver illnesses remain a substantial worldwide health issue, impacting more than 10% of the global population [4]. Up to 40% of people with cirrhosis may not show any symptoms and can remain without symptoms for long periods of time. However, when complications like variceal hemorrhage, ascites, or encephalopathy occur, there is often a progressive decline that can result in mortality or the need for a liver transplant [5].
The liver’s hepatocytes employ various hepatic defense mechanisms to protect against oxidative damage. These mechanisms include the synthesis of reduced glutathione (GSH), a natural antioxidant, and superoxide dismutase (SOD), an enzyme that converts the superoxide radical into molecular oxygen and hydrogen peroxide, thereby facilitating detoxification [6–10].
The antioxidant, antibacterial, anti-inflammatory, and cytotoxic capabilities of natural sources are significant [11–13]. The existence of secondary metabolites in their composition makes their ability to contribute to antioxidant defense and redox signaling possible. These secondary metabolites include flavonoids, phenolic acids, terpenoids, and volatile oils. These compounds act as scavengers of free radicals [14–18]. Various hepatoprotective compounds derived from plants function as effective pharmaceuticals and dietary supplements. Hepatoprotective drugs produced from natural sources are of great significance for such agents as glycyrrhizin, ellagic acid, and silymarin [19, 20].
Moricandia is a genus belonging to the family Brassicaceae and comprises approximately eight species that exhibit various biological activities such as cytotoxicity, antioxidant properties, and anti-inflammatory effects [21–23]. Regarding the health benefits, leaves of M. arvensis are frequently incorporated into traditional Tunisian cuisine, and M. sinaica has been utilized traditionally as a treatment for both pain and syphilis [24].
Moricandia sinaica (Boiss.) is a herbaceous plant that grows for one year and is endemic to the Mediterranean region of Europe and America. The species is predominantly cultivated globally as decorative plants, exhibiting flowers in a variety of shades, including violet, purple, and white [25]. Currently, there are limited studies on the biological activities or the chemical composition of M. sinaica. A previous study on the leaves of M. sinaica by Aly et al. (2023) reported the chemical composition of the volatile oil and n-hexane extract of the leaves; the results revealed their richness with aliphatic hydrocarbons. Additionally, the primary constituents of the hexane extract include α-amyrin, γ-sitosterol, β-amyrin acetate, and α-tocopherol. Conversely, the primary constituents of the essential oil were predominantly monoterpenes and sesquiterpenes. Besides, the oil showed promising antioxidant and cytotoxicity in vitro [21]. Another study reported the anti-inflammatory, analgesic, and antipyretic activities in vivo of the water extract of M. sinaica along with the characterization of its polyphenols where the extract was predominantly composed of flavonoid, namely isorhamnetin, quercetin, and kaempferol [24].
We conducted a study to investigate the potential antioxidant and hepatoprotective properties of M. sinaica leaves extract (MSLE) when exposed to a paracetamol-induced toxicity model. This research was prompted by the increased demand for new hepatoprotective medicines obtained from natural sources. In addition, we performed a phytochemical analysis of the extract components using HPLC/ESI/MS/MS in combination with molecular docking research.
2. Materials and methods
2.1. Plant material
Moricandia sinaica Boiss. (Brassicaceae) leaves were collected in February 2022 from South Sinai, Egypt, 27°57’43.2"N and 34°16’16.7"E. Dr. Mohammed El-Gebaly, a professor in botany department at the National Research Centre in Giza, Egypt, identified and verified the plant with gratitude. The voucher specimens, labelled BUC-PHG-MS-10, were submitted to the Pharmacognosy Department of the Faculty of Pharmacy, Badr University in Cairo.
2.2. Preparation of the plant extract
The air-dried leaves of Moricandia sinaica (100 g) were initially extracted with n-hexane. The leaves were soaked in n-hexane (3 x 1L), then the liquid was separated by filtration and evaporated under vacuum at 40°C until all the solvent evaporated, resulting in the production of 2.41 g of the n-hexane extract. The defatted leaves were macerated in 80% methanol (3 x 1L) and then filtered. The filtrate was subjected to drying using a Rotary evaporator (Hei-VAP Value, Heidolph) at a temperature of 45°C. The collected dried residue (MSL) (17.63 g) was kept in the refrigerator till used in the study [26].
2.3. HPLC-ESI-MS/MS analysis
The phytochemical characterization of the methanol extract of M. sinaica leaves was performed by employing HPLC coupled with ESI-MS following a recently published method [27]. Briefly, the extract was diluted in HPLC-grade methanol (100 μg/mL), filtered with a 0.2 μm membrane disc filter, and injected in 10 μL. The Acquity HPLC apparatus (Waters®, Milford, MA USA) utilized a reversed-phase Acquity UPLC-BEH C18 column (1.7 μm particle size, 2.1 x 50 mm). Over a 35-minute run, with a gradient of acidified water and methanol with 0.1% formic acid, mobile phase elution flow was increased to 0.2 mL/min. An XEVO TQD triple quadruple instrument was used to conduct ESI-MS in both positive and negative ion acquisition modes. The triple quadrupole mass spectrometer XEVO TQD (Waters Corporation, Milford, MA, USA) was utilized for mass spectrometric analysis under these conditions: Edwards® vacuum pump (Chandler, AZ, USA) delivers 30 eV cone voltage and 3 kV capillary voltage at 150° source and 440° desolvation temperatures. Maslynx 4.1 observed mass spectra in the ESI region between 100–1000 m/z, and the fragmentation pattern and mass spectra were used to tentatively identify the peaks with reported data.
2.4. Total phenolic and flavonoid content
The Folin-Ciocalteu and AlCl3 spectrophotometric techniques were used to measure the extracts’ total phenolic and flavonoid content. Our previous published work provides the experimental methods [27]. The total phenolic concentration was quantified in milligrams of gallic acid equivalents (GAE) per gram of dry extract [28]. The total flavonoid concentration was evaluated as mg quercetin equivalents (QE) per gram of dry extract [29].
2.5. In vitro antioxidant assays
2.5.1. DPPH and FRAP assays
The DPPH radical scavenging activity and FRAP ferric-reducing antioxidant power assays were performed based on previously published methods [30–32]. All measurements were done three times and averaged S1 and S2 Figs.
2.6. In vivo hepatoprotective activity
2.6.1. Animals
The National Research Centre in Cairo, Egypt, provided 30 mature male Wistar albino rats weighing 200–220 g and ten weeks old for the study. In regulated laboratory circumstances, rats were housed at 25 ± 2°C, 40–60% humidity, and a 12-hour light-dark cycle. Rats were fed a regular diet and given water freely. The rats were lab-acclimated for a week before the experiment began. Each animal received a temporary random identifier within its respective weight range category. Based on their placement on the rack, cages were assigned numerical labels. For each group, a cage was randomly chosen from the entire pool of cages. animals were then selected from each weight range category and assigned their permanent numerical identifiers within the cages. Subsequently, the cages were randomized within the designated exposure group. The Research Ethics Committee of Badr University’s in Cairo, Faculty of Pharmacy authorized the study protocols (PG-117-A) in accordance with the guidelines of the National Institutes of Health for the proper care and use of laboratory animals (NIH Publication No. 85–23, revised 2011).
In our study, we have prioritized the well-being of animals involved in scientific research to uphold the credibility and dependability of our findings. Through diligent monitoring and implementing humane practices, we have consistently evaluated the health conditions of rats. Our assessments, recorded in a comprehensive scoresheet, encompassed various factors such as physical appearance, weight changes, behavior, and locomotion. The majority of rats demonstrated normal scores, indicating overall good health [33].
2.6.2. Experimental design
In this study, a total of 30 rats were randomly allocated into five different weight groups according to a computer-generated randomization table (www.randomization.com) (n = 6 per group) as follows: Group I: served as a normal control and received 10% Tween 80 (10 mL/kg, po) for 14 days. Group II: received MSLE (250 mg/kg, p.o.) as a drug alone group [34]. Group III: received 10% Tween 80 via oral route for 14 days and then received PAR (2 g/kg, p.o.) [35, 36] suspended in 10% Tween 80 1 hour after the last dose, serving as the disease group. Group IV: received MSLE (250 mg/kg, p.o.) for 14 days and then received PAR (2 g/kg, p.o.) suspended in 10% Tween 80 1 hour after the last dose, serving as the treatment group. Group V: received (N-Acetylcysteine (NAC) at a dose of (100 mg/kg, po) dissolved in 10% Tween 80 and followed by PAR (2 g/kg, p.o.) suspended in 10% Tween 80 one hour after the last dose, serving as the standard group [36]. Power analysis method (power = 0.8, α = 0.05) was conducted to estimate group size.
2.6.3. Blood sampling and tissue preparation
After PAR intoxication, blood samples were obtained 4 hours later and then subjected to centrifugation at a speed of 3000 rpm for 5 minutes. The specimens were preserved at a temperature of -20ºC for subsequent examination. The animals were administered thiopental (50 mg/kg) for anesthesia after a period of 24 hours and then sacrificed by decapitation by well-trained personnel according to AVMA Guidelines for the Euthanasia of Animals: 2020 Edition [37]. The liver was expeditiously excised, quantified, rinsed with frigid saline solution, and partitioned into two segments. The initial portion was combined with phosphate buffer saline (0.1 M PBS, pH 7.4) and subjected to centrifugation at a rate of 10,000 revolutions per minute for a duration of 30 minutes at a temperature of 4ºC. The liquid portion of the mixture was subsequently preserved at a temperature of -80ºC for future biological analyses. The second portion of the liver was stored in a solution of 10% neutral buffered formalin for the purpose of conducting histological analysis. During the analysis of these measurements, the investigators were blinded to sample identity and an independent experimenter performed sample coding and decoding.
2.6.4. Biochemical analyses
2.6.4.1. Plasma biochemical parameters. The concentrations of AST and ALT in the blood samples were determined using commercially available kits from Bio-Diagnostic (Giza, Egypt) and a UV-visible spectrophotometer (V630; JASCO, Tokyo, Japan) at a wavelength of 505 nm. The data were evaluated using standard methods [38].
2.6.4.2. Tissue biochemical parameters. 2.6.4.2.1. Estimation of lipid peroxidation levels: Thiobarbituric acid (TBA) was used to test lipid peroxidation in homogenized liver tissue supernatant. The malondialdehyde (MDA) production can be measured based on this method. The TBA-MDA complex absorbance was measured at 530 nm, and liver MDA concentration was reported in nmol/mg protein [39].
2.6.4.2.2. Estimation of superoxide dismutase activity: The activity of superoxide dismutase (SOD) was determined using spectrophotometric measurement, as described by Robak et al. [40]. This method depended on the generation of superoxide ions by the oxidation of xanthine and oxygen by xanthine oxidase, resulting in the formation of uric acid and hydrogen peroxide. Superoxide anions convert nitroblue tetrazolium (NBT) into NBT-diformazan, which absorbs at a wavelength of 550 nm. Superoxide dismutase (SOD) was tested for its ability to reduce superoxide ions and prevent NBT-diformazan production. Reduced NBT-diformazan production measures Superoxide Dismutase (SOD) activity in the tested sample.
2.6.4.2.3. Estimation of reduced glutathione levels: The concentration of reduced glutathione (GSH) was determined based on [41, 42]. This approach relies on the interaction between DTNB [5,50-dithiobis-(2- nitrobenzoic acid)] and reduced glutathione (GSH), resulting in the creation of a yellow-colored molecule that exhibits its highest absorption at 412 nm. The GSH concentration was measured and expressed as nmol/mg protein.
2.6.4.2.4. Estimation of antioxidant enzyme activities: The Glutathione peroxidase (GPx) activity was evaluated using a colorimetric assay. Hydrogen peroxide is the substrate in this GSH-based test. The absorbance was measured at 420 nm, and GPx specific activity was reported as μmol GSH/mg protein [43].
2.6.5. Histopathological examination
Liver tissue samples were dissected, then preserved in 10% neutral buffered formalin (NBF) and dried using increasing concentrations of alcohol. The liver samples were immersed in paraffin to create solid blocks, which were then cut into thin sections measuring 4–6 μm in thickness. To assess histology, these slices were stained with hematoxylin and eosin [44]. Ordinary light microscopy [Olympus, Japan] was used to capture images at X400 magnification.
2.7. Statistical analysis
The acquired data were displayed as the mean ± standard deviation (SD) using one-way analysis of variance (ANOVA) to examine the variations between the groups. Tukey’s t-test was then employed as a post hoc test. The results were regarded as statistically significant if P ≤ 0.05.
2.8. Molecular docking
The X-ray crystallographic structures of NADPH oxidase, butyrylcholinesterase, and tyrosinase were obtained from the Protein Data Bank (www.pdb.org) on 15 August 2023. The structures were downloaded using the corresponding IDs: 2CDU, 6ESJ, and 5M8Q [45–47]. All of the docking studies were carried out with the help of the Vina autodock applications [48, 49]. After ChemDraw sketched the ligands, they were converted to 3D structures by the Biovia Discovery Studio visualizer. The MGL tools were used to prepare both the ligands and receptors in the pdbqt format as a prerequisite for the Vina autodock. The seven identified major compounds were subjected to docking analysis in the active site of each target. The active site was defined beforehand based on the binding of the relevant co-crystalized ligand. Ultimately, the docking outcomes were observed and examined according to the docking scores.
3. Results and discussion
3.1. Chemical composition of MSLE
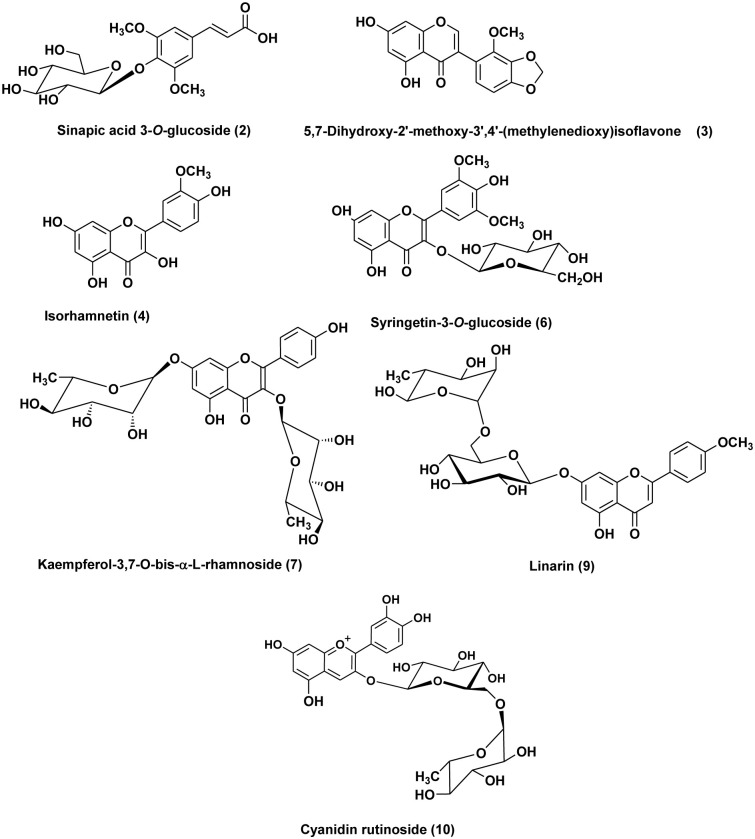
The M. sinaica leaves extract (MSLE) was subjected to analysis using HPLC-ESI-MS/MS to identify its phytoconstituents S3 Fig. The findings indicated the detection of thirteen phenolic components from various groups, including flavonoids, anthocyanins, and phenolic acids, in MSLE. Their MS properties and fragmentation pattern were compared to previous published data in order to make a tentative identification (Table 1). Among the identified secondary metabolites in MSLE, the flavonoid glycosides were found to be the most predominant class. The ion mass peaks at m/z 509.25, 577.35, 593.25, 535.25, 621.30 for the suggested molecular formulas C23H24O13, C27H30O14, C28H32O14, C25H25O12, C32H27O13, respectively, corresponding to five flavonoid glycosides; namely syringetin-3-O-glucoside, kaempferol-3,7-O-bis-α-L-rhamnoside, linarin, luteolin-O-diacetylhexoside, apigenin derivative (6, 7, 9, 11, 13). A previous study on the aqueous fraction of M. sinaica displayed a high percentage of polyphenols represented as flavonoid glycosides that are in correlation with our results, and the extract was reported for its anti-inflammatory activity [24]. Besides, Linarin, known as acacetin-7-O-β-D-rutinoside, was previously identified from Isatis indigotica (Brassicaceae) [50, 51].
Table 1. Characteristics of M. sinaica leaves extract (MSLE) phytoconstituents identified by HPLC-MS/MS in the positive and negative ionization mode.
| No. | Rt (min) | Tentative identified compound | [M-H] ¯/[M+H] + (m/z) | MS2 fragments (m/z) | Compound Class | Molecular Formula | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 0.69 | Caffeic acid derivative | 377.10 | 341 | Phenolic acid | C18H17O9 | [54] |
| 2 | 3.45 | Sinapic acid 3-O-glucoside | 409.15 [M+Na] + | - | Phenylpropanoid acid | C17H22O10 | [55] |
| 3 | 9.47 | 5,7-Dihydroxy-2’-methoxy-3’,4’-(methylenedioxy)isoflavone | 327.30 | 179 | Flavonoid | C17H12O7 | [56] |
| 4 | 10.89 | Isorhamnetin | 315.05 | 315, 300, 151 | Flavonoid | C16H12O7 | [57] |
| 5 | 11.19 | Trihydroxy Octadecenoic acid | 329.10 | 211, 229, 171, 139, 99, 155 | Fatty acid | C18H34O5 | [54] |
| 6 | 13.80 | Syringetin-3-O-glucoside | -/509.25 | - | Flavonoid glycoside | C23H24O13 | [56] |
| 7 | 13.83 | Kaempferol-3,7-O-bis-alpha-L-rhamnoside | 577.35 | 431, 285 | Flavonoid glycoside | C27H30O14 | [56] |
| 8 | 15.63 | 3′,3′′′-Binaringen methyl ether | 555.35 | 403, 237, 219, 151 | Biflavonoid | C31H24O10 | [58] |
| 9 | 23.27 | Linarin (Acacetin-7-O-β-D-rutinoside) | -/593.25 | - | Flavonoid glycoside | C28H32O14 | [59] |
| 10 | 23.82 | Cyanidin rutinoside | -/593.30 | 287, 434, 595 | Anthocyanin | C27H31O15+ | [60] |
| 11 | 24.69 | Luteolin-O-diacetylhexoside | -/535.25 | 490, 489, 285 | Flavonoid glycoside | C25H25O12 | [61] |
| 12 | 26.98 | Australisine A | -/607.25 | 286 | Flavonoid | C35H26O10 | [60] |
| 13 | 28.12 | Apigenin derivative | -/621.30 | 269 | Flavonoid glycoside | C32H27O13 | [62] |
Besides, three ion peaks values at m/z327.30, 315.05, and 607.25 with molecular formulas C17H12O7, C16H12O7, and C35H26O10, respectively were tentatively identified as flavonoid aglycones (3, 4, 12) 5,7-dihydroxy-2’-methoxy-3’,4’-(methylenedioxy) isoflavone, isorhamnetin, and australisine A. Biflavonoid was tentatively characterized as 3’,3‴-binaringen methyl ether (8), The compound displayed a deprotonated ion [M-H]- at m/z 555.35, and its MS2 pattern matched the previously documented data for biflavonoid 3’,3‴-binaringen methyl ether [52]. Moreover, anthocyanin is characterized as cyanidin rutinoside with a protonated ion peak value at m/z 593.30 in (10) that may improve the hepatoprotective potential of the extract through its antioxidant effect [53]. Phenolic acid and phenylpropanoic acid in MSLE are represented as caffeic acid derivative (1) and sinapic acid 3-O-glucoside (2). The chemical structures of the major components in MSLE are depicted in (Fig 1). Based on our results, we were encouraged to conduct an in vitro antioxidant and in vivo hepatoprotective study due to the identification of bioactive secondary metabolites in the methanol extract of M. sinaica leaves.
Fig 1. The chemical structures of major compounds of the M. sinaica leaves extract (MSLE) identified by HPLC-MS/MS.
3.2. Total phenolics (TP) and total flavonoids (TF)
The Folin-Ciocalteu and AlCl3 methods, which are spectrophotometric techniques, were employed to quantify the total phenolics and total flavonoids in the MSLE. Polyphenolics, a type of secondary metabolites, possess numerous biological activities such as antioxidant, cytotoxic, antidiabetic, and anti-inflammatory effects [63–65]. The methanol extract of M. sinaica leaves exhibited total phenolics and total flavonoid contents corresponding to 59.37 ± 2.19 mgGAE/g and 38.94 ± 2.72 mgQE/g, respectively. This suggests it is a suitable candidate for antioxidant and hepatoprotective applications.
3.3. In vitro antioxidant potential of MSLE
Regarding the antioxidant potential of MSLE, it was estimated using two techniques DPPH and FRAP assays (Table 2). The results revealed its potent activity with an IC50 value of 5.78 ± 0.67 μg/mL and 17.35± 1.28 μg/mL in comparison to ascorbic acid standard with an IC50 value of 10.22 ± 0.64 and 20.89 ± 1.25 μg/mL in DPPH and FRAP assays, respectively.
Table 2. Antioxidant potential, TPC and TPC of M. sinaica leaves extract (MSLE).
| Extract | DPPH assay IC50 (μg/mL) | FRAP assay IC50 (μg/mL) | Total phenolics (mg/g gallic acid equivalents) mgGAE/g | Total flavonoids (mg/g quercetin equivalents) mgQE/g |
|---|---|---|---|---|
| MSLE | 5.78 ± 0.67 | 17.35 ± 1.28 | 59.37 ± 2.19 | 38.94 ± 2.72 |
| Ascorbic acid | 10.22 ± 0.64 | 20.89 ± 1.25 | - | - |
Values are mean ± SEM, n = 3, IC50: Inhibitory concentration 50%.
Earlier studies have examined the antioxidant properties of plants from the genus Moricandia. The ethyl extract of M. arvensis shown significant ability to remove DPPH radicals, with an IC50 value of 171.9 ± 1.0 μg/mL. Additionally, it effectively protected linoleic acid from peroxidation in β-carotene bleaching tests, with an IC50 value of 35.69 ± 2.30 μg/mL [66–68].
3.4. In vivo hepatoprotective activity of MSLE
3.4.1. Effects on liver stress markers
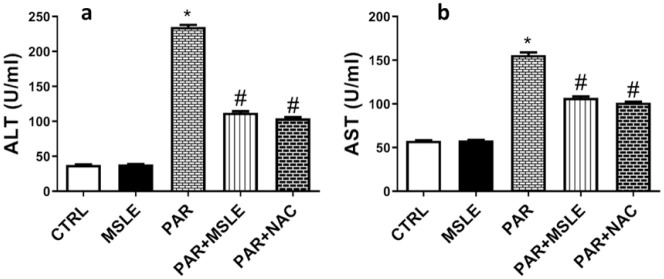
The administrated dose of PAR resulted in a substantial elevation of liver enzymes ALT and AST by 6-fold (P < 0.0001) and 2.7-fold (P < 0.0001), respectively, in contrast to the control group. Preliminary treatment with MSLE significantly mitigated liver injury caused by PAR, as shown in (Fig 2) by decreasing ALT and AST levels by (P < 0.0001; 52%) and (P < 0.0001; 30%), respectively, in comparison to PAR group. Notably, MSLE showed a similar effect as the standard drug NAC (N-Acetylcysteine), there was no notable difference observed between the two groups.
Fig 2. The effect of M. sinaica leaves extract (MSLE) identified on serum liver function markers in PAR-induced liver toxicity in rats.
(a) Alanine aminotransferase, (ALT) (b) Aspartate aminotransferase (AST). Data are presented as mean ± SD (n = 6); * p < 0.05 vs. control rats; # p < 0.05 vs. PAR-treated rats using Tukey’s post hoc test.
3.4.2. Effects on oxidative stress markers
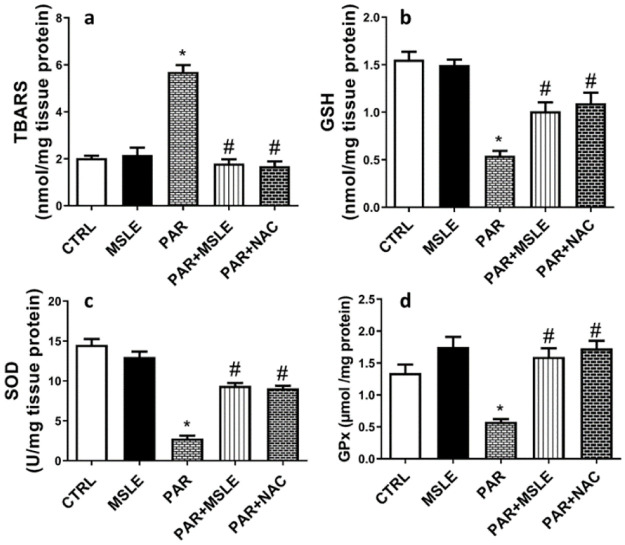
The administration of paracetamol resulted in a significant rise in the formation of reactive oxygen species (ROS), which subsequently led to a substantial elevation in the level of thiobarbituric acid reactive substances (TBARS) (P < 0.0001; 2.9-fold) relative to the control group. This was confirmed by a substantial reduction in the activity of SOD (P<0.0001; 5.4-fold), GSH (P <0.0001; 3-fold), and GPx (Glutathione peroxidase) enzyme activity (P < 0.0001; 57%) (Fig 3). Rats treated with MSLE demonstrated notable antioxidant activity and effectively restored the redox imbalance induced by PAR, as compared to the PAR group that demonstrated by lower levels of lipid peroxide (P <0.0001; 68%) and elevated levels of SOD (P< 0.0001; 3.4-fold), GSH (P = 0.0007; 2.2-fold), and GPx activity (P <0.0001; 2.6-fold). M. sinaica leaves extract had similar effects as NAC, with no statistically significant distinctions observed between the two groups.
Fig 3. The effect of M. sinaica leaves extract (MSLE) identified on oxidative stress markers in PAR-induced liver toxicity in rats.
(a) TBARs, (b) GSH, (c) SOD, and (d) GPx. Data are presented as mean ± SD (n = 6); * p < 0.05 vs. control rats; # p < 0.05 vs. PAR-treated rats using Tukey’s post hoc test.
The excessive consumption of Paracetamol (PAR) can be harmful to the liver when used within the recommended therapeutic dose for alleviating mild pain and headaches, it undergoes biotransformation and is eliminated from the body as harmless conjugates of sulphate and glucuronic acid [69]. The overdose of PAR can cause hepatic necrosis, liver lesions, and kidney injury, and may even result in fatalities in both humans and experimental animals [70]. Hepatic enzymes (ALT and AST) will be released from the injured liver into the bloodstream, increasing their levels in blood tests [71]. Additionally, the oxidative stress and ATP depletion caused by mitochondrial permeability transition may be the cause of the hepatic damage brought on by PAR [72]. In our study, the MSLE (250 mg/kg) was used to explore the extract’s potential for protection against PAR-induced toxicity in rats. Compared to the group that received only PAR treatment, the group that received MSL after PAR therapy had significantly lower levels of AST and ALT. The results align with the prior study conducted by Laraba et al. (2022), who demonstrated the hepatoprotective effects of n-butanol extract from M. arvensis in cases of doxorubicin-induced toxicity. The extract was administered at doses of 50 mg/kg and 100 mg/kg. Compared to the DOX-treated rats, it significantly reduced the amount of TBARS, elevated GSH, and improved GPx activity. Evidence of the liver’s hepatoprotective efficiency was demonstrated by the preservation of its architecture and the reduction of structural and functional changes [73]. Another study showed the protective effects of M. sinaica methanol and n-butanol extracts (100 mg/kg and 200 mg/kg) regarding the oxidative stress on the kidney and heart through carbon tetra chloride (CCl4)-induced nephron and cardiotoxicity. Also, it demonstrated a protective effect for MDA and NPSH (non-protein sulfhydryl) levels [74].
The antioxidant and hepatoprotective potency of MSLE would be correlated with the presence of flavonoids as the major identified components (Table 1) that were reported for their hepatoprotective and antioxidant effects [75, 76], besides the high TPC and TFC (Table 2). Syringetin-3-O-glucoside was reported for its antioxidant properties [77, 78]. Another flavonoid, kaempferol, showed a significant decrease in oxidative stress and lipid peroxidation as well as an increase in antioxidative defense activity [79]. Another investigation on kaempferol glycosides namely, kaempferol 3-O-rutinoside and kaempferol 3-O-glucoside, showed that they increased total protein levels and prevented the CCl4-induced elevations in AST, ALP, and MDA levels in the serum, and revealed normal catalase (CAT) and SOD activity and had greatly recovered GSH levels [80]. Furthermore, apigenin, a flavonoid found in MSLE, has been shown to possess hepatoprotective properties by mitigating liver damage through the reduction of oxidative stress and inflammation [81]. Eventually, the presence of bioactive secondary metabolites in the methanol extract of M. sinaica leaves clarified that providing MSLE to rats exposed to PAR-induced toxicity considerably reduced liver damage and eased fibrosis and was thus thought to be a possible herbal remedy for treating liver injury.
3.5. Histopathological investigation of the liver
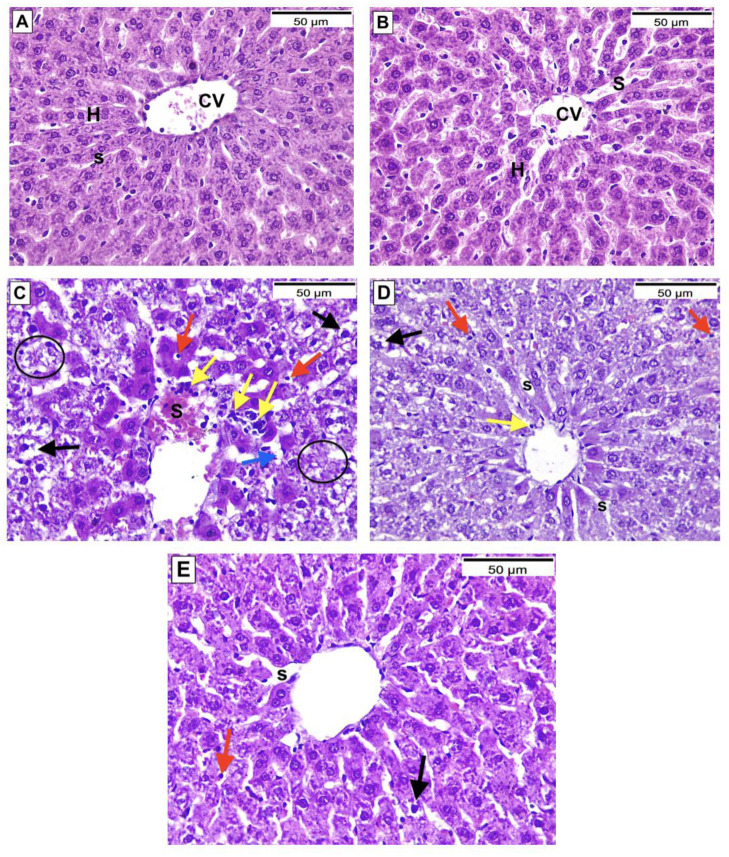
Histopathological assessment and grading of liver tissue injury were based upon previous study [82] as shown in (Table 3). Group I (control) & II (MSLE) showed normal liver histology with acidophilic hepatocytes arranged in regular cords radiating from the central vein [Grade 0]. Group III (PAR) showed disorganized liver architecture, severely degenerated hepatocytes with ballooning [Garde II], apoptotic cells, cytoplasmic lipid droplets [Grade III], focal lobular necrosis [Grade IV], markedly congested sinusoids, and heavy inflammatory cellular infiltrates. Group IV, which was treated with MSLE, showed improved liver histology with few ballooned hepatocytes [Grade II], fewer apoptotic cells, and mildly congested sinusoids. Group V treated with NAC showed improved liver histology with scarce ballooned hepatocytes [Grade II], rare apoptotic cells, and mild sinusoidal congestion (Fig 4).
Table 3. Histological grading of liver injury.
| Grade | Grade Description |
|---|---|
| 0 | No apparent injury by light microscopy |
| I | Swelling of hepatocytes |
| II | Ballooning of hepatocytes |
| III | Lipid droplets in hepatocytes |
| IV | Necrosis of hepatocytes |
Fig 4. Hematoxylin & eosin-stained sections from livers of all study groups, magnification X400.
(A & B) Group I (control) & II (MSLE) respectively. (C) Group III (PAR). (D) Group IV (Treatment with MSLE). (E) Group V (Treatment with NAC). C.V: Central vein, H: Hepatocytes, S: Blood Sinusoids, Black arrows: Ballooning of hepatocytes, Red arrows: Apoptotic hepatocytes, Blue arrows: Lipid droplets, Yellow arrows: Inflammatory cells. Circles: Lobular necrosis.
3.6. Molecular docking
The active site regions of three enzymes, specifically NADPH oxidase, butyrylcholinesterase, and tyrosinase, allowed for the binding of the seven major compounds. As shown in (Table 4), all compounds exhibited satisfactory binding scores when docked with the three selected targets.
Table 4. The docking scores of the seven major compounds against the three enzymes, NADPH oxidase, BChE and tyrosinase.
| Compound name | NADPH oxidase (Kcal/Mol) | Butyrylcholinesterase BChE (Kcal/Mol) | Tyrosinase (Kcal/Mol) |
|---|---|---|---|
| 5,7-Dihydroxy-2’-methoxy-3’,4’-(methylenedioxy)isoflavone | -12.02 | -11.74 | -14.49 |
| Sinapic acid 3-O-glucoside | -12.66 | -10.70 | -14.21 |
| Syringetin-3-O-glucoside | -15.37 | -13.20 | -12.71 |
| Isorhamnetin | -11.53 | -15.85 | -13.85 |
| Linarin | -14.41 | -13.81 | -18.94 |
| Kaempferol-3,7-O-bis-α-L-rhamnoside | -15.06 | -13.66 | -10.62 |
| Cyanidin rutinoside | -14.01 | -18.12 | -13.94 |
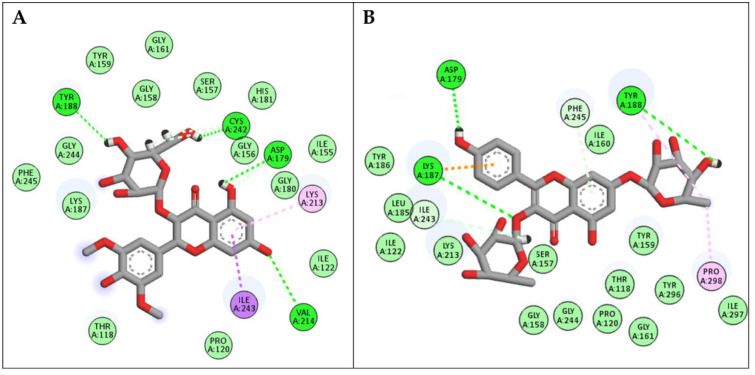
In the docking studies with NADPH oxidase, compounds syringetin-3-O-glucoside and kaempferol-3,7-O-bis-α-L-rhamnoside achieved the highest docking scores of -15.37 and -15.06 Kcal/mol, respectively. As seen in (Fig 5), syringetin-O-glucoside interacted with NADPH oxidase residues through hydrogen bond interactions with Asp179, Tyr188, Val214, and Cys242, in addition to various hydrophobic interactions with Lys213, Cys242, and Ile243. Similarly, Kaempferol-3,7-O-bis-α-L- rhamnoside interacted with Asp179, Lys187, Tyr188, Ile243, Phe245 and Pro298 through both hydrophilic and hydrophobic interactions.
Fig 5. 2D binding modes of (A) Syringetin-3-O-glucoside and (B) Kaempferol-3,7-O-bis-α-L-rhamnoside to the active binding sites of NADPH enzyme.
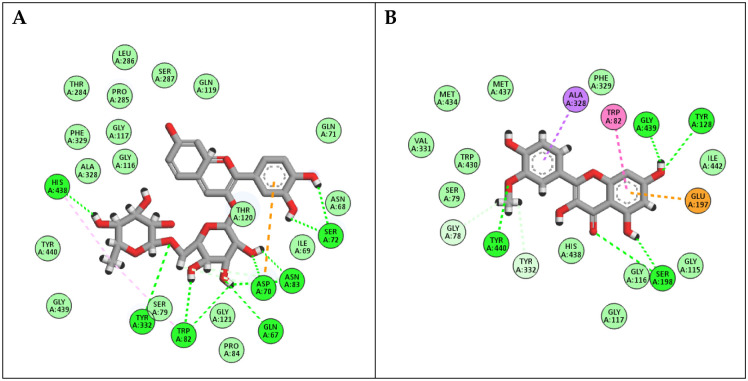
The docking investigations with BChE revealed that cyanidin rutinoside and isorhamnetin were the most efficient molecules, with docking scores of -18.12 and -15.85 Kcal/mol, respectively. As depicted in (Fig 6), cyanidin rutinoside was able to interact with BChE oxidase residues through hydrogen bond interactions with Gln67, Asp70, Ser72, Trp82, Asn83, Tyr332, and His438, in addition to various hydrophobic interactions with Asp70, Trp82, Asn83, and His438. In a similar way, isorhamnetin interacted with Gly78, Trp82, Tyr128, Glu178, Ser198, Ala328, Try332, Gly439 and Tyr440 through both hydrophilic and hydrophobic interactions.
Fig 6. 2D binding modes of (A) Cyanidin rutinoside and (B) Isorhamnetin to the active binding sites of BChE enzyme.
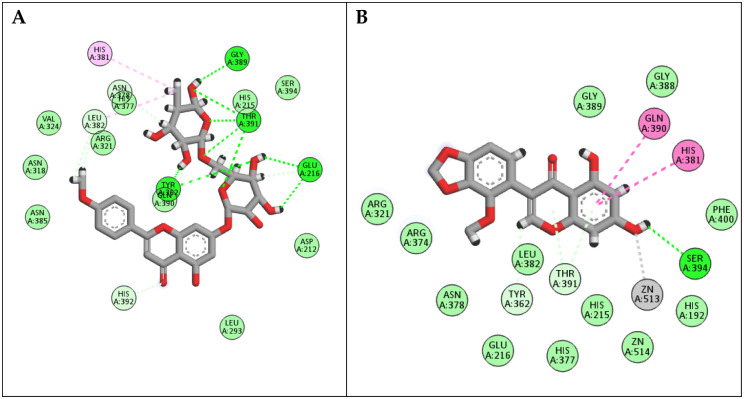
In the docking studies with tyrosinase, linarin and 5,7-dihydroxy-2’-methoxy-3’,4’-(methylenedioxy) isoflavone achieved docking scores of -18.92 and -14.49 Kcal/mol, respectively. As shown in (Fig 7) reveals, linarin could engage with tyrosinase active sites through forming hydrogen bond interactions with Glu216, Tyr362, Gly389, and Thr391; it formed various hydrophobic interactions with Glu216, His381, Leu382, Gln390, and Thr391. In the same way, 7-Dihydroxy-2’-methoxy-3’,4’-(methylenedioxy) isoflavone interacted with tyrosinase residues through both hydrophilic and hydrophobic interactions with His381, Gln391, Thr391, Ser394, and Zn513. In conclusion, all the compounds in the major extract demonstrated excellent docking scores with the three targets, suggesting the synergetic contribution of the whole extract.
Fig 7. 2D binding modes of (A) Linarin and (B) 5,7-Dihydroxy-2’-methoxy-3’,4’-(methylenedioxy) isoflavone to the active binding sites of Tyrosinase enzyme.
4. Conclusions
Based on HPLC-ESI-MS/MS, the methanol extract of M. sinaica has been investigated and identified a total of 13 secondary metabolites, which included flavonoids, anthocyanins, and phenolic acids such as syringetin, kaempferol, and apigenin derivatives. It exhibited high total phenolics and flavonoid contents that were directly associated with its antioxidant capabilities as measured by the DPPH and FRAP assays. Moreover, MSLE exhibited noteworthy hepatoprotective activities and ameliorated the harmful effects of paracetamol toxicity in rats. The findings suggest that M. sinaica could be regarded as a plant of biological significance and a promising candidate for antioxidant and hepatoprotective agents.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through Large Research Project under grant number (RGP2/285/45). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sheth K, Bankey P. The liver as an immune organ. Curr Opin Crit Care. 2001;7(2): 99–104. doi: 10.1097/00075198-200104000-00008 [DOI] [PubMed] [Google Scholar]
- 2.Huang B, Ban X, He J, Tong J, Tian J, Wang Y. Hepatoprotective and antioxidant activity of ethanolic extracts of edible lotus (Nelumbo nucifera Gaertn.) leaves. Food Chem. 2010;120(3): 873–878. doi: 10.1016/j.foodchem.2009.11.020 [DOI] [Google Scholar]
- 3.Sabir SM, Ahmad SD, Hamid A, Khan MQ, Athayde ML, Santos DB, et al. Antioxidant and hepatoprotective activity of ethanolic extract of leaves of Solidago microglossa containing polyphenolic compounds. Food Chem. 2012;131(3): 741–747. doi: 10.1016/j.foodchem.2011.09.026 [DOI] [Google Scholar]
- 4.Wood NJ. Nonobese individuals in the developing world are at risk of nonalcoholic fatty liver and liver disease. Nat Rev Gastroenterol Hepatol. 2010;7(7): 357–358. [DOI] [PubMed] [Google Scholar]
- 5.Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, et al. Morbidity and mortality in compensated cirrhosis type C: A retrospective follow-up study of 384 patients. Gastroenterology. 1997;112: 463–472. doi: 10.1053/gast.1997.v112.pm9024300 [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Dong H, Thompson DC, Shertzer HG, Nebert DW, Vasiliou V. Glutathione defense mechanism in liver injury: Insights from animal models. Food and Chemical Toxicology. 2013;60: 38–44. doi: 10.1016/j.fct.2013.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8(7): 579–591. doi: 10.1038/nrd2803 [DOI] [PubMed] [Google Scholar]
- 8.Elgindi MR, Singab AE-NB, Aly SH, Mahmoud II. Phytochemical investigation and antioxidant activity of Hyophorbe verschaffeltii (Arecaceae). J Pharmacogn Phytochem. 2016;5(2): 39–46. [Google Scholar]
- 9.Zhang C, Zhao J, Famous E, Pan S, Peng X, Tian J. Antioxidant, hepatoprotective and antifungal activities of black pepper (Piper nigrum L.) essential oil. Food Chem. 2021;346:128845. doi: 10.1016/j.foodchem.2020.128845 [DOI] [PubMed] [Google Scholar]
- 10.Zhao C, Fan J, Liu Y, Guo W, Cao H, Xiao J, et al. Hepatoprotective activity of Ganoderma lucidum triterpenoids in alcohol-induced liver injury in mice, an iTRAQ-based proteomic analysis. Food Chem. 2019;271: 148–156. doi: 10.1016/j.foodchem.2018.07.115 [DOI] [PubMed] [Google Scholar]
- 11.Aly SH, Elissawy AM, El Hassab MA, Majrashi TA, Hassan FE, Elkaeed EB, et al. Comparative metabolic study of the chloroform fraction of three Cystoseira species based on UPLC/ESI/MS analysis and biological activities. J Enzyme Inhib Med Chem. 2024;39(1): 2292482. [DOI] [PubMed] [Google Scholar]
- 12.Aly SH, Elbadry AMM, El-Shazly M, Hwang T-L. Exploring the potential role of genus Sophora in the management of osteoporosis: a phytochemical and biological review. Frontiers in Natural Products. 2023;2:1302371. doi: 10.3389/fntpr.2023.1302371 [DOI] [Google Scholar]
- 13.Goher SS, Aly SH, Abu-Serie MM, El-Moslamy SH, Allam AA, Diab NH, et al. Electrospun Tamarindus indica-loaded antimicrobial PMMA/cellulose acetate/PEO nanofibrous scaffolds for accelerated wound healing: In-vitro and in-vivo assessments. Int J Biol Macromol. 2024;258:128793. [DOI] [PubMed] [Google Scholar]
- 14.Aly SH, Elissawy AM, Eldahshan OA, Elshanawany MA, Singab ANB. Variability of the Chemical Composition of the Essential Oils of Flowers and the Alkaloid Contents of Leaves of Sophora secundiflora and Sophora tomentosa. Journal of Essential Oil-Bearing Plants. 2020;23(3):442–452. doi: 10.1080/0972060X.2020.1750489 [DOI] [Google Scholar]
- 15.Aly SH, Elissawy AM, Salah D, Alfuhaid NA, Zyaan OH, Mohamed HI, et al. Phytochemical Investigation of Three Cystoseira Species and Their Larvicidal Activity Supported with In Silico Studies. Mar Drugs. 2023;21(2): 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aly SH, El-Hassab MA, Elhady SS, Gad HA. Comparative Metabolic Study of Tamarindus indica L.’s Various Organs Based on GC/MS Analysis, In Silico and In Vitro Anti-Inflammatory and Wound Healing Activities. Plants. 2022;12(1): 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aly SH, Eldahshan OA, Al-Rashood ST, Binjubair FA, El Hassab MA, Eldehna WM, et al. Chemical Constituents, Antioxidant, and Enzyme Inhibitory Activities Supported by In-Silico Study of n-Hexane Extract and Essential Oil of Guava Leaves. Molecules. 2022;27(24): 8979. Academic doi: 10.3390/molecules27248979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevenson DE, Hurst RD. Polyphenolic phytochemicals–just antioxidants or much more? Cellular and Molecular Life Sciences. 2007;64(22): 2900–2916. doi: 10.1007/s00018-007-7237-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elebeedy D, Ghanem A, Aly SH, Ali MA, Faraag AHI, El-Ashrey MK, et al. Synergistic antiviral activity of Lactobacillus acidophilus and Glycyrrhiza glabra against Herpes Simplex-1 Virus (HSV-1) and Vesicular Stomatitis Virus (VSV): experimental and In Silico insights. BMC Microbiol. 2023;23(1): 173. doi: 10.1186/s12866-023-02911-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim TK. Edible Medicinal and Non-Medicinal Plants. Edible Medicinal and Non-Medicinal Plants. Springer; 2016. [Google Scholar]
- 21.Aly SH, Kandil NH, Hemdan RM, Kotb SS, Zaki SS, Abdelaziz OM, et al. GC/MS Profiling of the Essential Oil and Lipophilic Extract of Moricandia sinaica Boiss. and Evaluation of Their Cytotoxic and Antioxidant Activities. Molecules. 2023;28(5):2193. doi: 10.3390/molecules28052193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farooq M, Nasr FA, Almoutiri ND, Al-yahya N, Wadaan MA, Abutaha N. The phytochemical screening and antiangiogenic activity of audthan al himar (Moricandia sinaica Boiss.) extracts in zebrafish embryos and human umbilical vein endothelial cells. Journal of King Saud University-Science. 2020;32(4): 2370–2376. doi: 10.1016/j.jksus.2020.03.017 [DOI] [Google Scholar]
- 23.Farooq Khan M, Nasr FA, Baabbad AA, Alqahtani AS, Wadaan MA. Investigating the anticancer activity and characterization of bioactive constituents of Moricandia sinaica (Boiss.) Boiss through in vitro and in silico approaches in triple-negative breast cancer cell line. Applied Sciences. 2021;11(3):1244. doi: 10.3390/app11031244 [DOI] [Google Scholar]
- 24.El-Mekkawy S, Shahat AA, Alqahtani AS, Alsaid MS, Abdelfattah MAO, Ullah R, et al. A Polyphenols-Rich Extract from Moricandia sinaica Boiss. Exhibits Analgesic, Anti-Inflammatory and Antipyretic Activities In Vivo. Molecules. 2020;25(21):5049. doi: 10.3390/molecules25215049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warwick SI, Francis A, Gugel RK. Guide to wild germplasm of Brassica and allied crops (tribe Brassiceae, Brassicaceae). Canada: Agriculture and Agri-Food Canada. 2009;1. [Google Scholar]
- 26.Abdelazim E, Goher S, Aly SH, El-Nashar H, EL-Moslamy S, El-Fakharany E, et al. In vitro and in vivo studies of Syzygium cumini loaded electrospun PLGA/PMMA/collagen nanofibers for accelerating topical wound healing. RSC Adv. 2024;14(1): 101–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aly SH, Elissawy AM, Mahmoud AMA, El-tokhy FS, Mageed SSA, Almahli H, et al. Synergistic Effect of Sophora japonica and Glycyrrhiza glabra Flavonoid-Rich Fractions on Wound Healing: In Vivo and Molecular Docking Studies. Molecules. 2023;28(7): 2994. doi: 10.3390/molecules28072994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-ciocalteu reagent. Methods in enzymology. Elsevier; 1999. pp. 152–178. [Google Scholar]
- 29.Chang C-C, Yang M-H, Wen H-M, Chern J-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10(3). [Google Scholar]
- 30.Yen GC, Duh P Der. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J Agric Food Chem. 1994;42(3): 629–632. [Google Scholar]
- 31.Al Zahrani NA, El-Shishtawy RM, Elaasser MM, Asiri AM. Synthesis of novel chalcone-based phenothiazine derivatives as antioxidant and anticancer agents. Molecules. 2020;25(19): 4566. doi: 10.3390/molecules25194566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferreira ICFR, Baptista P, Vilas-Boas M, Barros L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: Individual cap and stipe activity. Food Chem. 2007;100(4): 1511–1516. doi: 10.1016/j.foodchem.2005.11.043 [DOI] [Google Scholar]
- 33.Morton, D.B., 1998. The use of score sheets in the implementation of humane end points. In Proceedings of the Joint ANZCCART/NAEAC Conference on Ethical Approaches to Animal-based Science (pp. 75–82). Adelaide, Australia and Wellington, New Zealand: ANZCCART.
- 34.Kim SM, Kang K, Jho EH, Jung Y, Nho CW, Um B, et al. Hepatoprotective effect of flavonoid glycosides from Lespedeza cuneata against oxidative stress induced by tert‐butyl hyperoxide. Phytotherapy Research. 2011;25(7): 1011–1017. [DOI] [PubMed] [Google Scholar]
- 35.Dkhil MA, Abdel Moneim AE, Hafez TA, Mubaraki MA, Mohamed WF, Thagfan FA, et al. Myristica fragrans kernels prevent paracetamol-induced hepatotoxicity by inducing anti-apoptotic genes and nrf2/ho-1 pathway. Int J Mol Sci. 2019;20(4): 993. doi: 10.3390/ijms20040993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El Morsy EM, Kamel R. Protective effect of artichoke leaf extract against paracetamol-induced hepatotoxicity in rats. Pharm Biol. 2015;53(2): 167–173. doi: 10.3109/13880209.2014.913066 [DOI] [PubMed] [Google Scholar]
- 37.Abdel Mageed SS, Ammar RM, Nassar NN, Moawad H, Kamel AS. Role of PI3K/Akt axis in mitigating hippocampal ischemia-reperfusion injury via CB1 receptor stimulation by paracetamol and FAAH inhibitor in rat. Neuropharmacology. 2022;207:108935.. doi: 10.1016/j.neuropharm.2021.108935 [DOI] [PubMed] [Google Scholar]
- 38.Elshibani FA, Alamami AD, Mohammed HA, Rasheed RA, El Sabban RM, Yehia MA, et al. A multidisciplinary approach to the antioxidant and hepatoprotective activities of Arbutus pavarii Pampan fruit; in vitro and in Vivo biological evaluations, and in silico investigations. J Enzyme Inhib Med Chem. 2024;39(1):2293639. doi: 10.1080/14756366.2023.2293639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buege JA, Aust SD. [30] Microsomal lipid peroxidation. In Methods in enzymology 1978. Jan 1 (Vol. 52, pp. 302–310). Academic press. [DOI] [PubMed] [Google Scholar]
- 40.Robak J, Gryglewski RJ. Flavonoids are scavengers of superoxide anions. Biochem Pharmacol. 1988;37(5): 837–841. doi: 10.1016/0006-2952(88)90169-4 [DOI] [PubMed] [Google Scholar]
- 41.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82: 70–77. doi: 10.1016/0003-9861(59)90090-6 [DOI] [PubMed] [Google Scholar]
- 42.Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11(3): 151–169. doi: 10.1159/000136485 [DOI] [PubMed] [Google Scholar]
- 43.Laouar A, Klibet F, Bourogaa E, Benamara A, Boumendjel A, Chefrour A, et al. Potential antioxidant properties and hepatoprotective effects of Juniperus phoenicea berries against CCl4 induced hepatic damage in rats. Asian Pac J Trop Med. 2017;10(3): 263–269. doi: 10.1016/J.APJTM.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 44.Bancroft JD, Gamble M. Theory and practice of histological techniques. 4th Ed. Churchill Livingstone, Edinburgh, London, Melbourne, New York.: Elsevier health sciences; 2013; ISBN 0443102791. [Google Scholar]
- 45.Rosenberry TL, Brazzolotto X, MacDonald IR, Wandhammer M, Trovaslet-Leroy M, Darvesh S, et al. Comparison of the binding of reversible inhibitors to human butyrylcholinesterase and acetylcholinesterase: A crystallographic, kinetic and calorimetric study. Molecules. 2017;22(12):2098. doi: 10.3390/molecules22122098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lai X, Wichers HJ, Soler-Lopez M, Dijkstra BW. Structure of Human Tyrosinase Related Protein 1 Reveals a Binuclear Zinc Active Site Important for Melanogenesis. Angew Chem Int Ed Engl. 2017;56(33): 9812–9815. doi: 10.1002/anie.201704616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lountos GT, Jiang R, Wellborn WB, Thaler TL, Bommarius AS, Orville AM. The crystal structure of NAD(P)H oxidase from Lactobacillus sanfranciscensis: insights into the conversion of O2 into two water molecules by the flavoenzyme. Biochemistry. 2006;45(32): 9648–9659. doi: 10.1021/bi060692p [DOI] [PubMed] [Google Scholar]
- 48.El Hassab MA, Ibrahim TM, Shoun AA, Al-Rashood ST, Alkahtani HM, Alharbi A, et al. In silico identification of potential SARS COV-2 2′-O-methyltransferase inhibitor: fragment-based screening approach and MM-PBSA calculations. RSC Adv. 2021;11(26): 16026–16033. doi: 10.1039/d1ra01809d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El Hassab MA, Eldehna WM, Al-Rashood ST, Alharbi A, Eskandrani RO, Alkahtani HM, et al. Multi-stage structure-based virtual screening approach towards identification of potential SARS-CoV-2 NSP13 helicase inhibitors. J Enzyme Inhib Med Chem. 2022;37(1): 563–572. doi: 10.1080/14756366.2021.2022659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao WT. Optimization of ultrasonic assisted extraction technology and antioxidant activity of Flavonoids from Isatis indigotica root by response surface methodology. Chin Modern Appl Pharm. 2016;33: 313–317. [Google Scholar]
- 51.Chen Q, Lan H-Y, Peng W, Rahman K, Liu Q-C, Luan X, et al. Isatis indigotica: a review of phytochemistry, pharmacological activities and clinical applications. J Pharm Pharmacol. 2021;73(9): 1137–1150. doi: 10.1093/jpp/rgab014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yao H, Chen B, Zhang Y, Ou H, Li Y, Li S, et al. Analysis of the total biflavonoids extract from Selaginella doederleinii by HPLC-QTOF-MS and its in vitro and in vivo anticancer effects. Molecules. 2017;22(2):325. doi: 10.3390/molecules22020325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tulio AZ Jr, Reese RN, Wyzgoski FJ, Rinaldi PL, Fu R, Scheerens JC, et al. Cyanidin 3-Rutinoside and Cyanidin 3-Xylosylrutinoside as Primary Phenolic Antioxidants in Black Raspberry. J Agric Food Chem. 2008;56(6): 1880–1888. doi: 10.1021/jf072313k [DOI] [PubMed] [Google Scholar]
- 54.Zengin G, Fahmy NM, Sinan KI, Uba AI, Bouyahya A, Lorenzo JM, et al. Differential Metabolomic Fingerprinting of the Crude Extracts of Three Asteraceae Species with Assessment of Their In Vitro Antioxidant and Enzyme-Inhibitory Activities Supported by In Silico Investigations. Processes. 2022;10(10): 1911. doi: 10.3390/pr10101911 [DOI] [Google Scholar]
- 55.Emam M, Abdel-haleem DR, Salem MM, Abdel-hafez LJM, Latif RRA, Farag SM, et al. Phytochemical Profiling of Lavandula coronopifolia Poir. Aerial Parts Extract and Its Larvicidal, Antibacterial, and Antibiofilm Activity against Pseudomonas aeruginosa. molecules. 2021;26(6): 1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eltamany EE, Elhady SS, Ahmed HA, Badr JM, Noor AO, Ahmed SA, et al. Chemical profiling, antioxidant, cytotoxic activities and molecular docking simulation of Carrichtera annua DC. (cruciferae). Antioxidants. 2020;9(12): 1–27. doi: 10.3390/antiox9121286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Güzel Y. Specialized natural product analysis and chemophenetics of some Turkish endemic Centaurea L. (Asteraceae) taxa by electrospray ionization mass spectrometry fingerprinting and liquid chromatography-tandem mass spectrometry. Biochem Syst Ecol. 2020;91: 104079. doi: 10.1016/j.bse.2020.104079 [DOI] [Google Scholar]
- 58.El-Din MIG, George MY, Youssef FS. Chemical characterization of the polyphenolic rich fraction of Thunbergia erecta and its therapeutic potential against doxorubicin and cyclophosphamide-induced cognitive impairment in rats. J Ethnopharmacol. 2023; 116213. doi: 10.1016/j.jep.2023.116213 [DOI] [PubMed] [Google Scholar]
- 59.Cheng Y, Schneider B, Oberthur C, Graf H, Adler S HM. Flavone C-glycosides from Isatis tinctoria leaves. Heterocycles-Sendai Institute of Heterocyclic Chemistry. 2005;65(7): 1655–1661. [Google Scholar]
- 60.Hamdan DI, Salah S, Hassan WHB, Morsi M, Khalil HMA, Ahmed-Farid OAH, et al. Anticancer and Neuroprotective Activities of Ethyl Acetate Fractions from Morus macroura Miq. Plant Organs with Ultraperformance Liquid Chromatography-Electrospray Ionization-Tandem Mass Spectrometry Profiling. ACS Omega. 2022; 7(18):16013–27. doi: 10.1021/acsomega.2c01148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El-Nashar HAS, Abbas H, Zewail M, Noureldin MH, Ali MM, Shamaa MM, et al. Neuroprotective Effect of Artichoke-Based Nanoformulation in Sporadic Alzheimer’s Disease Mouse Model: Focus on Antioxidant, Anti-Inflammatory, and Amyloidogenic Pathways. Pharmaceuticals. 2022;15(10): 1202. doi: 10.3390/ph15101202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bouzabata A, Montoro P, Gil KA, Piacente S, Youssef FS, Al Musayeib NM, et al. HR-LC-ESI-Orbitrap-MS-Based Metabolic Profiling Coupled with Chemometrics for the Discrimination of Different Echinops spinosus Organs and Evaluation of Their Antioxidant Activity. Antioxidants. 2022;11(3):453. doi: 10.3390/antiox11030453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aly SH, Elissawy AM, Fayez AM, Eldahshan OA, Elshanawany MA, Singab ANB. Neuroprotective effects of Sophora secundiflora, Sophora tomentosa leaves and formononetin on scopolamine-induced dementia. Nat Prod Res. 2021;35(24): 5848–5852. doi: 10.1080/14786419.2020.1795853 [DOI] [PubMed] [Google Scholar]
- 64.El-Nashar HAS, Aly SH, Ahmadi A, El-Shazly M. The Impact of Polyphenolics in the Management of Breast Cancer: Mechanistic Aspects and Recent Patents. Recent Pat Anticancer Drug Discov. 2021;17(4): 358–379. doi: 10.2174/1574892816666211213090623 [DOI] [PubMed] [Google Scholar]
- 65.Asif A, Ishtiaq S, Kamran SH, Youssef FS, Lashkar MO, Ahmed SA, et al. UHPLC-QTOF-MS Metabolic Profiling of Marchantia polymorpha and Evaluation of Its Hepatoprotective Activity Using Paracetamol-Induced Liver Injury in Mice. ACS Omega. 2023;8(21): 19037–19046. doi: 10.1021/acsomega.3c01867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Skandrani I, Boubaker J, Bhouri W, Limem I, Kilani S, Sghaier M Ben, et al. Leaf extracts from Moricandia arvensis promote antiproliferation of human cancer cells, induce apoptosis, and enhance antioxidant activity. Drug Chem Toxicol. 2010;33(1): 20–27. doi: 10.3109/01480540903376215 [DOI] [PubMed] [Google Scholar]
- 67.Braham H, Mighri Z, Jannet H Ben, Matthew S, Abreu PM. Antioxidant Phenolic Glycosides from Moricandia arvensis. J Nat Prod. 2005;68(4): 517–522. doi: 10.1021/np049581m [DOI] [PubMed] [Google Scholar]
- 68.Marrelli M, Morrone F, Gambacorta L, Argentieri MP, Conforti F, Avato P. Phytochemical and biological profile of Moricandia arvensis (L.) DC.: An inhibitor of pancreatic lipase. Molecules. 2018;23(11): 1–15. doi: 10.3390/molecules23112829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rotundo L, Pyrsopoulos N. Liver injury induced by paracetamol and challenges associated with intentional and unintentional use. World J Hepatol. 2020;12(4): 125–136. doi: 10.4254/wjh.v12.i4.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol. 2010;196: 369–405. doi: 10.1007/978-3-642-00663-0_12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gressner OA, Weiskirchen R, Gressner AM. Biomarkers of hepatic fibrosis, fibrogenesis and genetic pre‐disposition pending between fiction and reality. J Cell Mol Med. 2007;11(5): 1031–1051. doi: 10.1111/j.1582-4934.2007.00092.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Islam MT, Quispe C, Islam MA, Ali ES, Saha S, Asha UH, et al. Effects of nerol on paracetamol-induced liver damage in Wistar albino rats. Biomedicine & Pharmacotherapy. 2021;140: 111732. doi: 10.1016/j.biopha.2021.111732 [DOI] [PubMed] [Google Scholar]
- 73.Laraba M, Tachour SH, Belbache H, Boubekri N, Djebbari R, Benayache F, et al. Hepatoprotective potential of the n-butanol extract of Moricandia arvensis from Algeria against doxorubicin induced toxicity in Wistar albino rats. Advances in Traditional Medicine. 2022;22(4): 853–864. doi: 10.1007/s13596-022-00642-6 [DOI] [Google Scholar]
- 74.Shahat AA, Hassanein HD, Ullah R, Alqahtani AS, Husseiny HA, Kowalczyk A, et al. Renoprotective and Cardioprotective Potential of Moricandia sinaica (Boiss.) against Carbon Tetrachloride-Induced Toxicity in Rats. Evidence-based Complementary and Alternative Medicine. 2022.29;2022. doi: 10.1155/2022/8545695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mazumder A, Sharma A, Azad MAK. A Comprehensive Review of the Pharmacological Importance of Dietary Flavonoids as Hepatoprotective Agents. Evidence-Based Complementary and Alternative Medicine. 2023;2023. doi: 10.1155/2023/4139117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chin Y-W, Lim SW, Kim YC, Choi SZ, Lee KR, Kim J. Hepatoprotective flavonol glycosides from the aerial parts of Rodgersia podophylla. Planta Med. 2004;70(6): 576–577. [DOI] [PubMed] [Google Scholar]
- 77.Hu X, Chen Y, Dai J, Yao L, Wang L. Rhodomyrtus tomentosa Fruits in Two Ripening Stages: Chemical Compositions, Antioxidant Capacity and Digestive Enzymes Inhibitory Activity. Antioxidants. 2022;11(7):1390. doi: 10.3390/antiox11071390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chmiel M, Stompor-Gorący M. The Spectrum of Pharmacological Actions of Syringetin and Its Natural Derivatives—A Summary Review. Nutrients. 2022;14(23): 1–12. doi: 10.3390/nu14235157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang M, Sun J, Jiang Z, Xie W, Zhang X. Hepatoprotective effect of kaempferol against alcoholic liver injury in mice. The American journal of Chinese medicine. 2015;43(02):241–54. doi: 10.1142/S0192415X15500160 [DOI] [PubMed] [Google Scholar]
- 80.Wang Y, Tang C, Zhang H. Hepatoprotective effects of kaempferol 3-O-rutinoside and kaempferol 3-O-glucoside from Carthamus tinctorius L. on CCl4-induced oxidative liver injury in mice. J Food Drug Anal. 2015;23(2): 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yue S, Xue N, Li H, Huang B, Chen Z, Wang X. Hepatoprotective effect of apigenin against liver injury via the non-canonical NF-κB pathway in vivo and in vitro. Inflammation. 2020;43: 1634–1648. [DOI] [PubMed] [Google Scholar]
- 82.Plaa GL, Charbonneau M, Plante I. Detection and Evaluation of Chemically Induced Liver Injury. Sixth Edition: Hayes’ Principles and Methods of Toxicology. 2014; 1445–1488. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.