Abstract
Purpose of Review:
To offer a narrative review of literature and an update on RA multimorbidity research over the past five years as well as future directions.
Recent findings:
Patients with RA experience higher prevalence of multimorbidity (31-86% vs 18-71% in non-RA) and faster accumulation of comorbidities. Patients with multimorbidity have worse outcomes compared to non-RA multimorbid patients and RA without multimorbidity including mortality, cardiac events, and hospitalizations. Comorbid disease clusters often included: cardiopulmonary, cardiometabolic, and depression and pain-related conditions. High frequency comorbidities included: interstitial lung disease, asthma, COPD, cardiovascular disease, fibromyalgia, osteoarthritis, and osteoporosis, thyroid disorders, hypertension, and cancer. Furthermore, patients with RA and multimorbidity are paradoxically at increased risk of high RA disease activity but experience a lower likelihood of biologic use and more biologic failures.
Summary:
RA patients experience higher prevalence of multimorbidity and worse outcomes versus non-RA and RA without multimorbidity. Findings call for further studies.
INTRODUCTION
Rheumatoid arthritis (RA) is the most common systemic autoimmune inflammatory arthropathy that can have effects far beyond the joints. Recently, the importance of multimorbidity, defined as greater than or equal to two co-existing conditions in an individual, has been gaining attention as a growing global health challenge. Multimorbidity expands beyond consideration of comorbidity which focuses on the effect of chronic conditions to an index disease. Multimorbidity, in contrast, focuses on the cumulative impact of all chronic conditions on an individual or group of individuals with conditions such as RA.[1]
Prior studies have identified a higher mortality in RA compared to peers in the general population.[2, 3] Several specific comorbidities have higher incidence in RA compared to the general population, with cardiopulmonary diseases accounting for the largest proportion of excess mortality.[4–8] The association between RA and comorbidities is multifaceted; proposed mechanisms include shared common inflammatory pathways, shared risk factors, and treatment effects.[4, 5, 7]
Recently, there has been growing interest in investigating multimorbidity in patients with RA given its impact on outcomes, disease progression, and treatment selection. Prior studies investigating the relationship of multimorbidity in RA consistently report an association with increased mortality and healthcare utilization.[9, 10] This paper aims to offer a narrative review of the current literature and an update on the state of RA multimorbidity research over the past five years as well as applications and future directions.
Methods
We performed a review of primary English language articles published from January 1, 2018 through February 1, 2023. A PubMed search used a combination of the terms “multimorbidity”, “rheumatoid arthritis”, and “comorbidities.” Articles were limited to full text, observational studies with controls, or clinical trials that focused on multimorbidity in RA as a primary outcome. These search terms returned 188 potential citations. Articles were screened for relevance first, by title review then abstract review, resulting in 17 full text articles included in our narrative summary. Figure 1 provides a flow diagram detailing the breakdown of our study selection. Studies were categorized by their primary outcome related to multimorbidity: prevalence and clusters compared to controls, outcomes, disease activity, and treatment-related factors. Key features of the articles were abstracted with pertinent results related to multimorbidity and RA summarized in Tables 1–3.
Figure 1.
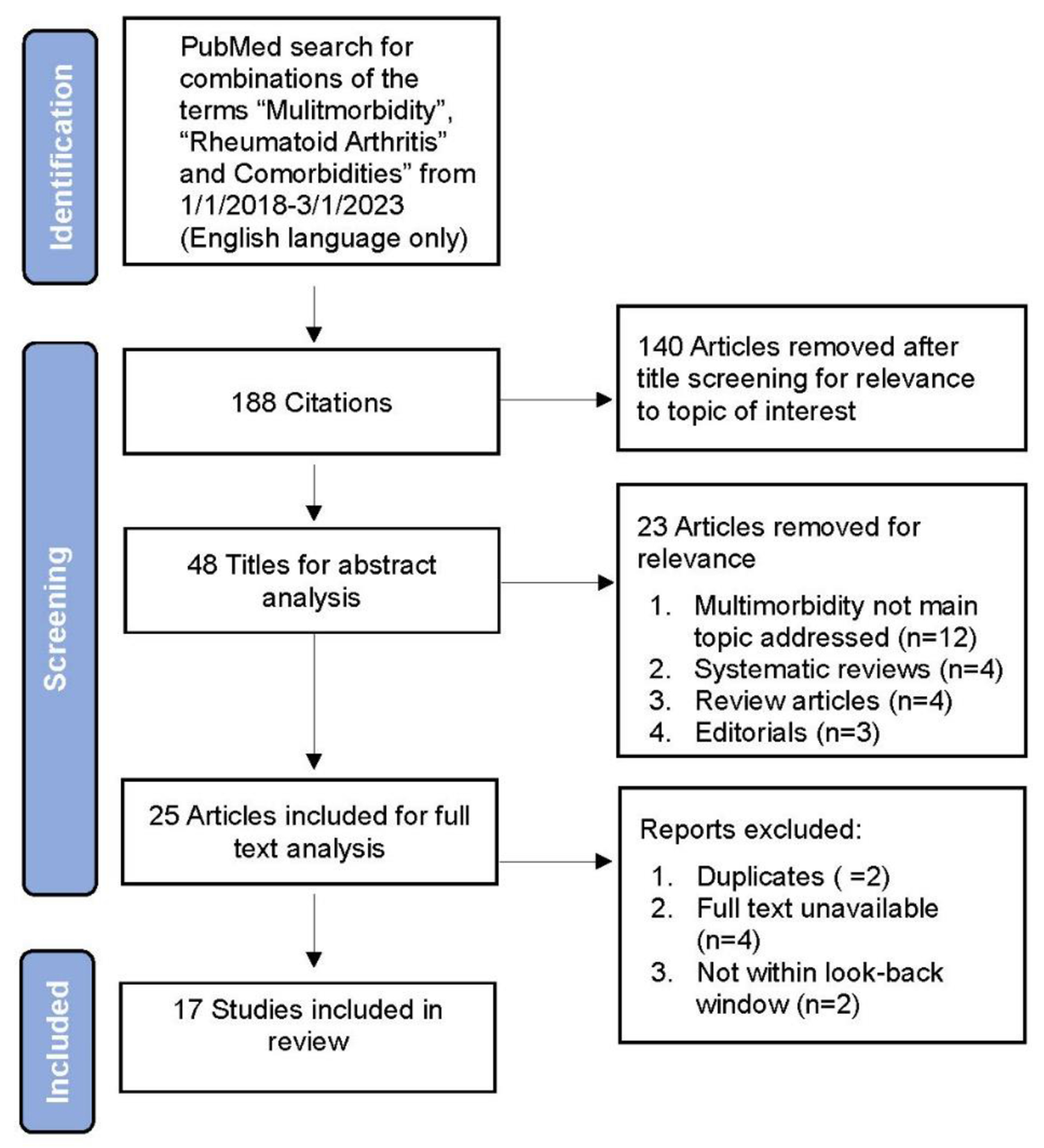
Flow diagram of article inclusion
Table 1.
Prevalence and Patterns of Multimorbidity in Rheumatoid Arthritis (RA)
| Study | Methods | Measures | Outcomes |
|---|---|---|---|
| Crowson et al, 2022 | Retrospective matched cohort study. Prevalent RA patients matched to non-RA. | LTC count. Prevalent multimorbidity: ≥ 2 Substantial Multimorbidity: ≥ 5 | Multimorbidity: 86% in RA and 71% in non-RA (p<0.001); OR RA 2.80 (2.33 to 3.38); Substantial Multimorbidity 55% RA and 38% non-RA (p<0.001) OR: 2.37 (2.03 to 2.77). Odds were greatest for Interstitial lung disease (ILD) 3.11 (2.03 to 4.90), fibromyalgia 3.11 (2.27 to 4.32), osteoarthritis 2.95 (2.51 to 3.48) and osteoporosis 2.92 (2.28 to 3.77). |
| England et al, 2022 | Retrospective cross-sectional matched cohort study. A) Cohort of RA and non-RA from commercial insurance (MarketScan) n=226,850 and B) Veterans Health Administration (VHA) n=120,780 matched 1:1 by sex | LTC count. Multimorbidity: ≥ 2 by machine learning algorithm to identify clusters | Patterns identified through machine learning from Marketscan: 1) Mental Health and Chronic Pain, 2) Cardiopulmonary, 3) Vascular and Metabolic. VHA: 1) Mental Health & Substance abuse, 2) Metabolic, 3) Cardiovascular & Chronic Pain. ORs of multimorbidity in RA vs non-RA by sex (F/M). MarketScan F: 1) Mental Health & Chronic Pain 2.56 (2.5, 2.63), 2) Cardiopulmonary 2.27 (2.17, 2.36), 3) Cardiometabolic 1.8 (1.76, 1.85). M:1) Mental Health & Chronic Pain 2.96 (2.81, 3.11), 2) Cardiopulmonary 2.03 (1.91, 2.16), 3) Mental Health & Substance Abuse 1.88 (1.58, 2.24), 4) Cardiometabolic 1.55 (1.49, 1.62). VHA F: 1) Mental health & chronic pain 2.16 (2.10, 2.31), 2) Cardiovascular 1.71 (1.43, 2.04), 3) Metabolic 1.71 (1.58, 1.85). M: 1) Chronic Pain 2.07 (2.01, 2.14), 2) Metabolic 1.48 (1.44, 1.52), 3) Cardiovascular 1.24 (1.18, 1.30), 4) Mental Health & Substance Abuse 1.17 (1.12, 1.21). |
| Kronzer et al, 2022 | Retrospective case-control design; data n=154,391 1:1 matched RA to controls; US commercial Optum Labs | LTC count. Multimorbidity: ≥ 2; Substantial Multimorbidity: ≥ 5 | 70% with RA had multimorbidity vs 53.6% non-RA. Substantial multimorbidity: 30.7% RA vs 18.3% (p<0.001). Adjusted odds ratio (AOR) for RA multimorbidity 2.19 (2.16–2.23); substantial multimorbidity aOR 2.06 (2.02–2.09). Higher asthma, COPD, CVD, fibromyalgia, ILD, osteoarthritis, & osteoporosis. |
| McLoone et al, 2022 | Longitudinal cohort study n= 5,658; latent class analysis correlated with outcomes over 11-year follow-up across the different LTC classes | LTC count. Multimorbidity: ≥ 2 Mortality (all cause MACE), Emergency hospital admissions | Latent class analysis found 5 significant clusters. Within each cluster MACE and emergency hospital admissions increased with increasing number of LTCs. RA deaths and hospitalizations with >/=2 LTC exceeded non-RA with >/=3 LTC. Comorbid hypertension and asthma most common in women with RA, cardiometabolic in men. |
| Huang et al, 2021 | Retrospective cohort study of RA (n=24,767) from Taiwan National Health Insurance matched to non--RA patients. | Annual change in indexes: CCI, EDI, MMI, RDCI | Incidence rate ratio for change in comorbidity index of RA vs Non-RA: CCI 1.80 (1.68–1.92), ECI 2.77 (2.67–2.87), MMI 1.33 (1.26–1.40), and RDCI 1.47 (1.41–1.54. Annual percent increases after RA diagnosis were 11.0%, 11.3%, 9.7%, and 6.8%, respectively |
| England et al, 2020 | Retrospective case-control; RA n= 277,782 incident RA n= 61,124 matched (1:1) using a US commercial insurance database (MarketScan). | LTC count. Prevalent multimorbidity: ≥ 2; Multimorbidity trajectory from RA onset | Overall, RA multimorbidity prevalence of 33.9% vs 21.2% of non-RA at baseline. At 1 year follow-up, rose to 51.8% vs 33%, multimorbidity AOR 2.29 (2.25 to 2.34). |
Abbreviations: CCI=Charlson Comorbidity Index; COPD=Chronic Obstructive pulmonary disease; CVD=Cardiovascular disease ECI=Elixhauser Comorbidity Index; F=Female; ILD=Interstitial Lung Disease; LTC=Long Term Conditions; M=Male; MACE=Major Adverse Cardiac Event; MMI=Multimorbidity Index; RA=Rheumatoid Arthritis; RDCI=Rheumatic Disease Comorbidity; VHA=Veterans Health Administration.
Table 3.
Disease Markers and Treatment-Related Factors in Multimorbidity in Rheumatoid Arthritis (RA)
| Study | Methods | Measures | Outcomes |
|---|---|---|---|
| Ruscitti et al, 2023 | Italian cross-sectional observational study n=757. Patients with cardiometabolic multimorbidity and long-term conditions were correlated with disease activity scores and compared to controls to evaluate rates of methotrexate prescriptions and biologic failures. | LTC count. Cardiometabolic multimorbidity: ≥2 of HTN, T2DM, and/or HLD | Cardiometabolic comorbidities odds of ACPA presence: Multivariate OR 1.47 [1.06, 2.04] (P = 0.020); Lower Odds of clinical remission: AOR: 0.61 [0.41, 0.96]. MTX prescription assoc. with cardio-metabolic comorbidities 65.5% vs without 80.2% (P = 0.009). Biologic failure associated with cardiometabolic mortality 78.4% vs without 40.4% (P < 0.001) |
| Busby et al, 2022 | UK prospective cohort study n=2,701. Associated functional outcomes in RA determined by 5 and 10 years health assessment questionnaire (HAQ)a | RDCI; HAQ | No association for baseline RDCI and HAQ >1.5 at 5 years: OR 1.06, [0.90, 1.26] or 10 years OR: 0.94, [0.73, 1.22] |
| Davis 3rd et al, 2020 | US cross-sectional cohort study n=192. Assessed fatigue with Bristol Rheumatoid Arthritis Fatigue Multidimensional Questionnaire (BRAF-MDQ)a with and without substantial multimorbidity | Count of 25 comorbidities per indexes: CCI, ECI, RDCI. Fatigue. Substantial multimorbidity (≥4 LTCs) | Higher fatigue Relative Risk Ratio for moderate fatigue 1.27 [1.11; 1.17] and severe fatigue 1.60 [1.42; 1.82] |
| Biggioggero et al, 2019 | Italian retrospective case-control n=346. Prevalence of comorbidities and stratification according to RDCI to assess role of comorbidities on TNFi choice, concomitant methotrexate, and clinical response | RDCI; Discontinuation of biologics | Higher RDCI assoc. with higher risk of biologic drug discontinuation HR 1.186 [1.01, 1.39] |
| Ramos et al, 2019 | German cross-sectional observational cohort study n=96,921. Assessed comorbidity prevalence and factors related to disease activity, patient reported outcomes (joint counts), and well being indexes (WHO-5 and FFbH)a | LTC count.Joint count. WHO-5; FFbH | Each additional comorbidity associated with increasing mean scores: swolllen joint count (0.4 units) and tender joint count (0.7 units). WHO-5 and FFbH worsened by 1.9 and 2.3 units per each additional comorbidity. |
| Tournadre et al, 2019 | French cross-sectional cohort study n=962. Assessed fatigue with Rheumatoid Arthritis Impact of Disease Score (RAID).a | Counted and weighted Multimorbidity index value. Outcomes RAID and Fatigue. | Relative Risk Ratio increased with counted multimorbidity for moderate fatigue 1.27 [1.11; 1.17]; severe fatigue 1.60 [1.42; 1.82] |
| Armagan et al, 2018 | Turkish retrospective cohort study n=998. Baseline demographics plus current and past synthetic and biological DMARDs. At initial visit and follow-up, multiple measures of disease activity recorded and compared to RA patients without multimorbidity. | LTC count. Multimorbidity ≥2; time to biologic use | Time to 1st biologic with Multimorbidity mean=72 mos [range 3–552] vs non-multimorbidity 60 mos [range 3–396] mos. |
Abbreviations: BRAF-MDQ= Bristol Rheumatoid Arthritis Fatigue Multidimensional Questionnaire; CCI=Charlson Comorbidity Index; DMARD= Disease Modifying Antirheumatic Drug; ECI= Elixhauser Comorbidity Index; FFbH = Hannover Functional Ability Questionnaire; HAQ= Health assessment questionnaire; LTC=Long Term Conditions; RA=Rheumatoid Arthritis; RAID = Rheumatoid Arthritis Impact of Disease Score; RDCI=Rheumatic Disease Comorbidity Index; TNFi=Tumor Necrosis Factor Inhibitors; WHO-5 = World Health Organization 5-item well-being Index.
Health assessment questionnaires: Score greater than >1.5 indicates significant levels of disability. BRAF-MDQ: Higher scores = greater fatigue severity. WHO-5 severity of depressive symptoms categorized as mild (29–50); moderate- severe (0–28); scores >50 considered to have no depressive symptoms. FFbH; range 0 (no functional capacity) to 100 (full functional capacity). Tender, swollen joints: 50 and 48 joint counts respectively in a patient-reported question/mannequin format. RAID question 3: Assess fatigue from RA in past 7 days. Score range 0 (no fatigue) to 10 (totally exhausted).
Results
The search terms “multimorbidity”, “rheumatoid arthritis”, and “comorbidities,” yielded 17 full text articles. Seven of the studies investigated prevalence of multimorbidity (Table 1).[11–17] A total of five studies investigated outcomes related to multimorbidity in RA (Table 2).[10, 15, 17, 18] Table 3 lists studies that investigated markers related to disease activity or quality of life measures in multimorbid RA (n=5) as well as studies that investigated treatment-related decisions (n=3).[19–25]
Table 2.
Mortality and Hospitalization Outcomes Related to Multimorbidity in Rheumatoid Arthritis (RA)
| Study | Method | Measures | Outcomes |
|---|---|---|---|
| Morton et al, 2023 | Longitudinal cohort study from the UK Biobank (n= 4,757) and SERA (n= 825). UK Biobank RA patients linked to 5 non-RA controls. SERA RA patients matched by age, sex, postcode | LTC count. RA only, RA + 1 LTC and RA + ≥ 2; Hospitalizations; Mortality (all cause) | Incidence rate ratios. Hospitalizations: RA + ≥ 2 LTCs vs RA alone (UK Biobank RA: 2.10, [1.91, 2.30]; SERA RA: 1.74, [1.23, 2.48]). Length of stay: RA + ≥ 2 LTCs (UK BIOBANK: 2.48, [2.17, 2.84]; SERA: 1.90, [1.07, 3.38]) vs RA alone |
| McLoone et al, 2022 | Longitudinal cohort study of UK Biobank n=5,658 for Multimorbidities | Long-term conditions (LTC) count. Multimorbidity: ≥ 2; Mortality (all cause); MACE | All-cause mortality risk by cluster compared to RA with no LTC; Class 1 HR 1.53 (1.04, 2.25), Class 3 HR 2.32 (1.73, 3.11), Class 4 1.64 (1.25, 2.16), Class 5 2.66 (1.91, 3.70). Hospitalizations and hospital duration increased in RA by LTC over peers without RA and higher LTC. |
| Huang et al, 2021 | Retrospective cohort study n=24,767 RA patients from Taiwan National Health Insurance compared to non-RA | Percentage of change in indexes: CCI; ECI; MMI; RDCI | 1-year and 5-year mortality rates by top 20% of comorbidity indexes vs lowest 20%. 1-year mortality risk across multimorbidity measures: HR 2.5–4.3; 5-year mortality risk adjusted HR 2.5–4.3. |
| Yoshida et al, 2021 | Prospective US observational cohort study n=10,070. Incident RA patient from Nurses’ Health Study matched to 10 non-RA comparators | Validated Multimorbidity Weighted Index (vMWI); Mortality: all-cause; cardiovascular; respiratory | RA patients’ total mortality: HR 1.46 [1.32, 1.62]. Cardiovascular: 1.54 [1.22, 1.94]. Respiratory: 2.75 [2.05, 3.71]. Adj HR mortality: 1.18 [1.05, 1.32]. Cardiovascular: adj HR 1.19 [0.94, 1.51]. Respiratory Adj HR 1.93 [1.42, 2.62] |
| McQueenie et al, 2020 | Longitudinal cohort study of UK Biobank n=56,580 compared to non-RA patients | LTC count; MACE; Mortality (all cause) | RA with >4 LTCs vs without LTCs risk of all-cause mortality: HR 3.30 (2.61 to 4.16); MACE HR 3.45 (2.66 to 4.49) |
Abbreviations: CCI=Charlson Comorbidity Index; ECI= Elixhauser Comorbidity Index; LTC=Long Term Conditions; MACE=Major Adverse Cardiac Event; MMI= Multimorbidity Index; RA=Rheumatoid Arthritis; RDCI=Rheumatic Disease Comorbidity Index; SERA=Scottish Early Rheumatoid Arthritis Biobank; vMWI= Validated Multimorbidity Weighted Index.
Overall, the studies used heterogeneous definitions for multimorbidity. Definitions included: counts of specific long-term conditions informed by prior studies (n=12 studies), comorbidity indexes, or combined methods. Four comorbidity indexes were used: Charlson Comorbidity Index (CCI) (n=2 studies); Elixhauser Comorbidity Index (ECI) (n=1 study); Multimorbidity Index counted or weighted (MMIc/MMIv) (n=3 studies); and the Rheumatic Disease Comorbidity Index (RCDI) (n=3 studies).[10–26]
Prevalence of Multimorbidity in RA
Seven of 17 studies investigated the prevalence of multimorbidity in RA compared to non-RA controls (Table 1).[11–17] Three studies distinguished multimorbidity (≥2 conditions) from substantial multimorbidity (≥5 conditions).[11, 14, 16] Reported multimorbidity prevalence ranged from 33.9% to 86% in RA, compared to 21.2%-71% in non-RA patients.[11, 12, 14, 16] Substantial multimorbidity prevalence in RA ranged from 5%-55%. Kronzer et al reported 55% substantial multimorbidity in RA, and Crowson et al noted significantly higher prevalence of substantial multimorbidity in RA but lower prevalence overall, 30.7% vs 18.3% using similar methods.[11, 16] Gunderson et al were the only authors who did not find a significant difference in substantial multimorbidity at study index date (5% RA vs 4% non-RA) nor at 10-year follow-up (26.8%, 95% CI [23.1, 31.1] vs 22.1% [18.7, 26.2]), but noted greater 10-year cumulative incidence in developing multimorbidity in RA compared to non-RA patients, 56.5%, 95% CI [51.3, 62.3] vs 47.9% [42.8-53.7].[14] Similarly, England et al reported a higher baseline prevalence of multimorbidity (33.9% vs 21.2%) and at one year follow-up (51.8% compared to 33.0%).[12] Additionally, RA patients had a faster rate of accumulating chronic conditions compared to non-RA patients.[12]
Likewise, Huang et al evaluated the relation of comorbidity indexes in RA patients compared to non-RA controls. Comparing RA to controls, they found increased baseline score in all indexes: Charlson Comorbidity Index (CCI); Elixhauser Comorbidity Index (ECI); Multimorbidity Index (MMI); Rheumatic Disease Comorbidity Index (RDCI).[15] The reported annual percentage of change in various comorbidity indexes after RA diagnosis was CCI: 11.0%, ECI: 11.3%, MMI: 9.7%, and RDCI: 6.8%. This was higher than before the RA diagnostic period.[15]
Cohorts often matched on age and sex, but few studies included extensive subgroup analyses for multimorbidity by social factors or social determinants of health. In selected secondary analysis, Kronzer et al noted that odds of multimorbidity were similar for each racial category for RA patients, in contrast to non-RA patients where there where notable discrepancies across racial categories.[16]
Patterns of Multimorbidity in RA
Crowson et al noted that the comorbidities with the greatest difference in prevalence in RA vs non-RA were interstitial lung disease (ILD) 3.11 (2.03 to 4.90), fibromyalgia 3.11 (2.27 to 4.32), osteoarthritis 2.95 (2.51 to 3.48) and osteoporosis 2.92 (2.28 to 3.77).[11] This was similar to findings by Kronzer et al with elevated rates of asthma, chronic obstructive pulmonary disease (COPD), coronary artery disease, fibromyalgia, ILD, osteoarthritis, and osteoporosis in RA compared to non-RA patients.[11]
Two studies focused on describing patterns of multimorbidity and their prevalence in patients with RA compared to controls.[13, 17] England et al used machine learning to identify specific patterns of multimorbidity in commercial claims data and Veterans Health Affairs (VHA) data.[13] In commercial claims, patterns (in order of prevalence) included mental health and chronic pain, cardiopulmonary, vascular, and metabolic patterns of RA multimorbidity. Veteran patients with RA demonstrated mental health disorders and substance abuse, metabolic, cardiovascular, and chronic pain patterns.[13] They found that RA patients often fit two or more different patterns of multimorbidity compared to non-RA patients, when stratifying by sex and cohort (VHA or commercial claims). Among the commercial claims cases, more than one multimorbidity pattern was identified in 15.7% vs 6.6% of females and 15.5% vs 9.8% males. This finding was greater still in Veteran females 26.3% vs 15.7% and males 26.7% vs 17.8%.[13]
McLoone et al used latent class analysis to fit models to ultimately identify five classes of significant multimorbidity clusters.[17] The classes identified were 1) thyroid disorders, 2) painful conditions, 3) asthma, 4) hypertension, and 5) cancer. The hypertension class was the largest with 38.3% of participants, 100% with hypertension, and diabetes and congestive heart disease were additionally present in 25% and 22.4%, respectively. Next, the asthma class represented 20% of the RA population, wherein 60.9% of patients had asthma. The asthma class was further defined by a high prevalence of chronic obstructive pulmonary disease (COPD) 30.4% of participants in the class) and congestive heart disease (23%). Overall, 16.7 % of the population fell into Class 2, painful conditions. Among these, a priori defined painful conditions were present in 55.8% with many also having dyspepsia (40.2%) and migraines (28.7%). In the thyroid condition class, 100% had a thyroid condition and 13.8% of all patients were in this class. Lastly, the cancer class represented 11.2% of the study population.[17]
Outcomes related to multimorbidity in RA
Five studies investigated outcomes related to multimorbidity and RA (Table 2).[10, 15, 17, 18, 26] Four of these studies investigated all-cause mortality related to multimorbidity in RA reporting hazard ratios ranging from 1.46-4.30.[10, 15, 17, 26] Yoshida et al further noted that adjustments to all-cause mortality by accumulating comorbidities attenuated the hazard ratio suggesting that excess mortality was in part related to the cumulative burden.[10] Additionally, McQueenie et al found synergistic effects of the combination of osteoporosis and RA on all-cause mortality and major adverse cardiovascular events (MACE) compared to other combinations of comorbidities and RA.[26] McLoone et al investigated all-cause mortality, MACE, and risk of hospitalizations for patients with multimorbidity in the predefined classes above.[17] They demonstrated hazard ratios ranging from 1.53-2.66 with patients in the cancer class having the highest risk of mortality. Morton et al investigated hospitalizations[18] noting an increase in multimorbid RA compared to RA alone (331 vs 160 per 1000 patient-years in the UK cohort; 382 vs 182 hospitalizations per 1000 patient years in the SERA cohort). Hospital stays were also longer in RA multimorbidity, averaging 2182 days vs 955 per 1000 patient-years in the UK cohort and with 2379 days vs 812 days per 1000 patient years in the SERA cohort.[18]
Measures of Disease Activity and Treatment-Related Decisions in Multimorbidity and RA
Five studies investigated predictors or markers of disease activity and three studies specifically investigated treatment effects (Table 3).[19–25] The methods varied for defining predictors and assessing disease activity among these studies. Ruscitti et al found higher rates of anti-citrullinated protein antibody (ACPA) positivity in patients with cardiometabolic multimorbidity (OR 1.47, 95% CI [1.0, 2.04[) and noted that cardiometabolic multimorbidity negatively predicted RA remission (OR 0.61, 95% CI [0.41, 0.9]).[24] Luque Ramos et al investigated comorbidities related to patient reported outcomes. Their work reported that each additional comorbidity was associated with an average increase in swollen joint count and tender joint count of 0.4 and 0.7, respectively.[23] Additionally, well-being, measured by World Health Organization Five-item Well-being Index (WHO-5) and Hannover Functional Ability Questionnaire (FFbH-R) fell by an average of 1.9 and 2.3 units for each additional comorbidity.[23] Both Davis et al and Tournadre et al investigated the association of RA multimorbidity with fatigue. Davis used the Bristol Rheumatoid Arthritis Fatigue Multidimensional Questionnaire to assess fatigue, noting that each additional comorbidity associated with a 2.3 increase in total fatigue score; substantial multimorbidity (≥4 comorbidities) associated with a 9.3 increase.[22] Tournadre et al also noted higher relative risk of moderate or severe fatigue in patients with multimorbidity for moderate (RR=1.27, 95% CI [1.11, 1.17]) and severe fatigue (1.60, 95% CI [1.42, 1.82]) using a Rheumatoid Arthritis Impact of Disease (RAID) question.[25] However, Busby et al found that baseline RDCI comorbidity score did not associate with worse health assessment questionnaire scores at 5 or 10 years.[21] Ruscitti et al found lower methotrexate prescription (65.5% vs 80.2%) and higher biologic failure (78.4% vs 40.4%) in cardiometabolic multimorbid RA patients compared to RA patients without cardiometabolic multimorbidity.[24] Ruscitti et al also noted that patients with cardiometabolic multimorbid RA where more likely to be older and less likely to be in clinical remission.[24] Biggioggero et al found that higher RDCI score was associated with increased risk of two-year timer necrosis factor inhibitor (TNFi) discontinuation (HR 1.186, 95% CI [1.011, 1.390]; P = 0.036).[20] Finally, Armagan et al showed that patients with multimorbidity had a longer median time to first biologic prescription (72 [range 3-552] vs 60 [range 3-396] months).[19]
Discussion
Underscoring the need for rheumatologists to better understand the complexities of multimorbidity, our review demonstrates higher prevalence of multimorbidity and substantial multimorbidity in RA patients compared to non-RA peers.[11–16] Findings support that RA predisposes to developing more chronic conditions, as has been previously suggested.[27] Interestingly, prevalence of multimorbidity varied ranging from 31% to 86%.[11, 12, 14, 16] Various ranges are likely due to differences in definitions, cohort age, shared risk factors, demographics, and lived experiences of the populations. Age was the most important predictor of multimorbidity across all studies. Few studies controlled for smoking or sociodemographic factors that may influence multimorbidity as pointed out in a recent editorial [28].
Outcomes and interpreting RA multimorbidity clusters
Like many prior studies, [29, 30] this review also demonstrated increased mortality and MACE in multimorbid RA patients compared to RA patients without multimorbidity or non-RA patients.[10, 15, 17, 26] This survival gap has improved with early treatments and the increased use of biologic therapies.[31, 32] However, the gap remains with a strong association with increasing comorbidities.[33] McLoone et al offer insights by defining important phenotypic clusters and their association with higher MACE and all-cause mortality.[17] This suggests to us that specific phenotypes may see more benefit with increased screening or comorbidity management.
The work by England et al and McLoone et al provides important insights in understanding the increased prevalence and mortality observed in patients with RA and multimorbidity by examining patterns. Both authors identify similar specific phenotypic patterns of multimorbidity across several cohorts. Specifically, McLoone et al demonstrated that the hypertension class was the most prevalent followed by asthma class, mirroring cardiopulmonary pattern among women with RA, and cardiometabolic pattern among men with RA in England et al’s commercial dataset.
Multimorbidity and next steps in RA
Expanding on their work, we can begin to use phenotypic patterns to develop process interventions at the clinical level to improve outcomes. Current and future approaches are shown in Figure 2. Different approaches include population health implementations to address risk modifiers for multimorbid RA through improved screening (e.g., for hypertension [34, 35] or smoking [36] or exercise).
Figure 2.
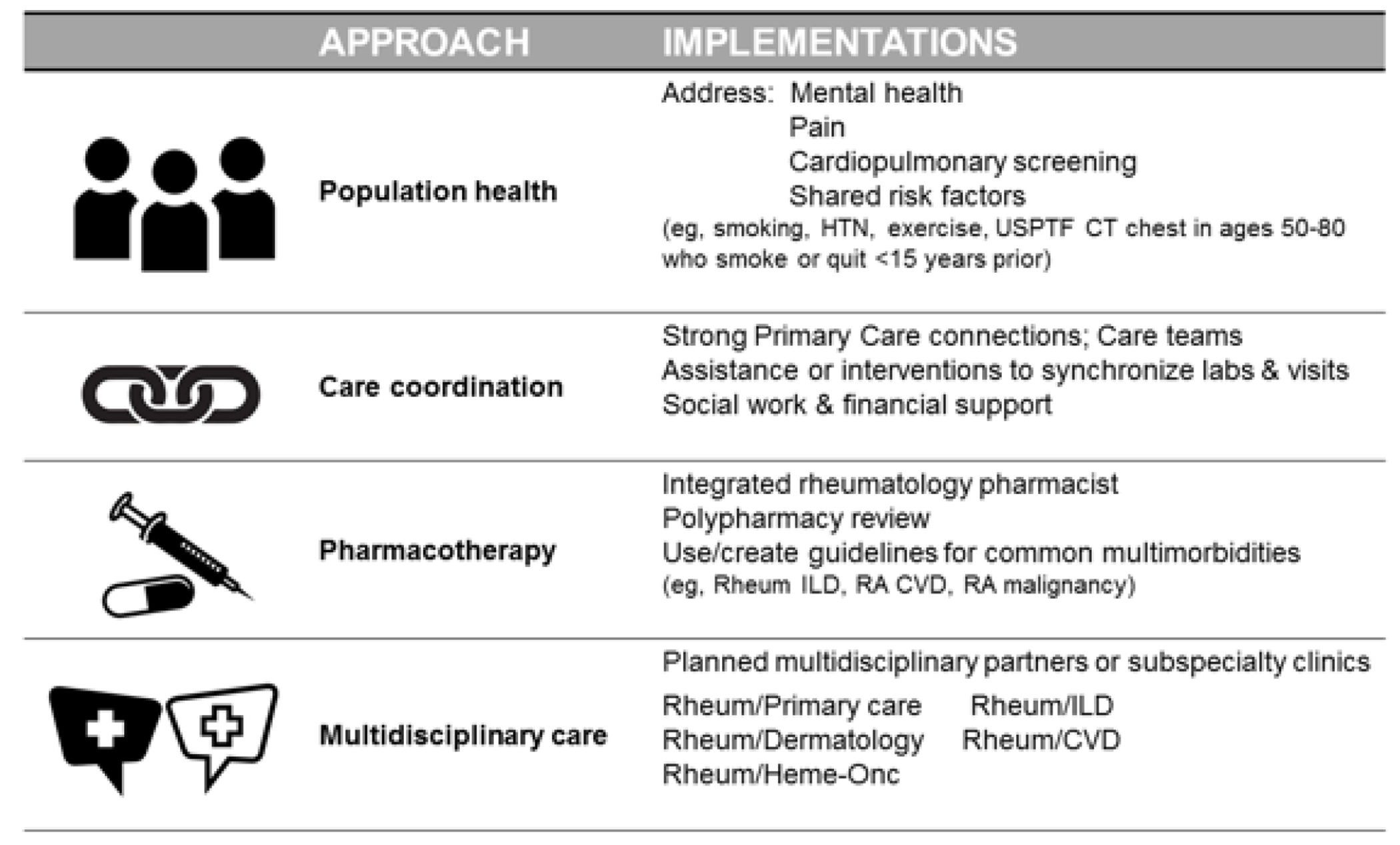
Approach and Ipmlementation to address multimorbidity in RA.
Likewise, phenotypic patterns may serve as a way to subcategorize multimorbid patients to define RA treatment algorithm pertinent to that cluster, such as the new 2023 rheumatology interstitial lung disease guideline for RA.[37] Coordinated care could likewise be tailored to patients with the highest risk or most common multimorbid phenotypes. Furthermore, increased medical contact for these patients in rheumatology could also serve as a way to monitor preexisting chronic conditions in conjunction with primary care. There have already been successful population based efforts to link blood pressure (BP) checks in rheumatology visits, [34] and smoking cessation interventions involving rheumatology medical assistants and nursing in brief interventions.[36] Such interventions would require dissemination and implantation support, and more studies are needed to inform optimal approaches for RA patients to treat and prevent multimorbidity. By identifying common clusters of comorbidities, clinicians could also streamline diagnostics, follow-up and referrals as part of a multidisciplinary approach (Figure 2).
Since multimorbidity focuses on the cumulative medical burden felt by an individual patient, a coordinated care approach is warranted. Engaging pharmacy, nursing, and social work may reduce patient-related barriers to care while also addressing social impacts and quality of life. For example, coordinating screening labs with routine labs for disease-modifying anti-rheumatic drugs (DMARDs) and biologic therapies to lab tests related to hemoglobin A1C for those with diabetes or metabolic disorders, or co-monitoring BP and cholesterol, could reduce burdens and improve care of conditions associated with cardiovascular disease, a major cause of mortality in RA patients. Lastly, some advocate collocated cardio-rheumatology or rheumatology and interstitial lung disease (ILD) clinics for instance,[38] or at least close partnerships with primary care and specialists.
RA specific considerations of in patients with multimorbidity
With respect to RA disease activity itself, while difficult to compare across studies because of different methodologies for assessing disease activity (BRAF-MDQ scores, patient reports, and RAID Scores), three of four studies associated multimorbidity with increased fatigue, and two noted lower likelihood of remission with multimorbidity.[21–25] Furthermore, multimorbid RA patients had increased fatigue, higher measures of disease activity, yet had longer time to biologic prescription with higher rates of biologic failure,[19–25] thus highlighting the need for further studies on optimal treatment approaches.
Etiologic considerations in RA and multimorbidity
While the vast majority of studies show increased prevalence of multimorbidity, the etiology remains unclear. Hypotheses include shared pathogenic processes exacerbated by systemic inflammation leading to earlier development of comorbid conditions such as cardiopulmonary disease.[39] Additionally, treatments in RA, specifically the cumulative exposure to exogenous steroids, are a known independent risk factor for diabetes and osteoporosis, which were highly prevalent comorbidities across the studies we reviewed.[11, 12, 14, 16, 26, 40] Likewise, immunosuppression can reduce host surveillance for malignancy and specific medications, such as JAK inhibitors, have risk for MACE as well as associations of TNF inhibitors and ILD.[41, 42] Finally, patients with advanced RA often suffer from decreased mobility and lower quality of life likely compounding these factors and limiting patient participation in health care. While the etiology may not yet have clear answers, current findings urge us to use patterns of multimorbidity to inform clinical care improvements, treatments, and outcomes in these complex patients. Few studies controlled for smoking or sociodemographic factors that may influence multimorbidity causal pathways and these should also be examined in future studies [28].
CONCLUSIONS
RA patients experience higher prevalence of multimorbidity (31-86% vs 18-71%) and worse outcomes compared to both non-RA multimorbid patients and RA patients without multimorbidity. Patterns in RA include high rates of cardiopulmonary, cardiometabolic, and depression and pain-related conditions as well as specific condition associations of ILD, asthma, cardiovascular disease, osteoarthritis, and osteoporosis. Furthermore, patients with RA and multimorbidity are paradoxically at increased risk of high disease activity but experience a lower likelihood of biologic use or biologic failure. Findings call for many further studies in this field.
Funding:
Supported by the University of Wisconsin-Madison School of Medicine and Public Health’s Institute for Clinical and Translational Research (NIH-CTSA Award 1UL1TR002373).
Disclosures:
Dr. Bartels has received institutional peer-reviewed grant funding from Independent Grants for Learning and Change (Pfizer) for unbranded tobacco cessation work. No other disclosures reported.
Footnotes
Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
REFERENCES
* *Of major importance
*Important
- 1.Skou ST, Mair FS, Fortin M, Guthrie B, Nunes BP, Miranda JJ, et al. Multimorbidity. Nature reviews Disease primers. 2022;8(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doran MF, Pond GR, Crowson CS, O'Fallon WM, Gabriel SE. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year period. Arthritis Rheum. 2002;46(3):625–31. [DOI] [PubMed] [Google Scholar]
- 3.Naz SM, Symmons DP. Mortality in established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21(5):871–83. [DOI] [PubMed] [Google Scholar]
- 4.Figus FA, Piga M, Azzolin I, McConnell R, Iagnocco A. Rheumatoid arthritis: Extra-articular manifestations and comorbidities. Autoimmun Rev. 2021;20(4):102776. [DOI] [PubMed] [Google Scholar]
- 5.Ruscitti P, Di Benedetto P, Berardicurti O, Liakouli V, Carubbi F, Cipriani P, et al. Adipocytokines in Rheumatoid Arthritis: The Hidden Link between Inflammation and Cardiometabolic Comorbidities. Journal of immunology research. 2018;2018:8410182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yurkovich M, Avina-Zubieta JA, Thomas J, Gorenchtein M, Lacaille D. A systematic review identifies valid comorbidity indices derived from administrative health data. J Clin Epidemiol. 2015;68(1):3–14. [DOI] [PubMed] [Google Scholar]
- 7.England BR, Thiele GM, Anderson DR, Mikuls TR. Increased cardiovascular risk in rheumatoid arthritis: mechanisms and implications. Bmj. 2018;361:k1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee EE, Shin A, Lee J, Lee JH, Ha YJ, Lee YJ, et al. All-cause and cause-specific mortality of patients with rheumatoid arthritis in Korea: A nation-wide population-based study. Joint Bone Spine. 2022;89(1):105269. [DOI] [PubMed] [Google Scholar]
- 9.Sambamoorthi U, Tan X, Deb A. Multiple chronic conditions and healthcare costs among adults. Expert review of pharmacoeconomics & outcomes research. 2015;15(5):823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshida K, Lin TC, Wei MY, Malspeis S, Chu SH, Camargo CA Jr., et al. Roles of Postdiagnosis Accumulation of Morbidities and Lifestyle Changes in Excess Total and Cause-Specific Mortality Risk in Rheumatoid Arthritis. Arthritis Care Res (Hoboken). 2021;73(2):188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Multimorbiidty was found to account for a substantial amount of the excess mortality seen in the RA patients.
- 11.Crowson CS, Gunderson TM, Dykhoff HJ, Myasoedova E, Atkinson EJ, Kronzer VL, et al. Comprehensive assessment of multimorbidity burden in a population-based cohort of patients with rheumatoid arthritis. RMD Open. 2022;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.England BR, Roul P, Yang Y, Sayles H, Yu F, Michaud K, et al. Burden and trajectory of multimorbidity in rheumatoid arthritis: a matched cohort study from 2006 to 2015. Ann Rheum Dis. 2021;80(3):286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]; * * Described how RA patients accumulate chronic conditions at a higher rate than non-RA patients.
- 13.England BR, Yang Y, Roul P, Haas C, Najjar L, Sayles H, et al. Identification of Multimorbidity Patterns in Rheumatoid Arthritis Through Machine Learning. Arthritis Care Res (Hoboken). 2023;75(2):220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]; * * One of two studies to describe phenotypic patterns of multimorbidity in RA patients, compared commercial and VHA data.
- 14.Gunderson TM, Myasoedova E, Davis JM, 3rd, Crowson CS. Multimorbidity Burden in Rheumatoid Arthritis: A Population-based Cohort Study. J Rheumatol. 2021;48(11):1648–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang YJ, Chen JS, Luo SF, Kuo CF. Comparison of Indexes to Measure Comorbidity Burden and Predict All-Cause Mortality in Rheumatoid Arthritis. Journal of clinical medicine. 2021;10(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kronzer VL, Dykhoff HJ, Stevens MA, Myasoedova E, Davis JM 3rd, Crowson CS. Racial Differences in Multimorbidity and Comorbidities in Rheumatoid Arthritis. Arthritis Care Res (Hoboken). 2023;75(1):76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Described how RA participants had an increased risk of multimorbidity and substantial multiorbidity compared to non-RA participants and that the odds of multimorbidity vary by race in non-RA patients but are similar across racial groups in RA.
- 17.McLoone P, Jani BD, Siebert S, Morton FR, Canning J, Macdonald S, et al. Classification of long-term condition patterns in rheumatoid arthritis and associations with adverse health events: a UK Biobank cohort study. Journal of multimorbidity and comorbidity. 2023;13:26335565221148616. [DOI] [PMC free article] [PubMed] [Google Scholar]; * * Found signficant phenotypic clusters in RA and reported that RA patients with these clusters had higher rates of adverse outcomes.
- 18.Morton FR, Jani BD, Mair FS, McLoone P, Canning J, Macdonald S, et al. Association between risk, duration and cause of hospitalisations in people with rheumatoid arthritis and multimorbidity in the UK Biobank and Scottish Early Rheumatoid Arthritis (SERA) cohorts: Longitudinal observational study. Semin Arthritis Rheum. 2023;58:152130. [DOI] [PubMed] [Google Scholar]
- 19.Armagan B, Sari A, Erden A, Kilic L, Erdat EC, Kilickap S, et al. Starting of biological disease modifying antirheumatic drugs may be postponed in rheumatoid arthritis patients with multimorbidity: Single center real life results. Medicine (Baltimore). 2018;97(13):e9930. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Identified how multimorbid RA patients experience longer time to biologic therapy compared to RA patients without multimorbidity.
- 20.Biggioggero M, Mesina F, Favalli EG. The Use of Rheumatic Disease Comorbidity Index for Predicting Clinical Response and Retention Rate in a Cohort of Rheumatoid Arthritis Patients Receiving Tumor Necrosis Factor Alpha Inhibitors. Biomed Res Int. 2019;2019:6107217. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Described how multimorbidity serves as a predictor of poor outcomes in RA and higher rates of tumor necrosis factor alpha inhibitor failure.
- 21.Busby AD, Wason J, Pratt AG, Young A, Isaacs JD, Nikiphorou E. Predictors of poor function in RA based on two prospective UK inception cohorts. Do comorbidities matter? Rheumatology (Oxford). 2022;61(4):1563–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis JM 3rd, Myasoedova E, Gunderson TM, Crowson CS. Multimorbidity and Fatigue in Rheumatoid Arthritis: A Cross-Sectional Study of a Population-Based Cohort. Rheumatol Ther. 2020;7(4):979–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luque Ramos A, Redeker I, Hoffmann F, Callhoff J, Zink A, Albrecht K. Comorbidities in Patients with Rheumatoid Arthritis and Their Association with Patient-reported Outcomes: Results of Claims Data Linked to Questionnaire Survey. J Rheumatol. 2019;46(6):564–71. [DOI] [PubMed] [Google Scholar]
- 24.Ruscitti P, Di Muzio C, Conforti A, Di Cola I, Pavlych V, Navarini L, et al. Cardiometabolic multimorbidity may identify a more severe subset of rheumatoid arthritis, results from a “real-life” study. Medicine (Baltimore). 2023;102(14):e33362. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Described a Cardiometabolic phenotype of multimorbidity that predicts worse outcomes in RA patients.
- 25.Tournadre A, Pereira B, Gossec L, Soubrier M, Dougados M. Impact of comorbidities on fatigue in rheumatoid arthritis patients: Results from a nurse-led program for comorbidities management (COMEDRA). Joint Bone Spine. 2019;86(1):55–60. [DOI] [PubMed] [Google Scholar]
- 26.McQueenie R, Nicholl BI, Jani BD, Canning J, Macdonald S, McCowan C, et al. Patterns of multimorbidity and their effects on adverse outcomes in rheumatoid arthritis: a study of 5658 UK Biobank participants. BMJ Open. 2020;10(11):e038829. [DOI] [PMC free article] [PubMed] [Google Scholar]; * * Described the adverse outcomes in multimorbid RA patients and the synergistic effects of some comorbidites and RA on all cause mortality.
- 27.Radner H Multimorbidity in rheumatic conditions. Wiener klinische Wochenschrift. 2016;128(21-22):786–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barber CEH, Bartels CM. Making Sense of Multimorbidity in Rheumatoid Arthritis. Arthritis Care & Research. 2023;75(2):207–9. [DOI] [PubMed] [Google Scholar]
- 29.Løppenthin K, Esbensen BA, Østergaard M, Ibsen R, Kjellberg J, Jennum P. Morbidity and mortality in patients with rheumatoid arthritis compared with an age- and sex-matched control population: A nationwide register study. Journal of comorbidity. 2019;9:2235042x19853484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Hoek J, Boshuizen HC, Roorda LD, Tijhuis GJ, Nurmohamed MT, van den Bos GA, et al. Mortality in patients with rheumatoid arthritis: a 15-year prospective cohort study. Rheumatol Int. 2017;37(4):487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lacaille D, Avina-Zubieta JA, Sayre EC, Abrahamowicz M. Improvement in 5-year mortality in incident rheumatoid arthritis compared with the general population-closing the mortality gap. Ann Rheum Dis. 2017;76(6):1057–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Listing J, Kekow J, Manger B, Burmester GR, Pattloch D, Zink A, et al. Mortality in rheumatoid arthritis: the impact of disease activity, treatment with glucocorticoids, TNFα inhibitors and rituximab. Ann Rheum Dis. 2015;74(2):415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Almutairi KB, Inderjeeth CA, Preen DB, Keen HI, Nossent JC. Mortality Trends Among Patients with Rheumatoid Arthritis in Western Australia. Rheumatol Ther. 2023;10(4):1021–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartels CM, Ramly E, Panyard D, Lauver DR, Johnson HM, Lewicki K, et al. BP Connect Toolkit Madison, WI: University of Wisconsin – Madison School of Medicine and Public Health; 2020. [Available from: https://www.hipxchange.org/BPConnectHealth.
- 35.Bartels CM, Ramly E, Johnson HM, Lauver DR, Panyard DJ, Li Z, et al. Connecting Rheumatology Patients to Primary Care for High Blood Pressure: Specialty Clinic Protocol Improves Follow-up and Population Blood Pressures. Arthritis Care Res (Hoboken). 2019;71(4):461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartels CM, Johnson L, Ramly E, Panyard DJ, Gilmore-Bykovskyi A, Johnson HM, et al. Impact of a Rheumatology Clinic Protocol on Tobacco Cessation Quit Line Referrals. Arthritis Care Res (Hoboken). 2022;74(9):1421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.American College of Rheumatology. Guideline for the Treatment of Interstitial Lung Disease in People with Systemic Autoimmune Rheumatic Disease 2023. [Available from: https://assets.contentstack.io/v3/assets/bltee37abb6b278ab2c/bltaedebda97a351d47/interstitial-lung-disease-guideline-summary-treatment-2023.pdf.
- 38.Lubell J Teamwork guides cardio-rheumatology clinics that care for unique patient population. MDedge/Rheumatology. 2023. [Available from: https://www.mdedge.com/rheumatology/article/260726/business-medicine/teamwork-guides-cardio-rheumatology-clinics-care.
- 39.Kronzer VL, Crowson CS, Sparks JA, Myasoedova E, Davis JM, 3rd. Comorbidities As Risk Factors for Rheumatoid Arthritis and Their Accrual After Diagnosis. Mayo Clin Proc. 2019;94(12):2488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Briot K, Roux C. Glucocorticoid-induced osteoporosis. RMD Open. 2015;1(1):e000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang Y, Lin W, Chen Z, Wang Y, Huang Y, Tu S. Effect of tumor necrosis factor inhibitors on interstitial lung disease in rheumatoid arthritis: angel or demon? Drug design, development and therapy. 2019;13:2111–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, Rivas JL, et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N Engl J Med. 2022;386(4):316–26. [DOI] [PubMed] [Google Scholar]


