Abstract
Background and Aims:
The use of intrathecal opioids is associated with high risk of pruritis and this may be decreased by adding a low dose of naloxone. This study evaluated the effect of the addition of 20 μg of naloxone to fentanyl–bupivacaine mixture on the incidence of pruritis in pregnant females scheduled for cesarean section (CS).
Material and Methods:
Eighty pregnant patients scheduled for CS under spinal anesthesia were randomized to receive either 10 mg of 0.5% hyperbaric bupivacaine (2 ml) plus 25 μg fentanyl (group F) or 10 mg of 0.5% hyperbaric bupivacaine (2 ml) plus 25 μg fentanyl and 20 μg naloxone (group FN). The incidence, onset, duration, site, and severity of pruritis were measured. Furthermore, the postoperative numerical rating scale (NRS) score, the total tramadol rescue analgesia, and the time for the first request of rescue analgesia were recorded.
Results:
Compared to the F group, the FN group showed a significant decrease in the incidence of pruritis (P = 0.022), prolongation of the onset of pruritis (P = 0.006), shortening of the duration of pruritis (P = 0.029), and decrease in the severity of pruritis (P = 0.039). Furthermore, the postoperative pain score, the rescue analgesic consumption, and the time for the first request of rescue analgesia were comparable between the two groups (P > 0.05).
Conclusions:
The addition of an ultra-low dose of naloxone (20 μg) to fentanyl–bupivacaine mixture in spinal anesthesia for pregnant females scheduled for CS significantly reduced the incidence of pruritis without having a significant effect on the postoperative analgesia.
Keywords: Cesarean section, fentanyl, intrathecal, naloxone, pruritis
Introduction
Regional anesthesia techniques, especially spinal anesthesia, are the most common anesthesia techniques that are used for cesarean section (CS) as they have the advantages of postoperative analgesia and avoidance of the risk of aspiration, awareness, and difficult airway control.[1,2] Opioids are commonly used as a local anesthetic adjuvant in intrathecal anesthesia to decrease the incidence of spinal-induced hypotension that may have serious effects on the mother and/or baby and to prolong analgesia.[3,4] On the other hand, pruritis is very common with intrathecal administration of opioids and can reach an incidence of 85%.[5] Its exact line of treatment is not fully explained despite the successful use of ondansetron and antihistamines. Intravenous (IV) naloxone may be required in severe and resistant cases.[6,7]
Naloxone, the opioid receptor antagonist, may have an analgesic effect and may decrease the incidence of opioid-related side effects. The mechanism of this action is not known. It may be due to the release of endogenous opioids or regulation of opioid receptors.[8] The use of ultra-low dose of naloxone to overcome the side effects of opioids is studied in many trials with conflicting outcomes.[9,10] In addition, the use of ultra-low doses of naloxone as an additive to opioids–bupivacaine mixture in spinal anesthesia was evaluated by certain trials.[11]
This clinical study hypothesized that the use of ultra-low dose of naloxone (20 μg) as an additive to fentanyl–hyperbaric bupivacaine mixture in spinal anesthesia for full-term pregnant females scheduled for elective CS may decrease the incidence of pruritis without having a significant effect on postoperative analgesia. This randomized clinical study was carried out to evaluate the effect of adding 20 μg of naloxone to 10 mg of hyperbaric bupivacaine and 25 μg fentanyl in spinal anesthesia for full-term pregnant females undergoing elective CS on the incidence of pruritis (primary outcome) and the postoperative pain score (secondary outcome).
Material and Methods
This clinical trial was approved by the local Research Ethical Committee of our Faculty of Medicine (approval no. of 33954/7/20) and registered on clinicaltrial.gov before enrollment of the first patient. The study lasted from September 11, 2020 (first patient enrolled) to March 7, 2021 (last patient enrolled). The benefits and the hazards (the possibility of increased postoperative pain) were explained to the enrolled patients in the study. Informed written consent was obtained from all participants.
Full-term pregnant females undergoing elective CS under spinal anesthesia were included in the study, while the exclusion criteria were as follows: refusal of patients to participate in the study, body mass index (BMI) >35 kg/m2, height less than 160 cm, gestational age less than 37 weeks, presence of diabetes mellitus (DM) or hypertensive disorders of pregnancy, coagulopathy, psychological disorders, neurological disorders, antepartum hemorrhage, and allergy to the used medications.
An independent data manager performed random distribution of the included patients into two groups based upon computer-generated software of randomization introduced in sealed opaque envelopes. Grouping was as follows:
Group F: in which patients received spinal anesthesia with 10 mg hyperbaric bupivacaine (2 ml) and 25 μg fentanyl (0.5 ml)
Group FN: in which spinal anesthesia was performed with 10 mg hyperbaric bupivacaine (2 ml), 25 μg fentanyl (0.5 ml), and 20 μg preservative-free naloxone
The local anesthetic mixtures were prepared in uniform syringes under complete aseptic precautions through the aid of an anesthesiology resident who was not participating in the study and had no subsequent role in the study. The two groups had nearly the same volume as the 20 μg of naloxone used in group FN is 0.05 ml. All patients underwent adequate preoperative assessment. Once the patient was admitted to the operating theater, she was attached to a monitor device consisting of pulse oximeter, three-lead electrocardiogram (ECG), and noninvasive blood pressure. Then, intravascular access was established through the insertion of an 18-gauge peripheral venous cannula with starting fluid preload of 7 ml/kg of lactated Ringer’s solution over 20 min. All patients were premedicated with ranitidine 50 mg IV, 2 h before surgery.
Under complete aseptic precautions and in a sitting position, spinal anesthesia was performed at L3–L4 or L4–L5 intervertebral space using a 25-gauge spinal needle with injection of the pre-prepared local anesthetic mixture. Then, the patient was turned to supine position with left lateral tilt of 15° to prevent aortocaval compression. Nasal cannula was used at a flow of 3–4 l/min to supply oxygen to the patient. Maternal heart rate was maintained above 50 beats/min by administration of atropine 0.3 mg IV. Also, maternal systolic blood pressure was maintained Above 90 mmHg and the mean arterial pressure below 65 mmHg by administering 100 μg IV phenylephrine and 250 ml bolus of lactated Ringer’s solution.
Pinprick test using a 27-gauge needle from the caudal to cranial direction was used to assess sensory blockade till the sensory block reached the level of T4. Moreover, the modified Bromage score[12] (0 = no paralysis, 1 = cannot raise an extended leg, 2 = cannot flex the knee, and 3 = cannot dorsiflex the ankle) was evaluated every 5 min to evaluate the motor blockade until it reached a score of 2 or 3. The patients had received general anesthesia and were excluded from the study if the desired sensory and motor blockade was not achieved within 20 min. After delivery of the fetus, 5 IU of oxytocin was administered slowly IV over 10 min. One gram of paracetamol was given as IV infusion every 6 h and 30 mg of ketorolac IV was given every 12 h as routine postoperative analgesia.
The measurement data were obtained with the aid of an assistant nurse who did not participate in the study and was blinded to its groups. The incidence, onset, duration, site, and severity of pruritis were measured. The incidence of pruritis (primary outcome) represents the number of patients who developed pruritis in the first 24 h after surgery. The onset of pruritis was the time interval from intrathecal injection till the first incidence of pruritis. The duration of pruritis represents the time elapsed between the first and last incidence of pruritis. Moreover, the severity of pruritis was estimated through a specific score Pruritis Visual Analogue Score (PVAS score). It is 10 cm long, where the left endpoint represents no itching and the right endpoint represents most severe pruritis. The patients were asked to grade their degree of pruritis using PVAS score on the next day after surgery. PVAS score less than 3 means mild pruritis, PVAS score 4–6 means moderate pruritis, PVAS score more 7–8 means severe pruritis, and PVAS score 9 or 10 means very severe pruritis. Patients who developed pruritis were managed by administration of ondansetron 8 mg IV and 45.5 mg pheniramine hydrogen maleate IV.
The postoperative pain was assessed by the numerical rating scale (NRS) score (metric score 0–10 for assessment of the severity of pain, where 0 = no pain and 10 = maximal pain) immediately postoperative, then every 2 h in the first 8 h, and then every 4 h till 24 h. Whenever the NRS score reached 4 or more, 50 mg of tramadol was given IV as rescue analgesia and was repeated whenever required. The time interval from the end of the surgery till the first administration of tramadol rescue analgesia was recorded (time for the first request of rescue analgesia); also, the total dose of tramadol consumed in the first 24 h after surgery was calculated. Furthermore, the incidence of maternal side effects such as hypotension, bradycardia, shivering, or nausea and vomiting was recorded. The fetal APGAR score was recorded 1 and 5 min after delivery. On the next day after surgery, the patients were asked to grade their degree of satisfaction using a 4-point scale (4 = very satisfied, 3 = satisfied, 2 = dissatisfied, and 1 = very dissatisfied).
Sample size calculation based upon the results of a previous study[13] revealed that at least 35 patients will be required in each group to detect a significant decrease in the incidence of pruritis from 70% to 35% (50% reduction) at a 0.05 alpha value, 85% power of the study, and with a ratio of cases to control of 1:1. Forty patients will be included in each group to overcome the possibility of dropout cases. The statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) computer program (SPSS Inc., Chicago, IL, USA; version 16.0). The Kolmogorov–Smirnov test was used for checking the assumption of normality. Categorical data were analyzed by Fisher’s exact test and expressed as number and percent, while the parametric data were analyzed by unpaired t-test and expressed as mean ± standard deviation. The nonparametric data were analyzed by Mann–Whitney U test and expressed as median with interquartile range. Data were considered statistically significant when the P value decreased to less than 0.05.
Results
Ninety-three full-term pregnant female patients were assessed for their eligibility to participate in this study; 13 patients were excluded (eight patients were not meeting the study inclusion criteria and the other five patients declined to participate in the study) and the other 80 patients were randomly distributed to the two study groups. Two patients in group F and one patient in group FN were dropped out from the study owing to failed spinal anesthesia with successful obtaining and analysis of the data of the other patients in the two groups [Figure 1].
Figure 1.
CONSORT flow chart of the study
Age, BMI, gravidity, and gestational age were comparable between the two study groups (P = 0.159, 0.231, 0.495, and 0.191, respectively). Moreover, the incidence of complications including hypotension, bradycardia, nausea and vomiting, and shivering was statistically insignificant between the two groups (P = 0.639, 0.620, 0.584, and 0.620, respectively) [Table 1].
Table 1.
Demographic data of the study groups
| Group F (38 patients) | Group FN (39 patients) | 95% CI | |
|---|---|---|---|
| Age (years) | 24.18±2.87 | 25.10±2.79 | −0.369, 2.206 |
| BMI (kg/m2) | 30.50±1.64 | 30.97±1.80 | −0.308, 1.256 |
| Gravidity | |||
| Primigravida | 22 (57.89%) | 19 (48.72%) | |
| Multigravida | 16 (42.11%) | 20 (51.28%) | |
| Gestational age (weeks) | 38.40±0.97 | 38.69±1.00 | −0.152, 0.747 |
| Complications | |||
| Hypotension | 15 (39.47%) | 13 (33.33%) | |
| Bradycardia | 12 (31.58%) | 10 (25.64%) | |
| N&V | 9 (23.68%) | 7 (17.95%) | |
| Shivering | 10 (26.32%) | 13 (33.33%) |
BMI=body mass index, CI=confidence interval, N&V=nausea and vomiting, SD=standard deviation. Group F (spinal anesthesia with fentanyl–bupivacaine), group FN (spinal anesthesia with fentanyl–naloxone–bupivacaine). Data are presented as mean±SD or number and %
The incidence of pruritis decreased significantly from 60.53% in group F to 33.33% in group FN (P = 0.022). Moreover, the PVAS score decreased significantly in group FN compared to group F (P = 0.006), with a significant decrease in the severity of pruritis in group FN in comparison to group F (P = 0.039). Furthermore, the onset of pruritis was prolonged significantly in group FN in comparison to group F (P = 0.006). In addition, the duration of pruritis was significantly shorter in group FN than group F (P = 0.029). On the other hand, the site of pruritis (nasal, back, buttocks, and arms) was statistically insignificant between the two groups (P = 0.948) [Table 2].
Table 2.
The criteria of perioperative pruritis
| Group F (38 patients) | Group FN (39 patients) | P | 95% CI | |
|---|---|---|---|---|
| Incidence of pruritis | 23 (60.53%) | 13 (33.33%) | 0.022* | 1.089, 2.800 |
| PVAS score | 3 (0–10) | 0 (0–8) | 0.013* | |
| Onset of pruritis (h) | 1.11±0.66 | 1.87±0.85 | 0.006* | 0.238, 1.294 |
| Duration of pruritis (h) | 2.92±2.98 | 1.46±2.80 | 0.029* | 0.147, 2.772 |
| Site of pruritis | ||||
| Nasal | 14/23 (60.87%) | 8/13 (61.54%) | 0.948 | |
| Back | 4/23 (17.39%) | 3 (23.08%) | ||
| Buttock | 3/23 (13.04%) | 1 (7.69%) | ||
| Arms | 2/23 (8.70%) | 1 (7.69%) | ||
| Severity of pruritis | ||||
| Mild | 3/23 (13.04%) | 7/13 (53.85%) | 0.039* | |
| Moderate | 11/23 (47.83%) | 5/13 (38.46%) | ||
| Severe | 5/23 (21.74%) | 1/13 (7.69%) | ||
| Very severe | 4/23 (17.39%) | 0/13 (0%) |
CI=confidence interval, SD=standard deviation. Group F (spinal anesthesia with fentanyl–bupivacaine), group FN (spinal anesthesia with fentanyl–naloxone–bupivacaine). Data are presented as mean±SD or number and %. P value represents comparison between the two groups. *Denotes significant changes
The time to first request of tramadol rescue analgesia and the total dose of tramadol consumed in the first 24 h after surgery were comparable between the two study groups (P = 0.496 and 0.615, respectively). Moreover, the postoperative NRS score was comparable between the two groups at all time intervals (P > 0.05) [Table 3]. The fetal outcome assessed by 1- and 5-min APGAR scores was statistically insignificant between the two groups (P = 0.614 and 0.642, respectively) [Table 4]. Furthermore, the maternal satisfaction with their postoperative analgesia was indifferent between the two study groups (P = 0.831) [Figure 2].
Table 3.
Postoperative analgesia in the two groups
| Group F (38 patients) | Group FN (39 patients) | 95% CI | |
|---|---|---|---|
| Time to first request for rescue analgesia (min) | 288.42±83.00 | 275.13±87.20 | −51.960, 25.375 |
| Postoperative 24-h tramadol consumption (mg) | 151.32±55.125 | 157.69±55.652 | −18.776, 31.529 |
| NRS | |||
| Immediately postoperative | 1 (0–2) | 1 (0–2) | |
| 2 h | 1 (0–3) | 1 (0–3) | |
| 4 h | 3 (2–6) | 4 (2–6) | |
| 6 h | 4 (2–6) | 4 (2–6) | |
| 8 h | 3 (2–5) | 3 (2–5) | |
| 12 h | 3 (2–5) | 3 (2–5) | |
| 16 h | 2 (1–3) | 2 (1–3) | |
| 20 h | 1 (0–3) | 1 (0–3) | |
| 24 h | 1 (0–3) | 1 (0–3) |
CI=confidence interval, NRS=numerical rating scale, SD=standard deviation. Group F (spinal anesthesia with fentanyl–bupivacaine), Group FN (spinal anesthesia with fentanyl–naloxone–bupivacaine). Data are presented as mean±SD or median and interquartile range
Table 4.
APGAR score at 1 min and 5 min in the study groups
| Group F (38 patients) | Group FN (39 patients) | |
|---|---|---|
| 1-min APGAR score | 8 (7–10) | 8 (7–10) |
| 5-min APGAR score | 9 (8–10) | 9 (8–10) |
Group F (spinal anesthesia with fentanyl–bupivacaine), Group FN (spinal anesthesia with fentanyl–naloxone–bupivacaine). Data are presented as median and interquartile range
Figure 2.
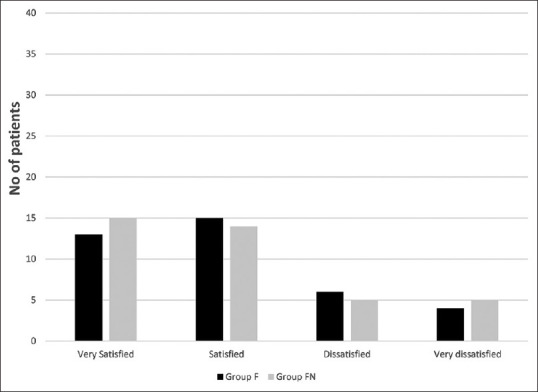
Maternal satisfaction in the studied groups. Group F (spinal anesthesia with fentanyl–bupivacaine: 38 patients), group FN (spinal anesthesia with fentanyl–naloxone–bupivacaine: 39 patients). Data are presented as the number of patients
Discussion
This randomized clinical trial evaluated the addition of 20 μg of naloxone to hyperbaric bupivacaine–fentanyl mixture in spinal anesthesia for patients undergoing CS and revealed that the use of such ultra-low dose of naloxone significantly decreased the incidence, the score, the severity, and the duration of postoperative pruritis without having a significant effect on the postoperative pain score, the need for rescue analgesia, or the maternal and fetal outcomes.
The mechanism of action of pruritis is not well known. It seems to be an adverse effect rather than an allergic reaction. Pruritis is very common in pregnant females who receive intrathecal opioids, which could be the result of the interaction between estrogen and opioids.[14,15] Other mechanisms may be the release of histamine[16] and stimulation of the spinal opioid receptors.[17]
Pruritis commonly occurs in the face due to cephalad spread of the opioid in cerebrospinal fluid (CSF) with subsequent interaction with trigeminal nucleus and nerve roots. Other sites for pruritis may include the neck, back, or trunk.[5] The onset and duration of opioid-induced pruritis are dependent upon the dose and the type of opioid. Lipid-soluble opioids (such as fentanyl) have a rapid onset and short duration of pruritis in contrast to lowlipid-soluble opioids (such as morphine), which is associated with prolonged duration of pruritis.[18] Several drugs can be used for the management of pruritis that develops after intrathecal opioid administration. They include diphenhydramine, ondansetron, nalbuphine, propofol, and naloxone. However, an effective drug for its management is still missing.[19]
The use of an ordinary dose of naloxone (0.1 mg/kg) may be helpful in the management of opioid-induced pruritis; however, it may act as an opioid receptor antagonist and reverse the analgesic effect. The use of intrathecal ultra-low dose of naloxone was approved to enhance the release of endogenous opioids and regulate the opioid receptor, hence it may have an analgesic effect.[20] So, the use of intrathecal ultra-low dose of naloxone may prevent or decrease the incidence of opioid-induced pruritis without affecting the analgesic effect.
To the best of our knowledge, there are no available clinical trials evaluating the effect of addition of an ultra-low dose of naloxone to bupivacaine–fentanyl mixture on the incidence of perioperative pruritis. The meta-analysis of He et al.[21] that included 13 studies (1138 patients) revealed that the use of naloxone through IV route decreases the incidence of opioid-related side effects (pruritis, and nausea and vomiting). Moreover, the systematic review of Kjellberg and Tramer[22] that included 22 trials (1477) concluded that the IV use of naloxone 0.25–0.4 μg/kg/h is an effective tool for the management of opioid-induced pruritis.
The randomized clinical study of Peivandi et al.[11] revealed that the use of naloxone 20 μg as an additive to bupivacaine and morphine in spinal anesthesia for CS significantly decreased the severity of pruritis and nausea and had no effect on the postoperative pain score. Furthermore, Ibrahim et al.[23] concluded that addition of 0.5 mg nalbuphine to hyperbaric bupivacaine and morphine 0.2 mg in spinal anesthesia for CS significantly decreased the incidence and severity of pruritis. Nalbuphine is an opioid mu receptor antagonist and an opioid kappa receptor agonist. This may suggest that the use of a low dose of intrathecal opioid antagonist can decrease the incidence and severity of pruritis.
On the other hand, Lockington and Fa’aea[24] studied 50 female patients scheduled for CS under spinal anesthesia with hyperbaric bupivacaine, 150 μg morphine, and 25 μg fentanyl and found that the use of naloxone 400 μg subcutaneously had an insignificant effect on the incidence of pruritis. This may be explained by the use of two opioids as local anesthetic adjuvants (morphine and fentanyl). Also, naloxone was administered subcutaneously and intrathecal opioid-induced pruritis may be mediated by the spinal opioid receptors.
There are many limitations to this study. First, pruritus is a subjective symptom. Second, the study did not evaluate the use of multiple doses of naloxone. Third, this study did not provide the optimal concentration of naloxone added to intrathecal bupivacaine with fentanyl, but we selected this dose based on its safety during pregnancy from other studies.
Conclusions
We conclude that the addition of an ultra-low dose of naloxone (20 μg) as an additive to fentanyl–hyperbaric bupivacaine mixture in spinal anesthesia for full-term pregnant females scheduled for elective cesarean delivery significantly reduced the incidence, duration, and severity of postoperative opioid-related pruritis without affecting the analgesic potency, incidence of complications, fetal outcome, or maternal satisfaction.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors would like to thank all the staff members of Department of Anesthesia and Intensive Care, Faculty of Medicine, Tanta University.
References
- 1.Tadevosyan M, Ghazaryan A, Harutyunyan A, Petrosyan V, Atherly A, Hekimian K. Factors contributing to rapidly increasing rates of cesarean section in Armenia: A partially mixed concurrent quantitative-qualitative equal status study. BMC Pregnancy Childbirth. 2019;19:1–10. doi: 10.1186/s12884-018-2158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reynolds F, Seed P. Anaesthesia for caesarean section and neonatal acid-base status: A meta-analysis. Anaesthesia. 2005;60:636–53. doi: 10.1111/j.1365-2044.2005.04223.x. [DOI] [PubMed] [Google Scholar]
- 3.Kinsella S, Carvalho B, Dyer R, Fernando R, McDonnell N, Mercier F, et al. International consensus statement on the management of hypotension with vasopressors during cesarean section under spinal anesthesia. Anaesthesia. 2018;73:71–2. doi: 10.1111/anae.14080. [DOI] [PubMed] [Google Scholar]
- 4.Bujedo BM, Santos SG, Azpiazu AU. A review of epidural and intrathecal opioids used in the management of postoperative pain. J Opioid Manag. 2012;8:177–92. doi: 10.5055/jom.2012.0114. [DOI] [PubMed] [Google Scholar]
- 5.Szarvas S, Harmon D, Murphy D. Neuraxial opioid-induced pruritus: A review. J Clin Anesth. 2003;15:234–9. doi: 10.1016/s0952-8180(02)00501-9. [DOI] [PubMed] [Google Scholar]
- 6.Abate SM, Belihu AE. Efficacy of low dose bupivacaine with intrathecal fentanyl for cesarean section on maternal hemodynamic: Systemic review and meta-analysis. Saudi J Anaesth. 2019;13:340–51. doi: 10.4103/sja.SJA_17_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gehling M, Tryba M. Risks and side-effects of intrathecal morphine combined with spinal anesthesia:a meta-analysis. Anaesthesia. 2009;64:643–51. doi: 10.1111/j.1365-2044.2008.05817.x. [DOI] [PubMed] [Google Scholar]
- 8.Block L, Lundborg C, Bjersing J, Dahm P, Hansson E, Biber B. Ultralow dose of naloxone as an adjuvant to intrathecal morphine infusion improves perceived quality of sleep but fails to alter persistent pain: A randomized, double-blind, controlled study. Clin J Pain. 2015;31:968–75. doi: 10.1097/AJP.0000000000000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koo C-H, Yoon S, Kim B-R, Cho YJ, Kim TK, Jeon Y, et al. Intraoperative naloxone reduces remifentanil-induced postoperative hyperalgesia but not pain: A randomized controlled trial. Br J Anaesth. 2017;119:1161–8. doi: 10.1093/bja/aex253. [DOI] [PubMed] [Google Scholar]
- 10.Firouzian A, Gholipour Baradari A, Ehteshami S, Zamani Kiasari A, Shafizad M, Shafiei S, et al. The effect of ultra–low-dose intrathecal naloxone on pain intensity after lumbar laminectomy with spinal fusion: A randomized controlled trial. J Neurosurg Anesthesiol. 2020;32:70–6. doi: 10.1097/ANA.0000000000000537. [DOI] [PubMed] [Google Scholar]
- 11.Peivandi S, Habibi MR, Baradari AG, Gholinataj A, Habibi A, Khademloo M, et al. The effect of adding low-dose naloxone to intrathecal morphine on postoperative pain and morphine related side effects after cesarean section: A double-blind, randomized, clinical trial. J Med Sci. 2019;7:3979–83. doi: 10.3889/oamjms.2019.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maggiore ULR, Silanos R, Carlevaro S, Gratarola A, Venturini P, Ferrero S, et al. Programmed intermittent epidural bolus versus continuous epidural infusion for pain relief during termination of pregnancy: A prospective, double-blind, randomized trial. Int J Obstet Anesth. 2016;25:37–44. doi: 10.1016/j.ijoa.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Prin M, Guglielminotti J, Moitra V, Li G. Prophylactic ondansetron for the prevention of intrathecal fentanyl-or sufentanil-mediated pruritus: A meta-analysis of randomized trials. Anesth Analg. 2016;122:402–9. doi: 10.1213/ANE.0000000000001046. [DOI] [PubMed] [Google Scholar]
- 14.Chen M-K, Chau S-W, Shen Y-C, Sun Y-N, Tseng K-Y, Long C-Y, et al. Dose-dependent attenuation of intravenous nalbuphine on epidural morphine-induced pruritus and analgesia after cesarean delivery. Kaohsiung J Med Sci. 2014;30:248–53. doi: 10.1016/j.kjms.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar K, Singh SI. Neuraxial opioid-induced pruritus: An update. J Anaesthesiol Clin Pharmacol. 2013;29:303–7. doi: 10.4103/0970-9185.117045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swegle JM, Logemann CD. Management of common opioid-induced adverse effects. Am Fam Physician. 2006;74:1347–54. [PubMed] [Google Scholar]
- 17.McNicol E, Horowicz-Mehler N, Fisk RA, Bennett K, Gialeli-Goudas M, Chew PW, et al. Management of opioid side effects in cancer-related and chronic noncancer pain: A systematic review. J Pain. 2003;4:231–56. doi: 10.1016/s1526-5900(03)00556-x. [DOI] [PubMed] [Google Scholar]
- 18.Reich A, Szepietowski J. Opioid-induced pruritus: An update. Clin Exp Dermatol. 2010;35:2–6. doi: 10.1111/j.1365-2230.2009.03463.x. [DOI] [PubMed] [Google Scholar]
- 19.Waxler B, Dadabhoy ZP, Stojiljkovic L, Rabito SF, Warltier DC. Primer of postoperative pruritus for anesthesiologists. Anesthesiology. 2005;103:168–78. doi: 10.1097/00000542-200507000-00025. [DOI] [PubMed] [Google Scholar]
- 20.Yang C-P, Cherng C-H, Wu C-T, Huang H-Y, Tao P-L, Wong C-S. Intrathecal ultra-low dose naloxone enhances the antinociceptive effect of morphine by enhancing the reuptake of excitatory amino acids from the synaptic cleft in the spinal cord of partial sciatic nerve–transected rats. Anesth Analg. 2011;113:1490–500. doi: 10.1213/ANE.0b013e31822d39c1. [DOI] [PubMed] [Google Scholar]
- 21.He F, Jiang Y, Li L. The effect of naloxone treatment on opioid-induced side effects: A meta-analysis of randomized and controlled trials. Medicine (Baltimore) 2016;95:e4729. doi: 10.1097/MD.0000000000004729. doi:10.1097/MD.0000000000004729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kjellberg F, Tramer M. Pharmacological control of opioid-induced pruritus: A quantitative systematic review of randomized trials. Eur J Anaesthesiol. 2001;18:346–57. doi: 10.1046/j.0265-0215.2000.00826.x. [DOI] [PubMed] [Google Scholar]
- 23.Ibrahim AS, Aly MG, Thabet ME, Abdelaziz MR. Effect of adding nalbuphine to intrathecal bupivacaine with morphine on postoperative nausea and vomiting and pruritus after elective cesarean delivery: A randomized double-blinded study. Minerva Anestesiol. 2019;85:255–62. doi: 10.23736/S0375-9393.18.12751-9. [DOI] [PubMed] [Google Scholar]
- 24.Lockington P, Fa'aea P. Subcutaneous naloxone for the prevention of intrathecal morphine-induced pruritus in elective caesarean delivery. Anaesthesia. 2007;62:672–6. doi: 10.1111/j.1365-2044.2007.05098.x. [DOI] [PubMed] [Google Scholar]



