Abstract
Background and Aims:
Pediatric upper gastrointestinal (GI) endoscopy is commonly performed under deep sedation, which is frequently associated with respiratory complications. The study compared the respiratory benefits of applying bilateral modified nasopharyngeal airways (NPAs) to conventional low-flow nasal cannula (LFNC).
Material and Methods:
Fifty patients scheduled for an upper GI endoscopy under deep sedation, with an American Society of Anesthesiologists physical status I/II, were enrolled in the study. The patients were randomly divided into bilateral NPA group and the LFNC group. Fentanyl and propofol were administered to both groups to maintain deep sedation. After the application of NPA or LFNC, the hypoxic incidents (oxygen saturation [SpO2] <90%) and airway interventions during the procedure were noted and recorded. Other outcomes such as nasopharyngeal injuries, gastroenterologist satisfaction, the incidence of hypotension or bradycardia, and postoperative nausea and vomiting were also compared.
Results:
No significant differences were noted in the demographic data. The incidence of hypoxemia was 16% (n = 4) in the NPA group versus 36% (n = 9) in the LFNC group (P = 0.634). Airway intervention was lower in the NPA group compared to the LFNC group, but the difference was not significant (P = 0.539). No significant differences were noted in the incidence of nasopharyngeal injuries, postoperative nausea and vomiting, bradycardia, and hypotension. The NPA group showed higher gastroenterologist’s satisfaction (P = 0.003).
Conclusion:
Double-modified NPA in pediatric endoscopy was noninferior to the standard LFNC for the incidence of hypoxemia and airway intervention rate, with greater gastroenterologist satisfaction.
Keywords: Endoscopy, hypoxia, nasal cannula, nasopharyngeal airway, pediatric
Introduction
Esophagogastroduodenoscopy (EGD) is an important diagnostic and therapeutic procedure that is commonly conducted in children under general anesthesia or deep sedation. Sedation during gastrointestinal (GI) endoscopy aims to induce amnesia and analgesia, while making the procedure easier and safer to complete and maintaining patient safety.[1]
Propofol, ketamine, fentanyl, pethidine, and midazolam are the most used drug combinations for pediatric procedural sedation. However, each of these medications can induce respiratory depression and hypoxemia.[2] These adverse respiratory events associated with procedural sedation may be caused by lower oxygen reserve and greater oxygen consumption in children compared to adults. Therefore, adequate respiratory care is required during pediatric procedural sedation. The incidence of hypoxemia may reach up to 5.5% with pediatric procedural sedation.[3]
Supplemental oxygen has been suggested by the American Society for Gastrointestinal Endoscopy and the American Society of Anesthesiologists (ASA) to help minimize the incidence of hypoxemia.[4,5]
Other major concerns with the endoscopy procedure are the shared airway, the need for its performance outside the operating room, and the need for a lateral or semi-prone position. Numerous guidelines recommend deep sedation with propofol during GI endoscopy because of its short-acting properties. This, however, might lead to respiratory depression and airway obstruction.[6] Deep sedation impairs protective airway reflexes and respiratory function. As a result, airway management using special equipment is required.[5]
Numerous airway devices have been utilized for endoscopic operations to improve patient outcomes by increasing airway control and minimizing hypoxia while eliminating the necessity for intubation. These devices include the endoscopy mask, the low-flow nasal cannula (LFNC), the laryngeal mask airway, the LMA® Gastro™ airway, the gastrolaryngeal tube, and the nasal positive pressure devices.[7,8,9,10,11]
By acting as a splint, the nasopharyngeal airway (NPA) maintains the airway open and keeps the tongue away from the posterior pharynx, thereby preventing airway obstruction.[12] Nevertheless, its application might be associated with the risk of harming the nasal mucosa and resulting in blood aspiration.[13]
The purpose of this study is to evaluate the effectiveness of inserting bilateral NPA during pediatric endoscopic sedation for upper GI endoscopy compared to the standard use of LFNC. We hypothesize that bilateral NFA is noninferior to LFNC in terms of the incidence of hypoxic events and respiratory instability.
The primary outcome measure included the number of patients who developed hypoxemia (oxygen saturation [SpO2] <90% for more than 15 s as identified by pulse oximetry, or apnea for more than 15 s as identified by end-tidal CO2 [EtCO2] waveform). Secondary outcomes included duration of the endoscopy, the gastroenterologist’s satisfaction at the end of the procedure, the incidence of nasopharyngeal injury, bradycardia (heart rate [HR] <60 beats/min), hypotension (systolic blood pressure <20% from baseline), and the occurrence of other side effects (e.g., nausea and vomiting).
Material and Methods
The study was registered at the Pan-African Clinical Trials Registry (https://pactr.samrc.ac.za; registration no. PACTR202205629173217; date of first registration: May 31, 2022) and approved (approval no. FMASU R 107/2022) by the institutional review board before recruitment of the first participant. The study enrolled 50 children of both sexes, aged between 1 and 18 years, with an ASA physical status of I–II, who were undergoing elective diagnostic EGD under deep sedation. Successive 50 children posted for EGD in the pediatric endoscopy unit were recruited.
According to the Declaration of Helsinki, children’s parents or legal guardians provided informed written consent for participation in the study. Children older than 6 years were asked for their assent by using a different age-specific assent form. Patients with upper airway anatomic disorders, malignancy, known allergies to the drugs used, or who required emergency procedures were excluded from the study.
Routine preoperative laboratory investigations, including complete blood count, coagulation profile, and liver function tests, were performed in all patients. Patients were asked to fast for solid foods and formula milk for 6 h, breast milk for 4 h, and clear fluids for 2 h. Examination and documentation of loose teeth and enlarged tonsils were done to avoid loose tooth dislodgement by accident, which may create airway complications. Premedication with oral midazolam at a dosage of 0.5 mg/kg was administered to all patients (maximum of 15 mg). HR, noninvasive mean arterial pressure (MAP), respiratory rate, EtCO2, and SpO2 were all monitored before the onset of sedation and then every 3 min throughout the procedure. Carbon dioxide (CO2) monitoring was performed by using micro-stream capnography to measure EtCO2 in patients who received O2 through the nasal cannula, while in patients with NPA, EtCO2 was measured using side-stream capnography connected to the junction between the double-lumen endotracheal tube connector and the anesthesia circuit. All patients received topical anesthesia to the posterior pharynx with two or three puffs of 10% lidocaine spray (10 mg/puff).
Anesthesia induction was performed by inhalational sevoflurane in 100% oxygen using a face mask connected to the Mapleson F circuit. Sevoflurane concentration was gradually increased from 1% to 6% until loss of consciousness and excitatory movements occurred. Following the insertion of a peripheral intravenous cannula, 1% propofol 0.5 mg/kg and fentanyl 1 μg/kg were slowly administered intravenously.[14] To maintain deep sedation throughout the procedure, a 100 μg/kg/min infusion of propofol was administered intravenously. The rate of propofol infusion was modified to keep the Richmond Agitation Sedation Scale (RASS) score of −4.
The patients were randomly allocated to two groups according to the methods of oxygen administration. The allocation was concealed by using sealed, opaque envelopes with a 1:1 allocation ratio (25 patients per group). Due to the nature of the study, the trial was conducted in a nonblinded manner. To guarantee the lowest level of bias, patients were enrolled in treatment assignments by an independent staff member who was not otherwise associated with the trial.
In group A (intervention group), the correct sizes of double well-lubricated (using lidocaine gel) NPAs were bilaterally inserted via nostrils once the patients had attained deep level of sedation (i.e., stoppage of active body movements). The correct size of the NPA was identified by measuring from the patient’s ala nasi to the tip of the earlobe. Maximum fitting diameter of the airway was used. To ensure this, we selected a size that was close to the diameter of the patient’s little finger. NPAs were advanced carefully with the bevel directed toward the nasal septum, along the floor of the nasopharynx until the flanges rested against the nostrils. The correct placement of the NPA should be distal to the soft palate and proximal to the epiglottis. NPAs were then attached to endotracheal tube connectors of the same size, which were subsequently coupled to a double-lumen endotracheal tube connector, enabling a simple connection to the anesthesia circuit [Figure 1]. At a rate of 5 l/min, 100% oxygen was supplied. Spontaneous ventilation was allowed, with periods of intermittent manual-assisted ventilation provided as needed.
Figure 1.
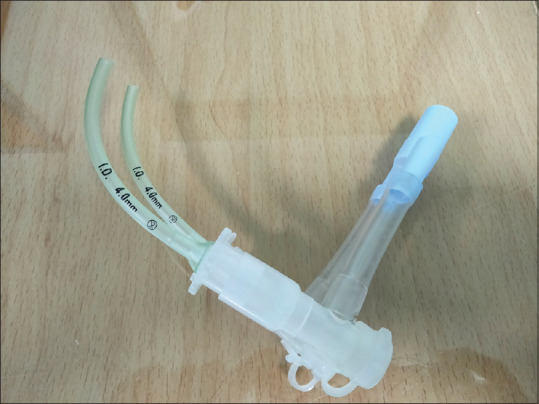
The bilateral NPA device. NPA = nasopharyngeal airway
In group B (control group), the patients received low-flow 100% supplemental oxygen (5 l/min) via a nasal cannula (pediatric size) throughout the endoscopy procedure.
The patients were then placed in the lateral decubitus position after the system’s proper operation was established. An endoscopic examination was conducted after the endoscope was introduced with the help of a mouth opener.
The technique was considered successful when the procedure could be completed without patients’ movement and respiratory complications, with an SpO2 of more than 92%. The duration of the procedure was calculated from the start of anesthesia until the recovery of consciousness. The gastroenterologist’s satisfaction was assessed at the end of the procedure using a four-point rating scale (1 = highly satisfactory, 2 = satisfactory, 3 = somewhat satisfactory, 4 = unsatisfactory).[15]
In both groups, certain respiratory measures were taken to help respiratory recovery, including head tilt, chin lift, and jaw thrust for airway obstruction, or bag–mask ventilation in case of apnea.
Statistical analysis
The sample size was calculated using Number Cruncher Statistical Systems Power Analysis and Sample Size (NCSS PASS) 11.0 and was based on a study carried out by Klotz et al.[16] Group sample sizes of 25 patients in group A and 25 patients in group B achieved 81% power to detect noninferiority using a one-sided, two-sample t-test. The margin of noninferiority was −0.010. The true difference between the means was assumed to be 0.430. The significance level (alpha) of the test was 0.05000. The data were drawn from populations with standard deviations of 0.600 and 0.500.
The Statistical Package for Social Science was used to conduct statistical analysis (IBM SPSS Statistics for Windows, version 23.0; IBM Corp., Armonk, NY, USA). Numbers and percentages were used to represent qualitative variables. Mean and standard deviation were used to express quantitative variables. The Kolmogorov–Smirnov test was used to determine whether the distribution was normal. For comparisons of continuous variables, we used nonparametric Mann–Whitney U tests or independent t-tests as necessary. Fisher’s exact test was also used to compare categorical variables. A two-tailed P value of 0.05 was used to define statistical significance.
Results
From June to December 2022, 50 eligible patients scheduled for elective EGD were screened and enrolled in the study. The patients were randomly assigned to NPA or LFNC groups. No patients were excluded from the trial. Therefore, all enrolled and randomized patients could be followed up for the primary outcome analysis [Figure 2].
Figure 2.
The Consolidated Standards of Reporting Trials (CONSORT) flow diagram
When comparing the patient characteristics in the two groups, there was no statistically significant difference in terms of age, sex, weight, height, body mass index (BMI), or ASA physical status (P > 0.05) [Table 1]. The procedure duration was 20.92 ± 4.75 min in group A versus 25.68 ± 5.72 min in group B (P = 0.193) [Table 1]. The total doses of fentanyl and propofol utilized throughout the procedure did not differ significantly between the two groups [Table 1].
Table 1.
Patients’ characteristics
| Variables | Group A (n=25) | Group B (n=25) | P |
|---|---|---|---|
| Age (years) | 4.59 (2.50) | 5.84 (3.50) | 0.552 |
| Sex (male/female) | 12/13 | 15/10 | 0.347 |
| Weight (kg) | 17.19 (7.07) | 18.22 (6.86) | 0.120 |
| Height (cm) | 102.72 (18.06) | 108.12 (17.91) | 0.431 |
| BMI (kg/m2) | 15.93 (1.82) | 15.07 (1.26) | 0.401 |
| ASA (I/II) | 15/10 | 16/9 | 0.627 |
| Total propofol dose (mg) | 15.72 (4.59) | 14.92 (4.68) | 0.629 |
| Total fentanyl dose (µg) | 17 (7.03) | 18.08 (6.86) | 0.157 |
| Procedure duration (min) | 20.92 (4.75) | 25.68 (5.72) | 0.193 |
| Indications for esophagogastroduodenoscopy | |||
| Dysphagia | 3 (12%) | 4 (16%) | 0.188 |
| Unexplained anemia | 6 (24%) | 7 (28%) | |
| Unexplained diarrhea | 5 (20%) | 5 (20%) | |
| Abdominal pain | 7 (28%) | 4 (16%) | |
| Weight loss | 4 (16%) | 5 (20%) |
ASA=American Society of Anesthesiologists, BMI=body mass index, SD=standard deviation. Data are expressed as mean (SD) or number (%). P>0.05 is considered nonsignificant
In group A, 4/25 (16%) patients suffered from episodes of O2 desaturation versus 9/25 (36%) patients in group B (P = 0.634) [Table 2]. In group A, two patients (8%) required head tilt and chin lift, two patients (8%) required jaw thrust, and no patient (0%) required bag–mask ventilation. On the other hand, in group B, four patients (16%) required head tilt and chin left, three patients (12%) required jaw thrust, and two patients (8%) required bag–mask ventilation. The difference between both groups was statistically nonsignificant (P = 0.539). The lowest SpO2 during the procedure (%) was 94.20 (3.12) in the NPA group versus 91.64 (4.09) in the LFNC group. The difference between both groups was statistically nonsignificant (P = 0.168) [Table 2].
Table 2.
Operative and postoperative complications
| Variables | Group A (n=25) | Group B (n=25) | P |
|---|---|---|---|
| Hypoxic attacks | 4 (16%) | 9 (36%) | 0.634 |
| Lowest SpO2 during the procedure (%) | 94.20 (3.12) | 91.64 (4.09) | 0.168 |
| Airway intervention | 4 (16%) | 9 (36%) | 0.539 |
| Head tilt, chin lift | 2 (8%) | 4 (16%) | |
| Jaw thrust | 2 (8%) | 3 (12%) | |
| Bag–mask ventilation | 0 (0%) | 2 (8%) | |
| PONV | 3 (12%) | 5 (20%) | 0.558 |
| Nasopharyngeal injury | 2 (8%) | 1 (4%) | 0.775 |
| Intraoperative bradycardia | 2 (8%) | 4 (16%) | 0.540 |
| Intraoperative hypotension | 3 (12%) | 4 (16%) | 0.442 |
Data are expressed as mean (SD) or numbers (%). P>0.05 is considered nonsignificant. PONV=postoperative nausea and vomiting, SD=standard deviation, SpO2=oxygen saturation
There were no statistically significant differences in the incidence of postoperative nausea and vomiting (PONV), bradycardia, and hypotension between both groups (P > 0.05) [Table 2].
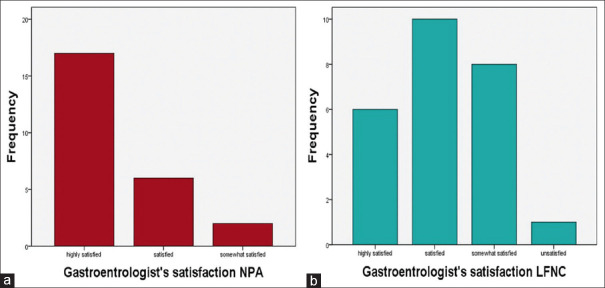
In group A, a nasopharyngeal injury occurred in 2/25 patients (8%), while only one patient (4%) in group B had a mild nasal injury that could not be directly linked to the nasal cannula but rather to the preexisting nasal crust (P = 0.775) [Table 2]. The nasal injuries in both groups were minimal and did not require any medical treatment. Group A’s score for gastroenterologists’ satisfaction was statistically higher than group B’s score (P = 0.003) [Figure 3].
Figure 3.
(a) Gastroenterologist’s satisfaction in the NPA group. (b) Gastroenterologist’s satisfaction in the LFNC group. LFNC = low-flow nasal cannula, NPA = nasopharyngeal airway
Discussion
This is a prospective, randomized, controlled, nonblinded, noninferiority, single-center trial comparing hypoxemic and apneic events during deep sedation for pediatric upper endoscopy procedures between two oxygenation methods – bilateral modified NPA and conventional LFNC.
The study aimed to offer an alternative oxygenation technique to shared airway procedures under deep sedation. This study will interest those who practice deep sedation.
The authors did not find a statistically significant difference in the number of patients who developed hypoxemic events (SpO2 < 90%) – 16% in the NPA group compared to 36% in the LFNC group. It may be a clinical significance in the difference in the proportion, but statistically, it is not significant. Apnea or airway obstruction can result in hypoxemia. Apnea in group A was managed with manually assisted ventilation, whereas in group B, apnea was managed with procedure interruption and bag and mask ventilation. Due to the splinting action of NPA in group A, hypoxemia from airway obstruction was more common in group B. NPA and LFNC are compared as both are considered oxygenation methods. No patient in either group required endotracheal intubation. The unprotected airway was guarded by proper preoperative fasting and limiting the endoscopy for the diagnostic purpose only without intervention.
Sedation is always required during pediatric GI endoscopy with special concerns about patient safety, comfort, and tolerance to the procedure, as well as providing good circumstances for the performance of the procedure.[17] Alongside the increased complexity of the diagnostic and therapeutic endoscopic procedure, a deep level of sedation is usually needed in pediatric gastrointestinal tract (GIT) endoscopic procedures.[18,19]
Pediatric patients are more vulnerable to respiratory complications during GIT endoscopic procedures, especially upper GI endoscopy, due to several factors, including but not limited to higher lung resistance, deeper level of sedation, anesthetic agents used (like propofol), and the procedure itself.
Oxygen supplementation during the pediatric endoscopic procedure is mandatory, but the best method to maintain adequate patient oxygenation and ventilation and ensure airway patency is still debatable.
Previous studies confirmed a higher incidence of hypoxemia during sedated digestive endoscopy with O2 supplementation through a standard nasal cannula.[20] Although many airway devices were used to maintain oxygenation and ventilation during GI endoscopic procedures, most of such devices were designed for adult populations or bigger children, like the gastrolaryngeal tube or LMA Gastro Airway (not recommended for patients weighing <30 kg),[21] or failed to increase respiratory stability in pediatric patients as High-flow nasal cannula (HFNC).[16]
Beattie[22] used a modified nasal airway in 35 adult patients who could not intubate/ventilate and who were with or without muscle relaxants, with successful ventilation and intubation and with fiberoptic bronchoscope assistance in all cases. He described that positive pressure ventilation was applied with an anesthesia bag, while the other nostril was occluded and the mouth and lips were closed tightly.
There was no statistically significant difference between the groups in the incidence of bradycardia, hypotension, and PONV. However, a previous study could demonstrate that in sedated patients, the pressure response following the insertion of NPAs was substantially higher. Mechanical stimulation of the nasopharynx, nose, or both may be the cause.[23] PONV could result from upper GI endoscopic complications or could be caused by the insertion of NPA.
The NPA group had a shorter procedure duration (20.92 [4.75] min vs. 25.68 [5.72] min). This can be explained by the fact that group B required stoppage of the procedure to perform airway support by mask ventilation to manage apnea and hypoxia.
Gastroenterologists’ satisfaction was statistically higher in the NPA group. This may be related to less procedural interruption resulting in shorter procedure duration and to a lower clinical incidence of hypoxemia. According to the literature, NPA was associated with a higher incidence of nasal damage. However, we detected a minor risk for nasopharyngeal injury with the application of soft, well-lubricated NPA in our trial. This might be because of the small sample size. Future research could lead to the creation of a device that could be used for any minor procedure requiring deep sedation, consisting of double NPA of various diameters connected to an oxygen catheter.
The lack of availability of successful airway tools during GI endoscopy attracts our attention to describe a new airway device possessing the advantage of maintaining a patent airway and allowing intermittent assisted ventilation if required. This piece of equipment has been described in a case report in 2021[24] as a successful oxygenation device used in a child with a difficult airway due to massive neck swelling requiring anesthesia. The characteristics of the device are original modifications of otherwise commonly accessible equipment, including NPAs, endotracheal connectors, the ability to attach anesthetic circuits, and the delivery of pressure-assisted breathing. This device will not change practice, but offers an alternative technique for oxygenation in this setting.
We used bilateral rather than unilateral NPA to allow more airway sealing, which helps in assisting ventilation. As the position of the bilateral NPA might be displaced by operator manipulations, which might occasionally cause airway obstruction, jaw thrust and head extension might be required in the NPA group.
Our study was limited by the difficulties in recruiting a larger number of patients. Larger sample sizes are needed to show the superiority of bilateral NPA over LFNC. Unfortunately, we did not use a bispectral index (BIS) monitor for the depth of sedation. Instead, we used a subjective assessment tool. Another limitation was that we did not record how many hypoxemic episodes each patient experienced. To validate our results, this airway device should be compared to other airway devices.
Conclusion
Routine use of double NPA in children undergoing endoscopic sedation was noninferior in the incidence of hypoxemia, airway intervention, and gastroenterologist satisfaction, compared to conventional LFNC.
Key message
The application of a bilateral nasopharyngeal airway device was comparable to the nasal cannula in eliminating periods of hypoxia and procedure interruption.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Scottish Intercollegiate Guidelines Network. SIGN guideline 58 Safe sedation of children undergoing diagnostic and therapeutic procedures. Paediatr Anaesth. 2008;18:11–12. doi: 10.1111/j.1460-9592.2007.02405.x. [DOI] [PubMed] [Google Scholar]
- 2.Jun J, Han JI, Choi AL, Kim YJ, Lee JW, Kim DY, et al. Adverse events of conscious sedation using midazolam for gastrointestinal endoscopy. Anesth Pain Med. 2019;14:401–6. doi: 10.17085/apm.2019.14.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malviya S, Voepel-Lewis T, Tait AR. Adverse events and risk factors associated with the sedation of children by non-anesthesiologists. Anesth Analg. 1997;85:1207–13. doi: 10.1097/00000539-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Standards of Practice Committee. Lichtenstein DR, Jagannath S, Baron TH, Anderson MA, Banerjee S, et al. Sedation and anesthesia in GI endoscopy. Gastrointest Endosc. 2008;68:815–26. doi: 10.1016/j.gie.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 5.American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004–17. doi: 10.1097/00000542-200204000-00031. [DOI] [PubMed] [Google Scholar]
- 6.Vargo JJ, Cohen LB, Rex DK, Kwo PY, et al. American Association for the Study of Liver Diseases;American College of Gastroenterology. Position statement: Nonanesthesiologist administration of propofol for GI endoscopy. Gastroenterology. 2009;137:2161–7. doi: 10.1053/j.gastro.2009.09.050. [DOI] [PubMed] [Google Scholar]
- 7.Cai G, Huang Z, Zou T, He M, Wang S, Huang P, et al. Clinical application of a novel endoscopic mask: A randomized controlled trial in aged patients undergoing painless gastroscopy. Int J Med Sci. 2017;14:167–72. doi: 10.7150/ijms.16919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orfei P, Ferri F, Panella I, Meloncelli S, Patrizio AP, Pinto G. The use of laryngeal mask airway in esophagogastroduodenoscopy in children. Minerva Anestesiol. 2002;68:77–82. [PubMed] [Google Scholar]
- 9.Tran A, Thiruvenkatarajan V, Wahba M, Currie J, Rajbhoj A, van Wijk R, et al. LMA® Gastro™Airway for endoscopic retrograde cholangiopancreatography: A retrospective observational analysis. BMC Anesthesiol. 2020;20:113. doi: 10.1186/s12871-020-01019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fabbri C, Luigiano C, Cennamo V, Polifemo AM, Maimone A, Jovine E, et al. The gastro-laryngeal tube for interventional endoscopic biliopancreatic procedures in anesthetized patients. Endoscopy. 2012;44:1051–4. doi: 10.1055/s-0032-1310159. [DOI] [PubMed] [Google Scholar]
- 11.Dimou F, Huynh S, Dakin G, Pomp A, Turnbull Z, Samuels JD, et al. Nasal positive pressure with the SuperNO2VA™device decreases sedation-related hypoxemia during pre-bariatric surgery EGD. Surg Endosc. 2019;33:3828–32. doi: 10.1007/s00464-019-06721-1. [DOI] [PubMed] [Google Scholar]
- 12.Swaika S, Ghosh S, Bhattacharyya C. Airway devices in paediatric anaesthesia. Indian J Anaesth. 2019;63:721–28. doi: 10.4103/ija.IJA_550_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts K, Whalley H, Bleetman A. The nasopharyngeal airway: Dispelling myths and establishing the facts. Emerg Med J. 2005;22:394–6. doi: 10.1136/emj.2004.021402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butterworth IV JF, Mackey DC, Wasnick JD. Pediatric anesthesia. In: Butterworth IV JF, Mackey DC, Wasnick JD, editors. Morgan and Mikhail's Clinical Anesthesiology. 6th ed. ch 42. McGraw Hill; 2018. pp. 1517–67. [Google Scholar]
- 15.Abu-Shahwan I, Mack D. Propofol and remifentanil for deep sedation in children undergoing gastrointestinal endoscopy. Paediatr Anaesth. 2007;17:460–3. doi: 10.1111/j.1460-9592.2006.02132.x. [DOI] [PubMed] [Google Scholar]
- 16.Klotz D, Seifert V, Baumgartner J, Teufel U, Fuchs H. High-flow nasal cannula vs standard respiratory care in pediatric procedural sedation: A randomized controlled pilot trial. Pediatr Pulmonol. 2020;55:2706–12. doi: 10.1002/ppul.24975. [DOI] [PubMed] [Google Scholar]
- 17.Van Beek EJ, Leroy PL. Safe and effective procedural sedation for gastrointestinal endoscopy in children. J Pediatr Gastroenterol Nutr. 2012;54:171–85. doi: 10.1097/MPG.0b013e31823a2985. [DOI] [PubMed] [Google Scholar]
- 18.Torino A, Martino DD, Fusco P, Collina U, Marullo L, Ferraro F. Hot topics in airway management during gastrointestinal endoscopy. J Gastrointest Dig Syst. 2016;6:377. [Google Scholar]
- 19.Bhavani SS, Abdelmalak B. Nonoperating Room Anesthesia: Anesthesia in the Gastrointestinal Suite. Anesthesiol Clin. 2019;37:301–6. doi: 10.1016/j.anclin.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Zhang YX, He XX, Chen YP, Yang S. The effectiveness of high-flow nasal cannula during sedated digestive endoscopy: A systematic review and meta-analysis. Eur J Med Res. 2022;27:30. doi: 10.1186/s40001-022-00661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor CL, Wilson SR, Burgoyne LL, Endlich Y. LMA® Gastro™: A paediatric experience. Anaesth Intensive Care. 2021;49:119–24. doi: 10.1177/0310057X20981591. [DOI] [PubMed] [Google Scholar]
- 22.Beattie C. The modified nasal trumpet maneuver. Anesth Analg. 2002;94:467–9. doi: 10.1097/00000539-200202000-00045. [DOI] [PubMed] [Google Scholar]
- 23.Tong JL, Smith JE. Cardiovascular changes following insertion of oropharyngeal and nasopharyngeal airways. Br J Anaesth. 2004;93:339–42. doi: 10.1093/bja/aeh207. [DOI] [PubMed] [Google Scholar]
- 24.Dogra N, Sharma A, Sidhu B. Modified double nasopharyngeal airway used with a double-lumen connector: A case report. Pediatr Anaesth. 2021;31:1364–5. doi: 10.1111/pan.14296. [DOI] [PubMed] [Google Scholar]