To the Editor:
In March 2024, highly pathogenic avian influenza (HPAI) virus H5N1 clade 2.3.4.4b was detected in mammalian livestock, mostly dairy milking cattle, in the USA, and has now been reported in dairy herds in nine states1. High infectious virus titers and genome copies of H5N1 virus have been observed in milk from these infected dairy cows.2 Molecular testing has also revealed the presence of H5N1 genetic material in ~20% of samples obtained from retail pasteurized milk products, but researchers have been unable to culture virus from these samples3. Here, we measure the stability of H5N1 virus in raw milk at 63 and 72°C, the temperatures used most commonly in commercial pasteurization4.
We diluted HPAI H5N1 virus A/mountain lion/MT/1/2024 (clade 2.3.4.4b, EPI_ISL_19083124) in raw (unpasteurized) cow milk to 106 TCID50/ml. We heat-treated the milk in a temperature-controlled heat-block at 63°C and 72°C. We quantified infectious virus via end-point titration in MDCK cells, using tenfold serial dilutions. H5N1 genome copies were determined by qRT-PCR.
We inferred individual titers and virus half-lives in a Bayesian framework as previously described5 (Supplement) modeling the virus titers observed by hemagglutination assay as Poisson-distributed. At 63°C, H5N1 virus was inactivated from initial titers of 106 TCID50/ml to undetectable within 2 minutes (Fig 1a). We estimated the half-life of infectious virus to be 4.5 [95% CrI 3.5–5.8] seconds at 63°C (Fig 1b, Table S1). At 72°C, we observed a drop from ~105 to ~101 TCID50/mL within 5 seconds, and then very low titers (<10 TCID50/mL, at the boundary of detectability) out to 20 seconds, and no viable virus at longer time points (Fig 1a). These data are not consistent with simple exponential decay, so we do not report a half-life. At both temperatures, H5N1 virus genome copy numbers measured by qPCR dropped less than one log over 30 minutes at 63°C or 15 seconds at 72°C (Figure S1).
Figure 1. Inactivation of H5N1 influenza virus in raw milk at 63°C and 72°C.
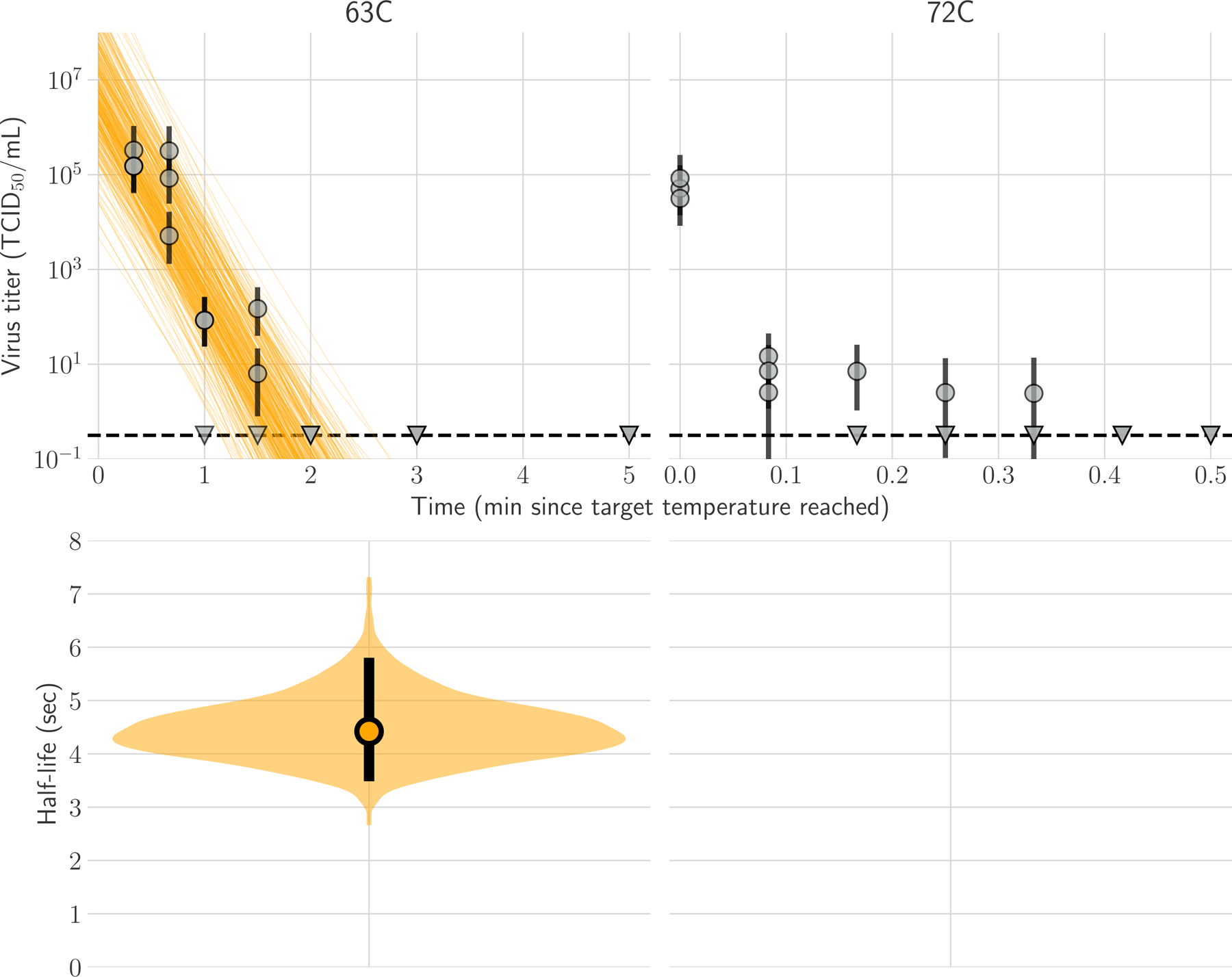
In panel A, regression plots indicate the predicted decay of virus titer over time; the titer is plotted on a logarithmic scale. Points show posterior median infectious titer values estimated from each replicate sample, and black bars show 95% credible intervals. Lines are random draws from the joint posterior distribution of the exponential decay rate (negative of the slope) and intercept (initial virus titer) to show the range of possible decay patterns for each experimental condition. There were 150 lines per panel, including 50 lines from each plotted replicate. The dashed lines indicate the limit of detection, which was 100.5 TCID50/mL of medium. In Panel B, violin plot indicates the posterior distribution for the half-life of viable virus based on the estimated exponential decay rates of the virus titer. The dot indicates the posterior median estimates, and the black line indicates the 95% credible interval; the width of the orange area indicates the posterior support for particular values of the half-life.
We emphasize that these measurements reflect experimental conditions, and direct measurements must be made of infected milk in commercial pasteurization equipment. Given our findings, however, heat treatment at 63°C would yield a 1010-fold decrease in infectious viral titer within 2.5 minutes [95% CrI 1.9–3.2 minutes], so standard bulk pasteurization of 30 minutes at 63°C has a large safety buffer. At 72°C, we measured a 104-fold decrease in infectious virus within 5 seconds; however, we also detected infectious virus just above the assay limit of detection after up to 20 seconds of heat treatment. This finding indicates the potential for a relatively small but detectable quantity of H5N1 virus to remain infectious in milk after 15 seconds at 72°C, if the initial titer is sufficiently high.
Our findings highlight the need for research on H5N1 influenza virus in dairy production. Replication of these findings and extension to other dairy products is needed including the study of milk from infected dairy cows with commercial pasteurization equipment, because treatment conditions can impact the effectiveness of heat inactivation5. One limitation of the current study is the use of raw-milk samples spiked with H5N1 virus, whereas raw milk from infected cows may have a different composition or contain cell-associated virus which might impact inactivation kinetics. Lastly, although gastrointestinal infections with HPAI H5N1 virus are reported in several mammalian species2, the dose-dependence of the probability of human infection via ingestion of HPAI H5N1 virus in milk is an unknown.
Supplementary Material
Acknowledgments
Supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH). JOL-S was supported by the U.S. National Science Foundation (DEB-2245631).
Footnotes
This is an Author Accepted Manuscript, which is the version after external peer review and before publication in the Journal. The publisher’s version of record, which includes all New England Journal of Medicine editing and enhancements, is available at https://www.nejm.org/doi/full/10.1056/NEJMc2405488.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
Contributor Information
Franziska Kaiser, National Institute of Allergy and Infectious Diseases, Hamilton, MT
Dylan H. Morris, University of California, Los Angeles, Los Angeles, CA
Arthur Wickenhagen, National Institute of Allergy and Infectious Diseases, Hamilton, MT
Reshma Mukesh, National Institute of Allergy and Infectious Diseases, Hamilton, MT
Shane Gallogly, National Institute of Allergy and Infectious Diseases, Hamilton, MT
Kwe Claude Yinda, National Institute of Allergy and Infectious Diseases, Hamilton, MT
Emmie de Wit, National Institute of Allergy and Infectious Diseases, Hamilton, MT
James O. Lloyd-Smith, University of California, Los Angeles, Los Angeles, CA
Vincent J. Munster, National Institute of Allergy and Infectious Diseases, Hamilton, MT
References
- 1.CDC. Current H5N1 Bird Flu Situation in Dairy Cows. Center for Disease Control and Prevention. 26.April.2024. (https://www.cdc.gov/flu/avianflu/mammals.htm). [Google Scholar]
- 2.Burrough ER MD, Petersen B, Timmermans SJ, Gauger PC, Zhang J, Siepker C, Mainenti M, Li G, Thompson AC, Gorden PJ, Plummer PJ, Main R. Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus Infection in Domestic Dairy Cattle and Cats, United States, 2024. Emerging Infectious Diseases 2024;30. DOI: 10.3201/eid3007.240508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FDA. Updates on Highly Pathogenic Avian Influenza (HPAI). (https://www.fda.gov/food/alerts-advisories-safety-information/updates-highly-pathogenic-avian-influenza-hpai). [Google Scholar]
- 4.Regulations CoF. Mandatory pasteurization for all milk and milk products in final package form intended for direct human consumption. (https://www.ecfr.gov/current/title-21/chapter-I/subchapter-L/part-1240/subpart-D/section-1240.61). [Google Scholar]
- 5.Gamble A, Fischer RJ, Morris DH, Yinda CK, Munster VJ, Lloyd-Smith JO. Heat-Treated Virus Inactivation Rate Depends Strongly on Treatment Procedure: Illustration with SARS-CoV-2. Appl Environ Microbiol 2021;87(19):e0031421. (In eng). DOI: 10.1128/aem.00314-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


