ABSTRACT
Objective
To address the current practice of liberating patients from invasive mechanical ventilation in pediatric intensive care units, with a focus on the use of standardized protocols, criteria, parameters, and indications for noninvasive respiratory support postextubation.
Methods
Electronic research was carried out from November 2021 to May 2022 in Ibero-American pediatric intensive care units. Physicians and respiratory therapists participated, with a single representative for each pediatric intensive care unit included. There were no interventions.
Results
The response rate was 48.9% (138/282), representing 10 Ibero-American countries. Written invasive mechanical ventilation liberation protocols were available in only 34.1% (47/138) of the pediatric intensive care units, and their use was associated with the presence of respiratory therapists (OR 3.85; 95%CI 1.79 - 8.33; p = 0.0008). The most common method of liberation involved a gradual reduction in ventilatory support plus a spontaneous breathing trial (47.1%). The mean spontaneous breathing trial duration was 60 - 120 minutes in 64.8% of the responses. The presence of a respiratory therapist in the pediatric intensive care unit was the only variable associated with the use of a spontaneous breathing trial as the primary method of liberation from invasive mechanical ventilation (OR 5.1; 95%CI 2.1 - 12.5). Noninvasive respiratory support protocols were not frequently used postextubation (40.4%). Nearly half of the respondents (43.5%) reported a preference for using bilevel positive airway pressure as the mode of noninvasive ventilation postextubation.
Conclusion
A high proportion of Ibero-American pediatric intensive care units lack liberation protocols. Our study highlights substantial variability in extubation readiness practices, underscoring the need for standardization in this process. However, the presence of a respiratory therapist was associated with increased adherence to guidelines.
Keywords: Respiration, artificial, Intensive care units, pediatric, Airway extubation, Noninvasive ventilation, Surveys and questionnaires
INTRODUCTION
Between 35.7 and 55% of patients admitted to pediatric intensive care units (ICUs) receive invasive mechanical ventilation (MV).( 1 - 4 ) Even in the context of various critical pathological processes, early liberation from invasive respiratory support remains a priority objective to avoid risks, complications, and increased health costs associated with prolonged invasive MV.( 5 - 8 )
Despite the broad consensus on minimizing invasive MV duration, the scarcity of evidence on pediatric liberation methods contributes to significant variability in practice.( 9 - 11 ) The first International Clinical Practice Guidelines for Pediatric Ventilator Liberation in Pediatrics were recently published. Three core recommendations emphasize the use of standardized protocols to identify patients who meet the criteria for liberation from the ventilator and the application of a bundle of measures to confirm readiness for extubation. These measures should include a spontaneous breathing trial (SBT).( 12 )
Although the implementation of these guidelines may contribute to standardizing liberation from MV in children, they have only recently begun to be disseminated, and previous studies have revealed substantial variability in care practices.( 1 - 5 , 7 - 11 )
We designed and conducted a survey with the general objective of examining current clinical practices related to liberation from invasive MV in Ibero-American pediatric ICUs. The specific objectives included the following: identify the use of standardized protocols; analyze the criteria and parameters used during liberation from invasive MV; and evaluate the use of noninvasive ventilation (NIV) postextubation. The survey results enabled us to identify areas for improvement and provide support for the development of future studies that contribute to generating evidence in this important area of intensive care medicine.
METHODS
Design and study population
The survey design was based on a previously published original document( 11 ) that was translated into Spanish, which was subsequently adapted by the Mechanical Ventilation Liberation Group of the Respiratory Committee of the Sociedad Latino-Americana de Cuidados Intensivos Pediátricos (SLACIP). The survey, initially published in Spanish and later translated into Portuguese in autumn 2021, was conducted through virtual meetings and distributed to all Ibero-American countries via email addresses provided by national coordinators affiliated with the Mechanical Ventilation Liberation Group. Each email included an invitation letter, and a reminder email was sent 2 - 4 weeks later. Upon agreeing to participate, a representative from each pediatric ICU completed the questionnaire, specifying their profession and role. The Google Form® platform (Google LLC, Mountain View, CA) was used, which required participants to enter a preassigned entry code.
Survey development
The survey comprised 25 questions related to the process of liberation from MV (Appendix 1 - Supplementary Material) and collected the following: demographic characteristics of the participating pediatric ICUs, including the type of health system, type of hospital and pediatric ICU and number and age range of patients admitted in 2019 (pre-coronavirus disease 2019 [COVID-19] pandemic); information on human resources, including the presence of a respiratory therapist (RT); information on clinical practice, including the definition of the process, the use of a standardized protocol, the use of an extubation readiness test (ERT) or bundle of measures to assess the eligibility of a patient to be liberated from invasive MV, including the SBT type and duration (Appendix 2 - Supplementary Material), the use of other tests, such as the air-leak test, the use of corticosteroids preextubation, the success/failure criteria for liberation, and the timeframe for conducting a new SBT in case of failure; and information on postextubation respiratory support.
All the surveys were completed between November 2, 2021, and May 12, 2022. Surveys lacking demographic data and those identified as high risk for duplicate responses were excluded. Duplications were determined by comparing identical responses regarding the profession of the person responsible for answering the survey, type of hospital, number of annual discharges from each unit and financier of the health care center. Exemption from informed consent was obtained by the Institutional Review Committee of the Hospital Universitario Austral in Buenos Aires (Argentina). This study complied with the ethical standards of the committee responsible for human experimentation, as well as the 1975 Helsinki Declaration, along with its most recent amendments.
Results and operational definitions
The primary outcome was the analysis of current practices related to liberation from MV in children within the Ibero-American critical care setting. This analysis focused on describing the characteristics of the participating pediatric ICUs, availability of human resources, implementation of the liberation process, and postextubation respiratory assistance. Specific details regarding the operational definitions can be found in Appendix 2 (Supplementary Material).
Statistical analysis
A univariate descriptive analysis was conducted. Categorical variables are presented as the absolute quantity (n) and percentage, whereas numerical variables are presented as the mean and standard deviation (SD) or median and interquartile range (IQR), as appropriate for the observed distribution. For comparisons of categorical results, either the chi-square test or Fisher’s exact test was used. For numerical variables, medians were compared via Kruskal-Wallis analysis of variance. A p value less than 0.05 indicated statistical significance. All analyses were performed via R (version 4.2.1; 2018 The R Foundation for Statistical Computing Platform).
RESULTS
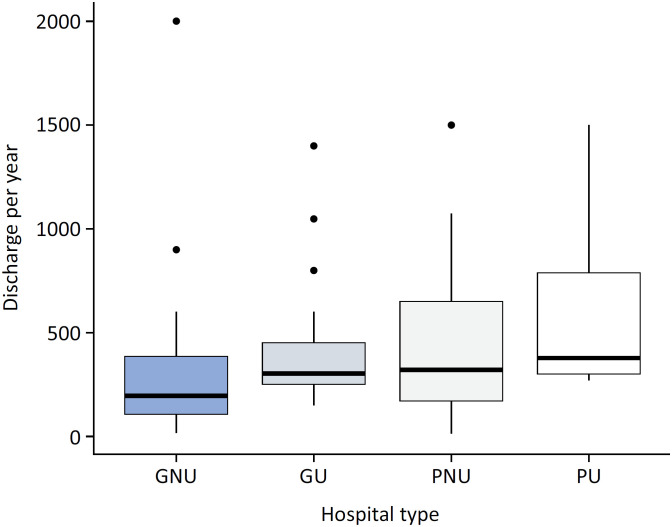
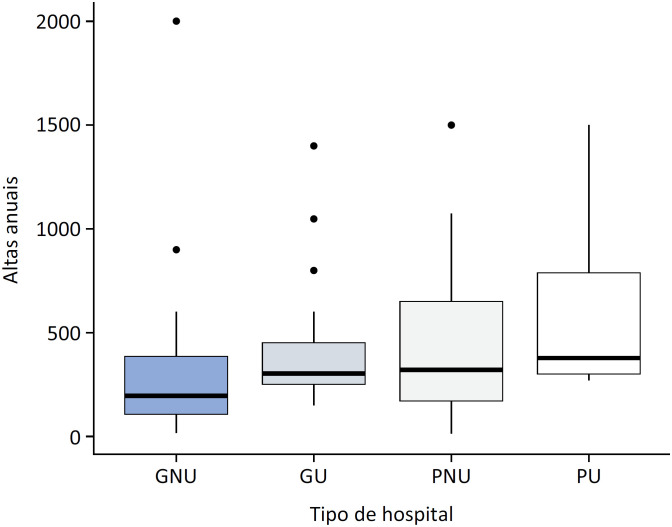
Invitations to participate were sent to 282 pediatric ICUs, and 138 completed surveys were received (response rate of 48.9%). Among the surveys, 91.3% (126/138) were completed by physicians, and 8.7% (12/138) were completed by RTs. Department heads, directors, or coordinators of pediatric ICUs completed 49.3% (68/138) of the surveys. Most of the responders were affiliated with public hospitals (73.9%, 102/38), and pediatric hospitals accounted for almost a third of them (31.2%, 43/138), with 76.8% (106/138) not being affiliated with a university. Among university-affiliated hospitals, 28.1% (9/32) were general hospitals. The pediatric ICU discharge rate was 300 patients/year (IQ 142 - 432, IQR) ( Figure 1 and Table 1 ).
Figure 1. Discharges per year according to the type of hospital.
GNU - general nonuniversity; GU - general university; PNU - pediatric nonuniversity; PU - pediatric university.
Table 1. Summary of key survey questions and response rates.
| Survey questions | Responses % | |
|---|---|---|
| Profession of respondent (n = 138) | ||
| Physician | 126/138 (91.7) | |
| Respiratory therapist | 30/138 (8.3) | |
| Health system to which the institution belongs (n = 138) | ||
| Public | 102/138 (73.9) | |
| Private | 30/138 (21.7) | |
| Oher type | 6/138 (4.3) | |
| Type of institution * (n = 138) | ||
| University | 32/138 (23.2) | |
| General | 65/138 (47.1) | |
| Pediatric | 43/138 (31.2) | |
| Other | 7/138 (5.1) | |
| Hospital classification by university affiliation (n = 32) | ||
| General university | 9/32 (28.1) | |
| Pediatric university | 23/32 (71.9) | |
| Type of pediatric ICU (n = 138) | ||
| Medical-surgical | 95/138 (68.8) | |
| Medical-surgical-cardiovascular | 35/138 (25.4) | |
| Other | 8/138 (5.7) | |
| Respiratory therapist availability (n = 138) | ||
| 24 × 7 | 61/138 (44.2) | |
| Partial (< 12 hours/day) | 58/138 (42) | |
| Not available | 19/138 (13.8) | |
| Method used to assess likelihood of extubation (n = 138) | ||
| SBT only * | 37/138 (26.8) | |
| PS | 26/37 (76.5) | |
| CPAP | 2/37 (5.9) | |
| T-tube | 13/37 (38.2) | |
| GRVS only | 36/138 (26.1) | |
| Both (SBT + GRVS) | 65/138 (47.1) | |
| Written protocol (n = 138) | ||
| Yes | 47/138 (34.1) | |
| No | 91/138 (65.9) | |
| ERT components * (n = 47) | ||
| Daily screening | 35/47 (74.5) | |
| Sedoanalgesia protocol | 31/47 (66) | |
| Criteria for defining ERT failure | 31/47 (66) | |
| SBT | 27/47 (57,4) | |
| Use of preplanned postextubation NIV/CPAP | 19/47 (40.4) | |
| Failure criteria checklist | 15/47 (31.9) | |
| SBT length * (n = 125) | ||
| < 30 minutes | 3/125 (2.4) | |
| 30 minutes | 38/125 (30.4) | |
| 60 - 120 minutes | 81/125 (64.8) | |
| > 120 minutes | 27/125 (21.6) | |
| Other duration | 12/125 (9.6) | |
| Use of an ETT with a cuff (n = 138) | ||
| Yes | 112/138 (81.2) | |
| No | 6/138 (4.3) | |
| Sometimes | 20/138 (14.5) | |
| Protocol-based ETT cuff pressure monitoring (n = 112) | ||
| Yes | 83/112 (74.1) | |
| No | 29/112 (25.9) | |
| Corticosteroids to prevent UAO (n = 125) | ||
| All patients | 49/125 (39.2) | |
| Patients at high risk of UAO | 70/125 (56) | |
| No indication | 3/125 (2.4) | |
| Unknown | 3/125 (2.4) | |
ICU - intensive care unit; SBT - spontaneous breathing trial; PS - pressure support; CPAP - continuous positive airway pressure; GRVS - gradual reduction of ventilatory support; ERT - extubation readiness testing; NIV - noninvasive ventilation, ETT - endotracheal tube; UAO - upper airway obstruction.
Multiple responses were allowed.
In terms of pediatric ICU characteristics, 68.8% (95/138) were identified as medical-surgical ICUs, and 25.4% (35/138) were identified as medical-surgical-cardiovascular ICUs. The youngest age at admission was 30 days of age in 64.5% (89/138) of the units, whereas in 29.7% (41/138) of the pediatric ICUs, the minimum age was 7 days. The oldest age at admission was 18 years in 47.1% (65/138), 16 years in 24.7% (34/138) and 14 years in 28.3% (39/138) of the pediatric ICUs.
With respect to the availability of RTs in pediatric ICUs, 44.2% (61/138) reported 24-hour availability, 7.2% (10/138) reported 12-hour availability, and 10.9% (15/138) reported 8-hour availability. In 23.9% (33/138) of the pediatric ICUs, RTs were only accessible for interconsultation, whereas 14% (19/138) of the pediatric ICUs did not have any RT availability.
Protocols for liberation from invasive mechanical ventilation: extubation readiness testing
Only 47 of the included pediatric ICUs (34.1%, 47/138) reported having a written protocol for liberation from invasive MV. The elements included in the ERT were a sedation and analgesia protocol (66%), criteria for defining ERT failure (66%), a standardized spontaneous ventilation test (57.4%), preestablished criteria for NIV or high-flow nasal cannula (HFNC) postextubation support (40.4%) and an extubation failure checklist (31.9%).
Other measures included in the ERT were the presence of a cough reflex (83.1%), swallowing (41.5%) and muscle strength (26.2%). A total of 66.9% of the professionals reported performing leak tests as part of the ERT via endotracheal tubes (ETTs) with deflated cuffs. With respect to the utilization of cuffed ETTs, a necessary condition for conducting the leak test, the prevalence was greater (91.5%) in units with an in-service RT than in those without (70.1%) an in-service RT (p = 0.0027).
In terms of corticosteroid usage, 56% of the professionals reported prescribing corticosteroids exclusively for high-risk patients prone to airway obstruction, whereas 39.2% reported prescribing corticosteroids exclusively for all patients. Additionally, 42.4% reported the routine administration of nebulized epinephrine.
Extubation readiness testing in pediatric ICUs was significantly associated with the presence of an in-service RT and MV liberation protocols (p = 0.0008; odds ratio [OR] 3.85; 95%CI 1.79 - 8.33). This association was not affected by the type of hospital (OR 3.70; 95%CI 1.72 - 8.33) or the type of pediatric ICU (OR 4.00; 95%CI 1.85 - 8.33).
Methods for evaluating the ability to ventilate spontaneously
The most common method for assessing extubation readiness was the gradual reduction of ventilatory support (GRVS) with an SBT (47.1%). An SBT alone was reported for 26.8% of the ICUs, and the GRVS alone was reported for 26.1%. The respondents could select more than one option for the method and duration. Among those who reported only SBTs, 47.1% used pressure support (PS) over positive end-expiratory pressure (PEEP), 38.2% used a T-piece, 29.4% used PS according to the diameter of the ETT, and 5.9% used continuous positive airway pressure (CPAP). Among the units that preferred the GRVS, 48.6% favored a gradual reduction in PS, 42.9% preferred a gradual reduction in the respiratory rate while on synchronized intermittent mandatory ventilation (SIMV), and 40% opted for volume support ventilation (VSV) ( Table 2 ).
Table 2. Methods for evaluating spontaneous ventilation ability.
| Methods | n (%) | IC95% | |
|---|---|---|---|
| Evaluation of the ability to breathe spontaneously * (n = 138) | |||
| GRVS + SBT | 65 (47.1) | 38.6 - 55.8 | |
| SBT | 37 (26.8) | 19.6 - 35.0 | |
| GRVS | 36 (26.1) | 19.0 - 34.2 | |
| Methods for SBT † (n = 37) | |||
| PS above PEEP | 16 (43.2) | 27.1 - 60.5 | |
| T-piece | 13 (27.0) | 20.2 - 52.5 | |
| PS according to the ETT diameter | 10 (27.0) | 13.8 - 44.2 | |
| CPAP | 2 (5.4) | 0.7 - 18.2 | |
| Methods for the GRVS † # n = 35 ‡ | |||
| Gradual reduction of PS | 17 (48.6) | 30.4 - 64.5 | |
| Respiratory rate gradual reduction while on SIMV | 15 (42.9) | 25.5 - 59.2 | |
| VSV | 14 (40.0) | 23.1 - 56.5 | |
| Others | 1 (2.8) | 0.7 - 14.5 | |
IC95% - 95% confidence interval; GRVS - gradual reduction in ventilatory support; SBT - spontaneous breathing trial; PS - pressure support; ETT - endotracheal tube; PEEP - positive end-expiratory pressure; CPAP - continuous positive airway pressure; SIMV - synchronized intermittent mandatory ventilation; VSV - volume support ventilation.
Total sample size;
multiple responses were allowed;
the sample size was 36, but one response was missing.
The reported duration of SBTs was follows: 120 minutes (43.2%), 30 minutes (30.4%), 60 minutes (21.6%), above 120 minutes (21.6%), other duration (9.6%) and less than 30 minutes (2.4%). Among the professionals who selected "other duration", seven out of twelve reported that it was based on patient characteristics ( Table 1 ).
The clinical parameters measured during SBTs included pulse oximetry saturation (92.8%), respiratory effort (89.9%), respiratory rate (86.2%), heart rate (82.6%), level of consciousness (82.6%), tidal volume (74.6%), the SpO2/FiO2 ratio (43.5%) and capnography (41.3%). For the second evaluation after the failure of the first SBT, the reported times for a new SBT were "after 24 hours" (69.3%), "after 48 hours" (17.5%), "after 12 hours" (10.2%) and "others" (3.6%).
Methods of evaluating the ability to achieve spontaneous ventilation and its relationship with pediatric intensive care unit characteristics
The distributions of the three types of evaluations did not differ between university-affiliated hospitals and non-university-affiliated hospitals (p = 0.7), between general hospitals and pediatric hospitals (p = 0.6), or among different types of pediatric ICUs. The highest proportion of SBT use (55.6%) was recorded in the subgroup of university-affiliated pediatric hospitals. However, owing to segmentation and the small sample size, this difference was not statistically significant (calculated power: 68%). The only feature that behaved as a good discriminator was RT availability, dichotomized as an RT on duty for 12 - 24 hours versus all others (p = 0.006).
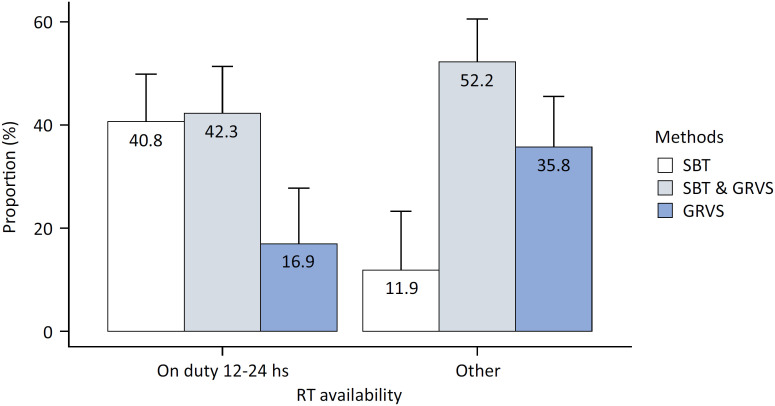
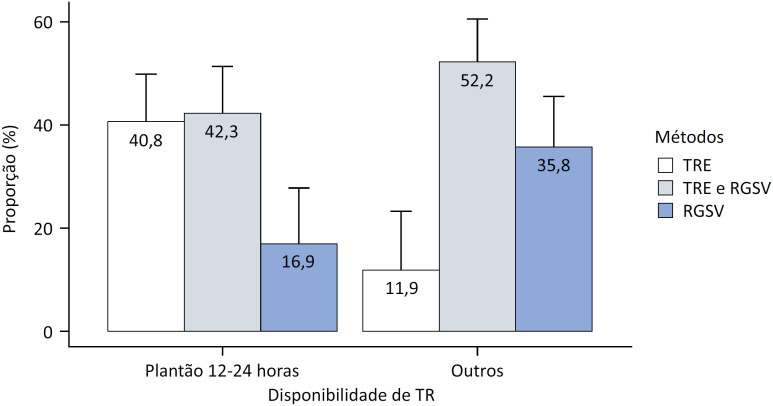
The distribution of methods between units with or without MV liberation protocols was 51.1% versus 14.3% for an SBT only, 14.9% versus 31.9% for the GRVS only, and 34.0% versus 53.8% for an SBT followed by the GRVS, respectively (p = 0.0001). These results did not differ between pediatric and general hospitals. The presence of an on-duty RT was associated with an OR of 5.1 (95%CI 2.1 - 12.5) for choosing an SBT as the main method. This result remained consistent after adjusting for pediatric versus general hospital status (OR 4.5; 95%CI 1.9 - 11.1) and university affiliation (OR 5.0; 95%CI 2.1 -12.5) ( Figure 2 ).
Figure 2. Distribution of methods according to the availability of respiratory therapists.
RT - respiratory therapist; SBT - spontaneous breathing trial; GRVS - gradual reduction of ventilatory support).
Use of postextubation noninvasive ventilation
Preestablished criteria for the indication of noninvasive respiratory support (NRS) were reported to be absent in 40.4% of the patients. The most commonly prescribed rescue therapy was NIV with bilevel positive airway pressure (BiPAP) (43.5%), followed by "at the discretion of the responsible team" (42.8%) and CPAP (4%). No one reported using HFNC as the most common support method.
DISCUSSION
This study is the first to examine the practices used during the process of liberation from invasive MV in the Ibero-American pediatric ICU setting. Only 34.1% (47/138) of the units used a standardized protocol. Nearly half of the units performed the GRVS followed by an SBT, whereas one-third of the units did not use an SBT prior to extubation. The most commonly employed SBT involved PS for 60 - 120 minutes. However, fewer than half of the units with a standardized protocol include criteria for the use of NRS postextubation. The only common characteristic among pediatric ICUs that used a standardized protocol and employed an SBT was the availability of an RT for at least 12 hours per day. The results of this survey highlight the need to standardize clinical practice for MV liberation in pediatric ICUs.
Five randomized clinical trials( 13 - 17 ) and three quality improvement projects( 18 - 20 ) support the use of MV liberation protocols. Additionally, recently published guidelines( 12 , 21 ) recommend adopting a bundle of measures to prepare for extubation or an ERT, aiming to reduce both the duration of MV and the risk of extubation failure. In our study, slightly more than 30% (47/138) of the pediatric ICUs used protocols, a percentage higher than that of 22% reported among 65 pediatric ICUs from 19 European countries,( 22 ) but lower than that of 46.8% reported in a survey among 380 international pediatric ICUs,( 10 ) and that of 57.5% reported in 146 pediatric ICUs in Brazil.( 11 ) The use of a standardized ERT over clinical judgment presents a valuable opportunity to enhance the care of children receiving invasive MV, and the first pediatric clinical practice guidelines on invasive MV liberation should serve as a supportive tool for implementing ERTs.
The results of this survey revealed high heterogeneity among Ibero-American pediatric ICUs. Fewer than one-third of the ICUs were affiliated with university hospitals, and only one-third were exclusively pediatric hospitals. The vast majority were classified as medical-surgical ICUs, with a low proportion being exclusively cardiovascular pediatric ICUs. Neither university affiliation, pediatric hospital affiliation, nor pediatric ICU type was associated with the use of standardized protocols for invasive MV liberation. No significant differences were observed when the cardiovascular pediatric ICUs were separated, considering that the effect of positive pressure may condition the liberation process on the basis of the underlying pathophysiology. The implementation of standardized protocols implies greater involvement of nurses and RTs in the weaning process, including the assessment of sedation.( 23 ) In our survey, the most common characteristic among pediatric ICUs with a standardized protocol was the presence of an RT for at least 12 hours per day. These findings contribute to existing evidence emphasizing the crucial role played by RTs and underscore the importance of multidisciplinary collaboration.( 17 - 20 ) Future multicenter trials should aim to demonstrate how adhering to clinical practice guidelines affects outcomes.
The international guidelines recommend the use of an SBT as part of the standardized liberation protocol. Surprisingly, almost one-third of the surveyed pediatric ICUs do not routinely incorporate SBT into their practices, despite other studies indicating more frequent SBT use in Latin America than in other geographical areas.( 10 ) Previously published studies conducted in Latin America may have already provided sufficient evidence for the widespread adoption of SBTs, including among postcardiovascular surgery patients.( 13 , 24 - 26 ) There is great variability in support methods used during SBTs, with half of the pediatric ICUs reporting the use of PS ranging from 5 - 7cmH2O and 30% reporting the use of a T-tube. Interestingly, the latter was chosen as the method of liberation from MV( 13 , 24 ) despite not being included in the international guidelines.( 12 )
The duration of SBTs varies from 10 to 120 minutes, likely due to the absence of comparative trials in pediatrics.( 27 - 29 ) In our survey, most pediatric ICUs performed SBTs for at least 30 minutes, with potentially longer trials for patients at a higher risk of extubation failure. The most frequently employed duration for SBT falls between 60 and 120 minutes. This is an area where guidelines lack evidence to support the duration of SBTs, and 60 - 120 minute tests are recommended for high-risk patients.( 12 ) Further clinical trials in this regard would be beneficial.
There are limited data on the benefit of postextubation NRS in preventing extubation failure. Noninvasive respiratory support treatment may prolong pediatric ICU and hospital stays, and there is no information in the pediatric literature concerning the use of preventive or rescue NRS. Forty percent of the respondents reported using preestablished criteria to support extubation with NIV or HFNC without being able to determine whether one type of support was superior to the other. An analysis of the postextubation NRS (FIRST-ABC trial) study( 30 ) concluded that HFNC, compared with CPAP, as postextubation support did not meet the noninferiority criterion for MV liberation. This study included patients under one year of age, and the international guidelines recommend CPAP over HFNC as for postextubation NRS in this age group.( 12 )
Our study has several limitations. First, the response rate was 48.9%, which reflects a lack of information from half of the Ibero-American pediatric ICUs. However, this response rate is consistent (44.1 - 52.3%) with that reported for other pediatric surveys that address the same topic.( 9 - 11 ) A higher response rate of 64% was only achieved in the European survey.( 22 ) Second, a common limitation shared with all surveys is that the data obtained rely on self-reports without data verification systems, resulting in estimates of the results in clinical practice. Finally, the heterogeneity of the sample, ranging from highly complex pediatric ICUs to those with limited resources, reflects practices that vary significantly. Nonetheless, we believe that the questionnaire provided a reliable assessment of the practices in each pediatric ICU, given that the completion of the survey was performed by a practitioner with enough knowledge of the liberation process.
CONCLUSION
A large proportion of Ibero-American pediatric intensive care units have not yet adopted extubation readiness testing, including spontaneous breathing trials, into their practice. These findings present an opportunity to optimize the mechanical ventilation liberation process by implementing standardized protocols conducted by a multiprofessional team and supported by new clinical practice guidelines. The presence of a respiratory therapist was associated with increased adherence to guidelines, suggesting a modifiable factor that could enhance patient outcomes during liberation from mechanical ventilation.
SUPPLEMENTAR MATERIAL
REFERENCES
- 1.Farias JA, Fernández A, Monteverde E, Flores JC, Baltodano A, Menchaca A, Poterala R, Pánico F, Johnson M, von Dessauer B, Donoso A, Zavala I, Zavala C, Troster E, Peña Y, Flamenco C, Almeida H, Nilda V, Esteban A, Latin-American Group for Mechanical Ventilation in Children Mechanical ventilation in pediatric intensive care units during the season for acute lower respiratory infection: a multicenter study. Pediatr Crit Care Med . 2012;13(2):158–164. doi: 10.1097/PCC.0b013e3182257b82. [DOI] [PubMed] [Google Scholar]
- 2.Wolfler A, Calderini E, Iannella E, Conti G, Biban P, Dolcini A, Pirozzi N, Racca F, Pettenazzo A, Salvo I, Network of Pediatric Intensive Care Unit Study Group Evolution of noninvasive mechanical ventilation use: a cohort study among Italian PICUs. Pediatr Crit Care Med . 2015;16(5):418–427. doi: 10.1097/PCC.0000000000000387. [DOI] [PubMed] [Google Scholar]
- 3.Silva DC, Shibata AR, Farias JA, Troster EJ. How is mechanical ventilation employed in a pediatric intensive care unit in Brazil? Clinics (Sao Paulo) . 2009;64(12):1161–1166. doi: 10.1590/S1807-59322009001200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balcells Ramírez J, López-Herce Cid J, Modesto Alapont V, Grupo de Respiratorio de la Sociedad Española de Cuidados Intensivos Pediátricos [Prevalence of mechanical ventilation in pediatric intensive care units in Spain] An Pediatr (Barc) . 2004;61(6):533–541. doi: 10.1016/s1695-4033(04)78440-4. Spanish. [DOI] [PubMed] [Google Scholar]
- 5.Newth CJ, Venkataraman S, Willson DF, Meert KL, Harrison R, Dean JM, Pollack M, Zimmerman J, Anand KJ, Carcillo JA, Nicholson CE, Eunice Shriver Kennedy National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network Weaning and extubation readiness in pediatric patients. Pediatr Crit Care Med . 2009;10(1):1–11. doi: 10.1097/PCC.0b013e318193724d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alonso-Ovies Á, Nin N, Martín MC, Gordo F, Merino P, Añón JM, Obón B, Magret M, Gutiérrez I, IVeMVA study investigators Safety incidents in airway and mechanical ventilation in Spanish ICUs: The IVeMVA study. J Crit Care . 2018;47:238–244. doi: 10.1016/j.jcrc.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Farias JA, Frutos-Vivar F, Casado Flores J, Siaba A, Retta A, Fernández A, Baltodano A, Ko IJ, Johnson M, Esteban A, Grupo Internacional de la Ventilación Mecánica en Niños [Factors associated with the prognosis of mechanically ventilated infants and children. An international study]. Med Intensiva . 2006;30(9):425–431. doi: 10.1016/s0210-5691(06)74565-x. Spanish. [DOI] [PubMed] [Google Scholar]
- 8.Newth CJ, Hotz JC, Khemani RG. Ventilator liberation in the pediatric ICU. Respir Care . 2020;65(10):1601–1610. doi: 10.4187/respcare.07810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mhanna MJ, Anderson IM, Iyer NP, Baumann A. The use of extubation readiness parameters: a survey of pediatric critical care physicians. Respir Care . 2014;59(3):334–339. doi: 10.4187/respcare.02469. [DOI] [PubMed] [Google Scholar]
- 10.Loberger JM, Campbell CM, Colleti J, Jr, Borasino S, Abu-Sultaneh S, Khemani RG. Ventilation liberation practices among 380 international PICUs. Crit Care Explor . 2022;4(6):e0710. doi: 10.1097/CCE.0000000000000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacci SL, Johnston C, Hattori WT, Pereira JM, Azevedo VM. Mechanical ventilation weaning practices in neonatal and pediatric ICUs in Brazil: the Weaning Survey-Brazil. J Bras Pneumol . 2020;46(4):e20190005. doi: 10.36416/1806-3756/e20190005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abu-Sultaneh S, Iyer NP, Fernández A, Gaies M, González-Dambrauskas S, Hotz JC, et al. Executive Summary: International Clinical Practice Guidelines for Pediatric Ventilator Liberation, A Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network Document. Am J Respir Crit Care Med . 2023;207(1):17–28. doi: 10.1164/rccm.202204-0795SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foronda FK, Troster EJ, Farias JA, Barbas CS, Ferraro AA, Faria LS, et al. The impact of daily evaluation and spontaneous breathing test on the duration of pediatric mechanical ventilation: a randomized controlled trial. Crit Care Med . 2011;39(11):2526–2533. doi: 10.1097/CCM.0b013e3182257520. [DOI] [PubMed] [Google Scholar]
- 14.Jouvet PA, Payen V, Gauvin F, Emeriaud G, Lacroix J. Weaning children from mechanical ventilation with a computer-driven protocol: a pilot trial. Intensive Care Med . 2013;39(5):919–925. doi: 10.1007/s00134-013-2837-8. [DOI] [PubMed] [Google Scholar]
- 15.Randolph AG, Wypij D, Venkataraman ST, Hanson JH, Gedeit RG, Meert KL, Luckett PM, Forbes P, Lilley M, Thompson J, Cheifetz IM, Hibberd P, Wetzel R, Cox PN, Arnold JH, Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network Effect of mechanical ventilator weaning protocols on respiratory outcomes in infants and children: a randomized controlled trial. JAMA . 2002;288(20):2561–2568. doi: 10.1001/jama.288.20.2561. [DOI] [PubMed] [Google Scholar]
- 16.Faustino EV, Gedeit R, Schwarz AJ, Asaro LA, Wypij D, Curley MA, Randomized Evaluation of Sedation Titration for Respiratory Failure (RESTORE) Study Investigators Accuracy of an extubation readiness test in predicting successful extubation in children with acute respiratory failure from lower respiratory tract disease. Crit Care Med . 2017;45(1):94–102. doi: 10.1097/CCM.0000000000002024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blackwood B, Tume LN, Morris KP, Clarke M, McDowell C, Hemming K, Peters MJ, McIlmurray L, Jordan J, Agus A, Murray M, Parslow R, Walsh TS, Macrae D, Easter C, Feltbower RG, McAuley DF, SANDWICH Collaborators Effect of a sedation and ventilator liberation protocol vs usual care on duration of invasive mechanical ventilation in pediatric intensive care units: a randomized clinical trial. JAMA . 2021;326(5):401–410. doi: 10.1001/jama.2021.10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abu-Sultaneh S, Hole AJ, Tori AJ, Benneyworth BD, Lutfi R, Mastropietro CW. An interprofessional quality improvement initiative to standardize pediatric extubation readiness assessment. Pediatr Crit Care Med . 2017;18(10):e463–e471. doi: 10.1097/PCC.0000000000001285. [DOI] [PubMed] [Google Scholar]
- 19.Krawiec C, Carl D, Stetter C, Kong L, Ceneviva GD, Thomas NJ. Challenges with implementation of a respiratory therapist-driven protocol of spontaneous breathing trials in the pediatric ICU. Respir Care . 2017;62(10):1233–1240. doi: 10.4187/respcare.05477. [DOI] [PubMed] [Google Scholar]
- 20.Loberger JM, Jones RM, Prabhakaran P. A respiratory therapist-driven pathway improves timeliness of extubation readiness assessment in a single PICU. Pediatr Crit Care Med . 2020;21(8):e513–e521. doi: 10.1097/PCC.0000000000002326. [DOI] [PubMed] [Google Scholar]
- 21.Abu-Sultaneh S, Iyer NP, Fernández A, Gaies M, González-Dambrauskas S, Hotz JC, Kneyber MC, López-Fernández YM, Rotta AT, Werho DK, Baranwal AK, Blackwood B, Craven HJ, Curley MA, Essouri S, Fioretto JR, Hartmann SM, Jouvet P, Korang SK, Rafferty GF, Ramnarayan P, Rose L, Tume LN, Whipple EC, Wong JJM, Emeriaud G, Mastropietro CW, Napolitano N, Newth CJ, Khemani RG, Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network Operational definitions related to pediatric ventilator liberation. Chest . 2023;163(5):1130–1143. doi: 10.1016/j.chest.2022.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tume LN, Kneyber MC, Blackwood B, Rose L. Mechanical ventilation, weaning practices, and decision making in European PICUs. Pediatr Crit Care Med . 2017;18(4):e182–e188. doi: 10.1097/PCC.0000000000001100. [DOI] [PubMed] [Google Scholar]
- 23.Elisa P, Francesca C, Marco P, Davide V, Laura Z, Fabrizio Z, et al. Ventilation weaning and extubation readiness in children in pediatric intensive care unit: a review. Front Pediatr . 2022;10:867739–867739. doi: 10.3389/fped.2022.867739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferreira FV, Sugo EK, Aragon DC, Carmona F, Carlotti AP. Spontaneous breathing trial for prediction of extubation success in pediatric patients following congenital heart surgery: a randomized controlled trial. Pediatr Crit Care Med . 2019;20(10):940–946. doi: 10.1097/PCC.0000000000002006. [DOI] [PubMed] [Google Scholar]
- 25.Farias JA, Retta A, Alía I, Olazarri F, Esteban A, Golubicki A, et al. A comparison of two methods to perform a breathing trial before extubation in pediatric intensive care patients. Intensive Care Med . 2001;27(10):1649–1654. doi: 10.1007/s001340101035. [DOI] [PubMed] [Google Scholar]
- 26.Farias JA, Alía I, Esteban A, Golubicki AN, Olazarri FA. Weaning from mechanical ventilation in pediatric intensive care patients. Intensive Care Med . 1998;24(10):1070–1075. doi: 10.1007/s001340050718. [DOI] [PubMed] [Google Scholar]
- 27.Chavez A, dela Cruz R, Zaritsky A. Spontaneous breathing trial predicts successful extubation in infants and children. Pediatr Crit Care Med . 2006;7(4):324–328. doi: 10.1097/01.PCC.0000225001.92994.29. [DOI] [PubMed] [Google Scholar]
- 28.Riou Y, Chaari W, Leteurtre S, Leclerc F. Predictive value of the physiological deadspace/tidal volume ratio in the weaning process of mechanical ventilation in children. J Pediatr (Rio J) . 2012;88(3):217–221. doi: 10.2223/JPED.2190. [DOI] [PubMed] [Google Scholar]
- 29.Nascimento MS, Rebello CM, Vale LA, Santos É, Prado CD. Spontaneous breathing test in the prediction of extubation failure in the pediatric population. Einstein (Sao Paulo) . 2017;15(2):162–166. doi: 10.1590/S1679-45082017AO3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramnarayan P, Richards-Belle A, Drikite L, Saull M, Orzechowska I, Darnell R, Sadique Z, Lester J, Morris KP, Tume LN, Davis PJ, Peters MJ, Feltbower RG, Grieve R, Thomas K, Mouncey PR, Harrison DA, Rowan KM, FIRST-ABC Step-Up RCT Investigators and the Paediatric Critical Care Society Study Group Effect of high-flow nasal cannula therapy vs continuous positive airway pressure therapy on liberation from respiratory support in acutely ill children admitted to pediatric critical care units: a randomized clinical trial. JAMA . 2022;328(2):162–172. doi: 10.1001/jama.2022.9615. [DOI] [PMC free article] [PubMed] [Google Scholar]