Abstract
Replication of the Epstein-Barr virus genome initiates at one of several sites in latently infected, dividing cells. One of these replication origins is close to the viral DNA maintenance element, and, together, this replication origin and the maintenance element are referred to as oriP. The replicator of oriP contains four binding sites for Epstein-Barr virus nuclear antigen 1 (EBNA-1), the sole viral protein required for the replication and maintenance of oriP plasmids. We showed previously that these EBNA-1 sites function in pairs and that mutational inactivation of one pair does not eliminate replicator function. In this study we characterized the contribution of each EBNA-1 site within the replicator and flanking sequences through the use of an internally controlled replication assay. We present evidence that shows that all four EBNA-1 sites are required for an oriP plasmid to be replicated in every cell cycle. Results from these experiments also show that the paired EBNA-1 binding sites are not functionally equivalent and that the low affinity of sites 2 and 3 compared to that of sites 1 and 4 is not essential for replicator function. Our results suggest that a host cell protein(s) binds sequences flanking the EBNA-1 sites and that interactions between EBNA-1 and this protein(s) are critical for replicator function. Finally, we present evidence that shows that the minimal replicator of oriP consists of EBNA-1 sites 3 and 4 and two copies of a 14-bp repeat that is present in inverse orientation flanking these EBNA-1 sites. EBNA-1 sites 1 and 2, together with an element(s) within nucleotides 9138 to 9516, are ancillary elements required for full replicator activity.
The Epstein-Barr virus (EBV) genome is maintained as an autonomously replicating, multicopy plasmid in most latently infected cells (9, 20, 27). Each plasmid is replicated once per cellular S phase, and the progeny plasmids are equally partitioned to daughter cells during mitosis (1, 38). Replication and maintenance of the genome during latency require three viral components: an origin of DNA replication, a DNA maintenance element (the family of repeats [FR]), and the EBV nuclear antigen 1 (EBNA-1) (reviewed in reference 45). FR is composed of 21 imperfect copies of a 30-bp repeat, 20 of which contain an EBNA-1 binding site (31). In addition to a dimerization and DNA binding domain, EBNA-1 contains multiple elements that mediate binding to mitotic host cell chromosomes (15, 16, 24, 43). It is believed that EBNA-1 performs an essential function in viral genome maintenance through simultaneously binding the reiterated sites in FR and host cell chromosomes, thereby tethering the EBV genome to the host cell chromosomes during cell division (15, 16, 24, 43). The analysis of replicative intermediates by electron microscopy and two-dimensional agarose gel electrophoresis revealed that latent cycle DNA replication can initiate at multiple sites on the EBV chromosome (8, 9, 21, 28). One of these origins is located close to FR, and, together, these two elements are referred to as oriP (Fig. 1) (8, 44). Recombinant plasmids bearing a 2.2-kb fragment of EBV DNA encompassing oriP are replicated in a cell cycle-regulated manner and maintained efficiently in cells expressing EBNA-1 (47, 48). Thus, small oriP plasmids provide an experimentally tractable system for studying EBV latent-cycle DNA replication and genome maintenance. Because all of the proteins that are required for the replication of oriP plasmids, with the exception of EBNA-1, are provided by the host cell, elucidation of the mechanism by which replication initiates at oriP may provide insight into the nature of mammalian origins of DNA replication.
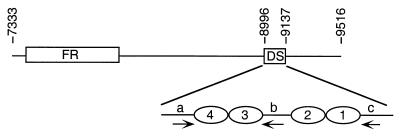
FIG. 1.
Organization of EBV oriP. The EBV DNA (nt 7333 to 9516) present in pHEBo-1 and pHEBo-1.1 and the locations of FR and DS are shown. Replication initiates within, or close to, DS (8). The arrangement of EBNA-1 binding sites (ovals) and repeat elements a, b, and c (arrows) within nt 8996 to 9137 are shown at the bottom.
The replicator of oriP is located ca. 1 kb from FR and is referred to as DS due to the presence of a 65-bp dyad symmetry element (10, 28, 32, 34, 46). DS contains four EBNA-1 binding sites arranged as two pairs with 21-bp center-to-center spacing and contains, or is close to, the site at which DNA replication initiates (Fig. 1) (8, 31). Plasmids bearing DS are replicated in an EBNA-1-dependent manner but are not stably maintained in the absence of FR (10, 34, 46). The observation that EBNA-1 differs from eukaryotic viral replication initiator proteins in that it lacks the ability to unwind DNA has led to the hypothesis that EBNA-1 participates in the recruitment of host cell factors required for the initiation of DNA replication from within oriP (7, 11). EBNA-1 interacts with a human single-stranded DNA binding protein (RPA) and a 32-kDa acidic host cell protein (p32/TAP) that may function in latent-cycle DNA replication, but interactions with a DNA helicase or other proteins required for DNA replication have not been reported (41, 42, 49). Data from our previous investigation of the EBNA-1 binding sites within DS showed that the EBNA-1 binding sites function as pairs and that replicator activity was not abolished by the inactivation of one pair of these binding sites (10). This raised the question of why the replicator of the EBV isolate used in our studies (B95-8) contains two pairs of EBNA-1 binding sites. To address this question, we utilized a quantitative short-term replication assay to assess the impact of inactivating individual and paired EBNA-1 binding sites within the oriP replicator. We found that all four binding sites are required for full replicator activity in an EBV-positive Burkitt's lymphoma cell line. These results are in agreement with the recently published findings of Yates et al. (46), and our studies further show that the paired binding sites are not functionally equivalent. We also measured the effects of deletions within oriP on replication efficiency, and the results suggest that interactions between EBNA-1 and a host cell protein bound to repeats flanking the EBNA-1 binding sites within DS are required for minimal replicator activity. Finally, we reinvestigated the role of sequences to the right of EBNA-1 binding site 1 and determined that one or more elements within this region are required for full oriP replicator function.
MATERIALS AND METHODS
Plasmids.
pHEBo-1 and pHEBo-1.1, plasmids bearing EBV (B95-8 strain) nucleotides (nt) 7333 to 9516 and a hygromycin resistance gene (hph) expression cassette, were described previously (11, 37). pHEBo-1.1Δ9138-9516 was derived from pHEBo-1.1 by deleting EBV nt 9138 to 9516 and 56 nonessential nucleotides from the 3′ end of the hph gene by partial digestion with BsaI followed by complete digestion with HpaI. The hph gene was restored upon ligation of a 471-bp PCR product that contained the appropriate hph sequences followed by BamHI and HpaI sites. The resulting plasmid contains a BamHI site immediately following EBV nt 9137. pHEBo-1.1Δ9138-9459 was constructed using the same basic strategy; however the PCR product ligated to the digested pHEBo-1.1 fragment contained EBV nt 9459 to 9516. pHEBo-1.1Δ9138-9459+M13 was created by introducing a 683-bp BamHI-BglII fragment from M13mp18 at the BamHI site of pHEBo-1.1Δ9138-9459. pHEBo-2.2 was derived by deleting sequences (EBV nt 8996 to 9135) between the EcoRV and HpaI sites of pHEBo-1.1Δ9138-9516. pHEBo-2.2.1 was constructed by substituting the EcoRV-HpaI fragment containing DS sequences from pHEBo-1.1(dpm1+2) (10) for the small EcoRV-HpaI fragment of pHEBo-1.1Δ9138-9516. pHEBo-2.2.5, -2.2.6, -2.2.7, and -2.2.8 were created by annealing oligonucleotides with 3′ complementary ends, synthesizing the complementary strands with the Klenow fragment of Escherichia coli DNA polymerase I, digesting the double-stranded products with BamHI and/or BglII, and inserting these fragments at the BamHI site of pHEBo-2.2. pHEBo-2.2.20 and -2.2.24 were generated by PCR amplification of DS sequences using pHEBo-1.1 as the template, digesting the products with BamHI and BglII (pHEBo-2.2.20) or EcoRV and BglII (pHEBo-2.2.24) and inserting the products at the BamHI site of pHEBo-2.2 (pHEBo-2.2.20) or between the EcoRV and BamHI sites of pHEBo-1.1Δ9138-9516 (pHEBo-2.2.24). pHEBo-1.1(dpm1), pHEBo-1.1(dpm2), pHEBo-1.1(dpm3), pHEBo-1.1(dpm4), pHEBo-1.1(dpm1+2), pHEBo-1.1(dpm3+4), pHEBo-1.1(in1/2[5]), pHEBo-1.1(in2/3[5]), and pHEBo-1.1(in3/4[5]) were described previously (10). pHEBo-1.1(dpm3+4;2=HAS) and pHEBo-1.1(dpm1+2;3=HAS) contain mutations that are predicted to increase the affinity of EBNA-1 for sites 2 and 3, respectively (2). Base pair transitions were introduced at the −3, +3, and +8 positions in site 2 in pHEBo-1.1(dpm3+4) or the −4 and +8 positions in site 3 in pHEBo-1.1(dpm1+2) by oligonucleotide-directed mutagenesis as previously described (11). pBS/KS[BclI] was made by inserting a BclI linker into the unique XhoI site of pBluescript KS (Stratagene). A SalI-EcoRV fragment from pHEBo-1.1 containing EBV nt 7333 to 8994 was introduced between the same sites in the polylinker region of pBS/KS[BclI] to create pBS/FR. Similarly, a SalI-BamHI fragment from pHEBo-1.1Δ9138-9516 containing EBV nt 7333 to 9137 was inserted between the same sites in the polylinker of pBS/KS[Bcl] to create pBS/FR.DS. The EBV nucleotides present in each of the plasmids created for this study are given in Table 1. Plasmid DNA was isolated from dam+ E. coli DH-1 or DH-5 cells and purified by isopycnic centrifugation on CsCl-ethidium bromide gradients. All plasmids were sequenced after purification to confirm their identity and to confirm that the intended mutations were the only mutations present within DS.
TABLE 1.
Description of plasmids constructed for this study
| Plasmid | EBV nucleotides |
|---|---|
| pHEBo-1.1Δ9138-9516 | 7333–9137 |
| pHEBo-1.1Δ9138-9459 | 7333–9137; 9460–9516 |
| pHEBo-1.1Δ9138-9459+M13 | 7333–9137; 9460–9516a |
| pHEBo-2.2 | 7333–8995 |
| pHEBo-2.2.1 | 7333–9137b |
| pHEBo-2.2.5 | 7333–8995; 9019–9085 |
| pHEBo-2.2.6 | 7333–8995; 9033–9069 |
| pHEBo-2.2.7 | 7333–8995; 9019–9069 |
| pHEBo-2.2.8 | 7333–8995; 9033–9085 |
| pHEBo-2.2.20 | 7333–8995; 9033–9123 |
| pHEBo-2.2.24 | 7333–9085 |
| pBS/FR | 7333–8994 |
| pBS/FR.DS | 7333–9137 |
Contains nt 6252 to 6935 from M13mp18 between EBV nt 7333 to 9137 and 9560 to 9516.
Contains base pair transversions at the −5 and +5 positions of EBNA-1 binding sites 1 and 2.
Sequence analysis of oriP from multiple EBV strains.
Whole-cell DNA was prepared from Raji (30), Namalwa (29), FF41 (6), Jijoye (12), and Akata (40) cells as previously described (23). Jijoye and Akata cells were induced to enter the EBV lytic cycle by adding either goat anti-human immunoglobulin M (IgM; Jijoye) or anti-human IgG (Akata) antibodies (HyClone Laboratories, Inc.) to the growth medium at a final concentration of 1%. Whole-cell DNA was isolated after 24 h of incubation in the presence of inducing antibodies. A 782-bp fragment of the EBV genome containing oriP DS and flanking sequences (B95-8 nt 8587 to 9363) was amplified by PCR using primers containing EcoRI and HindIII sites. The sequence between nt 8976 and 9142 was determined by direct sequencing of the PCR products or by sequencing pBluescript KS derivatives containing the amplified sequences.
Short-term replication assays.
Raji cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (HyClone Laboratories) and 100 U each of penicillin and streptomycin per ml at 37°C. Sixty-one micrograms each of the reference (pHEBo-1 or pKS/oriP) and test plasmid DNAs purified from dam+ E. coli were combined, precipitated with ethanol, and dissolved in 61 μl of 10 mM Tris-HCl (pH 8.0), 1 mM EDTA (TE). One microliter was reserved for determination of the ratio of test to reference plasmid DNA introduced into cells by quantitative Southern blot analysis. Logarithmic-phase Raji cells were washed with cold growth medium and suspended at 4 × 107 cells per ml in cold growth medium. Electroporations were performed in triplicate by mixing 107 cells with 20 μl of the combined test and reference plasmid DNA. This mixture was transferred to a 0.4-cm-wide cuvette and electroporated with a Bio-Rad Gene Pulser set at 250 V and 960 μF. Cells were returned to culture in 20% conditioned–80% fresh medium and maintained in an actively dividing state for 72 h (three population doublings), at which time low-molecular-weight DNA was isolated from 2 × 107 to 5 × 107 cells by the method of Hirt (13). Briefly, cells were washed twice with phosphate-buffered saline, suspended in 2 ml of 10 mM Tris-HCl (pH 8.0)–10 mM EDTA, mixed gently with an equal volume of 10 mM Tris-HCl (pH 8.0)–10 mM EDTA–1.2% sodium dodecyl sulfate and incubated for 20 min at room temperature. High-molecular-weight DNA was precipitated upon the addition of 800 μl of 5 M NaCl and incubation at 4°C for 12 to 16 h and pelleted by centrifugation at 27,000 × g for 30 to 45 min at 4°C. The supernatant was treated with 40 μg of RNase A per ml for 1 h at 37°C and, subsequently, 10 μg of proteinase K per ml at 37°C for 1 h. The samples were extracted once with phenol-chloroform-isoamyl alcohol (25:24:1) and once with chloroform-isoamyl alcohol (24:1), and the DNA was precipitated with ethanol. The DNA was pelleted by centrifugation at 25,000 × g for 45 min at 0°C, dissolved in 0.3 M sodium acetate (pH 7.5), and reprecipitated with ethanol. The precipitates were dissolved at 2 × 105 cell equivalents per μl in TE containing 20 μg of RNase A per ml.
Restriction enzyme digests containing 2 × 106 cell equivalents of low-molecular-weight DNA, 9 U each of EcoRV and PstI, and 5 U of DpnI in a solution containing 20 mM Tris-acetate, 10 mM magnesium acetate, 50 mM potassium acetate (pH 7.9), 100 mM NaCl, and 1 mM dithiothreitol (DTT) were incubated for 12 to 16 h at 37°C. Extracts containing pHEBo-2.2-derived plasmids that lack the EcoRV site at EBV nt 8992 were first digested with SacI in 20 mM Tris-acetate–10 mM magnesium acetate–50 mM potassium acetate (pH 7.9)–1 mM DTT and then adjusted to 100 mM NaCl and digested with DpnI, EcoRV, and PstI as described above. Under these salt conditions, fully methylated plasmid DNA is digested by DpnI while hemimethylated and nonmethylated plasmid DNA is resistant to DpnI digestion (25; M. Dodard and J. Hearing, unpublished data). Digested samples were electrophoresed on 0.8% agarose gels, transferred to Hybond-N+ (Amersham Pharmacia Biotech), cross-linked to the membrane with UV light, and probed with 32P-labeled probe DNA as previously described (11). A probe comprising EBV nt 8041 to 8996 was used for assays of plasmids derived from pHEBo-1.1 and -2.2, which lack sequences from within DS. A BamHI-EcoRV fragment from pBS/oriP encompassing EBV nt 7333 to 8992 was used to probe Southern blots of pHEBo-1.1 derivatives bearing point mutations within oriP DS or deletions to the right of DS. Southern blots for analysis of pBluescript KS-based plasmids were probed with pBS/KS[BclI].
Determination of replication efficiencies.
Southern blot quantitation was performed with a Molecular Dynamics Storm 860 phosphorimager. The replication efficiencies of test plasmids were calculated as the ratios of replicated test plasmids to the replicated reference plasmid (pHEBo-1 or pKS/oriP). These ratios were adjusted for the relative amounts of the two plasmids present at the time of electroporation and normalized to the amount of pHEBo-1.1, which was set at 100%. The efficiency with which plasmids replicated per cell generation was calculated using the formula Nt/Nr = [(1 + ɛ)i − (1 − ɛ)i]/2i, where Nt/Nr is the normalized ratio of replicated test plasmid molecules to replicated reference plasmid molecules after i cell generations and ɛ is the replication efficiency of the test plasmid per cell generation. This calculation assumes that the test plasmid is maintained in dividing cells with the same efficiency as the reference plasmid and that plasmids that are not replicated in one cellular S phase may be replicated in subsequent S phases. It also takes into account the elimination of plasmid DNA that is never replicated by digestion with DpnI. Statistical analysis of the short-term replication assay data was performed by one-way analysis of variance. Tukey's “honestly significant difference” test was used to test pairwise comparisons between the means, and P values of <0.05 were considered significant.
Long-term plasmid replication and maintenance assays.
The efficiency of transformation of Raji cells to hygromycin resistance by pHEBo-1.1 and its derivatives was determined by electroporating 107 cells with 2 μg of test plasmids as described above. Forty-eight hours after electroporation, cells were plated in 24-well dishes in growth medium supplemented with 300 μg of hygromycin B (Boehringer Mannheim) per ml at 104, 103, and 102 cells per well as previously described (46). Fresh medium containing 300 μg of hygromycin B per ml was added to the wells every 6 to 7 days, and the number of wells containing clones was determined 21 days after electroporation. Transformation frequencies were derived by applying the Poisson distribution (36). Two clones which were derived by plating the smallest number of electroporated cells per well were expanded under selection until 4.6 × 106 to 1.0 × 107 cells were available for analysis. Low-molecular-weight DNA was isolated as described above and was analyzed as either uncut or digested molecules by Southern blotting. Blots were probed with 32P-labeled pHEBo-1.1 (see Fig. 5) or the large SacII-EcoRV fragment of pHEBo-1.1 (see Fig. 3).
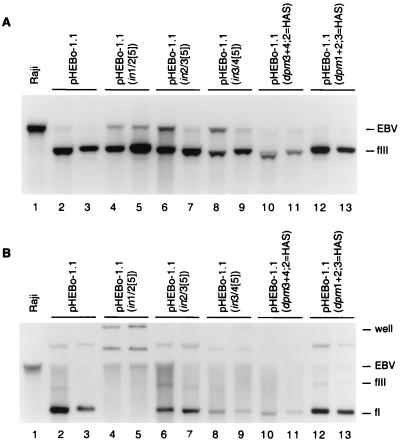
FIG. 5.
Analysis of plasmid DNA in Raji transformants established with pHEBo-1.1 and its derivatives bearing insertion and point mutations in oriP DS. Low-molecular-weight DNA isolated from Raji cells (lane 1) or hygromycin-resistant Raji clones transformed by pHEBo-1.1 (lanes 2 and 3), pHEBo-1.1(in1/2[5]) (lanes 4 and 5), pHEBo-1.1(in2/3[5]) (lanes 6 and 7), pHEBo-1.1(in3/4[5]) (lanes 8 and 9), pHEBo-1.1(dpm3+4;2=HAS) (lanes 10 and 11), or pHEBo-1.1(dpm1+2;3=HAS) (lanes 12 and 13) were analyzed by Southern blotting with radiolabeled pHEBo-1.1 DNA. The samples were either digested with SacII (A) or analyzed without prior restriction enzyme digestion (B). The positions of covalently closed circular (fI) and linear (fIII) pHEBo-1.1 DNA and Raji EBV DNA containing oriP that was generated by SacII digestion or mechanical shearing during isolation are indicated on the right. Nicked circular plasmid DNA is present in panel B, lanes 2, 3, 6, 7, 12, and 13, between the wells and the largest viral DNA fragments complementary to the probe.
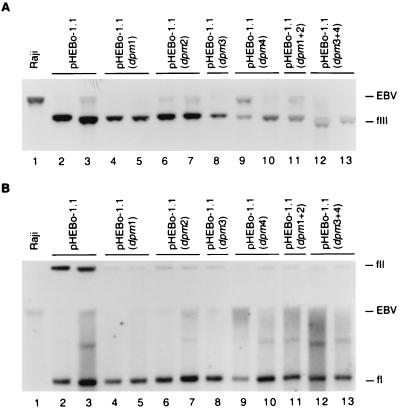
FIG. 3.
Analysis of plasmid DNA in Raji transformants established with pHEBo-1.1 and its derivatives bearing mutations within single and paired EBNA-1 binding sites. Low-molecular-weight DNA isolated from Raji cells (lane 1) or hygromycin-resistant Raji clones transformed by pHEBo-1.1 (lanes 2 and 3), pHEBo-1.1(dpm1) (lanes 4 and 5), pHEBo-1.1(dpm2) (lanes 6 and 7), pHEBo-1.1(dpm3) (lane 8), pHEBo-1.1(dpm4) (lanes 9 and 10), pHEBo-1.1(dpm1+2) (lane 11), or pHEBo-1.1(dpm3+4) (lanes 12 and 13) was analyzed by Southern blotting with radiolabeled pHEBo-1.1 DNA. The samples were either digested with SacII (A) or analyzed without prior restriction enzyme digestion (B). The positions of covalently closed circular (fI), nicked circular (fII), and linear (fIII) pHEBo-1.1 DNA are indicated on the right. Also indicated are the positions of Raji EBV DNA containing oriP that was generated by SacII digestion (A) or mechanical shearing during isolation of low-molecular-weight DNA (B).
RESULTS
Conservation of sequences in oriP DS.
Our previous analysis of EBNA-1 binding sites within oriP DS demonstrated that replicator function was not eliminated by inactivation of two sites provided the remaining sites were separated by 21 bp (10). However, four EBNA-1 binding sites, arranged as pairs with 21-bp center-to-center spacing, are present not only within the B95-8 strain oriP replicator used in our studies but also within the Raji and C15 EBV genomes (3, 14, 26, 31, 35). To determine if EBV isolates with fewer than four oriP DS EBNA-1 binding sites exist and to extend the comparisons further into flanking sequences, EBV (B95-8 isolate) nt 8587 to 9363 from four EBV isolates were amplified by PCR and the sequence of nt 8977 to 9142 was determined. These viral sequences were obtained from three Burkitt's lymphoma cell lines (Raji, Jijoye, and Akata) (12, 30, 40) and a marmoset cell line established by in vitro immortalization of B cells with virus from an infectious mononucleosis patient (FF41) (6). Two EBV types (EBV-1 and -2) have been distinguished (33), and both type 1 (B95-8, Raji, FF41, and Akata) and type 2 (Jijoye) viruses were included in this comparison.
Two of the viruses examined in this experiment, Jijoye and FF41, are identical to B95-8 between nt 8977 and 9142, while the Raji and Akata viral genomes differ in several places (Fig. 2). These differences were present within two independently derived PCR products and were therefore not the result of polymerase errors during PCR. Raji and Akata viral DNAs differ from each other and the B95-8 sequence at 2 and 3 nt, respectively, flanking the EBNA-1 binding sites. They also differ from B95-8 at 1 nt within EBNA-1 binding site 3, and Akata contains an additional difference within EBNA-1 binding site 2. Neither difference affects the ability of EBNA-1 to bind these sites. In vivo footprinting experiments are consistent with the occupation of site 3 by EBNA-1 in Raji cells (14, 26), and in vitro DNA binding experiments showed that the A-to-G transition within site 2 does not impair EBNA-1 binding (2, 11).
FIG. 2.
Sequence of oriP DS and flanking nucleotides from type 1 and type 2 EBV isolates. EBV nucleotides 8977 to 9142 from the B95-8 strain and the same sequence from the Raji, Jijoye, Akata, FF41, and C15 EBV strains are shown. The four EBNA-1 binding sites (boxes) and nonamer repeats a, b, and c (arrows) are indicated. Differences between the B95-8 sequence and the other viral sequences are underlined. The sequences from the B95-8 and C15 viral genomes were previously reported (3, 35).
The number and arrangement of DS EBNA-1 binding sites was also found by Snudden et al. to be the same within the genome of a nasopharyngeal carcinoma EBV isolate (C15) (35). The only differences between C15 and the isolates described here are within EBNA-1 binding sites 2 and 3 (Fig. 2). The A-to-C transversion within site 3, when present in the context of a high-affinity binding site, has no effect on EBNA-1 binding (2). The A-to-T transversion within site 2 was previously found to reduce the ability of EBNA-1 to bind an otherwise high-affinity site (2), but several observations argue that EBNA-1 can bind this site. This nucleotide difference is within only one of the two half sites of the recognition element, and the previous study which showed a reduction in binding affinity contained the transversion at both half sites (2). Also, the binding of EBNA-1 to site 1 facilitates binding to site 2 (11), and this interaction between adjacent EBNA-1 dimers likely stabilizes EBNA-1 bound to site 2 in the C15 genome. In summary, all six of the DS elements examined contain four EBNA-1 binding sites and, additionally, the center-to-center spacing of these sites was conserved among these EBV type 1 and type 2 isolates (Fig. 2).
Contribution of individual EBNA-1 binding sites in DS to replicator activity.
The results of this survey suggested that all of the EBNA-1 binding sites within DS, although individually not essential for replicator function, contribute to its activity. Two assays were performed to determine if derivatives of an oriP-bearing plasmid (pHEBo-1.1) with disabling mutations within individual or paired EBNA-1 binding sites in DS are replicated less efficiently. First, the frequencies with which pHEBo-1.1 and mutant derivatives transformed Raji cells to hygromycin resistance were determined (37, 46). The results of two independent experiments are shown in Table 2. No hygromycin-resistant clones were obtained with a pHEBo-1.1 derivative that lacks oriP DS (pHEBo-2.2) at 104 cells/well, yielding a transformation frequency of <3 × 10−6, while pHEBo-1.1 transformed Raji cells to hygromycin resistance with a frequency of 3 × 10−2 (experiment 1) to 2 × 10−3 (experiment 2). This high transformation efficiency is due to the ability of pHEBo-1.1 to be maintained as an autonomously replicating plasmid in cells expressing EBNA-1 and is similar to previously reported frequencies (37, 46). Each of the plasmids containing inactivating mutations within one of the four EBNA-1 binding sites transformed Raji cells at reduced frequency compared to pHEBo-1.1, suggesting that all four sites are required for full replicator activity. Because the binding of EBNA-1 to sites 1 and 4 enables binding to sites 2 and 3, respectively (10, 39), we expected pHEBo-1.1 derivatives with disabling mutations within site 1 or site 4 to resemble plasmids with mutations within both sites 1 and 2 or sites 3 and 4 in their ability to transform Raji cells. However, pHEBo-1.1(dpm1+2) and pHEBo-1.1 (dpm3+4) transformed Raji cells at even lower frequencies than pHEBo-1.1(dpm1) and pHEBo-1.1(dpm4) (30- and 300-fold reduction in transformation efficiency, respectively; Table 2).
TABLE 2.
Frequencies of transformation of Raji cells to drug resistance with plasmids bearing wild type or mutated oriP DS
| Expt | Plasmid | No. of positive wells/16 wellsg containing indicated no. of cells/well
|
Frequency | No. of plasmids/cella | ||
|---|---|---|---|---|---|---|
| 104 | 103 | 102 | ||||
| 1 | pHEBo-2.2 | 0, 0 | N.D.g | N.D. | <3 × 10−6 | NAb |
| pHEBo-1.1 | 16, 16 | 16, 16 | 15, 16 | 3 × 10−2 | 7.3, 7.0 | |
| pHEBo-1.1(dpm1) | 16, 16 | 16, 16 | 4, 9 | 5 × 10−3 | 3.3, 3.2 | |
| pHEBo-1.1(dpm2) | 16, 16 | 16, 16 | 6, 12 | 8 × 10−3 | 5.2, 4.9 | |
| pHEBo-1.1(dpm3) | 16, 16 | 15, 16 | 3, 0 | 3 × 10−3 | 2.2 | |
| pHEBo-1.1(dpm4) | 16, 16 | 16, 16 | 2, 3 | 2 × 10−3 | 1.4, 2.2 | |
| pHEBo-1.1(dpm1+2) | 16, 16 | 16, 16 | 2, 2 | 1 × 10−3 | 1.9 | |
| pHEBo-1.1(dpm3+4) | 16, 16 | 3, 0 | 0, 1 | 1 × 10−4 | 2.5, 1.7 | |
| pHEBo-1.1(dpm3+4;1/2[ATTTA]) | 16, 16 | 3, 0 | 0, 0 | 1 × 10−4 | 2.3, 3.1 | |
| pHEBo-1.1Δ9138-9516 | 16, 16 | 16, 15c | 9, 4c | 5 × 10−3 | 2.5, 2.5 | |
| 2 | pHEBo-2.2 | 0, 0 | N.D. | N.D. | <3 × 10−6 | NA |
| pHEBo-1.1 | 16, 16 | 16, 16 | 2, 4 | 2 × 10−3d | 3.8, 5.9 | |
| pHEBo-1.1(in1/2[5]) | 16, 16 | 2, 1 | 0, 0 | 1 × 10−4 | 2.3, 6.2e | |
| pHEBo-1.1(in2/3[5]) | 16, 16 | 14, 14 | 0, 0 | 2 × 10−3d | 5.2, 5.3 | |
| pHEBo-1.1(in3/4[5]) | 16, 16 | 2, 7 | 1, 0 | 3 × 10−4 | 1.8, 2.7f | |
| pHEBo-1.1(dpm3+4;2=HAS) | 16, 16 | 0, 1 | 0, 0 | 3 × 10−5 | 1.5, 1.0 | |
| pHEBo-1.1(dpm1+2;3=HAS) | 16, 16 | 6, 4 | 0, 0 | 4 × 10−4 | 6.1,3.0 | |
Average number of plasmids per cell for one or two Raji clones derived by transformation with the indicated plasmids.
NA, not applicable; no hygromycin-resistant clones obtained.
Much smaller clones 21 days postelectroporation than obtained with pHEBo-1.1.
pHEBo-1.1 gave rise to more positive wells at both 103 and 102 cells/well than pHEBo-1.1(in1/2[5]). The calculated frequencies are the same, however, because the error of this method becomes large when the calculation is based on a small number of negative or positive wells.
Determination of the copy number for these clones assumed that all the plasmids were dimers of pHEBo-1.1(in1/2[5]).
Actual copy number is lower due to the presence of both monomeric and dimeric forms of pHEBo-1.1(in3/4[5]) in these clones.
N.D., none detected.
One or two hygromycin-resistant Raji clones established with each plasmid were expanded under drug selection, and the plasmid DNA within the cells was analyzed by Southern blotting. All six plasmids with disabling mutations within individual or paired EBNA-1 binding sites were present as monomeric plasmids and at a lower copy number than pHEBo-1.1 (Fig. 3 and Table 2), further supporting the conclusion that all four EBNA-1 binding sites in DS are required for full replicator activity. However, the observation that pHEBo-1.1(dpm1+2) and pHEBo-1.1(dpm3+4) were maintained as monomeric circles without gross rearrangements and transformed Raji cells greater than 33-fold more efficiently than an oriP plasmid which lacks DS demonstrated that plasmids with only one of the two paired EBNA-1 binding sites intact exhibit significant replicator activity, as was previously reported (10).
Although wild-type oriP plasmids are replicated once per cell cycle and efficiently maintained in stably transformed cells, these plasmids are rapidly lost during the 2 weeks following introduction into EBNA-1-expressing cells. The establishment of an oriP-plasmid as a stable, autonomously replicating plasmid is dependent on an undefined epigenetic event that occurs in only a small percentage of the transfected cells (18). Clonal populations of Raji cells harboring derivatives of pHEBo-1.1 with inactivating mutations within individual or paired EBNA-1 sites grew noticeably more slowly than Raji cells containing wild-type pHEBo-1.1 when cultured in the presence of hygromycin to select for the presence of the plasmids. The impaired growth of these cells indicated that the mutations affected replicator function and not the epigenetic event that allows an oriP plasmid to avoid loss from the cell during the several weeks following its introduction. For example, the four cell clones containing pHEBo-1.1(dpm1) and pHEBo-1.1(dpm2) required 32 to 37 days following electroporation to expand for the analysis shown in Fig. 3 and cell clones containing pHEBo-1.1(dpm3) and pHEBo-1.1(dpm4) took 37 to 60 days for this expansion. In contrast, cells harboring wild-type pHEBo-1.1 took only 28 to 30 days of growth before they could be harvested and analyzed for plasmid copy number.
Mutations that inactivated sites 3 and 4 had a greater effect on transformation efficiency than mutations that inactivated sites 1 and 2, suggesting that the paired EBNA-1 binding sites in DS differ in their contributions to oriP replicator activity. This is not unexpected, as the paired sites differ in sequence context and the relative affinity of the EBNA-1 binding sites. Sites 4 and 3 are immediately flanked by 14-bp inverted repeats, each containing the 9-bp repeats referred to by Niller et al. as nonamer a and b (26) (Fig. 2). This 9-bp repeat is also found to the right of site 1 (nonamer c) in the same orientation as nonamer b. Differences in the relative affinities of the EBNA-1 binding sites within DS have been detected by an electrophoretic mobility shift assay (2) and by DNase I footprinting (10, 11, 31). Sites 1 and 4 exhibit the highest affinity for EBNA-1, while site 2 is the lowest-affinity binding site (10, 11).
To investigate further the relative contributions of individual and paired EBNA-1 binding sites in DS, the replication efficiencies of plasmids bearing mutations in these binding sites were determined using a quantitative, internally controlled replication assay (19) (see Materials and Methods for details). A representative Southern blot used for quantitation of replication efficiency is shown in Fig. 4, and the data are summarized in Table 3. In agreement with previous studies, an oriP plasmid lacking DS (pHEBo-2.2) replicated very inefficiently compared to pHEBo-1 (approximately 70-fold lower per cell generation) (10, 32, 34, 46). Mutation of any one EBNA-1 binding site within DS impaired replicator function, and mutations in site 3 or 4 reduced replicator activity to a greater extent than mutations in site 1 or 2 (P < 0.01). Furthermore, inactivation of sites 3 and 4 reduced replicator activity to a greater extent than inactivation of sites 1 and 2 (P < 0.01), and this result was consistent with the 10-fold-reduced transformation frequency obtained with pHEBo-1.1(dpm3+4) compared to that obtained with pHEBo-1.1(dpm1+2) (Table 2). In summary, these results showed that all four EBNA-1 binding sites within DS are required to ensure that replication will initiate once per cell cycle and that the contributions of the two pairs of EBNA-1 binding sites in DS to replicator function are not equivalent.
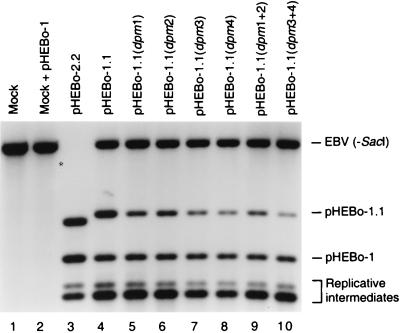
FIG. 4.
Short-term replication assay of pHEBo-1.1 derivatives containing inactivating mutations within EBNA-1 binding sites. Low-molecular-weight DNA isolated from Raji cells 72 h after electroporation with pHEBo-1 and a test plasmid [pHEBo-2.2 (lane 3), pHEBo-1.1 (lane 4), pHEBo-1.1(dpm1) (lane 5), pHEBo-1.1(dpm2) (lane 6), pHEBo-1.1(dpm3) (lane 7), pHEBo-1.1(dpm4) (lane 8), pHEBo-1.1(dpm1+2) (lane 9), or pHEBo-1.1(dpm3+4) (lane 10)] was digested with DpnI, EcoRV, and PstI (lanes 4 to 10) or DpnI, EcoRV, PstI, and SacI (lane 3) and analyzed by Southern blotting with a probe containing oriP FR. Low-molecular-weight DNA from Raji cells that had not been subjected to electroporation (lane 1) and that had been supplemented with 400 pg of pHEBo-1 DNA from dam+ E. coli (lane 2) was digested with DpnI, EcoRV, and PstI and included in the analysis. The positions of the diagnostic PstI-EcoRV (2,873 bp) and EcoRV-EcoRV (2,276 bp) fragments derived from pHEBo-1.1 and pHEBo-1, respectively, are indicated on the right. Also indicated are two fragments derived from pHEBo-1 and pHEBo-1.1 replicative intermediates containing DpnI-sensitive sites adjacent to oriP FR. EBV (-SacI), 4.4-kb PstI-EcoRV fragment from Raji EBV DNA containing oriP FR. Digestion of Raji EBV DNA with SacI, in addition to EcoRV and PstI, results in a 2.7-kb fragment bearing oriP FR which migrates slightly more rapidly than the pHEBo-1.1 diagnostic fragment (lane 3). This digestion allowed for the quantitation of DpnI-resistant pHEBo-2.2 DNA, which migrates slightly more rapidly than the Raji EBV DNA fragment generated by digestion with EcoRV and PstI (asterisk to the left of lane 3, fragment's expected position).
TABLE 3.
Replication efficiencies of pHEBo-1.1 derivatives with point or insertion mutations in oriP DS
| Expt. | Test plasmid | Repl. eff.a |
|---|---|---|
| 1 | pHEBo-2.2 | 1.4 ± 1.1 |
| pHEBo-1.1 | 100b | |
| pHEBo-1.1(dpm1) | 60 ± 2.2 | |
| pHEBo-1.1(dpm2) | 49 ± 3.0 | |
| pHEBo-1.1(dpm3) | 39 ± 3.9 | |
| pHEBo-1.1(dpm4) | 37 ± 3.7 | |
| pHEBo-1.1(dpm1+2) | 51 ± 5.2 | |
| pHEBo-1.1(dpm3+4) | 27 ± 3.1 | |
| 2 | pHEBo-2.2 | 2.8 ± 2.0 |
| pHEBo-1.1 | 100c | |
| pHEBo-1.1(dpm3+4;1/2[ATTTA]) | 26 ± 0.81 | |
| pHEBo-1.1(in1/2[5]) | 42 ± 2.7 | |
| pHEBo-1.1(in2/3[5]) | 57 ± 3.2 | |
| pHEBo-1.1(in3/4[5]) | 44 ± 1.9 | |
| pHEBo-1.1(dpm3+4;2=HAS) | 29 ± 0.43 | |
| pHEBo-1.1(dpm1+2;3=HAS) | 54 ± 1.7 |
Replication efficiencies (repl. eff.) are the percentages of replicated test plasmid DNA relative to replicated reference plasmid (pHEBo-1) DNA, normalized to pHEBo-1.1 (100%), per cell generation. The values are means ± standard deviations and were derived from three independent determinations.
Replication efficiency over three cell generations relative to pHEBo-1.1 was 141 ± 5.1%.
Replication efficiency over three cell generations relative to pHEBo-1.1 was 102 ± 15%.
The sequence context and spatial organization of EBNA-1 binding sites influence their contribution to replicator function.
EBNA-1 binds cooperatively to the paired sites within DS in vitro, and double point mutations that eliminate binding to site 4 also eliminate binding to site 3 (10, 39). We therefore expected the replication efficiencies of pHEBo-1.1(dpm4) and pHEBo-1.1(dpm3+4) to be the same. However, as suggested by the results of the Raji transformation assay (Table 2), pHEBo-1.1(dpm4) replicated more efficiently than pHEBo-1.1(dpm3+4) (P < 0.01) and the replication efficiencies of pHEBo-1.1(dpm3) and pHEBo-1.1(dpm4) were the same (P = 1; Table 3). These results suggest that, in vivo, either EBNA-1 can bind sites 3 and 4 independently or the mutations that eliminate EBNA-1 binding in vitro do not completely eliminate binding in vivo. Both scenarios could be explained by interactions between EBNA-1 and a host cell protein(s) bound to neighboring sequences in vivo, and interactions between EBNA-1 dimers could also facilitate the occupation of a crippled site. We expected the relative effects of mutations within EBNA-1 binding sites 1 and 2 to be the same as the effects of mutations in sites 3 and 4 but, instead, observed that the mutations within site 2 had a greater impact on replicator function than mutations within site 1 (P < 0.01). One notable difference between sites 1 and 2 is the presence of nonamer c adjacent to site 1 and the absence of a similarly positioned nonamer repeat next to site 2 (Fig. 1 and 2). Taken together, these results suggest that interactions between EBNA-1 and a host cell protein(s) bound to the nonamer repeats and between EBNA-1 dimers increase the ability of EBNA-1 to bind sites containing inactivating mutations.
We previously showed that the introduction of 5 or 10 bp between EBNA-1 binding sites 1 and 2 or sites 3 and 4 results in replicators with minimal activity when the other two sites are mutated to prevent EBNA-1 binding (10). These results indicated that two DNA-bound dimers of EBNA-1 with a specific spatial arrangement are required for an essential role of EBNA-1 in oriP replicator function. Because the binding of EBNA-1 to sites in DS does not exhibit the same spatial constraints (10) and because the above results suggested that EBNA-1 interacts with a host cell protein(s) bound to the nonamer repeats, it was of interest to determine if EBNA-1 contributes to replicator activity when bound to paired sites with altered spacing. pHEBo-1.1 derivatives with 5-bp insertions between adjacent EBNA-1 binding sites {pHEBo-1.1(in1/2[5]), pHEBo-1.1(in2/3[5]), and pHEBo-1.1(in3/4[5])} transformed Raji cells with reduced frequencies compared to pHEBo-1.1 (Table 2), and cell clones harboring these plasmids grew more slowly than cells containing wild-type pHEBo-1.1. The two cell clones containing pHEBo-1.1(in3/4[5]) grew particularly slowly and required 40 to 45 days following electroporation to expand for the experiment shown in Fig. 5 compared to 30 to 31 days for the clones harboring pHEBo-1.1. The slow growth of cells containing these pHEBo-1.1 derivatives under selective conditions for the plasmids is consistent with defects in replicator function. pHEBo-1.1(in1/2[5]) and pHEBo-1.1(in3/4[5]) were reduced in transformation frequency to a greater extent than pHEBo-1.1(in2/3[5]) and also differed in their abilities to be maintained as monomeric, unrearranged plasmids (Fig. 5). Forms of pHEBo-1.1(in1/2[5]) and pHEBo-1.1(in3/4[5]) with reduced mobility compared to supercoiled pHEBo-1.1 were detected when low-molecular-weight DNA from Raji clones was examined by Southern blotting without prior restriction enzyme digestion (Fig. 5B). Digestion of low-molecular-weight DNA from Raji cells harboring pHEBo-1.1(in1/2[5]) and pHEBo-1.1(in3/4[5]) with an enzyme that linearizes the founding plasmids yielded plasmid DNA that comigrated with linear pHEBo-1.1 DNA (Fig. 5A), suggesting that the reduced-mobility forms of pHEBo-1.1(in1/2[5]) and pHEBo-1.1(in3/4[5]) are multimers that were preferentially replicated. Plasmid DNA from one of the two Raji clones established with pHEBo-1.1(in1/2[5]) was recovered in E. coli and, of 18 isolates examined, all were determined to be dimers of pHEBo-1.1(in1/2[5]) (data not shown).
As predicted on the basis of differences in their transformation frequencies (Table 2), all three plasmids with insertions between adjacent EBNA-1 binding sites were reduced in replication efficiency compared to pHEBo-1.1 and pHEBo-1.1(in2/3[5]) replicated with greater efficiency than pHEBo-1.1(in1/2[5]) and pHEBo-1.1(in3/4[5]) (P < 0.01; Table 3). The impact of a 5-bp insertion between EBNA-1 sites 3 and 4 on replication efficiency was much less than the effect of mutating both sites, indicating that EBNA-1 bound to sites 3 and 4 with altered spacing can still contribute to replicator activity (P < 0.01). These results lead us to conclude that the spatial arrangement of EBNA-1 sites 3 and 4 in Raji cells is important, but not essential. The analysis of the effects of altering the spacing between EBNA-1 binding sites also underscored the functional difference(s) between the paired sites. The replication efficiencies of pHEBo-1.1(in3/4[5]), pHEBo-1.1(dpm3), and pHEBo-1.1(dpm4) did not differ (P ≥ 0.23), while the effect of altering the spacing of binding sites 1 and 2 was greater than the effect of mutating site 1 (P < 0.01) but not site 2 (P = 0.19).
An alternative explanation for these results is that the insertion mutations alter the sequences between the EBNA-1 sites so they can no longer participate in some unknown function such as interaction with a host cell protein. To investigate this possibility, the 5 bp between binding sites 1 and 2 were changed from 5′-ACCCG-3′ to 5′-ATTTA-3′ in pHEBo-1.1(dpm3+4). The transformation frequency and replication efficiency of this plasmid, pHEBo-1.1(dpm3 + 4;1/2[ATTTA]), did not differ from those of pHEBo-1.1(dpm3+4) (P = 1; Tables 2 and 3). Therefore, the replication defects of pHEBo-1.1(in1/2[5]) and, most likely, pHEBo-1.1(in3/4[5]) are due to an alteration in the spacing between the paired EBNA-1 binding sites. The replication defect of pHEBo-1.1(in2/3[5]) is also consistent with a strict spatial requirement for the two paired EBNA-1 binding sites. However, the 5-bp insertion within this plasmid disrupts the 14-bp sequence that is present in inverse orientation flanking EBNA-1 binding site 4. Additional experiments will be necessary to determine if the negative impact of the 5-bp insertion between EBNA-1 sites 2 and 3 is due to disruption of the spacing between these sites or the 14-bp repeat.
The low affinity of EBNA-1 binding sites 2 and 3 relative to that of sites 1 and 4 is not required for the paired sites to function in the initiation of oriP plasmid DNA replication.
We considered the possibility that the arrangement of higher-affinity (sites 1 and 4) and lower-affinity (sites 2 and 3) EBNA-1 binding sites within oriP DS is important for replicator function. To test this hypothesis, we changed site 2 within pHEBo-1.1(dpm3+4) and site 3 within pHEBo-1.1(dpm1+2) to high-affinity EBNA-1 binding sites. Base pair transitions were introduced at positions −4 and +8 within site 3 and at positions −3, +3, and +8 within site 2 (2). No differences in the ability of EBNA-1 to bind the altered sites or the adjacent intact sites in each pair were detected by DNase I footprinting performed with full-length EBNA-1 (data not shown). Both pHEBo-1.1(dpm3+4;2=HAS) and pHEBo-1.1(dpm1+2;3=HAS) were maintained as monomeric circles (Fig. 5) and yielded replication efficiencies in the short-term replication assay (Table 3) similar to those for pHEBo-1.1(dpm3+4) and pHEBo-1.1(dpm1+2) (P = 1). These results indicate that the pairing of higher- and lower-affinity EBNA-1 binding sites is not required for replicator function.
An element(s) located to the right of nonamer c contributes to oriP replicator activity.
Previous investigations of the boundaries of oriP relied on the ability of mutated oriP plasmids to replicate and be maintained over many cell generations (4, 32, 44). The reduced copy numbers observed with plasmids lacking the sequence to the right of EBV nt 9134 in several of these studies suggested that sequence elements in addition to EBNA-1 binding sites, though not essential for oriP function, may contribute to replicator activity (see Fig. 1 and 2 for the location of nonamer c and nucleotide coordinates) (32, 44). The oriP deletion derivatives examined in these previous studies lacked part or all of nonamer c in addition to missing all other right-hand EBV sequences. To determine if the sequence to the right of the EBNA-1 binding sites within DS is required for full replicator activity and to distinguish between a requirement for nonamer c and nucleotides to its right, a pHEBo-1.1 derivative lacking all EBV nucleotides to the right of nonamer c was tested for its ability to be replicated and maintained in Raji cells. This plasmid, pHEBo-1.1Δ9138-9516, transformed Raji cells to drug resistance with sixfold-reduced frequency compared to pHEBo-1.1, and its copy number in two Raji cell clones was also reduced in comparison to that of pHEBo-1.1 (average of 2.5 copies per cell for pHEBo-1.1Δ9138-9516 compared to 7.1 copies per cell for pHEBo-1.1; Table 2). In agreement with these results, the replication efficiency of pHEBo-1.1Δ9138-9516 was only 70% per cell generation (Table 4). The replication defect of pHEBo-1.1Δ9138-9516 may be due to the loss of an auxiliary element(s) that facilitates the initiation of DNA replication or, alternatively, a negative effect of placing plasmid sequences adjacent to DS (see, e.g., reference 4). To address the latter possibility, the right-hand flanking sequence was changed in two ways. A 683-bp fragment from phagemid M13mp18 was substituted for EBV nt 9138 to 9459 in pHEBo-1.1 and the ability of this plasmid and its parental plasmid lacking M13 DNA (pHEBo-1.1Δ9138-9459) to be replicated was determined. The EBV sequences present in pHEBo-1.1Δ9138-9516 (nt 7333 to 9137) were also introduced into pBluescript KS, and the replication efficiency of this plasmid (pBS/FR.DS) was compared to that of pBluescript derivatives which contain EBV nt 7333 to 9516 (pBS/oriP) or EBV nt 7333 to 8994 (pBS/FR). pBS/FR replicated very inefficiently, as expected for a plasmid lacking DS. pHEBo-1.1Δ9138-9516, pHEBo-1.1Δ9138-9459, pHEBo-1.1Δ9138-9459+M13, and pBS/FR.DS replicated with the same reduced efficiency (approximately 70 to 80% per cell generation), indicating that the reduced replication efficiency of pHEBo-1.1Δ9138-9516 was not likely due to changes in flanking sequences (Table 4). The effect of deleting EBV nt 9138 to 9516 from a plasmid with inactivating mutations within EBNA-1 binding sites 1 and 2 was also examined. If the right-hand sequence contributes to replicator activity independently of EBNA-1 binding sites 1 and 2, the replication efficiency of this plasmid (pHEBo-2.2.1; Fig. 6) would be expected to be lower than the replication efficiency of pHEBo-1.1(dpm1+2). The replication efficiencies of these two plasmids, however, were the same (P = 0.98). Taken together, these results suggest that the deletion of EBV nt 9138 to 9459 either disrupts or eliminates an element(s) required for full activity of the oriP replicator and this putative element functions in conjunction with EBNA-1 binding sites 1 and 2.
TABLE 4.
Replication efficiencies of pHEBo-1.1 and pBS/oriP derivatives with deletions of sequences flanking DS
| Test plasmid | Repl. eff.a |
|---|---|
| pHEBo-2.2 | 0.54 ± 0.21 (3) |
| pHEBo-1.1 | 100 (3) |
| pHEBo-1.1Δ9138-9516 | 66 ± 3.6 (3) |
| pHEBo-1.1Δ9138-9459 | 72 ± 5.1 (3) |
| pHEBo-1.1Δ9138-9459+M13 | 61 ± 3.1 (3) |
| pBS/FR | 11, 3.0 (7.9)b |
| pBS/FR.DS | 74 ± 14 (5) |
Replication efficiencies (repl. eff.) are the percentages of replicated test plasmid DNA relative to replicated reference plasmid (pHEBo-1 or pBS/oriP) DNA per cell generation. The replication efficiencies of pHEBo-1.1-derived plasmids are expressed relative to that of pHEBo-1.1, which was set at 100% (122 ± 5.1). Unless otherwise indicated, the values are the means ± sample standard deviations and the numbers of experiments are included in parentheses.
Replication efficiency of test plasmid per cell generation. The values are the results of two independent experiments, and the number in parentheses is the average of the two experiments.
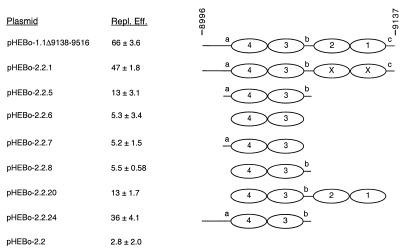
FIG. 6.
Replication efficiencies of pHEBo-1.1 and -2.2 derivatives with sequences in oriP DS deleted. The replication efficiencies (Repl. Eff.) of pHEBo-1.1Δ9138-9516 and pHEBo-2.2 derivatives lacking sequences in oriP DS were determined in triplicate and are given as percent per cell generation with standard deviations relative to that of pHEBo-1.1. These plasmids all contain oriP FR and the sequences normally present between FR and DS (EBV nt 7333 to 8995; see Fig. 1). The DS-derived sequences present in each plasmid are represented schematically at the right, and the nucleotide coordinates are at the top. Ovals, EBNA-1 binding sites; a, b, and c, nonamer repeats; EBNA-1 binding sites labeled X, sites containing inactivating mutations at the +5 and −5 positions (10). The replication efficiency of pHEBo-1.1 was 102 ± 15%, and that of pHEBo-1.1Δ9138-9516 was determined in the experiment presented in Table 4.
Minimal sequence requirements for the oriP replicator.
The results of our mutational analysis of EBNA-1 binding sites (10) showed that the paired sites are essential components of the oriP replicator, and data presented here suggest that at least one additional sequence element, located between EBV nt 9138 and 9516, is required to ensure that replication of oriP plasmids will initiate once per cell cycle. To identify the minimal fragment from oriP DS that provides replicator activity and to determine if there are additional, nonessential components that contribute to replicator function, we constructed derivatives of pHEBo-1.1Δ9138-9516 with mutations between EBV nt 8995 and 9137 and analyzed their ability to be replicated in the short-term assay. The approach taken was to consider the 14-bp repeats flanking EBNA-1 binding sites 4 and 3 (containing nonamers a and b) and nonamer c potential functional elements and to test the EBNA-1 binding sites in various combinations with the nonamer repeats and other flanking sequences. We chose to focus on sites 3 and 4 because they contribute more to replicator activity than sites 1 and 2 (Tables 2 and 3). The oriP DS sequences present in these plasmids are summarized in Table 1 and are shown schematically in Fig. 6. A plasmid containing EBNA-1 binding sites 3 and 4 but lacking all other sequences from DS and right-hand flanking sequences (pHEBo-2.2.6) resembled the parental plasmid lacking DS (pHEBo-2.2) in its inability to be replicated in Raji cells (P = 1), suggesting that one or more elements, in addition to these EBNA-1 binding sites, are required to constitute minimal replicator activity. Inclusion of either of the 14-bp repeats flanking sites 3 and 4 did not give rise to replicator activity (compare pHEBo-2.2 with pHEBo-2.2.7 and -2.2.8; P = 1), but a plasmid containing EBNA-1 binding sites 3 and 4 and both 14-bp repeats replicated, albeit inefficiently (compare pHEBo-2.2 with pHEBo-2.2.5; P < 0.01). Inefficient replication was also observed when all four EBNA-1 binding sites and the 17-bp between the paired sites containing nonamer b were present (compare pHEBo-2.2 with pHEBo-2.2.20; P < 0.01). Replication efficiency increased significantly when the 24 bp between nt 8994 and 9019 were restored (compare pHEBo-2.2.5 with pHEBo-2.2.24; P < 0.01). The latter result indicates that the inclusion of this sequence either provides an element that enhances the activity of EBNA-1 binding sites 3 and 4 and flanking repeats or places this minimal replicator at an appropriate distance from an auxiliary element located between oriP FR and DS.
DISCUSSION
Assessment of oriP replicator function.
Previous genetic analyses of the EBV oriP replicator carried out by this and other laboratories relied predominantly on quantitation of the ability of mutated oriP plasmids to transform EBNA-1-expressing cells to drug resistance and the copy number of the plasmids in stably transformed cells cultured over many cell generations (4, 10, 11, 22, 32, 44, 46). The transformation assays used in these previous experiments suffered from the absence of an internal control for transfection efficiency. Furthermore, the copy number of an oriP plasmid is determined not only by the replication efficiency of the plasmid but also by the amount of plasmid DNA introduced into a cell at the time of transfection (47). For these reasons, these experimental approaches have provided only a semiquantitative measure of replicator function and have been unable to allow accurate comparisons between plasmids with mutations that reduce but do not eliminate replicator activity (see, e.g., references 10 and 44). These limitations are highlighted by the results presented in this study. Although the transformation frequencies of plasmids reported here generally reflected relative replication efficiencies, there were several notable exceptions that suggested the influence of experimental variation [e.g., pHEBo-1.1(dpm3+4;2=HAS), pHEBo-1.1(dpm1+2), and pHEBo-1.1(in1/2 [5]); Tables 2 and 3]. Note, too, that the plasmid copy numbers for Raji clones transformed by pHEBo-1.1(dpm4) and pHEBo-1.1(dpm3+4) were similar and did not reflect the 20-fold difference in transformation efficiency (Table 2) and different replication efficiencies (Table 3) exhibited by these plasmids. In several previous studies, short-term replication assays were also used to characterize mutated oriP plasmids. These assays either lacked an internal control for transfection efficiency and experimental error in the isolation of plasmid DNA from transfected-cell populations (10) or compared the amount of replicated plasmid DNA to the total amount of plasmid DNA recovered from the cells (34). The latter comparison assumes that all of the plasmid DNA isolated from the cells is nuclear and available for replication and does not take into account possible contamination with extracellular plasmid DNA or plasmid DNA that is in an intracellular compartment separate from the nucleus. To solve these problems and enable the identification of genetic elements that are essential for replicator function or augment the activity of essential elements, we adopted a quantitative, internally controlled, short-term replication assay which compares the replication efficiency of a test plasmid to that of a previously characterized reference plasmid that is replicated once per S phase (19). Using this assay, we characterized the minimal sequences that are required for initiation of DNA replication from within oriP and identified additional elements that are required to ensure that replication will initiate once per cellular S phase.
Multiple functional elements of the oriP replicator.
Our analysis of pHEBo-1.1 derivatives bearing mutations in EBNA-1 sites that inhibit binding in vitro showed that all four EBNA-1 binding sites within oriP DS are required for full replicator activity in Raji cells. These findings provide an explanation for the conservation of all four EBNA-1 binding sites in EBV type 1 and type 2 isolates. Although we have not conducted quantitative analyses of the replication efficiencies of these plasmids in other EBNA-1-expressing cells, the reduced copy number of these plasmids in the D98/Raji hybrid cell line suggests that the requirement for all four sites is not restricted to B lymphocytes (10). Yates et al. reported that oriP plasmids bearing six-base substitutions near the center of each of the EBNA-1 binding sites in DS transformed Raji cells to drug resistance less efficiently than a plasmid containing four intact sites. In addition, the copy numbers of these mutated plasmids were reduced in an EBNA-1-expressing human osteosarcoma cell line as well as in Raji cells (46). Because the mutations created for their experiments may have altered genetic elements in addition to the EBNA-1 binding sites, it was not certain that the effects of the mutations were due to the disruption of the EBNA-1 binding sites. The data presented here substantiate previous results and support two conclusions: all four EBNA-1 binding sites are required for full replicator activity, and this requirement is likely universal and not cell type specific.
Despite the fact that all four EBNA-1 binding sites within oriP DS are required for full replicator activity, significant activity was observed when both sites within either pair were inactivated by point mutations or one pair was deleted (10, 46; this study). Thus, only one pair of EBNA-1 binding sites is required for minimal replicator activity. The similar transformation frequencies and copy numbers observed with plasmids bearing mutations within either pair of EBNA-1 binding sites in previous studies led to the conclusion that the paired sites contribute similarly to replicator activity (10, 46). However, data presented here clearly show that the paired EBNA-1 binding sites are not functionally equivalent. A plasmid bearing inactivating mutations within binding sites 1 and 2 replicated once every two cellular S phases, on average, while a plasmid with inactivating mutations within binding sites 3 and 4 replicated less than once every three S phases. Because EBNA-1 binding sites 3 and 4 contribute more to replicator activity than sites 1 and 2, we consider sites 3 and 4 to be components of the minimal replicator element and sites 1 and 2 to be components of an ancillary element.
Previous studies placed the right-hand boundary of the oriP replicator between EBNA-1 binding sites 1 and 2 and concluded that the sequence to the right of binding site 2 (EBV nt 9107 to 9516) is dispensable for replicator function (17, 32, 44). Notably, the copy numbers of oriP plasmids that lacked either nt 9107 to 9516 or 9135 to 9516 were reduced in comparison to that of a plasmid that contained this sequence, suggesting that an auxiliary element(s) is contained within this DNA fragment (32, 44). The results of experiments presented here showed that the deletion of nt 9138 to 9516 reduced replication efficiency approximately 30% per cell generation and that this effect was most likely due to the elimination of an element(s) that is required for full replicator activity. The deletion of nt 9138 to 9516 did not impinge on nonamer c and the 2 bp to its right, which preserved a purine and pyrimidine match with the repeats flanking EBNA-1 sites 3 and 4 (Fig. 2). Therefore, we conclude that the loss of replicator activity was due to the elimination of a functional element other than nonamer c.
Kirchmaier and Sugden identified an element that supports inefficient DNA replication in the absence of oriP DS (termed Rep*) within EBV nt 9364 to 9667. Plasmids bearing oriP FR and three copies of Rep* replicated more efficiently than a plasmid bearing only one copy (17). pHEBo-1.1Δ9138-9459 and pHEBo-1.1Δ9138-9516 lack 95 and 152 bp, respectively, of the DNA fragment harboring Rep*, and it is possible that the reduced replication efficiencies of these plasmids are due to the disruption of this functional element. However, Rep* was not found to improve the efficiency of replication of an oriP plasmid containing EBV nt 8995 to 9137 (17), suggesting that the auxiliary element(s) to the right of nonamer c may be distinct from Rep*. Additional experiments are necessary to define this element(s) and determine how it facilitates oriP plasmid DNA replication. Further work is also required to determine if another functional element of the oriP replicator is located to the left of nonamer a, as was suggested by the large difference in replication efficiencies of pHEBo-2.2.5 and pHEBo-2.2.20 (Fig. 6).
The minimal replicator.
EBNA-1 binding sites 3 and 4 did not support the initiation of DNA replication by themselves, but minimal replicator activity was observed when both of the 14-bp repeats that flank these EBNA-1 binding sites were also present. It is unlikely that this effect is due to an alteration in the position of the EBNA-1 binding sites relative to flanking sequences because the presence of only repeat a or repeat b adjacent to EBNA-1 binding sites 3 and 4 did not provide for detectable activity (Fig. 6) and because substitution mutations within the 14-bp repeats reduced plasmid copy number when EBNA-1 binding site 1 or sites 1 and 2 were deleted (46). Because the EBNA-1 binding sites are essential components of the oriP replicator (10) and because sites 3 and 4 contribute more to replicator activity than sites 1 and 2, we conclude that the minimal replicator is contained within the 65-bp fragment encompassing sites 3 and 4 and the flanking 14-bp repeats. Yates et al. showed that substitution mutations that substantially altered all three of the nonamer repeats had no apparent effect on plasmid maintenance. This result led them to conclude that the nonamer repeats are not part of the minimal replicator. These same mutations had a profound effect, however, when they were introduced into oriP plasmids that contained only “one-half” of the DS. Based on these and other results, they proposed that the minimal replicator of oriP is composed of two EBNA-1 binding sites with 21-bp center-to-center spacing (46). Although it is not clear why the mutation of all three nonamer repeats was found to have no apparent effect when assayed in the context of an otherwise wild-type replicator, results presented here demonstrate that the EBNA-1 binding sites do not provide for significant replicator activity in isolation.
EBNA-1 is bound to its recognition elements within the DS throughout the cell cycle, indicating that a cell cycle-regulated event distinct from the binding of EBNA-1 to the replicator is responsible for the regulated replication of oriP plasmids (14, 26). Results from in vivo dimethyl sulfate footprinting performed with synchronized Raji cells provided evidence for the cell cycle-regulated interaction of a protein with the nonamer repeats. Specifically, increased protection or reactivity of certain guanines within each of the nonamer repeats was observed in cells blocked in late G1, and this pattern of protection and reactivity differed from that observed in cells blocked in mitosis (26). These changes in methylation protection or reactivity suggest that a protein is bound to the nonamer repeats in late G1 but not in mitosis. This pattern is reminiscent of cell cycle-dependent alterations in the DNase I footprints at a yeast chromosomal origin of DNA replication that have been attributed to the formation and dissociation of the prereplication complex (5). The relative replication efficiencies of plasmids bearing substitution mutations within individual or paired EBNA-1 binding sites or 5-bp insertion mutations between paired sites strongly support the conclusion that protein-protein interactions occur not only between EBNA-1 dimers bound to the paired sites but also between EBNA-1 and an unidentified host cell protein(s) bound to the flanking repeats. These findings lead us to propose a model for the initiation of DNA replication at oriP in which EBNA-1 plays a crucial role by the recruitment of a host cell protein(s) to three sites in the DS.
ACKNOWLEDGMENTS
We thank K. Takada for providing Akata cells and L. Moore and W. Bauer for assistance with statistical analysis. We also thank A. Stenlund for many helpful discussions and M. Turner and P. Hearing for critical review of the manuscript.
This work was supported by grants from the National Cancer Institute (CA75992 and 5T32 CA09176).
REFERENCES
- 1.Adams A. Replication of latent Epstein-Barr virus genomes in Raji cells. J Virol. 1987;61:1743–1746. doi: 10.1128/jvi.61.5.1743-1746.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambinder R F, Shah W A, Rawlins D R, Hayward G S, Hayward S D. Definition of the sequence requirements for binding of the EBNA-1 protein to its palindromic target sites in Epstein-Barr virus DNA. J Virol. 1990;64:2369–2379. doi: 10.1128/jvi.64.5.2369-2379.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baer R, Bankier A T, Biggin M D, Deininger P L, Farrell P J, Gibson T J, Hatfull G, Hudson G S, Satchwell S C, Sequin C, Tuffnell P S, Barrell B G. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984;310:207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- 4.Chittenden T, Lupton S, Levine A J. Functional limits of oriP, the Epstein-Barr virus plasmid origin of replication. J Virol. 1989;63:3016–3025. doi: 10.1128/jvi.63.7.3016-3025.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diffley J F, Cocker J H, Dowell S J, Rowley A. Two steps in the assembly of complexes at yeast replication origins in vivo. Cell. 1994;78:303–316. doi: 10.1016/0092-8674(94)90299-2. [DOI] [PubMed] [Google Scholar]
- 6.Fisher D K, Miller G, Gradoville L, Heston L, Westrate M W, Maris W, Wright J, Brandsma J, Summers W C. Genome of a mononucleosis Epstein-Barr virus contains DNA fragments previously regarded to be unique to Burkitt's lymphoma isolates. Cell. 1981;24:543–553. doi: 10.1016/0092-8674(81)90345-7. [DOI] [PubMed] [Google Scholar]
- 7.Frappier L, O'Donnell M. EBNA1 distorts oriP, the Epstein-Barr virus latent replication origin. J Virol. 1992;66:1786–1790. doi: 10.1128/jvi.66.3.1786-1790.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gahn T A, Schildkraut C L. The Epstein-Barr virus origin of plasmid replication, oriP, contains both the initiation and termination sites of DNA replication. Cell. 1989;58:527–535. doi: 10.1016/0092-8674(89)90433-9. [DOI] [PubMed] [Google Scholar]
- 9.Gussander E, Adams A. Electron microscopic evidence for replication of circular Epstein-Barr virus genomes in latently infected Raji cells. J Virol. 1984;52:549–556. doi: 10.1128/jvi.52.2.549-556.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison S, Fisenne K, Hearing J. Sequence requirements of the Epstein-Barr virus latent origin of DNA replication. J Virol. 1994;68:1913–1925. doi: 10.1128/jvi.68.3.1913-1925.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hearing J, Mulhaupt Y, Harper S. Interaction of Epstein-Barr virus nuclear antigen 1 with the viral latent origin of replication. J Virol. 1992;66:694–705. doi: 10.1128/jvi.66.2.694-705.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinuma Y, Grace J T. Cloning of immunoglobulin-producing human leukemic and lymphoma cells in long term cultures. Proc Soc Exp Biol Med. 1967;124:107–111. doi: 10.3181/00379727-124-31677. [DOI] [PubMed] [Google Scholar]
- 13.Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh D J, Camiolo S M, Yates J L. Constitutive binding of EBNA1 protein to the Epstein-Barr virus replication origin, oriP, with distortion of DNA structure during latent infection. EMBO J. 1993;12:4933–4944. doi: 10.1002/j.1460-2075.1993.tb06187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hung S C, Kang M-S, Kieff E. Maintenance of Epstein-Barr virus (EBV) oriP-based episomes requires EBV-encoded nuclear antigen-1 chromosome-binding domains: which can be replaced by high-mobility group-I or histone H1. Proc Natl Acad Sci USA. 2001;98:1865–1870. doi: 10.1073/pnas.031584698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanda T, Otter M, Wahl G M. Coupling of mitotic chromosome tethering and replication competence in Epstein-Barr virus-based plasmids. Mol Cell Biol. 2001;21:3576–3588. doi: 10.1128/MCB.21.10.3576-3588.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirchmaier A L, Sugden B. Rep*: a viral element that can partially replace the origin of plasmid DNA synthesis of Epstein-Barr virus. J Virol. 1998;72:4657–4666. doi: 10.1128/jvi.72.6.4657-4666.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leight E R, Sugden B. Establishment of an oriP replicon is dependent upon an infrequent, epigenetic event. Mol Cell Biol. 2001;21:4149–4161. doi: 10.1128/MCB.21.13.4149-4161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J J, Peden K W C, Dixon R A F, Kelly T. Functional organization of the simian virus 40 origin of DNA replication. Mol Cell Biol. 1986;6:1117–1128. doi: 10.1128/mcb.6.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindahl T, Adams A, Bjursell G, Bornkamm G W, Kaschka-Dierich C, Jehn U. Covalently closed circular duplex DNA of Epstein-Barr virus in a human lymphoid cell line. J Mol Biol. 1976;102:511–530. doi: 10.1016/0022-2836(76)90331-4. [DOI] [PubMed] [Google Scholar]
- 21.Little R D, Schildkraut C L. Initiation of latent DNA replication in the Epstein-Barr virus genome can occur at sites other than the genetically defined origin. Mol Cell Biol. 1995;15:2893–2903. doi: 10.1128/mcb.15.5.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lupton S, Levine A J. Mapping genetic elements of Epstein-Barr virus that facilitate extrachromosomal persistence of Epstein-Barr virus-derived plasmids in human cells. Mol Cell Biol. 1985;5:2533–2542. doi: 10.1128/mcb.5.10.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 24.Marechal V, Dehee A, Chikhi-Brachet R, Piolot T, Coppey-Moisan M, Nicolas J C. Mapping EBNA-1 domains involved in binding to metaphase chromosomes. J Virol. 1999;73:4385–4392. doi: 10.1128/jvi.73.5.4385-4392.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melendy T, Sedman J, Stenlund A. Cellular factors required for papillomavirus DNA replication. J Virol. 1995;69:7857–7867. doi: 10.1128/jvi.69.12.7857-7867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niller H H, Glaser G, Knuchel R, Wolf H. Nucleoprotein complexes and DNA 5′-ends at oriP of Epstein-Barr virus. J Biol Chem. 1995;270:12864–12868. doi: 10.1074/jbc.270.21.12864. [DOI] [PubMed] [Google Scholar]
- 27.Nonoyama M, Pagano J S. Separation of Epstein-Barr virus DNA from large chromosomal DNA in non-virus-producing cells. Nat New Biol. 1972;238:169–171. doi: 10.1038/newbio238169a0. [DOI] [PubMed] [Google Scholar]
- 28.Norio P, Schildkraut C L, Yates J L. Initiation of DNA replication within oriP is dispensable for stable replication of the latent Epstein-Barr virus chromosome after infection of established cell lines. J Virol. 2000;74:8563–8574. doi: 10.1128/jvi.74.18.8563-8574.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pritchett R, Pedersen M, Kieff E. Complexity of EBV homologous DNA in continuous lymphoblastoid cell lines. Virology. 1976;74:227–231. [PubMed] [Google Scholar]
- 30.Pulvertaft R J V. Cytology of Burkitt's lymphoma (African lymphoma) Lancet. 1964;i:238–240. doi: 10.1016/s0140-6736(64)92345-1. [DOI] [PubMed] [Google Scholar]
- 31.Rawlins D R, Milman G, Hayward S D, Hayward G S. Sequence-specific DNA binding of the Epstein-Barr virus nuclear antigen (EBNA-1) to clustered sites in the plasmid maintenance region. Cell. 1985;42:859–868. doi: 10.1016/0092-8674(85)90282-x. [DOI] [PubMed] [Google Scholar]
- 32.Reisman D, Yates J, Sugden B. A putative origin of replication of plasmids derived from Epstein-Barr virus is composed of two cis-acting components. Mol Cell Biol. 1985;5:1822–1832. doi: 10.1128/mcb.5.8.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sculley T, Krauer K G, Kienzle N. Comparison of A-type and B-type Epstein-Barr virus. EBV Rep. 1999;6:1–6. [Google Scholar]
- 34.Shirakata M, Hirai K. Identification of minimal oriP of Epstein-Barr virus required for DNA replication. J Biochem. 1998;123:175–181. doi: 10.1093/oxfordjournals.jbchem.a021907. [DOI] [PubMed] [Google Scholar]
- 35.Snudden D K, Smith P R, Lai D, Ng M-H, Griffin B E. Alterations in the structure of the EBV nuclear antigen, EBNA1, in epithelial cell tumours. Oncogene. 1995;10:1545–1552. [PubMed] [Google Scholar]
- 36.Sokal R R, Rohlf F J. Biometry. San Francisco, Calif: W. H. Freeman and Company; 1981. pp. 82–88. [Google Scholar]
- 37.Sugden B, Marsh K, Yates J. A vector that replicates as a plasmid and can be efficiently selected in B-lymphoblasts transformed by Epstein-Barr virus. Mol Cell Biol. 1985;5:410–413. doi: 10.1128/mcb.5.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugden B, Warren N. Plasmid origin of replication of Epstein-Barr virus, oriP, does not limit replication in cis. Mol Biol Med. 1988;5:85–94. [PubMed] [Google Scholar]
- 39.Summers H, Barwell J A, Pfuetzner R A, Edwards A M, Frappier L. Cooperative assembly of EBNA1 on the Epstein-Barr virus latent origin of replication. J Virol. 1996;70:1228–1231. doi: 10.1128/jvi.70.2.1228-1231.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takada K. Cross-linking of cell surface immunoglobulins induces Epstein-Barr virus in Burkitt lymphoma lines. Int J Cancer. 1984;33:27–32. doi: 10.1002/ijc.2910330106. [DOI] [PubMed] [Google Scholar]
- 41.Van Scoy S, Watakabe I, Krainer A R, Hearing J. Human p32: a coactivator for Epstein-Barr virus nuclear antigen-1-mediated transcriptional activation and possible role in viral latent cycle DNA replication. Virology. 2000;275:145–157. doi: 10.1006/viro.2000.0508. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Finan J E, Middeldorp J M, Hayward S D. P32/TAP, a cellular protein that interacts with EBNA-1 of Epstein-Barr virus. Virology. 1997;236:18–29. doi: 10.1006/viro.1997.8739. [DOI] [PubMed] [Google Scholar]
- 43.Wu H, Ceccarelli D F J, Frappier L. The DNA segregation mechanism of Epstein-Barr virus nuclear antigen 1. EMBO Rep. 2000;1:140–144. doi: 10.1093/embo-reports/kvd026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yates J, Warren N, Reisman D, Sugden B. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc Natl Acad Sci USA. 1984;81:3806–3810. doi: 10.1073/pnas.81.12.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yates J L. Epstein-Barr virus DNA replication. In: DePamphilis M L, editor. DNA replication in eukaryotic cells. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 751–773. [Google Scholar]
- 46.Yates J L, Camiolo S M, Bashaw J M. The minimal replicator of Epstein-Barr virus oriP. J Virol. 2000;74:4512–4522. doi: 10.1128/jvi.74.10.4512-4522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yates J L, Guan N. Epstein-Barr virus-derived plasmids replicate only once per cell cycle and are not amplified after entry into cells. J Virol. 1991;65:483–488. doi: 10.1128/jvi.65.1.483-488.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yates J L, Warren N, Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. Nature. 1985;313:812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]
- 49.Zhang D, Frappier L, Gibbs E, Hurwitz J, O'Donnell M. Human RPA (hSSB) interacts with EBNA1, the latent origin binding protein of Epstein-Barr virus. Nucleic Acids Res. 1998;26:631–637. doi: 10.1093/nar/26.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]