Abstract
Background
Worldwide, countries are examining whether to implement 1-dose human papillomavirus (HPV) vaccination instead of using 2 doses. To inform policy, we sought to project the population-level impact and efficiency of switching from 2-dose to 1-dose gender-neutral routine HPV vaccination in Canada.
Methods
We used HPV-ADVISE, an individual-based transmission-dynamic model of HPV infections and diseases, to mathematically model vaccination programs in 2 provinces, Quebec, a province with high HPV vaccination coverage (around 85%), and Ontario, which has lower coverage (around 65%). We examined non-inferior and pessimistic scenarios of the efficacy (vaccine efficacy of 98% or 90%) and average vaccine duration (lifelong, 30 yr, or 25 yr) of 1 dose compared with 2 doses (98% vaccine efficacy, lifelong vaccine duration). Our main outcomes were the relative reduction in HPV-16 (by sex) and cervical cancers, and the number of doses needed to prevent 1 cervical cancer.
Results
Our model projected that 1-dose HPV vaccination would avert a similar number of cervical cancers as 2 doses in Canada, under various scenarios. Under the most pessimistic scenario (25-yr vaccine duration), 1-dose vaccination would avert fewer cervical cancers than 2 doses, by about 3 percentage points over 100 years. All 1-dose scenarios were projected to lead to elimination of cervical cancer (< 4 cervical cancers/100 000 female-years) and to be a substantially more efficient use of vaccine doses than a 2-dose scenario (1-dose v. no vaccination = 800–1000 doses needed to prevent 1 cervical cancer; incremental doses for 2-dose v. 1-dose vaccination > 10 000 doses needed to prevent 1 additional cervical cancer).
Interpretation
If the average duration of 1-dose protection is longer than 25 years, a 1-dose HPV vaccination program would protect those vaccinated during their peak ages of sexual activity and prevent a similar number of HPV-related cancers as a 2-dose program, while being a more efficient use of vaccine doses.
In April 2022, the World Health Organization (WHO) Strategic Advisory Group of Experts on Immunization (SAGE) announced that human papillomavirus (HPV) vaccination with a single dose could be considered for people aged 9–20 years.1 This advice was based on evidence from clinical trials indicating very high and sustained protection against HPV infections with a single dose.2–4 Since then, updated data from the Costa Rica HPV Vaccine Trial (CVT) showed stable antibodies up to 16 years after 1-dose vaccination5 and the Kenya single-dose HPV vaccine efficacy randomized controlled trial (KEN SHE RCT) showed 98% 1-dose efficacy after 36 months.6 The SAGE announcement was also based on modelling findings that suggested that 1-dose vaccination could lead to similar population-level reductions in cervical cancers as 2 doses in low- and middle-income countries, while being a more efficient use of vaccine doses, assuming that 1-dose protection lasts longer than 20 years.7,8
Most of the 35 countries or so that have adopted 1-dose HPV vaccination are low- and middle-income countries. As of May 2024, about half a dozen high-income countries have adopted a 1-dose vaccination strategy (e.g., United Kingdom, Australia), while some have decided to remain with 2 doses (e.g., Sweden) and many others are evaluating the issue.9,10 Several high-income countries, such as Canada, have been vaccinating against HPV for more than 15 years and are now observing substantial reductions in HPV-related diseases.11–14 Concerns have been raised about rebounds in the incidence of HPV infection or cervical cancer that could result from a switch to 1-dose vaccination if duration of protection of 1 dose is shorter than 2 doses.15
Our objective was to project the population-level impact and efficiency of switching from 2-dose to 1-dose HPV vaccination in Canada to help inform recommendations of the Canadian National Advisory Committee on Immunization and the Comité sur l’immunisation du Québec.16,17
Methods
Study design
We used mathematical modelling and up-to-date vaccine efficacy and durability data to project the population-level impact and efficiency of 1-dose, gender-neutral, routine HPV vaccination in Canada, compared with 2-dose vaccination, for different HPV-related outcomes among females and males. We examined non-inferior and pessimistic assumptions of 1-dose efficacy and duration of protection.
Model description
We used HPV-ADVISE (Agent-Based Dynamic Model for VaccInation and Screening Evaluation), an individual-based, transmission-dynamic model. This model has been extensively peer reviewed;18–21 validated to post-vaccination data from Canada17 and elsewhere,21,22 as well as to other models (Appendix 1, Figure A1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.240787/tab-related-content);23,24 and used to inform policy decisions on HPV vaccination in Canada and globally.25–30 Briefly, HPV-ADVISE simulates HPV transmission through sexual activity and type-specific progression from infection to cancers of the cervix, anus, oropharynx, vagina, vulva, and penis. Eighteen HPV types are modelled individually. The model can capture a wide variety of strategies of HPV vaccination and HPV or cervical cancer screening. For model projections, we used HPV-ADVISE Canada, which was calibrated to highly stratified Canadian sexual behaviour, HPV epidemiology, and cervical screening data from the pre-vaccination era, stratified by age and sex, as well as by HPV type for epidemiological outcomes. Details of HPV-ADVISE and its methodology are provided in its technical appendix (https://marc-brisson.net/HPVadvise-Can.pdf).
Settings and HPV vaccination strategies
Since 2007, all provinces and territories in Canada have implemented publicly funded HPV immunization programs. We modelled Quebec and Ontario as they have high and low HPV vaccination coverage in Canada, respectively.31 For model projections, we reproduced historical changes in HPV vaccination programs and coverage by age and sex for both provinces from 2007 to 2023 (Appendix 1, Figure A2).31 In 2007/08, both provinces introduced routine school-based HPV vaccination with the quadrivalent vaccine for females; by 2017/18 both had switched to gender-neutral programs with nonavalent vaccine.
The initial implementation of the routine HPV vaccination program in Quebec targeted 9-year-old females, with a 5-year catch-up period for those aged 14 years, with the vaccine provided free of charge for females aged 9–17 years. In 2019/20, Quebec switched to a 5-year extended schedule, with the first dose given at age 9 years and the second dose to be given at age 14 years. In Ontario, the inital program targeted 13-year-old females and switched to females and males aged 12 years in 2016. From 2007/08 to 2023, Quebec had a high mean vaccination coverage (with around 85% of people receiving 2 doses by age 14 yr); Ontario had lower vaccination coverage over this period (with around 65% of people receiving 2 doses by the end of grade 7).
From 2024 onward, we assumed that vaccination coverage was stable and was not affected by a potential switch to 1 dose (i.e., 85% coverage in Quebec, and 67% among females and 62% among males in Ontario). To estimate the population-level impact and efficiency of 1-dose HPV vaccination, we compared 1-dose gender-neutral HPV vaccination starting in 2024 with the status quo of 2-dose gender-neutral HPV vaccination. Given that the focus of the study was on HPV vaccination, all scenarios assumed that cytology-based cervical screening and participation rates were as per status quo.
Vaccine efficacy and duration scenarios
We assumed that 2 doses of the nonavalent HPV vaccine provides 98% efficacy against HPV types targeted by the vaccine and a lifelong duration of protection.32 For the 1-dose base-case scenario, we assumed a similar efficacy and duration of protection as the 2-dose scenario. The 98% 1-dose vaccine efficacy represented the final KEN SHE RCT results.6 The India International Agency for Research on Cancer (IARC) and CVT trials showed sustained protection for up to 12 and 16 years from 1-dose, respectively; for the 1-dose base case scenario, we assumed this protection extended lifelong.3,5 We also modelled different pessimistic 1-dose scenarios of lower vaccine efficacy (90%), with or without lower average duration of protection (25 yr or 30 yr) (Appendix 1, Table A2). The pessimistic scenario of 90% vaccine efficacy represented the lower bound of the 95% confidence interval of 1-dose vaccine efficacy in the KEN SHE RCT.6
We chose 25-year and 30-year average duration of protection as pessimistic scenarios because no evidence has indicated waning 1-dose protection after more than 12 and 16 years of follow-up, as in the India IARC study and the CVT, respectively.3–5,33 To reproduce these empirical data in the model, we used a normal distribution to allow people to have stable protection for a selected number of years. When assuming an average duration of protection of 25 years, individuals start losing protection after 15 years, half will have lost their protection after 25 years, and almost all will have lost protection after 35–40 years (Appendix 1, Figure A3).
Sensitivity analysis
We performed additional sensitivity analyses. Given that no 1-dose RCT has involved males, we modelled a scenario where 1 dose would be non-inferior for females, but pessimistic for males (90% vaccine efficacy and 25-year average duration of protection). We also modelled a mitigation strategy involving a switch back to 2-dose routine vaccination 10 years after the switch to 1-dose vaccination, should ongoing trials show waning 1-dose protection within the next 10 years. We chose the 10-year period as, in 2034, more than 25 years of follow-up data will be available from the current 1-dose studies (CVT and the India IARC study), which would be sufficient to detect whether protection is waning by 25 years or 30 years.
Outcomes
To evaluate the population-level impact of HPV vaccination, our main outcomes were the change in incidence of HPV-16 infection over time among females and males, compared with no vaccination; the change in the age-standardized incidence of cervical cancer over time, compared with no vaccination; and the percent change in the cumulative incidence of cervical cancer over 100 years (2- or 1-dose vaccination v. no vaccination and 2-dose v. 1-dose vaccination). We chose HPV-16 infection as a main outcome because it contributes most to HPV-related cancers worldwide34 and has the highest force of infection. Therefore, it is the hardest to control and would have the highest rebound after a switch to 1-dose vaccination, if a rebound were to happen.
To evaluate the efficiency of HPV vaccination, our main outcome was the number of doses needed to prevent 1 cervical cancer. We estimated this outcome by dividing the cumulative number of doses given in the population (assuming a switch to 1 dose) by the cumulative number of cervical cancers averted over 100 years, compared with no vaccination. We also calculated the incremental number of doses to prevent 1 additional cervical cancer by dividing the additional number of second doses given by the additional cancers prevented with 2-dose vaccination, compared with 1-dose vaccination.
Our secondary outcomes were the relative reduction in the incidence of high-risk HPV types (16, 18, 31, 33, 45, 52, and 58) targeted by nonavalent HPV vaccines and all other HPV-related cancers (among females and males) over time, compared with no vaccination.
For model projections, we identified 50 parameter sets that produced the best fit to data to represent the uncertainty and variability in HPV epidemiology and sexual behaviour. We presented results as the median and 10th and 90th percentiles (80% uncertainty interval) of model projections for the 50 best-fitting parameter sets.
Ethics approval
This mathematical modeling study did not require ethical approval, given that no individual-level data were used to simulate HPV vaccination in Quebec and Ontario.
Results
Impact on HPV-16 infection
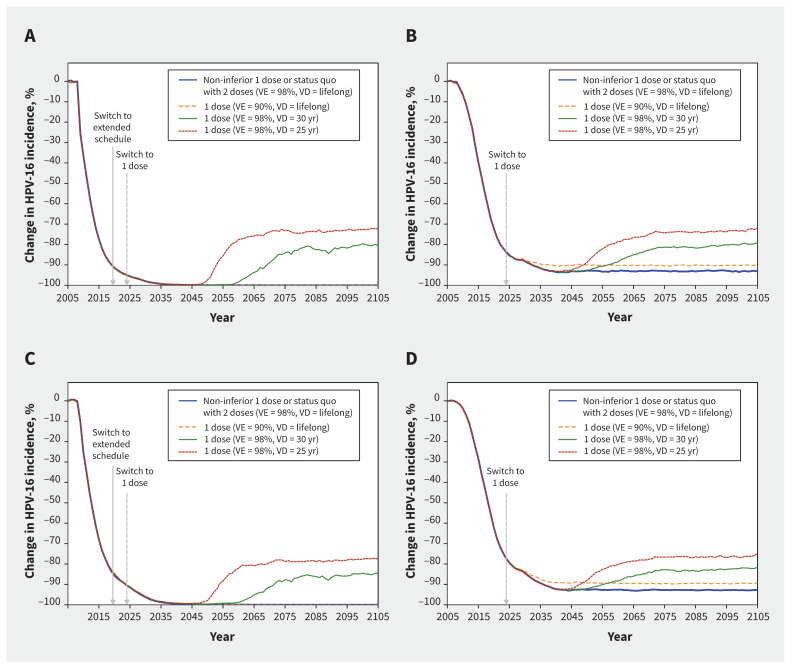
The model projected that gender-neutral nonavalent HPV vaccination with 2 doses or a non-inferior scenario of 1 dose would nearly eliminate HPV-16 infections among females and males by 2040–2045 in Quebec, and reduce HPV-16 infection by more than 90% in Ontario (Figure 1; Appendix 1, Table A3 and Table A4). Under the pessimistic assumption of 90% vaccine efficacy, 1-dose vaccination would produce a similar impact as 2 doses on the incidence of HPV-16 infections in both provinces. Under the most pessimistic scenario of a 25-year average duration of protection, 1 dose was projected to lead to around a 25– and 20–percentage point rebound among females and males from Quebec, respectively, and a 20– and 15–percentage point rebound among females and males from Ontario, respectively. However, when assuming a 30-year duration of protection, the rebound in infection was about 10 percentage points lower in both provinces. Finally, rebounds in Quebec and Ontario would start more than 25 years (in 2045–2050) or 30 years (in 2050–2055) after the switch to 1 dose, assuming a 25- or 30-year average duration of protection, respectively.
Figure 1:
Projected population-level impact of switching to 1-dose human papillomavirus (HPV) vaccination (v. no vaccination) on incidence of HPV-16 among (A) females in Quebec, (B) females in Ontario, (C) males in Quebec, and (D) males in Ontario, assuming different scenarios of 1-dose efficacy and duration (HPV vaccination coverage was 85% among females and males in Quebec, and 67% and 62% among females and males in Ontario). The lines are the median result of model projections using 50 parameter sets. Supporting data are presented in Appendix 1, Tables A3 and A4. All scenarios overlap during the first years after the start of vaccination. In Quebec, the scenarios in blue and orange led to the elimination of HPV-16 and overlap with the x-axis. Note: VD = vaccine duration of protection, VE = vaccine efficacy.
Impact on cervical cancer
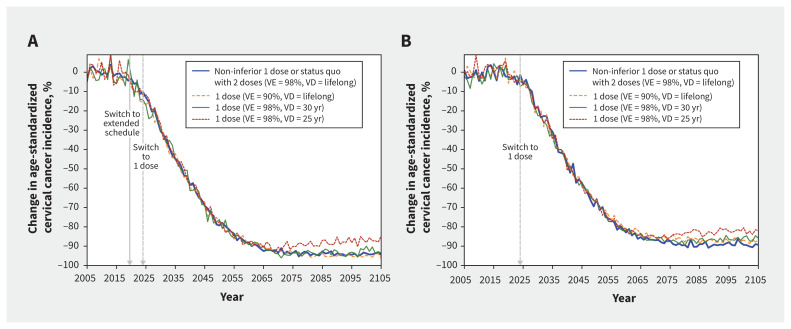
The model projected that gender-neutral HPV vaccination with 2 doses or a non-inferior scenario of 1 dose would reduce the age-standardized incidence of cervical cancer by 50% before 2040–2045 and by more than 85% before the end of the century in both provinces, with a faster and more pronounced reduction in Quebec (Figure 2; Appendix 1, Table A5, Table A6, and Figure A4).
Figure 2:
Projected population-level impact of switching to 1-dose human papillomavirus (HPV) vaccination (v. no vaccination) on incidence of cervical cancer (squamous cell carcinoma) in (A) Quebec and (B) Ontario, assuming different scenarios of 1-dose efficacy and duration (HPV vaccination coverage was 85% among females and males in Quebec, and 67% and 62% among females and males in Ontario). The lines are the median result of model projections using 50 parameter sets. Supporting data are presented in Appendix 1, Tables A5 and A6, and in Figures A4 and A5. Incidence of cervical cancer was standardized to the 2015 world population.35 Note: VD = vaccine duration of protection, VE = vaccine efficacy.
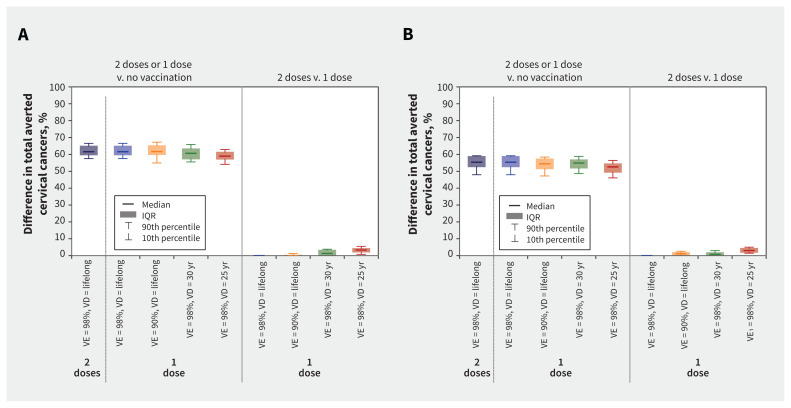
Over 100 years, HPV vaccination with 2 doses or a non-inferior scenario of 1 dose was projected to avert about 60% and 55% of all cervical cancers in Quebec and Ontario, respectively, compared with no vaccination (Figure 3; Appendix 1, Table A5 and Table A6). This percentage was estimated among all females since the beginning of vaccination and includes unvaccinated and vaccinated cohorts.
Figure 3:
Change in the cumulative number of cervical cancers (squamous cell carcinoma) averted over 100 years with 1-dose or 2-dose human papillomavirus (HPV) vaccination in (A) Quebec and (B) Ontario, assuming different 1-dose efficacy and duration scenarios. Boxplots represent model projections using 50 parameter sets, illustrating uncertainty related to sexual activity, screening behaviour, and the natural history and epidemiology of HPV infection and related diseases. This difference of averted cancer is estimated among all females of all ages since the beginning of vaccination and therefore includes unvaccinated and vaccinated cohorts. Supporting data are presented in Appendix 1, Tables A5 and A6. Note: IQR = interquartile range, VD = vaccine duration of protection, VE = vaccine efficacy.
Under the pessimistic 1-dose scenarios of 90% vaccine efficacy or 30-year average duration of protection, reductions in cervical cancer over time were projected to be similar to 2 doses (Figure 2 and Figure 3; Appendix 1, Table A5 and Table A6). However, under the most pessimistic scenario of a 25-year average duration of protection, 1-dose vaccination would avert fewer cervical cancers than 2 doses, by about 3 percentage points over 100 years.
Finally, all 1-dose scenarios, including the most pessimistic, were projected to lead to elimination of cervical cancer in both provinces, using the WHO elimination threshold of fewer than 4 cervical cancers per 100 000 female-years (Appendix 1, Figure A5).
Efficiency of HPV vaccination
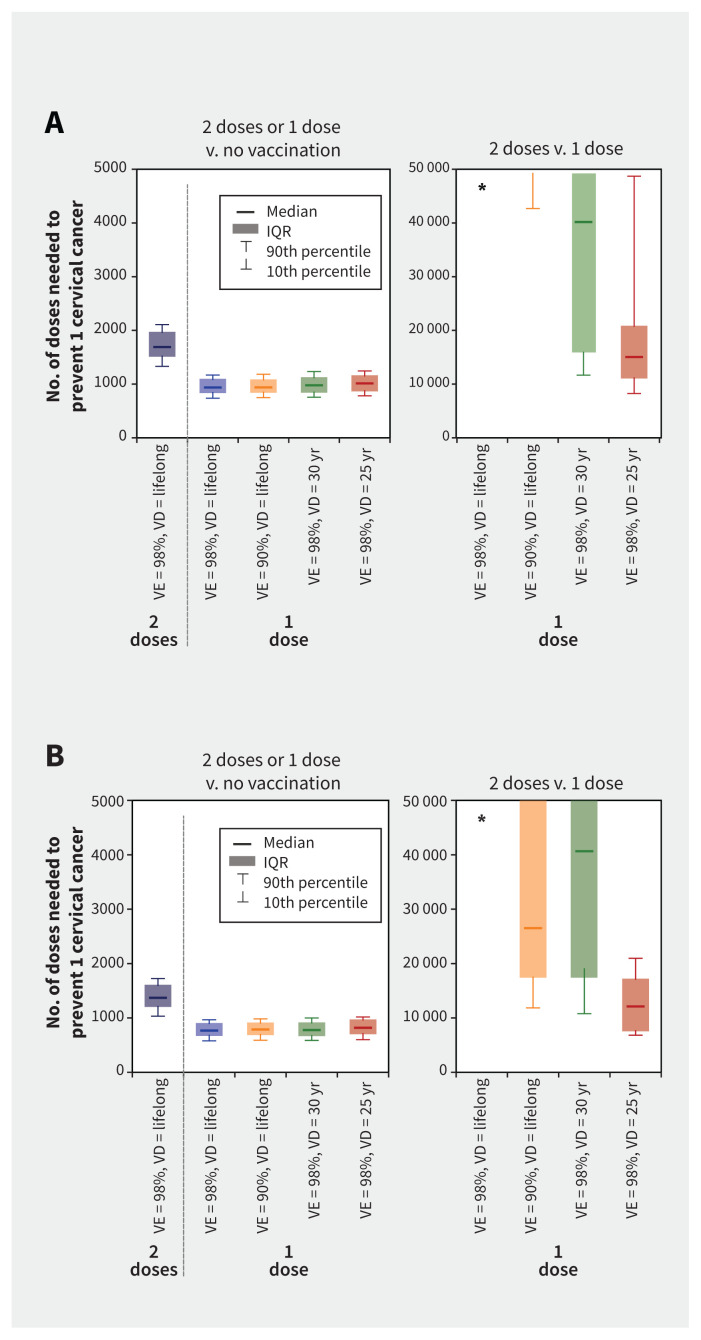
The model projected that 1-dose HPV vaccination would be a substantially more efficient use of vaccine doses than 2 doses, even under the most pessimistic 1-dose scenarios (Figure 4; Appendix 1, Table A5 and Table A6).
Figure 4:
Number of doses needed to prevent 1 cervical cancer (squamous cell carcinoma) with 1-dose or 2-dose human papillomavirus (HPV) vaccination in (A) Quebec and (B) Ontario, assuming different 1-dose efficacy and duration scenarios. Boxplots represent the model projections using 50 parameter sets, illustrating uncertainty related to sexual activity, screening behaviour, and the natural history and epidemiology of HPV infection and related diseases. *Model projections of the incremental number of doses needed to prevent 1 cervical cancer are higher than 50 000 for this scenario. Supporting data are presented in Appendix 1, Tables A5 and A6. Note: VD = vaccine duration of protection, VE = vaccine efficacy.
Secondary outcomes and sensitivity analyses
In pessimistic 1-dose scenarios, our model projected a very limited and delayed rebound in infections from high-risk HPV types targeted by nonavalent vaccines and other HPV-related cancers (v. HPV-16 and cervical cancer, respectively) (Appendix 1, Figure A6 and Figure A7).
In sensitivity analyses, assuming a pessimistic scenario of lower 1-dose vaccine efficacy and shorter duration of protection among males had little to no effect on the projected population-level impact of switching to 1-dose vaccination (Appendix 1, Figure A8). Finally, under the most pessimistic scenario of an average 25-year duration of protection, switching back to 2-dose routine vaccination (after 10 years of 1-dose vaccination) led to a similar impact on cervical cancer as remaining with a 2-dose strategy (Appendix 1, Figure A9).
Interpretation
Our modelling analysis projected that a 1-dose, gender-neutral, routine HPV vaccination program would avert a similar number of cervical and other HPV-related cancers as 2 doses over 100 years in Canada, using a variety of 1-dose vaccine efficacy and duration scenarios. Furthermore, all 1-dose vaccination scenarios, even the most pessimistic, were projected to be a substantially more efficient use of vaccine doses than 2-dose vaccination; these scenarios were also all projected to lead to elimination of cervical cancer in Canada between 2032 and 2040.
The key remaining uncertainty is the relative durability of 1- and 2-dose protection. Recent updates of the India IARC study and the CVT showed sustained efficacy and antibodies up to 12 and 16 years, respectively, after 1-dose vaccination.3,5,33 Even under the most pessimistic scenario of a 25-year average duration of protection, the model projected a limited and delayed rebound in HPV infections, cervical cancer, and other HPV-related cancers.
Four key factors can explain these results. First, even with an average duration of protection of 25 years, those vaccinated would be protected during their peak ages of sexual activity and would have, on average, few remaining new partners once the efficacy began to wane.36–38 Second, the high vaccination coverage and protection during peak ages of partner acquisition would provide herd immunity effects to unprotected people. Third, a potential rebound in HPV infections would start about 25 years after the switch to 1-dose vaccination, as the prevalence of HPV types targeted by vaccines is currently very low in Canada because of high vaccination coverage and time would be needed for the first 1-dose cohorts to start losing their protection. Fourth, the age at infection would shift from peak ages of sexual activity to the ages when vaccination efficacy would potentially decline. Hence, HPV infection would occur among older adults, leading to a reduced risk of cancer, given the long lag time between infection and development of cancer and fewer life years remaining to develop the cancers.
Our results have important policy implications in Canada, and in other similar high-income countries evaluating whether to switch to 1-dose HPV vaccination. First, our projections suggest that 1-dose vaccination is unlikely to result in a substantial rebound in HPV infection and related cancers among males and females, that the second dose provides small additional population-level benefits, and that 1-dose vaccination would not imperil cervical cancer elimination. Under the most pessimistic scenario of 1-dose duration (25-yr average duration of protection), a rebound in HPV-16 infection would occur between years 2045 and 2055. This leaves up to 20 years to detect any substantial waning of protection in ongoing studies and to introduce mitigation strategies, such as reverting to a 2-dose strategy after 10 years of 1-dose vaccination. Therefore, the CVT and India IARC studies should continue follow-up and HPV surveillance activities should be implemented in Canada to detect any early signs of increases in prevalence over time. Second, the COVID-19 pandemic affected HPV vaccination in Canada, particularly among vulnerable populations.39,40 The economic savings of switching to 1-dose vaccination and its programmatic flexibility could allow investments to increase vaccination uptake in regions where coverage is suboptimal and in subgroups with high HPV burden to mitigate the pandemic’s effects on programs and to reduce inequalities. Finally, our results are likely generalizable to the other provinces (as Quebec and Ontario cover the range of vaccination coverage in Canada) and to other high-income countries with similar HPV vaccination coverage, sexual activity patterns, and HPV epidemiology. 31,41–43
Limitations
As with all modelling studies, limitations are related to uncertainty in the data used for projections. We used 50 parameter sets for model projections to capture uncertainty related to sexual activity, screening behaviour, and the natural history and epidemiology of HPV infection and related diseases. More specifically, for 1-dose vaccination, 4 main sources of uncertainty should be considered, namely 1-dose duration of protection, absence of data on 1-dose efficacy for males, absence of data on 1-dose efficacy in preventing other HPV-related cancers, and the number of new sexual partnerships among adults older than 40 years (for whom protection could potentially have waned). To address the first source of uncertainty, we modelled a variety of scenarios for 1-dose duration based on the most recent immunogenicity and efficacy data.5,33 Second, although limited 1-dose data are available for males, our model projected that a pessimistic scenario of combined lower 1-dose efficacy (90%) and duration (25-yr average duration) for males would produce a similar population-level impact with 1-dose vaccination as a perfect 1-dose vaccine for males, assuming a non-inferior 1-dose scenario for females. If gender-neutral vaccination coverage is intermediate–high and vaccine efficacy for females is high and long lasting, herd effects would be sufficient to mitigate losses in vaccine efficacy for males. However, given that we assumed similar herd immunity effects for men who have sex with men (MSM), the rebound in HPV infection and other HPV-related cancers could be greater if duration of protection is limited. Third, even under the most pessimistic 1-dose vaccine scenarios, our model projected a more limited and delayed rebound for other HPV-related cancers (v. cervical cancer), given their slower progression from infection to cancer. Moreover, by preventing HPV infections at the genital sites, 1-dose vaccination would also lead to indirect prevention effects to other sites (similar to herd immunity effects), particularly given that the prevalence of high-risk HPV infection at other sites is generally lower than at the genital site.44–48 However, more work is required to better understand the natural history of other HPV-related cancers, as well as the potential impact of 1-dose vaccination on their transmission dynamics. Fourth, our model slightly overestimated the number of lifetime sexual partners for females older than 40 years, compared with recent North American data.36,38 Therefore, our projections may overestimate the rebound in HPV infections in pessimistic scenarios or capture potential future increases in sexual behaviour among older adults. Finally, we did not explore potential changes in screening policies. However, potential future improvements in screening tests and adherence could reduce the background incidence of cervical cancer, further limiting the potential consequences of pessimistic 1-dose scenarios and reducing the incremental benefit of a second dose.
Conclusion
Our model projected that, compared with 2 doses, 1-dose, gender-neutral, routine HPV vaccination would avert similar numbers of cervical cancers, HPV infections, and other HPV-related cancers among females and males in Canada, if vaccine protection remains high during the peak ages of sexual activity. In addition, 1-dose vaccination represents a more efficient use of vaccine doses and is projected to lead to elimination of cervical cancer in Canada. Continued monitoring of 1-dose protection over time is required to rapidly detect any signs of waning 1-dose efficacy. If steep declines in 1-dose protection were observed, policy-makers would have sufficient time to introduce mitigation strategies.
Footnotes
Competing interests: Chantal Sauvageau is a member of the Quebec Immunization Committee. Sarah Wilson is a member of the National Advisory Committee on Immunization. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Mélanie Drolet, Jean-François Laprise, and Marc Brisson contributed to the conception and design of the work. Chantal Sauvageau, Sarah Wilson, and Gillian Lim contributed to data acquisition. Jean-François Laprise and Éléonore Chamberland contributed to data analysis. All of the authors contributed to data interpretation. Mélanie Drolet and Marc Brisson drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Funding: This study was supported by the Public Health Agency of Canada, the Canadian Institutes of Health Research, the Bill and Melinda Gates Foundation (PATH/Single dose HPV vaccine evaluation consortium), and the Canadian Immunization Research Network.
Data sharing: No individual-level data were used in this study. Descriptions of the model structure, the parameters included in the model, and the empirical data used for calibration and validation are available in the technical appendix (https://marc-brisson.net/HPVadvise-Can.pdf).
References
- 1.World Health Organization. Meeting of the Strategic Advisory Group of Experts on Immunization, April 2022: conclusions and recommendations. Wkly Epidemiol Rec 2022;97:261–76. [Google Scholar]
- 2.Barnabas RV, Brown ER, Onono MA, et al. Efficacy of single-dose human papillomavirus vaccination among young African women. NEJM Evid 2022;1: EVIDoa2100056. doi: 10.1056/EVIDoa2100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basu P, Malvi SG, Joshi S, et al. Vaccine efficacy against persistent human papillomavirus (HPV) 16/18 infection at 10 years after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre, prospective, cohort study. Lancet Oncol 2021;22:1518–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kreimer AR, Sampson JN, Porras C, et al.; Costa Rica HPV Vaccine Trial (CVT) Group. Evaluation of durability of a single dose of the bivalent HPV vaccine: the CVT Trial. J Natl Cancer Inst 2020;112:1038–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romero B, Herrero R, Porras C, et al. Durability of HPV-16/18 antibodies 16 years after a single dose of the bivalent vaccine: the Costa Rica HPV vaccine trial. 35th International Papillomavirus Conference; 2023 Apr. 17–21; Washington, DC. [Google Scholar]
- 6.Barnabas RV, Brown ER, Onono MA, et al.; KEN SHE Study Team. Durability of single-dose HPV vaccination in young Kenyan women: randomized controlled trial 3-year results. Nat Med 2023;29:3224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bénard É, Drolet M, Laprise J-F, et al. Potential population-level effectiveness of one-dose HPV vaccination in low-income and middle-income countries: a mathematical modelling analysis. Lancet Public Health 2023;8:e788–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prem K, Choi YH, Bénard É, et al. Global impact and cost-effectiveness of one-dose versus two-dose human papillomavirus vaccination schedules: a comparative modelling analysis. BMC Med 2023;21:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.HPV dashboard. Geneva: Immunization, Vaccines and Biologicals Department, World Health Organization; 2024. Available: https://www.who.int/teams/immunization-vaccines-and-biologicals/diseases/human-papillomavirus-vaccines-(HPV)/hpv-clearing-house/hpv-dashboard (accessed 2024 Jan. 25). [Google Scholar]
- 10.Bloem P. Current landscape of HPV vaccination and the way towards elimination [technical meeting]. Accelerating HPV-related Cancer Elimination and HPV Faster; 2024. June 6; Antwerp, Belgium. [Google Scholar]
- 11.Drolet M, Bénard É, Pérez N, et al.; HPV Vaccination Impact Study Group. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet 2019;394:497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenblum HG, Lewis RM, Gargano JW, et al. Human papillomavirus vaccine impact and effectiveness through 12 years after vaccine introduction in the United States, 2003 to 2018. Ann Intern Med 2022;175:918–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kjaer SK, Dehlendorff C, Belmonte F, et al. Real-world effectiveness of human papillomavirus vaccination against cervical cancer. J Natl Cancer Inst 2021;113:1329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falcaro M, Castañon A, Ndlela B, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. Lancet 2021;398:2084–92. [DOI] [PubMed] [Google Scholar]
- 15.Daniels V, Saxena K, Patterson-Lomba O, et al. Modeling the health and economic implications of adopting a 1-dose 9-valent human papillomavirus vaccination regimen in a high-income country setting: an analysis in the United Kingdom. Vaccine 2022;40:2173–83. [DOI] [PubMed] [Google Scholar]
- 16.Summary of NACI statement of July 24, 2024: Updated recommendations on human papillomavirus vaccines. Ottawa: Public Health Agency of Canada; modified 2024 July 24. Available: https://www.canada.ca/en/public-health/services/publications/vaccines-immunization/national-advisory-committee-immunization-updated-recommendations-hpv-vaccines.html (accessed 2024 July 24). [Google Scholar]
- 17.Comité sur l’immunisation du Québec. Calendrier de vaccination contre les virus du papillome humain pour les personnes âgées de 20 ans et moins au Québec. Québec: Institut national de santé publique du Québec; 2024:1–16. Available: https://www.inspq.qc.ca/sites/default/files/2024-06/3495-calendrier-vaccination-virus-papillome-humain-20-ans-moins.pdf (accessed 2024 June 30). [Google Scholar]
- 18.Van de Velde N, Boily M-C, Drolet M, et al. Population-level impact of the bivalent, quadrivalent, and nonavalent human papillomavirus vaccines: a model–based analysis. J Natl Cancer Inst 2012;104:1712–23. [DOI] [PubMed] [Google Scholar]
- 19.Brisson M, Laprise J-F, Chesson HW, et al. Health and economic impact of switching from a 4-valent to a 9-valent HPV vaccination program in the United States. J Natl Cancer Inst 2015;108:djv282. doi: 10.1093/jnci/djv282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brisson M, Laprise J-F, Drolet M, et al. Comparative cost-effectiveness of the quadrivalent and bivalent human papillomavirus vaccines: a transmission-dynamic modeling study. Vaccine 2013;31:3863–71. [DOI] [PubMed] [Google Scholar]
- 21.Laprise J-F, Chesson HW, Markowitz LE, et al. Effectiveness and cost-effectiveness of human papillomavirus vaccination through age 45 years in the United States. Ann Intern Med 2020;172:22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drolet M, Laprise J-F, Brotherton JML, et al. The impact of human papillomavirus catch-up vaccination in Australia: implications for introduction of multiple age cohort vaccination and postvaccination data interpretation. J Infect Dis 2017;216:1205–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brisson M, Kim JJ, Canfell K, et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet 2020;395:575–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brisson M, Bénard É, Drolet M, et al. Population-level impact, herd immunity, and elimination after human papillomavirus vaccination: a systematic review and meta-analysis of predictions from transmission-dynamic models. Lancet Public Health 2016;1:e8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Comité sur l’immunisation du Québec. HPV immunization of Quebec pre-adolescents: Two or three doses? Québec: Institut national de santé publique du Québec; 2013:1–91. Available: https://www.inspq.qc.ca/en/publications/1837 (accessed 2023 Aug. 30). [Google Scholar]
- 26.Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2016;65:1405–8. [DOI] [PubMed] [Google Scholar]
- 27.Meites E, Szilagyi PG, Chesson HW, et al. human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2019;68:698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joint Committee on Vaccination and Immunisation (JCVI). JCVI statement on a one-dose schedule for the routine HPV immunisation programme [independent report]. London (UK): Public Health England; 2022. Available: https://www.gov.uk/government/publications/single-dose-of-hpv-vaccine-jcvi-concluding-advice/jcvi-statement-on-a-one-dose-schedule-for-the-routine-hpv-immunisation-programme (accessed 2024 Mar. 26). [Google Scholar]
- 29.Evidence based recommendations on human papilloma virus (HPV) vaccines schedules — background paper for SAGE discussions. Geneva: World Health Organization; 2014:1–68. Available: https://terrance.who.int/mediacentre/data/sage/SAGE_Docs_Ppt_Apr2014/7_session_hpv/Apr2014_session7_HPV_schedules.pdf (accessed 2023 Aug. 4). [Google Scholar]
- 30.World Health Organization. Meeting of the Strategic Advisory Group of Experts on Immunization, October 2019: conclusions and recommendations. Wkly Epidemiol Rec 2019;94:541–60. [Google Scholar]
- 31.Goyette A, Yen GP, Racovitan V, et al. Evolution of public health human papillomavirus immunization programs in Canada. Curr Oncol 2021;28:991–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joura EA, Giuliano AR, Iversen OE, et al.; Broad Spectrum HPV Vaccine Study. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med 2015;372:711–23. [DOI] [PubMed] [Google Scholar]
- 33.Basu P, Bhatla N, Muwonge R, et al. Multicentric cohort study to compare the long-term efficacy of a single-dose of 4-valent vaccine compared to two- and three-dose in 10–18 yr old females in India. Proceedings of the 35th International Papillomavirus Conference (IPVC); 2023 Apr. 17–21; Washington, DC. Geneva: IPVC; 2023. [Google Scholar]
- 34.Guan P, Howell-Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 2012;131:2349–59. [DOI] [PubMed] [Google Scholar]
- 35.World population prospects: the 2017 revision, custom data acquired via website. United Nations, Department of Economic and Social Affairs, Population Division. Available: https://www.un.org/en/desa/world-population-prospects-2017-revision (accessed 2024 Mar. 27). [Google Scholar]
- 36.Haderxhanaj LT, Leichliter JS, Aral SO, et al. Sex in a lifetime: sexual behaviors in the United States by lifetime number of sex partners, 2006–2010. Sex Transm Dis 2014;41:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Datta S, Mercer CH, Keeling MJ. Capturing sexual contact patterns in modelling the spread of sexually transmitted infections: evidence using Natsal-3. PLoS One 2018;13:e0206501. doi: 10.1371/journal.pone.0206501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brisson M, Fisman D, Kirwin E, et al. Measuring social and sexual contact patterns in Canada to improve the control of infectious diseases. Available: https://cirnetwork.ca/research-study/measuring-social-and-sexual-contact-patterns-in-canada-to-improve-the-control-of-infectious-diseases/ (accessed 2024 Mar. 27).
- 39.Immunization coverage report for school pupils in Ontario: 2019–20 to 2022–23 school years. Toronto: Public Health Ontario; 2024:1–25. Available: https://www.publichealthontario.ca/-/media/Documents/I/24/immunization-coverage-2019-2023.pdf?&sc_lang=en (accessed 2024 May 13). [Google Scholar]
- 40.Améliorer les couvertures vaccinales contre les virus du papillome humain (VPH) dans les programmes de vaccination en milieux scolaires au Québec. Québec: Institut National de Santé publique du Québec; 2023:1–50. Available: https://www.inspq.qc.ca/sites/default/files/2024-02/3450-ameliorer-couvertures-vaccinales-vph-programmes-scolaires.pdf (accessed 2024 Jan. 4). [Google Scholar]
- 41.Pingali C, Yankey D, Elam-Evans LD, et al. Vaccination coverage among adolescents aged 13–17 tears: National Immunization Survey-Teen, United States, 2022. MMWR Morb Mortal Wkly Rep 2023;72:912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.UK Health Security Agency. Human papillomavirus (HPV) vaccination coverage in adolescents in England: 2021 to 2022. Health Protection Report, 2022;16:1–16. [Google Scholar]
- 43.Historical human papillomavirus (HPV) immunisation coverage rates. Canberra (AU): Australian Government Department of Health and Aged Care. Available: https://www.health.gov.au/resources/publications/historical-human-papillomavirus-hpv-immunisation-coverage-rates (accessed 2024 Mar. 27). [Google Scholar]
- 44.Clifford GM, Gallus S, Herrero R, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet 2005;366:991–8. [DOI] [PubMed] [Google Scholar]
- 45.Wei F, Gaisa MM, D’Souza G, et al. Epidemiology of anal human papillomavirus infection and high-grade squamous intraepithelial lesions in 29 900 men according to HIV status, sexuality, and age: a collaborative pooled analysis of 64 studies. Lancet HIV 2021;8:e531–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tam S, Fu S, Xu L, et al. The epidemiology of oral human papillomavirus infection in healthy populations: a systematic review and meta-analysis. Oral Oncol 2018;82:91–9. [DOI] [PubMed] [Google Scholar]
- 47.Giuliano AR, Nyitray AG, Kreimer AR, et al. EUROGIN 2014 roadmap: differences in human papillomavirus infection natural history, transmission and human papillomavirus-related cancer incidence by gender and anatomic site of infection. Int J Cancer 2015;136:2752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serrano B, Brotons M, Bosch FX, et al. Epidemiology and burden of HPV-related disease. Best Pract Res Clin Obstet Gynaecol 2018;47:14–26. [DOI] [PubMed] [Google Scholar]