Abstract
A sustainable solution to prevent the waste of fruits and vegetables from spoilage is the use of edible coatings or films. This research project aimed to create a fresh coating recipe that could effectively extend the shelf life of mangoes. The coating was composed of chitosan, glycerol, and gum Arabic mixed with the extract obtained from the extraction of Cleistocalyx operculatus plant. The prepared exact has a total polyphenol content of 17% and showed potent free radical scavenging abilities in a dose-dependent manner. The chitosan/gum Arabic/Glycerol/extract edible coatings were analyzed using SEM and FTIR spectroscopy, revealing a smooth and uniform coating with a well-integration of components. Coating the mangoes with this formulation resulted in significant improvements in their appearance, brightness, weight loss, firmness, titratable acidity, and CO2 respiration rate compared to uncoated samples. The optimal concentration of the extract in the coating was determined to be 0.25% w/w for the best protective performance. After 21-day storage at room temperature, uncoated mangoes were found to be rotten, while coated mangoes remained fresh.
Keywords: Edible coating, Fruit preservation, Leaf extract, Postharvest mango, Shelf-life extension
1. Introduction
Mango is a tropical fruit that people worldwide enjoy because of its sweet and tangy flavour [1]. However, despite its popularity, mango is a highly perishable fruit that is prone to spoilage, leading to significant post-harvest losses [2]. The short shelf life of mangoes limits their availability in the market and makes it challenging for farmers to sell their products. Prolonging mango shelf life is, therefore, an essential requirement to reduce postharvest losses, increase the availability of the fruit, and improve the income of farmers. Various techniques have been established to prolong the storage duration of mangoes, encompassing the application of chemical preservatives, refrigeration, and controlled atmosphere storage [3–6]. Nevertheless, these approaches come with inherent limitations, such as potential health hazards, elevated expenses, and substantial energy demands. Recently, growing interest has been in developing natural and sustainable methods to prolong mango shelf life [7]. Edible coatings and plant extracts are among the promising solutions that have been studied extensively [8–10]. These natural methods have the potential to reduce post-harvest losses, increase the availability of mangoes, and improve the sustainability of the mango industry [11].
Using polysaccharides and intricate carbohydrates has garnered interest as a promising option in developing edible coatings for extending the lifespan of fruits and vegetables [12]. Edible coatings are thin layers of natural or synthetic substances applied to the surface of fresh produce to provide a protective barrier against external factors such as moisture loss, oxidation, and microbial growth [13]. Polysaccharide edible coatings are considered an attractive alternative to synthetic coatings due to their biodegradability, non-toxicity, and low cost. They can be obtained from various natural sources such as plants, algae, and microorganisms. Polysaccharides, such as chitosan, alginate, and starch, have been extensively studied for their effectiveness in preserving the quality and extending the shelf life of various fruits and vegetables [14,15]. Edible coatings can be created by combining one, two, or three types of coating materials and incorporating antioxidants and antimicrobial additives to improve their effectiveness [16–18].
Chitosan (CH), a biopolymer derived from chitin, is a cationic polysaccharide with excellent antimicrobial properties [19]. Studies have indicated that polysaccharides, such as chitosan, possess the ability to hinder the proliferation of diverse microorganisms, including bacteria and fungi, through the disruption of their cell membranes. Chitosan, in particular, can generate a robust film on the surface of fruits and vegetables, effectively safeguarding against moisture loss and external contaminants [20–24]. Gum arabic, a natural exudate from the stems and branches of Acacia senegal trees, is a water-soluble polysaccharide that has excellent film-forming properties [25]. It can be used to provide a barrier against moisture loss and to enhance the texture and appearance of fruits and vegetables. Gum Arabic (GA) has also been reported to exhibit antioxidant and antimicrobial properties, making it a potential natural preservative [26,27]. When used together, chitosan and gum arabic can form a strong and flexible film on the surface of fruits, providing a protective barrier against external factors such as moisture loss, oxidation, and microbial growth. To enhance the antimicrobial and mechanical properties of the coating, the chitosan can be combine with gum arabic to enable the synergistic effects, [9,10,21]. Studies have shown that the use of chitosan/gum arabic edible coating can extend the shelf life of mangoes by reducing weight loss, delaying ripening, and inhibiting microbial growth. Additives are frequently incorporated into the formula of edible coatings to enhance their mechanical properties. Glycerol (Gly) could be introduce to the formula of edible coatings to enhance their properties, such as their ability to adhere to the surface of fruits and vegetables, and to reduce their water vapor permeability [28]. This can help extend the shelf life of produce and maintain its quality during storage and transportation. Glycerol can also improve the edible coatings’ mechanical strength and elasticity, making them more resistant to cracking and breaking.
Cleistocalyx operculatus (CO), a tropical fruit, has attracted interest as a viable natural resource for deriving compounds suitable for the development of edible coatings with the purpose of extending the storage duration of fruits and vegetables [29,30]. The fruit and leaves of Cleistocalyx operculatus contain various bioactive compounds, including phenolics and flavonoids, that exhibit antioxidant and antimicrobial properties, which could be employed for green synthesis and edible coatings [31]. Studies have shown that extracts from the CO extract can be employed as a composition in edible coatings for fruits and vegetables [10]. Utilizing coatings derived from CO extract has shown promising results in enhancing the overall quality and extending the shelf life of fruits. These coatings effectively mitigate weight loss, delay ripening, and inhibit the growth of microorganisms. CO extract-based coatings offer several advantages over synthetic coatings, including their natural origin, biodegradability, and low cost. The CO extracts can be obtained from waste materials, providing a sustainable solution to reduce waste and maximize the utilization of resources.
The combination of chitosan, gum arabic, and glycerol, along with leaf extract, for the edible coating of mango is a relatively novel approach. While individual or combined use of chitosan, gum arabic, glycerol, and leaf extract has been explored for edible coatings in the past, there is limited research on their specific combination for preserving mangoes. This study focuses on developing a new formulation of edible coatings consisting of chitosan, gum arabic, glycerol, and CO extract to extend the shelf life of harvested bananas. The study extensively examines the effects of CO concentration on the physical and chemical properties of mangoes. Furthermore, the protective efficacy of the composite coating is also studied.
2. Experimental section
2.1. Materials
Mangoes of the same size and harvested from farms in Son La province, Vietnam, were collected. Tin Cay Joint Stock and Green Cosmetics companied provide chitosan and Gum Arabic, respectively. Various chemicals, including glycerol, ethanol, sodium carbonate, ammoniac solution, NaClO (99%), and CH3COOH (99%), were obtained from Xilong Chemicals (China). The mangoes were meticulously cleaned before utilization, while the remaining chemicals were used as received without the need for additional purification.
Cleistocalyx operculatus (CO) leaves were received from Ha Tay province (Vietnam) and authenticated. The CO leaves were completely dried and milled into a fine powder, then underwent a 48-h ethanol extraction process. This extraction process was repeated twice, and the obtained extract was dried in a vacuum rotary.
2.2. Determination of the CO extract’s polyphenols and flavonoid
The Folin Ciocalteu reagent described by Singleton and Rossi was employed to determine the total phenolic content of the ethanolic CE extract, with gallic acid being used as the standard [32]. Normally, a blend consisting of 5 mL Folin Ciocalteu reagent, 1 mL CO extract solution and 4 mL Na2CO3 solution (75 g/L) was stirred for 30 min. The total phenolic content of the mixture was then determined by measuring the mixture’s absorbance at a wavelength of 765 nm using gallic acid as the reference. The amount of polyphenols in milligrams per gram of gallic acid was then calculated.
2.3. Determination of the CO extract’s antioxidant activity
Its antioxidant activity was evaluated based on the extract’s capacity to neutralize free 1,1-diphenyl-2-picryl hydrazyl (DPPH) radicals. The extract was combined with a DPPH solution, resulting in the neutralization of the radicals. This reaction caused a color transition in the solution, shifting from purple to yellow. The extent of the color change served as an indicator of the strength of the antioxidant activity. Three mL of the extract was combined with 1 mL of DPPH solution (0.1 mM in ethanol) at various doses (5–30 μg/mL). After vigorously shaking the mixture for 30 min at room temperature, the absorbance was recorded at 517 nm. The experiment was carried out for three times using ascorbic acid as the reference standard. The logarithmic dosage inhibition curve was plotted to ascertain the mixture’s IC50 value. The formula used to calculate the DPPH scavenging effect was: DPPH scavenging effect (%) = (A0 – A1)/A0 × 100, where A0 represented the DPPH solution’s initial absorption intensity and A1 indicated the DPPH solution’s absorption intensity in the presence of the extract [33].
2.4. Preparation of edible coatings
The CH/GA/Gly/CO edible coatings were made in two steps by combining 1% chitosan, 2.5% gum Arabic, 0.3% glycerol, and different concentrations of the CO extract in the range of 0.05–0.5%. To begin, 5 g of gum Arabic was dissolved in 100 mL of water to create a solution known as solution A. The mixture was vigorously stirred for 2 h at a temperature of 40 °C. Secondly, 2 g of chitosan were dissolved in 100 mL of water and 0.67 g of glycerol and stirred for 2 h at room temperature to create solution B. The pH of the solution was adjusted to approximately 5.5 using either a diluted NaOH or H2SO4 solution. Subsequently, Solution A was combined with Solution B, and different concentrations of CO extract were incorporated into the mixture. This resulted in the creation of the edible coatings specifically formulated for the preservation of mangoes.
2.5. Application of the prepared edible coatings to mangoes
To get rid of any obvious surface dirt, the mangoes purchased from the farm were first cleaned with water. Subsequently, an additional cleaning step was performed using a 0.01% NaClO solution to ensure thorough cleanliness of the mangoes. Next, the mangoes were divided into 7 groups consisting of 18 mangoes each. These groups were treated with various coating solutions, including controlled samples, CH 1%/GA 2.5%, CH 1%/GA 2.5%/Gly 0.3%, and CH 1%/GA 2.5%/Gly 0.3%/CO solutions containing different CO extract proportions ranging from 0.05 to 0.5 w/w%. After coating, the mangoes were dried and stored under ambient conditions with controlled room temperature and relative humidity. At predetermined time intervals, typically every three days, individual mangoes from each group were selected and assessed to evaluate their physicochemical properties.
2.6. Study of color changing
Initially, the mango peel exhibited a green color, which later transitioned into a yellow hue. As the mangoes reached a deteriorated state, the color of the peel turned brown, indicating spoilage. To examine the interior of the mangoes, they were cut in half and observed. The International Commission on Illumination’s standard was used to objectively measure the mangoes’ lightness on the pericarp of the fruit [34]. The lightness property of mangoes was determined through the luminosity parameter (L value) measured with a colorimeter under common condition of light.
2.7. Determination of mangoes’ weight loss
The digital balance was used to weigh the mangoes in each group every 3 days during the storage period. The weight loss’ percentage was calculated based on the difference in weight of each mango during the storage time. The experiments were repeated three times to determine the average value and associated standard deviations.
2.8. Determination of mangoes’ firmness
The Instron Universal Testing Machine was employed to determine the firmness of the mangoes by puncturing them using an 8 mm diameter puncture tip. 20 mm per minute was consistently maintained during the piercing. The force needed to pierce the mango was expressed in Newtons (N). Each mango was punctured three times to obtain an average value.
2.9. Determination of mangoes’ titratable acidity
To determine the titratable acidity of mangoes, a titration method was used. The mangoes were initially peeled and mixed with 40 mL water for the experiment. The resulting blend underwent filtration through centrifugation for 15 min at 7000 rpm, followed by an additional filtration step using filter paper. The filtered sample was then mixed with 3 drops of a 0.1% phenolphthalein indicator in 5 mL of water. A dropwise addition of a 0.1 M NaOH solution was carried out up until a pink color change showed that the mangoes’ base and acids had reacted, suggesting that the reaction had occurred. By analyzing the amount of malic acid present in the mango pulp, the acidity of the mangoes was ascertained. To acquire an average value while accounting for the accompanying variances, the experiment was repeated three times.
2.10. Determination of mangoes’ CO2 respiration rate
The respiratory rate of mango fruit is determined using the ICA15 Dual CO2 gas measuring device. The respiratory rate is calculated using the following formula:
Where, X is the respiratory rate (mL CO2/kg.h), % CO2 is the measured concentration of CO2 in the device (%), V is the volume of the chamber (mL), w is the weight of the sample used in the experiment (g), t is the time of respiration (hours), and 1000 is the conversion factor from grams to kilograms.
2.11. Statistical analysis
The experiments were repeated three times, and the obtained data was averaged with standard deviation. A statistical analysis was conducted using Student’s t-test to ascertain any significant differences between the samples. A significance level of p < 0.05 was utilized to determine the presence of statistical significance.
3. Results and discussion
To assess the suitability of using CO extract as an additive in mango fruit preservation coating, its biological properties including total polyphenol content and antioxidant activity, were examined. As previously reported, the extract contained high levels of polyphenols, with flavonoids being the primary constituents [35]. The study showed that the extract had a polyphenol content of approximately 17% of the dry material. The results also demonstrated that the CE extract had strong free radical scavenging abilities in a dose-dependent manner, even at low concentrations. The extract exhibited over 60% activity in the DPPH assay at a 50 μg/mL concentration.
The effect of CH/GA ratios on the appearance of mangoes stored at different periods of time was investigated. The results are shown in Fig. S1. Through visual evaluation of the condition and color of the fruit, it can be observed that the control mangoes, after 15 days of storage under the same conditions, exhibited external signs of damage such as black spots, softness, wrinkled skin, and a generally deteriorated appearance. In contrast, the mangoes coated with the preservation film appeared fresher, with fewer black spots. Specifically, after 9 days of observation, the mangoes coated with the preservation film retained their freshness and shine. By the end of the 15-day observation period, mangoes coated with the CH 0.5%/GA 2.5%, CH 1.5%/GA 2.5%, and CH 2%/GA 2.5% formulations had more black spots, while mangoes coated with the CH 0.25%/GA 2.5% and CH 0.75%/GA 2.5% formulations had fewer black spots. No damage was detected in the mangoes coated with the CH 1%/GA 2.5% formulation. Therefore, the chitosan/gum arabic film-forming system with CH/GA ratio of 1%/2.5% was chosen as the optimized CH/GA ratio for the subsequent experiments.
The effect of glycerol content on the appearance of mangoes stored at different period of time. The results are shown in Fig. S2. When plasticizers are added to the edible coating formula, mangoes ripen more slowly, which may be due to the film now having more flexibility, elasticity, and better water retention properties. Fig. S2 shows mangoes have a shiny and smooth surface when using the preservation film. After 9 days of observation, mangoes coated with different formulations still maintain the freshness of the fruit. However, at the 15-day mark, the control group of mangoes showed signs of ripening with black spots, while mangoes coated with films containing 0.1%, 0.2%, 0.4%, and 0.6% additives also exhibited black spots, and some of the fruits began to rot. Mangoes coated with the CH 1%/GA 2.5%/Gly 0.3% and CH 1%/GA 2.5%/Gly 0.5% formulations did not show black spots or significant color changes after 15 days of observation under normal conditions. However, mangoes coated with the CH 1%/GA 2.5%/Gly 0.5% formulation displayed some wrinkling of the fruit’s skin due to water loss, and it was not as effective in terms of visual appearance. Based on visual observations, it can be concluded that after 15 days of preservation, the CH 1%/GA 2.5%/Gly 0.3% formulation was the most effective for preserving mangoes. The surface remained glossy, the fruit was firm, and no signs of spoilage were observed. Therefore, the edible coating formula of CH 1%/GA 2.5%/Gly 0.3% was selected for further investigation.
The study primarily focused on the color changes that occur during the aging of mangoes, which begin as green and ripen to yellow before ultimately turning brown and rotting. The researchers investigated the effect of various edible coatings on the color changes of mangoes after being preserved for 21 days at 25 °C and 70% relative humidity. The chlorophyll degradation in the mango skin causes the mangoes to turn yellow. The enzyme chlorophyllase, which breaks down chlorophyll, starts the enzymatic breakdown process. Yellow pigments like xanthophyll and carotene are created as a result. The browning of mangoes occurs as a result of enzymatic and non-enzymatic reactions. Enzymatic browning occurs when the enzyme polyphenol oxidase (PPO) present in the mango reacts with oxygen in the air, causing the fruit’s flesh to turn brown. Non-enzymatic browning, also known as Maillard browning, occurs when reducing sugars in the mango react with amino acids under high temperatures (the highest rate of this reaction occurs at the temperature range of 70–100 °C) [36]. At the room temperature, this reaction still slowly occurs, resulting in the formation of brown pigments and a change in the texture and flavor of the fruit [37,38]. Both enzymatic and non-enzymatic browning contribute to the degradation of mango quality and reduce the shelf life of the fruit, in which the enzymatic browning is dominantly responsible for the quality of mango at low temperatures (<25 °C).
The mangoes depicted in Fig. 1, without any coating, exhibited a significant transformation, appearing brown and rotten. On the other hand, the banana coated with films composed of 1% chitosan (CH) and 2.5% gum arabic (CA), as well as 1% chitosan (CH), 2.5% gum arabic (CA), and 0.3% glycerol (Gly), demonstrated signs of decay after 21 days of storage. Interestingly, adding the CO extract to the composite formula significantly reduces the mangoes’ color change. Specially, the mangoes coated with CH 1%/GA 2.5%/0.3% Gly/0.25%CO composite showed only partial yellowing after 21 days. These findings highlight the significant impact of the CH 1% + GA 2.5% + 0.3% Gly + 0.25% CO composite coating in extending the storage life of mangoes. This coating effectively slows down the mangoes’ respiration rate and reduces chlorophyll’s degradation. The study demonstrates that the application of this edible coating can successfully prolong the freshness of mangoes when stored under ambient conditions for more than 21 days.
Fig. 1.
The color changes of both uncoated mangoes and mangoes coated with various formulations of coatings.
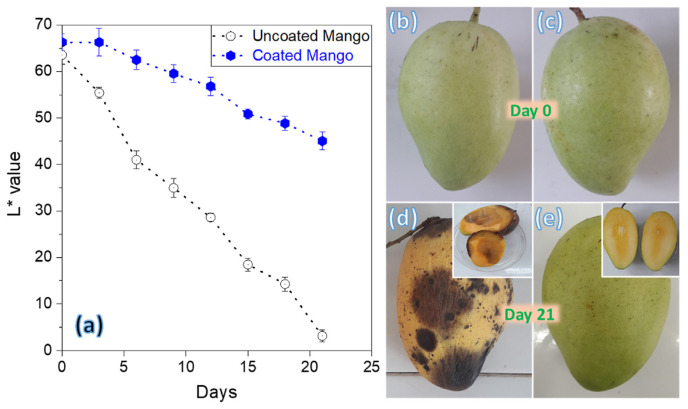
The color change was further accessed by the luminosity parameter. The luminosity parameter, indicated by the L value, is a reliable method to assess color changes in fruits, according to previous research [39]. The color changes of mangoes during the ripening process were evaluated using the L value as shown in Fig. 2. The findings showed that after 21 days of storage at room temperature without the use of an edible covering, the mangoes’ L values drastically dropped from 65 to 3. However, the L values of the mangoes significantly improved after applying the CH/GA/Gly/CO composite coating, reaching a value of 44 after the testing period. Due to the glossy appearance of the edible coating on the mangoes’ surface, the initial L value of the coated mangoes was roughly 67, which was greater than that of the uncoated samples. These results show that the mangoes with the edible coating maintained their brightness up to 66% under the same storage conditions, but the mangoes without the coating lost their brightness after just 21 days at ambient temperature. The exterior characteristics of the mangoes, as shown in Fig. 2b–e, provided visual corroboration of the findings. After 21 days of storage, the treated mangoes’ pericarp still showed a green tint, in contrast to the untreated mangoes’ wholly brown exterior. Assessing the freshness of a mango involves taking into account its internal appearance, which is crucial. The image in Fig. 2d’s insert illustrates that after 21 days, an uncoated mango appears grey and almost spoiled. In contrast, as seen in the inset in Fig. 2e, the inside of a mango coated with the CH/GA/Gly/CO composite coating remained rather fresh, exhibiting a yellowish tint and showing no symptoms of rotting. It wasn’t until after 30 days of storage that signs of spoilage were observed in the coated mango.
Fig. 2.
(a) The brightness parameter was assessed throughout the storage duration of both uncoated mangoes and mangoes coated with the CH/GA/Gly/CO edible coating. The appearances of the uncoated mangoes (b, d) were compared to those of the coated mangoes (c, e) after 21-day storage.
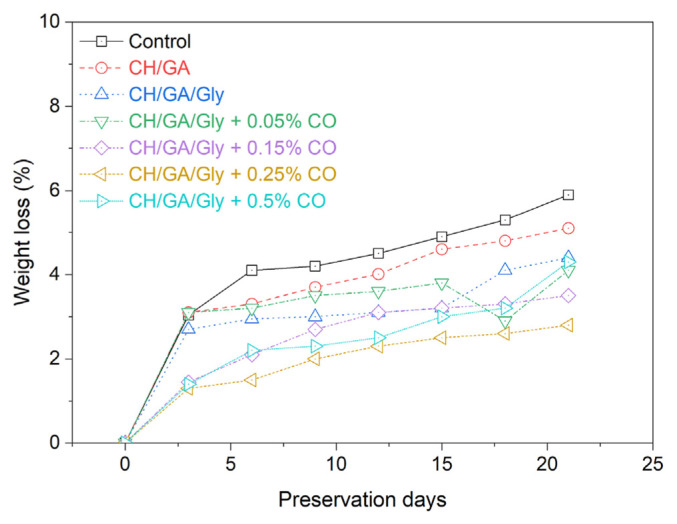
Mangoes lose moisture and/or carbon atoms during respiration, which results in a weight loss during the ripening process [10]. Hence, assessing the mass loss of mangoes serves as a crucial indicator in determining the efficacy of coatings in prolonging their shelf life. Fig. 3 illustrates the weight loss observed in uncoated mangoes compared to mangoes coated with various formulations and stored under ambient conditions. Mangoes don’t lose as much weight during the ripening process as other fruits do because of their thick pericarp. Fig. 3 clearly demonstrates that applying a film to the mangoes effectively mitigates weight loss. While the uncoated mangoes experienced a weight decrease of over 6% after 21 days of storage, this percentage was significantly reduced when the coating was applied. In the absence of the CO extract in the edible coating formulation, the mangoes experienced weight losses of 5% and 4.2% for the CH/GA and CH/GA/Gly coatings, respectively. It can be obvious from Fig. 3 that the weight loss of mangoes significantly decreases upon the addition of the CO extract to the coating composition. The most effective coating in minimizing mango mass loss is the one containing 0.25% CO extract with weight loss of only 2.3% after 21 days. Adding the CO extract improves mango weight loss by minimizing carbon atom loss during respiration, potentially due to CO’s antimicrobial properties [40]. Additionally, the concentration of polyphenols in the CO is notably high (around 12.6%), serving as effective scavengers of free radicals (these radicals, when in contact with mango, induce oxidative reactions that affect the fruit’s metabolism). It was noted that as the CO content increases, the weight loss of mago treated with the edible coatings decreases, reaching a minimum at a CO content of 0.25%. Any further increase in CO content beyond 0.25% showed a rise in weight loss of coated mango. Hence, the optimized concentration of CO extract in the CH/GA/Gly/CO composite coating edible coating for minimizing banana mass loss can be considered as 0.25%.
Fig. 3.
The amount of weight that uncoated mangoes lose compared to mangoes coated with different coatings formulations.
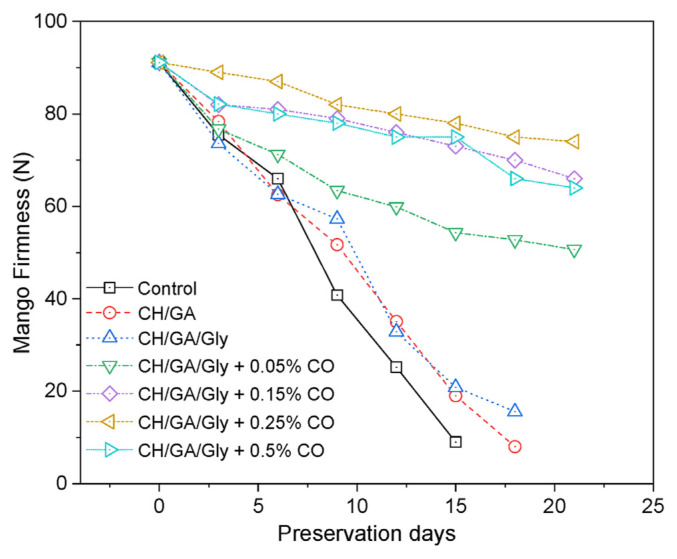
The wall, cell structure, chemical compounds, and intracellular components of the mango pericarp and its texture and internal makeup degrade during the ripening process. This degradation leads to a softening of the fruit [41]. Applying a coating to the fruit is a common practice to prevent oxygen and carbon dioxide from reacting with the enzymes present in the fruit, as these reactions are primarily responsible for the ripening process [42]. In order to assess the efficacy of edible coatings, the hardness of coated and untreated mangoes was assessed during storage at intervals of three days. The results are presented in Fig. 4. At the beginning of the experiment, the initial firmness of the mango was approximately 91 N. The uncoated mango quickly lost its firmness and became completely soft after only 15 days of storage. The application of CH/GA and CH/GA/Gly coatings is evident to slow the ripening process of mangoes, however, the firmness of the mangoes only retains for 18 days before completely ripening and soften. Intriguingly, the application of CH/GA/Gly/CO composite coatings to the mangoes resulted in a significant improvement in the retention of firmness, which was maintained for over 21 days of storage time. The best performance was observed with the mangoes coated with CH/GA/Gly/0.25% CO, which retained firmness for up to 21 days with firmness of 82 N. The coated mango showed complete loss of firmness after 30 days of storage.
Fig. 4.
The firmness of both uncoated mangoes and mangoes coated with different coating formulations.
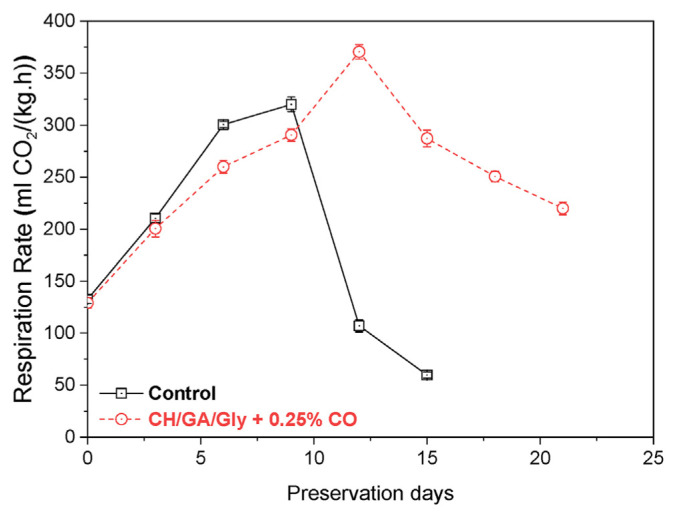
The respiration rate of fruits such as mangoes is a critical factor in determining their shelf life, and reducing this rate can help to extend their storage life. In which, CO2 is a by-product of mango respiration, and high levels of CO2 can lead to a reduction in the quality and shelf life of mangoes. In order to restrict the exchange of gases between the fruit and its surroundings and, in turn, the fruit’s respiration rate, edible coatings can act as a physical barrier [43]. This work also investigated the effects of various edible coatings on carbon dioxide (CO2) production rates in mangoes. The findings, presented in Fig. 5, demonstrate distinct climacteric peaks in coated mangoes on the twelfth day, whereas the climacteric trend is comparatively suppressed in these fruits. Conversely, control mangoes rapidly increased CO2 production rate, starting at an initial value of 125 mL CO2/(kg.h) and peaking at 325 mL CO2/(kg.h) on day 9, before decreasing to 50 on day 15. In contrast, coated fruits exhibited a gradual increase in CO2 production rate, starting at 120 mL CO2/(kg.h), reaching 375 mL CO2/(kg.h) on day 12, and subsequently decreasing to 224 mL CO2/(kg.h) on day 21. This indicates that the coating effectively delayed the occurrence of the respiratory climacteric peak and suppressed the climacteric trend. These factors collectively contribute to an extended shelf life and improve the mangoes’ storage quality.
Fig. 5.
The CO2 production rate of uncoated mangoes and mangoes coated with CH/GA/Gly/0.25% CO edible coating.
The edible coatings employed in this study demonstrated a dual effect by reducing the availability of oxygen for respiratory activity and impeding the diffusion of CO2 from the tissue. This caused beneficial secondary physiological adjustments to occur during the ripening phase. The elevated concentration of CO2 within the fruit’s internal atmosphere, facilitated by the coating, resulted in a decrease in respiration rate and delayed or prevented responses to ethylene. The observed inhibitory effect on mitochondrial activity and enzyme systems is generally attributed to the elevated CO2 concentration; however, the precise mechanism of action remains unclear [44]. Hence, the appropriate coating effectively slowed down the aerobic respiration of mangoes and extended their storage duration. However, in certain instances, this reduction in aerobic respiration triggered anaerobic respiration, negatively impacting the sensory qualities and visual appeal of the fruits. Our findings align with earlier studies in the literature, which suggest that coatings based on polysaccharides exhibit lower permeability to respiratory gases like O2 and CO2, while being comparatively more permeable to water vapor than waxes [45].
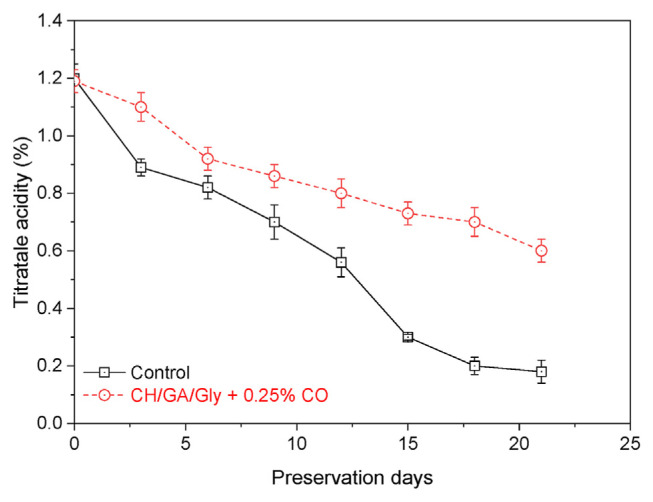
Malic acid is the predominant organic acid found in mangoes, which makes it a promising marker for assessing the effectiveness of coatings employed in mango preservation. The ripening process of mangoes is associated with variations in acidity, which can influence the fruit’s color. Fig. 6 illustrates the titratable acid (TA) values of both uncoated and coated mangoes treated with CH/GA/Gly/0.25% CO coating systems. The results demonstrate a significant decrease in TA values over time for uncoated mangoes, with a recorded value of approximately 0.16% after 21 days of storage, compared to the initial TA value of 1.2% before storage. However, the decrease in TA values is considerably slower with the use of CH/GA/Gly/0.25% CO coating, with calculated TA values of around 0.6% after 21 days of storage. The coatings on mangoes hinder the ripening process by providing a protective barrier, resulting in elevated titratable acid (TA) levels in coated mangoes compared to uncoated ones during storage. Since malic acid consumption is a primary indicator of fruit respiration, a decrease in acidity values indicates higher respiratory activity in the fruit [46].
Fig. 6.
The titratable acidity of uncoated mangoes and mangoes coated with CH/GA/Gly/0.25% CO edible coating.
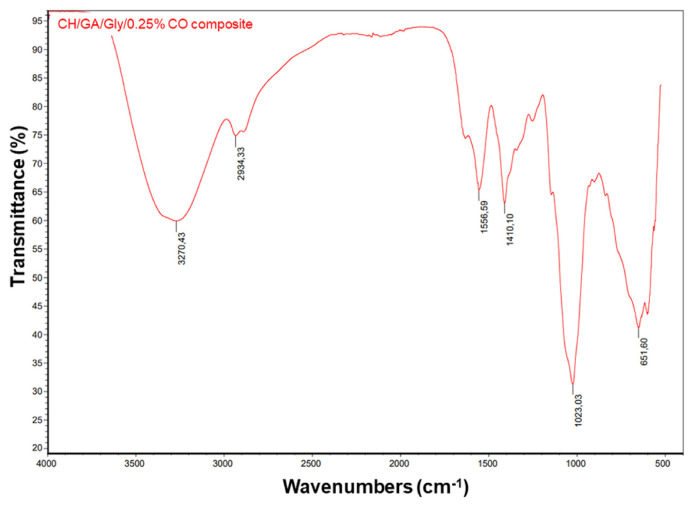
The characteristics and bonding nature of components in the CH/GA/Gly/0.25% CO edible coating were studied in the Fourier-transform infrared spectrum. Fig. S3 and Fig. 7 shows the FTIR spectrum of the CH/GA/Gly and CH/GA/Gly/0.25% CO edible coating. The composite material’s FTIR spectrum displayed a noticeable peak at about 3270 cm−1, caused by OH groups’ vibrations. This peak indicates the presence of absorbed moisture and OH bonding from glycerol, as well as alcohol and phenol groups from the CO extract [47,48]. The absorption band observed at approximately 3270 cm−1 in the FTIR spectrum is also in good agreement with the stretching group of NH in the chitosan molecule [49]. The FTIR spectrum also shows other characteristic groups of chitosan components in the edible coating. These include the C–H stretching in methylene observed at 2920 cm−1, the C–H stretching in methyl observed at 2820 cm−1, the C–O stretching in amide observed at 1601 cm−1, and the C–O–C stretching in glucosamine observed at 1023 cm−1 [50]. The C–H stretching bonds of alkynes exhibit a distinct vibration at 2934 cm−1 in the FTIR spectrum of the edible coating formula. Additionally, the C=O stretching group in ketones and aldehydes of the CO extract can be seen at 1677 cm−1. N–O asymmetric bonding in nitro compounds and the C–C bond in the ring of aromatics from the CO extract, which are seen at 1556 cm−1 and 1410 cm−1, respectively, are two other noteworthy peaks. The C–H stretching in alkanes is responsible for the absorption peak at 1320 cm−1. A characteristic of amines’ C–N bending is their presence of vibration bands at 1100 and 1023 cm−1. Finally, the bending of C–H in alkyl halides is ascribed to the characteristic FTIR peak at 651 cm−1. These results confirm the successful formation of the CH/GA/Gly/0.25% CO edible coating for preservation of mango fruits.
Fig. 7.
FTIR spectrum of CH/GA/Gly/0.25% CO edible coating.
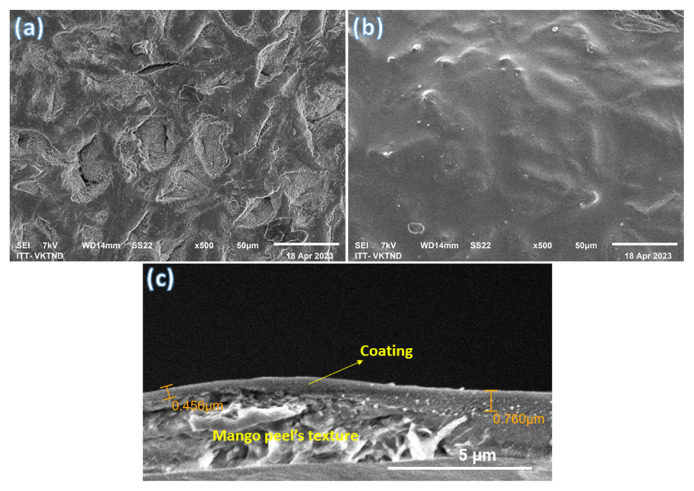
Before and after coating, the surface structure of mango peel was observed using scanning electron microscopy. Fig. 8 displays the SEM images of the mango’s surface, with and without the CH/GA/Gly/0.25% CO edible coating. Before coating, the surface was relatively rough with separated segments, as seen in Fig. 8a. However, after coating, the surface became smooth, and no segments were observed, as shown in Fig. 8b. Additionally, Fig. 8c exhibits scanning electron microscopy (SEM) images of the cross-section of a mango’s pericarp coated with a CH/GA/Gly/0.25% CO edible coating. The pictures show how the peel exhibits a tightly packed porous structure with an even dispersion of the edible film. The Fig. 8c indicates that the average thickness of the film is approximately 0.76 μm.
Fig. 8.
SEM images of (a) uncoated mango, (b) mango coated, and (c) cross-section SEM images of the pericarp surface of the mangoes coated with CH/GA/Gly/0.25% CO edible coating.
4. Conclusions
In short, Cleistocalyx operculatus leaves were successfully extracted with total polyphenols content of 17% of dried material and employed as an additive in an edible coating made of glycerol, chitosan, gum Arabic, and the CO extract. The resulting coating significantly extended the shelf life of mango, maintaining its appearance and freshness for up to 21 days at ambient temperatures, while uncoated mangoes spoiled after only 15 days. The coated mangoes had a smooth and uniform surface, without any cracks. The optimal concentration of the CO extract in the coating was found to be 0.25% w/w, providing the best protection for the mango fruit. The coating application also enhanced mangoes’ nutrient content and various physicochemical properties, including weight loss, brightness, firmness, titratable acidity, and CO2 respiration rate. These findings indicate that the coating has promising potential as an alternative technology for prolonging the shelf life of mangoes and other fruits and vegetables.
Supplementary Information
Acknowledgment
This work was supported by the Vietnam Ministry of Science and Technology (Contract number 01/2021/HĐ-ĐT/VPTV).
Appendix
The effect of CH/GA ratios on the appearance of mangoes stored at different period of time.
Effect of glycerol content on the appearance of mangoes stored at different period of time.
FTIR spectrum of CH/GA/Gly edible coating.
Funding Statement
This work was supported by the Vietnam Ministry of Science and Technology (Contract number 01/2021/HĐ-ĐT/VPTV).
Footnotes
Conflict of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1. Menon RR, Goswami T. Post-harvest handling and storage of mangoes-An overview. J Food Sci Technol. 2007;44:449–58. [Google Scholar]
- 2. Kaur K, Kaur G, Brar JS. Pre-harvest application of hexanal formulations for improving post-harvest life and quality of mango (Mangifera indica L.) cv. Dashehari. J Food Sci Technol. 2020;57:4257–64. doi: 10.1007/s13197-020-04464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yahia EM. Modified and controlled atmospheres for the storage, transportation, and packaging of horticultural commodities. CRC Press; 2009. [Google Scholar]
- 4. Kim Y, Brecht JK, Talcott ST. Antioxidant phytochemical and fruit quality changes in mango (Mangifera indica L.) following hot water immersion and controlled atmosphere storage. Food Chem. 2007;105:1327–34. [Google Scholar]
- 5. Tefera A, Seyoum T, Woldetsadik K. Effect of disinfection, packaging, and storage environment on the shelf life of mango. Biosyst Eng. 2007;96:201–12. [Google Scholar]
- 6.Thompson AK, Prange RK, Bancroft R, Puttongsiri T. Controlled atmosphere storage of fruit and vegetables. CABI; 2018. [Google Scholar]
- 7. Tavassoli-Kafrani E, Gamage MV, Dumée LF, Kong L, Zhao S. Edible films and coatings for shelf life extension of mango: a review. Crit Rev Food Sci Nutr. 2022;62:2432–59. doi: 10.1080/10408398.2020.1853038. [DOI] [PubMed] [Google Scholar]
- 8. Paidari S, Zamindar N, Tahergorabi R, Kargar M, Ezzati S, Shirani N, et al. Edible coating and films as promising packaging: a mini review. J Food Meas Char. 2021;15:4205–14. [Google Scholar]
- 9. Le KH, Nguyen MD-B, Dai Tran L, Thi HPN, Van Tran C, Van Tran K, et al. A novel antimicrobial ZnO nanoparticles-added polysaccharide edible coating for the preservation of postharvest avocado under ambient conditions. Prog Org Coat. 2021;158:106339. [Google Scholar]
- 10. La DD, Nguyen-Tri P, Le KH, Nguyen PT, Nguyen MD-B, Vo AT, et al. Effects of antibacterial ZnO nanoparticles on the performance of a chitosan/gum Arabic edible coating for post-harvest banana preservation. Prog Org Coat. 2021;151:106057. [Google Scholar]
- 11. Kundu P, Adhikary NK, Maji S. A critical review on use of edible coating to enhance shelf life of mango. Current J Appl Sci Technol. 2020;39:116–28. [Google Scholar]
- 12. Dhall R. Advances in edible coatings for fresh fruits and vegetables: a review. Crit Rev Food Sci Nutr. 2013;53:435–50. doi: 10.1080/10408398.2010.541568. [DOI] [PubMed] [Google Scholar]
- 13. Aloui H, Khwaldia K. Natural antimicrobial edible coatings for microbial safety and food quality enhancement. Compr Rev Food Sci Food Saf. 2016;15:1080–103. doi: 10.1111/1541-4337.12226. [DOI] [PubMed] [Google Scholar]
- 14. Zambrano-Zaragoza ML, González-Reza R, Mendoza-Muñoz N, Miranda-Linares V, Bernal-Couoh TF, Mendoza-Elvira S, et al. Nanosystems in edible coatings: a novel strategy for food preservation. Int J Mol Sci. 2018;19:705. doi: 10.3390/ijms19030705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prakash A, Baskaran R, Vadivel V. Citral nanoemulsion incorporated edible coating to extend the shelf life of fresh cut pineapples. LWT. 2020;118:108851. [Google Scholar]
- 16. Maqbool M, Ali A, Alderson P. A combination of gum Arabic and chitosan can control anthracnose caused by Colletotrichum musae and enhance the shelf-life of banana fruit. J Hortic Sci Biotechnol. 2010;85:432–6. [Google Scholar]
- 17. Maqbool M, Ali A, Alderson P, Mohamed MTM, Siddiqui Y, Zahid N. Postharvest application of gum arabic and essential oils for controlling anthracnose and quality of banana and papaya during cold storage. Postharvest Biol Technol. 2011;62:71–6. [Google Scholar]
- 18. Eom H, Chang Y, Lee ES, Choi HD, Han JJ. Development of a starch/gum-based edible coating for rice cakes to retard retrogradation during storage. LWT. 2018;97:516–22. [Google Scholar]
- 19.Ansorena MR, Marcovich NE, Pereda M. Food biopackaging based on chitosan Handbook of ecomaterials. Berlin, Germany: Springer; 2019. pp. 2057–83. [Google Scholar]
- 20. Vatankhah H, Taherian AR, Ramaswamy HS. High-pressure induced thermo-viscoelasticity and dynamic rheology of gum Arabic and chitosan aqueous dispersions. LWT. 2018;89:291–8. [Google Scholar]
- 21. Maqbool M, Ali A, Alderson P, Zahid N, Siddiqui Y. Effect of a novel edible composite coating based on gum Arabic and chitosan on biochemical and physiological responses of banana fruits during cold storage. J Agric Food Chem. 2011;59:5474–82. doi: 10.1021/jf200623m. [DOI] [PubMed] [Google Scholar]
- 22. Correa-Pacheco ZN, Bautista-Baños S, Ramos-García MdL, Martínez-González MdC, Hernández-Romano J. Physicochemical characterization and antimicrobial activity of edible propolis-chitosan nanoparticle films. Prog Org Coat. 2019;137:105326. [Google Scholar]
- 23. Martínez HG, Amodio ML, Colelli G. Carvacrol-loaded chitosan nanoparticles maintain quality of fresh-cut carrots. Innov Food Sci Emerg. 2017;41:56–63. [Google Scholar]
- 24. Nguyen TT, Thi Dao UT, Thi Bui QP, Bach GL, Ha Thuc CN, Ha Thuc H. Enhanced antimicrobial activities and physiochemical properties of edible film based on chitosan incorporated with Sonneratia caseolaris (L.) Engl. leaf extract. Prog Org Coat. 2020;140:105487. [Google Scholar]
- 25. Prakash A, Joseph M, Mangino M. The effects of added proteins on the functionality of gum Arabic in soft drink emulsion systems. Food Hydrocolloids. 1990;4:177–84. [Google Scholar]
- 26. Motlagh S, Ravines P, Karamallah K, Ma Q. The analysis of Acacia gums using electrophoresis. Food Hydrocolloids. 2006;20:848–54. [Google Scholar]
- 27. Çanga EM, Dudak FC. Characterization of cellulose acetate/gum Arabic fibers loaded with extract of Viburnum opulus L. fruit. LWT. 2019;110:247–54. [Google Scholar]
- 28. Lin D, Zhao Y. Innovations in the development and application of edible coatings for fresh and minimally processed fruits and vegetables. Compr Rev Food Sci Food Saf. 2007;6:60–75. [Google Scholar]
- 29. Silva-Weiss A, Ihl M, Sobral P, Gómez-Guillén M, Bifani V. Natural additives in bioactive edible films and coatings: functionality and applications in foods. Food Eng Rev. 2013;5:200–16. [Google Scholar]
- 30. Goldsmith CD, Vuong QV, Stathopoulos CE, Roach PD, Scarlett CJ. Ultrasound increases the aqueous extraction of phenolic compounds with high antioxidant activity from olive pomace. LWT. 2018;89:284–90. doi: 10.3390/antiox3040700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vu TT, Nguyen PTM, Pham NH, Le TH, Nguyen TH, Do DT, et al. Green synthesis of selenium nanoparticles using Cleistocalyx operculatus leaf extract and their acute oral toxicity study. J Composites Sci. 2022;6:307. [Google Scholar]
- 32. Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. AJEV (Am J Enol Vitic) 1965;16:144–58. [Google Scholar]
- 33. Leaves L. Antioxidant activity by DPPH radical scavenging method of ageratum conyzoides. Am J Ethnomed. 2014;1:244–9. [Google Scholar]
- 34. Chrisment A. Couleur & colorimétrie. 1998 [Google Scholar]
- 35. Dung NT, Bajpai VK, Yoon JI, Kang SC. Anti-inflammatory effects of essential oil isolated from the buds of Cleistocalyx operculatus (Roxb.) Merr and Perry. Food Chem Toxicol. 2009;47:449–53. doi: 10.1016/j.fct.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 36. Attri B, Singh A. Effect of different salt concentrations on drying and non-enzymatic browning of mango slices. Indian J Hortic. 2010;67:485–7. [Google Scholar]
- 37. Korbel E, Attal E-H, Grabulos J, Lluberas E, Durand N, Morel G, et al. Impact of temperature and water activity on enzymatic and non-enzymatic reactions in reconstituted dried mango model system. Eur Food Res Tech. 2013;237:39–46. [Google Scholar]
- 38. Alaka O, Aina J, Falade K. Effect of storage conditions on the chemical attributes of Ogbomoso mango juice. Eur Food Res Tech. 2003;218:79–82. [Google Scholar]
- 39. Saucedo-Pompa S, Rojas-Molina R, Aguilera-Carbó AF, Saenz-Galindo A, de La Garza H, Jasso-Cantú D, et al. Edible film based on candelilla wax to improve the shelf life and quality of avocado. Food Res Int. 2009;42:511–5. [Google Scholar]
- 40. Le KH, La DD, Nguyen PTM, Nguyen MD-B, Vo ATK, Nguyen MTH, et al. Fabrication of Cleistocalyx operculatus extracts/chitosan/gum Arabic composite as an edible coating for preservation of banana. Prog Org Coat. 2021;161:106550. [Google Scholar]
- 41.Seymour G, Taylor J, Tucker G. Biochemistry of fruit ripening. London, England: Chapman & Hall; 1993. pp. 327–41. [Google Scholar]
- 42. Yaman Ö, Bayoındırlı L. Effects of an edible coating and cold storage on shelf-life and quality of cherries. LWT–Food Sci Technol. 2002;35:146–50. [Google Scholar]
- 43. Moalemiyan M, Ramaswamy HS, Maftoonazad N. Pectin-based edible coating for shelf-life extension of ataulfo mango. J Food Process Eng. 2012;35:572–600. [Google Scholar]
- 44. Lieberman M. Biosynthesis and action of ethylene. Annu Rev Plant Physiol. 1979;30:533–91. [Google Scholar]
- 45. Baldwin E, Burns J, Kazokas W, Brecht J, Hagenmaier R, Bender R, et al. Effect of two edible coatings with different permeability characteristics on mango (Mangifera indica L.) ripening during storage. Postharvest Biol Technol. 1999;17:215–26. [Google Scholar]
- 46. El-Anany A, Hassan G, Ali FR. Effects of edible coatings on the shelf-life and quality of Anna apple (Malus domestica Borkh) during cold storage. J Food Technol. 2009;7:5–11. [Google Scholar]
- 47. Thi HPN, Thi KTP, Nguyen TT, Nguyen PT, Vu TT, Le HT, et al. Green synthesis of an Ag nanoparticle-decorated graphene nanoplatelet nanocomposite by using Cleistocalyx operculatus leaf extract for antibacterial applications. Nano Struct Nano Obj. 2022;29:100810. [Google Scholar]
- 48. Le NT, Dang T-D, Binh KH, Nguyen TM, Xuan TN, La DD, et al. Green synthesis of highly stable zero-valent iron nanoparticles for organic dye treatment using Cleistocalyx operculatus leaf extract. Sustain Chem Pharm. 2022;25:100598. [Google Scholar]
- 49. Rajabi H, Jafari SM, Rajabzadeh G, Sarfarazi M, Sedaghati S. Chitosan-gum Arabic complex nanocarriers for encapsulation of saffron bioactive components. Coll Surf A Phys Eng Asp. 2019;578:123644. [Google Scholar]
- 50. Puvvada YS, Vankayalapati S, Sukhavasi S. Extraction of chitin from chitosan from exoskeleton of shrimp for application in the pharmaceutical industry. Int Curr Pharmaceut J. 2012;1:258–63. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The effect of CH/GA ratios on the appearance of mangoes stored at different period of time.
Effect of glycerol content on the appearance of mangoes stored at different period of time.
FTIR spectrum of CH/GA/Gly edible coating.