Abstract
Protective immunity of BALB/c mice immunized with simian virus 40 (SV40) large T antigen (TAg) against SV40-transformed, TAg-expressing mKSA tumor cells is critically dependent on both CD8+ and CD4+ T lymphocytes. By depleting mice of T-cell subsets at different times before and after tumor challenge, we found that at all times, CD4+ and CD8+ cells both were equally important in establishing and maintaining a protective immune response. CD4+ cells do not contribute to tumor eradication by directly lysing mKSA cells. However, CD4+ lymphocytes provide help to CD8+ cells to proliferate and to mature into fully active cytotoxic T lymphocytes (CTL). Depletion of CD4+ cells by a single injection of CD4-specific monoclonal antibody at any time from directly before injection of the vaccinating antigen to up to 7 days after tumor challenge inhibited the generation of cytolytic CD8+ lymphocytes. T helper cells in this system secrete the typical Th-1 cytokines interleukin 2 (IL-2) and gamma interferon. Because in this system TAg-specific CD8+ cells secrete only minute amounts of IL-2, it appears that T helper cells provide these cytokines for CD8+ T cells. Moreover, this helper effect of CD4+ T cells in mKSA tumor rejection in BALB/c mice does not simply improve the activity of TAg-specific CD8+ CTL but actually enables them to mature into cytolytic effector cells. Beyond this activity, the presence of T helper cells is necessary even in the late phase of tumor cell rejection in order to maintain protective immunity. However, despite the support of CD4+ T helper cells, the tumor-specific CTL response is so weak that only at the site of tumor cell inoculation and not in the spleen or in the regional lymph nodes can TAg-specific CTL be detected.
CD8+ cytotoxic T lymphocytes (CTL) are potent mediators of antigen-specific tumor cell destruction. Therefore, most attempts to generate an immune response against tumor cells have focused on the induction of CTL, for example, by immunizing with peptides corresponding to major histocompatibility complex (MHC) class I-restricted epitopes. However, as this strategy fails to also stimulate T helper cells, which respond to MHC class II-restricted epitopes, the supportive role of these cells in tumor-specific immune responses may be neglected.
The importance of T helper cells for the outcome of immune responses against infectious pathogens has been recognized for quite some time. Recently, some groups (reviewed in reference 35) have presented evidence that the contribution of T helper cells also may be crucial in immune responses against tumors.
To ascertain more about the involvement of T helper cells in immune responses against tumors, we investigated the rejection of a well-characterized virus-induced tumor, the simian virus 40 (SV40) large-T-antigen (TAg)-expressing mKSA tumor, by BALB/c mice immunized with recombinant TAg. This particular system was chosen for the following reasons. (i) BALB/c mice immunized with TAg readily reject mKSA tumor cell inocula of 106 cells, corresponding to about 10,000 times the 50% lethal dose of these tumor cells in naive mice (54). (ii) BALB/c mice are considered to be low responders or nonresponders with respect to the generation of TAg-specific CTL (1, 2, 16, 17, 36, 41, 42, 46). This weak immune reaction resembles the immune responses against nonviral tumor-specific antigens. (iii) Tumor-associated lymphocytes (TAL) can be isolated very conveniently from BALB/c mice injected intraperitoneally (i.p.) with mKSA cells by peritoneal lavage. These cells can be tested in primary in vitro cytotoxicity assays as well as for cytokine secretion without the need for prolonged in vitro cultivation. Thus, their measured activity approximates that in vivo as closely as possible. (iv) From the site of tumor cell inoculation, TAg-specific CD8+ CTL which lyse TAg-expressing target cells in primary in vitro assays can be recovered (54). (v) Although these TAg-specific, MHC class I-restricted CTL appear to be the actual effector cells in eliminating mKSA tumor cells, previous experiments demonstrated that at least at some point in the immune response CD4+ T cells are also needed for protective immunity (54).
The requirement of CD4+ lymphocytes for mKSA cell rejection could be explained by the following, not mutually exclusive scenarios. First, CD8+ CTL activity might be too weak or too slow to completely eradicate mKSA tumor cells, and an antibody response that requires CD4+ T helper cells might provide the complementary part in tumor rejection. The possibility of such a combined cell-mediated and humoral anti-mKSA tumor response is supported by the report of Bright and coworkers (2), who proposed an antibody-dependent cell-mediated cytoxicity mechanism for mKSA tumor rejection by BALB/c mice. Second, CD4+ cells might provide help to CD8+ cells. This interpretation is supported by several studies of adoptive immunization in which CTL were effective against tumors only when coadministered either with T helper cells or with one of the major help-providing lymphokines of these cells, interleukin 2 (IL-2) (18, 27, 38, 39, 53). Third, CD4+ lymphocytes could lyse tumor cells via soluble factors (47) or via membrane-bound mechanisms (20, 21). Fourth, they could recruit and activate macrophages or other leukocytes to exert tumoricidal activity (10, 11, 15, 32, 45, 52).
Our results show that there is an absolute requirement for both CD4+ and CD8+ cells during the entire course of mKSA tumor rejection. During this time, the major function of CD4+ T lymphocytes is to enable CD8+ cells to proliferate and to mature into fully active effector CTL. However, despite the support of T helper cells, the expansion of TAg-specific CTL is so limited that ex vivo lytically active CTL can be isolated from the tumor site only, while both the spleen and the regional lymph nodes are devoid of them.
MATERIALS AND METHODS
Mice and tumor cells.
Specific-pathogen-free female BALB/cAnNCrlBR mice were purchased from Charles River Wiga, Sulzfeld, Germany. The mice were kept strictly under barrier conditions and were used when 8 to 12 weeks old. SV40-transformed mKSA (22) tumor cells, originating from BALB/c mice, and SV40-transformed BALB/c fibroblasts (BALB/c-SV40 fibroblasts) were routinely maintained in Dulbecco modified Eagle medium supplemented with 5% fetal calf serum (FCS).
Production and purification of recombinant TAg and production of SV40.
TAg was produced by infecting Sf 158 cells with recombinant baculovirus coding for full-length SV40 TAg (24), kindly provided by Ellen Fanning. TAg was purified from cell lysates by immunoaffinity chromatography using PAb108 (19) coupled to CNBr-Sepharose. The purified protein was dialyzed against phosphate-buffered saline (PBS)–40% glycerin and stored at −70°C. SV40 was harvested from cultures of TC7 African green monkey kidney cells infected with SV40.
Immunization of mice with recombinant TAg or with SV40.
Ten micrograms of purified TAg in PBS was injected twice i.p. at a 1-week interval (days −14 and −7). Seven days after the second immunization (day 0), the animals were challenged by i.p. injection of 106 viable mKSA cells. Alternatively, mice were immunized with SV40 by injection of 50 μl of SV40 suspension (virus titer, about 108 PFU/ml) into the left hind footpad.
Depleting mice of CD4+ or CD8+ T lymphocytes.
Groups of mice were treated intravenously (i.v.) with either a mixture of 250 μg each of rat monoclonal antibodies (Mab) YTS191.1 and YTA3.1 (37) or 500 μg of rat MAb YTS169.4 (9) to deplete CD4+ or CD8+ T lymphocytes, respectively. As a control, purified rat immunoglobulin G (IgG) (Sigma, Deisenhofen, Germany) was administered in some experiments. The time of the antibody injection relative to tumor challenge is given in Results. MAb were produced, purified, used, and efficacy controlled as described previously (13).
Magnetic cell sorting.
The magnetic cell sorting method has been described elsewhere (28). TAg-immunized or naive mice were inoculated i.p. with 106 mKSA tumor cells. Peritoneal exudate cells were recovered by rinsing the peritoneal cavities with cold PBS, and single-cell suspensions of spleens or mesenteric lymph nodes were prepared from the same animals. These cell preparations were incubated with CD4- or CD8-specific MAb attached to magnetic beads (Miltenyi Biotech, Bergisch Gladbach, Germany) and subsequently passed through MiniMACS columns in a magnetic field (Miltenyi). Flow cytometry revealed >95% purity of the CD4+ or CD8+ T-lymphocyte preparation.
In vitro restimulation of TAg-specific CTL.
Spleen cells (5 × 107) were cocultured with 5 × 106 irradiated (10 Gy) BALB/c-SV40 fibroblasts in 20 ml of complete medium (RPMI 1640, 10% FCS, 2 mM l-glutamine, 50 μM 2-mercaptoethanol, 100 U of penicillin/ml, 100 μg of streptomycin/ml) supplemented with 10% concanavalin A supernatant in upright TC-25 culture flasks for 6 days. The TAg-specific cytotoxicity of these cells was tested with standard chromium release assays.
In some experiments, T cells were restimulated three times at weekly intervals by removing the consumed medium and adding fresh medium supplemented with concanavalin A supernatant and stimulator cells as described above; cytotoxicity was measured 6 days after the third cycle of restimulation.
Chromium release assays.
CTL activity was measured as described by Brunner and colleagues (5) with modifications (13). Briefly, 51Cr-labeled mKSA or fetal BALB/c-SV40 fibroblast target cells were incubated at 37°C with effector cells at various ratios; control target cells were TAg-negative MethA cells (H-2d) (34) or TAg-positive SV40-transformed fetal C57BL/6J fibroblasts (H-2b). After 4 h, the release of 51Cr was measured and percent lysis was calculated as [(experimental counts per minute − spontaneous counts per minute)/(maximal counts per minute − spontaneous counts per minute)] × 100.
Measurement of cytokines.
CD4+ or CD8+ T cells were prepared from peritoneal exudate cells or splenic single-cell suspensions by magnetic cell sorting. Purified cells were kept for 24 h at a density of 106/ml in RPMI 1640–5% FCS in flat-bottom microtiter plates (200 μl/well) before supernatants were harvested (49). The supernatants were tested for their contents of IL-2, gamma interferon (IFN-γ), or tumor necrosis factor alpha (TNF-α) by a commercially available enzyme-linked immunosorbent assay (R&D Systems, Wiesbaden, Germany).
RESULTS
Detection of TAg-specific CD8+ CTL in TAg-immunized BALB/c mice.
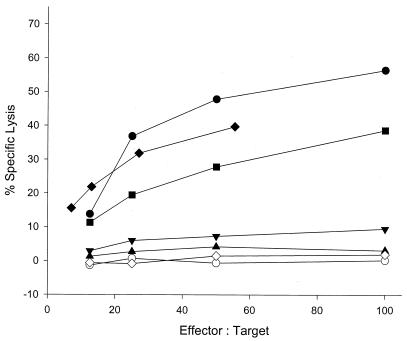
CD8+ TAL collected from the site of tumor cell inoculation by peritoneal lavage of BALB/c mice immunized twice with 10 μg of recombinant TAg and challenged i.p. with mKSA tumor cells lysed TAg-expressing, MHC class I-matched target cells in primary ex vivo cytotoxicity assays (Fig. 1) (54). However, CD8+ T cells prepared from spleens (Fig. 1) (54) or mesenteric lymph nodes (data not shown) from the same mice did not exhibit this cytotoxic activity. This finding indicates that only a limited number of TAg-specific CTL is activated in BALB/c mice and that these few cells are apparently recruited quantitatively to the site of the tumor.
FIG. 1.
TAg-specific cytotoxicity mediated by freshly prepared CD8+ cells or by spleen cell bulk cultures prepared from mice immunized with different protocols. TAL or spleen cells were recovered from mice pretreated as follows: twice immunized with TAg, challenged with mKSA cells, CD8+ TAL prepared from the peritoneal cavity 8 days after challenge (filled diamonds; the reduced effector/target ratio is due to the poor yield of CD8+ TAL from this group of five mice); TAg immunized, challenged with mKSA cells, CD8+ spleen cells prepared on day 8 (open diamonds); TAg immunized, challenged with mKSA cells, restimulated in the presence of TAg-expressing cells 4 weeks after challenge (filled circles); SV40 immunized (once), restimulated in the presence of TAg-expressing cells 2 weeks after immunization (filled squares); TAg immunized, restimulated 4 weeks after immunization for three weekly cycles (inverted filled triangles); TAg immunized, restimulated once 4 weeks after immunization (filled triangles); and naive, stimulated once in the presence of TAg-expressing cells (open circles). Lytic activity against MHC-matched, non-TAg-expressing MethA cells or against MHC-mismatched, TAg-expressing SV40-transformed C57BL/6 fibroblasts was below 7% (data not shown).
Aside from isolating CD8+ TAL, CTL activity that could be detected by standard in vitro restimulation was obtained only after enhanced priming of BALB/c mice by abortive infection with SV40 or after boosting of BALB/c mice immunized with recombinant TAg by challenge with TAg-expressing tumor cells (Fig. 1).
The data confirm that the TAg-specific response of CD8+ T cells in BALB/c mice is quantitatively and/or qualitatively so weak that it is not detected by standard restimulation protocols. In the past, this feature has led to the classification of BALB/c mice as low responders or even nonresponders with respect to a TAg-specific CTL response.
Both CD4+ and CD8+ cells are required during the entire course of mKSA tumor rejection.
It was previously reported that protective immunity in TAg-immunized BALB/c mice is dependent not only on CD8+ but also on CD4+ T lymphocytes (54). In order to find out at what time during the course of the immune response T helper cells were needed, groups of mice were depleted of either CD4+, CD8+, or both T-cell subsets by administering i.v. a single dose of the appropriate MAb at various times. The development of clinical signs of tumor progression (inactivity, ruffled fur, hunched posture, projection of the flanks due to ascites, or development of subcutaneous tumor nodes at the injection site) and the time of tumor-related death of these mice were monitored. Effective antitumor protection was defined as mice staying free of mKSA for life rather than some prolongation of survival compared to that of naive controls.
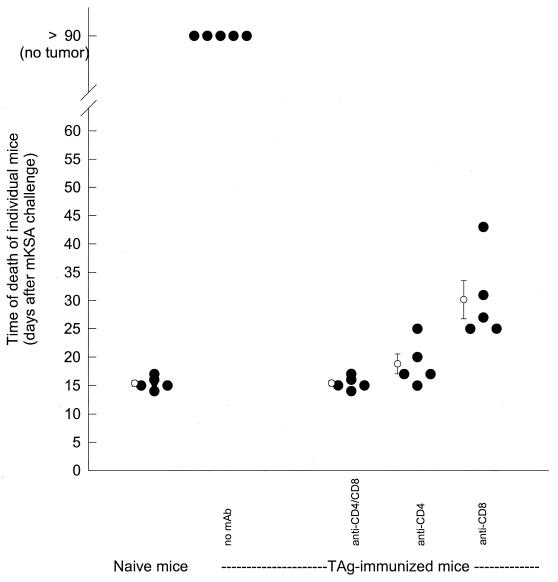
Figure 2 shows a plot of the time of death of mice injected with T-cell-depleting MAb prior to antigen injection. Animals treated simultaneously with CD4- and CD8-specific MAb died at about the same time after tumor challenge as naive mice. Thus, as expected, depletion of both T-cell subsets during the immunization phase rendered these mice completely incapable of restraining the growth of mKSA tumors. Treatment of mice with CD4-specific MAb 1 day prior to antigen injection also prevented the induction of protective immunity, with a mean survival time not different from that of naive mice. Elimination of CD8+ cells prior to antigen injection also resulted in the failure to eliminate tumor cells, leading to the death of all mice. However, the mean survival time was prolonged for about 10 days compared to those for the previously described groups.
FIG. 2.
Mortality of mice after depletion of T-lymphocyte subsets prior to immunization with TAg. Groups of five mice were injected i.v. with a combination of each CD4- and CD8-specific MAb, CD4-specific MAb, or CD8-specific MAb on days −15 and −8; 500 μg of each MAb was injected. A control group received no MAb treatment. One day after T-cell depletion (i.e., days −14 and −7), mice were immunized by i.p. injection of 10 μg of TAg; five control mice received neither TAg nor MAb. On day 0, all mice were challenged by i.p. inoculation of 106 mKSA cells. Development of tumors and death of the mice were monitored daily. Open circles and error bars indicate means and standard errors.
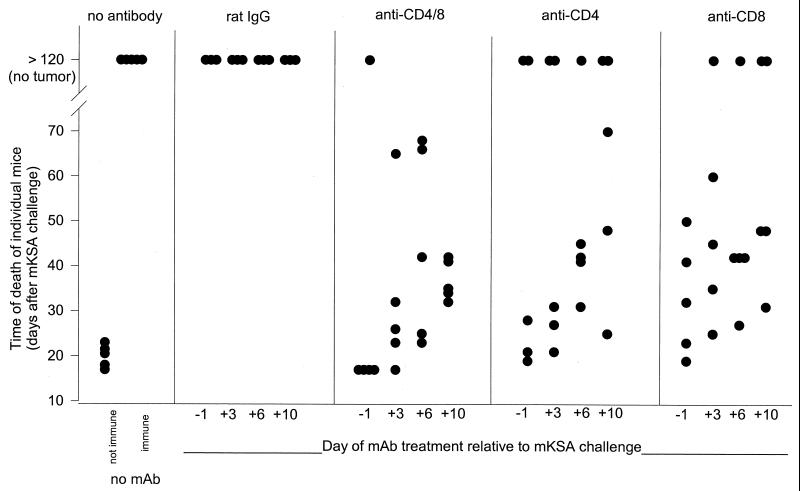
Figure 3 shows the survival times for TAg-immunized mice depleted of T-cell subsets on day −1, +3, +6, or +10 with respect to mKSA tumor cell challenge. For combined depletion of CD4+ and CD8+ cells, protection was abrogated by a single MAb injection at any of the indicated times. Depletion of either CD8+ or CD4+ T cells also severely affected protective immunity regardless of the time of MAb injection. However, this treatment did not abolish protection as effectively as eliminating both subsets, since we observed one or two surviving animals in nearly every group of five mice.
FIG. 3.
Dependence of course of mortality on T-cell subset depletion 1 day before or 3, 6, or 10 days after mKSA cell challenge. Mice immunized by two i.p. injections of 10 μg of TAg on days −14 and −7 were depleted of CD4+, CD8+ or both T-lymphocyte populations. Depletion was achieved by i.v. injection of 500 μg of the respective MAb as a single dose on day −1, +3, +6, or +10 relative to challenge with 106 mKSA cells on day 0. Tumor development and death of mice were monitored daily.
Efficacy and duration of CD4+ or CD8+ T-cell depletion by a single MAb injection.
To exclude the possibility that T cells that escaped depletion by a single treatment with 500 μg of MAb might be responsible for the survival of several T-cell-depleted mice (Fig. 3), we thouroghly investigated the efficacy and duration of the MAb treatment. We especially addressed concerns that in immunized and tumor cell-challenged mice, depletion of T-cell subsets might be incomplete due to the expansion of the respective T-cell population or due to the down-modulation of CD4 or CD8 coreceptors on activated T cells (6, 51). In Table 1, we show that regardless of whether the respective MAb was injected on the day of challenge or 4 or 7 days after challenge, CD4+ as well as CD8+ T cells were completely eliminated from the spleens. Moreover, the recovery of the T-cell compartment was so slow that the respective T-cell subset could not be detected up to 18 days after administration of the MAb. The data suggest that the minute numbers of T cells that escaped depletion most probably were not responsible for the survival of MAb-treated mice.
TABLE 1.
Efficacy and duration of depletion of splenic T-cell subsets by a single dose of CD4- or CD8-specific MAb administered at different times during TAg-specific rejection of mKSA tumorsa
| Mice | MAb specificity | Day of MAb treatment relative to challenge with mKSA cells | Mouse designation | % of respective T-cell subset within mononuclear spleen cells at the following time of analysis (day) relative to injection of mKSA cells:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1
|
8
|
14
|
22
|
||||||||
| CD4+ | CD8+ | CD4+ | CD8+ | CD4+ | CD8+ | CD4+ | CD8+ | ||||
| Naive | NA | NA | 1 | 23.1 | 4.0 | 17.3 | 4.4 | ND | ND | 12.7 | 2.4 |
| 2 | ND | ND | 20.9 | 5.9 | ND | ND | ND | ND | |||
| Naive mKSA challengedb | CoAbc | 0 | 1 | 17.4 | 3.3 | 14.3 | 3.8 | 13.2 | 2.9 | ND | ND |
| 2 | 19.7 | 3.4 | ND | ND | 12.9 | 2.5 | ND | ND | |||
| CD4d | 0 | 1 | 0.5 | 5.9 | 0.4 | 4.0 | 0.6 | 8.0 | ND | ND | |
| 2 | 0.7 | 4.0 | 0.3 | 4.4 | 0.6 | 5.0 | ND | ND | |||
| CD8e | 0 | 1 | 23.2 | 0.0 | 20.5 | 0.0 | 15.2 | 0.0 | ND | ND | |
| 2 | 24.3 | 0.1 | 18.1 | 0.1 | — | — | ND | ND | |||
| TAg immunized, mKSA challengedf | CoAb | 0 | 1 | 17.4 | 4.4 | 17.3 | 5.4 | 20.6 | 4.3 | 21.4 | 5.1 |
| 2 | 21.6 | 5.9 | 17.1 | 5.0 | 15.4 | 2.9 | 14.5 | 3.9 | |||
| 4 | 1 | NA | NA | 17.7 | 3.9 | ND | ND | ND | ND | ||
| CD4 | 0 | 1 | 1.0 | 7.1 | 0.3 | 7.1 | 0.7 | 4.6 | ND | ND | |
| 2 | 0.6 | 6.4 | 0.3 | 5.3 | 0.7 | 3.7 | ND | ND | |||
| 4 | 1 | ND | ND | 0.5 | 4.1 | ND | ND | 0.7 | 4.9 | ||
| 2 | ND | ND | 0.8 | 3.8 | ND | ND | 0.9 | 5.4 | |||
| 7 | 1 | ND | ND | 0.4 | 8.6 | 0.3 | 5.9 | 0.9 | 8.5 | ||
| 2 | ND | ND | 0.5 | 9.7 | 0.5 | 5.9 | 0.9 | 8.3 | |||
| CD8 | 0 | 1 | 23.2 | 0.1 | 19.5 | 0.1 | 22.6 | 0.1 | ND | ND | |
| 2 | 21.4 | 0.0 | 16.0 | 0.1 | 28.7 | 0.2 | ND | ND | |||
| 4 | 1 | ND | ND | 13.3 | 0.1 | ND | ND | 22.8 | 0.4 | ||
| 2 | ND | ND | 20.0 | 0.0 | ND | ND | ND | ND | |||
| 7 | 1 | ND | ND | 21.6 | 0.1 | 22.5 | 0.0 | ND | ND | ||
| 2 | ND | ND | 22.6 | 0.1 | 28.3 | 0.1 | ND | ND | |||
NA, not applicable; ND, not determined. —, mouse succumbed to tumor before analysis.
Mice were inoculated i.p. with 106 mKSA cells.
500 μg of rat IgG.
250 μg each of MAb YTS191.1 and YTA3.1.
500 μg of MAb YTS169.4.
Mice were immunized i.p. with 10 μg of TAg on days −14 and −7 and challenged i.p. with 106 mKSA cells on day 0.
CD4+ T lymphocytes are not directly cytolytic for mKSA cells.
As CD4+ T cells were as important for the protective TAg-specific immune response of BALB/c mice as CD8+ T cells, we considered the possibility that CD4+ T lymphocytes might directly attack tumor cells either by contact-mediated lytic mechanisms (12, 21, 25, 29, 40) or by the secretion of cytostatic or cytotoxic lymphokines (47). However, the specific recognition of target cells by CD4+ T cells usually requires the expression of MHC class II molecules on the target cells. As mKSA cells do not express MHC class II molecules (R. Schirmbeck, personal communication; our unpublished data), direct cytotoxic activity of CD4+ T cells against mKSA cells was rather unlikely. Nevertheless, we tested whether CD4+ T cells freshly prepared from immune mice on day 4, 6, or 8 after tumor challenge were able to lyse mKSA cells. Although IFN-γ does not induce or upregulate the expression of MHC class II molecules in mKSA cells (R. Schirmbeck, personal communication), we used native as well as IFN-γ-preincubated mKSA cells as target cells in these assays. In standard 4-h chromium release assays, we could never detect any specific destruction of native or IFN-γ-treated mKSA cells. In addition, overnight cytolytic assays also did not reveal any specific CD4+ cell-mediated lysis of mKSA cells (data not shown).
The development of CD8+ T-lymphocyte-mediated TAg-specific cytotoxicity is critically dependent on CD4+ T helper cells.
Since CD4+ T lymphocytes are not directly cytolytic for mKSA cells, we assumed that these cells might act as helper cells for the generation of TAg-specific CD8+ CTL.
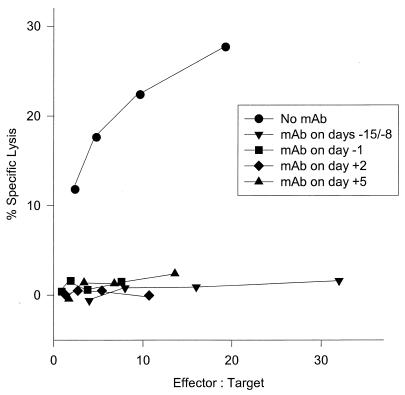
The ability to detect ex vivo lytically active TAg-specific CTL during the course of mKSA cell rejection provided a means to directly test the hypothesis that CD4+ T cells help CD8+ cells become active CTL. We did so by measuring the primary TAg-specific lytic activity of CD8+ TAL prepared from mice that had been depleted of CD4+ T cells at defined times during the immune response. CTL activity was determined on day 8 or 10 after tumor challenge because the lytic activity of CD8+ cells from immune control mice reaches peak levels approximately on these days.
Mice were depleted of CD4+ cells either 1 day prior to each antigen injection or 1 day before or 2 or 5 days after tumor challenge. CTL acitivity was measured on day 8 after tumor cell challenge. Depletion of CD4+ cells at any of the indicated times resulted in the complete absence of any cytolytic activity of CD8+ cells (Fig. 4). Purified rat IgG administered to control mice in replicate experiments never had any effect upon CTL activity, regardless of the time of injection (data not shown).
FIG. 4.
Primary cytotoxicity of CD8+ peritoneal exudate cells from TAg-immunized mice after depletion of CD4+ cells at different times during immunization or maturation. Mice were immunized on days −14 and −7 by i.p. injection of 10 μg of TAg and challenged on day 0 with 106 mKSA cells injected i.p. Depletion of CD4+ cells was achieved by treating groups of five mice on days −15 and −8, day −1, day +2, or day +5 with an i.v. injection of CD4-specific MAb. On day 8, the cytotoxic activity of CD8+ peritoneal exudate cells was determined with a standard 4-h chromium release assay using mKSA cells as targets. Variable effector/target ratios are due to various yields of CD8+ TAL in the groups.
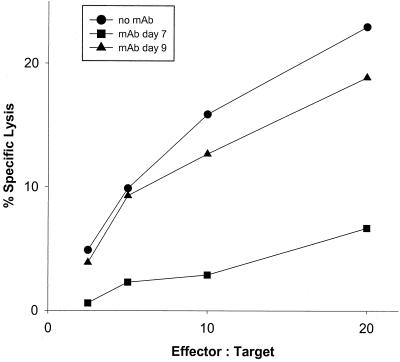
To elucidate whether TAg-specific CTL were dependent on T helper cells beyond induction and maturation and even during the effector phase, we measured the TAg-specific CTL activity of CD8+ cells harvested from mice which had been depleted of T helper cells later than 5 days after tumor cell inoculation (Fig. 5), namely, on day 7 or 9 after challenge. CD8+ TAL recovered on day 10 from mice depleted of T helper cells on day 7 showed markedly reduced lytic activity compared to cells recovered from control mice. This finding substantiates that the generation of CTL activity as late as 7 days postchallenge and thereafter is still critically dependent on T helper cells. However, depletion of T helper cells on day 9 only marginally reduced cytotoxicity measured on day 10, indicating that between days 9 and 10 after tumor challenge, the lytic activity of TAg-specific CD8+ CTL no longer seems to be dependent on T helper cells.
FIG. 5.
Primary cytotoxicity of CD8+ TAL after depletion of CD4+ cells during the effector phase. Mice were immunized and challenged with tumor as described in the legend to Fig. 4. CD4+ T cells were depleted on day 7 or 9. The cytotoxicity of CD8+ peritoneal exudate cells was determined on day 10 after tumor challenge. TAL from mice not treated with MAb were included as positive controls.
CD4+ T cells freshly isolated from TAg-immunized, mKSA-challenged mice secrete large amounts of IL-2 and IFN-γ.
CD4+ T cells mediate their helper functions via the secretion of cytokines. Therefore, we compared the ex vivo capacities of CD8+ and CD4+ cells to secrete cytokines that are known to support cell-mediated immunity. We determined the amounts of IL-2, IFN-γ, and TNF-α in the supernatants of either CD4+ or CD8+ cells freshly prepared from spleens or peritoneal exudate lymphocytes (PEL) of TAg-immunized mice at different times after mKSA cell inoculation.
In order to prove that neither the preparative procedure nor the culture conditions as such provided a stimulus for the release of cytokines, we also measured the secretion of these cytokines by cells prepared from naive mice. As shown in Table 2, CD4+ cells from spleens or PEL of naive mice secreted only small amounts of IL-2, while IFN-γ and TNF-α levels remained below the limits of detection. CD8+ cells from the same mice did not secrete detectable amounts of any of the three tested cytokines.
TABLE 2.
Cytokines secreted by CD4+ or CD8+ T cells freshly prepared from PEL or spleena
| Cytokine | Source of cells | Amt (pg/ml) of cytokine secreted by 106 cells in 24 h under the following conditions:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4+ T lymphocytes fromb:
|
CD8+ T lymphocytes fromb:
|
||||||||||
| Naive mice | TAg-immunized mice on the following day after mKSA inoculationc:
|
Naive mice | TAg-immunized mice on the following day after mKSA inoculationc:
|
||||||||
| 4 | 6 | 8 | 14 | 4 | 6 | 8 | 14 | ||||
| IL-2 | PEL | 90 | 285 | 520 | 3,250 | 4,000 | <15 | 40 | 50 | 125 | 80 |
| Spleen | 115 | 515 | 475 | 1,900 | ND | <15 | ND | ND | <15 | <15 | |
| IFN-γ | PEL | <15 | 345 | 1,190 | 3,150 | 2,700 | 50 | <15 | <15 | 1,900 | 80 |
| Spleen | <15 | 45 | 55 | 80 | ND | 50 | ND | ND | <15 | <15 | |
| TNF-α | PEL | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 |
| Spleen | <5 | <5 | <5 | <5 | ND | <5 | ND | ND | <5 | <5 | |
ND, not determined.
CD4+ or CD8+ T cells were enriched by magnetic cell separation using the MACS technique. Following magnetic enrichment, CD4+ or CD8+ T cells were kept for 24 h in RPMI 1640 medium at a density of 106 cells/ml. Supernatants were collected and tested by an enzyme-linked immunosorbent assay for the presence of IL-2, IFN-γ, and TNF-α. TNF-α was never detected in any of the samples tested (limit of detection, 5.0 pg/ml).
Mice were immunized with 10 μg of TAg via i.p. injection 14 and 7 days prior to challenge injection of mKSA cells. At the indicated days following injection of 106 viable mKSA cells, PEL were collected from groups of 5 to 10 mice by peritoneal lavage or single-cell suspensions of spleens were prepared.
In comparison to CD4+ cells prepared from naive mice, CD4+ cells prepared from TAg-immunized mice secreted elevated levels of IL-2 as early as 4 days after challenge with mKSA cells and further increased their secretory activity up to the end of the experiment on day 14. It is noteworthy that both PEL- and spleen-derived CD4+ T cells displayed elevated levels of IL-2 secretion, indicating that mKSA tumor rejection is a systemic immune reaction and not just a local response. In sharp contrast to CD4+ cells, CD8+ PEL prepared from TAg-immunized mice secreted only minute amounts of IL-2.
IFN-γ is another Th1 factor that has been shown to be involved in the activation of CTL in different systems (8, 14, 26, 43, 44, 50). As early as 4 days after tumor cell inoculation, CD4+ PEL released large amounts of IFN-γ. This activity remained at high levels up to at least day 14 after tumor challenge. In contrast to IL-2, which was released by both CD4+ PEL and CD4+ spleen cells, the secretion of IFN-γ by T helper cells was restricted to CD4+ cells from the tumor site, since hardly any IFN-γ secreted by splenic CD4+ cells was detectable.
IFN-γ secretion by CD8+ PEL was not detectable on days 4 and 6, peaked at high levels on day 8, and then declined. Splenic CD8+ T cells were completely devoid of IFN-γ secretion on days 8 and 14.
DISCUSSION
CD4+ T lymphocytes are often essential for humoral and cellular immune responses (18, 27, 39, 53), but only recently has the importance of CD4+ T helper cells in tumor-specific immune responses attracted some scientific interest (21, 32, 35, 48). The previous neglect of these cells is due at least in part to methodological problems. For example, in most experimental systems, it is not possible to determine the ex vivo activity of freshly prepared CD4+ or CD8+ tumor-infitrating lymphocytes (TIL) due to rather small quantities of these cells and difficulties in isolating them from the tumor tissue. Therefore, TIL usually can be studied only following prolonged in vitro cultivation.
TAg-immunized BALB/c mice challenged i.p. with TAg-expressing mKSA tumor cells provide a unique opportunity to analyze the in vivo and ex vivo activities of both CD4+ and CD8+ T lymphocytes during the entire course of antigen-specific tumor rejection, because T lymphocytes can easily be enriched from peritoneal exudate cells (54). However, since these lymphocytes are not derived from a solid tumor, we define them as TAL rather than as TIL. Studying such freshly isolated cells should provide data approximating the functional state of these lymphocytes in vivo as closely as possible. These data, in conjunction with in vivo experiments, should allow a detailed understanding of the mechanisms of antigen-specific immune responses against tumor cells in vivo.
Studying the contributions of CD4+ and CD8+ T lymphocytes to TAg-specific tumor elimination in BALB/c mice revealed two major findings. First, TAg-specific CD8+ CTL are absolutely dependent on antigen-specific CD4+ T helper cells not only during induction and maturation but also during the late effector phase. Second, despite the support of T helper lymphocytes, the expansion of specific CTL appears to be very limited, and the small numbers of TAg-specific CTL performing ex vivo detectable target cell lysis appear to migrate quantitatively to the tumor site so that ex vivo active CTL can be recovered neither from the spleen nor from the regional lymph nodes.
Dependence of CTL on T helper cells.
The possibility of measuring primary TAg-specific CTL activity in this system enabled us to disclose the dependence of CD8+ lytic effector cells on CD4+ T helper cells. The measurement of the lytic activity of CD8+ T lymphocytes subsequent to the depletion of CD4+ lymphocytes revealed that CD4+ T cells are absolutely required for the generation of lytically active TAg-specific CD8+ CTL, because elimination of CD4+ cells at any time point between 1 day prior to TAg injection and 5 days after tumor challenge completely prevented the emergence of CTL. Moreover, withdrawal of T helper cells at day 7 after tumor challenge stopped the augmentation of the already acquired low level of CTL-mediated lysis present at the moment of T helper cell depletion. However, depletion of T helper cells at day 9 did not significantly alter the lytic activity measurable on day 10 compared to the results for immune controls.
This latter finding seems to be in apparent contradiction to our in vivo data, because the depletion of CD4+ cells as late as 10 days after tumor challenge abrogated protective immunity in vivo. Since T-cell depletion by our MAb is very fast and effective (Table 1), we must conclude that CTL present at day 9 are able to maintain their lytic ability for at least 1 day without the presence of CD4+ cells. However, these CD8+ cells appear to be unable to eliminate the very low numbers (undetectable by cytological inspection) of mKSA cells which are still present at this time. Two major reasons might be proposed for these findings. Either CTL may be unable to stay in a lytically active state for a longer time without the support of T helper cells or, alternatively, because effector CTL may be exhausted by repeated encounters with target cells (30), a continuous supply of freshly activated and mature CTL is needed for complete tumor cell eradication.
We cannot easily explain the observation that the depletion of either CD4+ or CD8+ cells was not as effective as the combined elimination of both T-cell subsets (Fig. 3), as judged by the survival of up to 40% of mice in several groups, although depletion of the respective T-cell subpopulation was highly efficacious. One explanation for occasional survivors in T-cell-depleted groups of mice might be the elimination of the tumor by a second line of defense, namely, antibody-dependent cell-mediated cytoxicity, as proposed by Bright and coworkers (2). Another mechanism might be the recruitment and activation of macrophages or other leukocytes which are present in large quantities in the peritoneal cavity of mKSA-challenged mice (data not shown). These cells might mediate some non-antigen-specific effect against mKSA cells, as has been shown in other systems (10, 11, 15, 32, 45, 52).
Analysis of the capacity of CD4+ and CD8+ T cells to secrete cytokines known to be essential for the generation of cellular immune responses provided evidence for CD4+ T cells acting as classical T helper cells for CD8+ CTL. CD4+ T cells isolated from the site of tumor inoculation secreted large amounts of the typical Th1 factors IL-2 and IFN-γ between days 4 and 14 after tumor challenge. TNF-α was not detected in any of the samples. These data confirm and extend a report of Bright and coworkers (3), who determined the cytokine response of TAg-immunized BALB/c mice by stimulating splenocytes in vitro in the presence of TAg for 6 days. Splenocytes of immune mice were found to secrete IL-2 and IFN-γ but not IL-4 and IL-5 in response to TAg. Our analyses extend these data by the finding that CD4+ TAL were so strongly activated that they spontaneously secreted large amounts of IL-2 and IFN-γ but no TNF-α during the first 24 h of in vitro cultivation. In marked contrast, CD4+ splenocytes isolated from the same mice secreted only minute amounts of IFN-γ and no IL-2. As demonstrated in these analyses, the preparation of cells and the culture conditions as such do not stimulate the secretion of the tested cytokines. Thus, it is possible to determine with this system and ex vivo assays the tumor-specific activity not only of CD8+ CTL but also of CD4+ T helper cells.
In contrast to the high level of cytokine release by CD4+ TAL, CD8+ TAL were nearly devoid of any IL-2 secretion and only on day 8 released significant amounts of IFN-γ. CD8+ CTL release IFN-γ in response to MHC class I-restricted contact with antigen-expressing target cells (7, 31). We assume that IFN-γ was detectable only in supernatants of CD8+ TAL collected on day 8 because CTL activity is at maximal levels at this time. Thus, CTL freshly isolated from peritoneal cavities at the time of peak lytic activity continue to secrete IFN-γ for some time, despite missing target cells in vitro. CD8+ spleen cells, which had no target contacts in situ, were devoid of in vitro IFN-γ secretion.
Taken together, the kinetics of release of IL-2 and IFN-γ detectable in the supernatants of CD4+ and CD8+ TAL prepared from TAg-immunized, mKSA-challenged mice in conjunction with the differential secretory activities of TAL and splenic cells lead us to the following conclusions. CD8+ TAg-specific T cells have only a limited capacity to secrete IL-2, although they are—most probably in response to target cell contacts—able to secrete IFN-γ. The lack of sufficient autocrine IL-2 production renders CD8+ cells dependent on the supply of this important growth factor—and perhaps additional ones—by CD4+ T helper cells in order to acquire and maintain a TAg-specific tumoricidal CTL phenotype.
Accumulation of lytically active antigen-specific CTL at the tumor site.
The critical dependence of TAg-specific CTL in BALB/c mice on CD4+ cells and its consequences might explain why BALB/c mice, in contrast to C57BL/6 mice, have been denoted as low responders or even nonresponders with respect to their ability to mount an SV40 TAg-specific CTL response (1, 2, 4, 16, 23, 33, 42, 46). Beyond confirming this assessment in a previous study (54) and in this report (Fig. 1), as far as the detection of TAg-specific CTL by restimulation in vitro of cells from secondary lymphoid organs is concerned, we found that for the judgment of weak tumor-specific immune responses, topological aspects must be considered. Thus, we failed to detect primary ex vivo active TAg-specific CTL in the spleens or regional lymph nodes of immune mice rejecting i.p.-injected mKSA cells, while we easily detected lytically active CD8+ TAL in the peritoneal cavities of the same mice. Apparently, despite the help of CD4+ cells, only small numbers of TAg-specific CTL are generated, and these cells are quantitatively recruited to the tumor site.
It appears that TAg-specific CTL precursors present at a low frequency expand rather poorly despite the support of T helper cells. Moreover, mature effector CTL are quantitatively recruited to the tumor site, while the secondary lymphoid organs are virtually devoid of these effector cells. Apparently, a favorable microenvironment for the accumulation of TAg-specific CD8+ and CD4+ T cells is present at the site of tumor cell inoculation, allowing the vigorous activity of these cells—lytic activity as well as cytokine secretion—to be measured in primary assays. In contrast, the frequency or the activity of TAg-specific T cells in lymphoid organs remains below a critical threshold which precludes their detection.
The apparent discrepancy between almost nondetectable systemic and easily measurable primary local immune reactivity is of more general interest because in clinical settings, the success of tumor-specific immunotherapies can often be evaluated only by determining the antigen-specific reactivity of peripheral blood mononuclear cells. If, after immunization (and even during the phase of strong elimination of tumor cells) against a weak, but foreign viral tumor-specific antigen, specific CTL activity can be recovered exclusively from the tumor site but not from lymphatic organs, there is a considerable danger of underestimating the immune responses of tumor patients against weakly immunogenic altered self-tumor antigens. The response against such antigens might be judged falsely negative despite the presence of considerable immune reactivity at the tumor site. Therefore, we suggest study of the correlation between local and systemic immunological parameters in experimental systems like the one presented here in more detail in order to define reliable criteria for determining the immune responses of patients to tumor-specific immunotherapies.
ACKNOWLEDGMENTS
This work was supported by research grant Le 132/29-1 from Deutsche Forschungsgemeinschaft and research grant 93.048.2 from Wilhelm Sander-Stiftung.
We are grateful to Reinhold Schirmbeck for testing the MHC class II expression of mKSA cells. We sincerely thank H. Münd and J. Schreiner for expert work at the animal care facility of the Heinrich-Pette-Institut.
REFERENCES
- 1.Bright R K, Shearer M H, Kennedy R C. Comparison of the murine humoral immune response to recombinant simian virus 40 large tumor antigen: epitope specificity and idiotype expression. Cancer Immunol Immunother. 1993;37:31–39. doi: 10.1007/BF01516939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bright R K, Shearer M H, Kennedy R C. Immunization of BALB/c mice with recombinant simian virus 40 large tumor antigen induces antibody-dependent cell-mediated cytotoxicity against simian virus 40-transformed cells. An antibody-based mechanism for tumor immunity. J Immunol. 1994;153:2064–2071. [PubMed] [Google Scholar]
- 3.Bright R K, Shearer M H, Kennedy R C. Examination of lymphokines induced in mice following immunization with recombinant simian virus 40 large tumor antigen. Cancer Immunol Immunother. 1995;40:206–211. doi: 10.1007/BF01517353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bright R K, Beames B, Shearer M H, Kennedy R C. Protection against a lethal tumor challenge with SV40-transformed cells by the direct injection of DNA-encoding SV40 large tumor antigen. Cancer Res. 1996;56:1126–1130. [PubMed] [Google Scholar]
- 5.Brunner K T, Mauel J, Cerottini J C, Chapuis B. Qunatitative assay of the lytic action of immune lymphoid cells on 51Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology. 1968;14:181–196. [PMC free article] [PubMed] [Google Scholar]
- 6.Busch D H, Philip I M, Vijh S, Pamer E G. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity. 1998;8:353–362. doi: 10.1016/s1074-7613(00)80540-3. [DOI] [PubMed] [Google Scholar]
- 7.Butz E A, Bevan M J. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L, Tourvieille B, Burns G F, Bach F H, Mathieu-Mahul D, Sasportes M, Bensussan A. Interferon: a cytotoxic T lymphocyte differentiation signal. Eur J Immunol. 1986;16:767–770. doi: 10.1002/eji.1830160709. [DOI] [PubMed] [Google Scholar]
- 9.Cobbold S P, Jayasuriya A, Nash A, Prospero T D, Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984;312:548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- 10.Colombo M P, Ferrari G, Stoppaciaro A, Parenza M, Rodolfo M, Mavilio F, Parmiani G. Granulocyte colony-stimulating factor gene transfer suppresses tumorigenicity of a murine adenocarcinoma in vivo. J Exp Med. 1991;173:889–897. doi: 10.1084/jem.173.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colombo M P, Modesti A, Parmiani G, Forni G. Local cytokine availability elicits tumor rejection and systemic immunity through granulocyte-T-lymphocyte cross-talk. Cancer Res. 1992;52:4853–4857. [PubMed] [Google Scholar]
- 12.Frey A B. Rat mammary adenocarcinoma 13762 expressing IFN-γ elicits antitumor CD4+ MHC class II-restricted T cells that are cytolytic in vitro and tumoricidal in vivo. J Immunol. 1995;154:4613–4622. [PubMed] [Google Scholar]
- 13.Gegin C, Lehmann-Grube F. Control of acute infection with lymphocytic choriomeningitis virus in mice that cannot present an immunodominant viral cytotoxic T lymphocyte epitope. J Immunol. 1992;149:3331–3338. [PubMed] [Google Scholar]
- 14.Giovarelli M, Santoni A, Jemma C, Musso T, Giufrida A M, Cavallo G, Landolfo S, Forni G. Obligatory role of IFN-γ in induction of lymphokine-activated and T lymphocyte killer activity, but not in boosting of natural cytotoxicity. J Immunol. 1988;141:2831–2836. [PubMed] [Google Scholar]
- 15.Golumbek P T, Azhari R, Jaffee E M, Levitsky H I, Lazenby A, Leong K, Pardoll D M. Controlled release, biodegradable cytokine depots: a new approach in cancer vaccine design. Cancer Res. 1993;53:5841–5844. [PubMed] [Google Scholar]
- 16.Gooding L R. Specificities of killing by cytotoxic lymphocytes generated in vivo and in vitro to syngeneic SV40 transformed cells. J Immunol. 1977;118:920–927. [PubMed] [Google Scholar]
- 17.Gooding L R. Specificities of Killing by T lymphocytes generated against syngeneic SV40 transformants: studies empoying recombinants within the H-2 complex. J Immunol. 1979;122:1002–1008. [PubMed] [Google Scholar]
- 18.Greenberg P D. Adoptive T cell therapy of tumors: mechanisms operative in the recognition and elimination of tumor cells. Adv Immunol. 1991;49:281–355. doi: 10.1016/s0065-2776(08)60778-6. [DOI] [PubMed] [Google Scholar]
- 19.Gurney E G, Tamowski S, Deppert W. Antigenic binding sites of monoclonal antibodies specific for simian virus 40 large T antigen. J Virol. 1986;57:1168–1172. doi: 10.1128/jvi.57.3.1168-1172.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hahn S, Gehri R, Erb P. Mechanism and biological significance of CD4-mediated cytotoxicity. Immunol Rev. 1995;146:57–79. doi: 10.1111/j.1600-065x.1995.tb00684.x. [DOI] [PubMed] [Google Scholar]
- 21.Heike M, Schlaak J, Schulze-Bergkamen H, Heyl S, Herr W, Schmitt U, Schneider P M, Meyer zum Büschenfelde K-H. Specificities and functions of CD4+ HLA class II-restricted T cell clones against a human sarcoma. J Immunol. 1996;156:2205–2213. [PubMed] [Google Scholar]
- 22.Kit S, Kurimura T, Dubbs D R. Transplantable mouse tumor line induced by injection of SV40-transformed mouse kidney cells. Int J Cancer. 1969;4:384–392. doi: 10.1002/ijc.2910040403. [DOI] [PubMed] [Google Scholar]
- 23.Knowles B B, Koncar M, Pfizenmaier K, Solter D, Aden D P, Trinchieri G. Genetic control of the cytotoxic T cell response to SV40 tumor-associated specific antigen. J Immunol. 1979;122:1798–1806. [PubMed] [Google Scholar]
- 24.Lanford R E. Expression of simian virus 40 T antigen in insect cells: using a baculovirus expression vector. Virology. 1988;167:72–81. doi: 10.1016/0042-6822(88)90055-4. [DOI] [PubMed] [Google Scholar]
- 25.LeMay L G, Kan-Mitchell J, Goedebuure P, Harel W, Mitchell M S. Detection of melanoma-reactive CD4+ HLA-class I-restricted cytotoxic T cell clones with long-term assay and pretreatment of targets with interferon-γ. Cancer Immunol Immunother. 1993;37:187–194. doi: 10.1007/BF01525434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucin P, Pavic I, Polic B, Jonjic S, Koszinowski U H. Gamma interferon-dependent clearance of cytomegalovirus infection in salivary glands. J Virol. 1992;66:1977–1984. doi: 10.1128/jvi.66.4.1977-1984.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melief C J M. Tumor eradication by adoptive transfer of cytotoxic T lymphocytes. Adv Cancer Res. 1992;58:143–175. doi: 10.1016/s0065-230x(08)60294-8. [DOI] [PubMed] [Google Scholar]
- 28.Miltenyi S, Müller W, Weichel W, Radbruch A. High gradient magnetic cell separation with MACS. Cytometry. 1990;11:231–238. doi: 10.1002/cyto.990110203. [DOI] [PubMed] [Google Scholar]
- 29.Miyazaki A, Sato N, Takahashi S, Sasaki A, Kohama G, Yamaguchi A, Yagihashi A, Kikuchi K. Cytotoxicity of histocompatibility leukocyte antigen-DR8-restricted CD4 killer T cells against human autologous squamous cell carcinoma. Jpn J Cancer Res. 1997;88:191–197. doi: 10.1111/j.1349-7006.1997.tb00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moskophidis D, Lechner F, Pircher H, Zinkernagel R M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 31.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J D, Zajac A J, Miller J D, Slynsky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 32.Nagarkatti M, Clary S R, Nagarkatti P S. Characterization of tumor-infiltrating CD4+ T cells as Th1 cells based on lymphokine secretion and functional properties. J Immunol. 1990;144:4898–4905. [PubMed] [Google Scholar]
- 33.Newmaster R S, Mylin L M, Fu T M, Tevethia S S. Role of a subdominant H-2Kd-restricted SV40 tumor antigen cytotoxic T lymphocyte epitope in tumor rejection. Virology. 1998;244:427–441. doi: 10.1006/viro.1998.9148. [DOI] [PubMed] [Google Scholar]
- 34.Old L J, Boyse E A, Clarke D A, Carswell E A. Antigenic properties of chemically induced tumors. Ann N Y Acad Sci. 1962;101:80. [Google Scholar]
- 35.Ossendorp F, Toes R E M, Offringa R, van der Burg S H, Melief C J M. Importance of CD4+ T helper cell responses in tumor immunity. Immunol Lett. 2000;74:75–79. doi: 10.1016/s0165-2478(00)00252-2. [DOI] [PubMed] [Google Scholar]
- 36.Pfizenmaier K, Pan S-H, Knowles B B. Preferential H-2 association in cytotoxic T cell responses to SV40 tumor-associated specific antigens. J Immunol. 1980;124:1888–1891. [PubMed] [Google Scholar]
- 37.Qin S, Cobbold S, Tighe H, Benjamin R, Waldmann H. CD4 monoclonal antibody pairs for immunosuppression and tolerance induction. Eur J Immunol. 1987;17:1159–1165. doi: 10.1002/eji.1830170813. [DOI] [PubMed] [Google Scholar]
- 38.Rosenberg S A. Immunotherapy and gene therapy of cancer. Cancer Res. 1991;51:5074s–5079s. [PubMed] [Google Scholar]
- 39.Rosenstein M, Eberlein T, Rosenberg S A. Adoptive immunotherapy of established syngeneic solid tumors: role of T lymphoid subpopulations. J Immunol. 1984;132:2117–2122. [PubMed] [Google Scholar]
- 40.Schattner E J, Mascarenhas J, Bishop J, Yoo D-H, Chadburn A, Crow M K, Friedman S M. CD4+ T-cell induction of Fas-mediated apoptosis in Burkitt's lymphoma B cells. Blood. 1996;88:1375–1382. [PubMed] [Google Scholar]
- 41.Schirmbeck R, Zerrahn J, Kuhröber A, Deppert W, Reimann J. Immunization of mice with the N-terminal (1–272) fragment of simian virus 40 large T-antigen (without adjuvants) specifically primes cytotoxic T lymphocytes. Eur J Immunol. 1993;23:1528–1534. doi: 10.1002/eji.1830230720. [DOI] [PubMed] [Google Scholar]
- 42.Schirmbeck R, Böhm W, Reimann J. DNA vaccination primes MHC class I-restricted, simian virus 40 large tumor antigen-specific CTL in H-2d mice that reject syngeneic tumors. J Immunol. 1996;157:3550–3558. [PubMed] [Google Scholar]
- 43.Simon M M, Landolfo S, Diamantstein T, Hochgeschwender U. Antigen- and lectin-sensitized murine cytolytic T lymphocyte-precursors require both interleukin 2 and endogenously produced immune (γ) interferon for their growth and differentiation into effector cells. Curr Top Microbiol Immunol. 1986;126:173–185. doi: 10.1007/978-3-642-71152-7_21. [DOI] [PubMed] [Google Scholar]
- 44.Stuhler G, Walden P. Collaboration of helper and cytotoxic T lymphocytes. Eur J Immunol. 1993;23:2279–2286. doi: 10.1002/eji.1830230934. [DOI] [PubMed] [Google Scholar]
- 45.Tepper R I, Pattengale P K, Leder P. Murine interleukin-4 displays potent anti-tumor activity in vivo. Cell. 1989;57:503–512. doi: 10.1016/0092-8674(89)90925-2. [DOI] [PubMed] [Google Scholar]
- 46.Tevethia S S. Recognition of simian virus 40 T antigen by cytotoxic T lymphocytes. Mol Biol Med. 1990;7:83–96. [PubMed] [Google Scholar]
- 47.Tite J P. Evidence of a role for TNF-α in cytolysis by CD4+, class II MHC-restricted cytotoxic T cells. Immunology. 1990;71:208–212. [PMC free article] [PubMed] [Google Scholar]
- 48.Topalian S L. MHC class II restricted tumor antigens and the role of CD4+ T cells in cancer immunotherapy. Curr Opin Immunol. 1994;6:741–745. doi: 10.1016/0952-7915(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 49.Utermöhlen O, Tárnok A, Bönig L, Lehmann-Grube F. T lymphocyte-mediated antiviral immune responses in mice are diminished by treatment with monoclonal antibody directed against the interleukin-2 receptor. Eur J Immunol. 1994;24:3093–3099. doi: 10.1002/eji.1830241227. [DOI] [PubMed] [Google Scholar]
- 50.Utermöhlen O, Dangel A, Tárnok A, Lehmann-Grube F. Modulation by gamma interferon of antiviral cell-mediated immune responses in vivo. J Virol. 1996;70:1521–1526. doi: 10.1128/jvi.70.3.1521-1526.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Viola A, Salio M, Tuosto L, Linkert S, Acuto O, Lanzavecchia A. Quantitative contribution of CD4 and CD8 to T cell antigen receptor serial triggering. J Exp Med. 1997;186:1775–1779. doi: 10.1084/jem.186.10.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoneda Y, Yoshida R. The role of T cells in allografted tumor rejection: IFN-γ released from T cells is essential for induction of effector macrophages in the rejection site. J Immunol. 1998;160:6012–6017. [PubMed] [Google Scholar]
- 53.Zatloukal K, Schneeberger A, Berger M, Schmidt W, Koszik F, Kutil R, Cotten M, Wagner W, Buschle M, Maass G, Payer E, Stingl G, Birnstiel M L. Elicitation of a systemic and protective anti-melanoma immune response by an IL-2-based vaccine. Assessment of critical cellular and molecular parameters. J Immunol. 1995;154:3406–3419. [PubMed] [Google Scholar]
- 54.Zerrahn J, Utermöhlen O, Warnecke G, Deppert W, Lehmann-Grube F. Protective immunity in BALB/c mice against the Simian Virus 40-induced mKSA tumor resulting from injection of recombinant large T antigen. Requirement of CD8+ T lymphocytes. J Immunol. 1996;156:3919–3924. [PubMed] [Google Scholar]