Abstract
Background and Objectives
Spinal muscular atrophy (SMA) is an autosomal recessive disorder caused by biallelic variants of the Survival Motor Neuron 1 gene (SMN1) that affects approximately 1 in 15,000 live births. Availability of 3 SMN-enhancing treatments for SMA has led to urgency to review how clinicians and patients use these treatments for SMA, while additional research and real-world data and experience are being collected. This work describes important factors to assist with decision-making for SMN-enhancing treatments.
Methods
A systematic literature review was conducted on SMN-enhancing treatments for SMA and related studies. A working group of American and European health care providers with expertise in SMA care identified barriers and developed recommendations through a modified Delphi technique with serial surveys and feedback through virtual meetings to fill gaps for information where evidence is limited. A community working group of an individual living with SMA and caregivers provided insight and perspective on SMA treatments and support through a virtual meeting to guide recommendations.
Results
The health care provider working group and the community working group agreed that when determining whether to start, change, add, or discontinue a treatment, essential considerations include patient and family/caregiver perspective, and treatment safety and side effects. When initiating treatment for patients newly diagnosed with SMA, important patient characteristics are age and Survival Motor Neuron 2 gene (SMN2) copy number. Furthermore, when initiating, changing, or adding treatment, current clinical status and comorbidities drive decision-making. When considering a medication or treatment plan change, unless there is an urgent indication, a treatment and associated patient outcomes should be monitored for a minimum of 6–12 months. When determining a treatment plan with an adolescent or adult with SMA, consider factors such as quality of life, burden vs benefit of treatment, and reproductive issues. Access to care coordination and interdisciplinary/multidisciplinary care are essential to treatment success.
Discussion
Sharing information about current knowledge of treatments and shared decision-making between health care providers and patients living with SMA and caregivers are essential to overcoming barriers to providing SMN-enhancing treatments.
Introduction
SMA is an autosomal recessive disorder caused by biallelic variants of the Survival Motor Neuron 1 (SMN1) gene on chromosome 5q and affects approximately 1 in 15,000 live births.1 Across 6 major ethnic groups in the United States, carrier frequency and detection rates range from 1/47 and 94.8% (White population) to 1/72 and 70.5% (African American population), respectively.2 SMA is characterized by dysfunction and irreversible loss of alpha motor neurons in the spinal cord and brainstem, causing progressive muscular weakness and atrophy.3 SMA results in a wide range of clinical severity. The number of Survival Motor Neuron 2 (SMN2) gene copies, a low-functioning paralogue of SMN1,4,5 generally correlates inversely with disease phenotype with some overlap especially among individuals with 3 or 4 SMN2 copies. Historically, before SMN-enhancing disease-modifying therapies (DMTs), SMA was classified into types defined by age of symptom onset and maximum motor function achieved.3,6
In 2007, an International Conference convened to develop the first publication on SMA standards of care.6 In 2018, the SMA best practice recommendations for diagnosis and management were updated by convening an International Conference of experts in SMA7 and 2 publications were produced.8,9 To date, 3 SMN-enhancing treatments, nusinersen (Spinraza, Biogen, Cambridge, MA), onasemnogene abeparvovec-xioi (Zolgensma, Novartis Gene Therapies, Bannockburn, IL), and risdiplam (Evrysdi, Genentech/Roche, South San Francisco, CA), have been approved by multiple national health regulatory agencies, and treatment approval is heterogeneous by country.
The SMN-enhancing treatments have resulted in dramatic change in the natural history of SMA across all ages. The knowledge generated by clinical trials and commercial use of these interventions have led to a shift in disease management because suspected SMA is now recognized as a clinical emergency that requires (1) accurate genetic diagnosis, including SMN2 copy number quantitation in both symptomatic and presymptomatic individuals,8 and (2) rapid implementation of treatment. Furthermore, the historical classification of SMA by type no longer adequately characterizes prognosis or outcomes. With the availability of up to 3 SMN-enhancing treatments for children younger than 2 years and 2 treatments for older individuals with SMA, understanding the risk and benefits of each treatment, and selecting “best” treatment for a specific clinical scenario require additional real-world data collection research.
In addition, understanding treatment outcome expectations is essential to shared decision-making between patient/caregiver and health care provider (HCP). SMA is a progressive neurodegenerative disorder with rapid irreversible motor neuron loss and functional decline early in the disease course followed by a variable rate of further decline.10-14 Therefore, DMT outcome expectations include slowing disease progression which may present clinically as slower rates of functional decline as evaluated by longitudinal assessments or no change or restoring some function or increased function. Understanding SMA natural history and the range of outcomes following DMTs is important for HCPs, the SMA community, and treatment access policy makers. This knowledge will guide decision-making, best care practice and access to treatments, and identify gaps for future research.
The aim of this work was to provide best practice recommendations to facilitate shared decision-making between HCPs and patients living with SMA and caregivers when considering DMTs. This work is intended for HCPs, patients and caregivers, and policy makers.
Methodology
Systematic Literature Review
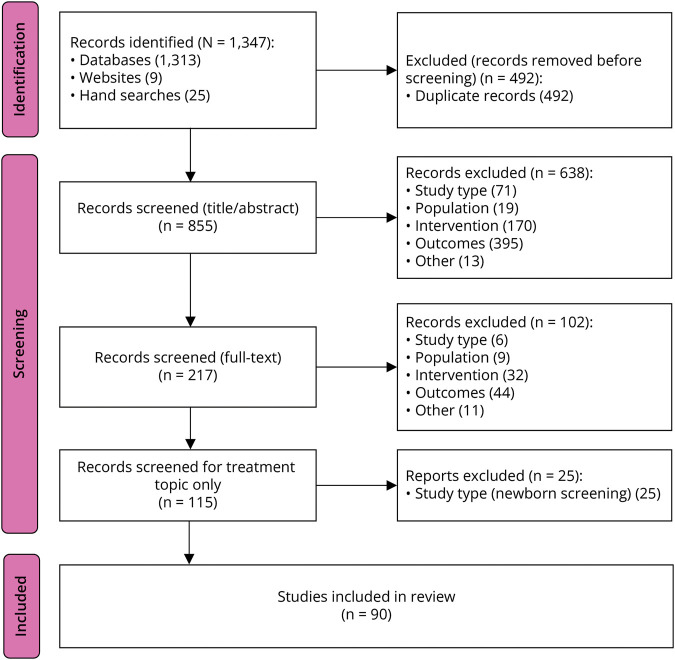
In November 2021, Cure SMA enlisted RTI Health Solutions (RTI-HS) to conduct a systematic literature review (SLR) to understand diagnostic/newborn screening and treatment landscape for SMA over the previous 10 years. The objective of the SLR was to review the SMA treatment evidence. Searches of electronic medical literature databases identified 1,347 records. After removing duplicates, 855 records (titles and abstracts) were manually screened, 217 articles were progressed to full-text review, and 115 articles met the predefined inclusion criteria. After excluding diagnostic/newborn screening articles, 90 articles were selected for inclusion in the SLR on SMA treatment landscape, see Figure (PRISMA Diagram)15,16 and eSAP 1. A second literature search was conducted to include more recent treatment evidence, used the same keywords and inclusion criteria, and was limited to peer-reviewed treatment data published through June 2023. This search yielded 8 additional articles for inclusion.
Figure. PRISMA Diagram.
PRISMA flow diagram for systematic literature review conducted in November 2021; see eSAP 1 for complete report. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-analyses.
SMA Diagnosis Working Groups
Health Care Provider Working Group
Participants included Cure SMA Care Center Network Center Directors, SMA Clinical Trial Investigators, and Cure SMA Medical Advisory Council members. SMA Europe, a partner patient advocacy organization, identified European providers. Respondents were invited to participate in a modified Delphi process. The health care provider working group (HCPWG) members, supported by Cure SMA, were invited to an introductory virtual meeting. The HCPWG included 18 members, plus 2 organizing and nonvoting Cure SMA staff members who moderated discussions and had no stake in the decisions. The HCPWG included 3 European physician neurologists, 14 US physician neurologists, and 1 US pediatric critical care physician. All HCPWG members participated voluntarily without compensation.
Achieving Consensus Through the Modified Delphi Technique
The HCPWG used a modified Delphi technique17,18 to reach consensus on recommendations. Data were collected using 3 iterative rounds19 of online surveys. The responses to the initial survey open-ended questions were consolidated and reported back to the participants through virtual meeting discussion. The second survey asked participants to rank order possible barriers to treatments, indicate factor importance contributing to treatment plan decision-making scenarios using a Likert scale, and select important characteristics for specific patient categories contributing to treatment plan decision-making using multiple choice responses. Compiled results were discussed among participants through virtual meeting. Based on discussion, a final survey requesting yes/no agreement response to specific recommendations was developed and completed by the HCPWG. Results with 90% or more agreement were considered highly significant. Results with 80%–89% agreement were considered significant.
Community Working Group
Individuals with SMA and caregivers were invited to participate in a virtual meeting to gain their perspective. One adult with SMA, a representative from SMA Europe, and 4 caregivers participated and discussed questions as a group. Questions focused on treatment, resources, and information that would be helpful at time of diagnosis to make informed decisions. The community working group (CWG) was asked comparable questions as the HCPWG. Consensus was achieved through CGW discussion. Qualitative responses were compiled.
Standard Protocol Approvals, Registrations, and Patient Consents
Not applicable.
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Current SMA Treatments
Three SMN-enhancing treatments are approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA): nusinersen, onasemnogene abeparvovec-xioi (OA), and risdiplam. See Table 1 for SMA treatment characteristics based on published European Public Assessment Reports20-22 and US Prescribing Information.23-25 Treatments are described briefly in chronological order of licensing.
Table 1.
SMA Treatment Characteristics for EU and US Prescribers
| Nusinersen (Spinraza®) | Onasemnogene abeparvovec-xiox (Zolgensma®) | Risdiplam (Evrysdi®) | |
| Description | Antisense oligonucleotide | Single-stranded SMN1 DNA through adeno-associated virus (AAV9) vector | Small molecule |
| Mechanism | SMN2 mRNA splicing modifier | SMN1 functional replacement with SMN1 DNA episome and own promotor | SMN2 mRNA splicing modifier |
| EU EMA-approved indications20-22 | 5q spinal muscular atrophy | 5q spinal muscular atrophy and SMA type 1 or up to 3 SMN2 gene copies up to 21 kg | 5q spinal muscular atrophy and SMA type 1, 2, or 3, or up to 4 SMN2 gene copies |
| US FDA-approved indications23-25 | 5q spinal muscular atrophy in pediatric and adult patients | Pediatric patients with 5q spinal muscular atrophy and <2 y of age | 5q spinal muscular atrophy in pediatric and adult patients |
| Dose | 12 mg/5 mL | 1.1 × 1014 vector genomes/kg body weight (vg/kg) | • 0.15 mg/kg/d up to 2 mo of age • 0.2 mg/kg/d 2 mo to <2 y • 0.25 mg/kg >/= 2 yo and <20 kg • 5 mg per day >/= 2 yo and >20 kg Concentration: 0.75 mg/mL |
| How given | Intrathecal bolus | Slow intravenous infusion over 60 min | Enteral liquid (oral or feeding tube) |
| How often | Four loading doses over 2 mo, then every 4 mo | One time only | Daily |
| Body distribution | Cerebrospinal fluid | Systemic and cerebrospinal fluid | Systemic and cerebrospinal fluid |
| Laboratory testing and monitoring | Blood and urine Obtain at baseline and before each dose: platelet count, prothrombin time (PT), activated partial thromboplastin time (PTT), quantitative spot urine protein testing |
Before OA infusion: Assess for illness and do not give if concurrent infection is suspected until the infection has resolved Obtain blood for anti-AAV9 antibody testing, liver function testing, creatinine, complete blood count, and troponin-I After OA infusion monitor blood tests for the following 3 mo after dosing: Liver function and platelet count weekly for the first month, then every other week for the second and third mo until back to baseline. Monitor troponin-I level weekly for 1 mo, then monthly for the second and third mo until back to baseline |
None required |
| Boxed warning | None | SERIOUS LIVER INJURY and ACUTE LIVER FAILURE Cases of sudden liver failure with fatal outcomes have been reported. Acute serious liver injury and elevated liver aminotransferase levels can occur with OA. Patients with preexisting liver impairment may be at higher risk. Before receiving OA, assess liver function of all patients by clinical examination and laboratory testing. Corticosteroids must be given to all patients before and after OA infusion. Continue to monitor liver function for at least 3 mo after infusion, and as clinically indicated |
None |
| Warnings and precautions | Thrombocytopenia and coagulation abnormalities Renal toxicity |
After OA infusion, instruct caregiver to monitor for fever, lethargy, decreased feeding, vomiting, decreased urination, seizures, or easy bruising or bleeding and report to health care provider immediately 1. Acute serious liver injury, acute liver failure including fatalities, have been reported with OA use. These effects are mitigated by administering systemic corticosteroids to all patients before giving OA and continued after dosing OA. Patients with preexisting liver impairment or acute hepatic viral infection may be at higher risk for serious liver injury/liver failure 2. Systemic immune response may occur if OA is given to a patient with an active infection. Avoid giving OA. When an infection of any kind is suspected until infection is resolved 3. Thrombocytopenia may be transient and was typically observed in the first 2 wk after OA. 4. Thrombotic microangiopathy (TMA) has been reported within the first 2 weeks after OA infusion. TMA is associated with thrombocytopenia, hypertension, microangiopathic hemolytic anemia, and acute kidney injury 5. Elevated troponin-I has been observed and should be monitored for at least 3 mo after dose |
None |
| Adverse reactions | Infantile onset SMA: Lower respiratory infection and constipation; later-onset SMA: fever, headache, vomiting and back pain | Elevated liver aminotransferases and vomiting | Later-onset SMA: fever, diarrhea, and rash; infantile-onset SMA: fever, diarrhea, rash, upper respiratory tract infection, lower respiratory tract infection, constipation, vomiting, and cough |
| Drug interactions | None | Adjust vaccination schedule to accommodate concomitant corticosteroid administration | Evrysdi may increase plasma concentration of drugs eliminated through multidrug and toxin extrusion (MATE) protein transporters |
| Associated treatment | None | One day before OA infusion begin systemic enteral prednisolone 1 mg/kg per day or equivalent for 30 d while monitoring as above followed by tapering dose as clinically indicated RSV prophylaxis treatment is recommended |
None |
| Use in specific populations | Pregnancy: insufficient data | Premature neonates: not recommended because concomitant treatment with corticosteroids may adversely affect neurologic development. Delay ZOLGENSMA infusion until full-term gestational age is reached | Pregnancy: no adequate data on developmental risk in pregnant women. In nonclinical animal studies, risdiplam during pregnancy or throughout pregnancy and lactation resulted in adverse effects on development Females of reproductive potential: Pregnancy testing before initiation of treatment is recommended and advising use of effective contraception Male fertility may be compromised by treatment with risdiplam based on animal toxicity studies Counsel male patients of reproductive potential receiving risdiplam about the potential effects on fertility. Male patients may consider sperm preservation before treatment |
Nusinersen is an antisense oligonucleotide approved by the United States in 2016, and in 2017 by the EU and in many other countries. Nusinersen acts on the SMN2 gene transcript to alter splicing of the premessenger RNA (pre-mRNA) and results in greater inclusion of exon 7 thus yielding increased full-length SMN protein production. Nusinersen is administered intrathecally with 4 loading doses over 2 months followed by maintenance dosing once every 4 months; see eTable 1 (Nusinersen Clinical Trials).
OA is a gene transfer therapy approved by the United States in 2019 and by the EU in 2020 and other countries variably. OA delivers a functional SMN1 gene using a transgene packaged in an adeno-associated viral vector serotype 9 (AAV9). OA is administered as a single intravenous dose to infants and toddlers per each country's health governing agency guidance; see eTable 2 (OA Clinical Trials).
Risdiplam is a small molecule that functions as an SMN2 splice modulator increasing exon 7 inclusion in SMN2 mRNA transcripts and thus enhancing production of full-length SMN protein. Risdiplam was approved by the United States in 2020 and with extended indications in 2022 and approved by the EU in 2021 and with extended indications in 2023. Risdiplam is an enteral liquid given by mouth or feeding tube once daily; see eTable 3 (Risdiplam Clinical Trials).
Clinical trials of SMN-enhancing treatments have demonstrated outcome benefit in motor function, event-free survival, and reduced need for ventilatory support compared with natural history.26-28 In addition, best outcomes are associated with early treatment as demonstrated by clinical trials with presymptomatic infants.29-32 Thus, treatment as soon as reasonably possible after diagnosis is essential. Each treatment has a unique route of administration, frequency, and potential side effects to monitor. To date, long-term follow-up is primarily clinical trial extension study data. None of the current treatments have resulted in a cure for SMA.
Treatment Barriers
The HCPWG identified the following barriers to providing DMT in rank order.
Access to Treatments Including Insurance/Payer Issues and National Clinical Criteria (Overall Rank 1)
Barriers to treatment access included insurance/payer issues, applicable in some countries, and regulations for access (not payment) defined by country health governing agencies and applicable in some countries. In addition, continued access to a treatment may be limited by the payer or health governing agency due to a requirement that patients demonstrate continued absolute improvement while on treatment and may not take into consideration the significance of improvement relative to SMA natural history and in the context of each patient's clinical status. Furthermore, current motor function assessments may be limited and may not accurately indicate slowed disease progression.
Time to Treatment Initiation for Newly Diagnosed With SMA (Overall Rank 2)
The first clinic visit with a patient suspected and/or diagnosed with SMA and their caregivers is tremendously important, and multiple goals must be achieved. These goals include evaluating the patient's clinical condition, providing culturally appropriate education to patients and caregivers with compassion and care to facilitate shared decision-making, and obtaining data and laboratory studies to confirm the diagnosis and submit for treatment approval. Variance at each step may contribute to treatment initiation delay. In addition, for all patients and especially those with 2 SMN2 copies, time to initiate treatment is critical due to ongoing associated progressive irreversible motor neuron loss.33,34
Insufficient Data (Overall Rank 3)
Insufficient data (see Table 2) may be contextual and may apply to different aspects of a treatment. For example, each treatment may have different levels of available data regarding short-term efficacy, timing of beneficial effect onset, side effects, and long-term outcomes. In addition, HCPs have variable experience and confidence with the available DMTs, and some may have limited staff and infrastructure resources to provide intrathecal treatment. Unknown long-term efficacy outcomes may contribute to subjective expectations of HCPs and patients and caregivers.
Table 2.
Treatment Barriers: Insufficient Data Survey
| Question | Responses (%) Yes |
No |
| Do you agree we have SUFFICIENT data for short-term safety and efficacy? | 89 | 11 |
| Do you agree we currently have INSUFFICIENT data for | ||
| • Long-term safety and efficacy? | 78 | 22 |
| • Safety and efficacy and potential efficacy of combination/sequential therapy? | 100 | 0 |
| • Comparing treatments? | 100 | 0 |
Expectations (Overall Rank 4)
Expectations about care and treatment, response to treatment, side effects, and long-term outcomes may be unknown and influence decision-making. However, expectations may be managed by HCP continuous self-education, providing education and information about SMA and treatment options to patients and caregivers, and providing reliable information resources. These conversations require repetition, curiosity, and being open to asking questions to facilitate understanding and perception. Core to conversations between HCPs and patients and caregivers is acknowledging what is not known and sharing a commitment to gather as much data and information as possible together. Shared expectations between HCP and patients and caregivers are essential to fostering a collaborative relationship and shared decision-making.
Recommendations
To facilitate shared decision-making regarding SMN-enhancing treatments, the following recommendations were developed with the HCPWG and CWG (see Table 3).
Table 3.
Spinal Muscular Atrophy Update in Best Practice: Recommendations for Treatment Considerations Summary
| Recommendation 1 When determining whether to start, change, add, or discontinue treatment, essential and consistent considerations include (1) patient and family perspectives and (2) treatment safety and side effects |
| Recommendation 2 When considering initiating treatment for patients newly diagnosed with SMA (either symptomatically or before symptom onset) SMN2 copy number and age are two important patient characteristics that guide treatment |
| Recommendation 3 When considering whether to start, change, or add a treatment for patients with SMA who are not newly diagnosed, a major factor driving decision-making is current clinical status (which may include comorbidities, complex spine anatomy, and/or decreased function following treatment) |
| Recommendation 4 A. When considering a medication or treatment plan change, unless there is an URGENT indication, a medication and associated patient outcomes should be monitored for a minimum of 6–12 mo before making a change (89% agreement) B. URGENT indications to consider changing a treatment plan outside of a 6–12 mo assessment period (100% agreement) include: • Significant side effects or intolerance to medication not acceptable to patient or HCP • Intolerance to medication administration route • Significant disease progression as determined by the health care provider and patient/caregiver • Loss of motor milestones (infancy and young child) |
| Recommendation 5 Factors that guide decision-making when determining treatment for adolescent and adult patients with SMA: • Treatment intolerance • Quality of life • Benefit vs burden • Treatment side effects • Loss of functionality • Reproductive concerns (male and female) • Pregnancy • Disease progression despite treatment • Patient perspective (tired, burnt out) |
| Recommendation 6 Access to care coordination and interdisciplinary/multidisciplinary care are essential to the success of providing SMN-enhancing treatment to patients living with SMA, benefits the patient and caregiver experience, and benefits the HCP experience |
Recommendation 1
When determining whether to start, change, add, or discontinue treatment, essential and consistent considerations include (1) patient and family perspective and (2) treatment safety and side effects.
The HCPWG achieved significant consensus (89%), and these considerations ranked high in all treatment plan scenarios (see Table 4). Treatment safety and side effects were discussed and considered separate from treatment efficacy. Central to this recommendation is that prescribing HCPs be educated and knowledgeable about all treatment options including expected and possible side effects, safety monitoring requirements, currently known efficacy, and best practice regarding each treatment. This information should be shared with patients and caregivers objectively and reliable information resources should be provided including websites of patient advocacy organizations. Additional knowledgeable team members may be important to support and reinforce information provided to patients and caregivers including, for example, genetic counselor and care coordinator.
Table 4.
Treatment Workgroup Consensus Survey Results
| Delphi questions | Responses (%) | (%) |
| Recommendation 1 | Yes | No |
| Do you agree with the following statement? The top 2 factors when determining whether to start, change, add, or stop a treatment are - Safety and side effects - Patient and family preference |
89% | 11% |
| Recommendation 2 | Yes | No |
| Do you agree with the following statement? SMN2 copy number and age are 2 important factors when considering initiating treatment for patients diagnosed with SMA (either symptomatic or without symptom) |
100 | 0 |
| Recommendation 3 | Yes | No |
| Do you agree with the following statement? When it comes to making a decision on whether to start, change, or add a treatment, a major factor for making this determination is current clinical status (which may include comorbidities, complicated spines, and decreased function following treatment) | 100 | 0 |
| Recommendation 4A | Yes | No |
| When considering a medication or treatment plan change: unless there is an URGENT reason, a medication and patient outcomes should be monitored for a minimum of 6–12 mo before making a change | 89 | 11 |
| Recommendation 4B | Yes | No |
| The following would be considered URGENT reasons to consider changing a treatment plan outside of a 6–12 mo assessment period: - Significant side effects/intolerance to medication - Intolerance to medication administration route - Significant disease progression - Loss of motor milestones |
100 | 0 |
| Recommendation 5 | Very important (%) | Somewhat important (%) |
| Please identify the level of importance of the following factors that guide decision making when determining treatment for adult patients with SMA | ||
| Using a Likert 5 level scale, the HCPWG identified the following factors as very important or somewhat important | ||
| • Treatment intolerance | 100 | 0 |
| • Quality of life | 91 | 9 |
| • Benefit vs burden | 82 | 18 |
| • Treatment side effects | 82 | 18 |
| • Loss of functionality | 82 | 18 |
| • Reproductive concerns (male and female) | 72 | 27 |
| • Pregnancy | 64 | 36 |
| • Disease progression despite treatment | 64 | 36 |
| • Patient perspective (tired, burnt out) | 55 | 45 |
The CWG agreed with this recommendation and reiterated the importance of receiving clear and reliable information regarding all treatment options including safety concerns, side effects, and monitoring so they are empowered to be active decision-making partners regarding treatment.35 Expectations about treatments should be shared collaboratively with patients and caregivers, including anticipated realistic goals of treatment—both known and unknown and that treatment is not a cure.36 Patients and caregivers require information about how each treatment is administered, clinic visit frequency, travel requirements to receive treatment, and ongoing testing requirements to monitor for side effects and efficacy. This information along with collaborative discussions between the HCP about their considerations and recommendations and the perspectives of the patient and caregiver and their capacity to comply with specific requirements will provide guidance to determine the specific treatment plan and overcome barriers around expectations. Providing a treatment is an agreement for continued care between the HCP and the patient and caregiver. The emphasis on shared decision-making between patient, caregiver, and HCP is essential to providing best care and achieving best outcomes.
Efficacy and safety information is available through completed clinical trials and extension trials on small well-defined clinical cohorts with limited variability to determine proof of concept for efficacy and safety for each treatment as outlined above. Although limited, real-world data are actively being collected across the SMA community to better understand treatment efficacy and safety, the changing SMA phenotype, and to guide development of treatment recommendations for specific clinical scenarios. Collection of real-world clinical data is essential to establish clinically meaningful outcomes and requires collection of high-quality agreed-upon measures across the SMA community for analyses. Furthermore, these data, which may include new findings and care practices, may be leveraged to advocate, educate, and engage with payers and health governing agencies to address barriers to treatment access and insufficient data.
Recommendation 2
When considering initiating treatment for patients newly diagnosed with SMA (either symptomatically or before symptom onset) SMN2 copy number and age are 2 important patient characteristics that guide treatment.
The HCPWG achieved highly significant consensus (100%) (see Table 4). In addition, the HCPWG agreed that SMN-enhancing treatment should be initiated as soon as feasible for newly diagnosed patients. SMN2 copy number and age at diagnosis and age at onset of SMA symptoms (when present) are important factors to consider when providing guidance around initiating treatment for newly diagnosed individuals with SMA. Infants with 2 SMN2 copies have an extremely short time during which rapid irreversible motor neuron loss occurs.33 Infants with 3 or 4 SMN2 copies diagnosed before symptom onset may have a somewhat longer yet highly variable time course for irreversible motor neuron loss and the appearance of symptoms. However, rapid loss of motor neurons occurs before symptom presentation33; thus, treatment cannot be delayed. Generally, a higher SMN2 copy number is associated with less severe disease although there are exceptions.5 Knowledge of the SMA natural history based on age and number of SMN2 copies relative to a patient's presentation, e.g., newborn screening or symptomatically, guides the education and information to provide about SMA to the patient and caregivers to guide expectations. Initiating treatment for newly diagnosed SMA is urgent for all infants with up to and including 4 SMN2 copies per consensus statement among US HCPs.37
Currently SMN2 copy number and patient age affect specific treatment access. For example, availability of intravenous OA is specified by each country's health governing agency and may be based on age, SMN2 copy number, and/or weight.21,24 Access to other treatments is heterogeneously available as per each country's policy makers. In some countries, the national health governing agency may not support treatment of patients with SMA and 4 SMN2 copies and who are without symptoms. Individuals with SMA and 5 or more SMN2 copies may not be eligible for SMN-enhancing treatments.
An additional consideration when initiating treatment for newly diagnosed patients with SMA is the results of screening laboratory studies. Abnormal initial laboratory test results may affect which initial treatment may be used, e.g., abnormal AAV9 titer or abnormal liver function evaluation in a child <2 years will delay or may eliminate OA treatment. For best outcomes, and to minimize time to first treatment barrier, treatment should be started as early as possible after diagnosis with any SMN-enhancing treatment. When abnormal test results normalize, the initial intended treatment may be considered.
Recommendation 3
When considering whether to start, change, or add a treatment for patients with SMA who are not newly diagnosed, a major factor driving decision-making is current clinical status (which may include comorbidities, complex spine anatomy, and/or decreased function following treatment).
The HCPWG achieved highly significant consensus (100%) that a patient's clinical status at the time of initiating, changing, or adding a treatment drives decision-making regarding treatment management (see Table 4). For example, due to physical route of administration, a patient with complex spine anatomy is at risk for or may have difficulty tolerating the administration of intrathecal DMT. Therefore, physical route of administration limitations may drive consideration of initial DMT and may also drive a treatment plan modification from intrathecal administration to a DMT with an alternative route of administration. Similarly, patients with underlying liver disease or elevated liver transaminases on initial screening are limited in treatment choice because treatment (OA) may exacerbate preexisting liver disease. Another example of clinical status considerations for treatment is a patient whose neurologic status stabilized on a treatment for several years and then lost function, e.g., lost the ability to walk independently. This delayed loss of function may be due to treatment slowing SMA disease progression while not preventing further progression. In this clinical scenario, an HCP and patient/caregiver may explore whether to change or add a treatment, although there is currently a lack of published data. Reproductive planning also affects DMT considerations due to insufficient data.
The CWG discussed the importance of receiving information about timeline expectations for treatment response and what the response may be. It is also helpful to discuss which options may be available if a treatment has unacceptable or intolerable side effects/symptoms or no longer seems to be providing benefit.38 In addition to discussing treatment and to optimize treatment effect, guidance and recommendations for supportive care is equally important and includes receiving multidisciplinary care for musculoskeletal, respiratory, and nutrition concerns. These conversations will reduce the barrier of expectations and facilitate shared decision-making.
Recommendation 4
When considering a medication or treatment plan change, unless there is an URGENT indication, a medication and associated patient outcomes should be monitored for a minimum of 6 to 12 months before making a change (89% agreement).
-
URGENT indications to consider changing a treatment plan outside of a 6-to-12 month assessment period (100% agreement) include:
Significant side effects or intolerance to medication not acceptable to patient or HCP
Intolerance to medication administration route
Significant disease progression as determined by the HCP and patient/caregiver
Loss of motor milestones (infancy and young child)
The HCPWG achieved significant (4.A.) and highly significant (4.B.) consensus (see Table 4). A treatment plan is defined as a collaborative effort to identify the patient's/caregiver's goals for treatment and what will be provided to treat or manage the disease. In general, the HCPWG recommended allowing 6 months to monitor response based on current knowledge of treatment onset of action and timeline to clinically observe response to a treatment. However, there are exceptions. Clinical examples that may lead to consider changing a treatment plan before 6 months include:
Intolerance to intrathecal injections
Intolerance to gastrointestinal side effects of oral medication
Abnormal results from monitoring laboratory studies
Within 2–3 months after starting a treatment, loss of motor milestone(s) in an infant or further loss of function in older individuals with SMA
A patient who becomes pregnant
Persistent symptoms after DMT and perceived lack of response
These scenarios require monitoring, review of patient journey, and consideration of a possible change in the treatment plan.
The CWG emphasized that patients and caregivers are integral to decisions around modifying a treatment plan. To be successful shared decision-makers, patients and caregivers require unbiased information about considerations and options for treatment changes, what is known and not known, and guidance for monitoring symptoms and possible side effects that may occur with a specific treatment plan. In addition, patients and caregivers should receive information about who to contact about concerns. Owing to limited data around long-term effect of overlapping or sequential treatments on larger numbers of patients from real-world data, shared decision-making between patients, caregivers, and their health care team is essential to providing best care.
Clinical judgment about significant disease progression is individualized and includes understanding the natural history of SMA relative to the individual patient's age at symptom onset (if present), age and development or functional status at DMT start, disease trajectory before DMT, and their capacity to respond to treatment which includes slowing progression of disease, stabilizing, or maintaining function, or improvement. For example, patients with SMA <5 years old have capacity for increased response to SMN-enhancing treatments with the greatest responsiveness in patients younger than 2 years.13,14,26-28,39-45 Older children, teens, and adults may increase function that assists with optimizing independence, e.g., continued ability to drive their own motorized wheelchair or ability to lift a drink in a cup from a surface.46,47 In addition, the response may include stabilization of disease progression and improved quality of life, e.g., improved tolerance to viral illness while continuing to require ventilation support or the ability to tolerate sitting upright for longer periods of time.48,49 Observations in natural history and clinical trials have shown that the outcomes response to DMTs is positive across all age groups and severity. If significant disease progression is the clinician's judgment, e.g., loss of motor milestones in an infant, as the treatment plan is being reviewed, questions to consider include whether the patient may have additional response with a change of the treatment plan.
Add-on treatment is being explored in clinical trials, and additionally, real-world data are being collected. Add-on treatment includes any SMN-enhancing treatment that is given after receiving OA and also includes risdiplam and nusinersen given concurrently. Unanswered questions concerning the possible benefits of add-on treatment (efficacy) include is it safe to add on treatment (safety), and when to add (timing). Discussion with the patient and caregiver is essential to achieve shared decision-making because there are insufficient data to provide recommendations regarding add-on treatment, and in many countries, this may not receive cost approval.
Real-world data collection of the patient journey will guide understanding of SMA treatments and reduce the barriers of insufficient data in SMA care and managing expectations. In addition, collaborative development of clinically meaningful outcomes for older children, teens, and adults is needed to reduce treatment access barriers and contribute to decision-making.
Recommendation 5
Factors that guide decision-making when determining treatment for adolescent and adult patients with SMA:
Treatment intolerance
Quality of life
Benefit vs burden
Treatment side effects
Loss of functionality
Reproductive concerns (male and female)
Pregnancy
Disease progression despite treatment
Patient perspective (tired, burnt out)
The HCPWG used a Likert 5-level scale to identify the importance of each of the above factors. All were identified as very important or somewhat important and factors are listed in decreasing order of percent of respondents indicating the factor was very important (see Table 4).
When providing care and considering treatment of adults with SMA, it is important to review the natural history of SMA disease without treatment which is progressive neuromotor loss over time.14 In addition, comorbidities play a significant role such as having complex spine anatomy or underlying renal disease, and reproductive planning also affects decision-making. The expectations for response to treatment for adults have a different focus compared with young children. Anticipated outcomes for adults are slowed progression of SMA disease, maintaining current motor function to perform activities of daily living, and optimizing independence. Thus, the effect of SMN-enhancing treatments may require a longer time course to observe, 12 months or more. In addition, patient age is a less important when considering treatment for adults. When considering treatment options with an adult living with SMA, it is essential to discuss the above listed topics and gain understanding of each patient's personal goals for their unique life and their baseline functional status and then engage in shared decision-making.
Recommendation 6
Access to care coordination and interdisciplinary/multidisciplinary care are essential to the success of providing SMN-enhancing treatment to patients living with SMA, benefits the patient and caregiver experience, and benefits the HCP experience.
The CWG identified the importance of patients and caregivers having access to a designated care coordinator(s) introduced early in the SMA journey to support and navigate local and specialty health care systems including:
Receiving and reinforcing information about SMA and available treatments
Understanding the process and expectations around access to treatments
Scheduling care visits with the SMA care team and interdisciplinary/multidisciplinary HCPs
Coordinating laboratory monitoring studies associated with SMA treatment
Information and access to community support services, e.g., counseling services, transportation, patient advocacy groups, patient and caregiver support services
The HCPWG discussed and agreed with the importance of care coordination to facilitate providing SMN-enhancing treatments (see Table 5) and addressing multiple barriers including time to treatment, access to treatment, and managing expectations. Coordinated care for rare conditions involves working together across multiple care processes to enable everyone involved in a patient's care to avoid duplication and achieve shared outcomes. Ideally, care coordination is patient-centered, holistic for the patient and caregiver, evidence-based, with equal access to coordinated care irrespective of patient circumstances and geographical location.50
Table 5.
Recommended SMA Education and Resources
| SMA diagnosis education | SMA treatments education | Support resources |
| Understanding SMA • Cause • Genetics • Pathophysiology ◦Adapted to patient clinical status |
Overview of treatments • Mechanism of action • Route and frequency of administration • Clinical trial information and results |
Identified care coordinator
Identified multidisciplinary/interdisciplinary team members |
| Symptoms • Impacted organ systems |
Risks and benefits • Efficacy • Side effects ◦ Known, anticipated and Possible • Required monitoring |
Psychosocial counseling services for patient/caregiver |
| Natural history of disease without treatment |
Access to treatment process • Health governing agency |
Support navigating process to access treatment • Health governing agency/payors • Communications |
| Health management • Supportive care of SMA disease symptoms ◦ Interdisciplinary care - Respiratory - Nutrition/bone health/gastrointestinal - Neuromusculoskeletal/rehabilitation - Orthopedic - Psychosocial - Acute care • Preventative (routine) health care immunizations ◦ Routine health care checkup ◦ Exercise/activities |
Expectations • Shared decision-making • Time and travel commitments • Medical compliance ◦ Safety ◦ Follow-up • Financial • Cultural considerations |
Patient advocacy organizations • Examples ◦ Cure SMA ◦ SMA Europe |
| Current knowledge limitations |
What is known and not known • Treatment outcomes |
Parent and patient support groups • Social media |
In addition, emphasizing the importance of and providing access to interdisciplinary and multidisciplinary supportive care optimizes DMT outcomes and facilitates assessment and anticipation of unique needs, e.g., possible need for respiratory support during illness, or musculoskeletal bracing and other assistive devices to optimize development, function, and independence. Owing to not having a cure for SMA, limited numbers of patients with SMA and limited long-term SMN-enhancing treatment outcomes data, continued interdisciplinary and multidisciplinary evaluations are necessary to understand and improve healthcare for individuals with SMA.
Discussion
This work provides recommendations for SMA treatment decision-making between HCPs and patients and caregivers in North America and Western Europe. Access to treatments and resources varies worldwide, reflecting a variety of barriers. The goals are to provide uniform early diagnosis, SMN-enhancing treatment, and ongoing interdisciplinary/multidisciplinary care for all people living with SMA. Despite many unanswered questions, this framework requires current knowledge of SMN-enhancing treatments and collaboratively sharing that knowledge with patients and caregivers to actively engage in shared decision-making. Barriers to providing DMTs may be addressed through collaborative and coordinated real-world data collection, analyses and dissemination to increase evidence and knowledge and leveraging this knowledge to advocate for treatment access, optimize time to treatment, and managing expectations.
Additional work is needed to establish a revised clinically meaningful classification of SMA, characterize the “new” natural history of SMA disease including presymptomatic to symptomatic SMA disease spectrum, and validate clinically meaningful outcomes for adolescents and adults with SMA. Use of SMA DMTs will continue to evolve, and further updates in SMA treatment best practice are needed.
TAKE-HOME POINTS
→ When determining whether to start, change, add, or discontinue SMA treatment, essential considerations include patient and family/caregiver perspective, and treatment safety and side effects.
→ When initiating treatment for patients newly diagnosed with SMA, important patient characteristics are age and SMN2 gene copy number. Furthermore, when initiating, changing, or adding treatment for patients not newly diagnosed with SMA, current clinical status and comorbidities drive decision-making.
→ When considering a medication or treatment plan change, unless there is an urgent indication, a treatment and associated patient outcomes should be monitored for a minimum of 6–12 months before making a change.
→ When determining a treatment plan with an adolescent or adult with SMA consider factors such as quality of life, burden vs benefit of treatment, and reproductive issues.
→ Access to care coordination and interdisciplinary/multidisciplinary care are essential to the success of providing SMN-enhancing treatment to patients living with SMA, benefits the patient and caregiver experience, and benefits the HCP experience.
Acknowledgment
The authors acknowledge the following additional Cure SMA Treatments Considerations' Working Group participants: Healthcare Provider Working Group: John Brandsema, MD; Emma Ciafaloni, MD; John Day, MD, PhD; Erika Finanger, MD; Richard Finkel, MD; Jana Haberlová, MD, PhD; Jennifer Kwon, MD; Susana Quijano-Roy, MD, PhD; Community Working Group: Amanda DeVay, Annie Heathcote, Beth Moore, Marie-Christine Ouillade, Rory Philstrom, and Kristen Resendez. The authors thank Nicole Gusset, SMA Europe for manuscript review and editing.
Appendix 1. Authors
| Name | Location | Contribution |
| Mary K. Schroth, MD | Cure SMA | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Jennifer Deans, MHA, MS, CCLS | Clinical Care Education, Cure SMA | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Diana X. Bharucha Goebel, MD | Neurology and Pediatrics, Children's National, and National Institute of Neurological Diseases and Stroke, National Institutes of Health | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| W. Bryan Burnette, MD, MS | Department of Pediatrics, Division of Neurology, Vanderbilt University | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Basil T. Darras, MD | Department of Neurology, Boston Children's Hospital, Harvard Medical School | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Bakri H. Elsheikh, MBBS, FRCP (Edin) | Department of Neurology, The Ohio State University Wexner Medical Center | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Marcia V. Felker, MD | Child Neurology, Indiana University | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Andrea Klein, MD | Division of Neuropediatrics, Development and Rehabilitation, Department of Pediatrics, Inselspital, Bern University Hospital | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Jena Krueger, MD | Pediatric Neuromuscular, Helen DeVos Children's Hospital | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Crystal M. Proud, MD | Neurology, Children's Hospital of the King's Daughters | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Aravindhan Veerapandiyan, MD | Department of Pediatrics, Division of Neurology, University of Arkansas for Medical Sciences, Arkansas Children's Hospital | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Robert J. Graham, MD | Department of Anesthesiology, Critical Care and Pain Medicine, Boston Children's Hospital, Harvard Medical School | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
Appendix 2. Coinvestigators
| Coinvestigators are listed at Neurology.org/cp. |
Study Funding
Funding was provided by the Cure SMA Real World Evidence Collaboration which includes Cure SMA, Novartis Gene Therapies, and Biogen. Authors had full control over the content and development of the manuscript. Novartis Gene Therapies and Biogen had no influence or control on the content or development of this manuscript.
Disclosure
M.K. Schroth is an employee of Cure SMA; J. Deans is an employee of Cure SMA; D.X. Bharucha-Goebel has received research support from Genentech/Roche; W.B. Burnette reports no disclosures; B.T. Darras has served as an ad hoc scientific advisory board member for AveXis/Novartis Gene Therapies, Biogen, and Roche/Genentech; Steering Committee Chair/Member for Roche FIREFISH and MANATEE studies, and has received research support from Biogen, Novartis Gene Therapies (AveXis), and Roche/Genentech; B.H. Elsheikh received research support from Biogen, Genentech, NMD Pharma, and served as a consultant for Biogen and Genentech; M.V. Felker served as an advisor for PHAR (Partnership for Health Analytic Research), which was hired by Genentech and also received honoraria serving as a consultant for Genentech; A. Klein received honoraria for serving on advisory boards and/or symposia for Biogen, Novartis, and Roche, has received research support from Biogen, Novartis, and Roche, and serves as clinical lead of the Swiss-Reg NMD; J. Krueger received research support and served as a primary investigator for clinical trials sponsored by Novartis, Genetech/Roche, and Scholar Rock; C.M. Proud has received research support and served as a site primary investigator for clinical trials sponsored by Biogen, Novartis Gene Therapies, and Scholar Rock, has received honoraria for serving on advisory board or Speaker's Bureaus for Biogen, Novartis Gene Therapies, and Roche; A. Veerapandiyan has received honoraria for serving on ad hoc advisory boards for Biogen and for Novartis, and receives research support from the Muscular Dystrophy Association and from Novartis; R.J. Graham reports no disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Aragon-Gawinska K, Mouraux C, Dangouloff T, Servais L. Spinal muscular atrophy treatment in patients identified by newborn screening-a systematic review. Genes (Basel). 2023;14(7):1377. doi: 10.3390/genes14071377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugarman EA, Nagan N, Zhu H, et al. Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: clinical laboratory analysis of >72,400 specimens. Eur J Hum Genet. 2012;20(1):27-32. doi: 10.1038/ejhg.2011.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold WD, Kassar D, Kissel JT. Spinal muscular atrophy: diagnosis and management in a new therapeutic era. Muscle Nerve. 2015;51(2):157-167. doi: 10.1002/mus.24497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lefebvre S, Burglen L, Reboullet S, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80(1):155-165. doi: 10.1016/0092-8674(95)90460-3 [DOI] [PubMed] [Google Scholar]
- 5.Feldkotter M, Schwarzer V, Wirth R, Wienker TF, Wirth B. Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am J Hum Genet. 2002;70(2):358-368. doi: 10.1086/338627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang CH, Finkel RS, Bertini ES, et al. Consensus statement for standard of care in spinal muscular atrophy. J Child Neurol. 2007;22(8):1027-1049. doi: 10.1177/0883073807305788 [DOI] [PubMed] [Google Scholar]
- 7.Finkel RS, Sejersen T, Mercuri E, Group ESWS. 218th ENMC International Workshop: revisiting the consensus on standards of care in SMA Naarden, The Netherlands, 19-21 February 2016. Neuromuscul Disord. 2017;27(6):596-605. doi: 10.1016/j.nmd.2017.02.014 [DOI] [PubMed] [Google Scholar]
- 8.Mercuri E, Finkel RS, Muntoni F, et al. Diagnosis and management of spinal muscular atrophy: part 1: recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. 2018;28(2):103-115. doi: 10.1016/j.nmd.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 9.Finkel RS, Mercuri E, Meyer OH, et al. Diagnosis and management of spinal muscular atrophy: part 2: pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul Disord. 2018;28(3):197-207. doi: 10.1016/j.nmd.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 10.Finkel RS, McDermott MP, Kaufmann P, et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014;83(9):810-817. doi: 10.1212/WNL.0000000000000741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Sanctis R, Pane M, Coratti G, et al. Clinical phenotypes and trajectories of disease progression in type 1 spinal muscular atrophy. Neuromuscul Disord. 2018;28(1):24-28. doi: 10.1016/j.nmd.2017.09.015 [DOI] [PubMed] [Google Scholar]
- 12.Kolb SJ, Coffey CS, Yankey JW, et al. Natural history of infantile-onset spinal muscular atrophy. Ann Neurol. 2017;82(6):883-891. doi: 10.1002/ana.25101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coratti G, Lucibello S, Pera MC, et al. Gain and loss of abilities in type II SMA: a 12-month natural history study. Neuromuscul Disord. 2020;30(9):765-771. doi: 10.1016/j.nmd.2020.07.004 [DOI] [PubMed] [Google Scholar]
- 14.Mercuri E, Finkel R, Montes J, et al. Patterns of disease progression in type 2 and 3 SMA: IMPLICATIONS for clinical trials. Neuromuscul Disord. 2016;26(2):126-131. doi: 10.1016/j.nmd.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. doi: 10.1186/s13643-021-01626-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rowe G, Wright G. Expert opinions in forecasting. Role of the Delphi technique. In: Armstrong JS, ed. Principles of Forecasting: A Handbook for Researchers and Practitioners. Springer; 2001:125-144. [Google Scholar]
- 18.Delbecq AL, Van de Ven AH, Gustafson CH. Group Techniques for Program Planning: A Guide to Nominal Group and Delphi Processes. Scott Foresman Company; 1975. [Google Scholar]
- 19.Hsu CC, Sandford BA. The delphi technique - making sense of consensus. Pract Assess Res Eval. 2007;12(10):1-8. [Google Scholar]
- 20.Spinraza (Nusinersen): European Public Assessment Report (EPAR). European Medicines Agency; 2017. (Revised 2018, Revised 2022). [Google Scholar]
- 21.Zolgensma (Onasemnogene Abeparvovec): European Public Assessment Report (EPAR). European Medicines Agency; 2020. (Revised 2023). [Google Scholar]
- 22.Evrysdi (Risdiplam): European Public Assessment Report (EPAR). European Medicines Agency; 2021. (Revised 2023). [Google Scholar]
- 23.Spinraza (Nusinsersen): Prescribing Information. US Food and Drug Administration; 2016. (Revised 2020, Revised 2023). [Google Scholar]
- 24.Zolgensma (Onasemonogene Abeparvovec-Xioi): Prescribing Information. US Food and Drug Administration; 2019. (Revised 2023). [Google Scholar]
- 25.Evrysdi (Risdiplam): Prescribing Information. US Food and Drug Administration; 2020. (Revised 2022, Revised 2023). [Google Scholar]
- 26.Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus Sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377(18):1723-1732. doi: 10.1056/NEJMoa1702752 [DOI] [PubMed] [Google Scholar]
- 27.Mendell JR, Al-Zaidy S, Shell R, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377(18):1713-1722. doi: 10.1056/NEJMoa1706198 [DOI] [PubMed] [Google Scholar]
- 28.Darras BT, Masson R, Mazurkiewicz-Beldzinska M, et al. Risdiplam-treated infants with type 1 spinal muscular atrophy versus historical controls. N Engl J Med. 2021;385(5):427-435. doi: 10.1056/NEJMoa2102047 [DOI] [PubMed] [Google Scholar]
- 29.De Vivo DC, Bertini E, Swoboda KJ, et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: interim efficacy and safety results from the phase 2 NURTURE study. Neuromuscul Disord. 2019;29(11):842-856. doi: 10.1016/j.nmd.2019.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crawford TO, Swoboda KJ, De Vivo DC, et al. Continued benefit of nusinersen initiated in the presymptomatic stage of spinal muscular atrophy: 5-year update of the NURTURE study. Muscle Nerve. 2023;68(2):157-170. doi: 10.1002/mus.27853 [DOI] [PubMed] [Google Scholar]
- 31.Strauss KA, Farrar MA, Muntoni F, et al. Onasemnogene abeparvovec for presymptomatic infants with two copies of SMN2 at risk for spinal muscular atrophy type 1: the phase III SPR1NT trial. Nat Med. 2022;28(7):1381-1389. doi: 10.1038/s41591-022-01866-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strauss KA, Farrar MA, Muntoni F, et al. Onasemnogene abeparvovec for presymptomatic infants with three copies of SMN2 at risk for spinal muscular atrophy: the phase III SPR1NT trial. Nat Med. 2022;28(7):1390-1397. doi: 10.1038/s41591-022-01867-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swoboda KJ, Prior TW, Scott CB, et al. Natural history of denervation in SMA: relation to age, SMN2 copy number, and function. Ann Neurol. 2005;57(5):704-712. doi: 10.1002/ana.20473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kong L, Valdivia DO, Simon CM, et al. Impaired prenatal motor axon development necessitates early therapeutic intervention in severe SMA. Sci Transl Med. 2021;13(578):eabb6871. doi: 10.1126/scitranslmed.abb6871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inhestern L, Brandt M, Driemeyer J, Denecke J, Johannsen J, Bergelt C. Experiences of health care and psychosocial needs in parents of children with spinal muscular atrophy. Int J Environ Res Public Health. 2023;20(7):5360. doi: 10.3390/ijerph20075360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gusset N, Erbas Y, Germanenko O, Rucinski K, Stumpe E, de Lemus M. A decision for life - treatment decisions in newly diagnosed families with spinal muscular atrophy (SMA). Eur J Paediatr Neurol. 2021;30:105-107. doi: 10.1016/j.ejpn.2020.11.003 [DOI] [PubMed] [Google Scholar]
- 37.Glascock J, Sampson J, Connolly AM, et al. Revised recommendations for the treatment of infants diagnosed with spinal muscular atrophy via newborn screening who have 4 copies of SMN2. J Neuromuscul Dis. 2020;7(2):97-100. doi: 10.3233/JND-190468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monnette A, Chen E, Hong D, et al. Treatment preference among patients with spinal muscular atrophy (SMA): a discrete choice experiment. Orphanet J Rare Dis 2021;16(1):36. doi: 10.1186/s13023-020-01667-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mercuri E, Darras BT, Chiriboga CA, et al. Nusinersen versus Sham control in later-onset spinal muscular atrophy. N Engl J Med. 2018;378(7):625-635. doi: 10.1056/NEJMoa1710504 [DOI] [PubMed] [Google Scholar]
- 40.Mercuri E, Lucibello S, Pera MC, et al. Long-term progression in type II spinal muscular atrophy: a retrospective observational study. Neurology. 2019;93(13):e1241-e1247. doi: 10.1212/WNL.0000000000008166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mercuri E, Deconinck N, Mazzone ES, et al. Safety and efficacy of once-daily risdiplam in type 2 and non-ambulant type 3 spinal muscular atrophy (SUNFISH part 2): a phase 3, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2022;21(1):42-52. doi: 10.1016/s1474-4422(21)00367-7 [DOI] [PubMed] [Google Scholar]
- 42.Mendell JR, Al-Zaidy SA, Lehman KJ, et al. Five-year extension results of the phase 1 START trial of onasemnogene abeparvovec in spinal muscular atrophy. JAMA Neurol. 2021;78(7):834-841. doi: 10.1001/jamaneurol.2021.1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mercuri E, Muntoni F, Baranello G, et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy type 1 (STR1VE-EU): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021;20(10):832-841. doi: 10.1016/S1474-4422(21)00251-9 [DOI] [PubMed] [Google Scholar]
- 44.Day JW, Finkel RS, Chiriboga CA, et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021;20(4):284-293. doi: 10.1016/S1474-4422(21)00001-6 [DOI] [PubMed] [Google Scholar]
- 45.Oskoui M, Day JW, Deconinck N, et al. Two-year efficacy and safety of risdiplam in patients with type 2 or non-ambulant type 3 spinal muscular atrophy (SMA). J Neurol. 2023;270(5):2531-2546. doi: 10.1007/s00415-023-11560-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duong T, Wolford C, McDermott MP, et al. Nusinersen treatment in adults with spinal muscular atrophy. Neurol Clin Pract. 2021;11(3):e317-e327. doi: 10.1212/CPJ.0000000000001033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hagenacker T, Wurster CD, Gunther R, et al. Nusinersen in adults with 5q spinal muscular atrophy: a non-interventional, multicentre, observational cohort study. Lancet Neurol. 2020;19(4):317-325. doi: 10.1016/S1474-4422(20)30037-5 [DOI] [PubMed] [Google Scholar]
- 48.Elsheikh B, Severyn S, Zhao S, et al. Safety, tolerability, and effect of nusinersen in non-ambulatory adults with spinal muscular atrophy. Front Neurol. 2021;12:650532. doi: 10.3389/fneur.2021.650532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brakemeier S, Stolte B, Thimm A, et al. Assessment of bulbar function in adult patients with 5q-SMA type 2 and 3 under treatment with nusinersen. Brain Sci. 2021;11(9):1244. doi: 10.3390/brainsci11091244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walton H, Hudson E, Simpson A, et al. Defining coordinated care for people with rare conditions: a scoping review. Int J Integr Care. 2020;20(2):14. doi: 10.5334/ijic.5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.