Abstract
Misdiagnosis of Mycobacterium heraklionense tenosynovitis is common due to the challenging identification and perceived rarity of the disease. This can result in delayed therapy initiation and potentially irreversible consequences. In this report, we present an additional case of hand tenosynovitis, which highlights the diagnostic and management challenges of Mycobacterium heraklionense tenosynovitis and provides further evidence of its emergence as a cause of tenosynovitis. Additionally, we provide a comprehensive summary of published case reports that describe Mycobacterium heraklionense tenosynovitis.
Keywords: Nontuberculous mycobacteria, Mycobacterium heraklionense, Tenosynovitis, Hand, Musculoskeletal diseases
1. Introduction
Nontuberculous mycobacteria (NTM) are ubiquitous microorganisms widely distributed in environmental habitats, including soil, water sources, and animal reservoirs [1]. NTM species are emerging causes of human diseases of global significance and have been increasingly reported as primary pathogens causing pulmonary and, less frequently, extrapulmonary infections [2], [3], [4], [5], [6], [7]. While NTM infections typically involve the lungs, NTM tenosynovitis represents a rare yet but potentially serious condition that often affects the hand and wrist and is typically caused by Mycobacterium marinum or Mycobacterium avium complex [8], [9]. NTM tenosynovitis is usually related to prior trauma, surgical interventions, local corticosteroid injections, or exposure to contaminated water sources [10]. However, exposure to these organisms is probably a constant occurrence, and infection requires a combination of environmental-related factors, such as a high organism load in hot tub aerosols, the introduction of the bacteria through surgical incisions [11], and host factors, such as genetic susceptibility and immunosuppression [12], [13], [14]. Diagnosing and managing NTM tenosynovitis poses significant challenges due to low suspicion, nonspecific clinical presentations, and suboptimal laboratory identification techniques [8]. Consequently, NTM tenosynovitis is frequently misdiagnosed, leading to delays in treatment initiation [8], [15]. Although tenosynovitis is rare, comprehensive epidemiological data is lacking. Recent advancements in the differentiation of Mycobacterium species using molecular methods have led to increased reporting and identification of additional NTM species [16]. These enhanced speciation methods play a crucial role in elucidating the clinical and epidemiological characteristics of rare NTM infections, offering novel insights into their clinical and epidemiological features.
In 2013, through genotypic analysis of 150 strains belonging to the Mycobacterium terrae complex, a previously unknown species named Mycobacterium heraklionense sp (M. heraklionense). Nov was identified based on a distinctive sequence in the 16S rRNA gene [1]. Among the 150 strains analysed, twenty-three strains were collected between 2002 and 2011 from Greece (including several strains from Heraklion in Crete), Italy, and India. In 2014, the first case of hand tenosynovitis caused by M. heraklionense, a member of the Mycobacterium terrae complex, was reported [17]. Since then, only a few cases have been documented [8], [17], [18], [19], [20], [21], [22], [23]. However, this entity should be considered in the differential diagnosis of chronic tenosynovitis, as it can lead to consequent aggressive and repetitive surgical interventions, prolonged antibiotic therapies with potential drug side effects, and irreversible sequelae. This report presents the clinical course of a patient diagnosed with hand tenosynovitis caused by M. heraklionense. This case contributes additional insights to our understanding of M. heraklionense infection and provides further evidence of the emergence of M. heraklionense as a cause of tenosynovitis. We also provide a comprehensive summary of published case reports describing M. heraklionense tenosynovitis.
2. Case report
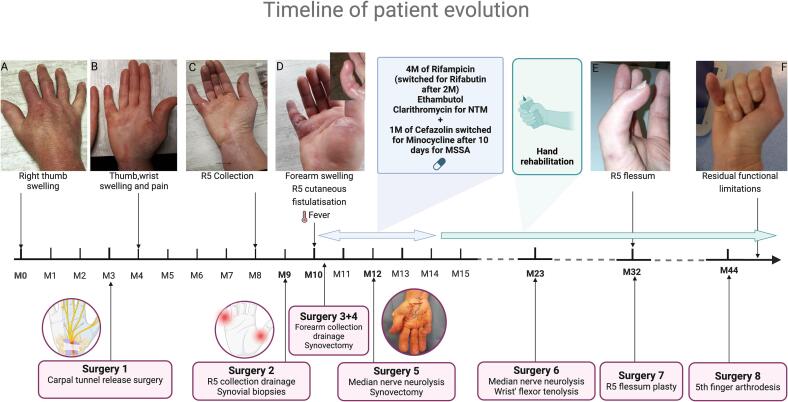
A 52-year-old woman was admitted for a painful inflammatory tumefaction of the right fifth finger. She had a nine-month history of gradual swelling and stiffness of her right thumb (Fig. 1A), which subsequently extended to her hand and wrist upon returning from a trip to Jamaica. During this time, she experienced a series of misdiagnosis, including successively severe idiopathic carpal tunnel syndrome, De Quervain's tenosynovitis, rheumatoid arthritis, and complex regional pain syndrome type II, leading to multiple unsuccessful treatments of non-steroidal anti-inflammatory drug, sulfasalazine, local corticosteroid (CS) injections and systemic CS (Fig. 1A–C). She had no fever, and the laboratory markers showed no abnormalities. Although she did not report any history of trauma, she was gardening in her usual capacity and sometimes sustained unnoticed minor injuries. The investigations did not reveal any cause of immunosuppression, including notably HIV infection and diabetes. Ultrasound of the hand revealed marked and diffuse synovitis with a low vascular flow and tenosynovitis of the common flexor tendons extending into the thumb and the fifth finger’s flexor tendon sheath. Subsequently, she underwent drainage of the collection in the 5th ray (R5) and limited debridement of the flexor tendon sheath. However, one month later, she was re-admitted for acute clinical deterioration with extension of the infection to the forearm (Fig. 1D). Histopathological analysis of the synovial tissue collected a month earlier showed intense chronic inflammation features with both necrotising and non-necrotizing granulomas. Cultures from perioperative samples, including aerobic culture and mycobacterial liquid media culture using the BACTEC MGIT 960 System, demonstrated the presence of methicillin-sensitive Staphylococcus aureus (MSSA) and mycobacteria after eight days of incubation. After the Ziehl-Neelsen staining, acid-fast bacilli (AFB) were observed. She was diagnosed with chronic NTM flexor tenosynovitis complicated by MSSA cellulitis. She was started empirically on rifampicin (600 mg once per day), clarithromycin (1000 mg once per day), ethambutol (1200 mg once per day), and cefazolin (six g/24 h). As specific detection of Mycobacterium tuberculosis Complex by Real-Time PCR was negative, species identification through 16S ribosomal RNA sequencing (16S rRNA) revealed M. heraklionense [1]. Antimycobacterial susceptibility testing (AST) was tested using broth microdilution method with Thermofisher ScientificTM Sensititre Slow Growing Mycobacteria (SLOMYCO2) Plate. Minimum inhibitory concentrations (MICs) were interpreted in accordance with the guidelines of the Clinical and Laboratory Standards Institute (CLSI) (document M24-A2) [24] and were reported in µg/mL as follows: clarithromycin, 0.25; rifabutin, ≤0.25; rifampin, >8; ethambutol, 2 (Table 1). Despite antibiotic therapy, two surgical interventions were necessary, including drainage of the abscessed collection and synovectomy. The procedures revealed a complete absence of pulleys and partial and complete ruptures of the flexor tendon sheaths of the R1 and R5, respectively. After a 10-day hospital stay, she was discharged on a combination of rifampicin, clarithromycin, and ethambutol. Cefazolin was substituted with minocycline (100 mg twice daily) for a month. Due to persistent flexor tenosynovitis in R4 and R5 after two months, she underwent a repeat synovectomy and neurolysis of the median nerve. Following negative per-operative mycobacterial culture, she continued the same antibiotic therapy for an additional four months, resulting in clinical improvement. Notably, rifampicin was replaced with rifabutin after two months due to gastrointestinal side effects. However, due to major functional impairment, subsequent management focused on hand rehabilitation and required serial reconstructive surgeries, including a second median nerve neurolysis, wrist flexors tenolysis, flessum plasty (Fig. 1E), and then arthrodesis of 5th finger (Fig. 1F). Currently, more than six years from the initial presentation, she has successfully returned to work despite experiencing residual stiffness and limitations in finger flexion movements.
Fig. 1.
Timeline of patient evolution. Abbreviations: M, months; R5, MSSA, Methicillin- susceptible Staphylococcus aureus; NTM, Nontuberculous Mycobacteria; R5, 5th rayon.
Table 1.
Characteristics of 19 previously reported cases from patients with tenosynovitis due to Mycobacterium heraklionense.
| First author | Gender |
Age (years) |
Underlying immune deficiency | History of trauma | Exposure History | Localisation | Method of diagnosis | Diagnosis delay (months) | Treatment before diagnosis | Susceptibility testing, MICs (ug/mL) |
Antibiotic therapy |
ATB duration (months) | Surgery | Number of surgery | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| El Moussaoui, 2023 | F | 52 | No | No | Gardening | R hand | Culture + in 8 days (liquid media culture, BACTEC MGIT 960 System) And 16S rRNA sequencing |
9 | NSAIDs, Oral + local CS injections | AMK, 32; RMP, >8; RBT, ≤0.25; CLA, 0.25; EMB, 2; CIP, >16; MOX, >8; DOX>16; LZD, 16; INH, >8; STR, 64; TMP-SMX, 2/38 | RMP (switch for RBT because of GI side effects) + CLA+EMB 1 month of MIN for MSSA infection |
4 | Yes | 4 | Favorable (4 months) with no relapse at 5 years, but limitation of his finger movements and residual stiffness |
| Dutronc, 20234 | M | 31 | No | Yes | Palm thorn | R PIP index finger | Culture + in 16–24 days and 16S rRNA Sequencing |
12 | NSAIDs | Unknown | RBT + CLA |
1,5 | Yes | 1 | Favorable (6 months) but lost follow-up after |
| Dutronc, 20234 | M | 52 | No | Yes | Gardening | L third finger | Culture + in 16–24 days and 16 s rRNA Sequencing |
7 | Oral + local CS injections | Unknown | RBT (switch for RMP because of stock-out) + CLA (switch for AZI due to GI side effects) |
6 | Yes | 1 | Complete resolution (6 months) |
| Dutronc, 20234 | M | 58 | No | Yes | Nail that fell on the ground into his right hand | R second and fifth fingers | Culture + in 16–24 days and 16 s rRNA Sequencing |
10 | CS | Unknown | RMP + CLA + EMB |
7 | Yes | 1 | Complete resolution (7 months) |
| Mason, 20215 | F | 58 | HTA, Epilepsy, Lupus treated by immune-modulating agents recently stopped | No | Potential injuries during seizures | R index finger | Culture + in 28 days and 16 s rRNA Sequencing | >2 | Local CS injections | S to AMK, RMP, RBT, CLA, EMB, LZD, and R to CIP, MOX, and TMP-SMX | RBT + AZI + EMB |
12 | Yes | 2 | Favorable (12 months) but residual stiffness up to 2 years after |
| Turner, 20219 | M | 66 | HTA, Coronary artery disease, Nephrolithiasis | Yes | Injury while handling water damaged plywood | R fifth finger | Culture + and 16S sequencing and WGS |
6 | Unknown | AMK, 16; CLA, 2; CLO, 0.12; Bedaquiline, 0.008 | AZI + DOX + LZD (changed to LEV for GI side effects) |
6 | Yes | 2 | Favorable (at 6 months) with no relapse at 1 year |
| Bouchet, 20178 | M | 41 | No | Yes | Working in his vines | L third fingertip | Culture + in 14 days and rpoB and hsp65 sequencing | 5.8 | Amoxicillin-clavulanic acid, TMP-SMX | AMK, 16; RMP, 2; RBT, < 0.25; CLA, 4; EMB 2; CIP, 16; MOX>8; LZD, 64; SMX, 9.5 | CLA | 6 | Yes | 2 | Favorable (at 6 months) |
| Aburjania, 20166 | M | 72 | No | No | Gardening and picking up golf balls | R middle finger | Some AFB on Ziehl-Neelsen-stained smears, No growth, rpoB sequencing | 9 | Local CS injections | No AST available due to negative culture | RMP + CLA (switched for AZI due to GI side effects) + EMB + 1 month of LEV for MSSA infection |
3 | Yes | 2 | Favorable (2–3 months) but limitation of his finger movements |
| Greninge, 20157 | F | 53 | Unknown | Yes | Right Achilles tendon surgery after a gardening accident 20 years before | R medial soft-tissue ankle mass | Culture + in 14 days and partial rpoB sequencing |
Unknown | Unknown | AMK, 2; RMP, >16; RBT, 0.25; CLA, 4; EMB, 1.25; CIP, >4; MOX, >2; KAN, 8; STR, 4 | Unknown | Unknown | Yes | 1 | Unknown |
| Abedalthagai, 20142 | M | 37 | No | Yes | Tree pruning | L middle finger | 16S rRNA sequencing | 4 | No | AMK, 8; RMP, ≤0.5; RBT, ≤0.12; CLA, ≤4; EMB, 2.5; CIP, >4; CLO, 0.5; STR, 8 | RBT + CLA (switched after 2 weeks for AZI due to GI side effects) + EMB |
3 | Yes | 2 | Recurrence at 2 months after discontinuing treatment → AZI+EMB |
| Vasiredy, 20163 | Unknown | Unknown | Unknown | Unknown | Unknown | R hand | 16S rRNA sequencing | Unknown | Unknown | AMK, 8; RMP, 2; CLA, 2; EMB, 4; CIP, 8; MIN, >32; SMX, 8 | Unknown | Unknown | Unknown | Unknown | Unknown |
| Vasiredy, 20163 | Unknown | Unknown | Unknown | Unknown | Unknown | Hand | 16S rRNA sequencing | Unknown | Unknown | AMK, 8; RMP, 8; CLA, 1; EMB, 16; CIP, >8; MIN, >32; SMX, 16 | Unknown | Unknown | Unknown | Unknown | Unknown |
| Vasiredy, 20163 | Unknown | Unknown | Unknown | Unknown | Unknown | Finger | 16S rRNA sequencing | Unknown | Unknown | AMK, 64; RMP, 8; RBT, ≤0.25; CLA, 1.025; EMB, 2; CIP, >16; MOX, >8; DOX, >16; LZD, 8; TMP-SMX, 1/19 | Unknown | Unknown | Unknown | Unknown | Unknown |
| Vasiredy, 20163 | Unknown | Unknown | Unknown | Unknown | Unknown | Finger | 16S rRNA sequencing | Unknown | Unknown | AMK, 64; RMP, >8; RBT, 1.1; CLA, 1; EMB, 4; CIP, >16; MOX, >8; DOX, >16; LZD, 32; TMP-SMX, 2/38 | Unknown | Unknown | Unknown | Unknown | Unknown |
| Vasiredy, 20163 | Unknown | Unknown | Unknown | Unknown | Unknown | R index finger | 16S rRNA sequencing | Unknown | Unknown | AMK, 16; RMP, 4; RBT, 0.5; CLA, 2; EMB, 2; CIP, >16; MOX, >8; DOX, >16; LZD, 64; TMP-SMX, 1/19 |
Unknown | Unknown | Unknown | Unknown | Unknown |
| Vasiredy, 20163 | Unknown | Unknown | Unknown | Unknown | Unknown | L hand | 16S rRNA sequencing | Unknown | Unknown | AMK, 16; RMP, 1; RBT, ≤0.25; CLA, 0.5; EMB, 2; CIP, >16; MOX, >8; DOX, >16; LZD, 32; TMP-SMX, 2/38 | Unknown | Unknown | Unknown | Unknown | Unknown |
| Vasiredy, 20163 | Unknown | Unknown | Unknown | Unknown | Unknown | R index finger | 16S rRNA sequencing | Unknown | Unknown | AMK, 64; RMP, 0.5; RBT, ≤0.25; CLA, 0.5; EMB, 8; CIP, 16; MOX, >8; DOX, >16; LZD, >64; TMP-SMX, ≤0.12/2.38 | Unknown | Unknown | Unknown | Unknown | Unknown |
| Vasiredy, 20163 | Unknown | Unknown | Unknown | Unknown | Unknown | R index finger | 16S rRNA sequencing | Unknown | Unknown | AMK, >64; RMP, 8; RBT, 0.5; CLA, 4; EMB, 2; CIP, >16; MOX, >8; DOX, >16; LZD, 64 | Unknown | Unknown | Unknown | Unknown | Unknown |
| Vasiredy, 20163 | Unknown | Unknown | Unknown | Unknown | Unknown | R index finger | 16S rRNA sequencing | Unknown | Unknown | AMK, 16; RMP, >8; RBT, ≤0.25; CLA, 0.5; EMB, 2; CIP, >16; MOX, >8; DOX, 8; LZD, 16; TMP-SMX, 1/19 | Unknown | Unknown | Unknown | Unknown | Unknown |
| Vasiredy, 20163 | Unknown | Unknown | Unknown | Unknown | Unknown | Index finger | 16S rRNA sequencing | Unknown | Unknown | AMK, 32; RMP, >8; RBT, 0.5; CLA, 0.5; EMB, 2; CIP, >16; MOX, >8; DOX, >16; LZD, 32; TMP-SMX, 1/19 | Unknown | Unknown | Unknown | Unknown | Unknown |
Abbreviations: M, male; F, female; HTA, hypertension; PIP, interphalangeal; R, right; L, left; CS, corticosteroid; NA, not available; S, susceptible; R, resistant; CLA, clarithromycin; AZI, azithromycin; EMB, ethambutol; RBT, rifabutin; RMP, rifampicin; AMK, amikacin; DOX, doxycycline; CIP, ciprofloxacin; MOX, Moxifloxacin; LZD, linezolide; LEV, levofloxacin; CLO, clofazimine; KAN, Kanamycin; STR, Streptomycin; TET, Tetracycline; MIN, minocycline; TMP-SMX, Trimethoprim-sulfamethoxazole; BDQ, bedaquiline; GI, gastrointestinal; NSAIDs, MSSA, methicillin-sensitive S. aureus; Non-steroidal anti-inflammatory drugs, WGS, whole genome sequencing.
3. Discussion
M. heraklionense is an intermediate-growing member of the Mycobacterium terrae complex (MTC), initially identified in 2013 from many strains in Heraklion [1]. M. heraklionense has been isolated from various human specimens, including musculoskeletal, sputum, broncho-alveolar lavage, and urine samples, notably in a case series involving 12 elderly patients with underlying health conditions at Heraklion Hospital in Greece [25]. However, despite its detection in various clinical contexts, a definitive link between M. heraklionense and patient mortality remains elusive, except for musculoskeletal involvement. In the environment, M. heraklionense has been detected in water treatment sludge (Makovcova J, Babak V, Slany M, Slana I. 2015) [26] and Bolaños and colleagues identified M. heraklionense in milk samples obtained from cows that tested positive for the tuberculin test, thereby emphasising the potential route of pathogen transmission from bovines to humans via milk or dairy products [27].
Since the first description of a patient with hand tenosynovitis in 2014, 19 additional patients have been described [8], [17], [18], [19], [20], [21], [22], [23]. Among these, ten were previously diagnosed as unspecified MTC tenosynovitis or osteomyelitis using nonmolecular methods between 1984 and 2015 [18]. Patients’ characteristics are displayed in Table 1. Most patients were male, with a median age of 52.5 years (range 31–72). Tenosynovial or osteoarticular infections caused by M. heraklionense typically manifest with an insidious course, presenting as long-standing pain and swelling involving either the wrist or the hand. Due to its challenging diagnosis, most patients experienced a delay between the onset of symptoms and diagnosis, with a median time of 7 months (range, 2–12). Consequently, some patients had received corticosteroids before the diagnosis, potentially promoting bacterial coinfection, such as the reported Staphylococcus aureus infection in two cases. While most patients showed no evidence of immunosuppression, all reported either a history of hand trauma or an occupation that increased the risk of trauma (typically gardening), suggesting that direct environmental inoculation was the infection mechanism.
A definitive diagnosis relies on a tissue specimen culture from liquid or solid media incubated at several temperatures. Mature growth is achieved after at least eight days of incubation (range 8–24 days, Table 1), and establishing a diagnosis may necessitate multiple tissue specimens and serial debridement [28]. Acid-Fast Bacilli (AFB) smear is often negative [18]. Molecular diagnostics using sequencing of 16S ribosomal RNA (16S rRNA) or sometimes beta subunit of RNA polymerase (rpoB), heat shock protein (hsp65), or even whole-genome sequencing, can be invaluable in establishing a diagnosis, particularly when the culture is negative despite a high suspicion of NTM tenosynovitis [18].
There are no standardised breakpoints or interpretative procedures for MTC, and the correlation between in vitro susceptibility and clinical efficacy remains unknown [28]. While some authors have extrapolated data from Mycobacterium kansasii for interpreting MIC [17], [23], [28], the current recommendation is to use broth microdilution method and report only the MIC without interpretation. As illustrated in Table 1, M. heraklionense strains are generally susceptible to rifabutin, clarithromycin, and ethambutol while displaying resistance to rifampin, trimethoprim-sulfamethoxazole, tetracycline, and quinolones.
Although one patient reported was treated only with a single agent [23], most patients were treated with a combination of three agents. Various antimicrobials were administrated, with the most common combinations being rifampin or rifabutin plus ethambutol and clarithromycin. The most common drug side effect reported involved the gastrointestinal system in 5 patients (clarithromycin; n = 2, rifampicin; n = 2, linezolide; n = 1) and required therapy modification.
The median duration of antibiotic therapy was six months, ranging from 1.5 to 12 months. Clinical improvement is reported after at least two months of treatment. Most patients were free from infection after a median follow-up of 6 months (ranging from 2 months to 5 years), with only one recurrence noted. At the last follow-up, three patients reported experiencing finger movement limitations and residual stiffness (Table 1). All patients underwent surgical treatment with an average of 1,8 surgeries per patient (range, 1–4).
Although the optimal duration of antibiotic therapy in NTM is unclear, prolonged treatment is frequently recommended, typically spanning a minimum of three to six months, with some cases necessitating therapy for up to 12 months, depending on the clinical severity [29], [30], [31]. The extended duration of treatment beyond 12 months does not appear to be associated with a better outcome. While microbiological cure is generally achieved, most long-term issues like persistent pain or stiffness are primarily mechanical, highlighting the significance of hand rehabilitation. Although antimycobacterial therapy alone may lead to complete remission in the early stages of the disease in rare case reports, delayed diagnosis is common, and an aggressive surgical approach surgery for source control remains a key component of managing NTM hand infection. This strategy seems crucial in locally advanced diseases to reduce the overall bacterial tissue load and give chemotherapy a better chance of eradicating the residual infection. It may also potentially shorten the duration of drug therapy and subsequently minimise drug side effects.
In conclusion, our report provides a detailed overview of M. heraklionense infection. The true epidemiology remains unknown, requiring more molecular methods for NTM identification. Due to challenging diagnosis and potential severe hand infections, M. heraklionense should be considered in chronic flexor tenosynovitis differential diagnosis to minimise sequelae. NTM infection should be suspected in patients who have a history of trauma or repeated steroid injections if their symptoms do not improve with conventional treatment. Future research is crucial to defining optimal therapeutic strategies for NTM tenosynovitis. Further research is essential to define the optimal therapeutic strategy for nontuberculous mycobacteria tenosynovitis.
4. Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
CRediT authorship contribution statement
Majdouline El Moussaoui: Writing – original draft, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Nicolas Lambert: Writing – review & editing. Patrick Massage: Writing – review & editing. Cécile Meex: Writing – review & editing. Marie-Pierre Hayette: Writing – review & editing, Investigation. Philippe Delvenne: Writing – review & editing, Investigation. Charline Rinkin: Writing – review & editing, Investigation. Michel Moutschen: Writing – review & editing. Gilles Darcis: Writing – review & editing. Olivier Malaise: Writing – review & editing, Validation, Investigation, Data curation. Jean-Baptiste Giot: Writing – review & editing, Validation, Supervision, Investigation, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the patient for her collaboration and her consent to publish this case report. The figure was made with biorender.com.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jctube.2024.100479.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Tortoli E., Gitti Z., Klenk H.P., et al. Survey of 150 strains belonging to the Mycobacterium terrae complex and description of Mycobacterium engbaekii sp. nov., Mycobacterium heraklionense sp. nov. and Mycobacterium longobardum sp. nov. Int J Syst Evol Microbiol. 2013;63(Pt 2):401–411. doi: 10.1099/ijs.0.038737-0. [DOI] [PubMed] [Google Scholar]
- 2.Simons S., van Ingen J., Hsueh P.R., et al. Nontuberculous mycobacteria in respiratory tract infections, eastern Asia. Emerg Infect Dis. 2011;17(3):343–349. doi: 10.3201/eid1703.100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Honda J.R., Hasan N.A., Davidson R.M., Williams M.D., Epperson L.E., Reynolds P.R., et al. Environmental nontuberculous mycobacteria in the Hawaiian Islands. PLoS Negl Trop Dis. 2016;10:e0005068. doi: 10.1371/journal.pntd.0005068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prevots D.R., Marshall J.E., Wagner D., Morimoto K. Global epidemiology of nontuberculous mycobacterial pulmonary disease: a review. Clin Chest Med. 2023;44(4):675–721. doi: 10.1016/j.ccm.2023.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nohrenberg M., Wright A., Krause V. Non-tuberculous mycobacterial skin and soft tissue infections in the Northern Territory, Australia, 1989–2021. Int J Infect Dis. 2023;135:125–131. doi: 10.1016/j.ijid.2023.07.031. [DOI] [PubMed] [Google Scholar]
- 6.Mejia-Chew C., Chavez M.A., Lian M., et al. Spatial epidemiologic analysis and risk factors for nontuberculous mycobacteria infections, Missouri, USA, 2008–2019. Emerg Infect Dis. 2023;29(8):1540–1546. doi: 10.3201/eid2908.230378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan Y., Deng Y., Yan X., et al. Nontuberculous mycobacterial pulmonary disease and associated risk factors in China: a prospective surveillance study. J Infect. 2021;83(1):46–53. doi: 10.1016/j.jinf.2021.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Turner N.A., Sweeney M.I., Xet-Mull A.M., Storm J., Mithani S.K., Jones D.B., et al. A cluster of nontuberculous mycobacterial tenosynovitis following hurricane relief efforts. Clin Infect Dis. 2021 Jun 15;72(12):e931–e937. doi: 10.1093/cid/ciaa1665. [DOI] [PubMed] [Google Scholar]
- 9.Holden I.K., Kehrer M., Andersen A.B., Wejse C., Svensson E., Johansen I.S. Mycobacterium marinum infections in Denmark from 2004 to 2017: a retrospective study of incidence, patient characteristics, treatment regimens and outcome. Sci Rep. 2018;8(1):6738. doi: 10.1038/s41598-018-24702-7. Published 2018 Apr 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falkinham J.O., 3rd. Environmental sources of nontuberculous mycobacteria. Clin Chest Med. 2015;36(1):35–41. doi: 10.1016/j.ccm.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Buser G.L., Laidler M.R., Cassidy P.M., Moulton-Meissner H., Beldavs Z.G., Cieslak P.R. Outbreak of Nontuberculous Mycobacteria Joint Prosthesis Infections, Oregon, USA, 2010–2016. Emerg Infect Dis. 2019;25(5):849–855. doi: 10.3201/eid2505.181687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honda J.R., Alper S., Bai X., Chan E.D. Acquired and genetic host susceptibility factors and microbial pathogenic factors that predispose to nontuberculous mycobacterial infections. Curr Opin Immunol. 2018;54:66–73. doi: 10.1016/j.coi.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Szymanski E.P., Leung J.M., Fowler C.J., et al. Pulmonary nontuberculous mycobacterial infection. a multisystem, multigenic disease. Am J Respir Crit Care Med. 2015;192(5):618–628. doi: 10.1164/rccm.201502-0387OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song J.H., Kim B.S., Kwak N., Han K., Yim J.J. Impact of body mass index on development of nontuberculous mycobacterial pulmonary disease. Eur Respir J. 2021;57(2):2000454. doi: 10.1183/13993003.00454-2020. Published 2021 Feb 4. [DOI] [PubMed] [Google Scholar]
- 15.Pennington K.M., Vu A., Challener D., et al. Approach to the diagnosis and treatment of non-tuberculous mycobacterial disease. J Clin Tuberc Other Mycobact Dis. 2021;24 doi: 10.1016/j.jctube.2021.100244. Published 2021 May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Opperman CJ, Singh S, Goosen W, Cox H, Warren R, Esmail A. Incorporating direct molecular diagnostics in management algorithms for nontuberculous mycobacteria: is it high time?. IJID Reg. 2023;10:140-145. Published 2023 Dec 13. doi:10.1016/j.ijregi.2023.12.003. [DOI] [PMC free article] [PubMed]
- 17.Abedalthagafi M., Rosenberg O., Miller S. First report of tenosynovitis in an immunocompetent person caused by Mycobacterium heraklionense. JMM Case Rep. 2014;1:e002071. [Google Scholar]
- 18.Vasireddy R., Vasireddy S., Brown-Elliott B.A., et al. Correction for Vasireddy et al., Mycobacterium arupense, Mycobacterium heraklionense, and a Newly Proposed Species, “Mycobacterium virginiense” sp. nov., but Not Mycobacterium nonchromogenicum, as Species of the Mycobacterium terrae Complex Causing Tenosynovitis and Osteomyelitis. J Clin Microbiol. 2017;55(3):985. doi: 10.1128/JCM.02290-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutronc H., Sawaya E., Poursac N., Desclaux A., Ménard A., Peuchant O. Mycobacterium heraklionense as an emerging cause of tenosynovitis. J Microbiol Immunol Infect. 2023;56(1):197–199. doi: 10.1016/j.jmii.2022.08.019. [DOI] [PubMed] [Google Scholar]
- 20.Mason C., Wong D., Lefebvre R. Flexor tenosynovitis caused by mycobacterium heraklionense. J Hand Surg Glob Online. 2022;4(3):184–188. doi: 10.1016/j.jhsg.2021.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aburjania N., Hammert W.C., Bansal M., Boyce B.F., Munsiff S.S. Chronic tenosynovitis of the hand caused by Mycobacterium heraklionense. Int J Mycobacteriol. 2016;5(3):273–275. doi: 10.1016/j.ijmyco.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Greninger AL, Cunningham G, Chiu CY, Miller S. Draft Genome Sequence of Mycobacterium heraklionense Strain Davo. Genome Announc. 2015;3(4):e00807-15. Published 2015 Jul 23. doi:10.1128/genomeA.00807-15. [DOI] [PMC free article] [PubMed]
- 23.Bouchet F., Martin B., Aubry A., Veziris N., Lavigne J.P., Sotto A. Should single antibiotic therapy be avoided for nontuberculous mycobacteria? Med Mal Infect. 2017 Dec;47(8):566–568. doi: 10.1016/j.medmal.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Woods GL, Brown-Elliott BA, Conville PS, Desmond EP, Hall GS, Lin G, Pfyffer GE, Ridderhof JC, Siddiqi SH, Wallace RJ Jr, Warren NG, Witebsky FG. Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes [Internet]. 2nd ed. Wayne (PA): Clinical and Laboratory Standards Institute; 2011 Mar. Report No.: M24-A2. PMID: 31339680. [PubMed]
- 25.Neonakis I.K., Spandidos D.A., Gitti Z. Mycobacterium heraklionense sp. nov.: A case series. Exp Ther Med. 2015;10(4):1401–1403. doi: 10.3892/etm.2015.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makovcova J., Babak V., Slany M., Slana I. Comparison of methods for the isolation of mycobacteria from water treatment plant sludge. Antonie Van Leeuwenhoek. 2015;107(5):1165–1179. doi: 10.1007/s10482-015-0408-4. [DOI] [PubMed] [Google Scholar]
- 27.Solaghani T.H., Nazari R., Mosavari N., Tadayon K., Zolfaghari M.R. Isolation and identification of nontuberculous mycobacteria from raw milk and traditional cheese based on the 16S rRNA and hsp65 genes, Tehran. Iran Folia Microbiol (Praha) 2024;69(1):81–89. doi: 10.1007/s12223-023-01073-9. [DOI] [PubMed] [Google Scholar]
- 28.Brown-Elliott BA, Woods GL. Antimycobacterial Susceptibility Testing of Nontuberculous Mycobacteria. J Clin Microbiol. 2019;57(10):e00834-19. Published 2019 Sep 24. doi:10.1128/JCM.00834-19. [DOI] [PMC free article] [PubMed]
- 29.Kim D.H., Park J.Y., Won H.C., Park J.S. Nontuberculous mycobacterial tenosynovitis of the hand: a 10-year experience at two centers in South Korea. Clin Orthop Surg. 2023;15(3):477–487. doi: 10.4055/cios22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hogan J.I., Hurtado R.M., Nelson S.B. Mycobacterial musculoskeletal infections. Infect Dis Clin North Am. 2017;31(2):369–382. doi: 10.1016/j.idc.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Hussam T., Humza Y.S., Karim B., Aaron J.T. Two decades of insights into nontuberculous mycobacterial hand infections. Open forum. Infect Dis. 2024;ofae152 doi: 10.1093/ofid/ofae152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.