Abstract
The objective of the present study is to provide reliable concentration values as assigned values for target pesticides in brown rice samples used in proficiency testing (PT) organized by the Hatano Research Institute (HRI). The test samples for PT were prepared by immersing brown rice in the pesticide solution and using a spray dryer by the HRI. Homogeneity and stability assessments were performed for PT samples, and the relative uncertainties due to inhomogeneity and instability were 0.58 %–0.78 % and 0 %–0.96 %, respectively. Quantification for the assigned values of target pesticides by the National Metrology Institute of Japan (NMIJ) was carried out using the multiple analytical methods including Japanese official analytical method, QuEChERS, and modified QuEChERS, which were combined with isotope dilution mass spectrometry, to ensure the reliability of the analytical results. The NMIJ assigned values were 0.065±0.004 mg/kg for chlorpyrifos, 0.217±0.012 mg/kg for diazinon, 0.138±0.008 mg/kg for fenitrothion, and 0.138±0.008 mg/kg for malathion.
Keywords: isotope dilution mass spectrometry, QuEChERS method, modified QuEChERS method, pesticide residue, brown rice, proficiency testing
Introduction
It is important to analyze the pesticide residues in food to monitor the contamination and to investigate the relationship between exposure and health risks. The complex sample pretreatments and highly selective instrumental analyses involved in analyzing pesticide residues in food necessitate quality control to obtain accurate analytical results.1,2) The quality of the analytical results can be evaluated effectively using proficiency testing (PT), which is also useful for improving the measurement quality and resolving analytical problems.1–4) The International Standards Organization (ISO)/International Electrotechnical Commission (IEC) 17025: 20055) recommends PT for testing and calibration laboratories, and the Guidelines of Codex Alimentarius (CAC/GL–27)6) requires that laboratories involved in the import and export control of food participate in appropriate PT.
In quantitative PT schemes, the participant’s results are generally compared with the assigned values for the evaluation of the participant’s analytical skills. Various procedures are available for the establishment of assigned values for target compounds in the PT samples. According to ISO/IEC 17043,7) the most common procedures for determining the assigned values are a) formulation, b) a certified reference material (CRM), c) results from one laboratory, d) consensus value from expert laboratories, and e) consensus value from participant results. In many cases, consensus values are calculated by using participant results (approach e)).8) However, since this consensus value is influenced by the skills of other participants,9) in some cases, it is more important to provide the assigned value by other procedures such as a), b), c), and d). So far, the National Metrology Institute of Japan (NMIJ) has developed five types of CRMs for the quantification of pesticide residues in food.2) Therefore, the assigned values for PT samples can be provided by using the same procedure as that for determining the certified reference values of CRMs. NMIJ analyzed pesticide residues in samples using isotope dilution mass spectrometry (IDMS) to provide highly reliable values for the pesticide residues contained in samples, which is a potential primary measurement method.10–13) This is useful for PT participants because it enables comparison with a reliable assigned value.
The objective of the present study was to provide reliable concentration values as assigned values for target pesticides (chlorpyrifos, diazinon, fenitrothion, and malathion; the combination of target pesticides was different from PT provided by NMIJ2)) in samples of brown rice used in PT organized by Hatano Research Institute (HRI), which can be used to evaluate the trueness of the participants’ analytical methods. The test samples for PT were prepared by immersing brown rice in the pesticide solution and using a spray dryer by HRI as described later. The assigned values were determined from the analytical results obtained by three independent procedures: the Japanese official multiresidue method (Multiresidue), Quick, Easy, Cheap, Effective, Rugged, and Safe (QuEChERS) method, and solid-phase extraction (SPE) technique with the QuEChERS method (known as “STQ” in Japan; hereafter referred to as “modified QuEChERS (STQ)”), to ensure reliability. To the best of our knowledge, this is the first demonstration for the determination of assigned values by these three methods. Furthermore, the NMIJ assigned values were compared with the results of the PT participants.
Materials and methods
1. Preparation and distribution of test samples for PT
The test samples for PT were prepared by HRI. The pesticide solution for producing test samples was prepared by dissolving in acetonitrile/water (1 : 4, v/v) four target pesticide reagents: chlorpyrifos, diazinon, fenitrothion, and malathion (for pesticide residue analysis grade, Dr. Ehrenstorfer GmbH, Augsburg, Germany), with respective concentrations of 0.025 µg/L, 0.1 µg/L, 0.05 µg/L, and 0.05 µg/L. Brown rice of 1,000 g, which was commercially available and pulverized by HRI (average particle size: about 220 µm), was immersed in the prepared pesticide solution (4,000 mL) as described above. After suspension, the brown rice sample was dried by a spray dryer (CL-8i, Ohkawara Kakohki, Kanagawa, Japan) operated as follows: inlet temperature of 100 °C; MC-50 atomizer disk; and rotation speed of 20,000 rpm. Obtained samples were homogenized by a rocking mixer (RM-10-3, Aichi Electric Co., Aichi, Japan), and then placed in a laminated film zipper bag (AL-9, Seisannipponsha, Tokyo, Japan).
A total of 22 Japanese testing laboratories, including public research organizations, food manufacturers, and others, have participated in the PT. Two test samples were sent by refrigerated car to each participant on February 16, 2024. The number of target pesticides for the analysis could be selected by participants; that is, even one target pesticide was allowable. The use of any kind of analytical methods were permitted by participants. The deadline of the report to the HRI by electronic submission was March 25, 2024.
2. Chemicals used at NMIJ
Acetonitrile, acetone, toluene, anhydrous sodium sulfate (for pesticide residue and PCB analysis grade), anhydrous magnesium sulfate, sodium chloride, trisodium citrate dihydrate (reagent grade), and disodium hydrogen citrate 1.5-hydrate (extra pure grade) were purchased from Kanto Chemical Co. (Tokyo, Japan). Phosphate buffer solution (pH 7.0) was prepared from dipotassium hydrogen phosphate (reagent grade; Kanto Chemical), potassium dihydrogen phosphate (reagent grade; Fujifilm Wako Pure Chemical Corporation, Osaka, Japan), and purified water (Puric α, UP-0090α-TU1, Organo Corp., Tokyo, Japan). Purified water (Organo) was also used for the water-soaking process. Primary–secondary amine (PSA) and C18 for QuEChERS were purchased from Agilent Technologies (Santa Clara, CA).
3. Preparation of surrogate and syringe spike solutions
The surrogate solutions were gravimetrically prepared by dissolving in acetone four isotope-labeled pesticides: chlorpyrifos-d10, fenitrothion-d6 (Hayashi Pure Chemical, Osaka, Japan), diazinon-d10, and malathion-d6 (Toronto Research Chemicals, Ontario, Canada), which concentrations were 912 ng/g, 2324 ng/g, 3891 ng/g, and 2261 ng/g, respectively. The syringe spike solution was gravimetrically prepared by dissolving in acetone 2-chloro-2′,6′-diethyl-N-(methoxymethyl) acetanilide (alachlor; GL Sciences, Tokyo, Japan), which concentration was 574 ng/g.
4. Preparation of calibration solutions
The calibration solutions were prepared by gravimetric mixing as follows: the pesticide solutions were prepared by mixing the individual pesticide reagents with acetone, followed by a combination of the solutions. Diazinon, fenitrothion, malathion (TraceSure grade; Fujifilm Wako Pure Chemical), and chlorpyrifos (Traceable Reference Material grade; Fujifilm Wako Pure Chemical) were used. The calibration solutions for the quantification of pesticides in brown rice samples were gravimetrically prepared by mixing this mixed pesticide solution with surrogate and syringe spike solutions. Furthermore, the matrix-matched calibration solutions were prepared by mixing the final mixed solution with cleaned-up extracts of blank brown rice (confirmed to have no detectable target pesticides). These solutions were prepared in a way to match as closely as possible with the final concentration of each pesticide in the cleaned-up extracts of the brown rice samples.
5. Analytical method for obtaining NMIJ reference values
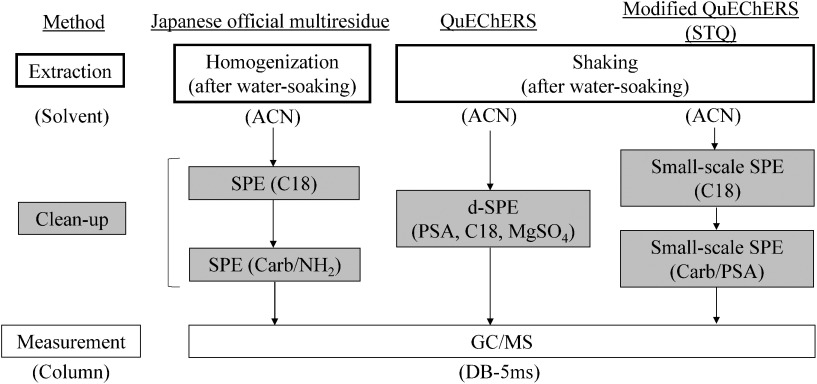
The analyses for obtaining the assigned values were carried out by NMIJ, and the scheme is shown in Fig. 1.
Fig. 1. Analytical scheme for the determination of NMIJ assigned values. QuEChERS, Quick, Easy, Cheap, Effective, Rugged, and Safe method; STQ, SPE technique with QuEChERS; ACN, acetonitrile; SPE, solid-phase extraction; d-SPE, dispersive SPE; Carb/NH2, graphite carbon/aminopropyl silanized silica gel; Carb/PSA, graphite carbon/primary–secondary amine.
5.1. Multiresidue with IDMS method
Analyses were carried out based on a modified Multiresidue method,1,14,15) which was also used for the development of NMIJ CRMs.15–17) Surrogate solution (0.4 mL) and purified water (10 mL) were added to 3 g of brown rice samples that had been weighed in a glass vial. After 15 min, the samples were homogenized (Polytron PT 1200 E (drive unit) with PT-DA 12/2EC-E157 (dispersing aggregates); Kinematica, Lucerne, Switzerland) for 2 min for extraction in acetonitrile and filtered with a cellulose filter (diameter: 60 mm; No. 5A, Kiriyama Glass Works, Tokyo, Japan). This crude extract was shaken with sodium chloride (10 g) and 0.5 mol/L phosphate buffer solution (pH 7.0, 20 mL) in a separatory funnel for 10 min. The acetonitrile layer was passed through a SPE cartridge (octadecylsilanized silica gel (1 g); Bond Elut MEGA BE-C18 1GM, Agilent Technologies; conditioned with 10 mL of acetonitrile). After dehydration by anhydrous sodium sulfate, the acetonitrile layer was concentrated and dried by a rotary evaporator, after which 2.0 mL of toluene/acetonitrile (1 : 3, v/v) was added. The crude extract was cleaned up with a SPE cartridge (500 mg/500 mg; ENVI-Carb/LC-NH2, Supelco Inc., Division of Sigma-Aldrich, St. Louis, MO; conditioned with 10 mL of toluene/acetonitrile (1 : 3, v/v)). Pesticides were eluted with toluene/acetonitrile (1 : 3, v/v; 20 mL) followed by concentration and drying processes using a rotary evaporator and nitrogen gas stream. Then, the syringe spike solution (0.5 mL) was added to this cleaned-up extract. The cleaned-up samples were measured by using a gas chromatograph with a mass spectrometer (GC/MS; Agilent 7890A GC equipped with a DB-5ms column (30 m×0.25 mm i.d., 0.25 µm film thickness) and an Agilent 5975C mass selective detector (MSD)). GC/MS measurement was performed by using the splitless injection mode, and the injection volume was 1.0 µL. Helium was used as the carrier gas (1.0 mL/min) and the injector temperature was 220 °C. The GC oven was programmed to remain at 50 °C for the initial 1 min, then increase to 125 °C at 25 °C/min, further increase to 300 °C at 10 °C/min, and then hold for 6.5 min. A quantitative analysis was conducted in SIM mode and the monitoring ions for quantification were as follows: chlorpyrifos, 314; chlorpyrifos-d10, 324; diazinon, 304; diazinon-d10, 314; fenitrothion, 277; fenitrothion-d6, 283; malathion, 285; malathion-d6, 291; and alachlor, 160.
Pesticides were quantified with IDMS and a matrix-matched calibration solution.1) Each sample was measured twice. NMIJ assigned values are given as the mean values of the results. The uncertainties of the NMIJ assigned values were estimated from the standard uncertainties due to characterization (u(char)), instability (u(stab)), and inhomogeneity of the material (u(hom)) as described in previous papers.1,15)
5.2. QuEChERS with IDMS method
Basically, the extraction and clean-up processes were performed according to the CEN Standard Method EN 1566218) and AOAC Official Method 2007.01,19) with some modifications. The surrogate solution (0.4 mL) and purified water (10 mL) were added to a weighed brown rice sample (5 g), which after 15 min was shaken by hand for 1 min with acetonitrile (10 mL). Trisodium citrate dihydrate (1 g), disodium hydrogen citrate 1.5-hydrate (0.5 g), sodium chloride (1 g), and anhydrous magnesium sulfate (4 g) were added to this crude extract, and the sample was shaken by hand for 1 min followed by centrifugation at 3500 rpm for 5 min. The acetonitrile layer (1 mL) was recovered and cleaned up by dispersive SPE (d-SPE) and shaking by hand (1 min) using 50 mg of PSA, 50 mg of C18, and 150 mg of anhydrous magnesium sulfate. The cleaned-up sample was centrifuged at 3500 rpm for 5 min, and the supernatant was recovered followed by a drying process with a nitrogen gas stream. Then, the syringe spike solution (0.08 mL) was added to the cleaned-up extract. This sample was measured using the GC/MS as described above.
5.3. Modified QuEChERS (STQ) with IDMS method
We modified the method reported in a previous study.20) Brown rice samples (5 g), weighed in a polypropylene centrifuge tube (50 mL; As One, Osaka, Japan), were spiked with the surrogate solutions (0.4 mL), after which purified water (10 mL) was added. After 15 min, extraction was performed according to the method described in the QuEChERS section. The acetonitrile layer (1 mL) was passed through a small-scale SPE (octadecylsilanized silica gel; Smart-SPE C18, 50 mg; AiSTI Science, Wakayama, Japan; conditioned with 2 mL of acetone and 2 mL of acetonitrile). Pesticides were eluted with 0.2 mL of acetonitrile, after which 0.4 mL of toluene was added to the obtained sample. This extract was cleaned up by a small-scale SPE, i.e., graphite carbon and primary–secondary amine silica gel cartridge (Smart-SPE GCS, 20 mg and PSA, 30 mg; AiSTI Science; conditioned with 2 mL of acetone and 2 mL of toluene/acetonitrile (1 : 3, v/v)). Pesticides were eluted with toluene/acetonitrile (1 : 3, v/v; 0.6 mL) followed by a drying process using a nitrogen gas stream. The syringe spike solution (0.05 mL) was then added to the cleaned-up extract. This sample was measured using the GC/MS as described above.
6. Validation of the methods by spiking test
All analytical methods were validated by spiking tests, and then used for determining the assigned values. To validate the methods, a mixed solution containing the target pesticides was spiked to a brown rice sample (confirmed to have no detectable target pesticides) so as to achieve similar concentrations as in a PT test sample (n=3). After 30 min,21) the target pesticides were analyzed by the three analytical methods described in Fig. 1.
7. Homogeneity and stability assessment
The between-bottle homogeneity of the test sample was evaluated in accordance with ISO Guide 35: 200622) in ten bottles randomly selected from the total set of 117 bottles, and two subsamples were taken to quantify the target pesticides (n=20). The stability of the target pesticides was assessed before and after the analytical period of PT by the participants (n=4 in each assessment; 76 days study) using bottles stored at a temperature between −20 °C and −30 °C in the dark. A modified Multiresidue method was used for these assessments as described above.
8. Consensus value calculation
Cochran and Grubbs tests were used to detect outliers. The Grubbs test returned two outliers in the diazinon and fenitrothion data and one outlier in the malathion data. The remaining participant data were used to estimate the consensus values of pesticides in the brown rice samples. The analytical results submitted by the participants could be assumed to be nearly normally distributed and the consensus values were taken as the median.23) The normalized interquartile range (NIQR) given by 0.7413×(quartile 3–quartile 1)24) was also calculated.
Results and discussion
1. Analytical methods used for determination of NMIJ assigned values
Different extraction and clean-up procedures were used for the determination of assigned values to avoid any bias associated with a certain analytical method. Analytical methods used for determination are shown in Fig. 1, including two simple methods, QuEChERS and modified QuEChERS (STQ). The QuEChERS method, which was developed in 2003,25) has been widely applied to various compounds. This is a simpler method26) that involves extraction/partitioning and clean-up with d-SPE.27) In addition, the modified QuEChERS (STQ) method has seen an increase in the number of users in Japan because it uses a smaller volume of solvent, the concentration process by an evaporator is not necessary, and quick analysis is possible.1,9,28) Therefore, we included these two simple methods to determine the NMIJ assigned values. To do so, it was necessary to validate the analytical methods.
The results of the validation of three analytical methods by the spiking test are shown in Table 1, described as percentages by the quantification results of IDMS (unit of mass) relative to the spiked amount of pesticides (unit of mass). Observed values by IDMS were nearly 100 % as the mean value for each pesticide, and the repeatability of the analysis, represented as standard deviations (SDs), was satisfactory.21) Thus, these results indicate that the analytical methods in Fig. 1 could be applied for the determination of assigned values. For pesticide analysis, it is suggested that the occurrence of matrix effects has a major impact on the quantitative value. Matrix effects can cause enhancement or suppression in observed chromatographic response for pesticide residues in a matrix extract compared with the same concentration in a matrix-free solution.29) In fact, a matrix effect was especially observed for fenitrothion, as shown in Table 1. It is suggested that the use of a matrix-matched standard for the calibration is effective for canceling out the matrix effects29); thus, this technique was applied for the determination of NMIJ assigned values.
Table 1. Results for the evaluation of accuracy by spike test.
| Pesticides | Japanese official multiresidue | QuEChERS | Modified QuEChERS (STQ) | |||
|---|---|---|---|---|---|---|
| MM (%) | not MM (%) | MM (%) | not MM (%) | MM (%) | not MM (%) | |
| Chlorpyrifos | 101.7±0.1 | 103.6±0.1 | 98.8±0.4 | 103.4±0.4 | 99.4±0.5 | 97.4±0.5 |
| Diazinon | 100.2±0.4 | 98.7±0.4 | 99.9±0.3 | 97.8±0.3 | 97.6±0.6 | 93.3±0.5 |
| Fenitrothion | 96.6±0.7 | 88.7±0.7 | 98.7±0.3 | 87.2±0.3 | 98.0±0.2 | 77.4±0.1 |
| Malathion | 103.3±0.7 | 98.2±0.6 | 100.5±1.4 | 102.5±1.4 | 101.1±2.9 | 93.8±2.7 |
The values represent the mean±standard deviation; the values are described as percentages based on the quantification results of IDMS relative to the spiked amount of pesticides; MM: matrix-matching calibration was used; not MM: matrix-matching calibration was not used; n=3.
2. NMIJ assigned values
The concentrations of the target pesticides were calculated using Eq. (1).30)
| (1) |
where C is the concentration of the analyte in the sample; Fext is a factor related to the extraction and clean-up step; Rsample, Rblank, and Rcal are the analyte/internal standard peak area ratios observed for the sample, blank, and calibration solutions, respectively; Mcal is the mass of the standard solution of analytes used for preparing the calibration solution; Ccal is the concentration of analyte in the calibration solution; and Mspike(sample), Msample, and Mspike(cal) are the mass of the internal standard solution added to the sample, the mass of the sample taken for analysis, and the mass of the internal standard solution used for preparing the calibration solution, respectively. The analytical results for the determination of the assigned values obtained by the respective methods (Fig. 1) are summarized in Table 2. The concentrations obtained by each method were in good agreement with each other.
Table 2. Analytical results for target pesticides in PT sample.
| Pesticides | Japanese official multiresidue (mg/kg) | QuEChERS (mg/kg) | Modified QuEChERS (STQ) (mg/kg) |
|---|---|---|---|
| Chlorpyrifos | 0.0638±0.0015 | 0.0662±0.0014 | 0.0652±0.0002 |
| Diazinon | 0.217±0.005 | 0.216±0.001 | 0.217±0.001 |
| Fenitrothion | 0.139±0.004 | 0.138±0.003 | 0.137±0.001 |
| Malathion | 0.136±0.003 | 0.138±0.002 | 0.140±0.001 |
The values represent the mean concentrations±standard deviations; n=4.
The assigned values were determined by using the weighted means of the analytical results obtained by the three methods for each pesticide, where 1/ui (ui: uncertainty of the result obtained by each method) was used as the weight, and these are shown in Table 3. The uncertainties of the certified values were calculated from uncertainties due to the respective factors, and these are also shown in Table 3. The uncertainty budget is summarized in Table 4. ISO Guide 3522) specifies that uncertainty is estimated from standard uncertainty due to characterization, u(char); standard uncertainty due to instability, u(stab); and inhomogeneity of the material, u(hom). The u(char) was estimated from u(Cind), u(Ccom), and u(Cbm).17) The u(Cind) associated with each analytical method was obtained from the uncertainty of Rsample, Rblank, Rcal, Fext, Msample, and Mspike(sample) of Eq. (1). The u(Ccom) that is common to analytical methods was estimated from the uncertainty of Mcal, Ccal, and Mspike(cal) (the uncertainty of Ccal was obtained by combining the uncertainty for the purity of neat pesticides and for weighing) of Eq. (1). The uncertainty for the between-method variance (u(Cbm)) was calculated by performing an analysis of variance (ANOVA) on the results obtained from the analytical methods in Fig. 1. The u(stab) was included for the uncertainties by using the results of the stability assessment before and after the analytical period of PT by the participants. A one-way ANOVA test revealed that the difference in the results of the stability assessment was not significant. The uncertainties of instability (u(stab)) calculated according to previous studies1,15) were 0 %, 0 %, 0 %, and 0.96 % for chlorpyrifos, diazinon, fenitrothion, and malathion, respectively. These values contributed to the combined uncertainty of the NMIJ assigned values. The u(hom) derived from the inhomogeneity of the material was estimated in the homogeneity assessment as described above. The target pesticide concentrations in the brown rice sample of the 10 randomly selected bottles did not show any statistically significant differences. The relative uncertainties due to inhomogeneity (u(hom)) for each pesticide were calculated according to ISO Guide 3522) and previous studies.1,15) The results were 0.58 %, 0.78 %, 0.71 %, and 0.75 % for chlorpyrifos, diazinon, fenitrothion, and malathion, respectively. The expanded uncertainty U of the certified value is equal to kuc, where uc is the combined standard uncertainty with a coverage factor of k=2, corresponding to a 95 % confidence interval. These expanded uncertainties were comparable with those of the past PT organized by NMIJ.9)
Table 3. NMIJ assigned values and expanded uncertainties for the pesticides in PT samples.
| Pesticides | NMIJ assigned value (mg/kg) | Expanded uncertainty (mg/kg) |
|---|---|---|
| Chlorpyrifos | 0.065 | 0.004 |
| Diazinon | 0.217 | 0.012 |
| Fenitrothion | 0.138 | 0.008 |
| Malathion | 0.138 | 0.008 |
The expanded uncertainty was determined by using a coverage factor (k=2), corresponding to a 95 % confidence interval.
Table 4. Uncertainty budget for the assigned values of target pesticides in PT samples.
| Uncertainty component | Values | |||
|---|---|---|---|---|
| Chlorpyrifos | Diazinon | Fenitrothion | Malathion | |
| Relative standard uncertainty (%) | ||||
| u(char): Combined u(Cind), u(Ccom), and u(Cbm) as calculated below | 3.01 | 2.71 | 2.74 | 2.71 |
| u(Cind) | 2.34 | 2.35 | 2.49 | 2.32 |
| u(Ccom) | 1.39 | 1.35 | 1.13 | 1.16 |
| u(Cbm) | 1.28 | 0 | 0 | 0.78 |
| u(stab) | 0 | 0 | 0 | 0.96 |
| u(hom) | 0.58 | 0.78 | 0.71 | 0.75 |
| Combined uncertainty, uc | ||||
| Relative standard uncertainty (%) | 3.06 | 2.81 | 2.82 | 2.97 |
| × certified value (mg/kg) | 0.002 | 0.006 | 0.004 | 0.004 |
| Expanded uncertainty, U (k=2) (mg/kg) | 0.004 | 0.012 | 0.008 | 0.008 |
u(char) was estimated from u(Cind), u(Ccom), and u(Cbm); uc was estimated from u(char), u(stab), and u(hom).
3. Comparison of NMIJ assigned and consensus values
NMIJ assigned values and consensus values calculated from participant results are compared in Table 5. In the past PT organized by NMIJ, the assigned values for most pesticides were up to approximately 30% greater than the corresponding consensus values from the participant results due to the different quantification method used: NMIJ used the IDMS method and most participants used an external standard method.2) The recovery yields of the target compounds will influence the results for an external standard method if not adequately corrected for, which is distinct from the IDMS method.9) Although most participants (about 70 %) used an external standard method in the present PT as well, the NMIJ assigned values and consensus values were comparable. An influence due to the difference in the test sample preparation method, i.e., by using the native-pesticide-contained material (such as in the past PT by NMIJ9)) or by adsorbing the target pesticides on blank brown rice (such as in the present PT), was considered since the extraction efficiency of target pesticides from the brown rice sample may change. However, this is not the reason; in a previous study,8) NMIJ assigned values were higher than those of the consensus values from participant results in the PT using test samples prepared by spiking (adsorbing) pesticides on the blank material. Many participants that obtained higher recovery yields may simply have participated in the present PT, but this is not clear because the investigation was not conducted for recovery yields. Multiple factors, such as matrix effects, can also be considered as reasons for this result.
Table 5. Comparison of assigned and consensus values.
| Pesticides | NMIJ assigned value (mg/kg)a) | Expanded uncertainty (mg/kg)a) | Consensus value (mg/kg)b) | NIQR (mg/kg)c) |
|---|---|---|---|---|
| Chlorpyrifos | 0.065 | 0.004 | 0.067 | 0.007 |
| Diazinon | 0.217 | 0.012 | 0.209 | 0.021 |
| Fenitrothion | 0.138 | 0.008 | 0.140 | 0.016 |
| Malathion | 0.138 | 0.008 | 0.141 | 0.021 |
a) The assigned values and expanded uncertainties were obtained by NMIJ. b) The consensus values were calculated from the analytical results reported by participants. c) NIQR=normalized interquartile range.
Conclusions
Reliable concentration values were provided as assigned values for target pesticides in brown rice samples used in PT organized by HRI. These values were obtained by three independent procedures: Multiresidue, QuEChERS, and modified QuEChERS (STQ) methods combined with IDMS, which were successfully validated by spiking tests. NMIJ assigned values can be used to evaluate the trueness of the participants’ analytical methods.
Acknowledgements
This work was supported by the program Research on Food Safety of the Ministry of Health, Labour and Welfare (Grant Number 23KA1002).
References
- 1) T. Otake, T. Yarita, Y. Aoyagi, M. Numata and A. Takatsu: Evaluation of the performance of 57 Japanese participating laboratories by two types of z-scores in proficiency test for the quantification of pesticide residues in brown rice. Anal. Bioanal. Chem. 406, 7337–7344 (2014). [DOI] [PubMed] [Google Scholar]
- 2) T. Otake and T. Yarita: Internationally harmonized certified reference materials and proficiency testings for pesticide residue analysis. J. Pestic. Sci. 46, 297–303 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3) P. Araujo and L. Froyland: Hierarchical classification designs for the estimation of different sources of variability in proficiency testing experiments. Anal. Chim. Acta 555, 348–353 (2006). [Google Scholar]
- 4) Y. Zhu, T. Kuroiwa, T. Narukawa, K. Inagaki and K. Chiba: Proficiency test in Japan for the elements in tea-leaf powder. Trends Analyt. Chem. 34, 152–160 (2012). [Google Scholar]
- 5) International Organization for Standardization: “ISO/IEC 17025: General requirements for the competence of testing and calibration laboratories,” ISO, Geneva, Switzerland, 2017.
- 6) Codex Alimentarius Commission: “Guidelines for the assessment of the competence of testing laboratories involved in the import and export control of food CAC/GL 27-1997,” Rome, Italy, 1997.
- 7) International Organization for Standardization: “ISO/IEC 17043: Conformity assessment—General requirements for the competence of proficiency testing providers,” ISO, Geneva, Switzerland, 2023.
- 8) T. Yarita, T. Otake, Y. Aoyagi, N. Takasaka, T. Suzuki and T. Watanabe: Comparison of assigned values from participants’ results, spiked concentrations of test samples, and isotope dilution mass spectrometric results in proficiency testing for pesticide residue analysis. J. AOAC Int. 101, 1199–1204 (2018). [DOI] [PubMed] [Google Scholar]
- 9) T. Otake, T. Yarita, Y. Aoyagi, N. Hanari and A. Takatsu: Proficiency testing by the National Metrology Institute of Japan for quantification of pesticide residues in grain samples from 2012 to 2018. J. Pestic. Sci. 44, 192–199 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10) P. De Bièvre and H. S. Peiser: Basic equations and uncertainties in isotope-dilution mass spectrometry for traceability to SI of values obtained by this primary method. Fresenius J. Anal. Chem. 359, 523–525 (1997). [Google Scholar]
- 11) T. J. Quinn: Primary methods of measurement and primary standards. Metrologia 34, 61–65 (1997). [Google Scholar]
- 12) W. Richter: Primary methods of measurement in chemical analysis. Accredit. Qual. Assur. 2, 354–359 (1997). [Google Scholar]
- 13) M. J. T. Milton and T. J. Quinn: Primary methods for the measurement of amount of substance. Metrologia 38, 289–296 (2001). [Google Scholar]
- 14) http://www.mhlw.go.jp/english/topics/foodsafety/positivelist060228/dl/060526-1a.pdf (Accessed 15 May, 2024).
- 15) T. Otake, N. Itoh, Y. Aoyagi, M. Matsuo, N. Hanari, S. Otsuka and T. Yarita: Development of certified reference material for quantification of two pesticides in brown rice. J. Agric. Food Chem. 57, 8208–8212 (2009). [DOI] [PubMed] [Google Scholar]
- 16) T. Otake, T. Yarita, Y. Aoyagi, Y. Kuroda, M. Numata, H. Iwata, K. Mizukoshi, M. Nakamura, M. Watai, H. Mitsuda, T. Fujikawa and H. Ota: Development of green onion and cabbage certified reference materials for quantification of organophosphorus and pyrethroid pesticides. J. Agric. Food Chem. 59, 8568–8574 (2011). [DOI] [PubMed] [Google Scholar]
- 17) T. Otake, T. Yarita, Y. Aoyagi, Y. Kuroda, M. Numata, H. Iwata, M. Watai, H. Mitsuda, T. Fujikawa and H. Ota: Development of apple certified reference material for quantification of organophosphorus and pyrethroid pesticides. Food Chem. 138, 1243–1249 (2013). [DOI] [PubMed] [Google Scholar]
- 18) European Committee for Standardization: “European Standard EN 15662: 2008, Foods of plant origin - Determination of pesticide residues using GC-MS and/or LC-MS/MS following acetonitrile extraction/partitioning and clean-up by dispersive SPE-QuEChERS-method,” European Committee for Standardization, Brussels, 2008.
- 19) https://nucleus.iaea.org/sites/fcris/Shared%20Documents/SOP/AOAC_2007_01.pdf (Accessed 15 May, 2024).
- 20) S. Hiramatsu, K. Nishiyama, S. Tokuhashi, T. Ashida, A. Kageyama, M. Takamiya, A. Nakamura, N. Takuma and K. Nishimori: Survey of pesticide residues in agricultural products from April 2010 to March 2013. Rep. Pub. Hlth. Kochi. 59, 47–52 (2013), in Japanese. [Google Scholar]
- 21) https://www.mhlw.go.jp/topics/bukyoku/iyaku/syoku-anzen/zanryu3/dl/101224-1.pdf (Accessed 15 May, 2024).
- 22) International Organization for Standardization: “Guide 35: Reference materials—General and statistical principles for certification (3rd ed.)” ISO, Geneva, Switzerland, 2006.
- 23) M. Thompson, S. L. R. Ellison and R. Wood: The International Harmonized Protocol for the proficiency testing of analytical chemistry laboratories (IUPAC Technical Report). Pure Appl. Chem. 78, 145–196 (2006). [Google Scholar]
- 24) K. Judprasong, P. Puwastien, J. Boonpor and N. Pinprapai: Laboratory performance on analysis of mandatory nutrients and preparation of nutrition labelling. Food Chem. 140, 598–607 (2013). [DOI] [PubMed] [Google Scholar]
- 25) M. Anastassiades, S. J. Lehotay, D. Stajnbaher and F. J. Schenck: Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 86, 412–431 (2003). [PubMed] [Google Scholar]
- 26) S. J. Lehotay, K. Maštovská and R. Lightfield: Use of buffering and other means to improve results of problematic pesticides in a fast and easy method for residue analysis of fruits and vegetables. J. AOAC Int. 88, 615–629 (2005). [PubMed] [Google Scholar]
- 27) A. Suganthi, K. Bhuvaneswari and M. Ramya: Determination of neonicotinoid insecticide residues in sugarcane juice using LCMSMS. Food Chem. 241, 275–280 (2018). [DOI] [PubMed] [Google Scholar]
- 28) T. Otake, T. Yarita, T. Sakamoto, M. Numata and A. Takatsu: Proficiency testing for quantification of pesticide residues in treated brown rice samples: Comparison of performance of Japanese official multiresidue, modified QuEChERS, and QuEChERS methods. J. AOAC Int. 99, 821–829 (2016). [DOI] [PubMed] [Google Scholar]
- 29) C. F. Poole: Matrix-induced response enhancement in pesticide residue analysis by gas chromatography. J. Chromatogr. A 1158, 241–250 (2007). [DOI] [PubMed] [Google Scholar]
- 30) T. Otake, Y. Aoyagi, T. Yarita and M. Numata: Characterization of certified reference material for quantification of polychlorinated biphenyls and organochlorine pesticides in fish. Anal. Bioanal. Chem. 397, 2569–2577 (2010). [DOI] [PubMed] [Google Scholar]