Abstract
Background
Tigecycline is considered one of the last resorts for treating infections caused by multidrug-resistant bacteria. Continuous renal replacement therapy (CRRT) is widely used in critically ill patients, especially those with acute kidney injury or severe infections. However, pharmacokinetic data for tigecycline in patients receiving CRRT are limited.
Methods
This was a single-center prospective clinical study with intensive sampling that included critically ill patients who received tigecycline and CRRT. A population pharmacokinetic (PPK) model was developed and evaluated by goodness-of-fit plots, bootstrap analysis, visual predictive checks, and numerical predictive checks. Pharmacokinetic/pharmacodynamic target attainment and cumulative fraction of response analyses were performed to explore the potential need for dose adjustments of tigecycline in CRRT.
Results
In total, 21 patients with 167 concentrations were included. A two-compartment model adequately described the tigecycline concentration–time points, but no covariates were found to adequately explain the viability in the pharmacokinetic parameters of tigecycline. The typical values of CL, Q, V1 and V2 were 4.42 L/h, 34.8 L/h, 30.9 L and 98.7 L, respectively. For most infections, the standard regimen of 50 mg/12 h was deemed appropriate, expect for skin and soft skin tissue infections and community-acquired pneumonia caused by Acinetobacter baumannii and Klebsiella pneumoniae, which required a dosage regimen of 100 mg/12 h or higher.
Conclusion
A tigecycline PPK model describing critically ill patients undergoing CRRT was successfully developed. The optimized dosage regimens for various infections are recommended.
Keywords: tigecycline, population pharmacokinetics, critically ill, continuous renal replacement therapy
Introduction
Tigecycline is a glycylcycline antimicrobial drug that is approved by the FDA for treating complicated intra-abdominal infections (cIAIs), skin and soft skin tissue infections (cSSSIs) and community-acquired pneumonia (CAP).1,2 Tigecycline exhibits broad-spectrum antimicrobial activity and demonstrates potent in vitro efficacy against multidrug-resistant (MDR) bacteria, including extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae, methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococci, and multidrug-resistant Acinetobacter baumannii.1 Thus, tigecycline is an alternative agent for difficult-to-treat infections in intensive care units (ICUs), especially for MDR bacterial infections that resistant to other antibiotics.3
Continuous renal replacement therapy (CRRT), as one of the three supportive techniques in critical care, is extensively utilized for the management of critically ill patients, especially those with severe acute kidney injury (AKI), hemodynamic instability or the need to remove inflammatory mediators.4,5 The use of CRRT may lead to increased antibiotic clearance and reduced exposure.6–8 Accordingly, a higher antibiotic dosage may be necessary in patients during CRRT.9
Although several small-sample studies have indicated that dose adjustment of tigecycline seems unnecessary during CRRT, there are still experts who believe that it is too early to draw this conclusion.10–13 Hence, in this prospective study, we aimed to develop a PPK model of tigecycline in critically ill patients undergoing CRRT, identify the factors influencing its pharmacokinetics, and utilize the final model to optimized tigecycline dosage regimens for different bacterial infections.
Methods
Study Design and Patient Inclusion
The detailed study protocol was published in our previous study, which complied with the Declaration of Helsinki.14 Briefly, we included critically ill patients who were treated with intermittent intravenous tigecycline and CRRT from July 2019 to July 2023. The patients’ physio-pathological data, such as age, sex, weight (WT), body mass index (BMI), infection site, pathogen status, 24-hour urine volume (UV), CRRT information, plasma tigecycline concentration, and other related laboratory test results, were collected. The exclusion criteria were as follows: (1) were < 18 years old, (2) lacked essential data, and (3) did not receive CRRT continuously during tigecycline treatment. All participants signed the informed consent form.
PPK Model Development
PPK modeling was performed with NONMEM 7.5.0 and PDx-Pop 5.3.1, and the results were visualized by the R program. The one- and two-compartment models with first-order eliminations were fitted to the data. The residual variability was modeled with additive, proportional, and combined error models, and the interindividual variability was modeled exponentially. The significant covariates were screened in a stepwise regression with forward inclusion (ΔOFV>3.84, p <0.05) and backward elimination (ΔOFV>10.83, p<0.001). The final model and parameter estimates were evaluated by goodness-of-fit plots, bootstrap analysis, visual predictive checks (VPCs) and numerical predictive checks (NPCs). For bootstrap analysis, a total of 1000 resampling iterations with replacement were conducted on the original dataset to create a resampled dataset to assess the uncertainty and variability associated with the estimated statistical parameters. The resulting PK parameter estimates were then compared with the original PK estimates derived from the final model. For VPCs, 1000 simulation replications were conducted to evaluate the predictive performance and adequacy of the model by comparing the observed data with simulated predictions as well as examining the quantiles of the simulated data (eg, 5th, 50th, and 95th percentiles). Complementing VPCs, NPCs were conducted to provide a quantitative assessment of the model’s predictive accuracy. More detailed modeling information can be found in our previous article.14
Monte Carlo Simulation and Dose Regimen Evaluation
The probability of target attainment (PTA) and cumulative fraction of response (CFR) analysis were performed using Monte Carlo simulation (1000 virtual patients per group). Three dose regimens were used for simulation: 50 mg, 100 mg and 150 mg every 12 hours. The PTAs corresponding to each MIC value were derived based on the PK/PD targets for different types of infections (AUC/MIC ≥17.9 for cSSSI, ≥12.8 for CAP, and ≥6.96 for cIAI).15–18 The CFRs, defined as the expected population PTA for a dose regimen against the whole population of microorganisms, were calculated according to the following equation:19,20
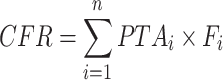 |
where PTAi is the probability of the target attainment value for the ith MIC level (n levels in total) and Fi is the percentage of bacteria counts at that MIC level.
The MICs were extracted from the European Committee on Antimicrobial Susceptibility Testing (EUCAST) MIC distribution website (https://mic.eucast.org/search). A PTA or CFR value ≥90% was considered the optimal dosing regimen.
Results
Patient Inclusion and Characteristics
In total, 21 patients with 167 tigecycline concentrations were included in the PPK model. Table 1 shows the main demographic characteristics of the included patients.
Table 1.
Demographic Characteristics of the Included Patients
| No. | SEX | AGE | WT (kg) | BMI | BUN (mmol/L) | SCr (μmol/L) | ALT (U/L) | AST (U/L) | ALP (U/L) | TP (g/L) | GGT (U/L) | TBIL (μmol/L) | ALB (g/L) | UV (mL) | CRRT Information | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRRT type | Intensity (mL/kg/h) | Anticoagulant | |||||||||||||||
| 1 | M | 62 | 72.6 | 23.7 | 6.42 | 60 | 150 | 106 | 64 | 43.2 | 33 | 99.1 | 30.3 | 6950 | CVVH | 28.7 | Heparin |
| 2 | M | 86 | 75 | 24.5 | 26.7 | 391 | 45 | 11 | 67 | 54.3 | 57 | 47.4 | 30.5 | 350 | CVVH | 29.3 | Heparin |
| 3 | M | 65 | 65 | 23.9 | 11.7 | 158 | 16 | 8 | 90 | 48.6 | 82 | 19.7 | 28.2 | 850 | CVVH | 31.5 | Heparin |
| 4 | M | 87 | 95 | 31.0 | 23.4 | 159 | 22 | 55 | 81 | 43.7 | 20 | 99.5 | 27.7 | 1000 | CVVH | 21.6 | None |
| 5 | F | 64 | 56 | 23.0 | 8.08 | 60 | 9 | 22 | 127 | 66.6 | 58 | 13.3 | 30.6 | 2800 | CVVH | 37.1 | Heparin |
| 6 | M | 42 | 55 | 20.2 | 17.1 | 257 | 4 | 13 | 61 | 50.1 | 23 | 26.7 | 30.2 | 160 | CVVH | 39.1 | Sodium citrate |
| 7 | M | 87 | 42 | 16.4 | 36.2 | 276 | 5 | 22 | 184 | 54.7 | 35 | 11.8 | 27.7 | 0 | CVVH | 51.2 | None |
| 8 | F | 66 | 53.5 | 20.9 | 4.91 | 115 | 13 | 19 | 101 | 49.3 | 108 | 90.5 | 25.9 | 1020 | CVVH | 37.4 | Heparin |
| 9 | M | 82 | 60 | 20.3 | 10.4 | 334 | 7 | 17 | 98 | 50.9 | 21 | 11.4 | 32.5 | 300 | CVVH | 34.7 | Heparin |
| 10 | M | 66 | 75 | 26.0 | 15.4 | 183 | 20 | 23 | 98 | 51.2 | 140 | 25.6 | 23.1 | 200 | CVVH | 28.0 | Heparin |
| 11 | F | 78 | 50.5 | 23.4 | 6.65 | 63 | 77 | 40 | 114 | 53.3 | 181 | 16.9 | 35.7 | 1600 | CVVH | 41.6 | None |
| 12 | F | 86 | 47.4 | 22.2 | 9.38 | 88 | 27 | 36 | 131 | 44.9 | 109 | 19.3 | 25.4 | 150 | CVVH | 42.2 | Heparin |
| 13 | M | 62 | 70 | 24.8 | 18.5 | 108 | 45 | 68 | 116 | 42.7 | 27 | 113.9 | 26.5 | 25 | CVVHDF | 30.0 | None |
| 14 | M | 22 | 100 | 30.9 | 33.4 | 469 | 6 | 15 | 59 | 52 | 16 | 18.8 | 27 | 1150 | CVVH | 31.0 | Heparin |
| 15 | M | 77 | 70 | 22.9 | 7.40 | 47 | 40 | 51 | 106 | 49.2 | 38 | 96.8 | 26.6 | 60 | CVVH | 29.3 | Sodium citrate |
| 16 | F | 71 | 55 | 22.0 | 7.24 | 64 | 9 | 29 | 79 | 56.5 | 51 | 18.9 | 31.5 | 850 | CVVH | 37.3 | Heparin |
| 17 | M | 86 | 75 | 24.5 | 37.3 | 218 | 47 | 34 | 69 | 45 | 16 | 29.3 | 26.3 | 4500 | CVVH | 27.3 | Heparin |
| 18 | M | 31 | 70 | 27.3 | 3.64 | 51 | 13 | 24 | 62 | 50.8 | 23 | 47.8 | 23 | 4000 | CVVH | 29.3 | Sodium citrate |
| 19 | M | 73 | 77.5 | 25.6 | 13.2 | 274 | 3 | 21 | 82 | 62.1 | 29 | 14.3 | 28.1 | 50 | CVVH | 26.5 | Sodium citrate |
| 20 | M | 58 | 100 | 31.6 | 7.78 | 107 | 12 | 21 | 122 | 53.8 | 53 | 63.8 | 30.5 | 1950 | CVVH | 21.0 | Sodium citrate |
| 21 | F | 73 | 90 | 37.5 | 14.9 | 144 | 24 | 29 | 159 | 55.4 | 137 | 21.6 | 25.5 | 70 | CVVH | 23.9 | Heparin |
Abbreviations: M, male; F, female; WT, body weight; BMI, body mass index; BUN, blood urea nitrogen; SCr, serum creatinine; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; TP, total protein; GGT, γ-glutamyl transpeptidase; TBIL, total bilirubin; ALB, albumin; UV, 24-hour urine volume; CVVH, continuous veno-venous hemofiltration;CVVHDF, continuous veno-venous hemodiafiltration.
PPK Model Development and Model Evaluation
The tigecycline concentration data could be well illustrated by a two-compartment model with first-order elimination (2425.331 and 2188.14 for the Akaike information criterion in one and two compartments, respectively). An exponential model and a proportional model were used to describe the interindividual variability and residual variability, respectively. The final PPK model and parameter estimates are shown in Table 2, and the key progression of covariate screening is shown in Table S1. We found that the inclusion of AST in the peripheral volume distribution led to a decrease in the OFV of 13.719. However, its inclusion had no impact on the PTA or CFR outcomes. Consequently, we ultimately decided to remove it from the final model.
Table 2.
Distribution Characteristics of CRRT or Non-CRRT Final Model Estimation Parameters and Bootstrap Analysis
| Parameter | Non-CRRT* | CRRT | ||
|---|---|---|---|---|
| Final Model | Bootstrap Analysis | |||
| Estimate [RSE (%)] | Estimate [RSE (%)] | Median Estimate [RSE (%)] | 95% CI | |
| CL (L/h) | 3.09 (26.3) | 4.22 (10.4) | 4.27 (9.51) | 3.47–5.07 |
| V1 (L) | 32.1 (8.38) | 30.9 (15.9) | 31.6 (16.7) | 22.4–43.1 |
| Q (L/h) | 39.7 (5.79) | 34.8 (8.94) | 35.0 (9.25) | 29.4–42.3 |
| V2 (L) | 113 (4.61) | 98.7 (11.8) | 98.5 (10.9) | 78.4–120 |
| Inter-individual variability | ||||
| ωCL (%) | 27.0 (15.0) | 22.4 (31.6) | 20.7 (33.0) | 9.93–36.3 |
| ωV1 (%) | 72.5 (13.9) | 55.2 (25.4) | 52.7 (25.3) | 28.4–81.0 |
| Residual variability | ||||
| σ (%) | 2.02 (13.8) | 1.58 (15.7) | 1.52 (15.0) | 1.11–1.98 |
Notes: * Parameters of non-CRRT patients were derivate from Su et al, Front Pharm. 2024, 15: 1342954.
Abbreviations: CRRT, continuous renal replacement therapy; CL, typical value of apparent clearance; V1, central volume of distribution; Q, inter-compartmental clearance; V2, peripheral volume of distribution; ωCL, fixed-effect parameter of CL; ωV1, fixed-effect parameter of V1; σ, residual variability for proportional error; RSE, residual standard error; CI, confidence interval.
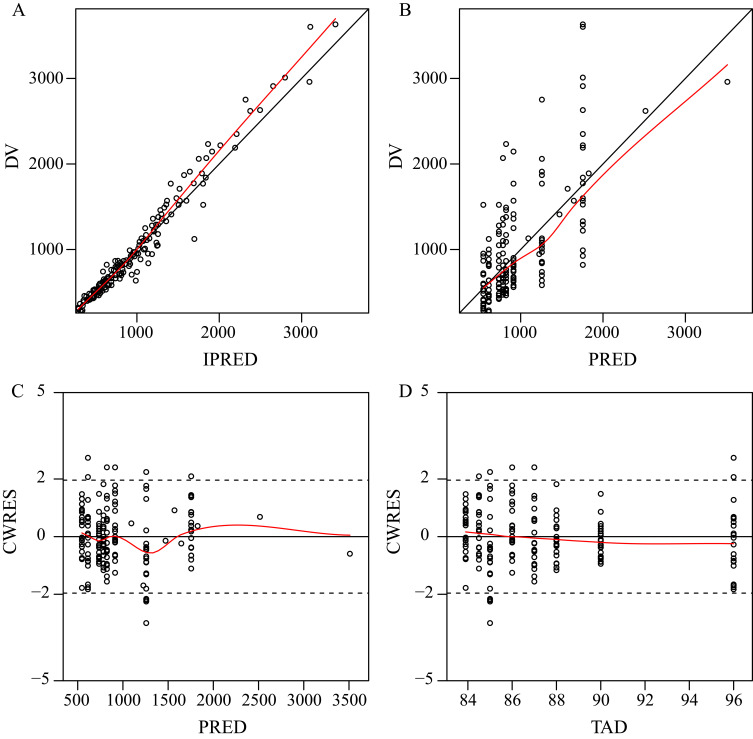
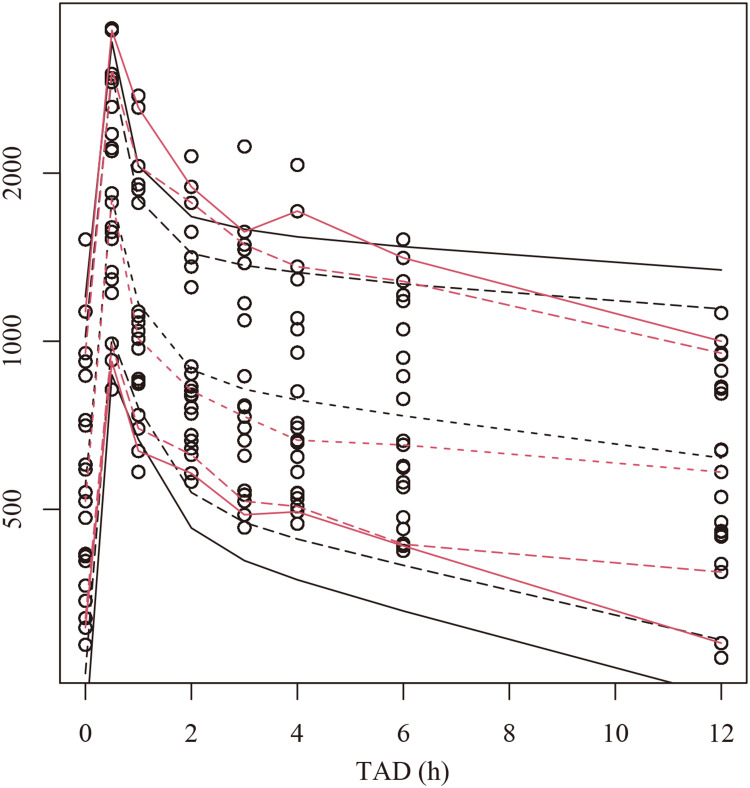
The final model was evaluated by goodness-of-fit plots (Figure 1), VPCs (Figure 2), 1000 bootstrap analyses (Table 2) and NPCs (Table 3), which showed that the model had good robustness and acceptable predictive performance.
Figure 1.
Goodness-of-fit plots for the final PPK model. (A) Observed tigecycline concentrations (DV) versus individual predictions (IPRED); (B) DV versus population predictions (PRED); (C) Conditional weighted residuals (CWRES) versus PRED; (D) CWRES versus time after dose (TAD).
Figure 2.
Visual predictive check (VPC) plots for the plasma tigecycline concentration. The Y-axis is the logarithm of the concentration. The open circles represent the observed concentrations. The hollow circles represent the observations. Observed (red lines) and predicted (black lines) 5th, 10th, 50th, 90th and 95th percentiles.
Table 3.
The Numerical Predictive Check (NPC) of the Final Population Pharmacokinetic Model
| PI | Points Below PI (Count) | Points Below PI (%) | 95% CI (%) | Points Above PI (Count) | Points Above PI (%) | 95% CI (%) |
|---|---|---|---|---|---|---|
| 0% | 101 | 60.5 | 32.3–66.5 | 66 | 39.5 | 33.5–67.7 |
| 20% | 86 | 51.5 | 23.4–56.9 | 52 | 31.1 | 24.0–55.7 |
| 40% | 58 | 34.7 | 14.4–46.7 | 50 | 29.9 | 15.6–44.9 |
| 50% | 47 | 28.1 | 10.8–40.1 | 43 | 25.7 | 11.4–38.9 |
| 60% | 37 | 22.2 | 7.19–34.1 | 39 | 23.4 | 7.78–32.3 |
| 80% | 9 | 5.39 | 1.80–20.4 | 28 | 16.8 | 1.80–19.8 |
| 90% | 3 | 1.80 | 0–12.6 | 16 | 9.58 | 0–13.2 |
| 95% | 1 | 0.60 | 0–8.38 | 10 | 5.99 | 0–8.98 |
Abbreviations: PI, prediction interval; CI, confidence interval.
Simulation and Dosing Regimen Optimization
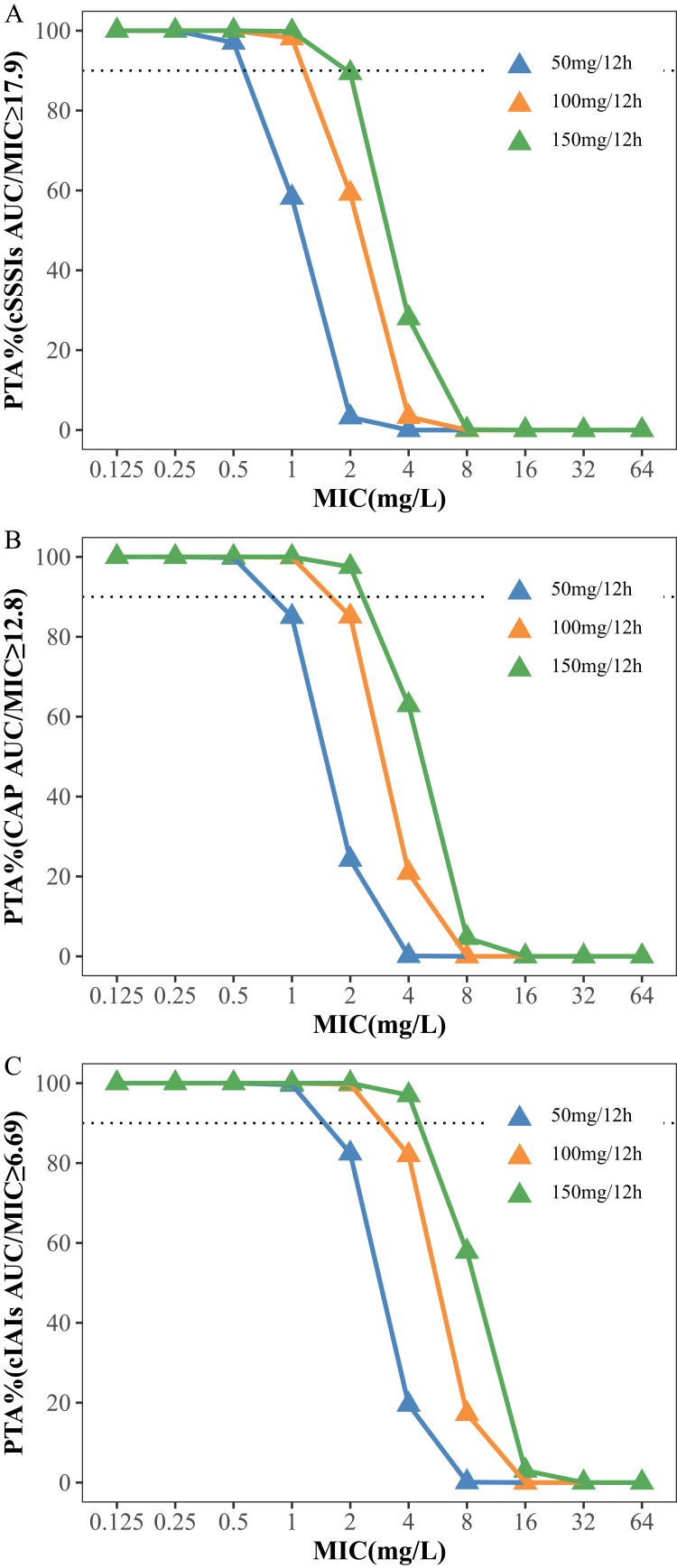
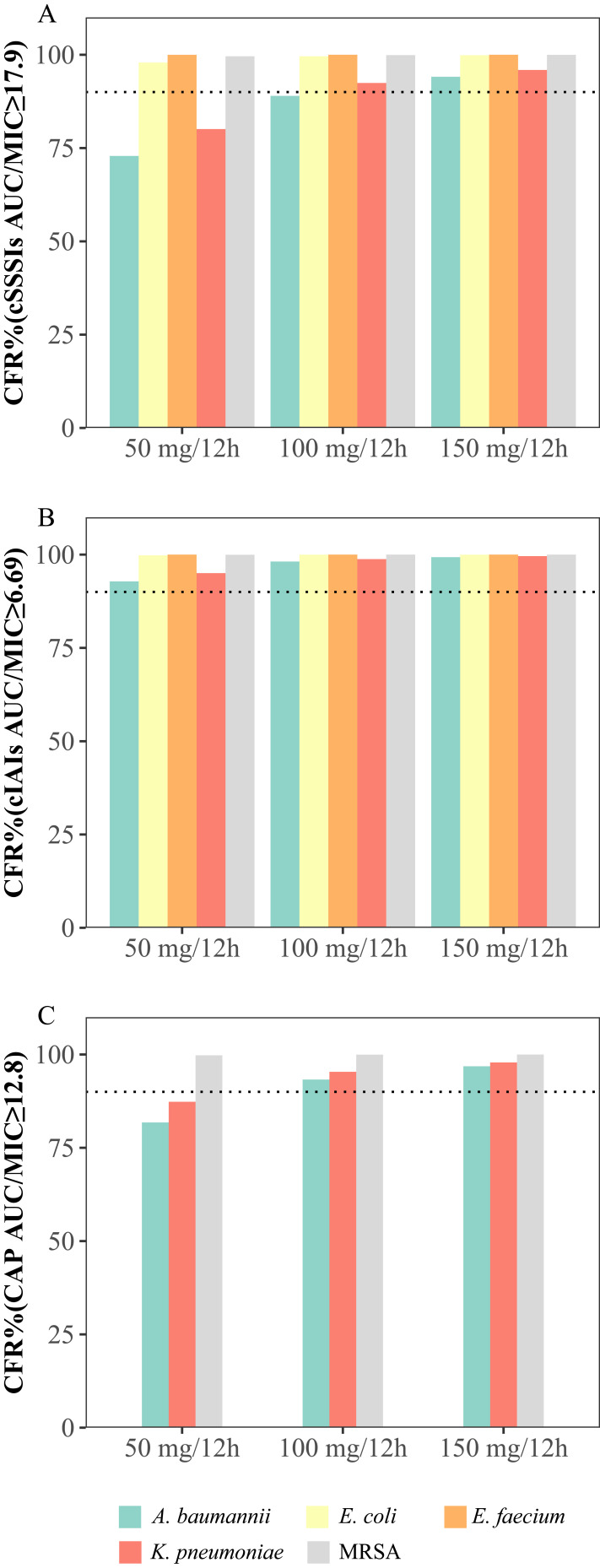
The PTAs and CFRs for the different dosages of tigecycline and the MIC distributions are shown in Figures 3 and 4 and Tables S2 and S3, respectively. The simulation results showed that for cSSSI, when the MIC was 1 mg/L, the PTAs of the standard and double-dose regimens (50 mg/12 h and 100 mg/12 h) were 58.2% and 98.1%, respectively, while for CAP, the PTA values were 85.0% and 99.9%, respectively. For cIAI, the PTAs were 82.4% and 99.7% at an MIC of 2 mg/L. The CFR results showed that all regimens exhibited a CFR > 90% for cIAI infection. In contrast, for cSSSI, the standard dosage regimens were 72.9% and 80.1% for Acinetobacter baumannii and Klebsiella pneumoniae infections, respectively. Similarly, for CAP, the CFR values at the 50 mg/12 h dose were 81.8% and 87.3% for these two pathogens, respectively.
Figure 3.
Probability of target attainment (PTA) for different dosage regimens of tigecycline for PK/PD targets with AUC/MIC ≥ 17.9 (A), 12.8 (B) and 6.96 (C). The dashed lines indicate a PTA of 90%.
Figure 4.
Cumulative fraction of response (CFR) for different dosage regimens of tigecycline for PK/PD targets with AUC/MIC ≥ 17.9 (A), 12.8 (B) and 6.96 (C). The MICs were determined based on the European Committee on Antimicrobial Susceptibility Testing (EUCAST) MIC distribution website. The dashed lines indicate a PTA of 90%.
Abbreviations: A. baumannii, Acinetobacter baumannii; E. faecium, Enterococcus faecium; E. coli, Escherichia coli; K. pneumoniae, Klebsiella pneumoniae; MRSA, methicillin-resistant Staphylococcus aureus.
Discussion
To our knowledge, this study was the only extensive sampled prospective study on the tigecycline pharmacokinetics exclusively in critically ill patients undergoing CRRT. Most notably, despite the use of an adequate base model, we were unable to identify any significant covariates influencing tigecycline pharmacokinetics. This surprising result highlights the complexity of drug deposition and excretion in critically ill patients receiving CRRT. Physicians and pharmacists worldwide could benefit from understanding the pharmacokinetics of tigecycline in these patients. Moreover, this study provided the optimized dosage regimens for common indications of tigecycline based on model simulation, which emphasized the need for individualized dosing strategies, the importance of therapeutic drug monitoring, and the necessity for further research to enhance clinical outcomes.
Currently, data on the pharmacokinetics of tigecycline during CRRT are insufficient. To the best of our knowledge, only two population pharmacokinetic studies have been conducted in patients undergoing CRRT with tigecycline.12,21 A population pharmacokinetics study on critically ill patients with IAIs conducted by Broeker incorporated bilirubin as a significant covariate for clearance.12 However, only 11 patients were enrolled in this study, and 6 of whom had liver failure, cirrhosis, or liver transplantation. Conversely, another study on the population pharmacokinetics of high-dose tigecycline in sepsis or septic shock patients did not identify any significant covariates, which included 31 patients who underwent CRRT and 6 patients who did not underwent CRRT.21 In our study, the various infections enrolled, aligning more closely with clinical characteristics.
The typical PK parameter value of clearance, intercompartment clearance, central and peripheral volumes of distribution (4.22 L/h, 34.8 L/h, 30.9 L and 98.7 L, respectively) observed in our cohort of patients undergoing CRRT was similar with the previously reported value (3.09 L/h, 39.7 L/h, 32.1 L and 113 L, respectively) in critically ill non-CRRT patients from our published study.14 However, these values significantly differ from those reported in other studies, such as Broeker’s findings of approximately 18.3 L/h, 56.4 L/h, 58.7 L and 154 L for relevant tigecycline pharmacokinetic parameters in CRRT patients.12 This observed discrepancy may be attributed to the inclusion of different patient populations, distinct pathophysiological conditions among patients, and variations in CRRT patterns. These findings indicate the substantial variability of tigecycline in CRRT patients and emphasize the important role of TDM for tigecycline in this specific population. In this study, we evaluated multiple potential covariates and observed that the AST could significantly affect the distribution of peripheral volumes. However, subsequent analysis revealed that changes in AST had minimal impact on the PTA and CFR, indicating that the changes in AST might be statistically significant but not clinically significant. Therefore, we decided to exclude this covariate from the final model. In addition, the pharmacokinetic parameters of tigecycline may also be affected by the specific setting of CRRT.11 A prospective study demonstrated that the type of CRRT had different effects on tigecycline clearance, with CL of 2.71 L/h for CVVHDF and 1.69 L/h for CVVHD.12 This discrepancy may arise from the differing saturation coefficients of tigecycline in the two modes of CRRT. Broeker et al determined that CVVHD exhibited an average saturation coefficient of 0.79, while CVVHDF demonstrated a higher average saturation coefficient of 0.90.12 Despite these findings, they still believed that the tigecycline dose would not require adjustment during CRRT due to a total clearance of 18.3 L/h. However, this result was derived from a small population (3 patients with CVVHDF and 8 patients with CVVHD). Moreover, the adsorption rate of tigecycline varies across different membranes. Tigecycline could be significantly adsorbed on polyethyleneimine-treated polyacrylonitrile membranes (over 90% of the administered dose), while it was less adsorbed on polysulfone filter membranes (up to 10%).22 In addition, tigecycline exhibits atypical nonlinear PPB behavior and can be affected by divalent metal ions, such as regional citrate anticoagulation (RCA).11,23 Thus, we also assessed the types and intensity of CRRT and the types of anticoagulants used. Regrettably, none of these factors had a significant impact on tigecycline pharmacokinetics. The insufficient sample size in our study may have contributed to this outcome, as only one individual underwent CVVHDF.
Our study therefore also assessed the probability of reaching the three PK/PD targets for various dose regimens. The findings revealed that standard dosing may be sufficient for the majority of patients with infections. Conversely, for patients with SSSIs or CAP caused by Acinetobacter baumannii and Klebsiella pneumoniae, the CFRs of the recommended dose regimens were less than 90%, necessitating higher tigecycline doses, such as 100 mg/12 h, or other available therapies to achieve optimal therapeutic efficacy.24–26 High-dose tigecycline therapy has been demonstrated to reduce all-cause mortality and improve microbial and clinical efficiency.27 Compared to monotherapy, tigecycline combination therapy has more advantages and may be a better choice for advanced-aged or severely ill patients.28,29
This study has several limitations. This model lacks external validation. The parameter settings for CRRT are not comprehensively recorded, making it challenging to accurately assess the actual intensity of CRRT. Consequently, this complicates the evaluation of how CRRT intensity influences the pharmacokinetics of tigecycline. Patients received CVVHDF was limited, and the impact of CRRT modality needs further data. Additionally, the most available data were derived from small cohorts, and it is imperative to conduct large-sample and well-designed randomized studies to ascertain the impact of the CRRT setting on the pharmacokinetics of tigecycline.
Conclusions
This single-center prospective study provided valuable population pharmacokinetic evidence for tigecycline in critically ill patients receiving CRRT. The pharmacokinetics of tigecycline in this population were well described by a two-compartment model. A dosage regimen of 50 mg every 12 h was suitable for most infected patients, while 100 mg every 12 h or higher dosage was needed for CAP and cSSSI caused by Acinetobacter baumannii and Klebsiella pneumoniae.
Funding Statement
This research was funded by Hangzhou Municipal Health Commission (B20231075) and Zhejiang Provincial Natural and Science Foundation of China (LYY21H310006).
Data Sharing Statement
The data are contained within the article or supplementary material. Further requirements can be requested from the corresponding author.
Institutional Review Board Statement
The study was approved by the ethics committee of Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University (reference number KEYAN20190108-9) and complied with the Declaration of Helsinki.
Informed Consent Statement
Written informed consent was obtained from the patient or his/her representative before enrollment.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Yaghoubi S, Zekiy AO, Krutova M, et al. Tigecycline antibacterial activity, clinical effectiveness, and mechanisms and epidemiology of resistance: narrative review. Eur J Clin Microbiol Infect Dis. 2022;41(7):1003–1022. doi: 10.1007/s10096-020-04121-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peterson LR. A review of tigecycline — the first glycylcycline. Int J Antimicrob Agent. 2008;32:S215–S222. doi: 10.1016/S0924-8579(09)70005-6 [DOI] [PubMed] [Google Scholar]
- 3.Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America 2023 Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections. Clin Infect Dis. 2023;2023:1 doi: 10.1093/cid/ciad428 [DOI] [PubMed] [Google Scholar]
- 4.Rachoin JS, Weisberg LS. Renal Replacement Therapy in the ICU. Crit Care Med. 2019;47(5):715–721. doi: 10.1097/CCM.0000000000003701 [DOI] [PubMed] [Google Scholar]
- 5.Tandukar S, Palevsky PM. Continuous Renal Replacement Therapy Who, When, Why, and How. Chest. 2019;155(3):626–638. doi: 10.1016/j.chest.2018.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Covajes C, Scolletta S, Penaccini L, et al. Continuous infusion of vancomycin in septic patients receiving continuous renal replacement therapy. Int J Antimicrob Agent. 2013;41(3):261–266. doi: 10.1016/j.ijantimicag.2012.10.018 [DOI] [PubMed] [Google Scholar]
- 7.Roberts JA, Joynt GM, Lee A, et al. The Effect of Renal Replacement Therapy and Antibiotic Dose on Antibiotic Concentrations in Critically Ill Patients: data From the Multinational Sampling Antibiotics in Renal Replacement Therapy Study. Clin Infect Dis. 2020;72(8):1369–1378. doi: 10.1093/cid/ciaa224 [DOI] [PubMed] [Google Scholar]
- 8.Yu Z, Liu J, Yu H, et al. Population pharmacokinetics and individualized dosing of vancomycin for critically ill patients receiving continuous renal replacement therapy: the role of residual diuresis. Front Pharmacol. 2023;14:1298397. doi: 10.3389/fphar.2023.1298397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, Li X, Xia Y, et al. Recommendation of Antimicrobial Dosing Optimization During Continuous Renal Replacement Therapy. Front Pharmacol. 2020;11:786. doi: 10.3389/fphar.2020.00786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honore PM, Jacobs R, De Waele E, Van Gorp V, Spapen HD. The blind spot in high-dose tigecycline pharmacokinetics in critically ill patients: membrane adsorption during continuous extracorporeal treatment. Crit Care. 2015;19(1):24. doi: 10.1186/s13054-015-0744-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honore PM, David C, Kugener L, et al. No dose adjustment of tigecycline is necessary during continuous renal replacement therapy: we are not sure. Crit Care. 2020;24(1):59. doi: 10.1186/s13054-020-2775-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broeker A, Wicha SG, Dorn C, et al. Tigecycline in critically ill patients on continuous renal replacement therapy: a population pharmacokinetic study. Crit Care. 2018;22(1):341. doi: 10.1186/s13054-018-2278-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang F, Cao WX, Yan YY, et al. Influence of continuous renal replacement therapy on the plasma concentration of tigecycline in patients with septic shock: a prospective observational study. Front Pharmacol. 2023;2023:14. doi: 10.3389/fphar.2023.1118788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su W, Song S, Liu J, et al. Population pharmacokinetics and individualized dosing of tigecycline for critically ill patients: a prospective study with intensive sampling. Front Pharmacol. 2024;15:1342947. doi: 10.3389/fphar.2024.1342947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meagher AK, Passarell JA, Cirincione BB, et al. Exposure-Response Analyses of Tigecycline Efficacy in Patients with Complicated Skin and Skin-Structure Infections. Antimicrob Agents Chemother. 2007;51(6):1939–1945. doi: 10.1128/AAC.01084-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Passarell JA, Meagher AK, Liolios K, et al. Exposure-Response Analyses of Tigecycline Efficacy in Patients with Complicated Intra-Abdominal Infections. Antimicrob Agents Chemother. 2008;52(1):204–210. doi: 10.1128/AAC.00813-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie J, Roberts JA, Alobaid AS, et al. Population Pharmacokinetics of Tigecycline in Critically Ill Patients with Severe Infections. Antimicrob Agents Chemother. 2017;61(8):1–10. doi: 10.1128/AAC.00345-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leng B, Yan G, Wang C, Shen C, Zhang W, Wang W. Dose optimisation based on pharmacokinetic/pharmacodynamic target of tigecycline. J Glob Antimicrob Resist. 2021;25:315–322. doi: 10.1016/j.jgar.2021.04.006 [DOI] [PubMed] [Google Scholar]
- 19.Asín E, Isla A, Canut A, Rodríguez Gascón A. Comparison of antimicrobial pharmacokinetic/pharmacodynamic breakpoints with EUCAST and CLSI clinical breakpoints for Gram-positive bacteria. Int J Antimicrob Agent. 2012;40(4):313–322. doi: 10.1016/j.ijantimicag.2012.06.005 [DOI] [PubMed] [Google Scholar]
- 20.Gumbo T, Alffenaar JWC. Pharmacokinetic/Pharmacodynamic Background and Methods and Scientific Evidence Base for Dosing of Second-line Tuberculosis Drugs. Clin Infect Dis. 2018;67(suppl_3):S267–S273. doi: 10.1093/cid/ciy608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moor A B-D, Rypulak E, Potręć B, et al. Population Pharmacokinetics of High-Dose Tigecycline in Patients with Sepsis or Septic Shock. Antimicrob Agents Chemother. 2018;62(4):e02273–17. doi: 10.1128/AAC.02273-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onichimowski D, Ziółkowski H, Nosek K, Jaroszewski J, Rypulak E, Czuczwar M. Comparison of adsorption of selected antibiotics on the filters in continuous renal replacement therapy circuits: in vitro studies. J Artif Organs. 2020;23(2):163–170. doi: 10.1007/s10047-019-01139-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh RSP, Mukker JK, Deitchman AN, Drescher SK, Derendorf H. Role of Divalent Metal Ions in Atypical Nonlinear Plasma Protein Binding Behavior of Tigecycline. J Pharm Sci. 2016;105(11):3409–3414. doi: 10.1016/j.xphs.2016.07.013 [DOI] [PubMed] [Google Scholar]
- 24.Katip W, Uitrakul S, Oberdorfer P. Clinical Efficacy and Nephrotoxicity of the Loading Dose Colistin for the Treatment of Carbapenem-Resistant Acinetobacter baumannii in Critically Ill Patients. Pharmaceutics. 2021;14(1):31. doi: 10.3390/pharmaceutics14010031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katip W, Rayanakorn A, Oberdorfer P, Taruangsri P, Nampuan T. Short versus long course of colistin treatment for carbapenem-resistant A. baumannii in critically ill patients: a propensity score matching study. J Infect Public Health. 2023;16(8):1249–1255. doi: 10.1016/j.jiph.2023.05.024 [DOI] [PubMed] [Google Scholar]
- 26.Katip W, Rayanakorn A, Sornsuvit C, et al. High-Loading-Dose Colistin with Nebulized Administration for Carbapenem-Resistant Acinetobacter baumannii Pneumonia in Critically Ill Patients: a Retrospective Cohort Study. Antibiotics. 2024;13(3):287. doi: 10.3390/antibiotics13030287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zha L, Pan L, Guo J, French N, Villanueva EV, Tefsen B. Effectiveness and Safety of High Dose Tigecycline for the Treatment of Severe Infections: a Systematic Review and Meta-Analysis. Adv Ther. 2020;37(3):1049–1064. doi: 10.1007/s12325-020-01235-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Pan Y, Shen J, Xu Y. The efficacy and safety of tigecycline for the treatment of bloodstream infections: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob. 2017;16(1):24. doi: 10.1186/s12941-017-0199-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai X, Yan H, Zhang W, et al. Intra-abdominal infection after tumor surgery: tigecycline combined with β-lactam antibiotics versus tigecycline alone. BMC Cancer. 2023;23(1):682. doi: 10.1186/s12885-023-11169-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are contained within the article or supplementary material. Further requirements can be requested from the corresponding author.