Abstract
To investigate whether assisted reproductive technology (ART) affects gross fetal movement. A prospective cohort study. 65 women who conceived with ART (ART group) and 211 women (control group) without ART recorded fetal movement with the fetal movement acceleration measurement recorder at night weekly after 28 weeks. The number ratio of 10 s epochs with fetal movement to all epochs was calculated as the fetal movement parameter. When no fetal movement was observed for more than 5 min, it was defined as a no fetal movement period, and the average number per hour, the average duration, and the longest duration of the no fetal movement periods were calculated as the no fetal movement parameters. Gestational weeks were classified into 28–33 and 34–39 weeks, and the fetal movement parameter and the no fetal movement parameters were compared using the Student’s t-test. The fetal movement parameters at 28–33 weeks were 17.43% (ART) and 16.58% (control) (p = 0.219), and those at 34–39 weeks were 11.72% (ART) and 11.96% (control) (p = 0.590). In the same way, for the no fetal movement parameters, the average numbers were 1.58 and 1.63 per hour (p = 0.357), and 2.36 and 2.30 per hour (p = 0.503). The average durations were 8.30 and 8.46 min (p = 0.712), and 9.20 and 9.51 min (p = 0.188). The longest durations were 16.26 and 17.02 min (p = 0.295), and 22.34 and 22.87 min (p = 0.534). ART does not affect gross fetal movement count.
Keywords: Fetal development, Fetal movement, Fetal movement acceleration measurement recorder, Assisted reproductive technology, In vitro fertilization
Subject terms: Developmental biology, Medical research
Introduction
Recently, more and more births have been conceived by assisted reproductive technologies (ART). There have also been many studies about the effects of ART on pregnancies, infants, and childhood development; however, there have been relatively few studies on the effect of ART on the fetuses themselves.
Spilliopoulos and Economides1 reported enhanced fetal growth during the first and second trimesters in ART fetuses, which might be due to the supplemental progesterone used in vitro fertilization (IVF). Eindhoven, et al.2 reported that embryonic and fetal growth trajectories and birth weight are not significantly different between pregnancies conceived with in IVF/intracytoplasmic sperm injection treatment and spontaneously conceived pregnancies. On the contrary, there have also been several reports that new born baby weight is greater in frozen embryo transfers. Pinborg, et al.3 reported that a sibling conceived after a frozen embryo transfer had an increased probability of being born large for gestational age compared with a sibling born after a fresh embryo transfer.
Other than fetal body size, Nau, et al.4 reported that thymic-thoracic ratios in fetuses were smaller in pregnancies conceived using IVF. Yin, et al.5 measured the cavum septum pellucidum width, transverse cerebellar diameter, cisterna magna depth, and lateral ventricle width of fetuses and concluded that the development of the fetal central nervous system was not different between IVF and natural conception pregnancies.
All of these reports were related to fetal morphology, but there have been far fewer reports studying fetal function. Joy, et al.6 reported that IVF fetuses demonstrated no differences in habituation suggesting that there was no neurodevelopmental delay.
ART fetuses may have different well-being state or functional development compared with non-ART ones. Fetal movement reflects fetal developments such as overall well-being and neurological development; however, there has been no study on the effect of IVF on fetal movement.
One reason for this deficiency was that there was no practical way of counting fetal movements. We have developed the fetal movement acceleration measurement recorder (FMAM recorder)7,8, http://e-mother.co-site.jp), which is designed to detect oscillations of the maternal abdominal wall caused by fetal movement and which can be used during overnight sleep at home. In a basic study7, gross fetal movements were counted by ultrasonography and maternal abdominal oscillations were simultaneously counted with the FMAM recorder, and this study confirmed that the two counts agreed almost perfectly. The FMAM recorder has enabled the long-term monitoring of gross fetal movement.
We hypothesized that there are any differences of gross movement counted by the FMAM recorder between ART fetuses and non-ART ones. Here we attempted to determine this.
Materials and methods
Participants
This was a prospective cohort study. For both groups, the number required to see a significant difference at 5% level of fetal movement with the following condition: (SD = 8%, alpha = 0.05, beta = 0.05) was 66. There were 276 singleton pregnant women who adequately recorded fetal movement with the FMAM recorder, and delivered a baby without major anomalies at 37–41 weeks between April 2010 and August 2017 at Teikyo University in Japan. Of these, 65 women conceived with ART (ART group), and the other 211women (control group) conceived without ART. Table 1 shows the characteristics of the mothers and newborns in both groups.
Table 1.
The characteristics of the mothers and the newborns.
| ART(n = 65) | Control(n = 211) | P-value | |
|---|---|---|---|
| Characteristics of the mothers | |||
| Age | 38 (29, 44) | 34 (19,44) | < 0.0001 |
| Body mass index | 21.4 (17.9, 32, 0) | 21.1 (14.7, 35.7) | 0.720 |
| Para/non-para | 18/47 | 93/117 | 0.017 |
| Vaginal/Cesarean delivery | 38/27 | 144/67 | 0.145 |
| Delivery weeks | 39 (37, 41) | 39 (37, 41) | 0.748 |
| Details of ART | |||
| ICSI/IVF | 35/30 | ||
| ET/BT | 21/44 | ||
| Fresh/Frozen-thawed | 8/57 | ||
| Characteristics of the newborns | |||
| Male/female | 26/39 | 107/104 | 0.130 |
| Newborn weight (g) | 2977 (1893, 3960) | 3006 (1948, 4236) | 0.431 |
| Umbilical artery pH | 7.274 (7.113, 7.476) | 7.295 (7.083, 7.564) | 0.072 |
| 1 min Apgar score 0–6/7–10 | 2/63 | 3/208 | 0.382 |
| 5 min Apgar score 0–6/7–10 | 1/64 | 1/210 | 0.376 |
Data are presented as number or median (minimum, maximum).
The two groups were compared using the Student’s t or χ2 test.
All the women were asked to record fetal movement by themselves with the FMAM recorder once a week after 28 weeks, because the accuracy of the FMAM recorder is unreliable before 28 weeks7. The women visited our hospital for routine examinations every two weeks from 28 to 35 weeks and every week from 36 weeks until birth. At every visit, they underwent fetal heart rate monitoring in order to confirm reassuring fetal status.
FMAM recorder
The FMAM recorder was explained in detail in our previous studies7,8. Here, it is described briefly. Figure 1 shows a scheme of a pregnant woman who are recording fetal movement with the recorder, and photograph of the recorder. The weight of the recorder is 290 g, and it can be used at home. It has two acceleration sensors: one is a fetal movement sensor attached to the mother’s abdominal wall; the other is a mother’s movement sensor attached to her thigh. The sensitivities of the fetal and maternal sensors are 700 mV/0.1G and 120 mV/0.1G, respectively. The fetal movement sensor picks up oscillations of the mother’s abdomen induced by gross fetal movement. Maternal movement also causes abdominal oscillations, and the recorder is unsuitable when the mother is active. That is why it is used mainly during night sleep. Even during sleep, though, mothers move occasionally. In principle, when the mother’s movement sensor detects no leg movement, and the fetal movement sensor detects oscillations of her abdominal wall, gross fetal movement is judged to have occurred.
Figure 1.

A scheme of a pregnant woman who are recording fetal movement with the recorder, and a photograph of the recorder with two sensors. One sensor is a fetal movement sensor placed on the maternal abdominal wall, and the other is a mother’s movement sensor placed on her thigh.
Fetal movement analysis and the parameters
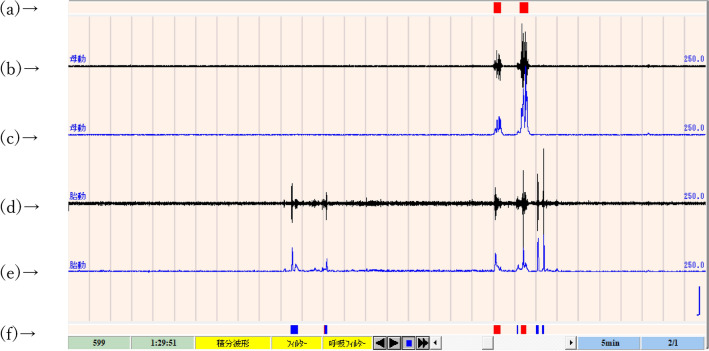
When data could be recorded for more than 4 h in one night, it was accepted as one valid record. Each record was analyzed and fetal movement was detected using a software system (Fetal movement analysis system, NoruPro Light Systems Inc., Tokyo, Japan), which was developed especially for the FMAM recorder9. A brief description of the system is as follows: 1. The low acceleration signals from both fetal and maternal sensors were filtered and changed to absolute integral values per 50 ms; 2. When the integral values were greater than twice the average amplitude during the 3 s just before and after measurement, they were judged to be positive for acceleration; 3. Any period in which the mother’s sensor detected positive accelerations more than four times per minute was deleted from the data because this usually indicated that the mother was active or awake; 4. When the fetal values were positive and maternal ones were negative, fetal movement was judged to have occurred; 5. Characteristic regular accelerations at 15–20 beats/minute detected by the fetal sensor were considered a sign of fetal hiccups and not counted as fetal movements. Figure 2 is an example of computer screen showing software analyzing for fetal movement.
Figure 2.
An example of computer screen showing software analyses for fetal movement. (b) Signals of the maternal sensor, (c) integral calculus value of (b). (a) Computer judgment of maternal movement. When values on (c) are positive, maternal movement is judged to be present, and red rectangles are shown. (d) Signals of the fetal sensor, (e) integral calculus value of (d). (f) Computer judgment of fetal movement. When values on (e) are positive, but values on (c) are negative, fetal movement is judged to be present, and blue rectangles are shown. When both values on (c) and (e) are positive, maternal movement is judged to be present, and red rectangles are shown.
From the fetal movements detected, the following parameters were calculated. First, the record was divided into intervals (epochs) of 10 s because usual duration of a series of gross fetal movement is several seconds, and an epoch contained a fetal movement was regarded as a positive epoch. The ratio of positive epochs to all epochs during one night was calculated as the fetal movement parameter. Second, a no fetal movement period was defined as when no fetal movement was observed for more than 5 min. If fetal movement occurred randomly and the fetal movement parameter was 20%, no fetal movement for 5 min (30 consecutive 10 s epochs) would be rare (0.8[1–0.2] to the 30th power: 0.12%), which can be considered non-random events controlled by the central nervous system. Thus, the average number per hour, the average duration, and the longest duration of the no fetal movement periods were calculated for each night as the no fetal movement parameters.
Examinations
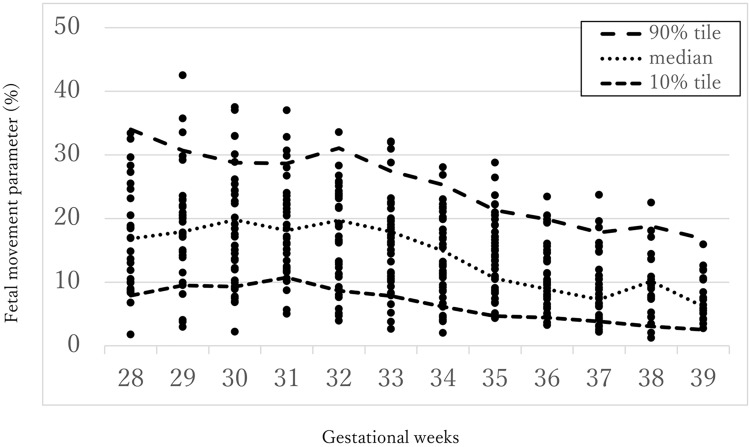
First, in order to see the big picture, the fetal movement parameters of the ART group were plotted over the normal reference curves for the FMAM recorder. The reference curves, as reported in our previous study8, are based on women with normal pregnancies and deliveries.
The fetal movement index changes little before 33 gestational weeks and decreases after that, as the normal reference curves in Fig. 3 show; therefore, we classified gestational weeks into an earlier period (28–33 weeks) and a later period (34–39 weeks). Then, the fetal movement and the no fetal movement parameters were compared for the ART and control groups using the Student’s t-test.
Figure 3.
Scatters of the fetal movement parameter for the fetuses in the ART group placed over the normal reference curves previously reported (reference8, Ryo E et al. 2018).
All data were analyzed with the JMP Pro 13. The statistically significant level was set at a p-value of less than 0.05.
Ethical approval
This study was carried out in accordance with the principles of the Declaration of Helsinki, and was approved by the ethics committee of Teikyo University (Teirin13-100-2, 2006/10/13). All method was performed in accordance with relevant guidelines and all women gave written informed consent before participating in the study.
Results
Not all women recorded weekly, and not all data were successfully recorded for more than four hours per night. As a result, we finished with a total of 2530.7 h of recording from 400 records for the ART group and 8093.1 h of recording from 1255 records for the control groups. For the ART group, we had 207 records at 28–33 weeks and 193 records at 34–39 weeks; for the control group, we had 661 records at 28–33 weeks and 594 records at 34–39 weeks.
The average number of records per woman at 28–33 weeks was 3.18 (ART) and 3.13 (control) (p = 0.903); the average number of records per woman at 34–39 weeks were 2.97 (ART) and 2.82 (control) (p = 0.552). In the same way, the average length of recording time per record was 6.39 h (ART) and 6.48 h (control) at 28–33 weeks (p = 0.159), and 6.25 h (ART) and 6.36 h (control) at 34–39 weeks (p = 0.199). There were no significant differences between the two groups in the number of records per woman and in the length of recording time per record.
Heart rate monitoring revealed a reactive pattern in every visit for every woman who delivered at term.
Figure 3 shows the plots of the fetal movement parameter for the fetuses in the ART group placed over the normal reference curves. Most of the parameters for the ART group seem to be within the normal reference range.
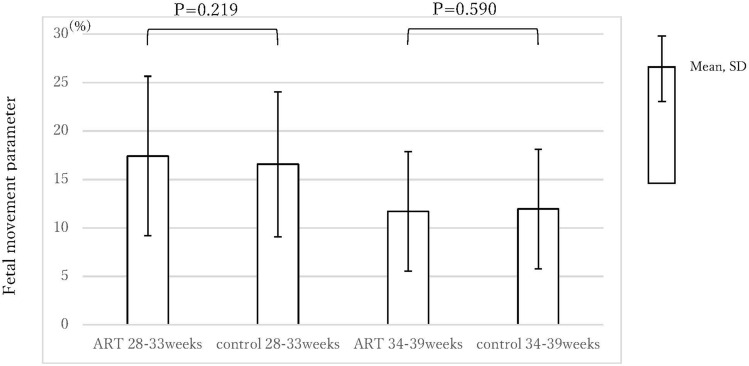
Figure 4 shows a comparison of the fetal movement parameters. The parameters (mean (SD)) at 28–33 weeks were 17.43 (8.23) % (ART) and 16.58 (7.48) % (control); those at 34–39 weeks were 11.72 (6.17) % (ART) and 11.96 (6.17) % (control). There were no significant differences for both periods (p = 0.219, 0.590, respectively).
Figure 4.
The fetal movement parameters of the ART group and the control group.
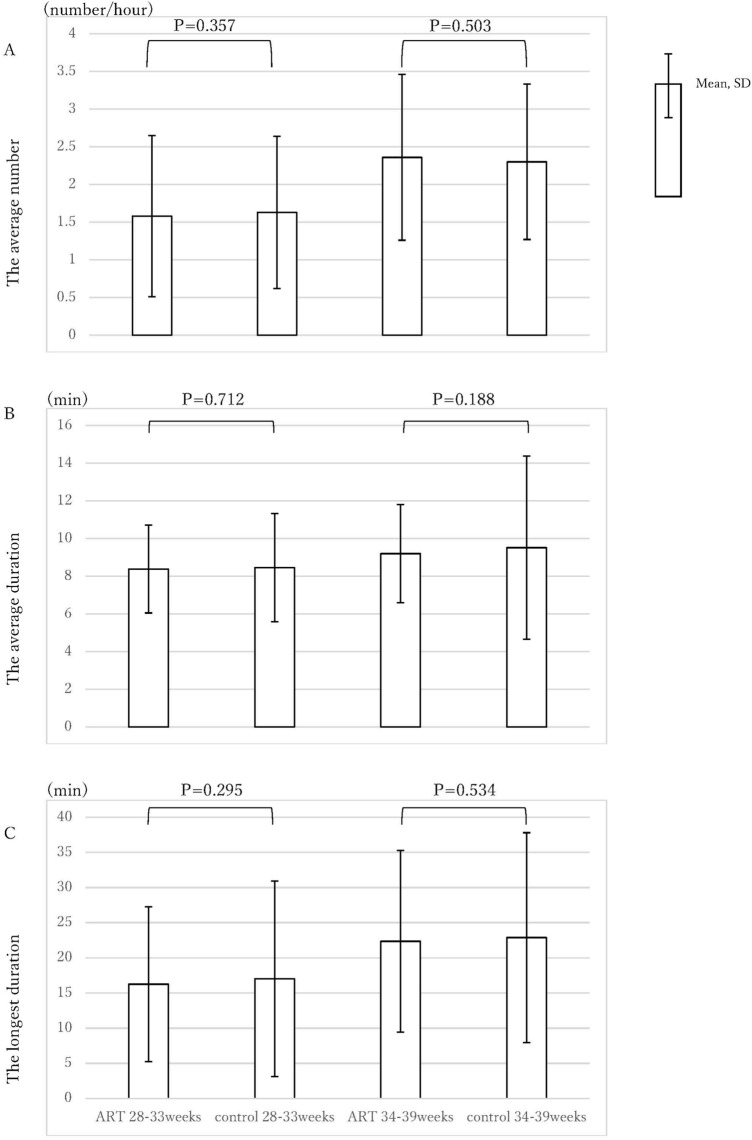
Figure 5 shows a comparison of the no fetal movement parameters. The average numbers of no movement occasions at 28–33 weeks were 1.58 (1.07) per hour (ART) and 1.63 (1.01) per hour (control) (p = 0.357); the averages at 34–39 weeks were 2.36 (1.10) per hour (ART) and 2.30 (1.03) per hour (control) (p = 0.503). In the same way, the average durations of no movement occasions were 8.38 (2.33) minutes (ART) and 8.46 (2.87) minutes (control) during the earlier period (p = 0.712), and 9.20 (2.60) minutes (ART) and 9.51 (4.86) minutes (control) during the later period (p = 0.188). The longest durations of no movement were 16.26 (11.02) minutes (ART) and 17.02 (13.88) minutes (control) during the earlier period (p = 0.295), and 22.34 (12.93) minutes (ART) and 22.87 (14.92) minutes (control) in the later period (p = 0.534). There were no significant differences between the two groups for any of the no movement parameters.
Figure 5.
The no fetal movement parameters of the ART group and the control group. (A) average numbers of no movement, (B) average durations of no movement, (C) longest durations of no movement.
Discussion
Fetal movement is assumed to reflect fetal well-being and neurological development. This study showed that there were no differences in the fetal movement parameter and no fetal movement parameters between the ART and the control groups.
In this study, all women in the ART group showed reactive fetal heart rate pattern every time they visited the hospital, which meant that ART did not deteriorate fetal well-being from the viewpoint of heart rate pattern.
A globally used biophysical profile score to assess fetal well-being includes fetal gross and respiratory movement10. In our previous report11, we counted gross fetal movement for fetuses with growth restriction using the FMAM recorder, which showed that fetal movement decreased even when the fetuses showed reactive fetal heart rate patterns. We suggested in that report that fetal growth restriction might have been an adaptation to a mildly insufficient environment resulting in decreased fetal movement, even though the heart rate patterns remained reactive.
On the other hand, the results of this study showed that there are no differences between ART fetuses and non-ART ones also from the viewpoint of gross fetal movement counting. In clinical practice, there seems to be no need for differentiating the counting method to assess fetal well-being for both fetuses in clinical practice.
As for the effect of ART on neurological development, there have been several reports studying long-term development. Hashimoto et al.12 reported that 238 babies born after IVF showed no significant differences compared with 365 naturally conceived babies in gross motor function at one, three, six, and 12 months of age. They also showed that linguistic and communication capacity were higher in IVF babies. Drenth et al.13 reported that IVF was not associated with less favorable neurological outcomes for 9-year-old singletons. A systematic review by Rumbold et al.14 showed there was no difference in cognitive outcomes among children conceived with conventional IVF and those conceived naturally. Another systematic review by Zhan et al.15 concluded that cognitive, motor, and language developments showed were comparable achievements; however, lower intelligence scores, worse visual-motor ability or locomotor development, and delayed receptive language competence were found in the ART group. A meta-analysis by Liu et al.16 indicated that use of ART may be associated with a higher risk of autism spectrum disorder. Some studies have shown that ART has a mild negative effect on development; however, it seems that no obvious negative effects have been demonstrated in most studies.
Development can be affected not only by factors before birth but also by the environments after birth. Considering the high cost of ART, many babies so conceived are likely to be brought up in economically fortunate circumstances. First, all ART babies are strongly wanted, and are assumed to grow up amid tender loving care. The age of the parents is probably older for an ART pregnancy. It is reasonable to assume there are some differences in home environment between ART and other children. Of course, actual physical development is most important; however, avoiding the biases after birth and examining the effect of ART only on the fetal development makes sense from a scientific viewpoint.
It has not been fully established yet, but fetal movement is assumed to reflect neurological development. Kurjak et al.17 introduced a scoring system to assess fetal neurodevelopment by observing fetal movement. Morokuma et al.18 reported that ultrasound evaluation of fetal behavior could adequately identify neurological impairment.
In normal reference value curves made with the FMAM recorder8, the fetal movement parameter decreased and the no fetal movement parameters increased as pregnancy progressed, which we think shows enhanced control of the central nervous system. This study found no differences in the fetal and no fetal movement parameters between the ART and control groups, which suggests the normal development of the central nervous system in ART fetuses.
This is the first report examining the effect of ART on fetal movement as pure fetal functioning, which could help eliminate the biases after birth; however, there are several limitations. One limitation was that we did not have data before 28 weeks because the FMAM recorder is unreliable before 28 weeks. Another limitation was that there are currently various methods of ART, and there were not enough participants for us to examine each method. However, we think that the results of this study would provide reassurance to many people involved in ART.
Conclusions
ART does not affect gross fetal movement as counted by the FMAM recorder.
Supplementary Information
Author contributions
ER wrote the main manuscript text and KY prepared Figs. 1–3 and HK and MM collected the data. All authors reviewed the manuscript.
Funding
This work was funded by Japan Society for the Promotion of Science, JP22K09627.
Data availability
The datasets generated and analyzed during the current study are available in the Supplementary File.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-70279-9.
References
- 1.Spiliopoulos, D. & Economides, D. L. Early fetal growth in progesterone-treated IVF pregnancies. Arch. Gynecol. Obstet.294, 63–69. 10.1007/s00404-015-3951-3 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Eindhoven, S. C. et al. The influence of IVF/ICSI treatment on human embryonic growth trajectories. Hum. Reprod.29, 2628–2636. 10.1093/humrep/deu271 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Pinborg, A. et al. Large baby syndrome in singletons born after frozen embryo transfer: Is it due to maternal factors or the cryotechnique?. Hum. Reprod.29, 618–627. 10.1093/humrep/det440 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Nau, T. G. et al. Foetal thymus size in pregnancies after assisted reproductive technologies. Arch. Gynecol. Obstet.298, 329–336. 10.1007/s00404-018-4795-4 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Yin, L. et al. Effect of assisted reproductive technology on fetal brain development assessed by prenatal ultrasonography. J. Perinat. Med.43, 103–109. 10.1515/jpm-2014-0020 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Joy, J., McClure, N., Hepper, P. G. & Cooke, I. Fetal habituation in assisted conception. Early Hum. Dev.88, 431–436. 10.1016/j.earlhumdev.2011.10.009 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Ryo, E., Nishihara, K., Matsumoto, S. & Kamata, H. A new method for long-term home monitoring of fetal movement by pregnant women themselves. Med. Eng. Phys.34, 566–572. 10.1016/j.medengphy.2011.09.001 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Ryo, E. et al. Reference values for a fetal movement acceleration measurement recorder to count fetal movements. Pediatr. Res.83, 961–968. 10.1038/pr.2017.328 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Nishihara, K., Ohki, N., Kamata, H., Ryo, E. & Horiuchi, S. Automated software analysis of fetal movement recorded during a pregnant woman’s sleep at home. PLoS One10(6), e0130503. 10.1371/journal.pone.0130503 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manning, F. A., Platt, L. D. & Sipos, L. Antepartum fetal evaluation: Development of a fetal biophysical profile. Am. J. Obstet. Gynecol.136(6), 787–795. 10.1016/0002-9378(80)90457-3 (1980). [DOI] [PubMed] [Google Scholar]
- 11.Morita, M., Ryo, E., Kamata, H., Seto, M. & Yatsuki, K. Counting fetal movements of small-for-gestational infants using a fetal movement acceleration measurement recorder. J. Matern. Fetal Neonat. Med.33, 3699–3705. 10.1080/14767058.2019.1583732 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto, K. et al. Gross motor function and general development of babies born after assisted reproductive technology. J. Obstet. Gynaecol. Res.42, 266–272. 10.1111/jog.12898 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Drenth Olivares, M. et al. IVF procedures are not, but subfertility is associated with neurological condition of 9-year-old off spring. Early Hum. Dev.129, 38–44. 10.1016/j.earlhumdev.2018.12.017 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Rumbold, A. R. et al. The impact of specific fertility treatments on cognitive development in childhood and adolescence: A systematic review. Hum. Reprod.32, 1489–1507. 10.1093/humrep/dex085 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Zhan, Q. et al. An overview of studies on psychological well-being in children born flowing assisted reproductive technologies. J. Zhejiang Univ. Sci. B14, 947–960. 10.1631/jzus.B1300101 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu, L. et al. Association between assisted reproductive technology and the risk of autism spectrum disorders in the offspring: a meta-analysis. Sci Rep7, 46207 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurjak, A. et al. New scoring system for fetal neurobehavior assessed by three and four-dimensional sonography. J. Perinat. Med.36, 73–81. 10.1515/JPM.2008.007 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Morokuma, S. et al. Ultrasound evaluation of fetal brain dysfunction based on behavioral patterns. Brain Dev.35, 61–67. 10.1016/j.braindev.2012.01.007 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available in the Supplementary File.