Abstract
Epithelioid glioblastoma (Ep-GBM) is a rare variant of glioblastoma characterized by a high recurrence rate and poor prognosis. Currently, there is no established standard treatment for Ep-GBM. Therefore, we identified 58 Ep-GBM cases to investigate these characteristics and identify the possible prognostic factors of survival. There were 30 male and 28 female patients with a median age of 39 years. Headaches and dizziness were the most common clinical symptom. The tumor is most frequently located in the temporal lobe (36.2%). The positivity rate for BRAF-V600E is 56.9% (33/58), for MGMT is 56.9% (33/58), and for INI-1 is 75% (30/40). Tumor recurrence was observed in 39 patients. The median progression-free survival (PFS) of all patients was 12.7 months, while the median overall survival (OS) was 29.1 months. Additionally, the median survival time after recurrence was 14.3 months. Both univariate and multivariate COX regression analyses revealed that individuals who received more than six cycles of adjuvant oral temozolomide experienced a longer median PFS compared to those who received fewer cycles. Characteristics associated with poorer PFS included tumor dissemination prior to initial surgery. Additionally, both analyses identified tumor dissemination, radiotherapy and adjuvant oral temozolomide as predictors of OS. Notably, for patients with recurrent Ep-GBM, reoperation was shown to significantly increase survival time after recurrence. In conclusion, the standard Stupp regimen is also applicable to patients with Ep-GBM, extending adjuvant oral temozolomide could further improve survival for Ep-GBM patients, reoperation may also prolong survival for recurrent Ep-GBM.
Keywords: Epithelioid glioblastoma, Chemoradiotherapy, Reoperation, Recurrent Ep-GBM
Introduction
Glioblastoma (GBM) stands as the most common malignant tumor of the central nervous system, with the highest occurrence rate among all brain tumors [1]. In 2016, a new GBM subtype, epithelioid glioblastoma (Ep-GBM), was added to the World Health Organization (WHO) Classification of Tumors of the Central Nervous System (CNS). In the 2021 WHO classification of CNS tumors [2], Ep-GBM was still recognized as a subtype of GBM. Ep-GBM distinguished it from other GBM types [3]. Histologically, Ep-GBM is manifests with abundant epithelioid and melanoma-like cells, which exhibit features such as abundant cytoplasm, eccentric nuclei, prominent nucleoli, and rhabdomyosin-like attributes [4]. Unlike conventional GBM, Ep-GBM is more prevalent in children and young adults and exhibits aggressive behaviors such as cerebrospinal fluid dissemination and central nervous system metastases. Ep-GBM presents a notably poor prognosis, with significantly worse outcomes than other GBM types [5]. Presently, therapeutic recommendations for Ep-GBM do not account for histological variations [6].
Recent studies suggest that Ep-GBM [7], as characterized by histopathological features, is not a singular diagnostic entity. Instead, it consists of at least three distinct tumor subtypes: PXA-like tumors, IDH-wildtype GBM-like tumors, and RTK1 pediatric GBM-like neoplasms. Each of these subtypes differs molecularly and biologically. They also vary significantly in their prevalence across different populations and exhibit distinct prognostic outcomes. Due to the relative rarity of Ep-GBM, comprehensive research on this tumor is limited. Most available literature consists of case reports or retrospective case series involving a relatively small number of cases. Therefore, the primary aim of this study was to thoroughly investigate and evaluate the clinicopathological features, treatment methods, and their impact on the prognosis of Ep-GBM. We anticipate that the findings of this study will provide valuable insights for facilitating the clinical diagnosis and treatment of Ep-GBM, as well as offer support and guidance to healthcare professionals and patients.
Materials and methods
Study participants
A retrospective review was conducted on glioma patients who received treatment at the Guangdong Sanjiu Brain Hospital and the First Affiliated Hospital of Jinan University. Progression-free survival (PFS) is defined as the period from surgery to the occurrence of postoperative tumor recurrence, metastasis, or the last follow-up date. Overall survival (OS) is typically defined as the duration from the surgical procedure to either the patient’s demise or the last follow-up visit. Recurrent survival time refers to the duration from surgery following recurrence to the patient’s death or last follow-up. For patients who were lost to follow-up midway through the study, those who died from causes unrelated to the study, or those who were still alive at the follow-up cutoff, we classified their survival time as censored data. Similarly, for patients who voluntarily withdrew from treatment, if they were still alive at the time of withdrawal, their data was also treated as censored. In this retrospective study, patient data were anonymized, eliminating the need for informed consent from the patients’ guardians. The study was conducted in accordance with the principles of the Declaration of Helsinki and received authorization from the hospital’s medical ethics committee.
Patient surveillance and follow-up
Patients with Ep-GBM underwent follow-up assessments involving brain MRI every 2 months after the initial radiotherapy for 2 or 3 years under the supervision of a multidisciplinary team. If a patient developed a new symptom or if the neurological symptoms deteriorated, then MRI was performed regardless of the scheduled follow-up period. When clinically indicated, surgery was performed to confirm the final diagnosis of a viable tumor. If surgery was not possible, then the viable tumor was determined using MRI in accordance with the RANO criteria and serial follow-up examinations with intervals of at least 3 months were performed. The clinicoradiological diagnosis was determined by a consensus reached during a multidisciplinary meeting involving two neuro-oncologists (all with 20 and 7 years of experience with neuro-oncology) and three neuroradiologists (with 18, 10 and 6 years of experience with neuro-oncologic imaging, respectively). When a contrast-enhancing lesion exhibited a steady increase in size during two or more successive follow-up MRI examinations within a 2- to 3-month interval. the patient was classified as having tumor recurrence. In contrast, when a contrast-enhancing lesion subsequently regressed or became stable without a change in treatment within 6 months of the index imaging, the patient was categorized as no recurrence.
The extent of tumor resection was determined by comparing a 24–72 h postoperative MRI to that of preoperative imaging. Gross total resection (GTR) was defined by the absence of visible residual tumors on postoperative T1-enhanced MRI findings, if only marginal enhancement of the resection cavity is observed on postoperative MRI imaging, it is classified as subtotal resection (STR), whereas the presence of residual tumor on these MRI results was designated as partial resection (PR). All cases were reviewed by a multidisciplinary neuro-oncology clinic comprised of neurosurgeons, neuro-oncologists, and radiation oncologists. Patients in good clinical condition with tumors that originate near the previous cavities and do not involve eloquent cortical areas, basal ganglia, diencephalic or brainstem structures, with a PFS of at least 6 months, are generally considered candidates for reoperation.
Pathological testing
Tumor tissue samples were fixed in 10% formalin and embedded in paraffin. Subsequently, the paraffin-embedded tumor sections, 3-μm thick, underwent staining using the standard hematoxylin and eosin staining method. Immunohistochemical staining was performed using the SP method and monoclonal antibodies against several markers, including glial fibrillary acidic protein (GFAP), methylguanine DNA methyltransferase (MGMT), oligodendrocyte transcription factor 2 (Olig-2), X-linked alpha-thalassemia mental retardation syndrome (ATRX), Integrase interactor (INI-1), BRAF-V600E (VE1), IDH-1, H3K27m, P53, and Ki-67 proliferation index.
Statistical analysis
Parametric data were expressed as means ± standard deviations and compared via the Student t-test. Nonparametric data were expressed as median values (interquartile range) and compared via the Mann–Whitney U-test. Percentages were compared via the chi-square test or Fisher exact test based on sample size. Survival analysis was conducted using the Kaplan–Meier method, with intergroup comparisons facilitated by the log-rank test. Factors influencing patient endpoint events were analyzed using both univariate and multivariate Cox regression methods. Factors with a p-value < 0.2 in the univariate analysis were subsequently included in the multivariate analysis. The significance level was set at p < 0.05. All statistical analyses were performed using SPSS 27.0.
Results
Patient demographics
From January 2017 to January 2024, Guangdong Sanjiu Brain Hospital and the First Affiliated Hospital of Jinan University diagnosed approximately 1,500 cases of glioblastoma. Within this period, 68 cases were specifically identified as Ep-GBM. Due to incomplete clinical data for 10 of these patients, they were excluded from our study, resulting in a final cohort of 58 Ep-GBM cases for analysis. The median age was 39 years, ranging from of 5 to 70 years. Regarding the gender distribution, 30 patients were male and 28 female. The pre-operative KPS scores for all patients averaged 80, with scores ranging from 30 to 100. The most frequently reported initial symptoms among the patients were headache and dizziness, which occurred in 42 cases, followed by limb weakness and sensory abnormalities in 6 cases, epilepsy in 6 cases, memory loss in 3 cases, and blurred vision in both eyes in 1 case.
Except for 1 case located in the left cerebellar hemisphere, patients with Ep-GBM predominantly exhibited tumor onset inside the cerebral hemispheres. There were 25 cases in the right cerebral hemisphere, with 10 cases located in the right temporal lobe, 5 in the right frontal lobe, and 10 cases involving multiple lobes. Additionally, 31 cases were found in the left cerebral hemisphere, including 11 cases in the left temporal lobe, 5 cases in the left parietal lobe, 3 cases in the left frontal lobe, 1 case in the left occipital lobe, 1 case in the left thalamus, and 10 cases involving multiple lobes. In addition, 1 case was mentioned in the callosal pressure Sect. 22 cases experienced tumor metastasis, of which 17 had leptomeningeal dissemination and 5 had cerebrospinal fluid dissemination. Clinical data are summarized in Table 1.
Table 1.
Demographics of patients with epithelioid glioblastoma
| Parameter | Parameter | ||
|---|---|---|---|
| Age (years) | Extent of resection (%) | ||
| Median age | 39 | GTR | 41(70.7%) |
| Range | 5–70 | STR | 8(13.8%) |
| Pre-operative KPS | PR | 9(15.5%) | |
| Median KPS | 80 | Stupp regimen (%) | 38(65.5%) |
| Range | 30–100 | Recurrence (%) | 39(67.2%) |
| Sex (%) | Local Recurrence | 30(51.7%) | |
| Male | 30(51.7%) | Distant Recurrence | 9(15.5%) |
| Female | 28(48.3%) | Recurrent KPS | |
| Presenting symptom (%) | Median KPS | 70 | |
| Headaches & dizziness | 42(72.4%) | Range | 50–90 |
| Limb weakness | 6(10.3%) | Treatment after recurrence (%) | |
| Epileptic | 6(10.3%) | Surgery & radiotherapy | 14(35.9%) |
| Diplopia | 1(1.7%) | Only Surgery | 4(10.3%) |
| Other | 3(5.2%) | Only radiotherapy | 5(12.8%) |
| Tumor location (%) | Supportive therapy | 16(41.0%) | |
| Temporal lobe | 21(36.2%) | Bevacizumab (%) | 10(17.2%) |
| Frontal lobe | 8(13.7%) | BRAF inhibitor (%) | 6(10.3%) |
| Parietal lobe | 5(8.6%) | State of survival (%) | |
| Other | 4(6.8%) | Censored | 23(39.7%) |
| Multiple lobes | 20(34.5%) | Mortality | 35(60.3%) |
| Tumor dissemination | 22(37.9%) | Median PFS (months) | 12.7 |
| Leptomeningeal dissemination | 17(29.3%) | Median OS (months) | 29.1 |
| CSF diffusion | 5(8.6%) | Median survival time after Recurrence (months) | 14.3 |
KPS Karnofsky Performance Scale, CSF Cerebrospinal Fluid, GTR Gross Total Resection, STR Subtotal Resection, PR Partial Resection, PFS Progression-free Survival, OS Overall Survival
Treatment
Initial treatment
In our study of patients diagnosed with Ep-GBMs, surgical resection was considered as the primary treatment approach. GTR was successfully completed in 41 patients, while 8 individuals underwent STR and 9 individuals underwent PR. Following the surgical procedures, 38 patients were treated using the Stupp regimen, which includes fractionated conformal three-dimensional radiotherapy to a total dose of 60 Gy in 30 daily fractions of 2 Gy each was delivered, using the entire T2/FLAIR hyperintense signal to define the clinical target volume (CTV). Concomitant chemotherapy consisted of oral temozolomide at a daily dose of 75 mg/m2 given 7 days per week from the first to the last day of radiotherapy, for at most 49 days. After a 4-week break, patients received adjuvant oral temozolomide (150–200 mg/m2) for 5 days every 28 days. Among the patients who received radiotherapy, only one child was treated with a radiation dose of 50 Gy in 25 fractions. 38 patients received adjuvant oral TMZ for up to 6 cycles, while 20 patients received it for 8 to 12 cycles. 2 participants were administered a BRAF inhibitor.
Treatment after recurrence
Throughout the follow-up period, tumor recurrence was observed in 39 patients, 30 individuals had local recurrence, and 9 patients had dissemination via cerebrospinal fluid. Among those who experienced recurrence, 19 patients received radiotherapy, including 12 patients reirradiation as part of their treatment. 14 patients underwent surgery combined with chemoradiotherapy, 4 patients chose surgery alone, 5 patients received radiation therapy alone, and 16 patients were solely treated with TMZ chemotherapy or other forms of supportive therapy. In the trial, 4 participants were administered a BRAF inhibitor, and 1 patient received combination therapy that included both BRAF and MEK inhibitors. The majority of patients were treated to a dose of 35 to 40 Gy in 10 total fractions. In special circumstances where overlap with prior radiation fields was minimal, doses were escalated to 50 to 60 Gy in conventional 2 Gy fractions.
Pathologic and immunohistochemistry
Microscopically, the tumor cells exhibited infiltrative growth with densely arranged regular or round cells. Some of these cells displayed an epithelioid or rhabdomyoid shape and lacked adhesion while maintaining a clear cellular membrane and eosinophilic cytoplasm. Nuclei were frequently enlarged and irregularly shaped, often accompanied by prominent nucleoli, indicating prevalent nuclear atypia, which occasionally led to the formation of multinucleated giant tumor cells. No intratumoral microvascular or glomeruloid-like vascular hyperplasia was observed. Tumor cells exhibited a pattern of arrangement around blood vessels, forming a pseudopapillary structure. Moreover, the presence of digitiform necrosis and pseudo-fenestrated necrosis features could be observed within the tissue. Immunohistochemistry findings revealed positive expression of various markers: BRAF-V600E (56.9%, 33/58), MGMT (56.9%, 33/58), GFAP (89.6%, 52/58), Olig-2 (93.1%, 54/58), ATRX (86.2%, 50/58), P53 (72.4%, 42/58), INI-1 (75%, 30/40). The Ki-67 proliferation index ranged from 3–80%, with a mean of 28.7%. Notably, IDH-1 and H3K27m were observed to be negative.
Analysis of survival prognostic factors
Progression-free Survival
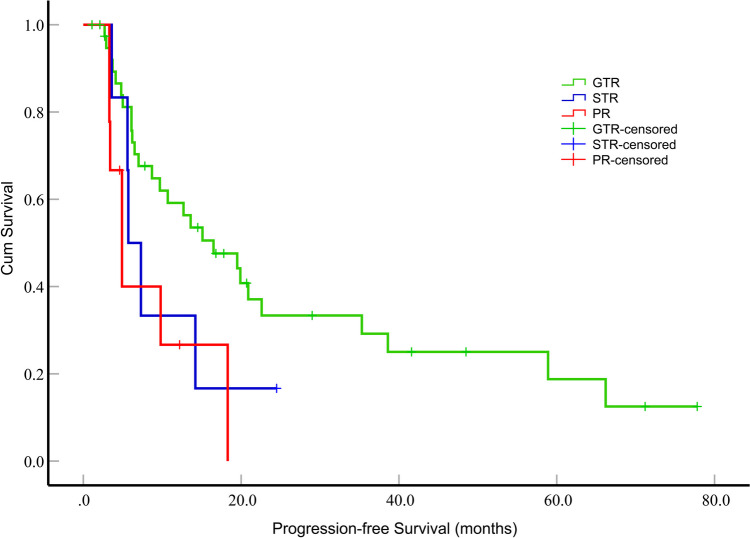
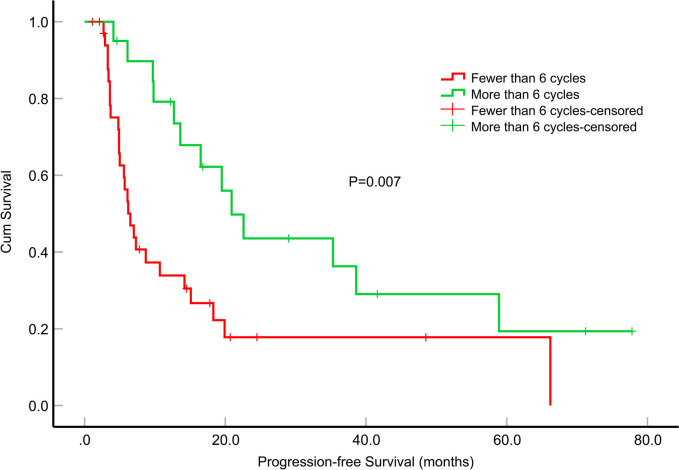
The median PFS for the patients was 12.7 months (95% confidence interval [CI], 6.744–18.656). Among these patients, those who underwent GTR exhibited a significantly longer median PFS than those who underwent PR (P = 0.017) (Fig. 1). However, the differences in median PFS between GTR and STR, as well as between STR and PR, were not statistically significant (P = 0.187 and P = 0.542, respectively). Additionally, patients who received more than 6 cycles of TMZ adjuvant chemotherapy demonstrated a significantly extended median PFS compared to patients who received fewer than 6 cycles (P = 0.007) (Fig. 2). In the univariate survival analysis, the PFS of the patients was examined in relation to various parameters. Notably, only the tumor dissemination (P = 0.038, hazard ratio [HR] = 1.974, 95% CI, 1.039–3.750), GTR (P = 0.019, HR = 2.848, 95% CI, 1.186–6.837) and the number of cycles of adjuvant chemotherapy with TMZ (P = 0.009, HR = 0.404, 95% CI, 0.204–0.799) were significantly associated with PFS. To further investigate these relationships, a multifactorial Cox proportional hazards model was constructed, which incorporated variables such as tumor dissemination, extent of tumor resection, the number of adjuvant chemotherapy cycles with TMZ, concomitant chemoradiotherapy, and BRAF-V600E mutation status. The tumor dissemination (P = 0.017, HR = 2.652, 95% CI, 1.189–5.916) and the number of cycles of adjuvant chemotherapy with TMZ (P = 0.011, HR = 0.354, 95% CI, 0.160–0.786) were significantly associated with PFS (Table 2).
Fig. 1.
Impact of the extent of resection on progression-free survival (months) in patients with epithelioid glioblastoma. The patient who underwent GTR exhibited a significantly longer median PFS compared to those who underwent PR (P = 0.017). In contrast, patients who underwent GTR showed a longer median PFS than those who underwent STR, but this difference was not statistically significant (P = 0.187)
Fig. 2.
Impact of the number of cycles of adjuvant oral temozolomide on progression-free survival (months) in patients with epithelioid glioblastoma
Table 2.
Survival analyses for prognosticators of progression-free survival in epithelioid glioblastoma patients
| Parameter | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% Confidence Interval | P | HR | 95% Confidence Interval | P | |
| Age | 1.007 | 0.985–1.029 | 0.524 | |||
| Sex | 1.247 | 0.659–2.362 | 0.498 | |||
| Tumor stroke | 0.886 | 0.387–2.027 | 0.774 | |||
| Tumor dissemination | 1.974 | 1.039–3.750 | 0.038 | 2.652 | 1.189–5.916 | 0.017 |
| Extent of resection | ||||||
| GTR | 1 (Reference) | 1 (Reference) | ||||
| STR | 1.873 | 0.708–4.957 | 0.206 | 0.653 | 0.202–2.110 | 0.477 |
| PR | 2.848 | 1.186–6.837 | 0.019 | 1.600 | 0.606–4.226 | 0.343 |
| Pre-operative KPS | 1.010 | 0.989–1.031 | 0.354 | |||
| Concomitant chemoradiotherapy | 0.572 | 0.284–1.154 | 0.119 | 0.676 | 0.302–1.515 | 0.341 |
| Adjuvant oral TMZ | 0.404 | 0.204–0.799 | 0.009 | 0.354 | 0.160–0.786 | 0.011 |
| Ki-67 | 0.612 | 0.277–1.354 | 0.225 | |||
| BRAF-V600E | 1.691 | 0.870–3.287 | 0.121 | 0.772 | 0.364–1.637 | 0.500 |
| MGMT | 0.920 | 0.484–1.748 | 0.799 | |||
| GFAP | 1.020 | 0.358–2.907 | 0.970 | |||
| ATRX | 1.257 | 0.481–3.281 | 0.641 | |||
| Olig-2 | 0.618 | 0.188–2.035 | 0.429 | |||
| P53 | 0.897 | 0.460–1.750 | 0.750 | |||
GTR Gross Total Resection, STR Subtotal Resection, PR Partial Resection, KPS Karnofsky Performance Scale, TMZ temozolomide, MGMT Methylguanine DNA Methyltransferase, GFAP Glial Fibrillary Acidic Protein, ATRX X-linked alpha-thalassemia mental retardation syndrome, Olig-2 Oligodendrocyte Transcription Factor 2
Overall survival
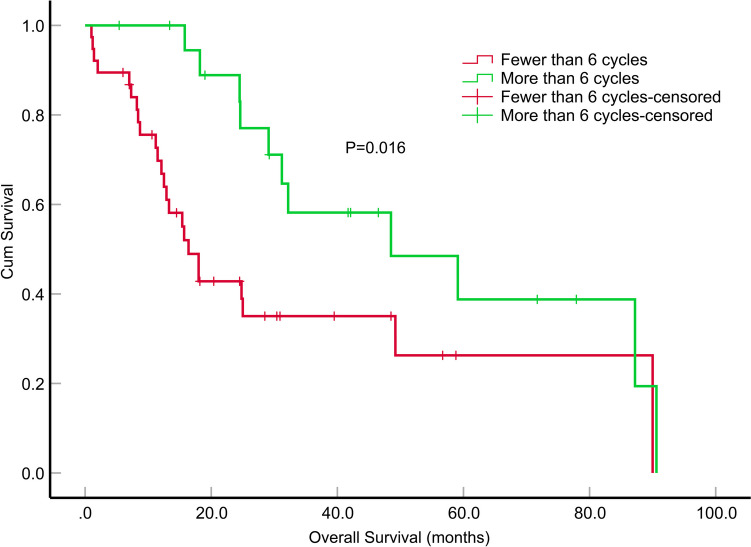
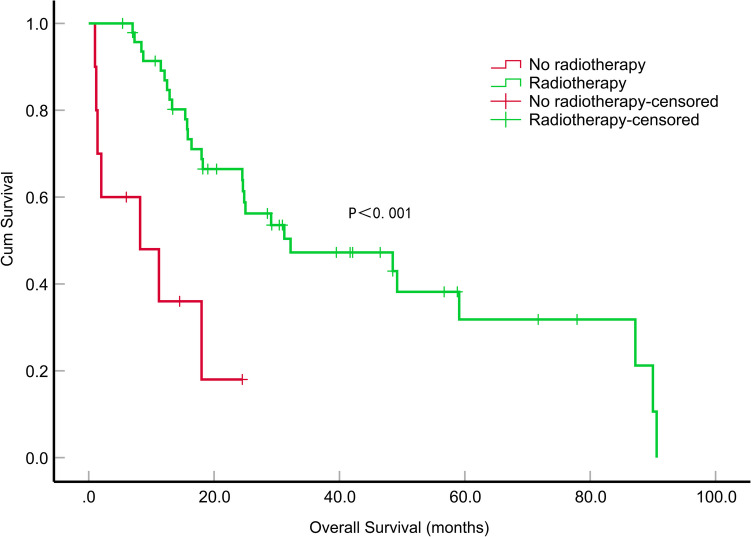
The median OS of the patients was 29.1 months (95% CI, 21.305–36.895). During the first year, the survival rate was 78.4%, which declined to 56.8% in the second year and further dropped to 18.9% by the fifth year. Among these patients, those who underwent GTR exhibited a significantly longer median OS than those who underwent STR (P = 0.030). However, the differences in median OS between GTR and PR, as well as between STR and PR, were not statistically significant (P = 0.710 and P = 0.146). Patients who received more than 6 cycles of TMZ adjuvant chemotherapy demonstrated a significantly extended median OS compared to patients who received fewer than 6 cycles (P = 0.016) (Fig. 3). A significant difference in the median OS was observed between patients who received radiotherapy and those who did not (P < 0.001) (Fig. 4). In the univariate survival analysis, GTR was associated with a longer median OS than STR (P = 0.029, HR = 2.861, 95% CI, 1.113–7.352), tumor dissemination (P = 0.049, HR = 1.984, 95% CI, 1.003–3.923), radiation therapy (P < 0.001, HR = 0.200, 95% CI, 0.081–0.495), the number of cycles of adjuvant chemotherapy with TMZ (P = 0.020, HR = 0.408, 95% CI, 0.192–0.868) showed a significant relation to OS. A multifactorial Cox proportional risk model was constructed including tumor dissemination, extent of tumor resection, administration of radiotherapy and the number of adjuvant chemotherapy cycles with TMZ. Similarly, tumor dissemination (P = 0.004, HR = 3.648, 95% CI, 1.507–8.975), radiotherapy (P = 0.019, HR = 0.223, 95% CI, 0.066–0.781), the number of cycles of adjuvant chemotherapy with TMZ (P = 0.007, HR = 0.254, 95% CI, 0.093–0.689) were significantly correlated with OS (Table 3).
Fig. 3.
Impact of the number of cycles of adjuvant oral temozolomide on overall survival (months) in patients with epithelioid glioblastoma
Fig. 4.
Impact of radiotherapy on overall survival (months) in patients with epithelioid glioblastoma
Table 3.
Survival analyses for prognosticators of overall survival in epithelioid glioblastoma patients
| Parameter | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% Confidence Interval | P | HR | 95% Confidence Interval | P | |
| Age | 1.008 | 0.985–1.032 | 0.513 | |||
| Sex | 1.863 | 0.911–3.809 | 0.288 | |||
| Tumor dissemination | 1.984 | 1.003–3.923 | 0.049 | 3.648 | 1.507–8.975 | 0.004 |
| Extent of resection | ||||||
| GTR | 1 (Reference) | 1(Reference) | ||||
| STR | 2.861 | 1.113–7.352 | 0.029 | 0.439 | 0.107–1.802 | 0.253 |
| PR | 1.206 | 0.451–3.224 | 0.708 | 0.517 | 0.163–1.641 | 0.263 |
| Tumor stroke | 0.919 | 0.397–2.129 | 0.844 | |||
| Pre-operative KPS | 1.015 | 0.991–1.039 | 0.221 | |||
| Radiotherapy | 0.200 | 0.081–0.495 | < 0.001 | 0.223 | 0.066–0.781 | 0.019 |
| Adjuvant oral TMZ | 0.408 | 0.192–0.868 | 0.020 | 0.254 | 0.093–0.689 | 0.007 |
| Ki-67 | 0.583 | 0.247–1.378 | 0.219 | |||
| BRAF inhibitor | 0.842 | 0.294–2.411 | 0.749 | |||
| BRAF-V600E | 1.034 | 0.502–2.130 | 0.927 | |||
| MGMT | 1.459 | 0.716–2.973 | 0.298 | |||
| ATRX | 1.504 | 0.555–4.075 | 0.423 | |||
| GFAP | 0.582 | 0.222–1.526 | 0.271 | |||
| Olig-2 | 0.403 | 0.119–1.368 | 0.245 | |||
| P53 | 1.027 | 0.488–2.160 | 0.944 | |||
GTR Gross Total Resection, STR Subtotal Resection, PR Partial Resection, KPS Karnofsky Performance Scale, TMZ temozolomide, MGMT Methylguanine DNA Methyltransferase, GFAP Glial Fibrillary Acidic Protein, ATRX X-linked alpha-thalassemia mental retardation syndrome, Olig-2 Oligodendrocyte Transcription Factor 2
Recurrent survival
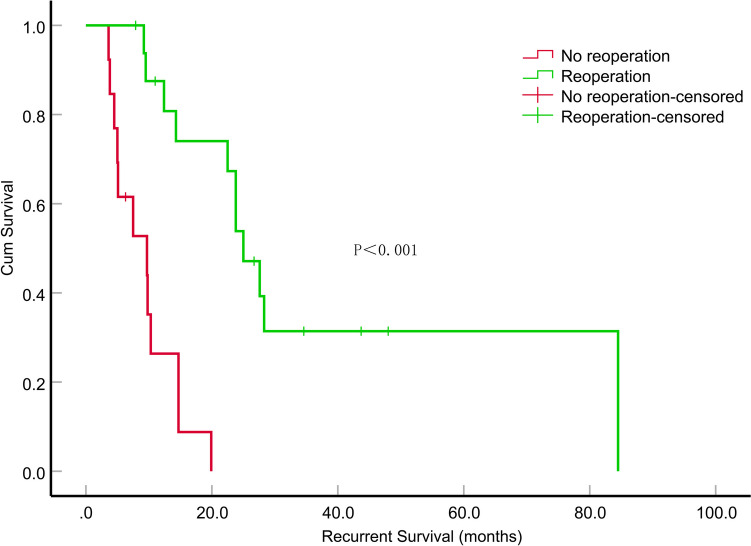
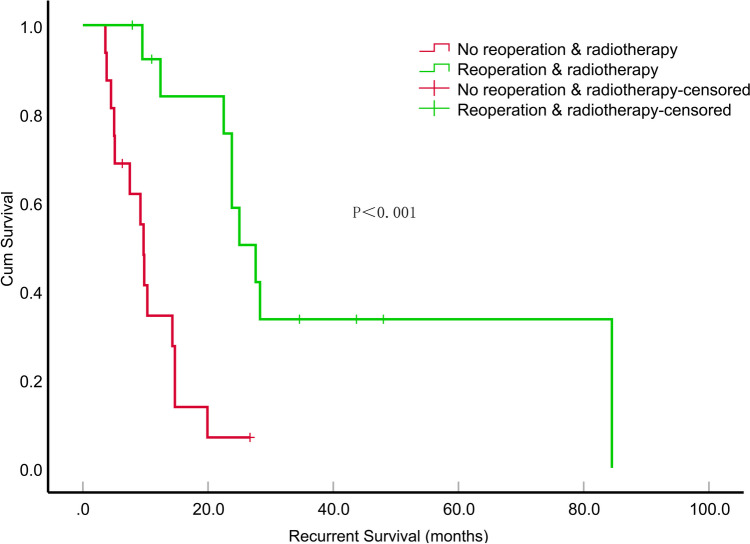
The median survival time after relapse was 14.3 months (95% CI, 8.201–20.399). We analyzed the local recurrence cases by dividing them into subgroups based on treatment type: surgical versus non-surgical and irradiated versus non-irradiated. Patients who underwent surgical treatment experienced a significantly longer median survival time compared to those in the non-surgical group (P < 0.001) (Fig. 5). Furthermore, patients who received a combination of reoperation and radiotherapy had an even longer median survival time than those in either of the other treatment groups (P < 0.001) (Fig. 6). In the univariate survival analyses, only sex (P = 0.047, HR = 2.451, 95% CI, 1.011–5.939) and reoperation (P < 0.001, HR = 0.116, 95% CI, 0.036–0.371) demonstrated a significant correlation with survival time after recurrence. A multifactorial Cox proportional risk model was constructed including sex recurrence-KPS, reoperation, re-irradiation, MGMT mutation status. Notably, reoperation (P = 0.006, HR = 0.179, 95% CI, 0.053–0.611) were significantly correlated with survival time after recurrence (Table 4).
Fig. 5.
Impact of reoperation on recurrent survival (months) in patients with recurrent epithelioid glioblastoma
Fig. 6.
The impact of combining reoperation with radiotherapy on the survival duration (in months) of patients with recurrent epithelioid glioblastoma
Table 4.
Survival analyses for prognosticators of survival in recurrence epithelioid glioblastoma
| Parameter | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% Confidence Interval | P | HR | 95% Confidence Interval | P | |
| Age | 0.989 | 0.958–1.021 | 0.495 | |||
| Sex | 2.451 | 1.011–5.939 | 0.047 | 3.010 | 0.993–9.127 | 0.052 |
| Recurrent-KPS | 0.967 | 0.929–1.007 | 0.103 | 1.007 | 0.964–1.053 | 0.744 |
| Bevacizumab | 1.021 | 0.414–2.517 | 0.964 | |||
| Reoperation | 0.116 | 0.03634–0.371 | < 0.001 | 0.179 | 0.053–0.611 | 0.006 |
| Re-irradiation | 0.542 | 0.220–1.337 | 0.183 | 0.494 | 0.177–1.380 | 0.179 |
| Ki-67 | 0.934 | 0.364–2.395 | 0.888 | |||
| BRAF inhibitor | 1.180 | 0.397–3.506 | 0.766 | |||
| BRAF-V600E | 1.259 | 0.539–2.940 | 0.594 | |||
| MGMT | 2.111 | 0.861–5.180 | 0.103 | 2.571 | 0.767–8.618 | 0.126 |
| ATRX | 1.625 | 0.541–4.876 | 0.387 | |||
| GFAP | 0.285 | 0.035–2.324 | 0.241 | |||
| Olig-2 | 0.744 | 0.217–2.546 | 0.634 | |||
| P53 | 1154 | 0.464–2.871 | 0.757 | |||
KPS Karnofsky Performance Scale, MGMT Methylguanine DNA Methyltransferase, GFAP Glial Fibrillary Acidic Protein, ATRX X-linked alpha-thalassemia mental retardation syndrome, Olig-2 Oligodendrocyte Transcription Factor 2
Discussion
Ep-GBM is a newly classified histological subtype of GBM included in the 2016 WHO Classification of Tumors of the Central Nervous System [8]. Ep-GBM accounts for approximately 3% of all GBM cases [9]. The course of Ep-GBM is an aggressive one and is often complicated by early recurrence, intratumoral hemorrhage and leptomeningeal spread [10]. Ep-GBM frequently exhibit BRAF V600E, TERT promoter mutations and CDKN2A/B homozygous deletions, these alterations tend to coexist in Ep-GBM [11]. Dramatic responses to BRAF inhibitors have been reported anecdotally in BRAF-V600E mutant examples, emphasizing that this variant may have several important differences from that of conventional GBM [10]. There is a scarcity of studies focusing on the clinical and pathological characteristics, as well as treatment outcomes related to Ep-GBM, especially regarding treatment options after Ep-GBM recurrence. Therefore, this study is unique and aims to provide more specific information on the prognosis and therapeutic choices for these cancers.
The literature presents varying prognoses for Ep-GBM. Chatterjee et al. [12] conducted a study where they reported a median survival time of 25.5 months among 24 patients diagnosed with Ep-GBM. However, Wang et al. [13] reported a significantly lower median survival time of only 10.6 months for Ep-GBM. These differences in the survival outcomes could be attributed to the treatment approach employed in their study. Specifically, Wang et al. [13] found that 48.4% of patients received concomitant chemoradiotherapy, compared with that of 67.2% in our study. Additionally, the absence of a defined treatment regimen for post-tumor recurrence in their study could also contribute to the disparity in survival times. Drexler et al. [14] demonstrate a survival benefit from maximized extent of resection for newly diagnosed and recurrent glioblastomas of the RTK I and RTK II. Similarly, in a study conducted by Lu et al. [5], patients with Ep-GBM who underwent GTR had longer PFS and OS than those who underwent PR. In our study, we found that patients who underwent GTR had longer survival times than those who did not receive GTR. We found that patients who received radiotherapy experienced a significant extension in median OS compared to those who did not. This aligns with the findings of Sun et al. [15] Standard treatment for GBM was radiation with concomitant and adjuvant TMZ for 6 cycles, although the optimal number of cycles of adjuvant TMZ had long been a subject of debate. The study by Balana et al. [16] demonstrated that extending adjuvant TMZ did not improve PFS or OS in any GBM patient subset. However, we found a correlation between an increased number of cycles of adjuvant chemotherapy with TMZ and improved PFS and OS.
Our study revealed a substantial recurrence rate of 67.2% among patients diagnosed with Ep-GBM, indicating a high likelihood of recurrence in this subtype. However, there is limited literature on the treatment of this specific subtype in its recurrent state. Previous research [17] supported the potential benefits of reoperation in managing recurrent GBM. Our study findings suggest that reoperation is equally relevant and applicable in the management of recurrent Ep-GBM, offering a potential avenue for improved outcomes and extended survival. Re-irradiation has been shown to be a feasible and effective treatment option for recurrent gliomas, as supported by published evidence [18, 19]. The study suggested that combining reoperation, chemotherapy, or re-irradiation as treatment modalities leads to a substantial improvement in survival compared to using individual treatments alone [20]. In addition, no clear survival advantages have been observed by other authors [21]. Our study focused on recurrent Ep-GBM and found that the combination of re-irradiation and reoperation resulted in a significant increase in patient survival. The median survival time was 28.6 months, compared to 9.2 months with other groups.
Among BRAF mutations, V600E is most frequently observed in gliomas [22]. Reports indicate that approximately 50% of Ep-GBM cases exhibit BRAF-V600E mutations, whereas conventional glioblastomas rarely show BRAF-V600E mutations [23, 24]. In our investigation, we observed BRAF-V600E mutant protein expression in 56.9% of cases. Previous studies have shown that gliomas with BRAF-V600E mutation have better prognoses than those without this mutation [25, 26]. Vemurafenib, a BRAF-V600E inhibitor, has been approved for treating malignant melanoma, papillary thyroid carcinoma and lung cancer. Strong clinical responses have been demonstrated in these settings, effectively reducing tumor development and progression caused by the BRAF-V600E mutation [27, 28]. The clinical efficacy of vemurafenib in the treatment of Ep-GBM has been active investigation [29–32]. According to Nakagomi et al. [33], vemurafenib has shown remarkable efficacy in reducing tumor cell survival and suppressing the phosphorylation of crucial intracellular signaling proteins. In our trial, 6 patients were treated with vemurafenib, 5 patients exhibited either steady or partial remission. Research [34] has demonstrated that the combination of BRAF and MEK inhibitors effectively inhibits tumor growth by dual-targeted activation of the MAPK pathway. This finding is supported by several recent clinical studies that established combination therapy with MEK inhibitors as a recognized therapeutic strategy for treating Ep-GBM [35, 36]. In our study, 1 patient developed resistance to vemurafenib after one year of treatment. To address this issue, the patient underwent BRAF-MEK inhibitors. Consequently, the patient experienced an additional 8 months survival benefit.
Limitations
Our study has several limitations. First, the retrospective nature of this study had inherent limitations. To overcome these limitations and provide more robust evidence, prospective studies are a more suitable approach for comparing therapeutic regimens for Ep-GBM. Additionally, only a few markers were analyzed using immunohistochemistry. Finally, the small sample size of our study should be noted. This limited sample size reduced the statistical power of our findings and may potentially limit the generalizability of the results.
Conclusions
In summary, our findings suggest that the standard Stupp regimen had demonstrated positive outcomes in extending the survival of patients with Ep-GBM. Extending adjuvant temozolomide could further improve survival for Ep-GBM patients. Reoperation may also prolong survival for those with recurrent Ep-GBM. Moreover, the development of targeted therapies promises to usher in a new era for the management of Ep-GBM.
Acknowledgements
The authors thank Juan Li, Li-chao Wang, and Min-ting Ye, doctors from Guangdong Sanjiu Brain Hospital, for their assistance with data collection. Additionally, we appreciate Dr. Yingxin Deng for her expertise in performing statistical analysis on the data. By leveraging her expertise, we ensure our analyses are thorough and accurate, leading to more reliable outcomes.
Author contributions
Conceptualization and design: MNS, DDF, MYL Acquisition of data: MNS, YG, JFZ, XYH, XJY. Analysis and interpretation of the data: SQL, YG, JFZ, XYH, XJY. Visualization: MNS, JFZ, XYH, XJY. Writing—original draft: MNS, SQL. Writing—review and editing: MNS, SQL, LBC, CZS. Final read and approval of the manuscript: all authors. Meng-nan Sun and Shao-qun Li contributed equally as the first authors of this study.
Funding
This study was supported by the Basic and Applied Basic Research Program of Guangzhou (Project No. 202201011741) and Guangzhou Science and Technology Project (Project Number: 2023A03J0609): Multimodal MRI Quantitative Analysis of Tumor Perfusion and Optimization of Anti-Angiogenesis Therapy Strategies Based on Tumor Microvascular Density.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval
This retrospective study was approved by the Ethics Committee of Guangdong Sanjiu Brain Hospital (No.202101017), and there was informed consent exemption for all patients.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lin-bo Cai, Email: cailinbo999@163.com.
Chang-zheng Shi, Email: tsczcn@jnu.edu.cn.
References
- 1.Stupp R, Taillibert S, Kanner A et al (2017) Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. Jama 318(23):2306–16. 10.1001/jama.2017.18718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Classification of Tumours Editorial Board (2021) Central nervous system tumours. Lyon (France): International Agency for Research on Cancer. (WHO classification of tumours series, 5th ed.; vol. 6). https://publications.iarc.fr/601
- 3.Matsumura N, Nakajima N, Yamazaki T et al (2017) Concurrent TERT promoter and BRAF V600E mutation in epithelioid glioblastoma and concomitant low-grade astrocytoma. Neuropathology 37(1):58–63. 10.1111/neup.12318 [DOI] [PubMed] [Google Scholar]
- 4.Perry A, Wesseling P (2016) Histologic classification of gliomas. Handbook Clin Neurol 134:71–95. 10.1016/B978-0-12-802997-8.00005-0 [DOI] [PubMed] [Google Scholar]
- 5.Lu VM, George ND, Brown DA et al (2019) Confirming Diagnosis and Effective Treatment for Rare Epithelioid Glioblastoma Variant: An Integrated Survival Analysis of the Literature. World Neurosurg 131:243–51.e2. 10.1016/j.wneu.2019.08.007 [DOI] [PubMed] [Google Scholar]
- 6.Weller M, Le Rhun E, Preusser M et al (2019) How we treat glioblastoma. ESMO Open 4(Suppl 2):e000520. 10.1136/esmoopen-2019-000520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korshunov A, Chavez L, Sharma T et al (2018) Epithelioid glioblastomas stratify into established diagnostic subsets upon integrated molecular analysis. Brain Pathol 28(5):656–62. 10.1111/bpa.12566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131(6):803–20. 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- 9.Zeng Y, Zhu X, Wang Y et al (2020) Clinicopathological, immunohistochemical and molecular genetic study on epithelioid glioblastoma: a series of fifteen cases with literature review. OncoTargets Ther 13:3943–52. 10.2147/OTT.S249317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexandrescu S, Korshunov A, Lai SH et al (2016) Epithelioid glioblastomas and anaplastic epithelioid pleomorphic Xanthoastrocytomas-Same entity or first cousins? Brain Pathol 26(2):215–23. 10.1111/bpa.12295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakajima N, Nobusawa S, Nakata S et al (2018) BRAF V600E, TERT promoter mutations and CDKN2A/B homozygous deletions are frequent in epithelioid glioblastomas: a histological and molecular analysis focusing on intratumoral heterogeneity. Brain Pathol 28(5):663–73. 10.1111/bpa.12572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatterjee D, Radotra BD, Aggarwal D et al (2021) Analysis of 24 cases of epithelioid glioblastoma: Experience from a tertiary centre of North India. Ann Diagn Pathol 50:151679. 10.1016/j.anndiagpath.2020.151679 [DOI] [PubMed] [Google Scholar]
- 13.Wang S, He Q, Zhang Q et al (2020) Clinicopathologic features and prognosis of epithelioid glioblastoma. Int J Clin Exp Pathol 13(7):1529–39 [PMC free article] [PubMed] [Google Scholar]
- 14.Drexler R, Schüller U, Eckhardt A et al (2023) DNA methylation subclasses predict the benefit from gross total tumor resection in IDH-wildtype glioblastoma patients. Neuro-oncology 25(2):315–25. 10.1093/neuonc/noac177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun K, Zhou X, Li T et al (2021) Clinicopathological characteristics and treatment outcomes of epithelioid glioblastoma. Neurosurg Rev 44(6):3335–48. 10.1007/s10143-021-01492-7 [DOI] [PubMed] [Google Scholar]
- 16.Balana Carmen, Vaz Maria Angeles, Sepúlveda Juan Manuel et al (2020) A phase II randomized, multicenter, open-label trial of continuing adjuvant temozolomide beyond 6 cycles in patients with glioblastoma (GEINO 14–01). Neuro-oncology 22(12):1851–61. 10.1093/neuonc/noaa107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suchorska B, Weller M, Tabatabai G et al (2016) Complete resection of contrast-enhancing tumor volume is associated with improved survival in recurrent glioblastoma-results from the DIRECTOR trial. Neuro-oncology 18(4):549–56. 10.1093/neuonc/nov326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navarria P, Pessina F, Clerici E et al (2022) Re-irradiation for recurrent high grade glioma (HGG) patients: Results of a single arm prospective phase 2 study. Radiother Oncol 167:89–96. 10.1016/j.radonc.2021.12.019 [DOI] [PubMed] [Google Scholar]
- 19.Minniti G, Niyazi M, Alongi F et al (2021) Current status and recent advances in reirradiation of glioblastoma. Radiat Oncol 16(1):36. 10.1186/s13014-021-01767-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azoulay M, Santos F, Shenouda G et al (2017) Benefit of re-operation and salvage therapies for recurrent glioblastoma multiforme: results from a single institution. J Neuro-Oncol 132(3):419–26. 10.1007/s11060-017-2383-2 [DOI] [PubMed] [Google Scholar]
- 21.Chapman CH, Hara JH, Molinaro AM et al (2019) Reirradiation of recurrent high-grade glioma and development of prognostic scores for progression and survival. Neuro-oncol Pract 6(5):364–74. 10.1093/nop/npz017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Nunno V, Gatto L, Tosoni A et al (2022) Implications of BRAF V600E mutation in gliomas: Molecular considerations, prognostic value and treatment evolution. Front Oncol 12:1067252. 10.3389/fonc.2022.1067252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleinschmidt-Demasters BK, Aisner DL, Birks DK et al (2013) Epithelioid GBMs show a high percentage of BRAF V600E mutation. Am J Surg Pathol 37(5):685–98. 10.1097/PAS.0b013e31827f9c5e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Behling F, Barrantes-Freer A, Skardelly M et al (2016) Frequency of BRAF V600E mutations in 969 central nervous system neoplasms. Diagn Pathol 11(1):55. 10.1186/s13000-016-0506-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korshunov A, Ryzhova M, Hovestadt V et al (2015) Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol 129(5):669–78. 10.1007/s00401-015-1405-4 [DOI] [PubMed] [Google Scholar]
- 26.Zhang RQ, Shi Z, Chen H et al (2016) Biomarker-based prognostic stratification of young adult glioblastoma. Oncotarget. 7(4):5030–41. 10.18632/oncotarget.5456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bollag G, Hirth P, Tsai J et al (2010) Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 467(7315):596–9. 10.1038/nature09454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chapman PB, Hauschild A, Robert C et al (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. New Engl J Med 364(26):2507–16. 10.1056/NEJMoa1103782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown NF, Carter T, Kitchen N et al (2017) Dabrafenib and trametinib in BRAFV600E mutated glioma. CNS Oncol 6(4):291–6. 10.2217/cns-2017-0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ceccon G, Werner J M, Dunkl V, et al (2018) Dabrafenib Treatment in a Patient with an Epithelioid Glioblastoma and BRAF V600E Mutation. Int J Mol Sci 19(4). 10.3390/ijms19041090 [DOI] [PMC free article] [PubMed]
- 31.Kanemaru Y, Natsumeda M, Okada M et al (2019) Dramatic response of BRAF V600E-mutant epithelioid glioblastoma to combination therapy with BRAF and MEK inhibitor: establishment and xenograft of a cell line to predict clinical efficacy. Acta Neuropathol Commun 7(1):119. 10.1186/s40478-019-0774-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith-Cohn M, Davidson C, Colman H et al (2019) Challenges of targeting BRAF V600E mutations in adult primary brain tumor patients: a report of two cases. CNS Oncol 8(4):Cns48. 10.2217/cns-2019-0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakagomi N, Sakamoto D, Hirose T et al (2020) Epithelioid glioblastoma with microglia features: potential for novel therapy. Brain Pathol 30(6):1119–33. 10.1111/bpa.12887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schreck KC, Morin A, Zhao G et al (2021) Deconvoluting mechanisms of acquired resistance to RAF inhibitors in BRAF(V600E)-Mutant human glioma. Clin Cancer Res 27(22):6197–208. 10.1158/1078-0432.CCR-21-2660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bahrami A, Hesari A, Khazaei M et al (2018) The therapeutic potential of targeting the BRAF mutation in patients with colorectal cancer. J Cell Physiol 233(3):2162–9. 10.1002/jcp.25952 [DOI] [PubMed] [Google Scholar]
- 36.Dratkiewicz E, Simiczyjew A, Pietraszek-Gremplewicz K, et al (2019) Characterization of Melanoma Cell Lines Resistant to Vemurafenib and Evaluation of Their Responsiveness to EGFR- and MET-Inhibitor Treatment. Int J Mol Sci 21(1). 10.3390/ijms21010113 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.