Abstract
The study investigated the effect of soilless media (burlap), on the morphological traits and antioxidant activities of microgreens from Brassicaceae, Amaranthaceae, and Linaceae families. The results revealed significant variations were observed in the selected morphological, biochemical composition, and antioxidant capacity of the microgreens. The radish sango and microgreens showed superior morphological characteristics compared to other microgreens. The elemental composition analysis revealed consistent moisture, ash, fat, fiber, and protein content across all families. The results revealed significant variations in the biochemical composition and antioxidant capacity of the microgreens, depending on the growing medium and between microgreens. Notably, microgreens differed in photosynthetic pigment profiles, with flaxseed and cabbage showing the highest chlorophyll content of 26.59 to 27.18 µg/g, FW and carotenoid content in a range of 3.74 to 6.39 µg/g, FW was observed in microgreens. The radish sango and beetroot microgreens exhibited elevated anthocyanin levels of 27.94–28.25 µmol/100 g, FW. Biochemical analysis indicated varying levels of ascorbic acid (177.58 to 256.46 mg/100 g, FW), total glucosinolate content (4.09 to 47.38 µmol/g, FW), phenolic content (131.44 to 298.56 mg GAE/100 g, FW), and flavonoid content (10.94 to 18.14 mg QUE/100 g, FW) were observed in selected microgreens families. Radish sango microgreens demonstrated the highest DPPH (76.82%, FW) and ABTS (88.49%, FW) radical scavenging activities, indicating superior antioxidant potential. The study showed that Brassicaceae microgreens are particularly rich in bioactive and antioxidant properties. Additionally, studies could assess the economic feasibility and scalability of soilless cultivation methods for microgreens to support their inclusion in sustainable agricultural practices and health-promoting diets.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-73973-w.
Subject terms: Plant physiology, Diseases
Introduction
The practice of cultivating various types of vegetable crops without soilless cultivation (comprising hydroponic and substrate-based) growing method, has become a practical alternative to conventional agriculture, especially in areas where soil quality is poor or there’s limited arable land1. This approach offers more benefits over soil based cultivation, including its help to enhance yields, optimize nutrient utilization, extend harvesting periods, and decrease vulnerability to soil-borne pathogens. Furthermore, the increasing impacts of climate change on agriculture farming practices and the diminishing availability of freshwater reserves emphasize the critical need to adopt effective farming techniques like hydroponic systems. These systems have the potential to mitigate the difficulties arising from water scarcity in agriculture, as evidenced by several recent studies1–3.
Microgreens, which are immature versions of leafy vegetables, are gaining attention in the culinary world due to their distinctive flavors and rich nutrient profiles4. They are identified by their early developmental stage, usually showcasing two developed cotyledon leaves and the beginnings of the first set of true leaves5,6. Commercial cultivation of microgreens dates back to the 1980s, but in recent years, they have surged in popularity among both restaurants and health-conscious individuals. As per the findings of, Allied Market Research, the microgreens industrial market was worth $1.3 billion in 2019 and is expected to climb to $2.2 billion by 2028, experiencing a compound annual growth rate exceeding 11% between 2021 and 20287. Microgreens, derived from various species of crop seeds including vegetables, herbs, grains, legumes, and beans, represent a burgeoning category of leafy greens. They offer superior bioavailability of minerals, vitamins, flavonoids, phenolic compounds, dietary fibers, enzymes, chlorophylls, and antioxidants, all of which contribute to reduced risks of cardiovascular diseases, cancer, and other health issues8.
The production of microgreens utilizes a wide range of growing techniques, such as indoor, outdoor, and controlled environments, incorporating both soil based and soilless systems9,10. Their rapid growth cycle (7–21 days), minimal spatial requirements, and rich nutritional profile render microgreens an ideal choice for urban and peri-urban agriculture as well as home cultivation, delivering essential nutrients to urban populations while minimizing transportation distances3. The numerous factors influencing the commercialization and home cultivation of microgreens include the selection of suitable species, growing methods, substrate types, seed quality and purity, sowing methods, germination processes, irrigation practices, fertilizer application, harvesting techniques, phytosanitary standards, post-harvest storage conditions6,11,12. Although there is limited comprehensive data on the nutritional composition of microgreens, multiple studies suggest that microgreens exhibit higher levels of antioxidant compounds in comparison to mature plants of similar varieties5,13.
Numerous studies have addressed effects of the cultivation of specific microgreens using hydroponic systems or soilless mediums supplemented with nutrients2,3,9. The peat and synthetic mats growing medium were used for the cultivation of rapini microgreens14. For the cultivation of mustard, leaf mustard, radish, and cabbage microgreens tissue media (tissue paper with water), foam media (foam with water), soil media, and soil + cow dung (1:1) media were used15. In another study, different formulated soilless media such as PitMoss, vermicast, sawdust, mushroom compost, perlite, and Pro-mix BX™ were used for the kale, swiss chard, arugula, and pak choi microgreens16. Apart from these the agriculture and food waste materials used for the cultivation of sunflower and water spinach microgreens17. The morphological parameters of microgreens are crucial in understanding physiology for several reasons. The overall growth and development of microgreens is a key indicator factor to determine the harvesting stage of microgreens18,19. The fresh weight and dry weight of these parameters generally indicate nutrient uptake and utilization during the selected growth period. Apart from these, it provides insights into the water content of plants, which is vital for understanding their water status and drought tolerance20. Moreover, leaf length and width are closely related to the plant’s photosynthetic capacity. A higher surface area of a leaf typically has more pigment content and can capture more sunlight enhancing the photosynthetic activity and promoting better growth21. The yield and biomass are directly linked to the economic value of microgreens in the market. The morphological parameters of microgreens are crucial from a food industrial perspective as they influence marketability, shelf life, yield, nutritional content, and product consistency22.
However, there is limited research examining the physical, nutrient, and antioxidant properties of these microgreens. Meanwhile, there is a dearth of research studies in the literature regarding the comparative analysis of various genotypes within microgreen families, as well as evaluating how growing conditions impact its quality parameters. The current study aims to utilize a soilless growing medium (burlap) for the commercial cultivation of high-quality microgreens, with emphasis on aspects such as biomass yields, biochemical properties, and antioxidant activity, all aimed at enhancing consumer nutrition (Fig. 1).
Fig. 1.
The different families selected microgreens grown in soilless growing medium.
Result and discussion
Effect of growing medium on morphological parameters
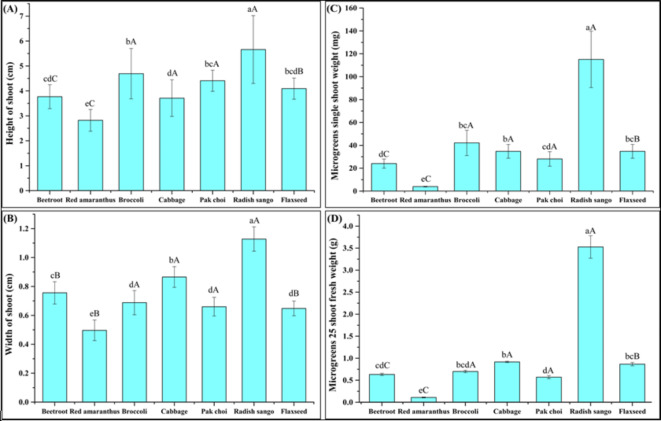
The morphological parameters are related to microgreens shoot (weight, height, and width), leaf (length, and width), and yield showed a significant (P < 0.05) effect on microgreens in soilless growing media as shown in (Figs. 2, 3 and 4). Among all the microgreens, radish sango microgreens were shown a higher shoot height (5.66 ± 1.36 cm) and width (1.33 ± 0.08 mm) in the soilless growing medium. The Brassicaceae families of microgreens such as broccoli, cabbage, pak choi, and radish sango microgreens showed better results in shoot height and width of microgreens as compared to other microgreens families Linaceae ˃ Amaranthaceae. Likewise, according to a study by Ntsoane et al.9 used the different microgreens of the Brassicaceae family (cabbage, rocket, and radish) were grown in different mediums (Hygromix, and Promix) with different seed densities (4, 8, and 12 seeds per cavity). This study showed a significant (P < 0.05) effect on the growing medium and seed densities on microgreen shoot height (22.3–47.97 mm). In another study different selected crops including mustard (6 to 11 cm), pearl millet (7 to 12 cm), mungbean (7 to 14 cm), red radish (6 to 9 cm), lentil (8 to 12 cm), and red cabbage (7 to 9 cm), microgreens shoot height were observed on the ninth days after sowing23. The red cabbage, broccoli, mizuna, green mustard, and pak choi microgreens were grown in substrates including (sand, organic soil, coco coir, rice husk, white sphagnum peat, and vermiculite) with different formulations, the shoot height of 5.8 to 7.2 cm were observed24. Similar findings of shoot length of microgreens were reported for, different formulated soilless media such as PitMoss, vermicast, sawdust, mushroom compost, perlite, and Pro-mix BX™ were used for the kale, swiss chard, arugula, and pak choi microgreens16. Previous studies showed significant differences in microgreen shoot height, mostly due to the variation in harvest times and some at the cotyledon stage, others at first true leaves, or after a set number of days18. These differences, along with microgreens seed sowing density25 and light irradiance12, significantly influenced this parameter results. Furthermore, the results also suggested that the variations in plant height of microgreens to the different media were dependent on genotypic differences16.
Fig. 2.
Effect of soilless growing medium on morphological parameters of selected microgreens families. The results were presented as mean ± standard deviations. The different lowercase letters within the bar indicate significant (P<0.05) differences among all the microgreens and uppercase letters in the bar indicate significant (P <0.05) differences among the different families (Amaeanthaceae, Brassicaceae, and Linaceae). Where, (A) Microgreens height of shoot (B) Microgreens width of shoot, (C) Microgreens single shoot weight (D) Microgreens 25 shoot fresh weight.
Fig. 3.
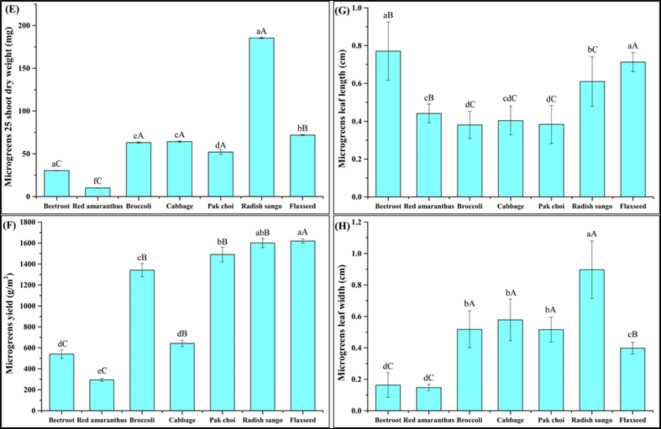
Effect of soilless growing medium on morphological parameters of selected microgreens families. The results were presented as mean ± standard deviations. The different lowercase letters within the bar indicate significant (P<0.05) differences among all the microgreens and uppercase letters in the bar indicate significant (P<0.05) differences among the different families (Amaeanthaceae, Brassicaceae, and Linaceae). Where (E) Microgreens 25 shoots dry weight, (F) Microgreens yield, (G) Microgreens leaf length, (H) Microgreens leaf width.
Fig. 4.
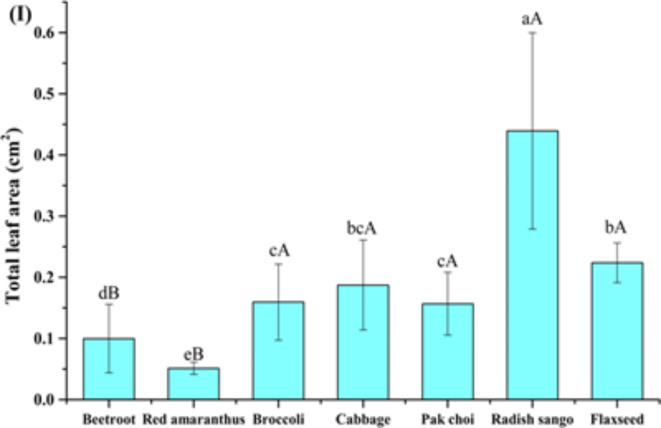
Effect of soilless growing medium on morphological parameters of selected microgreens families. The results were presented as mean ± standard deviations. The different lowercase letters within bar indicate significant (P<0.05) differences among all the microgreens and uppercase letters in bar indicate significant (P<0.05) differences among the different families (Amaeanthaceae, Brassicaceae, and Linaceae). Where, (I) Microgreens total leaf area.
The shoot width showed a significant effect (P < 0.05) on microgreen shoot width in selected microgreens families (Fig. 2B). The Brassicaceae families radish sango microgreens (1.13 ± 0.08 mm) showed higher shoot width compared to cabbage (0.87 ± 0.07 mm), beetroot (0.76 ± 0.08 mm), broccoli (0.69 ± 0.08 mm), pak choi (0.66 ± 0.06 mm), flaxseed (0.65 ± 0.05 mm) and red amaranthus (0.50 ± 0.07 mm) were observed. The Linaceae (flaxseed) and Amaranthaceae (red amaranthus and beetroot) microgreens showed a non-significant effect on the shoot width of microgreens. The morphological growth-related parameters of microgreens including shoot height, shoot width, and shoot weight these parameters are compared with soil based growing media revealing distinct differences in growth characteristics observed in both growing mediums. The comparison between both growing media is shown in (ESI Table 1). The soil based growing medium showed better results for shoot height, shoot width, and shoot weight of selected microgreens.
Table 1.
Selected microgreens families used for study and their specifications. Note: The above-mentioned microgreen seeds were provided by Seed Delivery LLP., Chandigarh, India. The germination percentage, physical purity, and genetic purity values were obtained from the information provided on the packaging materials by the producer.
| Families | Microgreens | Scientific name | Germination percentage (%) | Physical purity (%) | Genetic purity (%) | Sowing rate (g/m2) |
|---|---|---|---|---|---|---|
| Amaranthaceae | Beetroot | Beta vulgaris L. | 60 | 98 | 98 | 388.26 |
| Amaranthaceae | Red amaranthus | Amaranthus cruentus L. | 70 | 98 | 98 | 88.03 |
| Brassicaceae | Cabbage | Brassica oleracea var. capitate L. | 70 | 99 | 98 | 149.33 |
| Brassicaceae | Broccoli | Brassica oleracea var. italica | 70 | 98 | 98 | 190.20 |
| Brassicaceae | Pak choi | Brassica rapa | 75 | 98 | 98 | 125.75 |
| Brassicaceae | Radish sango | Raphanus sativus L. | 70 | 99 | 98 | 292.37 |
| Linaceae | Flaxseed | Linum usitatissimum L. | 80 | 99 | 98 | 314.38 |
The morphological parameters such as leaf length, leaf width, and total leaf area, with significant differences (P < 0.05) were observed among the different microgreens and their respective families, as shown in (Fig. 3G, and H and 4). The beetroot microgreens showed a leaf length (0.77 ± 0.15 cm), a leaf width (0.16 ± 0.08 cm), and a total leaf area (0.10 ± 0.06 cm²), while red amaranthus microgreens had a shorter leaf length (0.44 ± 0.05 cm), a similar leaf width (0.15 ± 0.02 cm), and a smaller total leaf area (0.05 ± 0.01 cm²) were observed. The Brassicaceae family radish sango microgreens showed the highest leaf width (0.90 ± 0.18 cm), and a total leaf area (0.16 ± 0.06 cm²) was observed. The significant differences (P < 0.05) within the selected families underline the diverse growth pattern of Brassicaceae microgreens in a soilless growing medium. The results reveal that the soilless medium effectively supported the growth of various microgreens, with significant differences observed across species and families. These findings are compared with the control growing medium (soil) (ESI Table 1a) to understand the growth performance of different microgreens in both growing mediums. The morphological parameters (leaf length, leaf width, and leaf area) of microgreens grown in soilless and soil based growing media revealed distinct differences in growth characteristics. The soilless growing medium showed better results as compared to the control growing medium.
The microgreens from the Brassicaceae family, particularly radish sango, exhibited the highest individual shoot weight, while red amaranthus, showed the lowest, as depicted in (Fig. 2C). Radish sango displayed the highest single-shoot weight among various microgreens, followed by broccoli, cabbage, flaxseed, beetroot, and red amaranthus, respectively. The (Figs. 2 and D and 3 and E) illustrate the results of fresh and dry shoot weights of randomly selected 25 shoots (n = 3) of the selected microgreens, showing a progressive increase in weight across different families. Specifically, radish sango microgreens from the Brassicaceae family demonstrated significantly higher fresh weight (3.53 ± 0.26 g) and dry weight (185.37 ± 0.54 mg) compared to other microgreens from the Linaceae and Amaranthaceae families. These findings are consistent with previous research on the fresh and dry weight of radish, cabbage, and rocket microgreens cultivated under varied conditions of selective media and seed density ranges9.
The highest microgreens fresh yield was obtained in the Linaceae family, specifically for flaxseed microgreens, while the lowest yield was observed in the Amaranthaceae family for specifically red amaranthus microgreens as shown in (Fig. 3 F). Following radish sango, pak choi, broccoli, and cabbage provided the highest fresh yield of microgreens. In assessing both the yield of microgreens, the sustainability of the production process heavily relies on the characteristics of the growing medium. The composition and properties of soilless growing mediums significantly impact nutrient content, crop growth, and yield26. The microgreens industry faces persistent challenges with lower yields, which remain a limiting factor22. The data concerning fresh yields of microgreens indicate similarity or slight improvement compared to the results reported for seventeen microgreen species in the range of (409.3 to 2258.8 g/m2)27 and for Brassicaceae microgreens families including kale, cabbage, arugula, and mustard for (535.6 to 871.2 g/m2) fresh yield of microgreens was observed in different growing medium containing sand, organic soil, coco coir, rice husk and humic acid in different percentage28. The morphological parameters including microgreens fresh yield, fresh weight, and dry weight of 25 microgreens shoots of microgreens grown in soilless and soil based growing media showed a significant effect (P < 0.05) for the 25 shoots dry weight of microgreens. Additionally, for the microgreens fresh yield and microgreens 25 shoot dry weight, no significant differences were observed as shown in (ESI Table 1b). The fundamental properties of a growing medium, such as nutrients, water-holding capacity, medium stability, aeration, and sufficient pH levels, are crucial for plant growth during the development stage29. Our experiment revealed a noteworthy impact stemming from the interplay of selected microgreens and growing medium on various morphological parameters. These results underscore the critical role of the growth medium in influencing microgreens selected morphological parameters. Additionally, our experimental findings suggest that soilless growing media offer a viable, cost-effective, and space-efficient alternative to traditional soil based cultivation. This method can be particularly beneficial in urban agriculture settings, where space and resources are limited. The easy, cheap, and fast-growing nature of soilless cultivation makes it an attractive option for producing high-quality microgreens across various species1.
Effect of growing medium on proximate composition
The proximate composition of microgreens belonging to the families (Amaranthaceae, Brassicaceae, and Linaceae), are shown in (Table 2). The various kinds of factors influencing moisture content include environmental parameters, method of processing, and harvesting and storage conditions. Our investigation indicates that there is no significant effect (P˃0.05) was observed in microgreens. Among the selected families, Amaranthace (beetroot) microgreens exhibited the highest moisture content (94.29 ± 0.71 g/100 g, FW), while Brassicaceae broccoli microgreens showed the lowest moisture (90.72 ± 0.21 g/100 g, FW). In another study by Kowitcharoen et al.30 moisture content (87.29–94.67 g/100 g, FW) was reported for fourteen microgreen crops. The effect of cultivation substrate on different sprouts of sorghum, wheat, horse gram, cowpea, mungbean, and fenugreek the moisture content similar range (90.28–90.74 g/100 g, FW), was observed31. In radish sango microgreens the moisture content was 90.84 to 92.20%, FW was grown in four distinct growing substrates containing soil, vermicompost, and cocopeat growing medium32. Another investigation was done on mustard, leaf mustard, radish, and cabbage microgreens grown in various soilless media and discovered nearly the same moisture content of 88.0 to 92.0%, FW was observed15. Our findings closely align with this study finding results.
Table 2.
Effect of soilless growing medium on proximate composition of selected microgreens families. The results were presented as mean ± standard deviations;(n=3). The different small letters within the column indicate significant (P<0.05) differences among the microgreens and different capital letters within the column indicate significant (P<0.05) differences among the families (Amaeanthaceae, Brassicaceae, and Linaceae) among the parameters. Where, BSA: Bovine serum albumin; FW: Fresh weight basis; DW: Dry weight basis. .
| Families | Microgreens | Moisture content (g/100 g, FW) | Ash content (g/100 g, FW (dry sample) | Fat content (g/100 g, FW (dry sample) | Fiber content (g/100 g, FW (dry sample) | Protein content (g/100 g, FW (dry sample) |
|---|---|---|---|---|---|---|
| Amaranthaceae | Beetroot | 94.29 ± 0.71aA | 12.11 ± 0.25bA | 6.08 ± 0.19bA | 11.03 ± 0.52aA | 2.89 ± 0.11bC |
| Red amaranthus | 92.54 ± 2.13aA | 14.92 ± 0.25aA | 5.71 ± 0.04aA | 8.28 ± 0.11cA | 2.04 ± 0.07cC | |
| Brassicaceae | Broccoli | 90.72 ± 0.21aA | 7.12 ± 0.47dB | 6.39 ± 0.53aB | 9.41 ± 0.40bcA | 2.87 ± 0.26bA |
| Cabbage | 91.20 ± 0.28aA | 8.73 ± 0.74cB | 6.03 ± 0.08cB | 10.69 ± 0.20abA | 3.86 ± 0.04aA | |
| Pak choi | 93.69 ± 0.88aA | 8.60 ± 0.13cB | 3.35 ± 0.15bB | 10.81 ± 0.39aA | 2.34 ± 0.04cA | |
| Radish sango | 91.82 ± 0.26aA | 6.20 ± 0.08deB | 2.13 ± 0.03cB | 10.56 ± 0.20abA | 4.28 ± 0.04aA | |
| Linaceae | Flaxseed | 93.02 ± 0.45aA | 4.91 ± 0.18eC | 5.50 ± 0.24aA | 9.79 ± 0.28abA | 2.86 ± 0.13bB |
The ash content of various microgreens families ranged from 4.91 ± 0.18 to 14.92 ± 0.25 g/100 g, FW with the highest concentration found in Amaranthaceae (red amaranthus) and the lowest in Linaceae (flaxseed) microgreens were observed. This range aligns closely with findings in other studies involving fenugreek, mungbean, cowpea, horse gram, wheat, and sorghum (9.12 to 9.71 g/100g)31, as well as white cabbage and red cabbage (16.06 to 25.60 g/100 g, DW)33, and black gram, mung bean, and chickpea microgreens (9.31 to 13.63 g/100g)34. In another study, ash content in radish microgreens in the range of 2.16 to 2.41 g/100 g, DW was observed in different formulated growing mediums32. The variations in the ash content of microgreens could be attributed to genotypic differences4.
The fat content varied among all families of microgreens ranging from 2.13 ± 0.03 g/100 g, FW (radish sango) to 6.39 ± 0.53 g/100 g, FW (broccoli) microgreens. Our analysis revealed significant differences (p˂0.05) in fat content among the Amaranthaceae (beetroot, and red amaranthus) and Linaceae (flaxseed) microgreens compared to Brassicaceae (broccoli, cabbage, pak choi, and radish sango) microgreens. The fat content is within a similar range to that reported in white cabbage and red cabbage microgreens (3.78 to 7.97 g/100 g) as per Podsędek et al.33. In the Brassicaceae (broccoli, Chinese kale, purple radish, radish rat-tailed radish, and red cabbage) and Fabaceae (lentil) microgreens the fat content was observed in the range of 0.38 to 0.50 g/100 g, FW and 0.43 g/100 g, FW observed in soilless growing medium30. Our findings suggest that microgreens, particularly those from the Amaranthaceae and Linaceae families, may have higher moisture content and lower fat content, may help prevent type-2 diabetes and weight gain because of their higher moisture and lower fat content15. Foods with a higher moisture content have more volume and weight without adding more calories, which helps with weight management by encouraging satiety and lowering caloric consumption overall. A lower-fat diet, especially one low in saturated fat, is also linked to a lower chance of insulin resistance and type 2 diabetes. Lower fat diets help to enhance insulin sensitivity and lipid profiles, both of which are important for both controlling and preventing type-2 diabetes35.
The fiber content of various microgreen families ranges from 8.28 ± 0.11 to 11.03 ± 0.52 g/100 g, FW as indicated in (Table 2). Beetroot microgreens from the Amaranthaceae family showed the highest fiber content, while red amaranthus microgreens exhibit the lowest fiber content. Additionally, our results are notably lower than those reported by Devi et al.36 for dried wheatgrass powder, which had higher fiber content. Dietary fiber plays a crucial role in supporting digestive, cardiovascular, and skin health, as well as in lowering blood cholesterol and glucose levels15.
The protein content in various families of microgreens varied, with Brassicaceae (radish sango) exhibiting the highest content and Amaranthaceae (red amaranthus) showing the lowest protein content. Among these families, Brassicaceae had the highest overall protein content, followed by Linaceae and Amaranthaceae. Similar protein ranges were observed in specific types of microgreens within these families: Brassicaceae (including broccoli, Chinese rat-tailed radish, radish, purple radish, kale, and red cabbage), Fabaceae (fenugreek, green pea, lentil, and mung bean), and other microgreens (black sesame, buckwheat, morning glory, and red roselle), as reported by Kowitcharoen et al.30. Ghoora et al.37 also found comparable protein content in ten culinary microgreens, ranging from 1.8 to 4.4 g/100 g.
The comparison of selected microgreens grown in soilless and soil media highlighted significant differences (P < 0.05) in their proximate parameters (ESI Table 2). The selected microgreens grown in a soilless growing medium exhibited higher moisture content compared to soil based growing medium. Similarly, soil grown microgreens showed higher ash, fat, fiber, and protein contents compared with soilless growing medium. The data concerning the proximate composition of microgreens indicate similarity between microgreens grown in both growing mediums. These findings suggest that soil media generally enhance the proximate content (ash, fat, fiber, and protein) of microgreens, potentially making them a more effective medium for their cultivation. The physicochemical properties of growing medium are crucial for plant growth during the development stage29. The variances observed in the proximate composition including moisture, ash, fat, fiber, and protein of microgreens can be attributed to disparities in their cultivation techniques6, growing environments31, microgreen varieties29,37, and nutrient solutions15. Additionally, the nutritional profile of microgreens is affected by factors like light intensity, consistent provision of specific supplements in nutrient solutions, and issues related to insects or pests38.
Effect of growing medium on photosynthetic pigments
As shown in (Table 3), the chlorophyll levels in various microgreens. Linaceae (flaxseed) microgreens exhibited the highest chlorophyll a content (15.82 ± 0.20 µg/g, FW), as well as the highest total chlorophyll (27.18 ± 0.01 µg/g, FW). Conversely, Amaranthaceae (beetroot) microgreens showed the lowest chlorophyll a content (3.44 ± 0.62 µg/g, FW), accompanied by minimal chlorophyll b levels (1.95 ± 0.04 µg/g, FW). The order of chlorophyll content across selected families was Linaceae > Brassicaceae > Amaranthaceae. Significant variations (p˂0.05) were noted in photosynthetic pigments content among different families and microgreens. Similar findings were reported by Altuner39 for various legume microgreens, indicating a range of chlorophyll content. Additionally, Fayezizadeh et al.40 explored the impact of Hoagland’s nutrient solution on basil cultivars and observed higher chlorophyll levels compared to our study. In another investigation the chlorophyll content in mustard, leaf mustard, radish, and cabbage microgreens ranged from 0.81 to 0.92 mg/100 g, FW, these findings values are lower than our finding result15. These differences in chlorophyll content highlight the influence of genotype and microgreen species. Evidence suggests that the concentrations of chlorophylls in younger leaves of the same species are lower compared to mature leaves, likely due to physiological factors. Additionally, some studies have not observed a significant impact of the growing media on phytochemical compounds when the harvesting cycle is short17.
Table 3.
Effect of soilless growing medium on photosynthetic pigment contents of selected microgreens families. The results were presented as mean ± standard deviations;(n=3). The different small letters within the column indicate significant (P<0.05) differences among the microgreens and different capital letters within the column indicate significant (P<0.05) differences among the families (Amaeanthaceae, Brassicaceae, and Linaceae) among the parameters. Where, FW: Fresh weight basis.
| Families | Microgreens | Chlorophyll a (µg/g, FW) | Chlorophyll b (µg/g, FW) | Chlorophyll a + b (µg/g, FW) | Total carotenoid (µg/g, FW) |
|---|---|---|---|---|---|
| Amaranthaceae | Beetroot | 3.44 ± 0.62eC | 1.95 ± 0.04eC | 11.72 ± 1.31dC | 6.12 ± 0.76aA |
| Red amaranthus | 4.51 ± 0.10eC | 2.25 ± 0.03eC | 13.69 ± 0.01dC | 4.68 ± 0.02bcA | |
| Brassicaceae | Broccoli | 9.77 ± 0.29cB | 4.27 ± 0.08bB | 20.72 ± 0.46bB | 4.39 ± 0.26bcA |
| Cabbage | 14.23 ± 0.19bB | 5.68 ± 0.08aB | 26.59 ± 0.40aB | 6.05 ± 0.10aA | |
| Pak choi | 6.13 ± 0.21dB | 3.00 ± 0.04dB | 15.99 ± 0.16cB | 3.74 ± 0.08cA | |
| Radish sango | 10.06 ± 0.12cB | 3.61 ± 0.13cB | 20.58 ± 0.16bB | 6.39 ± 0.36aA | |
| Linaceae | Flaxseed | 15.82 ± 0.20aA | 4.47 ± 0.22bA | 27.18 ± 0.01aA | 5.48 ± 0.04abA |
In this study, microgreens exhibited differing levels of carotenoid content, ranging from 3.74 ± 0.08 to 6.39 ± 0.36 µg/g, FW. The lower content was recorded in microgreens pak choi, while radish sango microgreens displayed the highest. Significant variations (p˂0.05) were noted in carotenoid content among families and microgreens. The seven microgreens were grown in soil and cocopeat medium and showed a carotenoid content range of 3.47 ± 0.04 to 8.09 ± 0.39 µg/g, FW. Additionally, carrot and dill microgreens were cultivated hydroponically with protein hydrolysate in the nutrient solution and carotenoid levels ranging from 0.291 to 0.340 µg/g, FW, were recorded6,41.
The comparison of pigment content in microgreens grown in soilless and soil growing mediums reveals significant differences in chlorophyll and carotenoid levels as shown in (ESI Table 3). The microgreens grown in soil exhibited significantly (p˂0.05) higher chlorophyll a and chlorophyll b content compared to those grown in soilless medium, indicating that soil medium enhances pigment content. Apart from these, the total carotenoid content showed no significant (p˃0.05) difference. These variations in photosynthetic pigment content, including chlorophyll and carotenoids may be attributed to the rapid growth rate of microgreens during cultivation, disrupting the balanced development of organelles such as chloroplasts and overall microgreen yield. Since photosynthetic pigments, particularly those present in leaves, play a crucial role in safeguarding the photosynthetic system, a correlation between them exists. Furthermore, the biosynthesis and accumulation of these pigments are predominantly regulated by genetic factors40.
Effect of growing medium on biochemical parameters
The determination of anthocyanin content was conducted across seven different types of microgreens, resulting in values ranging from 2.91 ± 0.45 to 28.25 ± 0.67 µmol/100 g, FW, as shown in (Table 4). Radish sango exhibited the highest anthocyanin levels, whereas flaxseed microgreens had the lowest. Notably, significant variations in anthocyanin content were observed among the selected crop families (Amaranthaceae, Brassicaceae, and Linaceae) (P < 0.05). Martínez-Ispizua et al.42 and Gunjal et al.6, reported the different varied anthocyanin levels ranged from 2.04 to 72.34 µmol/100 g, FW, across different microgreens crops. The pH, acidity, and composition of the growing medium these parameters influence pigment accumulation, particularly anthocyanins43.
Table 4.
Effect of soilless growing medium on biochemical parameters of selected microgreens families. The results were presented as mean ± standard deviations;(n=3). The different small letters within the column indicate significant (P<0.05) differences among the microgreens and different capital letters within the column indicate significant (P<0.05) differences among the families (Amaeanthaceae, Brassicaceae, and Linaceae) among the parameters. Where, GAE: Gallic acid; QUE: Quercetin; FW: Fresh weight basis; DW: Dry weight basis.
| Families | Microgreens | Anthocyanin (µmol/100 g, FW) | Ascorbic acid (mg/100 g, FW) | Total glucosinolate content (µmol/g, FW) | Total phenol content (mg GAE/100 g, FW) | Total flavonoid content (mg QUE/100 g, FW) |
|---|---|---|---|---|---|---|
| Amaranthaceae | Beetroot | 27.94 ± 0.18aA | 195.40 ± 1.48dB | 5.35 ± 0.42fC | 248.56 ± 1.27eA | 17.84 ± 0.14bB |
| Red amaranthus | 9.86 ± 0.34bA | 192.74 ± 2.28dB | 4.09 ± 0.17fC | 284.60 ± 1.28cA | 13.44 ± 0.14dB | |
| Brassicaceae | Broccoli | 3.94 ± 0.13cB | 256.46 ± 1.14bA | 34.33 ± 0.25cA | 298.56 ± 0.64bB | 10.94 ± 0.28eB |
| Cabbage | 3.63 ± 0.51cB | 243.14 ± 1.25cA | 24.47 ± 0.76dA | 318.83 ± 1.27aB | 15.44 ± 0.14cB | |
| Pak choi | 4.11 ± 0.18cB | 189.52 ± 2.28dA | 38.18 ± 1.10bA | 131.44 ± 1.27gB | 18.14 ± 0.28aB | |
| Radish sango | 28.25 ± 0.67aB | 266.29 ± 1.37aA | 47.38 ± 0.51aA | 260.27 ± 1.27dB | 16.14 ± 0.28bcB | |
| Linaceae | Flaxseed | 2.91 ± 0.45cC | 177.58 ± 0.91eC | 17.26 ± 0.59eB | 226.49 ± 0.64fC | 16.64 ± 0.14bA |
Ascorbic acid concentrations in microgreens cultivated in a soilless medium ranged from 177.58 ± 0.91 to 266.29 ± 1.37 mg/100 g, FW were observed, as shown in (Table 4). Radish sango microgreens exhibited the highest concentration, followed by broccoli, cabbage, beetroot, pak choi, and flaxseed. These findings align with cabbage and rocket microgreens, reporting concentrations between 246.3 and 390.6 mg/100g41. Xiao et al.44 observed that a microgreen contains a high level of ascorbic acid content 20.40 to 147.00 mg/100 g compared to its mature counterparts. Various factors such as environmental conditions, growing medium, and light influence the accumulation of ascorbic acid in soilless grown leafy vegetables. Notably, a lower pH level in the growth medium was found to enhance ascorbic acid content in plants9.
The total glucosinolate content in selected families of microgreens ranged from 4.09 to 47.38 µmol/g, FW with the radish sango exhibiting the highest value and red amaranthus the lowest, as shown in (Table 4). These findings align with recent research findings on radish (35.31 to 45.61 µmol/g, FW), cabbage (22.04 to 30.24 µmol/g, FW), and rocket (24.02 to 39.83 µmol/g, FW) microgreens9. Another study observed total glucosinolate content ranging from 1.0 to 535.5 µmol/100 g fresh weights across 30 Brassicaceae microgreen varieties21. Our research revealed the highest total glucosinolate content in Brassicaceae, followed by Linaceae and Amaranthaceae families of microgreens. The concentration of total glucosinolate content in different crops is significantly affected by factors such as developmental stage, environmental conditions (temperature and light), nutrient availability in the growing medium, plant variety, and density45,46.
The microgreens showed considerable variability in their total phenol content, as shown in (Table 4). Among them, cabbage microgreens exhibited the highest total phenol content at 318.83 mg GAE/100 g, FW, while pak choi microgreens showed the lowest at 131.44 mg GAE/100 g, FW. Significant differences in total phenolic content were observed across the three microgreen families: Amaranthaceae, Brassicaceae, and Linaceae. Within the Brassicaceae family, consisting of 14 microgreen crops substantial variations in total phenolic content were observed ranging from 9.22 to 268.99 mg GAE/100 g, FW30. Similarly, in another study focusing on 30 Brassicaceae microgreens varieties, total phenolic content ranged from 88.6 to 811.2 mg GAE/100 g, FW21, these results align with our findings. The agriculture and food waste materials were used for the cultivation of sunflower and water spinach microgreens, and the total phenolic content ranged from 8.89 to 13.72 mg GAE/100 g, DW observed17. The total phenol content in radish (314.11 to 590.07 mg GAE/100 g, FW), cabbage (336.21 to 500.83 mg GAE/100 g, FW), and rocket (312.88 to 461.94 mg GAE/100 g, FW) microgreens was observed in different seedling media (Hygromix, Promix, and TS1). The soilless substrate exhibited superior moisture and water retention capabilities compared to other substrates. This likely induced physiological stress to the roots triggered enhanced metabolic activity, and activated defensive responses in microgreens, leading to an increased accumulation of total phenol content9.
Furthermore, the concentrations of total flavonoid content across selected microgreens ranged from 10.94 to 18.14 mg QUE/100 g, FW. Pak choi microgreens exhibited the highest mean flavonoid content, followed by beetroot, flaxseed, and others, while broccoli microgreens showed the lowest. Flavonoid content also varied across different microgreen types, such as mungbean, lentil, and mustard, with values ranging from 0.98 to 6.5 mg/100 g, FW37,47. Moreover, sprouts like broccoli and red radish exhibited flavonoid content ranging from 25.16 to 25.36 mg CE/100 g, FW48. These findings underscore the significant influence of intrinsic and extrinsic factors, including species, growing conditions, harvesting days, and pre-harvest/postharvest conditions, on the variation in total phenolic and flavonoid content across microgreens6,9,11,21,30,38.
The above-mentioned results are compared with different microgreens grown in soilless and soil mediums revealing significant differences (P < 0.05) across various biochemical parameters. This data is represented in (ESI Table 4), the beetroot microgreens showed the highest anthocyanin, ascorbic acid, total glucosinolate content, total phenol, and total flavonoid contents compared to the soilless medium. Similarly, broccoli microgreens grown in soil showed elevated levels of ascorbic acid, total glucosinolate content, total phenol, and total flavonoid contents, although anthocyanin content was slightly lower observed. The cabbage microgreens grown in soil demonstrated a significant increase in ascorbic acid, total glucosinolate content, total phenol, and total flavonoid contents, highlighting the growing medium influence on these parameters. The physicochemical properties of soil provide a more conducive environment for these biochemical parameters synthesis22,49.
Effect of growing medium on antioxidant activities (DPPH, ABTS, and FRAP)
The different types of methods were used to assess the antioxidant activities of plants and foods47. In (Table 5) illustrates the impact of a soilless growing medium on the antioxidant activity of various microgreens. In the DPPH radical scavenging assay, which measures the ability of a substance to neutralize the DPPH radical, a higher percentage shows more antioxidant activity. Among the Brassicaceae family, radish sango microgreens demonstrated the highest DPPH radical scavenging activity at 76.82 ± 0.54%, FW while broccoli exhibited a lower activity at 60.38 ± 0.18%, FW. A diverse range of DPPH activity was observed in other microgreens such as spinach, carrot, bathua, and Bengal gram, with percentages ranging from 46.30 to 90.60%, FW38, as well as in other microgreens including mungbean, lentil, pearl millet, red radish, mustard, and red cabbage, with percentages ranging from 72 to 87%, FW23. Additionally, spinach and fenugreek showed DPPH activity at 39.16%, FW and 41.19%, FW respectively50,51.
Table 5.
Effect of soilless growing medium on antioxidant activity of selected microgreens families. The results were presented as mean ± standard deviations;(n=3). The different small letters within the column indicate significant (P<0.05) differences among the microgreens and different capital letters within the column indicate significant (P<0.05) differences among the families (Amaeanthaceae, Brassicaceae, and Linaceae) among the parameters. Where, FW: Fresh weight basis; TE: Trolox.
| Families | Microgreens | DPPH Radical scavenging activity (%, FW) | ABTS (%, FW) | FRAP (µmol TE/g, FW) |
|---|---|---|---|---|
| Amaranthaceae | Beetroot | 71.09 ± 0.90bA | 81.51 ± 0.51cA | 4.25 ± 0.50cB |
| Red amaranthus | 64.21 ± 0.90deA | 85.33 ± 0.40aA | 3.78 ± 0.50cB | |
| Brassicaceae | Broccoli | 60.38 ± 0.18fA | 79.50 ± 0.51dB | 5.55 ± 0.33bcA |
| Cabbage | 67.84 ± 0.45cA | 72.16 ± 0.30eB | 7.14 ± 0.91bA | |
| Pak choi | 66.31 ± 0.28cdA | 85.04 ± 0.21bB | 4.01 ± 0.34cA | |
| Radish sango | 76.82 ± 0.54aA | 88.49 ± 0.41aB | 12.62 ± 0.33aA | |
| Linaceae | Flaxseed | 62.74 ± 0.63efB | 83.60 ± 0.61bA | 4.13 ± 0.17cB |
The ABTS assay evaluates a substance’s capacity to counteract ABTS radicals, with greater inhibition percentages indicating stronger antioxidant properties. Among the selected microgreens, notable variations in ABTS free radical scavenging activity were observed. Radish sango, belonging to the Brassicaceae family, demonstrated the highest ABTS scavenging activity at 88.49 ± 0.41%, FW, followed by red amaranthus, pak choi, flaxseed, beetroot, broccoli, and cabbage, which also exhibited ABTS scavenging activity. Additionally, comparable findings of ABTS scavenging activity ranging from 29.36 to 81.85%, FW were documented for spinach, carrot, bathua, and Bengal gram38. In other findings the various wheatgrass juices (black, purple, and blue) were the ABTS scavenging activity ranging from 24.18 to 51.40%, FW was observed52. These outcomes were inferior to our findings, possibly attributable to variations in microgreen types.
The FRAP assay evaluates the capacity of a substance to reduce ferric ions, with higher values indicating stronger reducing abilities. In this study, the antioxidant activity of various microgreens cultivated in a soilless medium ranged from 3.78 to 12.62 µmol TE/g, FW observed, as shown in (Table 5). Radish sango microgreens exhibited the highest FRAP antioxidant activity, followed by cabbage, broccoli, beetroot, flaxseed, pak choi, and red amaranthus microgreens. Similarly, summer leafy greens microgreens showed FRAP values ranging from 2.15 to 17.17 µmol TE/g, FW13, mungbean microgreens ranged from 5.7 to 20.22 µmol TE/g, FW and lentil microgreens ranged from 14.7 to 55.00 µmol TE/g, FW47, P. frutescens var. crispa (red) and var. frutescens (green) microgreens exhibited FRAP values ranging from 3.62 to 5.53 µmol TE/g, FW8. The seedling soilless media (Hygromix, Promix, and TS1) were used for the cultivation of radish, cabbage, and rocket microgreens, and the FRAP antioxidant activity in ranged 1.2 to 7.0 mM TEAC/g, FW was observed9. The FRAP antioxidant activity in leafy greens is influenced by multiple factors such as horticultural practices, including the type of substrate utilized, the growing conditions, the plant species and cultivar, the stage of maturity, and the extraction methods employed. Each of these factors can potentially change the FRAP antioxidant content53.
In the comparison between soilless and soil based growing mediums for the antioxidant activity of microgreens, soil grown microgreens again demonstrated slightly superior results as shown in (ESI Table 5). The beetroot and broccoli grown in soil exhibited higher DPPH radical scavenging activity and FRAP values compared to those grown in soilless mediums, indicating stronger antioxidant properties. Broccoli microgreens grown in soil had higher ABTS values compared to those grown in soilless mediums and this pattern was observed across most microgreens. Also, soil grown radish sango microgreens had the highest FRAP values observed. The slightly higher values for antioxidant activity in soil grown microgreens can be attributed to better nutrient availability and root-soil interactions that promote the synthesis of antioxidant compounds. The variations in antioxidant activity values indicate the diverse antioxidant activity of these microgreens, which can be influenced by factors such as plant species13,38,47, growing conditions6, harvesting stage11, pre-harvest and postharvest conditions54,55 and processing method36,38.
Effect of growing medium on antinutrients
Antinutrients are the chemical compounds found in diverse types of foods that can interfere with the absorption of nutrients and these compounds show adverse effects on health when consumed in large amounts56. The different types of antinutrient contents present in microgreens are shown in (Table 6). Tannins are naturally occurring plant compounds that possess both positive and negative properties. On the positive side, tannins exhibit strong antibacterial properties, helping to inhibit the growth of harmful pathogens, and they possess potent antioxidant properties that can reduce oxidative stress and inflammation, contributing to overall health. However, tannins can also act as antinutrients by binding to proteins and minerals, potentially reducing their absorption and bioavailability57. The level of tannin content in all the microgreens ranged from 48.26 to 136.98 mg/100 g, FW. The cabbage microgreens exhibit higher levels of tannin content and flaxseed microgreens were shown the lowest concentration for tannin content. Similar trends were observed in the tannin content for the lotus seed sprouts in water at different temperatures (25 and 35 °C) i.e., 74 to 93 mg/100 g, FW58. In another study, the tannin content in lentil sprouts grown under different germination times (3 to 9 days) ranged from 0.60 to 77% was observed. From this study was shown that the increase in the germination days significantly reduced the tannin content59.
Table 6.
Effect of soilless growing medium on antinutrient content of selected microgreens families. The results were presented as mean ± standard deviations;(n=3). The different small letters within the column indicate significant (P<0.05) differences among the microgreens and different capital letters within the column indicate significant (P<0.05) differences among the families (Amaeanthaceae, Brassicaceae, and Linaceae) among the parameters. Where, FW: Fresh weight basis. .
| Families | Microgreens | Tannin (mg/100 g, FW) | Phytic acid (mg/100 g, FW) | Oxalic acid (mg/100 g, FW) |
|---|---|---|---|---|
| Amaranthaceae | Beetroot | 109.84 ± 0.45bA | 473.66 ± 1.29cA | 28.33 ± 0.09fC |
| Red amaranthus | 76.91 ± 0.56dA | 507.46 ± 0.86aA | 19.16 ± 0.04gC | |
| Brassicaceae | Broccoli | 72.46 ± 0.34eB | 414.00 ± 1.29eC | 55.04 ± 0.06bA |
| Cabbage | 136.98 ± 1.12aB | 480.82 ± 0.21bC | 51.57 ± 0.54cA | |
| Pak choi | 53.18 ± 0.67fB | 452.97 ± 0.86dC | 49.55 ± 0.04dA | |
| Radish sango | 97.54 ± 0.79cB | 145.32 ± 0.26fC | 58.94 ± 0.06aA | |
| Linaceae | Flaxseed | 48.26 ± 0.90gC | 416.34 ± 1.01eB | 42.78 ± 0.06eB |
The (Table 6) presents the phytic acid content found in various types of soilless grown microgreens. Phytic acid content varied between 145.32 and 507.46 mg/100 g, FW, with the highest concentration detected in red amaranthus microgreens and the lowest in radish sango microgreens. Notably, pea and sunflower microgreens exhibited lower phytic acid levels compared to unsoaked and soaked (1.06 to 0.91 and 1.91 g/100 g) for peas, and unsoaked (1.67 to 1.91 g/100 g) sunflower seeds. Additionally, research by Aktaş et al.59 on lentil sprouts cultivated over varying germination periods (3 to 9 days) revealed phytic acid content ranging from 0.59 to 80% was observed.
Oxalic acid presents a significant level in green leafy vegetables and it is recognized as a major antinutritional compound. Its primary action involves obstructing the absorption of divalent cations like calcium through the formation of insoluble salts60. The microgreens had oxalate content ranging from 19.16 to 58.94 mg/100 g, FW in radish sango microgreens exhibited the highest, and red amaranthus microgreens showed the lowest concentration of oxalic acid content. The level of oxalic acid in microgreens is lower than its mature counterpart61. Similar findings were reported in ten cultivated culinary microgreens ranging from 14.3 to 68.2 mg/100 g, FW oxalic acid5. The phytase enzyme activities were on the rise and the reduction in phytic acid content at the germination process stage was observed62. Similarly, the decrease in tannin content of seeds during the germination process may be due to the leaching of tannins into water and subsequent binding of polyphenols with different organic substances59. The growing medium fertility exerts effects on the oxalate levels of some plants. High-growing medium fertility associated with high carbon, nitrogen-available phosphorus, and potassium is associated with high total oxalate in Amaranthus and Basella63.
The above-mentioned findings data for antinutritional parameters, such as tannins, oxalates, and phytates, reveal significant differences between soil grown and soilless grown microgreens as shown in (ESI Table 6). The microgreens cultivated in soil based growing medium exhibited higher levels of these antinutritional factors. For instance, cabbage microgreens cultivated in the soil had higher tannin content compared to those grown in a soilless medium. This suggests that soil may promote tannin synthesis due to better nutrient availability and complex root-soil interactions that enhance secondary metabolite production64. Similarly, the oxalate content was higher in soil grown microgreens than in its soilless cultivated microgreens, likely influenced by soil composition that affects oxalate synthesis. Furthermore, soil grown microgreens showed higher phytate levels compared to those grown in soilless mediums. These findings indicate that soil cultivation enhances the antinutritional factors, necessitating proper processing techniques to mitigate their impact on nutrient absorption65.
Principal component analysis (PCA) of selected parameters in response to growing medium for microgreens
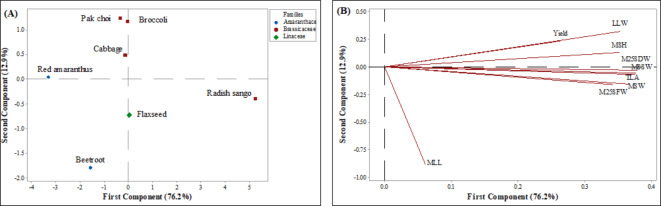
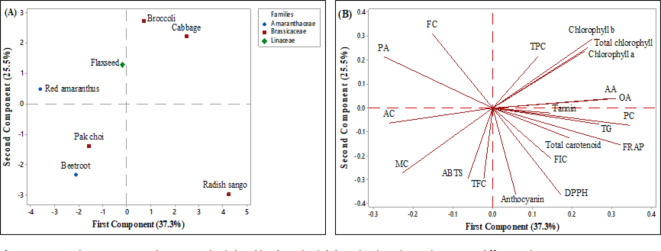
The current investigation utilized Principal Component Analysis (PCA) to examine various selected parameters (including morphological, proximate, biochemical, antioxidant, and antinutrient parameters) to identify potential clusters among microgreens from different families cultivated in soilless growing mediums. The PCA provided a condensed overview of the relationships between microgreen families crops performance and nine assessed morphological parameter variables, as illustrated in (Fig. 5 A and B). The eigenanalysis of the correlation matrix revealed that the first principal component (PC1) accounts for 76.2% of the total variance. The second principal component (PC2) explains an additional 12.9% these two components account for 89.1% of variations observed among microgreens and their respective families in the data. The loadings of the morphological parameters on the principal components provide insights into the contributions of each trait. For PC1 the microgreen shoots height (0.352), microgreen shoot width (0.342), microgreen single shoot weight (0.376), microgreen leaf width (0.353), total leaf surface (0.376), microgreens 25 shoots fresh weight (0.367), and microgreens 25 shoot dry weight (0.379) exhibit high positive loadings, indicating these parameters are the primary sources of variability in the given data analysis. In PC2, the microgreens leaf length (-0.876) shows a high negative loading, suggesting it significantly contributes to the second dimension of variability, highlighting differences in leaf length among the selected microgreens.
Fig. 5.
Principal component analysis score plot (A) and loading plot (B) describe the relationship among different morphological parameters of microgreens obtained from selected microgreens families varieties grown under soilless growing conditions. (MSH, Microgreens shoot height; MSW, Microgreens shoot width; MSSW, Microgreens single shoot weight; MLL, Microgreens leaf length; LLW, Microgreens leaf width; TLA, Total leaf area; M25SFW, Microgreens 25 shoot fresh weight; M25SDW, Microgreens 25 shoot dry weight).
The PCA score plot and loading plot (Fig. 5A and B) illustrate the relationships among the microgreen varieties and their morphological parameters. The Amaranthaceae families of microgreens such as beetroot and red amaranthus microgreens indicate similar morphological traits, particularly in terms of shoot height and width. This clustering suggests that these microgreens may share genetic or cultivation characteristics that influence these parameters. The Brassicaceae families of microgreens including broccoli, cabbage, pak choi, and radish sango microgreens reflect commonalities in leaf dimensions (length and width) and total leaf area. The close grouping suggests that Brassicaceae microgreens might have similar physiological and growth patterns under soilless cultivation conditions. Apart from these flaxseed microgreens from the Linaceae family stand apart from the other microgreens, particularly in terms of shoot weight and dry weight. This separation highlights the unique morphological characteristics of flaxseed microgreens.
Another Principal Component Analysis (PCA) was conducted on a dataset comprising twenty dependent variables encompassing proximate, biochemical, and antioxidant parameters across various microgreen families and their crops cultivated in a soilless medium. Utilizing the criterion of eigenvalue exceeding 1, two principal components (PCs) were derived, capturing over 85% of the cumulative contribution. The resultant score plots for the selected parameters, derived from PCA of microgreens harvested on the 9th day (at the true leaf stage), are depicted in (Fig. 6 A), while (Fig. 6 B) illustrates the spatial distribution of these parameters in the PCA-defined space of the first and second dimensions. Collectively, PC1 and PC2 elucidated 62.8% of the variance among the selected microgreens and the growing medium. The first principal component (PC1) accounted for 37.3% of the variance and was significantly influenced by high loadings on protein content, ascorbic acid, total glucosinolate content, and total phenol content. This suggests that these variables are correlated and contribute similarly to the variability captured by PC1. The second principal component (PC2), which explained 25.5% of the variance, was influenced by fat content, chlorophyll b, and total chlorophyll, indicating a relationship between these parameters. The PCA score plot and loading plot provided a visual representation of the relationships among the different parameters. In conclusion, PCA provides a comprehensive understanding of the complex relationships among various biochemical parameters in selected microgreens.
Fig. 6.
Principal component analysis score plot (A) and loading plot (B) describe the relationship among different elementary compositions, photosynthetic pigments, biochemical parameters, anti-nutrient concentrations, and, degree of antioxidant capacities parameters of microgreens obtained from selected microgreens families varieties grown under soilless growing conditions. (MC, Moisture content; AC, Ash content; FC, Fat content; FIC, Fiber content; PC, Protein content; AA, Ascorbic acid; TG, Total glucosinolate content; TPC, Total phenol content; TFC, Total flavonoid content; PA, Phytic acid; OA, Oxalic acid).
Methods
Chemicals, standards, and reagents
All chemicals utilized in this study were procured from LobaChemie (Mumbai, India), including calcium hypochlorite (M.W.: 142.99; Purity: extra pure), sodium hydroxide (M.W.: 40.00; Purity: 97% extra pure), petroleum ether (Purity: AR, extra pure), ethyl alcohol (M.W.: 46.07), sulfuric acid (M.W.: 98.08; Purity: 98% extra pure), acetic acid (M.W.: 58.08; Purity: 99.8%), methanol (M.W.: 32.04; Purity: 99.7%), hydrochloric acid (M.W.: 36.46; Purity: 37% extra pure), trichloroacetic acid (M.W.: 163.39; Purity: 98% extra pure), Folin & Ciocalteu’s Phenol Reagent, L-ascorbic acid (M.W.: 176.12; Purity: 99% extra pure), sodium carbonate (M.W.: 105.99; Purity: 99.5% extra pure), sodium nitrite (M.W.: 69.00; Purity: 97% extra pure), aluminum chloride (M.W.: 133.34; Purity: 98% extra pure), gallic acid (M.W.: 188.14; Purity: 99% extra pure), sodium acetate (M.W.: 82.03; Purity: 99% extra pure), ferric chloride (M.W.: 162.21; Purity: 99% extra pure), sodium potassium tartrate (M.W.: 282.22; Purity: 98% extra pure), and additional sodium carbonate (M.W.: 105.99; Purity: 99% extra pure). Additionally, chemicals sourced from Sigma-Aldrich (Steinheim, Germany) included 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) (M.W.: 250.29; Purity: 97% extra pure), 2,2-diphenyl-1-picrylhydrazyl (DPPH) (M.W.: 394.32), quercetin (M.W.: 302.24), and 2,4,6-Tripyridyl-S-triazine (TPTZ) (M.W.: 312.32).
Experimental details
The present experiment was carried out at the Lovely Professional University, Jalandhar - Delhi, Grand Trunk Rd, Phagwara, Punjab-144,001. The study was conducted on different families of microgreens including broccoli, cabbage, pak choi, radish sango, beetroot, red amaranthus, and flaxseed were used and its specification is shown in (Table 1). The above-selected microgreen seeds were provided by the Seed Delivery LLP., Chandigarh, India. The selected microgreens were grown in a soilless growing medium (burlap) with three replications. The seeds used for this study were purchased in January 2023, and the period from seed production to sowing was approximately six months. The jute bag was cut into 8 × 8 cm pieces and after this cut pieces were sterilized for 15 min at 121℃ and 15 psi. All the selected microgreen seeds were cultivated using distilled water and pieces of sterilized jute bag. The 2% calcium hypochlorite seed treatments were used for all the seeds after that distilled water was used three times to rinse and wash all microgreen seeds. The seeds were then immersed in distilled water for 8 to 12 h at room temperature. Following soaking, all of the seeds water was drained, and they were all distributed among growing media in different layers of jute bags cut pieces (approximately 10–12 pieces/containers). During the experimental study period, the temperature was 20 to 22℃, and relative humidity (RH) of 65 to 67% was maintained. After growing for 3 days in the dark condition in the growing chamber then level of carbon dioxide (0.45 g/L), photoperiod (12/12 hrs; light/dark), and light intensity (55 µmol m−2s−1) were retained. The changes in the growth of different microgreens families during the selected growth period are shown in (Fig. 1). Harvesting of microgreens was done after the 9th day of sowing followed by washing with ultra-pure water and packed in a polypropylene container.
Growth-related morphological parameters
At harvest time, 9th day after germination, the various morphological parameters, including the fresh and dry weight of 25 microgreen shoots were measured according to the methods described by Gunjal et al.6. A total of 25 microgreen shoots were randomly chosen for analysis (n = 3 number of observations). Measurements of the shoot and leaf dimensions, including length and width were taken using a digital vernier caliper (Model: B0CHFVZN1M, Brand: SKADIOO, Perfect Sales India, India) for this randomly microgreens shoot was selected for analysis (n = 25 number of observation). To determine the dry weight of the microgreen samples, each sample was dried at a consistent temperature of 55 ± 2℃ for two days until reaching a constant weight and this experiment was performed in triplicates (n = 3). The fresh yield of microgreens was measured in triplicates according to the method described by Di Gioia et al.27 and the results were presented for yield in g/m2.
Proximate analysis
Proximate analysis of microgreens like moisture, ash, fat, and fiber content were estimated following standard AOAC methods66 and each test was performed in triplicate (n = 3) to ensure accuracy and reproducibility of the results. The hot air drying method was used for moisture content determination by placing the known amount of fresh sample by subjecting at 105℃ temperature until a consistent weight was achieved. Ash content was determined by placing 2 g of dried samples in a muffle furnace at 550℃ for 5–6 h until white ash was obtained. The crude fat content was determined using a Soxhlet extraction process and fiber content in the microgreen samples was determined using acid-alkali treatments. The soluble protein content in the sample was estimated using the Lowry et al.67 method by placing the known quantity of supernatant and standard curves were generated using Bovine Serum Albumin (BSA). The obtained result for protein content was expressed as g BSA (Bovine Serum Albumin equivalent)/100 g on a fresh weight basis (FW) of the dried sample.
Photosynthetic pigments determination
The photosynthetic pigments such as chlorophyll and carotenoid content were determined by following the method described by Martnez-Ispizua et al.42. Briefly, microgreen samples of a known amount were homogenized in 10 mL of 80% acetone solution. The prepared extracts were centrifuged at 6000×g for 5 min and the absorbance (Abs) values were taken at different wavelengths (470, 646.6, and 663.6 nm) using a UV-Vis spectrophotometer (LB-925, Labcare, Mumbai, India). The chlorophyll a, chlorophyll b, total chlorophyll, and total carotenoid content were calculated by using Eqs. (01, 02, 03, and 04) and the obtained result was expressed as µg/g on a fresh weight basis (FW).
| Equation numbers | Compound names | Equation | Units |
|---|---|---|---|
| (01) | Chlorophyll a content |
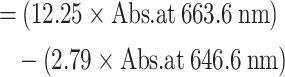
|
(µg/g, FW) |
| (02) | Chlorophyll b content |
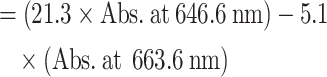
|
(µg/g, FW) |
| (03) | Total carotenoid content |
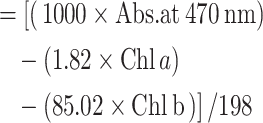
|
(µg/g, FW) |
| (04) | Total chlorophyll content |
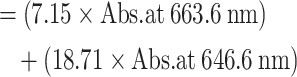
|
(µg/g, FW) |
Quantifications of bioactive compounds, and antioxidant activity
To detect various antioxidant compounds in a fresh microgreen sample, 1.00 g of the sample was weighed and subsequently blended with 100 mL of 80% ethanol. Followed by centrifuging the extract at 6000 g for 15 min. The ethanol-based supernatant was stored in glass vials at − 20 °C and quantified within 24 h to ensure the stability of the antioxidant compounds.
The quantifications of total phenolic content (TPC) were determined by following the method described by Dhaka et al.23. Briefly, 1.0 mL of ethanolic extract was dissolved in 0.5 mL of Folin–Ciocalteu (FC) reagent followed by 1.5 mL of 20% Na2CO3, and then 7 mL of deionized water was added to the test sample. This prepared solution was then allowed to incubate at room temperature for 10 min, following which the absorbance was measured at 765 nm using a UV-Vis spectrophotometer. The obtained results were represented in mg GAE (Gallic Acid Equivalents)/100 g fresh weight basis.
The total flavonoid concentration in samples was assessed following the protocol outlined by Alam & Sharma68. Briefly, 0.3 mL of 5% sodium nitrite and 0.3 mL of 10% aluminum chloride were added to 1 mL of the ethanolic extract. The resulting solution was then diluted to a final volume of 10 mL with distilled water. Subsequently, the absorbance of the prepared test sample was measured at a wavelength of 510 nm using a UV-Vis spectrophotometer. The total flavonoid content was determined in mg QUE (Quercetin Equivalents)/100 g by referencing a calibration curve constructed with standard quercetin.
The radical scavenging potential of the sample was evaluated using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, following the procedure outlined by Kowitcharoen et al.30. Briefly, ethanoic extracts of 0.1 mL were mixed with a DPPH solution (2.9 mL), and the absorbance of the resultant solution was measured at wavelength (517 nm) using a UV-Vis spectrophotometer after a 30 min incubation period. The inhibition percentage of DPPH radical scavenging activity was determined using Eq. (05). The ferric-reducing antioxidant potential of the sample was assessed by following the method proposed by Priti et al.47. Results were quantified as µmol Trolox equivalent (TE)/g by constructing a calibration curve using standard Trolox. Additionally, the 2,2-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) free radical scavenging activity of the microgreens was determined following the procedure described by Sanyukta et al.38. Briefly, ethanol extract (0.1 mL) was mixed with ABTS solution (2.9 mL) and vortexed. Subsequently, the absorbance of the sample was measured at 734 nm using a UV-Vis spectrophotometer after a 6 min incubation period. The percentage of ABTS scavenging activity was calculated using Eq. (06).
| Equation numbers | Compound names | Equation | Units |
|---|---|---|---|
| (05) | DPPH radical scavenging activity |
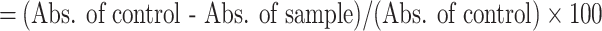
|
(%, FW) |
| (06) | ABTS scavenging activity |
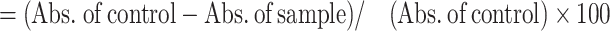
|
(%, FW) |
The anthocyanin content was spectrophotometrically determined as described by Martnez-Ispizua et al.42. Briefly, microgreen fresh samples of 0.1 g were homogenized in 5 mL of solution containing Methanol: HCl: H2O (90:1:9). The prepared samples mixture was vortexed and stored in the dark place for 60 min at room temperature. Then, they were centrifuged at 6000×g for 10 min and the supernatant was used for the analysis. The obtained supernatant solution absorbance (Abs.) was measured at 534, 643, and 661 nm wavelength using a spectrophotometer, with the methanol: HCl: H2O solution used as the blank. The anthocyanin content was calculated using Eq. (07) and expressed in µmol/100 g, FW.
| Equation numbers | Compound names | Equation | Units |
|---|---|---|---|
| (07) | Anthocyanin content |
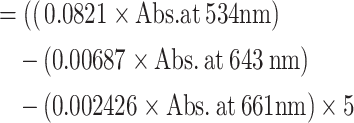
|
(µmol/100 g, FW) |
The determination of total glucosinolate content in microgreen samples following the procedure outlined by Mawlong et al.46. The 0.1 g of microgreen powder was blended with 10 mL of 80% methanol, then the resulting mixture was centrifuged at 3000 rpm for 4 min and left to settle for 12 h at room temperature. A methanol-based extract (0.1 mL) was mixed with double distilled water (0.3 mL) in test tubes, followed by the addition of 3 mL of 2 mM sodium tetrachloropalladate. After that, the prepared test sample was incubated for 60 min and the absorbance was taken at 425 nm. The findings were represented in µmol/g of fresh weight.
The ascorbic acid content in the microgreens was quantified using the protocol outlined by Jagota & Dani69. The samples 0.1 g were homogenized in 2 mL of 10% trichloroacetic acid and then the prepared mixture was centrifuged at 6000 rpm for 5 min. After that, 0.1 mL of extract was added to 2 mL of 10% trichloroacetic acid and 0.3 mL of Folin Ciocalteu’s reagent. Furthermore, test samples were incubated at room temperature for 20 min and absorbance was taken spectrophotometrically at 760 nm wavelength. The ascorbic content was calculated in mg/100 g on a fresh weight basis by using the calibration curve (10–50 µg/mL) prepared using standard L-ascorbic acid.
Quantifications of antinutrients
The tannins in microgreen samples were determined using the Folin Ciocalteu’s method, employing tannic acid as a reference standard concentration (100–500 µg/mL) and the results were quantified in mg/100 g, as outlined by Sirisangsawang & Phetyim70. For oxalic acid content analysis, microgreens underwent potassium permanganate titration following the protocol by Bok et al.71. The 1 mL of extract was added to 5 mL of 2.00 N sulfuric acid and 2.00 mL of 3 µmol KMnO4 reagent. Furthermore, test samples were incubated at room temperature for 10 min and absorbance was taken spectrophotometrically at 520 nm. The oxalic acid was calculated in mg/100 g by using the calibration curve (100–500 µg/mL) prepared using standard oxalic acid. The phytic acid content in samples was determined as per the method described by Adegbusi et al.72. The microgreens sample (75 mg) was homogenized with (10 mL) of 2.4% HCl solvent and incubated for 30 min at 25℃. After all the test samples were centrifuged at 3000 rpm for 30 min and from the obtained supernatant 3 mL aliquots and different concentrations of hydrated sodium phytates salt (0–40 µg/mL) were taken after that 1 mL of Wade reagent were added. The absorbance was taken at 500 nm using a UV-Vis spectrophotometer after 10 min. The total phytic acid content was calculated by using the below Eq. (08) and results were expressed in mg/100 g on a fresh weight basis.
| Equation numbers | Compound names | Equation | Units |
|---|---|---|---|
| (08) | Phytic acid content |
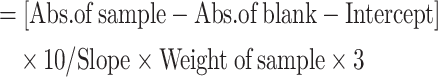
|
(µg/g, FW) |
Statistical analysis
The data collected from the experiment underwent a Two-way ANOVA analysis within each type of microgreen and their respective families utilizing SPSS version 23.0 (Chicago, USA). The factors considered in the two-way ANOVA were the type of growing medium (soilless and soil), species of microgreens and microgreens families. All data were subjected to two-way ANOVA, and significant differences among mean ± standard deviations were determined by the Tukey post hoc test (P < 0.05). Additionally, a principal component analysis (PCA) was conducted on various experimental study parameters using Minitab 17 software.
Conclusion
The findings of this study results demonstrate that the selected families of microgreens morphological, nutritional, and antioxidant parameters are significantly impacted by a soilless growing medium. The choice of growing medium significantly influences the yield and morphological characteristics of microgreens. Among the microgreens families, Brassicaceae microgreens families showed good results in various selected parameters followed by Amaranthaceae and Linaceae families. Radish sango microgreens from Brassicaceae families showed good results in morphological and bioactive parameters compared to other varieties investigated. The study highlights the significant influence of the growing medium on the biochemical attributes of microgreens. The results indicate that soilless grown microgreens showed similar levels of total phenolics, flavonoids, anthocyanin, and ascorbic acid, as well as superior antioxidant activities measured by DPPH, FRAP, and ABTS assays. The soil grown microgreens showed higher antinutrient content compared to those grown in soilless mediums. Soilless media is providing a practical and sustainable option for microgreens cultivation, especially in urban and resource limited backgrounds. By optimizing the growing medium, it is possible to enhance the productivity and quality of microgreens, thereby supporting the growth of the microgreens industry and contributing to sustainable food systems transformation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The first author received a Ph.D. fellowship from Chhatrapati Shahu Maharaj Research, Training and Human Development Institute (SARTHI), Pune, Maharashtra under the Chhatrapati Shahu Maharaj National Research Fellowship Program (CSMNRF-2021). The authors wish to thank Researchers Supporting Project Number (RSPD2024R706) at King Saud University Riyadh Saudi Arabia for financial support.
Author contributions
M.G: formal analysis; visualization; writing – original draft. P.R: supervision, conceptualization; writing – review and editing; project management. J.S and R.U: writing – review and editing; formal analysis; visualization. S.K: formal analysis; writing – review and editing; illustrations; co-supervision. V.N: writing – review and editing; resources; co-supervision. Z.I and S.E: writing – review and editing, data analysis.
Data availability
All the required data for the work is provided with the manuscript.
Materials availability
The selection of plant materials and all procedures were conducted in compliance with institutional, national, and international guidelines and legislation.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Çeki̇N, D. et al. Comparative analysis of closed hydroponic systems and planting seasons for Lettuces. Turk. J. Agric. For.48, 344–353. 10.55730/1300-011x.3186 (2024). [Google Scholar]
- 2.Chen, H., Tong, X. & Tan, L. Consumers’ acceptability and perceptions toward the consumption of hydroponically and soil grown broccoli microgreens. J. Agric. Food Res.2, 100051 (2020). [Google Scholar]
- 3.Tavan, M. et al. Optimizing sensor-based irrigation management in a soilless vertical farm for growing microgreens. Front. Sustainable Food Syst. Food Syst.4, 622720 (2021). [Google Scholar]
- 4.Ghoora, M. D., Babu, D. R. & Srividya, N. Nutrient composition, oxalate content and nutritional ranking of ten culinary microgreens. J. Food Compos. Anal.91, 103495 (2020). [Google Scholar]
- 5.Singh, M. et al. Comparison of mineral composition in microgreens and mature leaves of celery (Apium graveolens L). Biol. Trace Elem. Res.201, 4156–4166 (2022). [DOI] [PubMed] [Google Scholar]
- 6.Gunjal, M. et al. Comparative analysis of morphological, nutritional, and bioactive properties of selected microgreens in alternative growing medium. S Afr. J. Bot.165, 188–201 (2024). [Google Scholar]
- 7.Kopsell, D. A., Sams, C. E., Metallo, R. M., Waterland, N. L. & Kopsell, D. E. Biomass, carbohydrates, pigments, and mineral elements in kale (Brassica oleracea var acephala) microgreens respond to LED blue-light wavelength. Sci. Hortic.328, 112929 (2024). [Google Scholar]
- 8.Dimita, R. et al. Volatile compounds and total phenolic content of perilla frutescens at microgreens and mature stages. Horticulturae. 8, 71 (2022). [Google Scholar]
- 9.Ntsoane, M. L. L. et al. The phytonutrient content and yield of Brassica microgreens grown in soilless media with different seed densities. Horticulturae. 9, 1218 (2023). [Google Scholar]
- 10.Sharma, A. et al. Controlled Environment Ecosystem: a plant growth system to combat climate change through soilless culture. Crop Des.3, 100044 (2024). [Google Scholar]
- 11.Acharya, J., Gautam, S., Neupane, P. & Niroula, A. Pigments, ascorbic acid, and total polyphenols content and antioxidant capacities of beet (Beta vulgaris) microgreens during growth. Int. J. Food Prop.24, 1175–1186 (2021). [Google Scholar]
- 12.Luo, L. et al. Effects of LED light quality on broccoli microgreens plant growth and nutrient accumulation. J. Plant. Growth Regul. 1–9 (2024).
- 13.Yadav, L. P., Koley, T. K., Tripathi, A. & Singh, S. Antioxidant potentiality and mineral content of summer season leafy greens: comparison at mature and microgreen stages using chemometric. Agric. Res.8, 165–175 (2018). [Google Scholar]
- 14.Di Gioia, F., De Bellis, P., Mininni, C., Santamaria, P. & Serio, F. Physicochemical, agronomical and microbiological evaluation of alternative growing media for the production of rapini (Brassica rapa L.) microgreens. J. Sci. Food Agric.97, 1212–1219 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Polash, M. A. S., Sakil, A., Sazia, S. & Hossain, A. Selection of suitable growing media and nutritional assessment of microgreens. Agric. Res. J.56, 752 (2019). [Google Scholar]
- 16.Saleh, R. et al. Growth and biochemical composition of microgreens grown in different formulated soilless media. Plants. 11, 3546 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thepsilvisut, O. et al. Efficacy of agricultural and food wastes as the growing media for sunflower and water spinach microgreens production. Horticulturae. 9, 876 (2023). [Google Scholar]
- 18.Senevirathne, G. I., Gama-Arachchige, N. S. & Karunaratne, A. M. Germination, harvesting stage, antioxidant activity and consumer acceptance of ten microgreens. Ceylon J. Sci.48, 91 (2019). [Google Scholar]
- 19.Frąszczak, B. et al. Morphological and photosynthetic parameters of green and red kale microgreens cultivated under different light spectra. Plants. 12, 3800 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bulgari, R., Baldi, A., Ferrante, A. & Lenzi, A. Yield and quality of basil, Swiss chard, and rocket microgreens grown in a hydroponic system. N Z. J. Crop Hortic. Sci.45, 119–129 (2016). [Google Scholar]
- 21.Xiao, Z. et al. Microgreens of Brassicaceae: genetic diversity of phytochemical concentrations and antioxidant capacity. LWT. 101, 731–737 (2019). [Google Scholar]
- 22.Singh, A. et al. Emergence of microgreens as a valuable food, current understanding of their market and consumer perception: a review. Food Chem. X. 23, 101527 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhaka, A. S. et al. Evaluation of growth conditions, antioxidant potential, and sensory attributes of six diverse microgreens species. Agriculture. 13, 676 (2023). [Google Scholar]
- 24.Hoang, G. M. & Vu, T. T. Selection of suitable growing substrates and quality assessment of Brassica microgreens cultivated in greenhouse. Acad. J. Bio. 44, 133–142 (2022). [Google Scholar]
- 25.Signore, A., Somma, A., Leoni, B. & Santamaria, P. Optimising sowing density for microgreens production in rapini, kale and cress. Horticulturae. 10, 274 (2024). [Google Scholar]
- 26.Bhaswant, M., Shanmugam, D. K., Miyazawa, T., Abe, C. & Miyazawa, T. Microgreens-a comprehensive review of bioactive molecules and health benefits. Molecules. 28, 867 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Gioia, F. et al. Yield performance, mineral profile, and nitrate content in a selection of seventeen microgreen species. Front. Plant. Sci.14, (2023). [DOI] [PMC free article] [PubMed]
- 28.Jones-Baumgardt, C., Llewellyn, D., Ying, Q. & Zheng, Y. Intensity of sole-source light-emitting diodes affects growth, yield, and quality of Brassicaceae microgreens. HortScience. 54, 1168–1174 (2019). [Google Scholar]
- 29.Kyriacou, M. C. et al. Functional quality in novel food sources: genotypic variation in the nutritive and phytochemical composition of thirteen microgreens species. Food Chem.277, 107–118 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Kowitcharoen, L., Phornvillay, S., Lekkham, P., Pongprasert, N. & Srilaong, V. Bioactive composition and nutritional profile of microgreens cultivated in Thailand. Appl. Sci.11, 7981 (2021). [Google Scholar]
- 31.Eswaranpillai, U., Murugesan, P. & Karuppiah, P. Assess the impact of cultivation substrates for growing sprouts and microgreens of selected four legumes and two grains and evaluation of its nutritional properties. Plant. Sci. Today. 10, 160–169 (2023). [Google Scholar]
- 32.Khatoon, S. & Singh, M. Impact of various substrates on the physicochemical properties of radish microgreens. Annals Phytomedicine. 11, 591–596 (2022). [Google Scholar]
- 33.Podsędek, A. et al. LED light quality affected bioactive compounds, antioxidant potential, and nutritional value of red and white cabbage microgreens. Appl. Sci.13, 5435 (2023). [Google Scholar]
- 34.Kaur, N., Singh, B., Kaur, A. & Yadav, M. P. Impact of growing conditions on proximate, mineral, phenolic composition, amino acid profile, and antioxidant properties of black gram, mung bean, and chickpea microgreens. J. Food Process. Preserv46, (2022).
- 35.Ordovas, J. M., Ferguson, L. R., Tai, E. S. & Mathers, J. C. Personalised nutrition and health. BMJ (2018). [DOI] [PMC free article] [PubMed]
- 36.Devi, C. B., Bains, K. & Kaur, H. Effect of drying procedures on nutritional composition, bioactive compounds and antioxidant activity of wheatgrass (Triticum aestivum L). J. Food Sci. Technol.56, 491–496 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghoora, M. D., Haldipur, A. C. & Srividya, N. Comparative evaluation of phytochemical content, antioxidant capacities and overall antioxidant potential of select culinary microgreens. J. Agric. Food Res.2, 100046 (2020). [Google Scholar]
- 38.Sanyukta, N. et al. Comprehensive analysis of physicochemical, functional, thermal, and morphological properties of microgreens from different botanical sources. ACS Omega. 8, 29558–29567 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Altuner, F. Determination of biochemical composition and pigment content in legume and cereal microgreens. Legume Res.44, 1018–1025 (2021). [Google Scholar]
- 40.Fayezizadeh, M. R., Ansari, N. A., Sourestani, M. M. & Hasanuzzaman, M. Balancing yield and antioxidant capacity in basil microgreens: an exploration of nutrient solution concentrations in a floating system. Agriculture. 13, 1691 (2023). [Google Scholar]
- 41.El-Nakhel, C. et al. Nutrient supplementation configures the bioactive profile and production characteristics of three Brassica L. microgreens species grown in peat-based media. Agronomy. 11, 346 (2021). [Google Scholar]
- 42.Martínez-Ispizua, E. et al. The nutritional quality potential of microgreens, baby leaves, and adult lettuce: an underexploited nutraceutical source. Foods. 11, 423 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khandaker, M. M., Rahmat, S., Alias, N., Mohd, K. S. & Mat, N. The effects of different growing media on growth, flowering and quality of Petunia Grandiflora. Tarım Bilim Derg 373–383 (2019).
- 44.Xiao, Z., Lester, G. E., Luo, Y. & Wang, Q. Assessment of vitamin and carotenoid concentrations of emerging food products: edible microgreens. J. Agric. Food Chem.60, 7644–7651 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Ishida, M., Hara, M., Fukino, N., Kakizaki, T. & Morimitsu, Y. Glucosinolate metabolism, functionality and breeding for the improvement of Brassicaceae vegetables. Breed. Sci.64, 48–59 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mawlong, I., Kumar, S., Gurung, B., Singh, K. H. & Singh, D. A simple spectrophotometric method for estimating total glucosinolates in mustard de-oiled cake. Int. J. Food Prop.20, 3274–3281 (2017). [Google Scholar]
- 47.Priti et al. Diversity in phytochemical composition, antioxidant capacities, and nutrient contents among mungbean and lentil microgreens when grown at plain-altitude region (Delhi) and high-altitude region (Leh-ladakh), India. Front. Plant. Sci.12, (2021). [DOI] [PMC free article] [PubMed]
- 48.Lee, S. et al. LED lights influenced phytochemical contents and biological activities in kale (Brassica oleracea L. var. acephala) microgreens. Antioxidants 12, 1686 (2023). [DOI] [PMC free article] [PubMed]
- 49.Partap, M. et al. Microgreen: a tiny plant with superfood potential. J. Funct. Foods. 107, 105697 (2023). [Google Scholar]
- 50.Sharma, A. et al. Optimization of a process for a microgreen and fruit based ready to serve beverage. Int. J. Food Stud. 41–56 (2021).
- 51.Sharma, P. et al. Optimization of a process for microgreen and fruit-based functional beverage. An. Acad. Bras. Cienc.92, (2020). [DOI] [PubMed]
- 52.Sharma, N. et al. Evaluation of anthocyanin content, antioxidant potential and antimicrobial activity of black, purple and blue colored wheat flour and wheat-grass juice against common human pathogens. Molecules. 25, 5785 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kullaj, E. New insights on postharvest ecophysiology of fresh horticultural crops. Elsevier eBooks. 1–38. 10.1016/b978-0-12-804313-4.00001-3 (2016).
- 54.Vaštakaitė-Kairienė, V., Jurkonienė, S., Rasiukevičiūtė, N., Karklelienė, R. & Samuolienė, G. The influence of pre-harvest leds on phytochemical constituents and antioxidant activity of microgreens during short-term storage. Agronomy. 13, 2188 (2023). [Google Scholar]
- 55.Patil, M. et al. Effect of postharvest treatments and storage temperature on the physiological, nutritional, and shelf-life of broccoli (Brassica oleracea) microgreens. Sci. Hortic.327, 112805 (2024). [Google Scholar]
- 56.Petroski, W. & Minich, D. M. Is there such a thing as anti-nutrients? A narrative review of perceived problematic plant compounds. Nutrients. 12, 2929 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang, Q., Liu, X., Zhao, G., Hu, T. & Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr.4, 137–150 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sathithon, N. P. Effect of sprouting on the chemical and nutritional qualities and phenolic alkaloid content of lotus (Nelumbo nucifera Gaertn.) Seeds. Afr. J. Food Sci.6, (2012).
- 59.Aktaş, Ş., Köse, Ö. D. E., Kardeş, Y. M. & Mut, Z. The effect of different germination times on some nutritional and anti-nutritional properties of green lentil sprouts. Soil. Stud.11, 7–11 (2022). [Google Scholar]
- 60.Gemede, H. F. Antinutritional factors in plant foods: potential health benefits and adverse effects. Int. J. Nutri Food Sci.3, 284–289 (2014). [Google Scholar]
- 61.Longvah, T., An̲antan̲, I., Bhaskarachary, K., Venkaiah, K. & Longvah, T. Indian food composition tables. Hyderabad: National Institute of Nutrition, Indian Council of Medical Research. 2–58 (2017).
- 62.Poudel, P., Di Gioia, F., Lambert, J. D. & Connolly, E. L. Zinc biofortification through seed nutri-priming using alternative zinc sources and concentration levels in pea and sunflower microgreens. Front. Plant. Sci.14, 1177844 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Syed, S., Buddolla, V. & Lian, B. Oxalate carbonate pathway-conversion and fixation of soil carbon-a potential scenario for sustainability. Front. Plant. Sci.11, 591297 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Akula, R. & Ravishankar, G. A. Influence of abiotic stress signals on secondary metabolites in plants. Plant. Signal. Behav.6, 1720–1731 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Samtiya, M., Aluko, R. E. & Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: an overview. Food Prod. Process. Nutr.2, (2020).
- 66.A.O.A.C. Official Methods of Analysis of AOAC International, 17th edn. AOAC International, AOAC. MD, USA. (2010).
- 67.Lowry, O. H., Rosebrough, N. J., Farr, A. L. & Randall, R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem.193, 265–275 (1951). [PubMed] [Google Scholar]
- 68.Alam, N. N. & Sharma, K. R. Estimation of phenolic content, flavonoid content, antioxidant, and alpha-amylase inhibitory activity of some selected plants from Siraha district Nepal. Asian J. Pharm. Clin. Res. 18–23 (2020).
- 69.Jagota, S. K. & Dani, H. M. A new colorimetric technique for the estimation of vitamin C using Folin phenol reagent. Anal. Biochem.127, 178–182 (1982). [DOI] [PubMed] [Google Scholar]
- 70.Sirisangsawang, R. & Phetyim, N. Optimization of tannin extraction from coconut coir through response surface methodology. Heliyon. 9, e13377 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bok, V. V., Šola, I. & Rusak, G. Lemon juice formulations modulate in vitro digestive recovery of spinach phytochemicals. Food Technol. Biotech.60, 293–307 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adegbusi, H. S., Ismail, A., Esa, N. M. & Daud, Z. A. M. Effects of formulated Nigerian yellow maize, soybean, and crayfish blends on some growth performance and physiological status. Food Prod. Process. Nutr.5, (2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the required data for the work is provided with the manuscript.
The selection of plant materials and all procedures were conducted in compliance with institutional, national, and international guidelines and legislation.