The authors regret that the printed version of the above article contained a number of errors. The authors would like to apologise for any inconvenience caused.
In Section 3.5, there was a typographical error in the formula related to pH. The correct formula is: –COOH + H2O→-COO- + H3O+
In Fig. 4, some of the SEM micrographs have been put in a wrong order. The SEM images for samples P:P:B1 and P:P:B2 were inadvertently swapped in the original figure. This has been corrected in the updated image below:
Fig. 4.
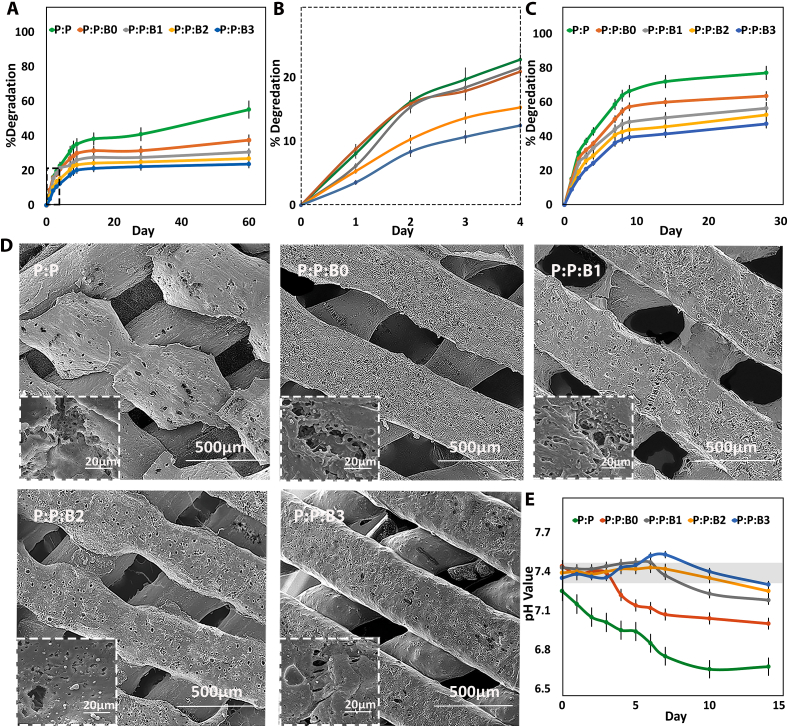
The degradation rate of the synthesized vascular grafts over 60 days. (A) Weight loss percentage in PBS at 37 °C on a logarithmic scale, which was revealed to be linear degradation. (B) Weight loss percentage of grafts in the first 4 days of degradation test in PBS at 37 °C. (C) Weight loss rates of grafts during enzymatic degradation in vitro. (D) Representative SEM micrographs of the vascular grafts degradation after 60 days incubation in PBS at 37 °C (E) pH value versus incubation time. Gray box indication of the normal physiological level (pH between 7.35 and 7.45).
In Table 3., P:P:B3 data has been corrected as follows:
| Vascular graft | (%) | UTS (MPa) | Suture strength (N) | n () |
|---|---|---|---|---|
| P:P:B3 | 231 8 | 8.5 0.4 | 5.2 0.5 | 110.4 |
Also, the regarding text has been corrected in page 12, section 3.6, last paragraph, as follows: “The cross-linking density of the vascular grafts increased by more than four-fold. For the P:P grafts, the cross-linking density was calculated as 26.4 ± 5.4 mol/m3, while for the P:P:B3 grafts, it was 110.4 ± 7.3 mol/m3. This suggests that the modified BGs, and the ions released from them (particularly Cu), contributed not only to physical entanglement but also to chemical cross-linking of the polymeric chains. Cross-linking density has a significant impact on the mechanical properties of elastomers [31]. This finding demonstrates that by incorporating an active filler, such as the modified BGs, which can generate new binding sites for the polymeric chains, it is possible to enhance the cross-linking density and, consequently, improve the mechanical properties of the vascular grafts.”
In Fig. 6 (B), cell viability for P:P:B3 (day 7) in MTT cell viability test was reported with an error that has been corrected.
Fig. 6.
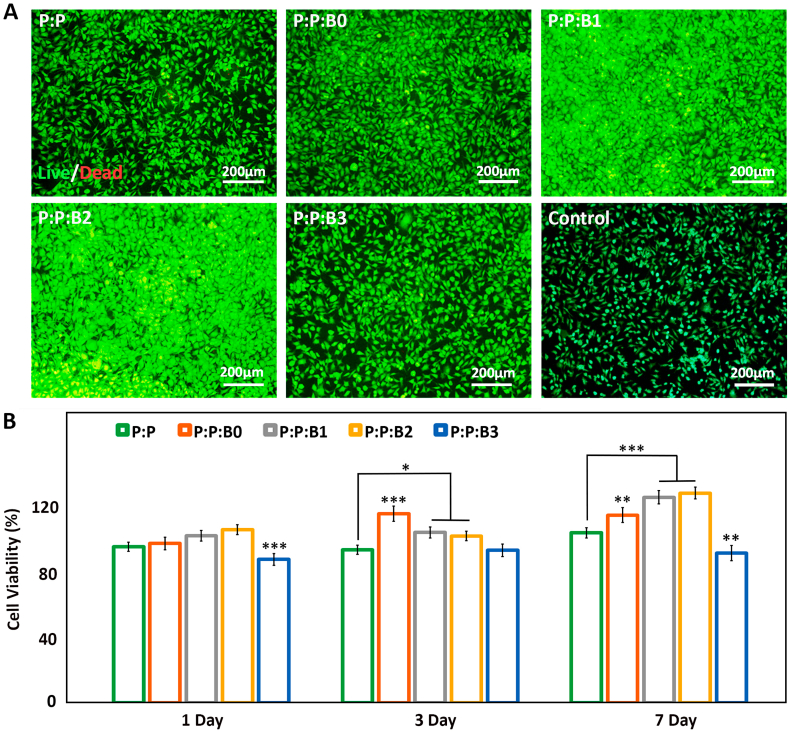
(A) HUVECs proliferation and morphology after 1 day of incubation with leachate the vascular grafts and control group obtained by fluorescence microscopy, indicating Cu-releasing vascular grafts stimulated proliferation of HUVECs (scale bar: 200 μm). (B) Vascular grafts' cytocompatibility evaluation. MTT assay measured EC viability after incubation for 1, 3, and 7 days with P:P:Bs vascular grafts and control graft. Data presented as mean ± SD, n = 3, all statistical significance shown in comparison to P:P graft, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Also, the text regarding Fig. 6 (Page 12, Subtitle 3.8.1. Second paragraph) has been corrected as follows:
“It is vital to evaluate the cytotoxicity of new products because biomaterials and chemical agents employed in cell treatment may influence cell viability and metabolism. MTT test was used to evaluate the cytotoxicity of P:P:Bs and control graft. The results in Fig. 6B demonstrate the non-cytotoxic effects of produced grafts over seven days. This conclusion is consistent with other studies, which found that scaffolds containing BGs had no harmful effects [53,54]. Additionally, the results showed that, compared to the control groups, the EC proliferation rate was significantly higher after one day of incubation with P:P:B1 and P:P:B2 grafts. The proliferation of ECs was also much higher after 3 and 7 days than after one day, showing that the presence of synthetic vascular grafts supports cell proliferation. The higher rate of EC proliferation caused by the addition of Cu doped in composites and previous reports of biocompatibility are both supported by our findings.
However, compared to the control group, in presence of P:P:B3 grafts, the viability of HUVECs has been reduced by 87 % after 3 and 7 days. Reactive oxygen species (ROS) forming, which is one of the primary mechanisms that induce Cu cytotoxicity in cell monolayers in vitro, can explain this phenomenon. Increase in ROS generation at high Cu concentrations might potentially cause DNA damage and mutations due to interactions with nuclear chromatin and peroxidation of biomolecules, including lipids and proteins in cell membranes [55].”
In Fig. 7., some of the migration test results have been put in wrong order. The control sample image for the 24h time point was inadvertently replaced with a wrong image. This has been corrected in the updated figure bellow. In addition, in Fig. 7 caption, part (B), the word “Cell Covered” has been spelled incorrectly.
Fig. 7.
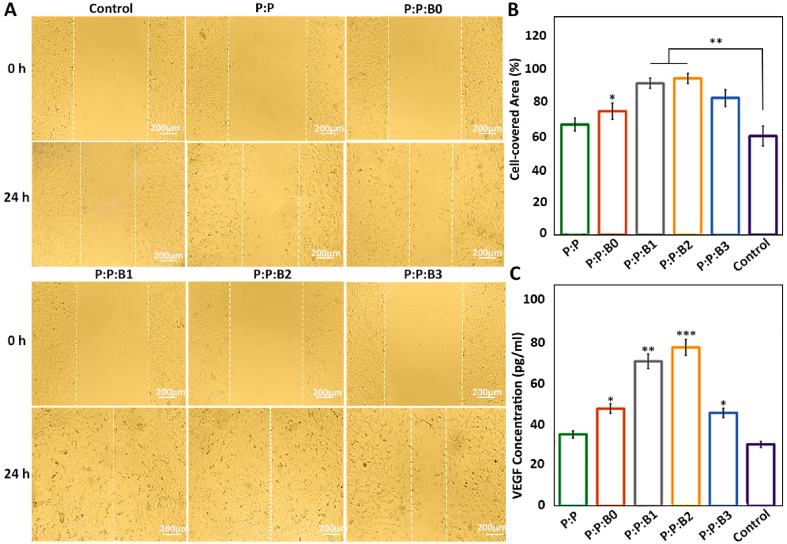
HUVECs Migration after 1 day of incubation with different culture mediums or leachates. (A) HUVECs monolayer was scratched using a tip of a 100 μl pipette. The position of the initial scratch edge is illustrated with dotted lines, at the beginning of the experiment and after a day of incubation with different culture mediums or leachates. (B) The ratio (%) of cell-covered area was evaluated after 1 day compared to the initial gap between scratch borders. (C) Quantification of secreted VEGF from HUVEC for different groups on day 5. Note: scale bars 200 μm. Data presented as mean ± SD, n = 3, all statistical significance shown in comparison to control group unless otherwise stated, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
There have been some errors in the reference list. The corrected list is shown here as follows:
[12] (No page number)
Ege, Duygu, Kai Zheng, and Aldo R. Boccaccini. "Borate bioactive glasses (BBG): bone regeneration, wound healing applications, and future directions." ACS Applied Bio Materials 5.8 (2022): 3608–3622, https://doi.org/10.1021/acsabm.2c00384.
[24] (No page number)
Schmitt, Phillip R., Kiera D. Dwyer, and Kareen LK Coulombe. "Current applications of polycaprolactone as a scaffold material for heart regeneration." ACS Applied Bio Materials 5.6 (2022): 2461–2480, https://doi.org/10.1021/acsabm.2c00174.
[39] (No doi.)
Chu, Ju Chin, K. C. Burridge, and Frank Brown. "The viscosity of pseudo-plastic fluids." Chemical Engineering Science 3.6 (1954): 229–247, https://doi.org/10.1016/0009-2509(54)80006-6.
[45] (No doi. and page number)
Alasvand, N. et al. "Synthesis and characterization of novel copper-doped modified bioactive glasses as advanced blood-contacting biomaterials." Materials Today Chemistry 29 (2023): 101465. https://doi.org/10.1016/j.mtchem.2023.101465.
[81] (No page number)
Fujii, Takeshiro, and Yoshinori Watanabe. "Multidisciplinary treatment approach for prosthetic vascular graft infection in the thoracic aortic area." Annals of Thoracic and Cardiovascular Surgery 21.5 (2015): 418–427, https://doi.org/10.5761/atcs.ra.15-00187.