Abstract
Introduction
Lumbar disc herniation (LDH) and disc degeneration (DD) are associated with low back pain (LBP) and sciatica, which are common health problems. Emerging evidence suggests a link between vascular health, specifically abdominal aortic calcification (AAC) and systemic lipid profiles, and these spinal conditions.
Research question
This study investigates the associations between AAC, systemic lipid profiles, lumbar Modic Changes (MC), DD/LDH, and the occurrence of LBP or sciatica.
Material and methods
A literature search was performed (up to August 2023) in PubMed, Embase, Web of Science, Emcare, Cochrane Library, and Academic Search Premier utilizing a sensitive search strategy. Studies were chosen based on predefined criteria and assessed for bias using an adapted Cochrane checklist. Specifically, studies exploring the relationship between AAC or lipid status and DD/LDH and/or LBP/Sciatica were included.
Results
Twenty-seven studies were included. Eight studies assessed the association between atherosclerosis or lipid status and clinical LBP/sciatica, with four showing a positive association between AAC/lumbar artery stenosis and these conditions. Twenty-one studies assessed atherosclerosis and DD/LDH, with seven showing a positive association between AAC and DD/LDH. Eight trials found a positive association between lipid status and DD/LDH, and two trails identified ApoL1 as a biomarker for LDH recovery.
Discussion and conclusion
Evidence supports the hypothesis that inadequate blood supply contributes to disc degeneration, inflammation and clinical symptoms. Both local vascular issues and systemic lipid profiles appear to influence lumbar degeneration, highlighting the need for further research to better understand these relationships and develop preventive and therapeutic strategies.
Keywords: Aortic calcification, Lipids, Low back pain, Sciatica, Disc degeneration
Highlights
-
•
Severe AAC and dyslipidemia are linked to clinical LBP, sciatica, and disc degeneration.
-
•
Inadequate blood supply to the lumbar spine contributes to disc degeneration and LBP.
-
•
Evidence supports vascular health and systemic lipid profiles influencing lumbar degeneration.
-
•
Further studies are needed to explore vascular and lipid contributions to lumbar spine disease.
1. Introduction
Low back pain (LBP), with or without leg pain (sciatica), is a common health problem with a lifetime incidence of 49–70% in the general population, and is the leading cause of absence from work (Heliövaara et al., 1987; van Tulder et al., 2002). A common cause of low back pain and sciatica is the occurrence of lumbar disc herniation. A herniated disc may cause these symptoms through compressing the adjacent nerve root. However, decompression of the nerve through surgical disc removal doesn't consistently alleviate symptoms (Albert et al., 2008; Parker et al., 2015), suggesting that other factors such as degeneration and inflammation, key factors in herniation, might also contribute to radicular symptoms and LBP (Djuric et al., 2021).
While the exact triggers of disc inflammation and degeneration remain unclear, recent findings suggested that a trigger could be the presence of compromised vascularization of vertebral endplates (Battié et al., 1991; Ratcliffe, 1982; Kauppila et al., 1994; Shi et al., 2020); a phenomenon that potentially coinciding with systemic atherosclerosis. This prompts an investigation into the co-occurrence of atherosclerosis indicators with lumbar disc degeneration (DD), lumbar disc herniation (LDH) and/or sciatica and/or LBP (Kauppila, 2009). In addition, cardiovascular and lifestyle risk factors such as obesity, reduced exercise, and abnormal serum cholesterol are considered to be signs of systemic atherosclerosis (Han et al., 1997; Kauppila et al., 1993) and could thus also impact the occurrence of DD, LDH, LBP and sciatica.
As early as the 1980s, Ratcliffe et al. (Ratcliffe, 1980, 1982) proposed the atherosclerosis hypothesis. Extensive research supports the notion that abdominal aortic atherosclerosis that began early in life often occurs near the orifices of lumbar arteries (Cluroe et al., 1992). This atherosclerosis can result not only in abdominal aorta calcification (AAC) but also in stenosis or occlusion of branching arteries, compromising blood supply to the corresponding lumbar spine segment (Turgut et al., 2008; Kurunlahti et al., 2004; Kauppila et al., 2004).
The reduced blood supply to the corresponding segments of the lumbar spine makes the lumbar endplate more susceptible to defects and annulus fibrosus tears, which can induce the infiltration of inflammatory factors, trigger autoimmune reactions, and even lead to bacterial infections. These pathological and physiological processes, resulting from decreased blood flow, may be the underlying cause of Modic Changes observed on MRI (Albert et al., 2008; Viswanathan et al., 2020). These alterations may ultimately lead to DD/LDH, followed by the onset of LBP/Sciatica symptoms (Kauppila, 1994, 2009; Kauppila et al., 1993; Crock et al., 1976; Chiras et al., 1979).
In order to elucidate the relationship indicated by the abovementioned studies; the purpose of this systematic literature review is to focus on the correlation between AAC or its related parameters and degenerative disc diseases, lumbar disc herniation, and LBP/Sciatica.
2. Methods
2.1. Search strategy and study selection
Up to August 2023, electronic databases PubMed, Embase, Web of Science, Cochrane Library, Emcare and Academic Search Premier were searched using the search strategies displayed in Supplementary (Fig. 1). Two of the authors (CVL & WL) separately evaluated the articles based on predefined selection criteria. Reference screening and citation tracking were performed on the selected articles. Selection criteria were stated as followed:
-
•
the article was published in English;
-
•
the study contains data on atherosclerosis (either radiological imaging of the lumbar spine or laboratory investigations of lipid-related biomarkers);
-
•
the study included studies that reported on patients with lumbar radiculopathy and/or low back pain due to a radiologically proven lumbar disc herniation or disc degeneration;
-
•
the study reported on a minimum of 10 patients;
-
•
the study reported a correlation between the two topics;
-
•
The study was published in a peer reviewed journal.
Fig. 1.
Search strategy.
The exclusion criteria included studies in which patients with merely backpain without data on radiculopathy and/or DD were included as well as case reports, letters, comments, and opinion papers.
Any discrepancy in selection between the two reviewers was resolved in open discussion. This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviewers and Meta-analysis (PRISMA) 2015 Checklist (Moher et al., 2015).
2.2. Quality assessment
The methodological quality of all studies was assessed by two independent reviewers, using an adjusted version of the scoring criteria by Cowley (1995). Any discrepancy in selection between the two reviewers was resolved in open discussion.
The four items reviewed in the assessment of risk of bias (RoB) were: definition of patient group, selection bias, outcome bias, and attrition bias. For each item a maximum of two points could be attributed, and thus a maximum of total 8 points could be awarded. Studies were scored as low (≥5 points), intermediate (3–4 points) or high (<3 points) risk of bias according to the method adapted from Furlan (Furlan et al., 2009).
2.3. Data collection and extraction
Sample size, gender, age, and study design were summarized. Outcomes were extracted separately by two independent reviewers (WL & ND) for the articles that described the association of atherosclerosis signs (AAC and/or laboratory lipid status) and clinical aspects of lumbar degeneration (sciatica with or without LBP), and articles that described the association of atherosclerosis signs and radiological aspects of lumbar degeneration (DD or LDH). Outcome data were extracted concerning clinical aspects (back pain, BMI, etc.), radiological data concerning DD and/or LDH, radiological data concerning atherosclerosis (calcification of aorta or lumbar arteries), and laboratory data concerning lipid status. The correlations between these aspects, as evaluated in the papers, were represented as well as the conclusions drawn by the authors.
2.4. Level of evidence
The quality of evidence for all outcome parameters was evaluated using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach (according to Atkins et al. (2004) and adapted from Furlan et al. (2009)). Scoring was completed by one author (WL) and checked by a second author (CVL). Any discrepancies in scoring were resolved through discussion.
3. Results
3.1. Characteristics of studies
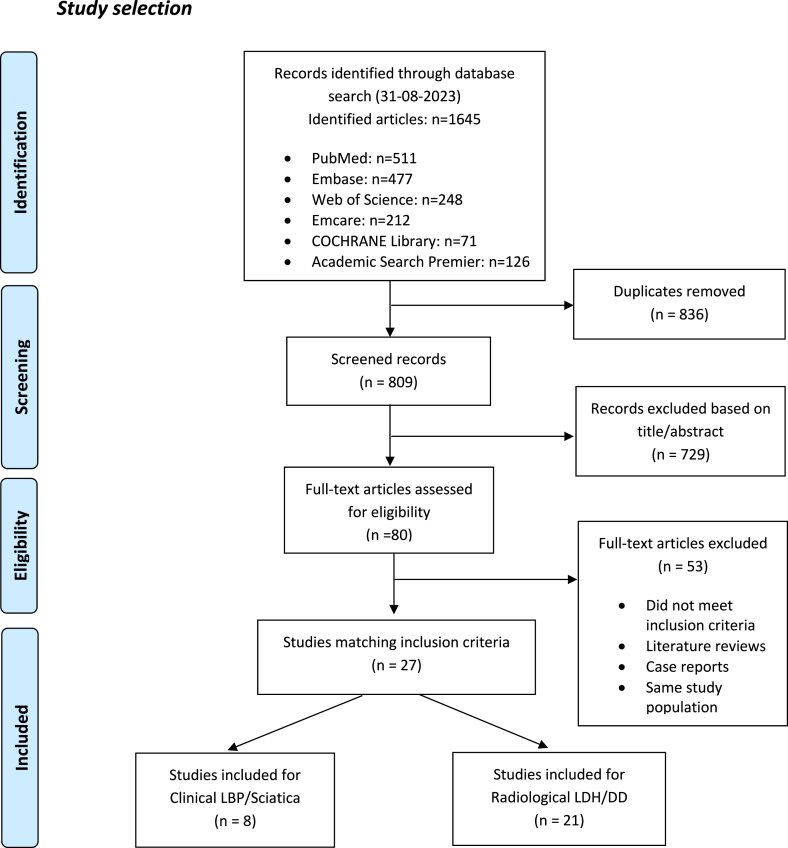
The search strategies identified 809 records after duplicates were removed. Titles and abstracts were screened, resulting in 80 eligible articles. After reading the full text, 27 articles suited the in- and exclusion criteria (Fig. 2). The characteristics and main findings of the included studies were summarized in Table 1. Atherosclerosis of patients was studied as AAC on CT or fluoroscopy, as arterial stenosis studied on 2D TOF-MRA, and as serum lipid status on laboratory situation.
Fig. 2.
Flow diagram-Studies selection progress.
Table. 1.
Characteristics of eligible studies.
| Author, year | Study population | Sample size (N) | Gender (M: F) | Age (Mean ± SD) | Study Design | Clinical parameters | Radiological evaluation LDH/DD | Radiological evaluation AAC/Stenosis | Laboratory situation | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Kauppila et al., (1997) | Framingham cohort | 606 | 35:65 | 54 ± 5 | Cross-sectional; Follow-up |
Back pain- “yes or no” BMI |
DD: 4 grades EP sclerosis:0 or 1 |
Radiograph AAC:4 grades | Serum cholesterol | 25 years |
| Kurunlahti et al., (1999) | Severe LBP | LBP: 29 Control: 52 |
62:38 | Range 20-63 | Cross-sectional | No specific parameters evaluated | CT discography: 4 grades | CT AAC: 4 grades | NA | NA |
| Kurunlahti et al., (2004) | Non-operated sciatica | 147 | 60:40 | Range 43-45 | Prospective; Follow-up | Leg-back pain (VAS); Disability (OLBQ); Physical ability (SEQ) |
NA | 2D TOF-MRA | NA | 3 years |
| Leino-Arjas et al., (2008) | ≥30 years old (Finland) | 5644 | 67:33 | 53 ± 15 | Cross-sectional | Physical work; sciatica; hyperlipemia; BMI | NA | NA | TC; LDL; HDL; Chol; TG | NA |
| Korkiakoski et al., (2009) | Healthy Caucasian males | 227 | 100:0 | Range 36-56 | Cross-Sectional | Leg-pain: VAS LBP: yes/no |
NA | 2D TOF-MRA | NA | NA |
| Bikbov et al., (2020) | ≥40 years old (Russia) | 5397 | 45:55 | 59 ± 11 | Cross-sectional | Pain: GPAQ | NA | NA | HDL; LDL; TG; Chol; CRP | NA |
| Kauppila, (1994) | Males (sudden unexpected death) | 86 | 100:0 | Range 36-69 | Cross-sectional | NA | Radiographs; X-ray: DD 4 grades | Stenosis of sacral arteries: 3 grades | NA | NA |
| Jhawar et al., (2006) | Female nurses (U.S.) | 98,407 | 0:100 | Range 30-55 | Prospective; Follow-up | BMI | MRI or CT | NA | Chol | 16 years |
| Hangai et al., (2008) | General population | 270 | 36:64 | Range 51-86 | Cross-sectional | Experience of LBP/smoke/alcoholic | DD on MRI | NA | LDL-C; TG | NA |
| Turgut et al., (2008) | LBP | 81 | <45 years: 23:77 45–65 years: 16:84 >65 years: 21:79 |
<45 years: 22 45–65 years: 45 >65 years: 14 |
Cross-sectional | Self-reported LBP | DD: 4 grades | AAC: 4 grades | NA | NA |
| Longo et al., (2011) | LDH surgery; arthroscopic meniscectomy | Case: 169 Control: 169 | 68:32 | Range 26-86 | Cross-sectional | No specific parameters evaluated | LDH on MRI | NA | TC; TG | NA |
| Suri et al., (2012) | Framingham Heart Study | 435 | 55:45 | 58 ± 13 | Cross-sectional | BMI | DHL:4 grades | Agatston score AAC: 3 grades | TC | NA |
| Xie et al., (2014) | LDH; Healthy subjects |
Proteomics: 30 for each ELISA: 10 |
50:50 | 28 ± 2 | Cross-sectional | No specific parameters evaluated | LDH on MRI | NA | APO-L1; APO-M; TN; IGL |
NA |
| Jin et al., (2015) | Healthy Center visitors' spine CT | 980 | 55:45 | 45 ± 5; Range 33-60 | Cross-sectional | Back leg pain/LDH/sciatica | LDH on CT | NA | TC; TG; HDL; LDL; FPG | NA |
| Estublier et al., (2015) | Men (50–85 years) | 766 | 100:0 | 64 ± 7 70 ± 8 |
Prospective; Cross-sectional |
Disability (5 physical tests), strength of the knee muscles, static balance, and dynamic balance. | Osteophytes and DSN: 4 scales EP sclerosis: absent/present |
Semiquantitative radiographic score: 24-point scale | TC, HDL, LDL, Triglycerides | 10 years |
| Huang et al., (2016) | LDH surgery; Healthy, SFR, spondylolisthesis, vertebral malformation and scoliosis |
LDH: 30 Control: 30 for each category |
50:50 | LDH: 45 ± 1 Control: 40 ± 1; 40 ± 1; 40 ± 1; 65 ± 2 |
Retrospective | No specific parameters evaluated | Unspecified confirmed LDH | NA | APO-L1: MWNT-based probe and conventional ELISA |
1 day, 7 days, 1 month, 3 months, 6 months, 12 months |
| Karabag et al., (2016) | LDH; Healthy subjects |
LDH: 48 Control: 50 |
LDH 50:50 Control: 64:36 |
35 ± 14 | Cross-sectional | No specific parameters evaluated | Unspecified confirmed LDH | NA | TG; TC; HDL-C; LOOH; TOS; PON1; TAS |
NA |
| Yuedong et al., (2016) | LDH; Trauma |
LDH:396 Trauma: 394 |
LDH 59:41 Trauma 57:43 |
41 ± 11 | Retrospective | NA | LDH on CT/MRI | NA | TC; TG; LDL-C; HDL-C | NA |
| Keser et al., (2017) | LDH surgery; Headache |
LDH:50 Headache: 50 |
50:50 | 41 ± 9 | Cross-sectional | No specific parameters evaluated | LDH on MRI | NA | TC; TG; LDL-C; HDL-C | NA |
| Beckworth et al., (2018) | Consecutive CTAs | 300 LAs; 120 disks |
NA | 58 ± 17 | Retrospective | No specific parameters evaluated | DD on CT: 4-point scale | CTA for LAs: 5 grades | NA | NA |
| Shi et al., (2020) | General population (China) | 678 | 47:53 | 48 ± 8 | Cross-sectional | BMI, WHR | Pfirrmann, Weishaupt scale |
NA | TG; TC; HDL-C; LDL-C | NA |
| Maurer et al., (2022) | General population, KORA cohort |
385 | 58:42 | 56 ± 9 | Cross-sectional | BMI, Back pain: Five levels |
DD on MRI: Pfirrmann; LDH on MRI |
NA | HDL-C; LDL; TG | NA |
| Coyle et al., (2021) | Community-dwelling, cognitively intact older adults | LBP: 21 Control: 21 |
38: 62 | Range 60-85 | Cross-sectional | low back pain–related disability (OLBQ); BMI | NA | NA | TC; HDL-C; LDL-C; TG; | NA |
| Huang et al., (2022) | Symptomatic hospitalized patients (LDH + LBP) | Group1 (no underlying diseases): 188 Group2 (underlying diseases): 114 |
Group1 60:40 Group2 45:55 |
Group1 52 ± 16 Group2 63 ± 11 |
Retrospective | No specific parameters evaluated | Pfirrmann, Weishaupt grading systems | NA | TC, TG, HDL-C, LDL-C, and LDLC/HDL-C | NA |
| Perera et al., (2022) | English population-based cohort | 3328 | 56:54 | 64 ± 7 | Cross-sectional | Self-reported back pain, BMI, WHR | NA | NA | TG, HDL-C | NA |
| Yuan et al., (2023) | General population (China) | 1035 | 52:48 | 50 ± 11 | Cross-sectional | BMI | IDD on MRI: Pfirrmann; MCs on MRI |
NA | TC, TG, LDL-C, HDL-C | NA |
| Schonnagel et al., (2023) | LBP | 217 | 48:52 | Range 48-68 | Cross-sectional | BMI | MRI; TEPS: 6 points; DD: Pfirrmann | Radiograph AAC: 3 grades |
NA | NA |
SD: Standard deviation, AAC: abdominal aortic calcification, LBP: Lower back pain, LDH: Lumbar disc herniation, DD: Disc degeneration, BMI: Body mass index, EP: Endplate, CT: computed tomography, VAS: visual analogue scale, OLBQ: Oswestry Low Back Questionnaire, SEQ: Self-Efficacy questionnaire, 2D-TOF: Two-dimensional time-of-flight, MRA: Magnetic resonance angiography, TC: Total cholesterol, LDL: Low-density lipoprotein, HDL: High-density lipoprotein, Chol: Cholesterol, TG: Triglycerides, GPAQ: Global physical activity questionnaire, CRP: C-reactive protein, MRI: Magnetic resonance imaging, LDL-C: Low-density lipoprotein cholesterol, DHL: Disc high loss, ELISA: enzyme-linked immunosorbent assay, APO-L1: Apolipoprotein-L1, APO-M: Apolipoprotein-M, TN: Tetranectin, IGL: Immunoglobulin light chain, FPG: Fasting plasma glucose, DSN: Disc space narrowing, SFR: Spinal fracture patients, MWNT: Multiwalled carbon nanotubes, HDL-C: High-density lipoprotein cholesterol, LOOH: lipid hydroperoxide, TOS: Total oxidative status, PON1: Paraoxonase 1, TAS: Total antioxidative status, CTA: Computed tomography angiography, LAs: lumbar arteries, WHR: waist to hip, TEPS: Total endplate score, KORA: Cooperative Health Research in the Region Augsburg, IDH: Intervertebral disk herniation, Apo AI: apolipoprotein AI, Apo B: apolipoprotein B, Lp(a): lipoprotein(a), WHR: Waist-to-height ratio, MCs: Modic changes.
3.2. Quality assessment in all included studies
A total of eight studies involved atherosclerosis/lipid status and clinical LBP/sciatica. Four articles were assessed as low risk of bias (Kurunlahti et al., 2004; Kauppila et al., 1997; Bikbov et al., 2020; Coyle et al., 2021), and the other four articles scored with an intermediate risk of bias (Kurunlahti et al., 1999; Leino-Arjas et al., 2008; Korkiakoski et al., 2009; Perera et al., 2022) (Table 2a).
Table. 2a.
Risk-of-bias analysis of Studies---Clinical LBP/Sciatica.
| Study (year of publication) | Total risk of bias (8) |
Patient group and study goal (2) | Absence of selection bias (2) | Outcome properly examined (2) | Absence of attrition bias (2) |
|---|---|---|---|---|---|
| Kauppila et al. (1997) | Low 5∗ | ∗∗ | – | ∗∗ | ∗ |
| Kurunlahti et al. (1999) | Inter 3∗ | ∗ | – | ∗ | ∗ |
| Kurunlahti et al. (2004) | Low 6∗ | ∗∗ | – | ∗∗ | ∗∗ |
| Leino-Arjas et al. (2008) | Inter 4∗ | ∗ | – | ∗∗ | ∗ |
| Korkiakoski et al. (2009) | Inter 4∗ | ∗ | – | ∗∗ | ∗ |
| Bikbov et al. (2020) | Low 8∗ | ∗∗ | ∗∗ | ∗∗ | ∗∗ |
| Coyle et al. (2021) | Low 5∗ | ∗∗ | – | ∗∗ | ∗ |
| Perera et al. (2022) | Inter 4∗ | ∗ | – | ∗∗ | ∗ |
The methodological quality of studies was evaluated according to an adjusted version of the Cowley Criteria (Cowley, 1995).
Low risk: ≥5∗; Intermediate risk: 3–4∗; High risk: ≤3∗.
1 score.
2 scores.
Twenty-one studies described the correlation between atherosclerosis/lipid status and radiological LDH/DD. Fifteen articles were assessed as low bias risk (Kauppila et al., 1994, 1997; Beckworth et al., 2018; Hangai et al., 2008; Karabag et al., 2016; Suri et al., 2012; Jhawar et al., 2006; Jin et al., 2015; Keser et al., 2017; Xie et al., 2014; Estublier et al., 2015; Maurer et al., 2022; Huang et al., 2022; Yuan et al., 2023; Schonnagel et al., 2023), while five articles with intermediate risk of bias (Shi et al., 2020; Turgut et al., 2008; Kurunlahti et al., 1999; Longo et al., 2011; Yuedong et al., 2016). Finally, only one article was assessed as high risk of bias (Huang et al., 2016) (Table 2b).
Table. 2b.
Risk-of-bias analysis of Studies---Radiological LDH/DD.
| Study (year of publication) | Total risk of bias (8) | Patient group and study goal (2) | Absence of selection bias (2) | Outcome properly examined (2) | Absence of attrition bias (2) |
|---|---|---|---|---|---|
| Kauppila et al. (1994) | Low 6∗ | ∗ | ∗∗ | ∗∗ | ∗ |
| Kauppila et al. (1997) | Low 5∗ | ∗∗ | – | ∗∗ | ∗ |
| Kurunlahti et al. (1999) | Inter 3∗ | ∗ | – | ∗ | ∗ |
| Jhawar et al. (2006) | Low 6∗ | ∗ | ∗∗ | ∗∗ | ∗ |
| Hangai et al. (2008) | Low 7∗ | ∗∗ | ∗∗ | ∗∗ | ∗ |
| Turgut et al. (2008) | Inter 4∗ | ∗ | – | ∗∗ | ∗ |
| Longo et al. (2011) | Inter 4∗ | ∗∗ | – | ∗ | ∗ |
| Suri et al. (2012) | Low 7∗ | ∗∗ | ∗∗ | ∗∗ | ∗ |
| Xie et al. (2014) | Low 5∗ | ∗∗ | – | ∗∗ | ∗ |
| Jin et al. (2015) | Low 6∗ |
∗∗ | ∗∗ | ∗ | ∗ |
| Estublier et al. (2015) | Low 5∗ | ∗∗ | ∗∗ | - | ∗ |
| Huang et al. (2016) | High 2∗ | ∗ | – | ∗ | – |
| Karabag et al. (2016) | Low 7∗ | ∗ | ∗∗ | ∗∗ | ∗∗ |
| Yuedong et al. (2016) | Inter 4∗ | ∗ | – | ∗∗ | ∗ |
| Keser et al. (2017) | Low 5∗ | ∗∗ | – | ∗∗ | ∗ |
| Beckworth et al. (2018) | Low 7∗ | ∗∗ | ∗∗ | ∗∗ | ∗ |
| Shi et al. (2020) | Inter 3∗ | ∗ | – | ∗ | ∗ |
| Maurer et al. (2022) | Low 5∗ | ∗∗ | – | ∗∗ | ∗ |
| Huang et al. (2022) | Low 5∗ | ∗∗ | – | ∗∗ | ∗ |
| Yuan et al. (2023) | Low 5∗ | ∗∗ | – | ∗∗ | ∗ |
| Schonnagel et al. (2023) | Low 5∗ | ∗∗ | – | ∗∗ | ∗ |
The methodological quality of studies was evaluated according to an adjusted version of the Cowley Criteria (Cowley, 1995).
Low risk: ≥5∗; Intermediate risk: 3–4∗; High risk: ≤3∗.
1 score.
2 scores.
The two articles that addressed the association of atherosclerosis with aspects of both clinical and radiological signs, were assessed as low to intermediate risk of bias (Kauppila et al., 1997; Kurunlahti et al., 1999).
3.3. Association of atherosclerosis with clinical signs of LBP/sciatica
Four studies evaluated the association between atherosclerosis and clinical aspects of low back pain and/or sciatica. Two of these studies showed that there was a positive correlation between AAC and LBP (Kauppila et al., 1997; Korkiakoski et al., 2009). In addition, the remaining two studies evaluated atherosclerotic changes of the lumbar arteries and demonstrated a positive association with LBP and sciatica (Kurunlahti et al., 1999, 2004) (Table 3).
Table. 3.
Results and conclusion---Correlation between Atherosclerosis with Clinical LBP/Sciatica.
| Author, year | Clinical Condition | Radiological outcome AAC/Stenosis | Correlation AAC/Stenosis and LBP/Sciatica | Conclusion | Correlation Age-adjusted |
|---|---|---|---|---|---|
| Kauppila et al., (1997) | Back pain: 74% M; 69% F BMI (Baseline): M 26.5 ± 3.1; F 24.9 ± 3.6; BMI (Follow-up): M 26.2 ± 3.3; F 25.6 ± 4.2 |
AAC (Baseline): 39% M and 25% F AAC (Follow-up): 91% M and 89% F |
Grade 3 AAC was correlated with back pain (OR 1.56 [1.10–2.21], P = 0.014); No correlation between Grade 1 or 2 AAC and back pain. |
Severe AAC was associated with back pain | Yes |
| Kurunlahti et al., (1999) | 29 patients with LBP | <50 years: 48% AAC + LBP+; 8% AAC + LBP-. >50 years: 83% AAC + LBP+; 50% AAC + LBP-. Total ages: 55% AAC + LBP+; 21% AAC + LBP-. |
Atherosclerosis was associated with LBP: <50 years (P = 0.00051, OR 10.08 [2.40–42.45]), Total ages (P = 0.00185, OR 4.59 [1.71–12.34]). |
AAC was associated with LBP. | NA |
| Kurunlahti et al., (2004) | No specific data available on LBP/sciatica | Narrowed or occluded lumbar arteries at Baseline: 19% 3 years Follow-up: 27% |
1. Arterial stenosis at baseline were associated with LBP at 1 year (P = 0.036) and leg pain at 2 years (P = 0.006); 2. Newly formed arterial stenosis were associated with medical consultations due to LBP (the year preceding the baseline) (P = 0.03), prolonged LBP (≥3 months, baseline-1 year) (P = 0.02) and prolonged sciatica (baseline-1 year) (P = 0.05) |
Narrowing or occlusion of lumbar arteries were associated with LBP, leg pain, and sciatica | NA |
| Korkiakoski et al., (2009) | LBP: 38%; Sciatica: 49% |
43% no stenosis; 27% stenosis in 1 lumbar artery; 30% stenosis in >2 lumbar arteries. |
Stenosis in >2 lumbar arteries were associated with duration of sciatica (OR 2.70 [1.18–6.20]), intensity of LBP (OR 1.38 [1.07–1.73]), and intensity of leg pain (OR 1.32 [1.02–1.67]) | lumbar arterial stenosis was associated with Sciatica and LBP | Yes |
AAC: abdominal aortic calcification, LBP: Lowe back pain, BMI: Body mass index, OR: Odds Ratio.
The level of evidence is high because the four included randomized controlled trials (RCT) had low or moderate risk and all concluded a positive association. Besides, two of the studies corrected for age as a confounder (Kauppila et al., 1997; Korkiakoski et al., 2009) (Appendix).
3.4. Association of BMI/lipid status with clinical signs of LBP/sciatica
A total of five studies investigated the association of serum lipid status with LBP or sciatica (Kauppila et al., 1997; Bikbov et al., 2020; Coyle et al., 2021; Leino-Arjas et al., 2008; Perera et al., 2022) (Table 4). Of these studies, two reported that serum lipid status was not associated with LBP (Kauppila et al., 1997; Coyle et al., 2021). However, another two studies demonstrated that atherogenic serum lipids in men and pharmacologically treated hyperlipidemia in women was positively associated with sciatica (Leino-Arjas et al., 2008) and high TG was also associated with more severe back pain (Perera et al., 2022). Notably, the study by Bikbov et al. found that high levels of Cholesterol and high-density lipoproteins but not low-density lipoproteins were associated with LBP (Bikbov et al., 2020).
Table. 4.
Results and conclusion---Correlation between BMI/lipid status and Clinical LBP/Sciatica.
| Author, year | Clinical Condition | Laboratory data | Correlation BMI and LBP/Sciatica | Correlation Lipid status and LBP/sciatica |
Conclusion | Correlation Age-adjusted |
|---|---|---|---|---|---|---|
| Kauppila et al., (1997) | Back pain:74% M; 69% F BMI (Baseline, Kg/m2): M 26.5 ± 3.1; F 24.9 ± 3.6; BMI (Follow-up, Kg/m2): M 26.2 ± 3.3; F 25.6 ± 4.2 |
Serum Cholesterol (Baseline): M 5.8 ± 1.0; F 6.1 ± 1.1 Serum Cholesterol (Follow-up): M 5.9 ± 1.0; F 6.3 ± 1.1 |
No correlation between BMI and back pain | No correlation between serum cholesterol and back pain | No correlation | No |
| Leino-Arjas et al., (2008) | Sciatica: 2.7% (3.3% M, 2.2% F); Pharmacologically treated hyperlipidemia: 6.2% (6.7% M, 5.8% F); BMI >30 kg/m2 (2.2%). |
TChol: M 5.97 ± 1.10; F 5.89 ± 1.11 LDL: M 3.82 ± 1.26; F 3.79 ± 1.07 HDL: M 1.20 ± 0.33; F 1.44 ± 0.38 TG: M 1.77 ± 1.23; F 1.39 ± 0.71 |
Pharmacologically treated hyperlipidemia was associated with sciatica in women (OR 2.02 [1.01–4.04]), but not in men. | High TChol (>6.31) (OR 2.28 [1.14–4.55]), LDL (OR 2.12 [1.11–4.05]), and TG (OR 1.92 [ 1.04–3.55]) in men were associated with sciatica, but not in women. | Sciatica was associated with lipid status in men and pharmacologically treated hyperlipidemia in women. | Yes |
| Bikbov et al., (2020) | LBP: 54.0% BMI (No specific data) |
No specific data available on values of HDL, LDL, TG, and Cholesterol | LBP was associated with higher BMI (OR 1.02 [1.01–1.03], P = 0.002) and history of CV disease (OR 1.23 [1.07–1.42], P = 0.004) | LBP was associated with higher HDL (OR 1.10 [1.03–1.18], P = 0.004) | LBP was correlated with higher HDL, BMI and history of CV disease | NA |
| Coyle et al., (2021) | BMI (CLBPR, Kg/m2): 26.7 ± 2.8 BMI (No Pain, Kg/m2): 24.0 ± 5.0 |
(CLBPR, Control, mg/dL) TC: 185.7 ± 34.7, 190.7 ± 33.6 HDL-C: 62.3 ± 18.8, 66.1 ± 17.0 LDL-C: 99.5 ± 25.8, 103.9 ± 27.7 TG: 119.3 ± 60.3, 103.0 ± 42.7 |
Older adults with CLBPR exhibited higher BMIs (P = 0.004) | There were no differences in TC(P = 0.647), HDL-C(P = 0.503), LDL-C(P = 0.609), or TG (P = 0.324) between groups. | LBP was correlated with higher BMI, but not blood lipid. | NA |
| Perera et al., (2022) | Back pain: Yes 20.2%, Severity 5.01 ± 2.36 BMI: 27.8 ± 4.71 kg/m2 WHR: 0.57 ± 0.07 |
TG: 1.5 ± 0.4 mmol/L HDL-C: 5.0 ± 0.9 mmol/L |
BMI was associated with back pain (OR 1.07 [1.05–1.09]) and more severe pain (Beta 0.05 [0.02–0.08]). WHR was associated with back pain in middle-aged women (OR 1.88 [1.34–2.64]) and older men (OR 2.18 [1.42–3.36]). |
High TG (OR 1.49 [1.05–2.12]) was associated with back pain in older women. TG (Beta 0.55 [0.20–0.90]) was associated with more severe back pain. | BMI, WHR and TG were associated with back pain. | Yes |
BMI: Body mass index, LBP: Lowe back pain TChol: Total cholesterol, LDL: Low density lipoprotein, HDL: High density lipoprotein, TG: Triglycerides, OR: Odds Ratio, CV: Cardiovascular, CLBPR: Chronic low back pain with radiculopathy, WHR: Waist-to-height ratio.
Regarding cardiovascular risk factors, four studies investigated the relation between BMI and LBP, of which three positively associated LBP with higher BMI, waist-to-height ratio (WHR) and a history of cardiovascular disease (Bikbov et al., 2020; Coyle et al., 2021; Perera et al., 2022), but the other did not find an association with BMI (Kauppila et al., 1997) (Table 5).
Table. 5.
Association between atherosclerosis markers and clinical LBP/Sciatica.
| Study (year of publication) | AAC | Lumbar arterial stenosis | BMI/WHR | History of CV disease | TChol | LDL | HDL | TG |
|---|---|---|---|---|---|---|---|---|
| Kauppila et al. (1997) | Yes (Grade 0 vs 3) | No | No | |||||
| No (Grade 0 vs 1 or 2) | ||||||||
| Kurunlahti et al. (1999) | Yes (<50yr, Total age) | |||||||
| Kurunlahti et al. (2004) | Yes (Newly formed) | |||||||
| Leino-Arjas et al. (2008) | Yes (men) | Yes (men) | Yes (men) | |||||
| Korkiakoski et al. (2009) | Yes (>2 lumbar arteries) | |||||||
| Bikbov et al. (2020) | Yes | Yes | Yes | No | Yes (Higher HDL) | |||
| Coyle et al. (2021) | Yes | No | No | No | No | |||
| Perera et al. (2022) | Yes | Yes |
BMI: Body mass index, LBP: Lowe back pain, TChol: Total cholesterol, LDL: Low density lipoprotein, HDL: High density lipoprotein, TG: Triglycerides, CV: Cardiovascular, WHR: Waist-to-height ratio.
As observational studies they all had a low level of evidence, but three of the five studies adjusted for the effects of confounders such as age, gender, and BMI, and can therefore be increased by moderate (Kauppila et al., 1997; Leino-Arjas et al., 2008; Perera et al., 2022) (Appendix).
3.5. Association of atherosclerosis and radiological DD/LDH
A total of eight studies investigated the association between arterial stenosis (either in aorta or lumbar arteries) or AAC and radiological DD (Table 6). Seven of those studies reported a positive correlation (Kauppila et al., 1994, 1997; Turgut et al., 2008; Beckworth et al., 2018; Suri et al., 2012; Estublier et al., 2015; Schonnagel et al., 2023), with four studies remained positive after adjusting for age (Kauppila et al., 1994, 1997; Beckworth et al., 2018; Estublier et al., 2015). In Schönnagel's study, the correlation between AAC and DD was no longer significant after adjusting for age confounders but was only associated with endplate changes (Schonnagel et al., 2023). The remaining study by Kurunlahti et al. found no association between the quantity of aortic plaques and the degree of DD, without adjustment for age (Kurunlahti et al., 1999).
Table. 6.
Results and conclusion---Correlation between Atherosclerosis and Radiological DD/LDH.
| Author, year | Clinical Condition | Radiological outcome AAC/Stenosis | Radiological outcome DD/LDH | Correlation AAC/Stenosis and DD/LDH | Conclusion | Correlation Age-adjusted |
|---|---|---|---|---|---|---|
| Kauppila et al., (1994) | NA | (Lumbar/Middle Sacral Arteries) No stenosis: 52%; Slight stenosis: 30%; Severe stenosis or occlusion: 18% |
DD grade 1: 12%, grade 2: 42%, grade 3: 33%, grade 4: 13%. |
DD scores were associated with the grade of arterial stenosis (P: 0.002–0.013) | Lumbar and Middle Sacral arterial stenosis due to atherosclerosis is associated with LDD | Yes |
| Kauppila et al., (1997) | Back pain: 74% M; 69% F BMI (Baseline): M26.5 ± 3.1; F24.9 ± 3.6; BMI (Follow-up): M 26.2 ± 3.3; F25.6 ± 4.2 |
AAC (Baseline): 39% M and 25% F AAC (Follow-up): 91% M and 89% F |
Baseline: anterior osteophytes 85% M, 63% F; DSN 19% M, 12% F; EP sclerosis 6% M, 2% F Follow-up: anterior osteophytes 97% M, 92% F; DSN 60% M, 60% F; EP sclerosis 29% M,26% F. |
Baseline: AAC was associated with DD (OR 1.59 [1.03–2.45], P = 0.036) Follow-up: AAC was associated with DD (OR 2.02 [1.16–3.51], P = 0.013). |
AAC was correlated with DD. | Yes |
| Kurunlahti et al., (1999) | NA | Atheromatous lesions grade 0: 37, grade 1: 31, grade 2: 11, grade 3: 7. |
DD grade 0: 6, grade 1: 25, grade 2: 31, grade 3: 24. |
No correlation between the DD grades and atheromatous lesion grades. | No correlation | NA |
| Turgut et al., (2008) | NA | AAC ≤45 years: 0.05 ± 0.05; 45–65 years: 0.53 ± 0.12; ≥65 years: 1.50 ± 0.29 |
DD ≤ 45 years: 0.37(X-ray), 0.26(CT); 45–65 years: 1.61 (X-ray), 0.53 (CT); ≥65 years: 2.71 (X-ray), 0.69 (CT). |
AAC was correlated with DD in X-ray study (r = 0.565, P < 0.001), and in CT study (r = 0.341, p < 0.05) | AAC was correlated with DD. | NA |
| Suri et al., (2012) | Overweight (BMI 25–30): 41.4% Obese (BMI≥30): 29.1% |
No AAC 34.9%; Low AAC 32.6% High AAC 32.6% (Agatston score >949.39) |
Moderate (grade 2) DHL: 61.8% | low AAC (OR 2.05 [1.27–3.30], p = 0.003) and high AAC (OR 2.24 [1.38–3.62], p = 0.001) were both associated with DHL. | AAC was correlated with DHL. | No |
| Estublier et al., (2015) | BMI (Survivors): 28.0 ± 3.6; BMI (Non survivors): 27.5 ± 3.6 |
Severe AAC (score >5): 29% |
Grade 3 osteophytes: 69%, Grade 3 DSN: 16%, Grade 2 overall changes at ≥1 vertebral level: 86% |
Severe AAC was associated with total DSN (OR 1.44 per 1 SD [1.11–1.87], P < 0.05); Lower probability of long-term AAC stability was associated with osteophyte score (OR 0.50 [0.30–0.84]) | Severe DSN was associated with severe AAC; Severe osteophytes were associated with faster AAC progression. | Yes |
| Beckworth et al., (2018) | NA | lumbar artery stenosis: Normal DDD: 0; Mild DDD: 4; Moderate DDD: 6.5; Severe DDD: 8.5 |
Mild DDD 35 (29%); Moderate DDD 12 (10%); Severe DDD 24 (20%). |
Lumbar artery stenosis was corelated with DDD (OR 0.80 [0.68–0.84], P < 0.0001). | Atherosclerotic disease of lumbar arteries and aorta were correlated with lumbar DDD | Yes |
| Schonnagel et al., (2023) | Median BMI: 26.2 | In total AAC 39%; Moderate AAC 21%; Severe AAC 18% |
DDD: L1/2 21%, L2/3 29%, L3/4 37%, L4/5 47%, L5/S1 44%; TEPS: L1/2 4, L2/3 4, L3/4 4, L4/5 5, L5/S1 5 |
AAC was associated with DDD (OR 3.64 [2.36–5.63], p < 0.001) and TEPS (β 0.94 [1.97–2.17], p < 0.001) | AAC was correlated with DD and endplate changes | No (only AAC and the severity of endplate changes) |
AAC: Abdominal aortic calcification LDH: Lumbar disc herniation, DD: Disc degeneration, LDD: Lumbar disc degeneration, BMI: Body mass index, DSN: Disc space narrowing, EP: Endplate, CT: computed tomography, DHL: Disc height loss, DDD: degenerative disk disease, TEPS: Total endplate score.
According to the GRADE assessment, the level of evidence is classified as high due to the inclusion of studies that demonstrate a low risk of bias, inconsistency, indirectness, and inaccuracy (Appendix).
3.6. Association of BMI/lipid status and radiological DD/LDH
The association between serum lipid status and/or BMI and DD or LDH was studied in fourteen articles (Table 7). A total of eight studies explored the correlation between BMI and DD/LDH, with five demonstrating a positive association (Shi et al., 2020; Hangai et al., 2008; Jhawar et al., 2006; Jin et al., 2015; Maurer et al., 2022), while three reported no significant correlation (Keser et al., 2017; Yuan et al., 2023; Yuedong et al., 2016). Of these, Maurer's study showed that BMI was only associated with DD and not with disk herniation (Maurer et al., 2022). Abdominal obesity (WHR) linked to DD in one study (Shi et al., 2020).
Table. 7.
Results and conclusion---Correlation between BMI/lipid status and Radiological DD/LDH.
| Author, year | Clinical Condition | Radiological outcome LDH/DD | Laboratory data | Correlation BMI and LDH/DD | Correlation lipid status and LDH/DD | Conclusion | Correlation Age-adjusted |
|---|---|---|---|---|---|---|---|
| Jhawar et al., (2006) | BMI <21.9: 603; 22-25: 703; 25–27: 352; 27–29: 246; >29: 524. |
LDH 2734 (3%) |
High cholesterol (no specified): 294 | Risk on LDH increasing with increasing BMI (P = 0.01) | LDH was associated with high cholesterol (RR: 1.26, [1.10–1.44]) | BMI and High cholesterol were associated with symptomatic LDH. | Yes |
| Hangai et al., (2008) | BMI: 24.0 ± 2.8 <25 64%, ≥25 36% |
DD: 57% M, 60% F |
LDLc (mg/dl): ≤140 68%, ≥140 32% TG (mg/dl): <150 86%, ≥150 14% |
BMI≥25 was correlated with DD (OR: 2.32–3.58, P < 0.05). | LDLc≥140 was correlated with DD only at L4/5 (OR 2.65 [1.33–5.52], P = 0.007). TG ≥ 150 was not correlated with DD. | DD was associated with high BMI and high LDLc but not with TG. | NA |
| Longo et al., (2011) | BMI: LDH 26.6 M, 24.1 F Control 26.7 M, 24.1 F |
LDH: 169 | (LDH, Control, mmol/L) TG: 1.8 ± 1.5, 1.5 ± 0.8 TC: 5.6 ± 1.2, 5.3 ± 1.0 |
No specific data available | Patients with LDH had higher TG (>4.5) (P = 0.02) and TC (>6.2) (P = 0.01). | Higher TG and TC were associated with symptomatic LDH. | NA |
| Suri et al., (2012) | Overweight (BMI 25–30): 41.4% Obese (BMI≥30): 29.1% |
Moderate DHL: 61.8% | Hypercholesterolemia (TC > 240 mg/dL): 24.8% | No specific data available | AAC or Hypercholesterolemia was not associated with DHL | DHL was not associated with AAC or hypercholesterolemia. | No |
| Xie et al., (2014) | NA | Proteomics: 30 LDH, 30 Control ELISA: 10 LDH, 10 Control |
APO-L1 was only found in LDH patients; APO-M, TN and IGL in LDH patients were all downregulated (by 22 ± 3%, 37 ± 5%, 27 ± 3%) | NA | Proteomic: APO-M, TN and IGL from patients with LDH were all downregulated (P < 0.01 for each) ELISA: APO-M, TN and IGL were lower in LDH (P < 0.05). APO-L1 was higher in LDH (P < 0.01) |
Serum APO-L1, TN, APO-M and IGL may serve as LDH biomarkers. | NA |
| Jin et al., (2015) | BMI (LDH): 25.7 ± 2.2 | LDH: 490 | (LDH, mmol/L) TC: 4.8 ± 0.9 TG: 1.7 ± 1.8 HDL:1.4 ± 1.5LDL:3.2 ± 0.7 |
LDH was correlated with BMI (P < 0.001) | LDH was associated with TG (P = 0.014), LDL (P < 0.001) and Lower HDL (P < 0.001) | BMI, TG, LDL, and Lower HDL was associated with LDH | NA |
| Huang et al., (2016) | NA | LDH: 30 | (Absorption intensity of ApoL1) MWNT: LDH vs others = 17 times; ELISA: LDH vs others = 10 times. | NA | LDH patients demonstrated much higher level of ApoL1 than in controls. (MWNT P < 0.001) The level of ApoL1 decreased with time after surgery for LDH | ApoL1 was a potential biomarker for early diagnosis of LDH and tracking the recovery of LDH. | NA |
| Karabag et al., (2016) | NA | LDH: 42 | (LDH, Control, mmol/L) TG: 260 ± 110, 198 ± 49; LDL:121 ± 33, 120 ± 39; PON.: 108 ± 35,128 ± 35; LOOH: 14 ± 5, 9 ± 2; TAS: 1.3 ± 0.2, 1.1 ± 0.2 |
NA | TG and LDL were not associated with LDH. Serum PON-1 level was lower (P = 0.008), TAS and LOOH levels were higher (both P < 0.001) in the LDH group. | LDH is associated with atherosclerosis | NA |
| Yuedong et al., (2016) | BMI LDH: 24.2 ± 3.6, Control: 24.6 ± 3.6 |
LDH: 396 | (LDH, Control, mmol/L) TC: 4.8 ± 1.0, 4.4 ± 0.9, TG: 1.5 ± 0.9, 1.4 ± 0.9, LDL-C: 2.9 ± 0.9,2.6 ± 0.7, HDL-C:1.3 ± 0.3; 1.3 ± 0.5 |
BMI was not associated with LDH (P = 0.144) | Higher TC (OR 2.05 [1.04–4.04]), LDL-C (OR 1.46 [1.18–1.81]), High TG (OR 2.97 [1.49–5.95]) and borderline High LDL-C (OR 1.63 [1.01–2.61]) were associated with LDH | BMI was not associated with LDH. Higher TC, LDL-C and TG were associated with LDH. |
NA |
| Keser et al., (2017) | BMI: LDH: 28.0 ± 0.7; Control: 28.8 ± 0.6 |
LDH: 50 | (LDH, mmol/L) TC: 198 ± 41, TG: 133 ± 66 LDL-C: 132 ± 35; HDL-C:40.4 ± 10; TC/HDL-C: 5.1 ± 1.3 |
BMI was not associated with LDH (P = 0.332) | There were no association between TC, TG, LDL-C, HDL-C, TC/HDL-C and LDH |
BMI and lipid status (TC, TG, LDL, HDL, TC/HDL) were not associated with symptomatic LDH. | NA |
| Shi et al., (2020) | Subjects: 14.9% LH-NO,11.4% LH-O, 18.1% LA-NO,55.6% LA-O BMI: LH-NO 22.01, LH-O 24.15, LA-NO 22.61, LA-O 24.88 WHR: LH-NO 0.82, LH-O 0.91, LA-NO 0.82, LA-O 0.91 |
DD: 4.2–13.5% |
(LDH, mmol/L) TG: 0.9–2.3, TC: 4.6–5.4 HDL-C: 1.2–1.5 LDL-C: 2.6–3.4 |
WHR was associated with DD (OR 1.05–1.12, P < 0.01). LA-NO demonstrates a high incidence for DD (P < 0.05), LH-O confers a severe DD grade (P < 0.05). | Elevated TG was associated with DD (OR 1.243–1.629, P < 0.01). | Elevated TG and abdominal obesity (WHR) were associated with DD | Yes |
| Maurer et al., (2022) | BMI: 28.1 ± 5.0 | DD: 76.4% | HDL-C (mg/dl): 62.0 ± 17.8 LDL-C (mg/dl): 139.8 ± 32.7 TG (mg/dl): 131.3 ± 83.0 |
BMI was associated with DD (P = 0.003) but not LDH (P = 0.98) | There were no association between LDL-C(P = 0.11), HDL-C (P = 0.99), TG (P = 0.81), and DD; There were no association between LDL-C(P = 0.21), HDL-C (P = 0.63), TG (P = 0.34), and LDH | BMI was associated with DD but not LDH; Lipid status (LDL, HDL, TG) was not associated with DD or LDH. | NA |
| Huang et al., (2022) | BMI (kg/m2) Group1: 23.54 ± 3.0 Group2: 24.06 ± 3.2 |
IVDD Group1: 188 Group2: 114 |
(Group1, Group2, mmol/L) TC: 5.4 ± 1.1, 5.7 ± 1.3; TG: 1.5 ± 0.9, 1.7 ± 0.9; LDL-C: 3.4 ± 0.8, 3.6 ± 0.9; HDL-C: 1.4 ± 0.9, 1.3 ± 0.3; LDL-C/HDL-C: 2.7 ± 0.8, 2.9 ± 0.9 | (Machine learning model) Contributing factors for the severity of IVDD: Group2 BMI (15.3%) | (Machine learning model) Contributing factors for the severity of IVDD: Group1 HDL-C (20.7%), TG (11.8%) Group2 LDL-C/HDL-C (13.9%) |
HDL-C and TG are related to the severity of IVDD | NA |
| Yuan et al., (2023) | BMI (kg/m2) Non-IDD group: 24.38 ± 2.93 IDD group: 25.09 ± 13.30 |
IDD: 446 MC1: 84 MC2: 244 MC3: 27 |
(IDD, Control, mmol/L) TC: 5.7 ± 1.2, 5.4 ± 1.1, TG: 1.6 ± 1.3, 1.7 ± 1.6, LDL: 3.6 ± 1.0, 3.4 ± 0.9, HDL: 1.7 ± 6.7, 1.3 ± 0.3 |
BMI was not significantly different between the two groups (p = 0.207). | TC (adjusted OR 1.775 [1.209–2.606]) and LDL-C (adjusted OR 1.818 [1.123–2.943]) were associated with IDD; There was no associations between serum lipid and any type of MCs after adjustment for age (P > 0.05). | High TC (≥6.2 mmol/L) and high LDL-C (≥4.1 mmol/L) were independent risk factors for IDD. Serum lipid was not correlated with MCs. | Yes (DD) No (MCs) |
BMI: Body mass index, LDH: Lumbar disc herniation, DD: Disc degeneration, LDLc: Low-density lipoprotein cholesterol, TG: Triglycerides, TC: Total cholesterol, DHL: Disc height loss, ELISA: enzyme-linked immunosorbent assay, APO-L1: Apolipoprotein-L1, APO-M: Apolipoprotein-M, TN: Tetranectin, IGL: Immunoglobulin light chain, HDL: High-density lipoprotein, LDL: Low-density lipoprotein, MWNT: Multiwalled carbon nanotubes, PON.: Paraoxonase, LOOH: lipid hydroperoxide, TAS: Total antioxidative status, WHR: waist to hip ratio, LH-NO: lipid healthy and non-obese, LH-O: lipid healthy but obese, LA-NO: lipid abnormal but not obese, LA-O: lipid abnormal and obese, IDH: Intervertebral disk herniation, IVDD: Intervertebral disc degeneration, Apo AI: apolipoprotein AI, Apo B: apolipoprotein B, Lp(a): lipoprotein(a).
Among all fourteen studies that investigated the correlation between serum lipid and DD/LDH, seven studies provided compelling evidence of a positive correlation (Jhawar et al., 2006; Jin et al., 2015; Xie et al., 2014; Huang et al., 2016, 2022; Longo et al., 2011; Yuedong et al., 2016), while four studies reached negative conclusions (Karabag et al., 2016; Suri et al., 2012; Keser et al., 2017; Maurer et al., 2022). Three other independent studies have demonstrated that only some, but not all, of the common lipid markers (total cholesterol, triglycerides, LDL and HDL) are associated with DD or LDH (Shi et al., 2020; Hangai et al., 2008; Yuan et al., 2023). Two laboratory studies discerned elevated serum APO-L1 levels in LDH patients, thereby considering it a promising candidate as a potential biomarker for LDH diagnosis (Xie et al., 2014; Huang et al., 2016). Moreover, the study by Karabag et al. elucidated that LOOH associated with oxidative stress is increased in patients with LDH, whereas the antioxidant response of PON1 is reduced, and these results were suggested as possible indirect evidence of an increased subclinical atherosclerotic and inflammatory state in patients with LDH (Karabag et al., 2016).
The level of evidence is lowered with 2 levels because of one trial with high risk of bias and three trials focused on indirect evidence of the study subjects (2 Apo-L1, 1 PON-1). Therefore, the overall level of evidence is rated as low according to the GRADE assessment (Appendix).
4. Discussion
In summary, four out of the four studies supported an association between atherosclerosis and low back pain and/or sciatica (Kurunlahti et al., 1999, 2004; Kauppila et al., 1997; Korkiakoski et al., 2009). Regarding the association between serum lipid levels and low back pain, three of the five included studies supported a positive association (Kauppila et al., 1997; Bikbov et al., 2020; Coyle et al., 2021; Leino-Arjas et al., 2008; Perera et al., 2022), and three of the four studies on BMI and LBP found a positive association (Kauppila et al., 1997; Bikbov et al., 2020; Coyle et al., 2021; Perera et al., 2022) (Table 5). Regarding the radiological data: six of the eight studies on atherosclerosis markers (AAC and lumbar arterial stenosis) demonstrated a positive correlation (Kauppila et al., 1994, 1997; Turgut et al., 2008; Kurunlahti et al., 1999; Beckworth et al., 2018; Suri et al., 2012; Estublier et al., 2015; Schonnagel et al., 2023). Five of the eight studies on BMI supported a positive association (Shi et al., 2020; Hangai et al., 2008; Jhawar et al., 2006; Jin et al., 2015; Keser et al., 2017; Maurer et al., 2022; Yuan et al., 2023; Yuedong et al., 2016), and ten of fourteen studies found a positive correlation between at one or more serum lipid markers and radiological DD (Shi et al., 2020; Hangai et al., 2008; Karabag et al., 2016; Suri et al., 2012; Jhawar et al., 2006; Jin et al., 2015; Keser et al., 2017; Xie et al., 2014; Maurer et al., 2022; Huang et al., 2016, 2022; Yuan et al., 2023; Longo et al., 2011; Yuedong et al., 2016) (Table 8).
Table. 8.
Association between atherosclerosis markers and radiological DD/LDH
| Study (year of publication) | AAC | Lumbar arterial stenosis | BMI/WHR | TC | TG | LDL | HDL | ApoL1 | Others |
|---|---|---|---|---|---|---|---|---|---|
| Kauppila et al. (1994) | Yes | ||||||||
| Kauppila et al. (1997) | Yes | ||||||||
| Kurunlahti et al. (1999) | No | ||||||||
| Jhawar et al. (2006) | Yes | Yes | |||||||
| Turgut et al. (2008) | Yes | ||||||||
| Hangai et al. (2008) | Yes | No | Yes | ||||||
| Longo et al. (2011) | Yes | Yes | |||||||
| Suri et al. (2012) | No | No | |||||||
| Xie et al. (2014) | Yes | ||||||||
| Jin et al. (2015) | Yes | Yes | Yes | Yes (lower HDL) | |||||
| Estublier et al. (2015) | Yes | ||||||||
| Huang et al. (2016) | Yes | ||||||||
| Karabag et al. (2016) | No | No | No | Yes (PON, LOOH, TAS) N, LOOH, TAS) |
|||||
| Yuedong et al. (2016) | No | Yes | Yes | Yes | |||||
| Keser et al. (2017) | No | No | No | No | No | ||||
| Beckworth et al. (2018) | Yes | Yes | |||||||
| Shi et al. (2020) | Yes | No | Yes | No | Yes (lower HDL) | ||||
| Maurer et al., (2022) | Yes (DD) No (LDH) |
No | No | No | |||||
| Huang et al. (2022) | Yes | Yes | Yes | ||||||
| Yuan et al. (2023) | No | Yes | No | Yes | No | ||||
| Schonnagel et al., (2023) | Yes |
BMI: Body mass index, LDH: Lumbar disc herniation, DD: Disc degeneration, LDLc: Low-density lipoprotein cholesterol, TG: Triglycerides, TC: Total cholesterol, DHL: Disc height loss, APO-L1: Apolipoprotein-L1, LDL: Low-density lipoprotein, PON.: Paraoxonase, LOOH: lipid hydroperoxide, TAS: Total antioxidative status, WHR: waist to hip ratio, Apo AI: apolipoprotein AI, Apo B: apolipoprotein B, Lp(a): lipoprotein(a).
This systematic overview of the literature concerning the associations between atherosclerosis-related risk factors and DD displays that there is high level evidence linking aortic calcification and stenosis with LBP and DD, while weak evidence supporting the correlation of BMI and lipid status with LBP and DD. Previous studies have concluded that the correlation between atherosclerotic risk factors and lumbar spine degeneration is largely attributable to age confounders (Suri et al., 2012; Shcherbina et al., 2017). Of the studies included in this systematic review, four of the five studies investigating the association of atherosclerosis and its risk factors with clinical low back pain still yielded positive associations after adjustment for age (Kauppila et al., 1997; Leino-Arjas et al., 2008; Korkiakoski et al., 2009; Perera et al., 2022). Similarly, a positive association between atherosclerosis and radiological DD/LDH was obtained in 7 of 10 studies that adjusted for age confounders (Kauppila et al., 1994, 1997; Shi et al., 2020; Beckworth et al., 2018; Jhawar et al., 2006; Estublier et al., 2015; Yuan et al., 2023). Hence these findings illustrate that the abovementioned association is not largely attributed to age. Atherosclerosis-related risk factors such as severe AAC and dyslipidemia are likely to play a role in the occurrence and development of degenerative disc disease, which may represent clinically as LBP or sciatica. Theoretically, an insufficient blood supply to the lumbar spine leads to malnutrition of the vertebral body and the endplate, resulting in micro-injuries in the endplate and annulus fibrosis. Consequently, the injury and recovery process can trigger an inflammatory response, irritate the spinal nerve root and finally lead to pain in the lower back (Kauppila, 2009; Kauppila et al., 1993; Walker, 2000).
Kauppila, 2009 systematic review highlighted a connection between atherosclerosis, narrowed lumbar arteries, high cholesterol, and lumbar disc degeneration (DD) and low back pain (LBP) (Kauppila, 2009). However, this link was predominantly evident in elderly populations and larger studies. The review included just one follow-up study, indicating that lumbar artery stenosis precedes LBP, which can persist for the first follow-up year. To strengthen the causal relationship between these common conditions, five longitudinal follow-up studies were included in our systematic review (Kurunlahti et al., 2004; Kauppila et al., 1997; Jhawar et al., 2006; Estublier et al., 2015; Huang et al., 2016), with all consistently supporting a positive correlation between LBP, DD, and atherosclerosis, lipid levels, and related risk factors. A noteworthy 25-year follow-up study found that severe AAC (Grade 3) was linked to LBP, while mild AAC (Grade 1 or 2), BMI, and cholesterol were not significantly associated with LBP (Kauppila et al., 1997). Another three-year follow-up study found that newly formed lumbar artery stenosis was positively associated with medical consultation for LBP, prolonged LBP (more than 3 months) and prolonged sciatica (Kurunlahti et al., 2004). These long-term findings corroborate the cross-sectional studies, suggesting that arterial stenosis not only intensifies pain but also prolongs its duration.
Regarding the clinical symptom, this review has unveiled a significant association between AAC or lumbar artery stenosis and the presence of clinical LBP and sciatica (Kurunlahti et al., 1999, 2004; Kauppila et al., 1997; Korkiakoski et al., 2009). Cardiovascular risk factors such as serum lipid levels and BMI were generally associated with LBP (Bikbov et al., 2020; Leino-Arjas et al., 2008; Perera et al., 2022). Intriguingly, the studies also revealed a nuanced relationship, where not all levels of artery calcification and stenosis were causative factors for pain; rather, it appeared to be a severity-dependent and dynamic correlation. More severe AAC (grade 3) (Kauppila et al., 1997) or stenosis in >2 lumbar arteries (Korkiakoski et al., 2009) were more strongly associated with the intensity of LBP and duration of sciatica. Conversely, there was no significant correlation observed between mild to moderate aortic calcification (grade 1 or 2) or the presence of any arterial stenosis (at least one artery stenosed) and LBP or sciatica symptoms.
A prospective study followed up for 3 years showed that arterial stenosis has nothing to do with past or future LBP, but newly formed arterial stenosis is related to medical consultation and prolonged LBP (>3 months) or prolonged sciatica due to LBP (Kurunlahti et al., 2004). A plausible hypothesis emerges: given that atherosclerosis is a chronic degenerative disease, its progression, leading to arterial stenosis, takes considerable time. Slow blood supply promotes the development of collateral circulation vessels. When arterial stenosis progresses slowly, the compensatory function of collateral circulation may sufficiently maintain the baseline blood supply around the lumbar spine (Kauppila, 2009). Hence, the presence of arterial stenosis does not invariably result in LBP. However, newly formed arterial stenosis often signifies a rapid advancement of atherosclerosis and coincides with reduced blood supply in the corresponding segment of the lumbar vertebral artery. In cases where collateral circulation has not been adequately established, it can trigger LBP or exacerbate pre-existing pain due to ischemia.
With regard to disc degeneration, four of the included studies supported a positive correlation between AAC or lumbar artery stenosis and DD, even after adjusting for age (Kauppila et al., 1994, 1997; Beckworth et al., 2018; Estublier et al., 2015). Conversely, Kurunlahti's findings revealed no correlation between the number of atherosclerotic plaques and DD (Kurunlahti et al., 1999). A possible plausible explanation for this discrepancy is that some of the smaller arteries (lumbar arteries) are already compromised before the AAC can be detected radiologically, making DD and AAC asynchronous in some individuals. Furthermore, while AAC can be detected in its early stages through CT or X-ray examinations, verifying its blockage of the lumbar artery opening and its impact on blood supply to the lumbar spine remains challenging. Lumbar arteriography, an invasive procedure, is typically deferred unless necessary, thereby delaying the exploration of AAC's effects on lumbar blood supply in the early stages of AAC development. These potential assumptions can complicate the observational study of the correlation between AAC and DD/LBP.
The correlation between serum lipid status and their potential impact on LBP and DD is intricate and characterized by a lack of consensus. Among the studies conducted on this subject, a total of twelve investigations identified at least one lipid marker associated with LBP or DD. However, six other studies did not detect a significant correlation between serum lipid status and LBP or DD, suggesting that lipids might not directly instigate LBP or DD. Interestingly, one study found that high level of high-density lipoproteins, rather than low-density lipoproteins, were correlated with LBP, challenging conventional expectations and adding complexity to the relationship between lipids and LBP (Bikbov et al., 2020). Similarly, the lack of association may be explained by the fact that dyslipidemia tends to precede arterial injury. Therefore, taking into account the duration and severity of the presence of dyslipidemia and possibly including parameters related to lumbar artery injury would be worthwhile in future studies.
Moreover, three studies proposed that increased ApoL1 levels, along with the presence of an inflammatory and oxidative environment during atherosclerosis, may be linked to DD, LDH, and LBP (Karabag et al., 2016; Xie et al., 2014; Huang et al., 2016). These findings suggest that inflammation could serve as a potential mechanism underlying lumbar degenerative changes and LBP induced by atherosclerosis. Consequently, further research on lipoproteins and lumbar degenerative diseases, including radiculopathy, is imperative.
A previous review by Shcherbina et al. (2017) (Shcherbina et al., 2017) reported that there is no conclusive evidence that atherosclerosis is related to “discogenic” LBP. However, the definition of LBP in the twenty-six included articles was extensive. The authors themselves highlighted that the lack of a consistent clinical definition of “discogenic” LBP leads to bias in subject selection. Discogenic LBP patients included patients with spondylolisthesis, spinal stenosis, and even lateral LBP, suggesting a possible origin of the articular process or sacroiliac joint. Moreover, the conclusion did not include age-corrected data, while it is likely that the age range of the study population also affects the prevalence of atherosclerosis, lumbar disc degeneration (LDD), and LBP.
5. Limitation
It is important to acknowledge the limitations of our study. Firstly, the radiological selection criteria were based on the patients of lumbar disc herniation or disc degeneration. Nevertheless, it remains difficult to ascertain whether the condition is exclusively discogenic, given that patients with spinal pathology frequently present with non-discogenic pain. Hence, even despite the implementation of more selective inclusion criteria than those employed in previous reviews, this limitation may still persist in our population.
Furthermore, a meta-analysis was not conducted due to the considerable heterogeneity among the included studies for each subanalysis. The discrepancies in study design, population characteristics, definitions of low back pain (LBP), and outcome measures rendered a quantitative meta-analysis inappropriate. As with other reviews that were confronted with substantial heterogeneity, we elected to pursue a qualitative synthesis approach in order to provide a comprehensive overview of the extant evidence. This approach permits a more detailed comprehension of the intricate interrelationship between lumbar spondylosis, lumbar disc herniation, degenerative spinal disease, and their associated clinical manifestations.
6. Conclusion
The association between serum lipid status, atherosclerosis risk factors like BMI/WHR, and DD or LBP remains uncertain. Atherosclerosis-related risk factors, such as severe AAC and dyslipidemia, appear to significantly contribute to the onset and progression of clinical LBP, sciatica, and degenerative disc disease. Inadequate blood supply to the lumbar spine leads to malnutrition of the vertebral body, endplate, and intervertebral disc, potentially resulting in micro-injuries and inflammatory responses as the underlying mechanisms. To gain a more comprehensive understanding of these interactions and mitigate the influence of age-related confounding factors, further prospective clinical studies are warranted.
Furthermore, optimizing the assessment method for aortic calcification is essential to facilitate a more precise examination of the relationship between atherosclerosis, ischemia, lumbar degeneration, and back pain. In the study and treatment of lumbar degenerative diseases and the clinical outcomes of low back pain, it is imperative to consider the adjustment of blood supply status and systemic lipid levels. This holistic approach could provide valuable insights into potential preventive and therapeutic strategies.
Statements and declarations
This work was supported by China Scholarship Council and the Department of Neurosurgery, Leiden University Medical Center, The Netherlands. Wensen Li received support from both funding agencies. The authors have no financial or proprietary interests in any material discussed in this paper.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Wensen Li reports a relationship with China Scholarship Council that includes: funding grants. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We sincerely thank Jan W. Schoones (Walaeus Library, Leiden University Medical Center) for assisting with search strategy.
Handling Editor: Prof F Kandziora
Appendix. GRADE Profile adapted from Furlan et al
| Quality assessment | ||||||
|---|---|---|---|---|---|---|
| No of studies | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall quality of evidence |
| Atherosclerosis - Clinical LBP/Sciatica (range of scores: Better indicated by less) | ||||||
| 4 | Not serious | Not serious | Not serious | Not serious | none | ⊕⊕⊕⊕High |
| BMI/lipid status - Clinical LBP/Sciatica (range of scores: Better indicated by less) | ||||||
| 5 | Not serious | Serious1 | Not serious | Serious2 | none | ⊕⊕ΟΟLow |
| Atherosclerosis - Radiological LDH/DD (range of scores: Better indicated by less) | ||||||
| 8 | Not serious | Not serious | Not serious | Not serious | none | ⊕⊕⊕⊕High |
| BMI/lipid status - Radiological LDH/DD (range of scores: Better indicated by less) | ||||||
| 14 | Serious3 | Not serious | Serious4 | Not serious | none | ⊕⊕ΟΟLow |
1 One study found no association, and another showed that LBP was associated with HDL (contrary to the subjects evaluated).
2 Few events.
3 One trial with high risk of bias (unsure of randomization, concealment, co-interventions; no blinding).
4 Three trials focused on indirect evidence of the study subjects (2 Apo-L1, 1 PON-1).
References
- Albert H.B., et al. Modic changes, possible causes and relation to low back pain. Med. Hypotheses. 2008;70(2):361–368. doi: 10.1016/j.mehy.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Atkins D., et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battié M.C., et al. 1991 Volvo Award in clinical sciences. Smoking and lumbar intervertebral disc degeneration: an MRI study of identical twins. Spine. 1991;16(9):1015–1021. [PubMed] [Google Scholar]
- Beckworth W.J., et al. Atherosclerotic disease and its relationship to lumbar degenerative disk disease, facet arthritis, and stenosis with computed tomography angiography. Physical Medicine & Rehabilitation. 2018;10(4):331–337. doi: 10.1016/j.pmrj.2017.09.004. [DOI] [PubMed] [Google Scholar]
- Bikbov M.M., et al. Prevalence of and factors associated with low Back pain, thoracic spine pain and neck pain in Bashkortostan, Russia: the Ural Eye and Medical Study. BMC Muscoskel. Disord. 2020;21(1):64. doi: 10.1186/s12891-020-3080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiras J., Morvan G., Merland J.J. The angiographic appearances of the normal intercostal and lumbar arteries. Analysis and the anatomic correlation of the lateral branches. J. Neuroradiol. 1979;6(3):169–196. [PubMed] [Google Scholar]
- Cluroe A.D., Fitzjohn T.P., Stehbens W.E. Combined pathological and radiological study of the effect of atherosclerosis on the ostia of segmental branches of the abdominal aorta. Pathology. 1992;24(3):140–145. doi: 10.3109/00313029209063161. [DOI] [PubMed] [Google Scholar]
- Cowley D.E. Prostheses for primary total hip replacement. A critical appraisal of the literature. Int. J. Technol. Assess. Health Care. 1995;11(4):770–778. doi: 10.1017/s026646230000920x. [DOI] [PubMed] [Google Scholar]
- Coyle P.C., et al. Markers of cardiovascular health in older adults with and without chronic low back and radicular leg pain: a comparative analysis. Pain Med. 2021;22(6):1353–1359. doi: 10.1093/pm/pnaa426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crock H.V., Yoshizawa H. The blood supply of the lumbar vertebral column. Clin. Orthop. Relat. Res. 1976;(115):6–21. [PubMed] [Google Scholar]
- Djuric N., et al. Study protocol: effect of infection, Modic and inflammation on clinical outcomes in surgery for radiculopathy (EIMICOR) BMC Neurol. 2021;21(1):379. doi: 10.1186/s12883-021-02377-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estublier C., Chapurlat R., Szulc P. Association of severe disc degeneration with all-cause mortality and abdominal aortic calcification assessed prospectively in older men: findings of a single-center prospective study of osteoporosis in men. Arthritis Rheumatol. 2015;67(5):1295–1304. doi: 10.1002/art.39055. [DOI] [PubMed] [Google Scholar]
- Furlan A.D., et al. 2009 updated method guidelines for systematic reviews in the Cochrane back review group. Spine. 2009;34(18):1929–1941. doi: 10.1097/BRS.0b013e3181b1c99f. [DOI] [PubMed] [Google Scholar]
- Han T.S., et al. The prevalence of low back pain and associations with body fatness, fat distribution and height. Int. J. Obes. Relat. Metab. Disord. 1997;21(7):600–607. doi: 10.1038/sj.ijo.0800448. [DOI] [PubMed] [Google Scholar]
- Hangai M., et al. Factors associated with lumbar intervertebral disc degeneration in the elderly. Spine J. 2008;8(5):732–740. doi: 10.1016/j.spinee.2007.07.392. [DOI] [PubMed] [Google Scholar]
- Heliövaara M., et al. Lumbar disc syndrome in Finland. J. Epidemiol. Community Health. 1987;41(3):251–258. doi: 10.1136/jech.41.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.F., et al. Colorimetric detection and efficient monitoring of a potential biomarker of lumbar disc herniation using carbon nanotube-based probe. Sci. China Chem. 2016;59(4):493–496. [Google Scholar]
- Huang Z., et al. Impact of dyslipidemia on the severity of symptomatic lumbar spine degeneration: a retrospective clinical study. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.1033375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhawar B.S., et al. Cardiovascular risk factors for physician-diagnosed lumbar disc herniation. Spine J. 2006;6(6):684–691. doi: 10.1016/j.spinee.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Jin G., et al. Physical activity is associated with elevated arterial stiffness in patients with lumbar disk herniation. J. Spinal Disord. Tech. 2015;28(1):E30–E34. doi: 10.1097/BSD.0000000000000140. [DOI] [PubMed] [Google Scholar]
- Karabag H., Hatice S. The relation of lumbar disc herniation with increased lipid hydroperoxide, paraoxonase 1 and total oxidative status. Crescent J. Med. Biol. Sci. 2016;3(3):86–90. [Google Scholar]
- Kauppila L.I. Blood supply of the lower thoracic and lumbosacral regions. Postmortem aortography in 38 young adults. Acta Radiol. 1994;35(6):541–544. [PubMed] [Google Scholar]
- Kauppila L.I. Atherosclerosis and disc degeneration/low-back pain--a systematic review. Eur. J. Vasc. Endovasc. Surg. 2009;37(6):661–670. doi: 10.1016/j.ejvs.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Kauppila L.I., Tallroth K. Postmortem angiographic findings for arteries supplying the lumbar spine: their relationship to low-back symptoms. J. Spinal Disord. 1993;6(2):124–129. [PubMed] [Google Scholar]
- Kauppila L.I., et al. Lumbar disc degeneration and atherosclerosis of the abdominal aorta. Spine. 1994;19(8):923–929. doi: 10.1097/00007632-199404150-00010. [DOI] [PubMed] [Google Scholar]
- Kauppila L.I., et al. Disc degeneration/back pain and calcification of the abdominal aorta. A 25-year follow-up study in Framingham. Spine. 1997;22(14):1642–1647. doi: 10.1097/00007632-199707150-00023. discussion 1648-9. [DOI] [PubMed] [Google Scholar]
- Kauppila L.I., et al. MR aortography and serum cholesterol levels in patients with long-term nonspecific lower back pain. Spine. 2004;29(19):2147–2152. doi: 10.1097/01.brs.0000141168.77393.b8. [DOI] [PubMed] [Google Scholar]
- Keser N., et al. Is there a relationship between blood lipids and lumbar disc herniation in young Turkish adults? Arch. Med. Sci. Atheroscler. Dis. 2017;2(1):e24–e28. doi: 10.5114/amsad.2017.68651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkiakoski A., et al. Association of lumbar arterial stenosis with low back symptoms: a cross-sectional study using two-dimensional time-of-flight magnetic resonance angiography. Acta Radiol. 2009;50(1):48–54. doi: 10.1080/02841850802587862. [DOI] [PubMed] [Google Scholar]
- Kurunlahti M., et al. Association of atherosclerosis with low back pain and the degree of disc degeneration. Spine. 1999;24(20):2080–2084. doi: 10.1097/00007632-199910150-00003. [DOI] [PubMed] [Google Scholar]
- Kurunlahti M., et al. Three-year follow-up of lumbar artery occlusion with magnetic resonance angiography in patients with sciatica: associations between occlusion and patient-reported symptoms. Spine. 2004;29(16):1804–1808. doi: 10.1097/01.brs.0000134576.77709.64. ; discussion 1809. [DOI] [PubMed] [Google Scholar]
- Leino-Arjas P., et al. Serum lipids in relation to sciatica among Finns. Atherosclerosis. 2008;197(1):43–49. doi: 10.1016/j.atherosclerosis.2007.07.035. [DOI] [PubMed] [Google Scholar]
- Longo U.G., et al. Symptomatic disc herniation and serum lipid levels. Eur. Spine J. 2011;20(10):1658–1662. doi: 10.1007/s00586-011-1737-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer E., et al. Association between cardiovascular risk factors and degenerative disc disease of the thoracolumbar spine in the general population: results from the KORA MRI Study. Acta Radiol. 2022;63(6):750–759. doi: 10.1177/02841851211010391. [DOI] [PubMed] [Google Scholar]
- Moher D., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker S.L., et al. Incidence of low back pain after lumbar discectomy for herniated disc and its effect on patient-reported outcomes. Clin. Orthop. Relat. Res. 2015;473(6):1988–1999. doi: 10.1007/s11999-015-4193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera R.S., et al. Age- and sex-specific effects of obesity, metabolic syndrome and its components on back pain: the English Longitudinal Study of Ageing. Joint Bone Spine. 2022;89(5) doi: 10.1016/j.jbspin.2022.105366. [DOI] [PubMed] [Google Scholar]
- Ratcliffe J.F. The arterial anatomy of the adult human lumbar vertebral body: a microarteriographic study. J. Anat. 1980;131(Pt 1):57–79. [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe J.F. The anatomy of the fourth and fifth lumbar arteries in humans: an arteriographic study in one hundred live subjects. J. Anat. 1982;135(Pt 4):753–761. [PMC free article] [PubMed] [Google Scholar]
- Schonnagel L., et al. Abdominal aortic calcification is independently associated with lumbar endplate degeneration. Eur. Spine J. 2023;32(10):3387–3393. doi: 10.1007/s00586-023-07871-6. [DOI] [PubMed] [Google Scholar]
- Shcherbina A., Longacre M. The association between atherosclerosis and low back pain: a systematic review. Physical Medicine & Rehabilitation. 2017;9(11):1144–1156. doi: 10.1016/j.pmrj.2017.04.007. [DOI] [PubMed] [Google Scholar]
- Shi S., et al. The impact and distinction of 'lipid healthy but obese' and 'lipid abnormal but not obese' phenotypes on lumbar disc degeneration in Chinese. J. Transl. Med. 2020;18(1):211. doi: 10.1186/s12967-020-02382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri P., et al. Quantitative assessment of abdominal aortic calcification and associations with lumbar intervertebral disc height loss: the Framingham Study. Spine J. 2012;12(4):315–323. doi: 10.1016/j.spinee.2012.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgut A.T., et al. Pineal gland calcification, lumbar intervertebral disc degeneration and abdominal aorta calcifying atherosclerosis correlate in low back pain subjects: a cross-sectional observational CT study. Pathophysiology. 2008;15(1):31–39. doi: 10.1016/j.pathophys.2007.12.001. [DOI] [PubMed] [Google Scholar]
- van Tulder M., Koes B., Bombardier C. Low back pain. Best Pract. Res. Clin. Rheumatol. 2002;16(5):761–775. doi: 10.1053/berh.2002.0267. [DOI] [PubMed] [Google Scholar]
- Viswanathan V.K., Shetty A.P., Rajasekaran S. Modic changes - an evidence-based, narrative review on its patho-physiology, clinical significance and role in chronic low back pain. J. Clin. Orthop. Trauma. 2020;11(5):761–769. doi: 10.1016/j.jcot.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker B.F. The prevalence of low back pain: a systematic review of the literature from 1966 to 1998. J. Spinal Disord. 2000;13(3):205–217. doi: 10.1097/00002517-200006000-00003. [DOI] [PubMed] [Google Scholar]
- Xie P., et al. Comparative analysis of serum proteomes: identification of proteins associated with sciatica due to lumbar intervertebral disc herniation. Biomed. Rep. 2014;2(5):693–698. doi: 10.3892/br.2014.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L., et al. The impact of dyslipidemia on lumbar intervertebral disc degeneration and vertebral endplate modic changes: a cross-sectional study of 1035 citizens in China. BMC Publ. Health. 2023;23(1):1302. doi: 10.1186/s12889-023-16224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuedong Z., et al. Serum lipid levels are positively correlated with lumbar disc herniation—a retrospective study of 790 Chinese patients. Lipids Health Dis. 2016;15:1–8. doi: 10.1186/s12944-016-0248-x. [DOI] [PMC free article] [PubMed] [Google Scholar]