Abstract
Chimeric poliovirus RNAs, possessing the 5′ nontranslated region (NTR) of hepatitis C virus in place of the 5′ NTR of poliovirus, were used to examine the role of the poliovirus 5′ NTR in viral replication. The chimeric viral RNAs were incubated in cell-free reaction mixtures capable of supporting the sequential translation and replication of poliovirus RNA. Using preinitiation RNA replication complexes formed in these reactions, we demonstrated that the 3′ NTR of poliovirus RNA was insufficient, by itself, to recruit the viral replication proteins required for negative-strand RNA synthesis. The 5′-terminal cloverleaf of poliovirus RNA was required in cis to form functional preinitiation RNA replication complexes capable of uridylylating VPg and initiating the synthesis of negative-strand RNA. These results are consistent with a model in which the 5′-terminal cloverleaf and 3′ NTRs of poliovirus RNA interact via temporally dynamic ribonucleoprotein complexes to coordinately mediate and regulate the sequential translation and replication of poliovirus RNA.
The single-stranded positive-sense RNA genome of poliovirus functions sequentially as an mRNA for viral protein synthesis and then as a template for viral negative-strand RNA synthesis (33). Cytoplasmic extracts from HeLa cells support the sequential translation and replication of poliovirus RNA (5, 31). In the presence of 2 mM guanidine HCl, cell-free translation-replication reactions support the translation of viral RNA and the accumulation of viral preinitiation RNA replication complexes (4, 6). Guanidine HCl reversibly blocks the initiation of negative-strand RNA synthesis by interfering with the function of viral protein 2CATPase (3, 38, 40). When preinitiation RNA replication complexes are resuspended in reaction mixtures in the absence of guanidine HCl, the preinitiation RNA replication complexes synchronously initiate the synthesis of viral negative-strand RNA (6). Ribosomes translating the viral mRNA within preinitiation RNA replication complexes prevent the synthesis of negative-strand RNA (8, 15). Thus, the conversion of viral ribonucleoprotein complexes into preinitiation RNA replication complexes is an important temporally regulated event in the replication of poliovirus RNA.
Contemporary models of eukaryotic mRNA translation suggest that the 5′ and 3′ nontranslated regions (NTRs) of mRNAs communicate via RNA binding proteins bound to both termini of the mRNA (14, 19, 42). In particular, poly(A) binding protein bound to 3′-terminal poly(A) interacts with eukaryotic initiation factors eIF4G I and II anchored to the 5′ NTR of mRNA, bringing the 5′ and 3′ NTRs into proximity (49). During the course of a poliovirus infection, viral protein 2APro mediates the cleavage of eIF4G I and II and poly(A) binding proteins (11, 18, 23, 24). This leads to the shutoff of cap-dependent host protein synthesis and precludes the ability of eIF4G I and II and poly(A) binding protein to form protein-protein bridges between the 5′ and 3′ termini of mRNAs, including poliovirus RNA. Therefore, it remains to be established whether poliovirus mRNA, in conjunction with host cell translation machinery, assumes a conformation in which the 5′ and 3′ NTRs are proximally located. Although conventional studies suggested that the poliovirus 3′ NTR and poly(A) tail function as the cis-active sequences for poliovirus negative-strand RNA synthesis (1, 21, 39), recent studies (9, 20, 48) suggest that the 5′ and 3′ termini of poliovirus RNA coordinately mediate poliovirus negative-strand RNA synthesis.
VPg, and/or one of its precursors, is thought to prime the initiation of RNA replication (16, 47). VPgpUpUOH is synthesized by poliovirus RNA replication complexes in vitro (45, 46) and accumulates as a product of replication in vivo (13). In reconstitution experiments, it has been shown that VPg can be uridylylated in reactions containing a poly(A) template, UTP, and 3Dpolymerase (37). More recent experiments suggest that an RNA stem-loop structure (CRE) within the 2CATPase coding region of poliovirus RNA also functions as a template for VPg uridylylation (17, 36, 41). In this report, we demonstrate that the viral open reading frame, 3′ NTR, and poly(A) tail of poliovirus RNA were insufficient to mediate the formation of functional preinitiation RNA replication complexes. The 5′-terminal cloverleaf of poliovirus RNA was required in cis to form functional preinitiation RNA replication complexes capable of uridylylating VPg and initiating negative-strand RNA synthesis.
MATERIALS AND METHODS
cDNA and cloning.
Plasmids pT7-PV1(A)80, encoding an infectious cDNA clone of poliovirus RNA, pT7-PV1(A)80G2474+CTAG, encoding DJB1 RNA, pT7-PV1(A)80ΔC1175-C2956, encoding poliovirus RNA with an in-frame deletion in the capsid genes, and pT7-PV1(A)80ΔC630-T6011ΔT6061-A6516, encoding DJB14 RNA, were kindly provided by James B. Flanegan, University of Florida College of Medicine, Gainesville, Fla. (7, 12). A plasmid, pNCR-C(AUG), encoding the hepatitis C virus (HCV) 5′ NTR, was kindly provided by Aleem Siddiqui, University of Colorado Health Sciences Center, Denver, Colo.
i. pDNVR1.
PCR-based cloning was used to remove nonviral nucleotides between the T7 promoter of pNCR-C(AUG) and the 5′ NTR of HCV in this plasmid. PCR primers (5′ CTGTAATACGACTCACTATAGGCCAGCCCCCTGAG 3′ and 5′ CTGGCCATTGAGGTTTAGGATTCGTGCTCATGG 3′) were used to amplify the HCV 5′ NTR from pNCR-C(AUG). pNCR-C(AUG) was cut with PvuII and EcoRI to remove the unwanted T7 promoter and HCV 5′ NTR. The 395-bp PCR product encoding the T7 promoter immediately upstream of the HCV 5′ NTR was blunt ligated into the PvuII- and EcoRI-cut pNCR-C(AUG).
ii. pDNVR2.
pT7-PV1(A)80 and pDNVR1 were used as parental plasmids to create pDNVR2. The MscI to PvuI fragment of pDNVR1 was ligated to the SnaBI to PvuI fragment of pT7-PV1(A)80 to make pDNVR2.
iii. pDNVR1b.
pDNVR1 was used as a parental plasmid to make pDNVR1b. The 5′-terminal 110 nucleotides of poliovirus cDNA were inserted between the T7 promoter and the HCV 5′ NTR of pDNVR1 to make pDNVR1b. To do this, 5′-phosphorylated PCR primers (5′ GATATCTAATACGACTCACTATAGG 3′ and 5′ GGGGCTGGCTCTAAGTTACGGGAAGGGAG 3′ ) were used to amplify the 5′-terminal 110 nucleotides of poliovirus from pT7-PV1(A)80ΔC630-T6011ΔT6061-A6516. The PCR product was then blunt ligated into the XcmI to PvuII fragment of pDNVR1.
iv. pDNVR12.
The FspI to NheI fragment of pDNVR1b was ligated to the NheI to FspI fragment of pDNVR2 to make pDNVR12.
All plasmids were confirmed by restriction enzyme analyses and DNA sequencing when necessary.
Viral mRNAs.
T7 RNA polymerase was used to synthesize viral mRNAs by runoff transcription of MluI-linearized plasmids. A T7 transcription kit (Epicentre, Madison, Wis.) was used according to the manufacturer's recommendations. When indicated, 5′ 7-methylguanosine cap analogue (m7G5′ppp5′G) was used to make viral mRNAs with 5′-terminal 7-methylguanosine caps. The cap analogue was used at a fourfold excess to GTP in the T7 transcription reaction mixtures to ensure that >60% of the viral mRNAs were capped. T7 RNA transcripts were purified either by phenol-chloroform-isoamyl alcohol extraction and G-50 column desalting or by sequential precipitation in 2.5 M LiCl and 66% ethanol.
HeLa S10 translation-replication reactions.
HeLa cell S10 extract (S10) and HeLa cell translation initiation factors (IFs) were prepared as previously described (7). HeLa S10 translation-replication reaction mixtures contained 50% by volume S10, 20% by volume IFs, 10% by volume 10× nucleotide reaction mix (10 mM ATP, 2.5 mM GTP, 2.5 mM CTP, 2.5 mM UTP, 600 mM KCH3CO2, 300 mM creatine phosphate, 4 mg of creatine kinase/ml, and 155 mM HEPES-KOH [pH 7.4]), 2 mM guanidine HCl, and viral mRNA at 25 to 75 μg per ml. Reaction mixes were incubated at 34°C.
mRNA stability.
Poliovirus mRNA stability was assayed by incubating 32P-labeled poliovirus mRNA in HeLa S10 translation-replication reaction mixtures. Portions of the reactions were solubilized in 0.5% sodium dodecyl sulfate (SDS) buffer (0.5% SDS [Sigma], 10 mM Tris-HCl [pH 7.5], 1 mM EDTA, and 100 mM NaCl) after incubation at 34°C for the times indicated in the figure legends. The reaction mixtures were extracted with phenol-chloroform, and the RNA from the reactions was ethanol precipitated. The RNA from the reactions was then denatured in 50 mM methylmercury hydroxide and separated by electrophoresis in 1% agarose. The RNAs in the gels were stained with ethidium bromide and visualized by UV light. The gels were then dried, and radiolabeled poliovirus mRNA was detected by autoradiography and quantified using a phosphorimager (Molecular Dynamics, Sunnyvale, Calif., or Bio-Rad, Hercules, Calif.).
mRNA translation.
Poliovirus mRNA translation was assayed by including [35S]methionine (1.2 mCi per ml; Amersham) in HeLa S10 translation-replication reaction mixtures. Incorporation of [35S]methionine into acid-precipitable material was assayed by collecting 1-μl samples of the HeLa S10 translation-replication reaction materials in 100 μl of 0.1 N KOH–1% Casamino Acids at the times indicated in the figure legends. Samples were then precipitated with 5% trichloroacetic acid (5 ml) on ice for 10 min. Precipitated proteins were collected by filtration on 2.5-cm-diameter nitrocellulose filters (Millipore), and the radiolabel retained on the filters was quantified by scintillation counting. The counts per minute of acid-precipitable [35S]methionine were plotted versus time of incubation.
[35S]methionine-labeled proteins synthesized in HeLa S10 translation-replication reaction mixtures were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE). Samples (4 μl) of the HeLa S10 translation-replication reaction mixtures containing [35S]methionine were solubilized in SDS-PAGE sample buffer (2% SDS [Sigma], 62.5 mM Tris-HCl [pH 6.8], 0.5% 2-mercaptoethanol, 0.1% bromophenol blue, 20% glycerol) after incubation for the times indicated in the figure legends. The samples were heated at 100°C for 5 min and separated by gel electrophoresis in 10% or 9 to 18% SDS-polyacrylamide gels as previously described (7). The gels were fixed by soaking them in 50% trichloroacetic acid. The gels were then fluorographed using dimethyl sulfoxide–2,5-dipheyloxazole, dried, and [35S]methionine-labeled proteins were detected on film (Kodak XL1-Blue).
Negative-strand RNA synthesis.
Poliovirus negative-strand RNA synthesis was assayed using preinitiation RNA replication complexes formed in HeLa S10 translation-replication reaction mixtures, as previously described (6). Viral RNAs (50 to 100 μg/ml) were incubated in HeLa S10 translation-replication reaction mixtures containing 2 mM guanidine HCl for 3 h at 34°C. Then, preinitiation RNA replication complexes were isolated from the reactions by centrifugation at 13,000 × g for 15 min at 4°C. Pellets containing preinitiation RNA replication complexes were then resuspended in 50-μl labeling reaction mixtures containing [α-32P]CTP and incubated at 37°C for 30 min as previously described (method 4 of reference 6). Under these conditions, radiolabel is incorporated into nascent negative-strand RNA as it is synthesized by the viral RNA replication complexes (6). The two nonviral G residues at the 5′ terminus of the T7 RNA transcripts used in this study (Fig. 1) prevent viral positive-strand RNA synthesis (8). Therefore, the products of these reactions are exclusively of negative polarity (8). The reactions were terminated by the addition of 0.5% SDS buffer. The products of the reaction were phenol-chloroform extracted, ethanol precipitated, denatured with 50 mM methylmercury hydroxide, and separated by electrophoresis in 1% agarose (7). RNA in the gels was stained with ethidium bromide and visualized by UV light. 32P-labeled poliovirus negative-strand RNA was detected by autoradiography and quantified using a phosphorimager (Molecular Dynamics or Bio-Rad).
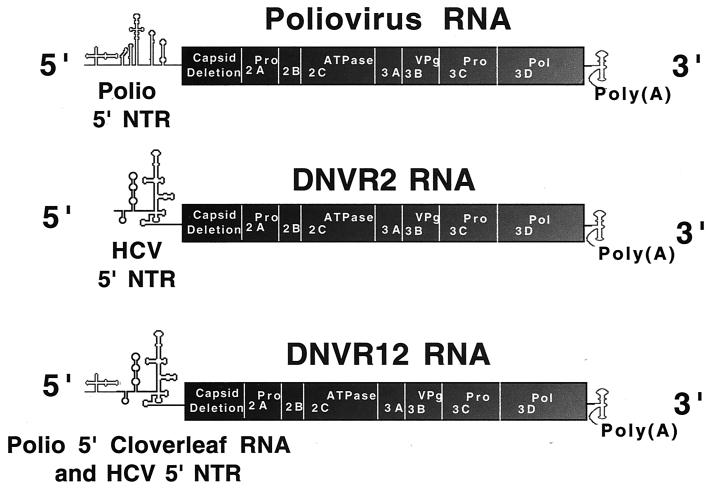
FIG. 1.
Viral RNAs analyzed in these studies. They were synthesized by T7 transcription of MluI-linearized plasmids: poliovirus RNA from pT7PV1(A)80ΔC1175-C2956; DNVR2 RNA from pDNVR2; and DNVR12 RNA from pDNVR12. Poliovirus RNA, diagrammed at the top, is a subgenomic poliovirus RNA replicon containing an in-frame deletion within the capsid genes (RNA2 in reference 12). The capsid gene deletion in each of these viral RNAs is advantageous for two reasons. First, it renders the subgenomic RNA replicons noninfectious, thereby reducing biosafety concerns, and second, the deletion of capsid genes renders the viral RNAs shorter, allowing for the synthesis of higher-quality T7 transcripts which replicate better than genome-length transcripts in HeLa S10 translation-replication reactions. Poliovirus RNA (with the in-frame capsid deletion) is the wild-type RNA in the following experiments. DNVR2 RNA is a chimeric viral RNA possessing the 5′ NTR of HCV in place of the 5′ NTR of poliovirus. DNVR12 RNA is a chimeric viral RNA possessing the 5′ terminal 110 nucleotides of poliovirus and the 5′ NTR of HCV. The three viral RNAs are 3′ coterminal, encoding the poliovirus replication proteins, the 3′ NTR of poliovirus, and a 3′-terminal poly(A) tail 83 bases in length. Two nonviral G residues are present on the 5′ terminus of these T7 transcripts. These 5′-terminal G residues prevent positive-strand RNA synthesis in HeLa S10 translation-replication reaction mixtures (8).
VPg uridylylation.
VPg uridylylation was assayed using preinitiation RNA replication complexes. Preinitiation RNA replication complexes were isolated from HeLa S10 translation-replication reaction materials in the same way as those used for negative-strand RNA synthesis described above (6). The preinitiation RNA replication complexes were resuspended in reaction mixtures containing [α-32P]UTP rather than [α-32P]CTP as described for negative-strand RNA synthesis (6). The reaction mixes were incubated for 60 min at 37°C. Following incubation, the reaction mixtures were centrifuged at 13,000 × g to pellet the viral RNA replication complexes. Radiolabeled VPgpUpUOH and radiolabeled viral RNA remained in the replication complexes and were not released into the soluble portion of the reaction mixtures (data not shown). The supernatant, containing unincorporated radiolabel, was discarded. The pellets, containing radiolabeled viral RNA and uridylylated VPg, were solubilized in SDS sample buffer (1.5% SDS [Sigma], 62.5 mM Tris-HCl, pH 6.8, 2.5% β-mercaptoethanol, 5% glycerol, 0.05% bromophenol blue). The samples were fractionated by electrophoresis (75 mA of constant current for 7 min followed by 13 mA of constant current for 15 h) in a 0.75-mm-thick polyacrylamide (29:1 ratio of acrylamide-bis acrylamide)-Tris-Tricine gel system (4% polyacrylamide stacking gel [4% polyacrylamide, 0.7 M Tris-HCl, pH 8.45, 0.08% SDS], 12% polyacrylamide separating gel [1 M Tris-HCl, pH 8.45, and 0.1% SDS]) by using a Tris-Tricine running buffer (0.1 M Tris-Tricine, pH 8.25, 0.3% SDS). Radiolabeled products in the gel were detected by phosphorimaging.
RESULTS
Replacing the 5′ NTR of poliovirus with the 5′ NTR of HCV.
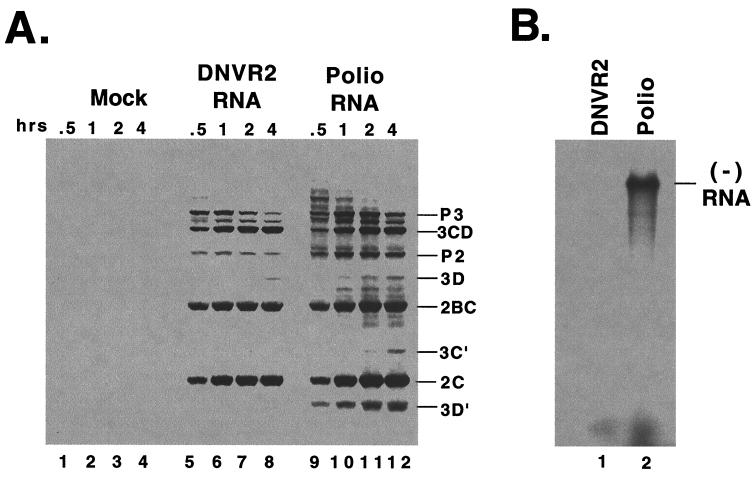
To determine the role of the poliovirus 5′ NTR in poliovirus RNA replication, we constructed a chimeric viral RNA molecule composed of the 5′ NTR of HCV fused to the open reading frame and 3′ NTR of poliovirus RNA (Fig. 1, DNVR2 RNA). The ability of DNVR2 RNA to express viral replication proteins and to function as a template for viral negative-strand RNA synthesis was examined using cell-free HeLa S10 translation reactions and preinitiation RNA replication complexes (Fig. 2). DNVR2 RNA translated efficiently, though not as efficiently as poliovirus RNA, leading to the synthesis of poliovirus replication proteins (Fig. 2, lanes 5 to 8 and lanes 9 to 12, respectively). DNVR2 preinitiation RNA replication complexes, however, were unable to synthesize negative-strand RNA when compared to poliovirus preinitiation RNA replication complexes (Fig. 2B, lane 1 versus lane 2). These results indicated that the HCV internal ribosome entry complex (IRES) within the 5′ NTR of DNVR2 RNA was able to functionally replace the IRES of poliovirus to drive viral protein expression. The 5′ NTR of HCV, however, did not functionally replace the 5′ NTR of poliovirus when negative-strand RNA synthesis was assayed.
FIG. 2.
Translation and replication of DNVR2 RNA. (A) Viral RNAs were incubated in HeLa S10 translation-replication reaction mixtures containing [35S]methionine to measure viral mRNA translation. Reaction mixes containing no viral mRNA (Mock), DNVR2 RNA, and poliovirus RNA were incubated at 34°C. Samples from each reaction mixture were solubilized in SDS-sample buffer after 0.5, 1, 2, and 4 h of incubation. The samples were separated by electrophoresis in SDS–10% PAGE, and radiolabeled viral proteins were detected by fluorography. (B) Preinitiation RNA replication complexes were isolated from a reaction mixture containing DNVR2 RNA (lane 1) and from a reaction mixture containing poliovirus RNA (lane 2). After incubating the preinitiation RNA replication complexes in mixtures containing [α-32P]CTP, the RNA products were denatured with methylmercury hydroxide and separated by electrophoresis in 1% agarose and viral negative-strand RNA was detected by autoradiography.
Viral RNA stability.
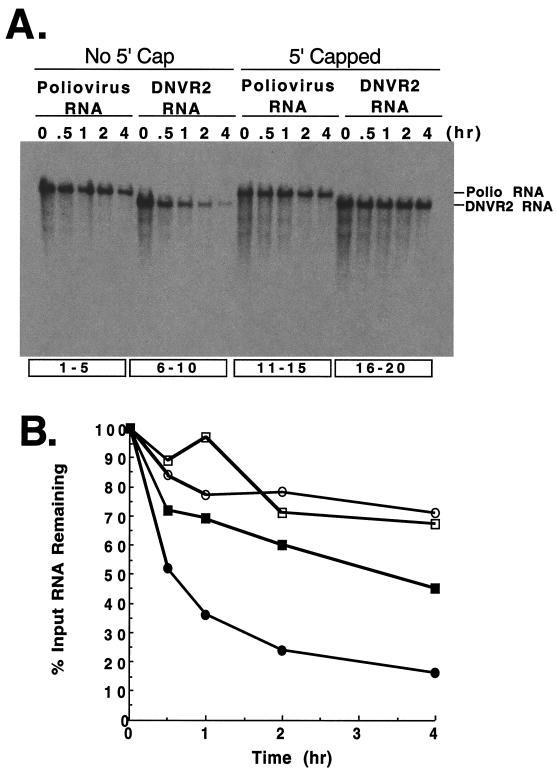
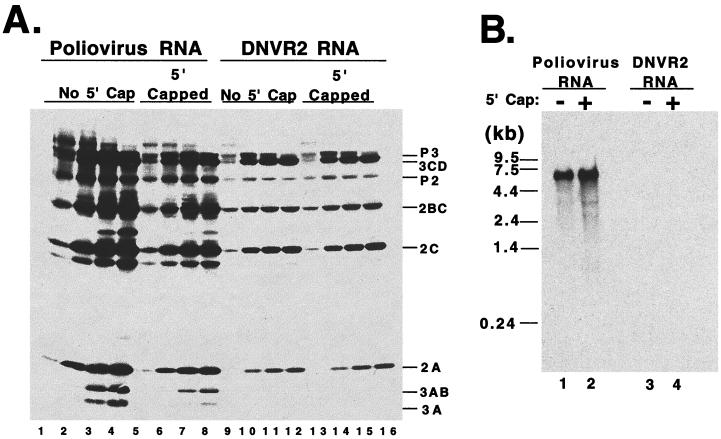
In previous studies, we observed that the 5′-terminal cloverleaf of poliovirus RNA mediated the stability of poliovirus RNA (9). Therefore, we examined the stability of DNVR2 RNA, which lacks the 5′-terminal cloverleaf of poliovirus RNA, to determine whether the 5′ NTR of HCV functionally replaced that of poliovirus to mediate viral RNA stability (Fig. 3). DNVR2 RNA was significantly less stable than poliovirus RNA in HeLa S10 translation reactions (Fig. 3A, lanes 6 to 10 versus 1 to 5 and 3B). A 5′-terminal 7-methylguanosine cap restored stability to DNVR2 RNA (Fig. 3A, lanes 16 to 20 versus 6 to 10 and 3B). These results support the conclusion that DNVR2 RNA is more susceptible to 5′ exonuclease digestion than poliovirus RNA. Thus, the inability of DNVR2 RNA to function as a template for viral negative-strand RNA synthesis in the previous experiment (Fig. 2B) could have been due to the degradation of the DNVR2 RNA templates required for negative-strand RNA synthesis. To rule out this possibility, we examined the ability of 5′-capped DNVR2 RNA to function as a template for negative-strand RNA synthesis (Fig. 4B). 5′-capped DNVR2 RNA was stable in HeLa S10 translation-replication reaction mixtures (Fig. 3) and translated efficiently to yield the viral replication proteins needed for RNA replication (Fig. 4A, lanes 13 to 16). 5′-capped DNVR2 RNA did not translate more efficiently than uncapped DNVR2 RNA (Fig. 4A, lanes 13 to 16 versus 9 to 12) despite its improved stability in the HeLa S10 translation-replication reactions (Fig. 3). Furthermore, despite the improved stability of the 5′-capped DNVR2 RNA template, 5′-capped DNVR2 RNA did not serve as a functional template for viral negative-strand RNA synthesis (Fig. 4B, lane 4). In contrast, poliovirus RNA functioned as an efficient template for viral negative-strand RNA synthesis both with and without a 5′ cap structure (Fig. 4B, lanes 1 to 2). These results support the conclusion that DNVR2 RNA lacks one or more cis-active RNA structures required for viral negative-strand RNA synthesis.
FIG. 3.
Viral RNA stability. The stability of DNVR2 and poliovirus RNA templates was compared in HeLa S10 translation-replication reactions. (A) 32P-radiolabeled DNVR2 and poliovirus RNAs, both without and with 5′ 7-methylguanosine caps, were incubated in HeLa S10 translation-replication reaction mixtures at 34°C. Samples of each reaction were solubilized in SDS buffer after incubation for 0, 0.5, 1, 2, and 4 h. The samples were separated by electrophoresis in 1% agarose, and the viral RNAs were detected by autoradiography. (B) 32P-labeled viral RNA bands were quantitated by phosphorimaging. The percent of input viral RNA detected at time zero in the reactions was plotted versus time. Poliovirus RNA (▪), DNVR2 RNA (●), 5′-capped poliovirus RNA (□), and 5′-capped DNVR2 RNA (○).
FIG. 4.
Translation and replication of 5′ 7-methylguanosine capped DNVR2 RNA. (A) Poliovirus RNA and DNVR2 RNAs, without and with 5′ 7-methylguanosine caps, were incubated in HeLa S10 translation-replication reaction mixtures containing [35S]methionine. Samples of each reaction mix were solubilized in SDS sample buffer after 0.5, 1, 2, and 4 h of incubation. Samples were analyzed by SDS-PAGE in 9 to 18% polyacrylamide. Radiolabeled viral proteins were detected by fluorography. (B) Preinitiation RNA replication complexes were isolated from reaction mixtures containing poliovirus RNA (lane 1), 5′-capped poliovirus RNA (lane 2), DNVR2 RNA (lane 3), and 5′-capped DNVR2 RNA (lane 4). After incubating the preinitiation RNA replication complexes in reaction mixes containing [α-32P]CTP, the RNA products were denatured with methylmercury hydoxide and separated by electrophoresis in 1% agarose and viral negative-strand RNA was detected by autoradiography. The image presented represents a 38-min exposure of film. In a 64-h exposure (100-fold longer than 38 min), no bands of DNVR2 negative-strand RNA were detected (data not shown).
trans replication of poliovirus RNA.
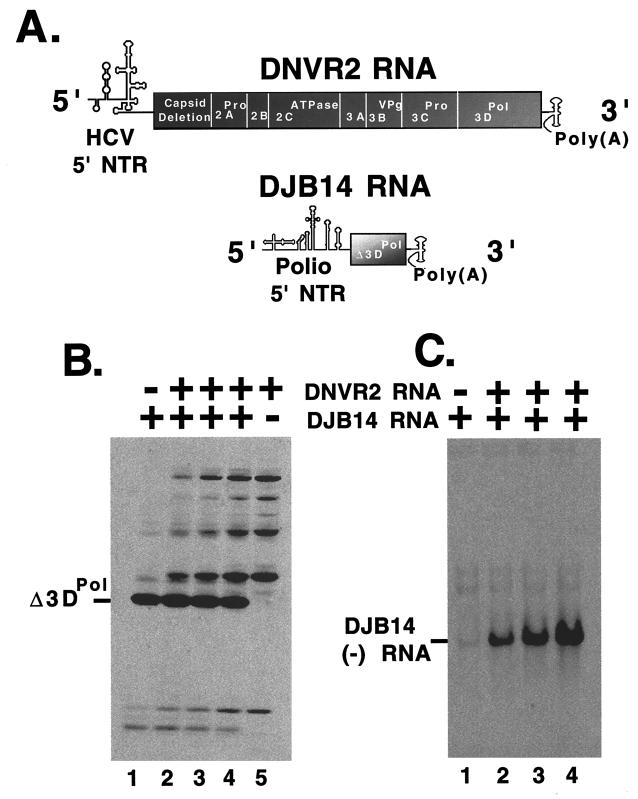
To determine whether the viral replication proteins expressed from DNVR2 RNA were functional, we performed trans-replication experiments in which 5′ 7-methylguanosine-capped DNVR2 RNA was used as a helper mRNA to provide replication proteins in trans to replicate a target RNA incapable of expressing its own replication proteins (Fig. 5). Although poliovirus replication proteins function in trans very inefficiently in vivo (33), they function in trans very efficiently in HeLa S10 cotranslation reactions as shown in Fig. 5. DJB14 RNA is a poliovirus RNA with two large internal deletions encompassing the majority of the viral open reading frame. DJB14 RNA consists of the 5′-terminal 629 nucleotides of poliovirus RNA, poliovirus nucleotides 6012 to 6056, and the 3′-terminal 1,007 nucleotides of poliovirus RNA with a poly(A) tail 83 bases in length (Fig. 5A). DJB14 RNA possesses a functional IRES, and a small fragment corresponding to the COOH terminus of 3DPol, Δ3DPol, was expressed from DJB14 mRNA (Fig. 5B, lane 1). DNVR2 RNA expressed the poliovirus replication proteins (Fig. 5B, lane 5). DNVR2-expressed replication proteins formed functional preinitiation RNA replication complexes capable of copying DJB14 RNA into negative-strand RNA (Fig. 5C, lanes 2 to 4). In the absence of replication proteins, DJB14 RNA was not copied into negative-strand RNA (Fig. 5C, lane 1). As the molar ratio of DJB14 RNA to DNVR2 RNA was decreased from 3:1 to 1:1 in the HeLa S10 translation-replication reactions, corresponding increases were seen in the amount of DNVR2-expressed viral replication proteins (Fig. 5B, lanes 2 to 4) and DJB14 negative-strand RNA synthesis (Fig. 5C, lanes 2 to 4). Furthermore, DNVR2 RNA was not used as a template for viral negative-strand RNA synthesis in reactions containing both DNVR2 RNA and DJB14 RNA (Fig. 5C, lanes 2 to 4). Thus, DNVR2 RNA was able to express the poliovirus replication proteins required for negative-strand RNA synthesis but was unable to use them in cis to synthesize negative-strand RNA. DJB14 RNA, a molecule containing both the 5′ and 3′ NTRs of poliovirus was functional as a template for viral negative-strand RNA synthesis. DJB14 RNA, however, did not complement DNVR2 RNA replication in trans (Fig. 5C, lanes 2 to 4). These results are consistent with the conclusion that one or more RNA sequences or structures within the 5′ NTR of poliovirus RNA are required in cis for viral negative-strand RNA synthesis.
FIG. 5.
trans complementation of poliovirus negative-strand RNA synthesis. (A) Diagrams of DNVR2 RNA and DJB14 RNA. In this experiment, DNVR2 RNA and DJB14 RNA were cotranslated in HeLa S10 translation-replication reactions to examine the cis-active and trans-active elements necessary for viral negative-strand RNA synthesis. (B) Viral protein synthesis. Viral protein synthesis was assayed in reaction mixtures containing [35S]methionine and DJB14 RNA alone (lane 1), DJB14 RNA and DNVR2 RNA at a 3:1 molar ratio (lane 2), a 1:1 molar ratio (lane 3), a 1:3 molar ratio (lane 4), and DNVR2 RNA alone (lane 5). Samples of each reaction were removed after 3 h of incubation and analyzed by SDS-PAGE. Radiolabeled viral proteins were detected by fluorography. (C) Negative-strand RNA synthesis. Preinitiation RNA replication complexes were isolated from reactions containing DJB14 RNA alone (lane 1), DJB14 RNA and DNVR2 RNA at a 3:1 molar ratio (lane 2), a 1:1 molar ratio (lane 3), and a 1:3 molar ratio (lane 4). Following incubation in reaction mixtures containing [α-32P]CTP, the RNA products of each reaction were denatured with methylmercury hydroxide and separated by electrophoresis in 1% agarose and viral negative-strand RNA products were detected by autoradoiography.
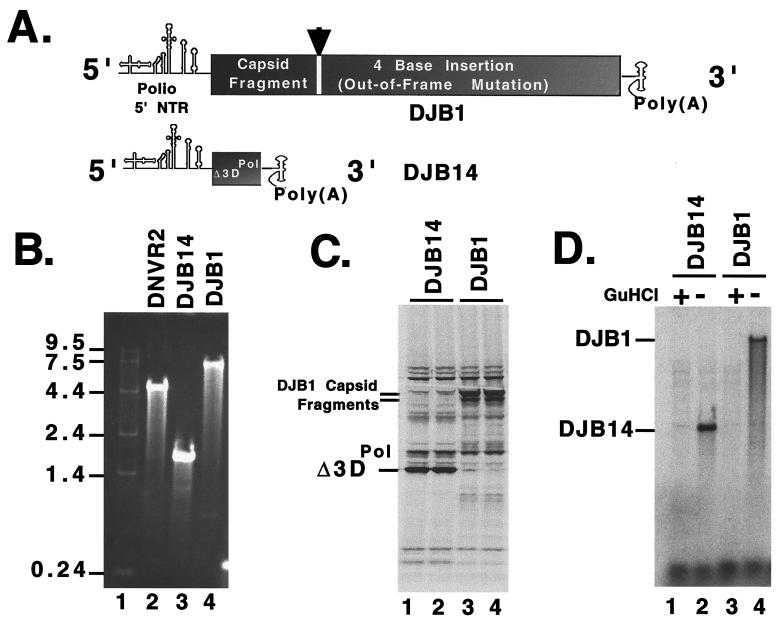
Because reduced protein expression could potentially lead to a defect, specifically in the replication of larger RNA templates, we examined the ability of DNVR2 RNA to function as a helper mRNA for both large and small RNA templates (Fig. 6). DJB1 RNA is a genome-length poliovirus RNA with a 4-base insertion in the capsid coding region (Fig. 6A and Materials and Methods). A 4-base insertion in the capsid coding region prevents expression of the downstream viral replication proteins (data not shown). When cotranslated with DNVR2 mRNA, equal amounts of viral replication proteins were provided in trans to DJB14 and DJB1 RNA templates (Fig. 6C). Proteins encoded by DJB14 and DJB1 mRNAs were also observed as predicted (Fig. 6C). Preinitiation RNA replication complexes formed during cotranslation with DNVR2 mRNA supported negative-strand RNA synthesis on DJB14 and DJB1 RNA templates (Fig. 6D). As before, DNVR2 RNA did not function as a template for negative-strand RNA synthesis (Fig. 6D, lanes 2 and 4, note that no RNA product is detected above DJB14 RNA nor below DJB1 RNA). When quantified by phosphorimaging, the amount of negative-strand RNA synthesized on the DJB1 RNA template was only slightly smaller than the amount of negative-strand RNA synthesized on the DJB14 RNA template. Thus, the reduced levels of viral replication proteins expressed from DNVR2 mRNA were sufficient to support negative-strand RNA synthesis on both small and large RNA templates. Therefore, the inability of DNVR2 RNA to function as a template for viral negative-strand RNA synthesis is not due to reduced expression of viral replication proteins.
FIG. 6.
trans complementation of negative-strand RNA synthesis on both small and large poliovirus RNA templates. (A) Diagrams of DJB1 and DJB14 RNAs. (B) Sizes of viral RNAs. The size and quality of the viral RNAs were determined by gel electrophoresis, ethidium bromide staining, and visualization by UV light. (C) Viral protein synthesis. DJB14 and DJB1 RNAs were cotranslated with DNVR2 RNA in HeLa S10 translation-replication reactions containing 2 mM guanidine HCl. Viral protein synthesis was assayed in reaction mixes containing [35S]methionine. Reaction mixtures contained DNVR2 and DJB14 RNAs (lanes 1 and 2) or DNVR2 and DJB1 RNAs (lanes 3 and 4) at a 1:1 molar ratio. Samples of each reaction were removed after 3 h of incubation and analyzed by SDS-PAGE. Radiolabeled viral proteins were detected by fluorography. (D) Negative-strand RNA synthesis. Preinitiation RNA replication complexes were isolated from reactions containing DJB14 and DNVR2 RNAs (lanes 1 and 2) or DJB1 and DNVR2 RNAs (lanes 3 and 4) at a 1:1 molar ratio. Following incubation in reaction mixes containing [α-32P]CTP, in the presence (lanes 1 and 3) or absence (lanes 2 and 4) of 2 mM guanidine HCl, the RNA products of each reaction were denatured with methylmercury hydroxide and separated by electrophoresis in 1% agarose and viral negative-strand RNA products were detected by autoradiography.
The 5′-terminal cloverleaf of poliovirus RNA was required in cis for viral negative-strand RNA synthesis.
We predicted, based on the results of previous investigations (D. J. Barton and J. B. Flanegan, unpublished observations) (29), that the 5′-terminal cloverleaf of poliovirus would be sufficient to restore template activity to DNVR2 RNA. Lu and Wimmer (29)previously demonstrated that chimeric viral RNAs composed of the poliovirus cloverleaf-HCV IRES-poliovirus open reading frame-3′ terminus were viable. Therefore, we constructed DNVR12 RNA; a chimeric viral RNA composed of poliovirus nucleotides 1 to 110 fused to the 5′ terminus of DNVR2 RNA (Fig. 1 and Materials and Methods). DNVR12 RNA was incubated in HeLa S10 translation-replication reaction mixtures to assay viral protein synthesis (Fig. 7), viral negative-strand RNA synthesis (Fig. 8), and VPg uridylylation (see Fig. 10).
FIG. 7.
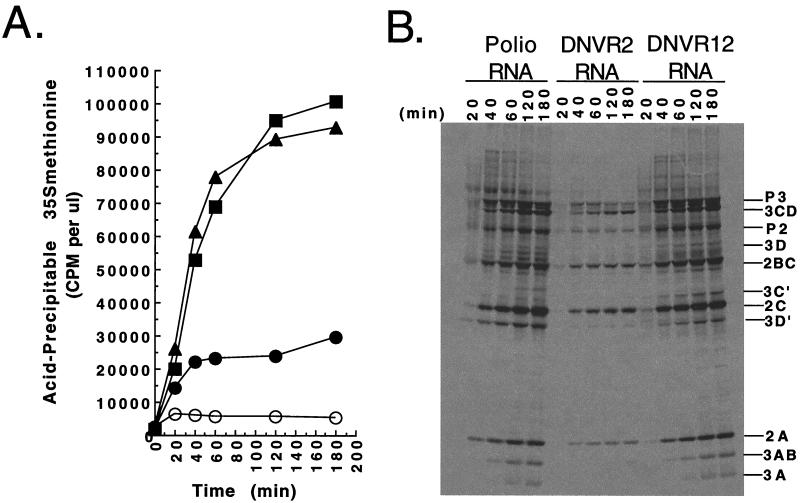
Kinetics and magnitude of viral RNA translation. Poliovirus RNA, DNVR2 RNA, and DNVR12 RNA were incubated in HeLa S10 translation-replication reaction mixtures containing [35S]methionine. (A) Acid-precipitable incorporation of radiolabel. One-microliter samples of each reaction were removed at the indicated times, precipitated with 5% trichloroacetic acid, collected by filtration on Millipore nitrocellulose filters, and quantitated by scintillation counting. Incorporation of radiolabel into acid-precipitable material was plotted versus time. Symbols: ▪, poliovirus RNA; ●, DNVR2 RNA; ▴, DNVR12 RNA; ○, mock. (B) SDS-PAGE. Samples collected at the indicated times from each reaction were separated in a 9 to 18% polyacrylamide gel. The radiolabeled viral proteins were detected by fluorography.
FIG. 8.
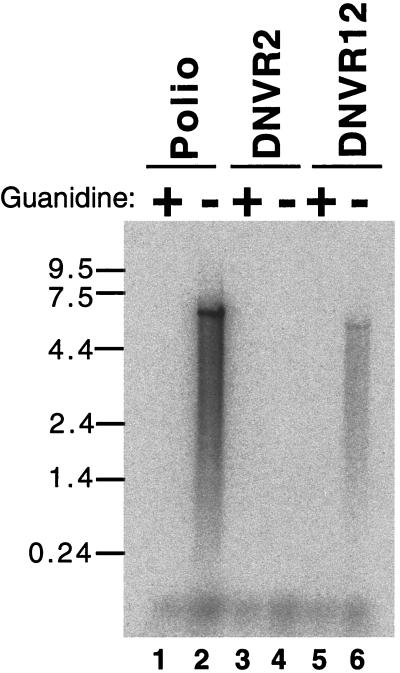
Negative-strand RNA synthesis. Preinitiation RNA replication complexes were isolated from HeLa S10 translation-replication reaction mixes containing poliovirus RNA (lanes 1 and 2), DNVR2 RNA (lanes 3 and 4), and DNVR12 RNA (lanes 5 and 6). The preinitiation RNA replication complexes were incubated in reactions containing [α-32P]CTP in the presence (lanes 1, 3, and 5) or absence (lanes 2, 4, and 6) of 2 mM guanidine HCl as described in Materials and Methods. RNA products from these reactions were denatured with 50 mM methylmercury hydroxide and separated by electrophoresis in 1% agarose, and the radiolabeled negative-strand RNA was detected by phosphorimaging. The mobility of single-stranded RNA size markers (in kilobases), detected by ethidium bromide staining, is indicated on the left side of the image.
FIG. 10.
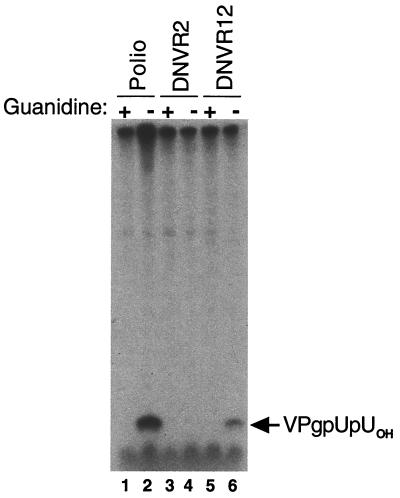
5′ Cloverleaf RNA and VPg uridylylation. Preinitiation RNA replication complexes were isolated from HeLa S10 translation-replication reactions containing poliovirus RNA (lanes 1 and 2), DNVR2 RNA (lanes 3 and 4), and DNVR12 RNA (lanes 5 and 6). The preinitiation RNA replication complexes were incubated in reaction mixtures containing [α-32P]UTP in the presence (lanes 1, 3, and 5) and absence (lanes 2, 4, and 6) of 2 mM guanidine HCl as described in Materials and Methods. Radiolabeled RNA products were fractionated by electrophoresis in a 10% polyacrylamide Tris-Tricine gel. Radiolabeled VPgpUpUOH was detected by phosphorimaging.
The kinetics of DNVR12 mRNA translation, relative to those of DNVR2 mRNA, were intriguing (Fig. 7A). DNVR12 RNA translated four times more efficiently than DNVR2 RNA (Fig. 7A) despite both RNAs possessing identical HCV IRESs (Fig. 1). The initial rate of DNVR2 mRNA translation was comparable to that of DNVR12 mRNA (Fig. 7A, 20 min). Following 20 min of incubation, however, the rate of DNVR2 mRNA translation quickly declined such that the accumulation of viral proteins ceased by 40 min of incubation (Fig. 7A). In contrast, the rate of DNVR12 mRNA translation remained robust after 20 min of incubation, with viral proteins accumulating until 120 min of incubation (Fig. 7A). The time at which the rate of DNVR2 mRNA translation declined corresponded with the time required for ribosomes to transit the viral RNA open reading frame. Viral protein 3CD, the viral protease responsible for proteolytically processing the viral polyprotein, is encoded at the 3′ terminus of the viral RNA open reading frame (Fig. 1). As soon as ribosomes complete the synthesis of viral polyprotein, viral protein 3CD, in the context of the viral polyprotein, begins to proteolytically process the viral polyprotein. After 20 min of incubation, neither full-length viral polyprotein nor viral protein 3CD was present in the products of the in vitro translation reactions (Fig. 7B). After 40 min of incubation, the viral polyprotein and each of the viral replication proteins were detected (Fig. 7B). Thus, DNVR2 mRNA ceased functioning as an mRNA at a time corresponding to that in which the ribosomes transited the viral mRNA open reading frame, while DNVR12 mRNA, with the 5′-terminal cloverleaf of poliovirus RNA, continued to function as an mRNA for another 80 min. These results suggest that the 5′-terminal cloverleaf of poliovirus RNA may affect the ability of viral mRNA to reutilize ribosomes from the 3′ terminus of the viral mRNA open reading frame.
We measured viral negative-strand RNA synthesis using preinitiation RNA replication complexes isolated from HeLa S10 translation-replication reaction mixtures containing poliovirus RNA, DNVR2 RNA, and DNVR12 RNA. As previously reported (6), guanidine HCl (2 mM) inhibited negative-strand RNA synthesis (Fig. 8, lane 1). In the absence of guanidine HCl, poliovirus RNA served as a functional template for the synthesis of negative-strand RNA (Fig. 8, lane 2). As shown previously (Fig. 2B, 4B, 5C, and 6D), DNVR2 RNA did not function as a template for viral negative-strand RNA synthesis (Fig. 8, lane 4). In contrast, DNVR12 RNA served as a functional template for viral negative-strand RNA synthesis (Fig. 8, lane 6). DNVR12 RNA, however, was not as efficient a template as poliovirus RNA, making only 10% as many negative-strand RNA molecules as poliovirus RNA (Fig. 8, compare lane 6 with lane 2). This lack of wild-type activity may be due to the reported complementarity between RNA sequences in the 5′ cloverleaf of poliovirus and HCV 5′ NTR (53) or to RNA sequences in the poliovirus IRES which are not present on these RNA templates (10). Nonetheless, this experiment demonstrated that the 5′-terminal 110 nucleotides of poliovirus RNA, corresponding to the 5′-terminal cloverleaf of poliovirus, when added to the 5′ terminus of DNVR2 RNA, rendered DNVR12 RNA capable of functioning as a template for viral negative-strand RNA synthesis.
The 5′-terminal cloverleaf of poliovirus RNA was required in cis for the uridylylation of VPg.
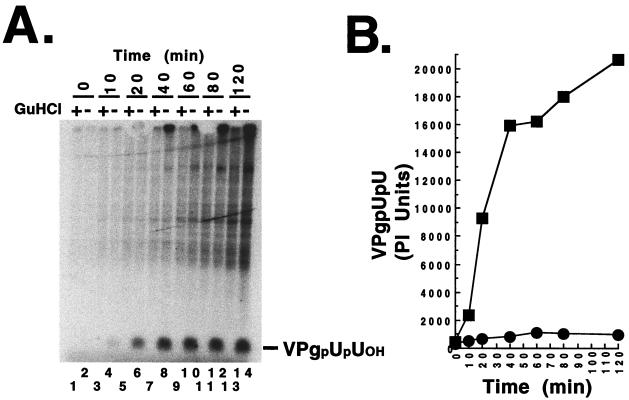
Next, we assayed the synthesis of VPgpUpUOH, using preinitiation RNA replication complexes (Fig. 9 and 10). Guanidine HCl (2 mM) inhibited the synthesis of VPgpUpUOH by preinitiation RNA replication complexes (Fig. 9A, odd-numbered lanes). In the absence of guanidine HCl, poliovirus RNA served as an efficient template for the uridylylation of VPg (Fig. 9A, even-numbered lanes). Radiolabeled VPgpUpUOH was detected at the earliest time points examined (10 min of incubation) and accumulated over 2 h of incubation (Fig. 9). In contrast, DNVR2 RNA did not function as a template for the uridylylation of VPg (Fig. 10, lane 4). DNVR12 RNA served as a functional template for the uridylylation of VPg at 10% the level of poliovirus RNA (Fig. 10, lane 6). Thus, template activity for VPg uridylylation was proportional to that for viral negative-strand RNA synthesis for the three viral RNAs tested (compare Fig. 8 with Fig. 10). The 5′-terminal cloverleaf of poliovirus RNA was required in cis for the uridylylation of VPg (Fig. 10, compare lane 6 with lane 4) and for the synthesis of viral negative-strand RNA (Fig. 8, compare lane 6 with lane 4).
FIG. 9.
VPg uridylylation by preinitiation RNA replication complexes. Preinitiation RNA replication complexes were isolated from HeLa S10 translation-replication reactions containing poliovirus RNA. The preinitiation RNA replication complexes were incubated for the indicated periods of time in reaction mixtures containing [α-32P]UTP in the presence (odd-numbered lanes) and absence (even-numbered lanes) of 2 mM guanidine HCl as described in Materials and Methods. Radiolabeled RNA products were fractionated by electrophoresis in a 10% polyacrylamide Tris-Tricine gel. (A) Radiolabeled VPgpUpUOH was detected by phosphorimaging. (B) The amount of VPgpUpUOH synthesized in the presence (●) and absence (▪) of 2 mM guanidine was plotted versus time.
DISCUSSION
In this report, we demonstrated that the 5′-terminal cloverleaf of poliovirus RNA is required in cis for viral RNA to function as a template for both the uridylylation of VPg and the synthesis of negative-strand RNA in the context of preinitiation RNA replication complexes. In addition, the 5′-terminal cloverleaf RNA dramatically affected the kinetics and magnitude of mRNA translation of chimeric mRNAs possessing the HCV IRES. These experiments highlight the importance of the 5′ cloverleaf RNA structure in the formation of functional mRNP complexes and viral RNA replication complexes.
5′ Cloverleaf RNA.
The 5′-terminal cloverleaf of poliovirus RNA is now associated with a number of interesting functions during viral replication, including viral mRNA stability (32), viral mRNA translation (this report and reference 44), VPg uridylylation (this report), viral negative-strand RNA synthesis (9, 20, 48), and potentially viral positive-strand RNA synthesis (2). To mediate these functions, the cloverleaf RNA structure interacts with both cellular proteins and viral replication proteins. Because poliovirus RNA functions sequentially as an mRNA for viral protein expression and then as a template for viral negative-strand RNA synthesis, it is likely that temporally dynamic ribonucleoprotein structures form on the 5′-terminal cloverleaf to mediate sequential activities. Poliovirus mRNA stability is mediated by interactions between poly(rC) binding proteins (PCBPs) and the 5′-terminal cloverleaf RNA (32). PCBPs bound to the 5′ cloverleaf of poliovirus RNA may simultaneously interact with poly(A) binding protein (PABP) bound to the poly(A) tail of poliovirus mRNA, effectively circularizing poliovirus mRNA within the messenger ribonucleoprotein (mRNP) complex (20). The potentiation of DNVR12 mRNA translation by the 5′ cloverleaf relative to DNVR2 mRNA translation reported in Figure 7 could be explained in the context of such a model. Eukaryotic cap-dependent mRNA translation is thought to occur in the context of interactions between eIF4G I and II at the 5′ end of mRNA and PABP at the 3′ end of mRNAs (49). The expression of poliovirus 2APro leads to the cleavage of eIF4G I and II, the shutoff of host protein synthesis, and the inability of eIF4G I and II to circularize mRNAs via interactions with PABP (because PABP binds to the NH terminus of eIF4Gs released by 2APro cleavage). PCBPs interacting with the 5′ cloverleaf of poliovirus mRNA could provide a mechanism for the restoration of interactions between the 5′ and 3′ termini of poliovirus mRNA within mRNP complexes (20). The abrupt cessation of translation of DNVR2 mRNA coordinates in time with the arrival of ribosomes at the 3′ end of the viral open reading frame (Fig. 7) and is consistent with a defect in the reutilization of ribosomes from the 3′ terminus of the viral open reading frame. A proximal orientation of the 5′ and 3′ ends of viral mRNA within mRNP complexes, mediated by protein-protein bridges between the 5′ cloverleaf RNA and poly(A) tail, could underlie the process of efficient reutilization of ribosomes from the 3′ terminus of poliovirus mRNA. As viral replication proteins accumulate, viral mRNP complexes are converted into viral RNA replication complexes. Viral protein 3CD binds to the 5′ cloverleaf RNA during this process and appears to be required for the subsequent initiation of viral negative-strand RNA synthesis (9).
Preinitiation RNA replication complexes.
Preinitiation RNA replication complexes represent an intermediate between viral mRNP complexes and viral RNA replication complexes. Preinitiation RNA replication complexes accumulate during the translation of poliovirus RNA in the presence of 2 mM guanidine HCl. Guanidine HCl inhibits the ATPase activity of viral protein 2C (38). The guanidine-inhibited activity of viral protein 2C is required for the initiation of viral negative-strand RNA synthesis (6, 48). Within preinitiation RNA replication complexes, cis-active RNA structures must align viral replication proteins along the viral RNA template near the site of RNA synthesis initiation. Thus, cis-active structures in poliovirus RNA must align VPg (or VPgpUpUOH) and 3DPol along the 3′-terminal poly(A) tail of the viral RNA template. Poliovirus protein 2CATPase and/or 2BCATPase may be involved in the alignment of the proteins along the poly(A) tail as guanidine HCl inhibits the uridylylation of VPg (Fig. 9) and the initiation of negative-strand RNA synthesis (Fig. 7 and 10) (6). Protein 2CATPase may alter protein-RNA interactions within preinitiation RNA replication complexes in a manner analogous to that described for vaccinia virus NPH II (22). 2C CRE is not present within DJB14 RNA and, therefore, is not required in cis for negative-strand RNA synthesis in trans-replication reactions (Fig. 5 and 6). The role of this RNA structure in VPg uridylylation within preinitiation RNA replication complexes requires further analysis. A cis-active 5′ RNP complex containing viral protein 3CD may deliver protease to cleave 3AB, the membrane-associated precursor of VPg, at the site of VPg uridylylation and/or the site of negative-strand synthesis initiation along the poly(A) tail.
5′-3′ communication.
The topology of poliovirus RNA, predicted by optimal and suboptimal free energy folding, favors a proximal orientation in space for the 5′ and 3′ termini (34). Furthermore, contemporary models of eukaryotic mRNA translation suggest that the 5′ and 3′ termini of mRNAs are held in a proximal conformation by RNA binding proteins (14, 42, 49). Finally, poliovirus RNA exists as an mRNA before it functions as a template for viral negative-strand RNA synthesis (33). We observed that the 5′-terminal cloverleaf of poliovirus RNA stimulated the translation of viral mRNA containing the HCV IRES (Fig. 7). As described above, we concur with Herold and Andino (20) that the 5′-terminal cloverleaf of poliovirus mRNA provides a mechanism for interactions between the 5′ and 3′ NTRs of poliovirus mRNA, namely a protein-protein bridge between PCBPs on the 5′ cloverleaf RNA and PABP on the 3′-terminal poly(A) tail. Our data demonstrate that both the 5′ and 3′ termini of poliovirus RNA are required in cis for viral RNA to function as a template for the uridylylation of VPg and the synthesis of viral negative-strand RNA. Therefore, it is possible that poliovirus preinitiation RNA replication complexes contain poliovirus RNA in a conformation in which the 5′-terminal cloverleaf of poliovirus RNA is in proximity to both the 2C-CRE element required for VPg uridylylation and the 3′ NTR and poly(A) tail where negative-strand RNA synthesis initiates.
Proximal RNA termini: a common strategy for RNA animal viruses?
RNA animal viruses appear to utilize communication between the 5′ and 3′ termini of their viral RNAs as a common strategy to regulate gene expression and RNA replication. As described above for poliovirus, communication between the cis-active RNA sequences and structures at the 5′ and 3′ termini of its viral RNA allows for coordinate regulation of viral mRNA translation and the initiation of negative-strand RNA synthesis. Sindbis virus RNA replication occurs via a genome-length negative-strand RNA molecule made from the genome-length viral RNA template. The Sindbis virus subgenomic viral mRNA, which is 3′ coterminal with the genome-length viral mRNA, does not serve as a functional template for viral negative-strand RNA synthesis. Thus, Sindbis virus RNA must possess cis-active RNA elements 5′ terminal to the subgenomic viral mRNA to mediate, in part, template recognition and the initiation of viral negative-strand RNA synthesis. An analysis of defective-interfering RNAs of Sindbis virus demonstrated that only RNA sequences from the very 5′ and 3′ termini of Sindbis virus RNA were required for replication and packaging (28). Coronavirus RNA transcription and RNA replication, though more complex, may subscribe to the same theme (43). The coronavirus RNA genome possesses 5′-terminal cis-active RNA sequences that are not present on subgenomic mRNAs (25, 26). Coronavirus mRNAs lacking these 5′-terminal cis-active RNA elements are incapable of serving as templates for negative-strand RNA synthesis. HCV RNA possesses complementarity between RNA sequences in its 5′ and 3′ termini (27), although this complementarity has not been demonstrated to be functionally relevant to any steps of viral replication. Negative-strand rhabdovirus and orthomyxovirus RNAs form panhandle structures due to complementarity in RNA sequences at their 5′ and 3′ termini (30, 51, 52). These panhandle RNA structures are required for viral RNA transcription and replication (30, 51, 52). Finally, rotaviruses appear to utilize complementarity at the termini of their viral RNA template to mediate negative-strand RNA synthesis (35, 50). Thus, the 5′ and 3′ termini of various viral RNA molecules exhibit a predilection for proximal orientations, and these proximal RNA termini seem to facilitate the regulation of viral gene expression and replication.
ACKNOWLEDGMENTS
We thank Rebecca Hoogstraten and Laura Hays for excellent technical assistance. We thank James B. Flanegan and Aleem Siddiqui for cDNAs of poliovirus and HCV, respectively. We thank Aleem Siddiqui, Naushad Ali, and members of our laboratory for critically reviewing the manuscript.
This work was supported by funds from the Howard Hughes Medical Institute and by National Institutes of Health grant AI 42189.
REFERENCES
- 1.Agol V I, Paul A V, Wimmer E. Paradoxes of the replication of picornaviral genomes. Virus Res. 1999;62:129–147. doi: 10.1016/s0168-1702(99)00037-4. [DOI] [PubMed] [Google Scholar]
- 2.Andino R, Rieckhof G E, Achacoso P L, Baltimore D. Poliovirus RNA synthesis utilizes an RNP complex formed around the 5′-end of viral RNA. EMBO J. 1993;12:3587–3598. doi: 10.1002/j.1460-2075.1993.tb06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baltera R F, Jr, Tershak D R. Guanidine-resistant mutants of poliovirus have distinct mutations in peptide 2C. J Virol. 1989;63:4441–4444. doi: 10.1128/jvi.63.10.4441-4444.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barton D J, Black E P, Flanegan J B. Complete replication of poliovirus in vitro: preinitiation RNA replication complexes require soluble cellular factors for the synthesis of VPg-linked RNA. J Virol. 1995;69:5516–5527. doi: 10.1128/jvi.69.9.5516-5527.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barton D J, Flanegan J B. Coupled translation and replication of poliovirus RNA in vitro: synthesis of functional 3D polymerase and infectious virus. J Virol. 1993;67:822–831. doi: 10.1128/jvi.67.2.822-831.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barton D J, Flanegan J B. Synchronous replication of poliovirus RNA: initiation of negative-strand RNA synthesis requires the guanidine-inhibited activity of protein 2C. J Virol. 1997;71:8482–8489. doi: 10.1128/jvi.71.11.8482-8489.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barton D J, Morasco B J, Flanegan J B. Assays for poliovirus polymerase, 3DPol, and authentic RNA replication in HeLa S10 extracts. Methods Enzymol. 1996;275:35–57. doi: 10.1016/s0076-6879(96)75005-x. [DOI] [PubMed] [Google Scholar]
- 8.Barton D J, Morasco B J, Flanegan J B. Translating ribosomes inhibit poliovirus negative-strand RNA synthesis. J Virol. 1999;73:10104–10112. doi: 10.1128/jvi.73.12.10104-10112.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barton D J, O'Donnell B J, Flanegan J B. 5′ cloverleaf in poliovirus RNA is a cis-acting replication element required for negative-strand synthesis. EMBO. 2001;20:1439–1448. doi: 10.1093/emboj/20.6.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borman A M, Deliat F G, Kean K M. Sequences within the poliovirus internal ribosome entry segment control viral RNA synthesis. EMBO J. 1994;13:3149–3157. doi: 10.1002/j.1460-2075.1994.tb06613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bovee M L, Lamphear B J, Rhoads R E, Lloyd R E. Direct cleavage of elF4G by poliovirus 2A protease is inefficient in vitro. Virology. 1998;245:241–249. doi: 10.1006/viro.1998.9172. [DOI] [PubMed] [Google Scholar]
- 12.Collis P S, O'Donnell B J, Barton D J, Rogers J A, Flanegan J B. Replication of poliovirus RNA and subgenomic RNA transcripts in transfected cells. J Virol. 1992;66:6480–6488. doi: 10.1128/jvi.66.11.6480-6488.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crawford N M, Baltimore D. Genome-linked protein VPg of poliovirus is present as free VPg and VPg-pUpU in poliovirus-infected cells. Proc Natl Acad Sci USA. 1983;80:7452–7455. doi: 10.1073/pnas.80.24.7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallie D R. A tale of two termini: a functional interaction between the termini of an mRNA is a prerequisite for efficient translation initiation. Gene. 1998;216:1–11. doi: 10.1016/s0378-1119(98)00318-7. [DOI] [PubMed] [Google Scholar]
- 15.Gamarnik A V, Andino R. Switch from translation to RNA replication in a positive-stranded RNA virus. Genes Dev. 1998;12:2293–2304. doi: 10.1101/gad.12.15.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giachetti C, Semler B L. Role of a viral membrane polypeptide in strand-specific initiation of poliovirus RNA synthesis. J Virol. 1991;65:2647–2654. doi: 10.1128/jvi.65.5.2647-2654.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodfellow I, Chaudhry Y, Richardson A, Meredith J, Almond J W, Barclay W, Evans D J. Identification of a cis-acting replication element within the poliovirus coding region. J Virol. 2000;74:4590–4600. doi: 10.1128/jvi.74.10.4590-4600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gradi A, Svitkin Y V, Imataka H, Sonenberg N. Proteolysis of human eukaryotic translation initiation factor eIF4GII, but not eIF4GI, coincides with the shutoff of host protein synthesis after poliovirus infection. Proc Natl Acad Sci USA. 1998;95:11089–11094. doi: 10.1073/pnas.95.19.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hentze M W. eIF4G: a multipurpose ribosome adaptor? Science. 1997;275:500–501. doi: 10.1126/science.275.5299.500. [DOI] [PubMed] [Google Scholar]
- 20.Herold J, Andino R. Poliovirus RNA replication requires genome circularization through a protein-protein bridge. Mol Cell. 2001;7:581–591. doi: 10.1016/S1097-2765(01)00205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobson S J, Konings D A M, Sarnow P. Biochemical and genetic evidence for a pseudoknot structure at the 3′ terminus of the poliovirus RNA genome and its role in viral RNA amplification. J Virol. 1993;67:2961–2971. doi: 10.1128/jvi.67.6.2961-2971.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jankowsky E, Gross C H, Shuman S, Pyle A M. Active disruption of an RNA-protein interaction by a DExH/D RNA helicase. Science. 2001;291:121–125. doi: 10.1126/science.291.5501.121. [DOI] [PubMed] [Google Scholar]
- 23.Joachims M, Van Breugel P C, Lloyd R E. Cleavage of poly(A)-binding protein by enterovirus proteases concurrent with inhibition of translation in vitro. J Virol. 1999;73:718–727. doi: 10.1128/jvi.73.1.718-727.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerekatte V, Keiper B D, Badorff C, Cai A, Knowlton K U, Rhoads R E. Cleavage of poly(A)-binding protein by coxsackievirus 2A protease in vitro and in vivo: another mechanism for host protein synthesis shutoff? J Virol. 1999;73:709–717. doi: 10.1128/jvi.73.1.709-717.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim Y-N, Jeong Y S, Makino S. Analysis of cis-acting sequences essential for coronavirus defective interfering RNA replication. Virology. 1993;197:53–63. doi: 10.1006/viro.1993.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim Y-N, Makino S. Characterization of a murine coronavirus defective-interfering RNA internal cis-acting replication signal. J Virol. 1995;69:4963–4971. doi: 10.1128/jvi.69.8.4963-4971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolykholov A A, Feinstone S M, Rice C M. Identification of a highly conserved sequence element at the 3′ terminus of hepatitis C virus genome RNA. J Virol. 1996;70:3363–3371. doi: 10.1128/jvi.70.6.3363-3371.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levis R, Weiss B G, Tsiang M, Huang H, Schlesinger S. Deletion mapping of Sindbis virus DI RNAs derived from cDNAs defines the sequences essential for replication and packaging. Cell. 1986;44:137–145. doi: 10.1016/0092-8674(86)90492-7. [DOI] [PubMed] [Google Scholar]
- 29.Lu H-H, Wimmer E. Poliovirus chimeras replicating under the translational control of genetic elements of hepatitis C virus reveal unusual properties of the internal ribosomal entry site of hepatitis C virus. Proc Natl Acad Sci USA. 1996;93:1412–1417. doi: 10.1073/pnas.93.4.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luytjes W, Krystal M, Enami M, Pavin J D, Palese P. Amplification, expression, and packaging of foreign gene by influenza virus. Cell. 1989;59:1107–1113. doi: 10.1016/0092-8674(89)90766-6. [DOI] [PubMed] [Google Scholar]
- 31.Molla A, Paul A V, Wimmer E. Cell-free, de novo synthesis of poliovirus. Science. 1991;254:1647–1651. doi: 10.1126/science.1661029. [DOI] [PubMed] [Google Scholar]
- 32.Murray K E, Roberts A W, Barton D J. Poly(rC) binding proteins mediate poliovirus mRNA stability. RNA. 2001;7:1126–1141. doi: 10.1017/s1355838201010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novak J E, Kirkegaard K. Coupling between genome translation and replication in an RNA virus. Genes Dev. 1994;8:1726–1737. doi: 10.1101/gad.8.14.1726. [DOI] [PubMed] [Google Scholar]
- 34.Palmenberg A C, Sgro J-Y. Topological organization of picornaviral genomes: statistical prediction of RNA structural signals. Semin Virol. 1997;8:231–241. [Google Scholar]
- 35.Patton J T, Wentz M, Xiaobo J, Ramig R F. cis-acting signals that promote genome replication in rotavirus mRNA. J Virol. 1996;70:3961–3971. doi: 10.1128/jvi.70.6.3961-3971.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paul A V, Rieder E, Kim D W, van Boom J H, Wimmer E. Identification of an RNA hairpin in poliovirus RNA that serves as the primary template in the in vitro uridylylation of VPg. J Virol. 2000;74:10359–10370. doi: 10.1128/jvi.74.22.10359-10370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paul A V, van Boom J H, Filippov D, Wimmer E. Protein-primed RNA synthesis by purified poliovirus RNA polymerase. Nature. 1998;393:280–284. doi: 10.1038/30529. [DOI] [PubMed] [Google Scholar]
- 38.Pfister T, Wimmer E. Characterization of the nucleoside triphosphatase activity of poliovirus protein 2C reveals a mechanism by which guanidine inhibits poliovirus replication. J Biol Chem. 1999;274:6992–7001. doi: 10.1074/jbc.274.11.6992. [DOI] [PubMed] [Google Scholar]
- 39.Pilipenko E V, Poperechny K, Maslova S V, Melchers W J G, Bruins Slot H J, Agol V I. Cis-element, oriR, involved in the initiation of (−) strand poliovirus RNA: a quasi-globular multi-domain RNA structure maintained by tertiary ('kissing') interactions. EMBO J. 1996;15:5428–5436. [PMC free article] [PubMed] [Google Scholar]
- 40.Pincus S E, Diamond D C, Emini E A, Wimmer E. Guanidine-selected mutants of poliovirus: mapping of point mutations to polypeptide 2C. J Virol. 1986;57:638–646. doi: 10.1128/jvi.57.2.638-646.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rieder E, Paul A V, Kim D W, van Boom J H, Wimmer E. Genetic and biochemical studies of poliovirus cis-acting replication element cre in relation to VPg uridylylation. J Virol. 2000;74:10371–10380. doi: 10.1128/jvi.74.22.10371-10380.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sachs A B, Sarnow P, Hentze M W. Starting at the beginning, middle, and end: translation initiation in eukaryotes. Cell. 1997;89:831–838. doi: 10.1016/s0092-8674(00)80268-8. [DOI] [PubMed] [Google Scholar]
- 43.Sawicki S G, Sawicki D L. A new model for coronavirus transcription. Adv Exp Med Biol. 1998;440:215–219. doi: 10.1007/978-1-4615-5331-1_26. [DOI] [PubMed] [Google Scholar]
- 44.Simoes E A, Sarnow P. An RNA hairpin at the extreme 5′ end of the poliovirus RNA genome modulates viral translation in human cells. J Virol. 1991;65:913–921. doi: 10.1128/jvi.65.2.913-921.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeda N, Yang C F, Kuhn R J, Wimmer E. Uridylylation of the genome-linked protein of poliovirus in vitro is dependent upon an endogenous RNA template. Virus Res. 1987;8:193–204. doi: 10.1016/0168-1702(87)90015-3. [DOI] [PubMed] [Google Scholar]
- 46.Takegami T, Kuhn R J, Anderson C W, Wimmer E. Membrane-dependent uridylylation of the genome-linked protein VPg of poliovirus. Proc Natl Acad Sci USA. 1983;80:7447–7451. doi: 10.1073/pnas.80.24.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takegami T, Semler B L, Anderson C W, Wimmer E. Membrane fractions active in poliovirus RNA replication contain VPg precursor polypeptides. Virology. 1983;128:33–47. doi: 10.1016/0042-6822(83)90316-1. [DOI] [PubMed] [Google Scholar]
- 48.Teterina N L, Egger D, Bienz K, Brown D M, Semler B L, Ehrenfeld E. Requirements for assembly of poliovirus replication complexes and negative-strand RNA synthesis. J Virol. 2001;75:3841–3850. doi: 10.1128/JVI.75.8.3841-3850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wells S E, Hillner P E, Vale R D, Sachs A B. Circularization of mRNA by eukaryotic translation initiation factors. Mol Cell. 1998;2:135–140. doi: 10.1016/s1097-2765(00)80122-7. [DOI] [PubMed] [Google Scholar]
- 50.Wentz M J, Zeng C Q, Patton J T, Estes M K, Ramig R F. Identification of the minimal replicase and the minimal promoter of (−)-strand synthesis, functional in rotavirus RNA replication in vitro. Arch Virol. 1996;12(Suppl.):59–67. doi: 10.1007/978-3-7091-6553-9_7. [DOI] [PubMed] [Google Scholar]
- 51.Wertz G W, Whelan S, LeGrone A, Ball L A. Extent of terminal complementarity modulates the balance between transcription and replication of vesicular stomatitis virus RNA. Proc Natl Acad Sci USA. 1994;91:8587–8591. doi: 10.1073/pnas.91.18.8587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whelan S P J, Wertz G W. Regulation of RNA synthesis by the genomic termini of vesicular stomatitis virus: identification of distinct sequences essential for transcription but not replication. J Virol. 1999;73:297–306. doi: 10.1128/jvi.73.1.297-306.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao W D, Lahser F C, Wimmer E. Genetic analysis of a poliovirus/hepatitis C virus (HCV) chimera: interaction between the poliovirus cloverleaf and a sequence in the HCV 5′ nontranslated region results in a replication phenotype. J Virol. 2000;74:6223–6226. doi: 10.1128/jvi.74.13.6223-6226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]