Abstract
Pavlovian fear conditioning is a widely used tool that models associative learning in rodents. For decades the field has used predominantly male rodents and focused on a sole conditioned fear response: freezing. However, recent work from our lab and others has identified darting as a female-biased conditioned response, characterized by an escape-like movement across a fear conditioning chamber. It is also accompanied by a behavioral phenotype: Darters reliably show decreased freezing compared to Non-darters and males and reach higher velocities in response to the foot shock (“shock response”). However, the relationship between shock response and conditioned darting is not known. This study investigated if this link is due to differences in general processing of aversive stimuli between Darters, Non-darters and males. Across a variety of modalities, including corticosterone measures, the acoustic startle test, and sensitivity to thermal pain, Darters were found not to be more reactive or sensitive to aversive stimuli, and, in some cases, they appear less reactive to Non-darters and males. Analyses of cFos activity in regions involved in pain and fear processing following fear conditioning identified discrete patterns of expression among Darters, Non-darters, and males exposed to low and high intensity foot shocks. The results from these studies further our understanding of the differences between Darters, Non-darters and males and highlight the importance of studying individual differences in fear conditioning as indicators of fear state.
1. Introduction
Pavlovian fear conditioning is a commonly used paradigm that models associative learning in rodents. Freezing, the total lack of all movement except that required by respiration (Fanselow, 1984), is the most common conditioned response (CR) used to indicate fear learning. The reliance on a single response as the sole indicator of learned fear reduces the likelihood of capturing and understanding the contribution of individual differences to fear learning outcomes. For example, darting in rats is a female-biased CR that is characterized by a quick, escape-like movement across the fear conditioning chamber in response to the conditioned stimulus (CS) (Gruene et al., 2015). Our lab has found that females in general, and Darters in particular, show heightened unconditioned responses, such as shock response (how quickly the animal moves in response to the shock) to the unconditioned stimulus (US) (Gruene et al., 2015; Mitchell et al., 2022). This heightened shock response predictably emerges before darting itself does (Mitchell et al., 2022), suggesting that sex differences in shock processing may underlie the tendency of female rats to engage in conditioned darting.
As the recognition of darting as a conditioned response has grown, questions have arisen asking if Darters are innately more stressed or hyperreactive than Non-darters and males. If so, this increased stress or hyperreactivity could be the cause of Darters’ heightened shock response, and darting could simply be one reflection of sex differences in aversive stimuli processing, which have been shown to exist across a large range of behavioral assays (Borkar et al., 2020; Greiner et al., 2019; Johnston and File, 1991; Kokras and Dalla, 2014; Laine et al., 2022; Lopez-Aumatell et al., 2008; Toufexis et al., 2016; Zambetti et al., 2019). The relationships between behavior following aversive stimuli exposure and conditioned darting have not been studied.
Sex differences in conditioned and unconditioned responses in rats during Pavlovian fear conditioning could be a result of differences in the processing of pain from the foot shock. Unfortunately, most preclinical studies of pain sensitivity and reactivity have focused on behaviors exhibited by male rodents (Gregus et al., 2021; Mogil and Chanda, 2005), even though there are known differences in pain processing across the sexes (Bartley and Fillingim, 2013; Berkley, 1997; Blanton et al., 2021; Fillingim, 2003; Fullerton et al., 2018; Girard-Tremblay et al., 2014; Linnman et al., 2012; Wiesenfeld-Hallin, 2005; Yu et al., 2021). Although our lab and others have observed conditioned darting in multiple experimental scenarios (Borkar et al., 2020; Colom-Lapetina et al., 2019; Greiner et al., 2019; Manzano Nieves et al., 2023; Mitchell et al., 2022; Pellman et al., 2017), the underlying biological contributors to darting are unknown. A better understanding of how brain regions known to be involved in processing aversive stimuli might differ in their recruitment during fear conditioning could give insight into the networks that drive sex differences in conditioned fear behavior.
The goals of the current study were three-fold: 1) determine if Darters exhibit higher physiological stress responses during fear conditioning; 2) determine how behavior during fear conditioning relates to behavior on other measures of aversive responding; and 3) investigate neural correlates of darting. To address these aims we first investigated rats’ change in corticosterone levels before and after fear conditioning. In another cohort of animals, we performed an acoustic startle test prior to classic fear conditioning and retrospectively compared startle responses in Darters, Non-darters, and males. In a third cohort, animals were exposed to a hot plate test one-week before fear conditioning to assess thermal pain responding in Darters, Non-Darters, and males. Finally, we quantified cFos expression following fear conditioning in brain regions known to be involved in the processing of pain and fear, including the dorsal horn of the lumbar spine (DHL) (Choi et al., 2020; Coghill, 2020; Coghill et al., 1991), the lateral parabrachial nucleus (lPbN) (Chiang et al., 2019), the sub columns of the periaqueductal gray (PAG) (Benarroch, 2012; Keay and Bandler, 2015), the lateral amygdala (LA) (Janak and Tye, 2015; Kuner and Kuner, 2021), the central amygdala (CeA) (Ciocchi et al., 2010; LeDoux, 2000; Maren and Quirk, 2004), and the basolateral amygdala (BLA) (Corder et al., 2019; Gale et al., 2004; Gore et al., 2015). We compared activation of these regions across sexes and between Darters, Non-darters and males.
2. Methods
2.1. Subjects
All experiments were conducted in young adult (8–10 weeks) male (325–350g) and female (225–250g) Sprague Dawley rats (Charles River). A greater number of females were used in each of the described experiments to ensure a substantial proportion of Darters to perform appropriately powered statistical analyses. Animals were same-sex, pair-housed at Northeastern University in a 12:12 light:dark cycle and had access to food and water ad libitum. Animals acclimated to the facility, undisturbed, for one week prior to testing. Testing was conducted during the light phase between the hours of 10AM and 3PM. To account for any stress that transport to and from the behavior rooms may cause, animals were placed on a rolling cart and rolled into the behavior rooms the day immediately prior to testing in addition to being handled for 5 min a day the two days prior to testing. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Northeastern University Institutional Animals Care and Use Committee.
2.2. Blood collection for corticosterone analysis
Blood was collected immediately before and 15 min after fear conditioning from the lateral tail vein. Rats were placed in a restraint tube and a 25G needle was used to create a small stick hole to allow for blood effusion from the penetration point. A total volume of ∼100 μL blood sample was collected using an EDTA coated microvette tube (Kent Scientific, KMIC-EDTA). After collection, blood samples were then centrifuged at 2000 g for 20 min at 4 °C. The supernatant was collected and immediately stored at −80 °C until analyzed. Plasma corticosterone levels were measured using a commercially available ELISA kit (R&D Systems, KGE009) according to the manufacturer's instructions. The experimenters involved in this assay represent people identifying as either female or male.
2.3. Acoustic startle assay
Acoustic startle was run identical to that in Granata et al. (2022). Briefly, Med Associates’ acoustic startle hardware and software package (product number: MED-ASR-PROQ) was used in sound-proof cabinets. In each cabinet, there was an animal holder with a grid rod on top of a startle platform containing the load cell. The load cell and amplifier converted force on to the platform to the voltage representing startle response. One inch behind the animal holder, speakers delivered white noise background sound (70 dB constant) and startle noise bursts (50 ms each at decibels of 95 db, 100 db, 105 db, 110 db, presented in random order). Sexes were counterbalanced between chambers. There were 100 trials presented in 30-s intervals. After each round, the chambers were cleaned with 70% ethanol. The experimenters involved in this assay represent people identifying as female.
2.4. Static hot plate assay
The hot plate assay was conducted using a Corning PC 520 hot plate, heated to 52C. The temperature of the hot plate was measured before each animal underwent the assay, using an Etekcity Lasergrip 1080 infrared thermometer. The hot plate was turned on to its lowest setting and allowed to heat up for 45 min before a glass cylinder (12″ high, 7″ in diameter) was placed on top. Animals were brought into the testing room and allowed to habituate to the room for 30 min, after which the test began. The first rat was placed at the bottom of the cylinder and left there for 45 s. The built-in webcam of a 27-inch, iMac Desktop computer was used for recording the duration of the trial, recording any nociceptive behaviors the rat may display. After the rat was removed from the cylinder, the recording was stopped, and the cylinder was cleaned with 70% ethanol. The temperature of the cylinder was maintained between each rat, and if the cylinder went above desired temperature, it was removed from the plate and allowed to cool before the next rat's test began. At the end of all the trials, the animals were placed back into the vivarium. The experimenters involved in this assay represent people identifying as either male or female.
2.5. Fear conditioning
Fear Conditioning (FC) was conducted in one of four identical 20-cm2 chambers constructed of aluminum and Plexiglass walls (rat test cage; Coulbourn Instruments) with metal stainless steel rod flooring attached to a shock generator (Coulbourn Instruments model H13-15). The chambers were lit with a single house light, and each chamber was enclosed within a sound isolation cubicle (Coulbourn Instruments model H10-24A). An overhead, infrared digital camera recorded each trial at a frame rate of 30 frames/second. Before the animals were placed in the room for habituation, a decibel meter measured the decibel level of the CS and a shock meter measured the unconditioned stimulus (US) mA, to ensure consistency across trials. After the boxes were set up for a trial, animals were placed in the room in their cages to habituate for 30 min. At the start of each FC session, animals were given 5 min to explore the arena before the first CS-US presentation. The CS was a 30-s, 4 kHz, 80 dB SPL sine wave tone which co-terminated with a 0.5 s foot shock US. In experiments 1 and 2, animals were exposed to a 0.5 mA shock or no shock for a tone-only control condition (CS-only). In experiment 3, animals were assigned to a 1 mA (high) shock group, 0.3 mA (low) shock group, or a CS-only control group. We used different shock intensities in experiment 3 to parse apart the behavioral and neural changes that are a result of darting specifically. There were 7 CS-US pairings throughout the trial with a mean intertrial interval of 3 min and a range of 1.5–5 min. Total test duration across all experiments was 30 min. After each trial, chamber walls, ceilings and the grid floors were cleaned with 70% ethanol and trays were cleaned with water and soap. Chambers were used for both male and female animals, but each test session was restricted to a single sex. Four animals ran per session, except for Experiment 3 where only two animals ran per session. The experimenters involved in this assay represent people identifying as either male or female.
2.6. Behavior tracking and behavioral data processing
Recorded videos from the hot plate assay were hand scored for nociceptive behaviors, such as time to lick hind paw, jumping, and fore paw attending and lifting (Carter, 1991; Minett et al., 2011). For behavior during fear conditioning, we used Noldus Ethovision software to track the animals and generate raw velocity data sheets from all video files at a sample rate of 30 frames per second. ScaredyRat, a custom python tool developed by our lab to analyze raw Ethovision data files (Mitchell et al., 2022), extracted freezing, darting, and velocity data from each animal during the tone and shock epochs. As in previous research from our lab (Gruene et al., 2015; Mitchell et al., 2022), Darters were defined as any animal that engaged in a movement across the fear conditioning chamber at or exceeding 20 cm/s in velocity during CS 3–7.
2.7. Euthanasia & tissue preparation
Animals in experiments 1 and 2 were euthanized via thoracotomy. Animals in experiment 3 were anesthetized 90 min after the completion of fear conditioning and euthanized via transcardial perfusion with 1% saline followed by 4% paraformaldehyde (PFA) in 0.1M phosphate buffer (PBS, PH 7.4). Brains and spinal cords were extracted and post-fixed in PFA for 24 h, and then placed in 0.1% sodium azide in PBS at 4C until slicing for storage.
2.8. Immunohistochemistry
All IHC procedures were conducted by an experimenter blind to the shock intensity group and Darter identity of the rats.
2.8.1. Spinal cord, amygdala, and lPbN sections: diaminobenzadine (DAB) staining
Once ready for slicing, spinal cords and brain tissue were placed into a 30% sucrose in PBS and left in 4C until the tissue sunk to the bottom of the sucrose solution. Brain tissue was blocked prior to submersion in sucrose, while spinal cords were sectioned after. Once the tissue sank, it was embedded in Optimal Cutting Temperature (OCT) compound and frozen for slicing on a cryostat (Leica 6800) in 30 μm thick sections. Sections containing the central, basolateral, and lateral amygdala, the lateral parabrachial nucleus, and spinal Lumbar sections 2, 3, 4 were collected and washed in PBS three times, 10 min each time. After the washes, endogenous peroxidase activity was quenched for 15 min in a 0.5% hydrogen peroxide solution in PBS, after which the slices were mounted into microscope slides. Once sufficiently dry, slides were rinsed three times with PBS, 10 min each time. All washes were performed on a shaker and all incubations took place in an opaque incubation chamber, both at room temperature. They were then blocked in a 10% Normal Goat Serum (NGS, S26, Millipore) in 0.1% PBS-T for 1 h at room temperature. Following blocking, slides were incubated in a Rabbit anti cFos primary antibody (Rabbit IgG Fos Abcam - ab190289) with 10% NGS in 0.1% PBS-T, overnight at room temperature. Eighteen to 24 h later, slides were rinsed in PBS for 10 min, three times before secondary antibody incubation in biotinylated goat anti-rabbit (PK-6101, 1:200) in 1.5% NGS in PBS for 2 h. An ABC solution was prepared using the Vectastain Elite ABC Kit (Vector Laboratories) 30 min prior to use to allow complex to form. After secondary antibody incubation, slides were rinsed three times, 5 min each in PBS and then incubated for 45 min in the ABC solution. After ABC, slides were rinsed three times, 5 min each, in 0.1M phosphate buffer and then incubated in DAB solution. The spinal cord and amygdala sections incubated for 20 min and the lPbN incubated for 2 min, due to differences in staining intensity. After incubation, slides were rinsed in PBS three times, 5 min each and then dried and cover-slipped for imaging. Images were taken at 10X magnification and stitched together using a Keyence BZX 710 at 1/500s exposure.
2.8.2. Fluorescent immunohistochemistry
Fifty micrometer PAG sections were collected using a vibrating microtome (Leica VT 1000S). PAG sections −8.04 from Bregma from each animal were isolated and washed three times for 10 min each in 0.1% Triton-X 100 in PBS (PBS-T), and then incubated in a blocking buffer containing 10% Normal Donkey Serum (NDS) in PBS-T for 1 h at room temperature. Sections were then incubated overnight at 4C in a polyclonal rabbit anti-cfos 1:2000 (ABCam AB190289). Eighteen to 24 h later, slices were rinsed in 0.1% PBS-T three times, 10 min each, before incubating for 1.5 h in a donkey anti-mouse secondary antibody 1:1000 Alexa 647 (Jackson Immuno product #715-605-150) at room temperature. After secondary incubation, sections were rinsed three times, 10 min each in 0.1% PBS. At the conclusion of the washes, sections were mounted on microscope slides, cover slipped, and dried overnight. Slides were imaged at 10X magnification on the same Keyence as DAB-stained sections and the PAG sections were imaged in a 3×3 grid at 3.5s exposure.
2.9. Cell quantification
Images from the brain and spinal cord were imported to ImageJ software and number of cFos + cells were quantified using a customized cell counting macro by an experimenter blind to animal shock intensity condition and Darter identity. We used one section per region, as regional functions can vary on a rostral/caudal gradient. We chose to focus on sections that were previously identified as being involved in pain or fear processing. Sections used were as follows: laminae i-iii in lumbar spinal cord sections 2, 3, 4 (Westlund and Willis, 2015), lateral parabrachial nucleus bregma −8.76 (Raver et al., 2020), periaqueductal gray columns bregma −8.04 (Carrive et al., 1997), central amygdala bregma −2.40 (Prusator & Greenwood-Van Meerveld, 2017), lateral amygdala bregma −2.76 (Swanson and Petrovich, 1998), basolateral amygdala bregma −3.0 (Vazdarjanova and Mcgaugh, 1999). For each region, a trained research associate outlined the whole brain region according to the Paxinos and Watson Rat Brain in Stereotaxic Coordinates, 7th Edition (Paxinos and Watson, 2013).
2.10. Statistical analysis
All statistical analyses were conducted using GraphPad Prism software version 10.2.3 (one and two-way ANOVAs, chi-squared tests) and SPSS version 28 (three-way ANOVAs). When appropriate, post hoc tests (Dunnett's multiple comparisons for comparisons to a single control group or Sidak's multiple comparisons for all other comparisons) were used on main effects and interactions. In standard cohorts, there are not sufficient N's to run statistics on male Darters so they are not split by Darter identity. Outliers were removed if they were±two standard deviations away from a group's mean and were calculated for each individual group analyzed (e.g. 0.3 mA females, 0.3 mA female Non-darters, 0.3 mA female Darters: outliers were calculated at each level).
In experiment 1, a 2 × 2 × 2 mixed model three-way analysis of variance (ANOVA) was performed to examine between subject factors of testing condition (shock exposure or CS only) and sex (male, female) and the within subject factor of time (CORT levels pre and post fear conditioning). For females, a 2x2 mixed model ANOVA was performed on the between subject factor of Darter identity (Darter, Non-darter) and the within subject factor of time (CORT levels pre and post fear conditioning).
In experiment 2, a 3x4 mixed model, two-way ANOVA was conducted with the between subject factor of Darter identity (Darter, Non-darter, males) and decibel intensity (95, 100, 105, 110 dB) for both peak startle amplitude and latency to startle.
In experiment 3, for nociceptive behavior, a one-way ANOVA was conducted on the between subject factor of Darter identity (Darter, Non-darter, male) for hind withdraw and fore paw attending. A 3x2 mixed model, two-way ANOVA was conducted on the between subject factor of Darter identity (Darter, Non-darter, male) and the within subject factor of paw withdraw latency (hind paw, fore paw). For fear conditioning behavior, chi-squared tests were conducted to determine the differences in Darter percentages between shock intensity groups in males and females. A 2 × 3 × 7 mixed-model, three-way ANOVA was conducted on the between subject factors of sex (male, female) and shock intensity (CS only, 0.3 mA, 1 mA) and the within subject factors of tone or shock (tones/shocks 1 thru 7), on percent of time spent freezing and shock response (cm/s). In females, a 3 × 2 × 7, mixed model 3-way ANOVA of the between subject factors of shock intensity (CS only, 0.3 mA, 1 mA), Darter identity (Darter, Non-darter), and tone or shock (tones/shocks 1 thru 7), was conducted for freezing and shock response.
To avoid cohort effects for cFos cell counts in experiment 3, we normalized data to each cohort's control condition (CS only) by taking the average of the controls from each cohort, and then dividing each individual animals' counts by their respective cohort's control average. For each region, 2x2 between-subjects ANOVAs (shock intensity, 0.3 mA and 1 mA and sex, male and female) were conducted on cFos expression in regions of interest. Females were further broken down and 2x2 between-subjects ANOVAs (shock intensity 0.3 mA and 1 mA and Darter identity, Darter, Non-Darter), were conducted on cFos expression in the same regions.
3. Results
3.1. Experiment 1
A graphical representation of experiment 1 can be found in Fig. 1A. Twenty-seven females (19 exposed to CS plus shock, 8 CS-only controls) and 17 males (10 shock, 7 controls) were used in this experiment. One female non-Darter was removed from analysis because their CORT levels were greater than two standard deviations from the mean and was deemed an outlier.
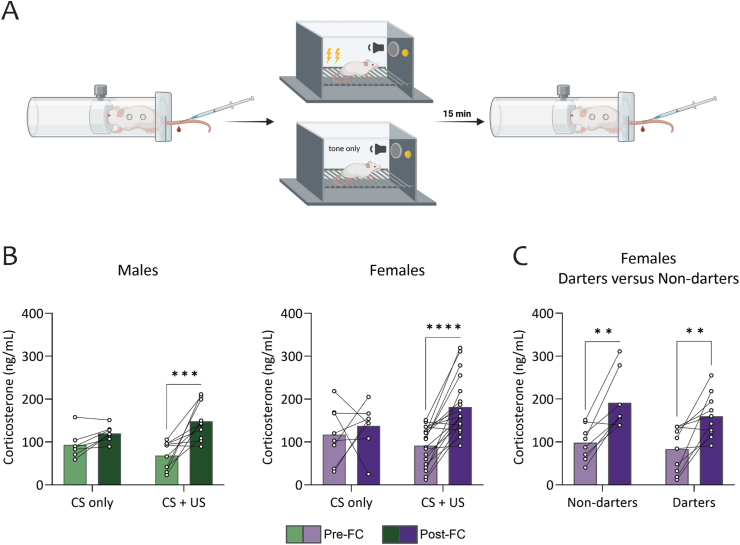
Fig. 1.
Fear conditioning induces comparable increases in corticosterone levels in all animals. A. Experimental design consisted of blood plasma samples taken immediately before and 15 min after a 7 CS-US fear conditioning paradigm. B. Corticosterone levels increased in fear conditioned males and females, but not in CS-only males and females. C. Corticosterone levels increased in all Darters and Non-darters following fear conditioning. (∗∗) p < 0.01, (∗∗∗) p < 0.001, (∗∗∗∗) p < 0.00001. Design figure in panel A created in Biorender.
3.1.1. Corticosterone levels do not differ between Darters and Non-darters
Measurements of blood plasma levels of corticosterone taken immediately before and 15 min after fear conditioning show that corticosterone levels increased in response to fear conditioning in males and females (Fig. 1B). CS-only controls did not show an increase in corticosterone levels (Fig. 1B). A mixed model ANOVA comparing the between subjects factors of shock exposure and sex with the within subject factor of pre and post conditioning corticosterone levels (pre/post timing) found a significant main effect for pre/post timing (F(1, 31) = 13.95, p = 0.0008). There was also a significant interaction between pre/post timing and shock exposure (F(1,31) = 5.72, p = 0.023), Sidak's multiple comparison test showed a significant increase in CORT levels following fear conditioning in the shock exposure group in both males (p = 0.01) and females (p = 0.006). No difference was found in the groups exposed to CS-only. Because corticosterone only increases in the fear conditioned group, this validates that increases in corticosterone levels following fear conditioning are indeed due to shock exposure and not stress from manipulation/testing.
Of the fear conditioned females, eight were Darters and ten were Non-darters. In both female Darters and Non-darters, corticosterone levels increased from baseline following fear conditioning (Fig. 1C). A mixed model ANOVA comparing the between subject factor of Darter identity (Darter, Non-darter) and the within subject factor of pre and post conditioning corticosterone levels in females found a significant main effect for pre/post timing (F(1,16) = 34.09, p < 0.0001). Sidak's multiple comparisons showed significant differences for Darters (p = 0.0023) and Non-darters (p = 0.0011). There was no main effect of Darter identity.
To ensure that blood collection prior to fear conditioning in Experiment 1 did not affect behavior during fear conditioning, we compared conditioned freezing and shock response in animals from experiment 1 and experiment 2 (in which experimental parameters were identical) and found comparable levels of freezing and shock response (Supplemental Fig. 1). Two-way ANOVAs (experiment, sex), revealed no significant main effects of experiment (F(1,80) = 0.440, p = 0.51) or sex (F(1,80) = 2.293, p = 0.134) on conditioned freezing, and no significant main effects of experiment (F(1,80) = 1.271, p = 0.263) on shock response. There was a significant main effect of sex (F(1,80) = 54.65, p < 0.0001) on shock response, and Sidak's multiple comparisons revealed that this was driven by females having higher shock responses than males across both experiments (p < 0.0001). The lack of main effects because of experiment suggests that blood collection prior to fear conditioning did not affect behavior during fear conditioning.
3.2. Experiment 2
A graphical representation of experiment 2 can be found in Fig. 2A. Twenty-four females and 19 males were used in this experiment.
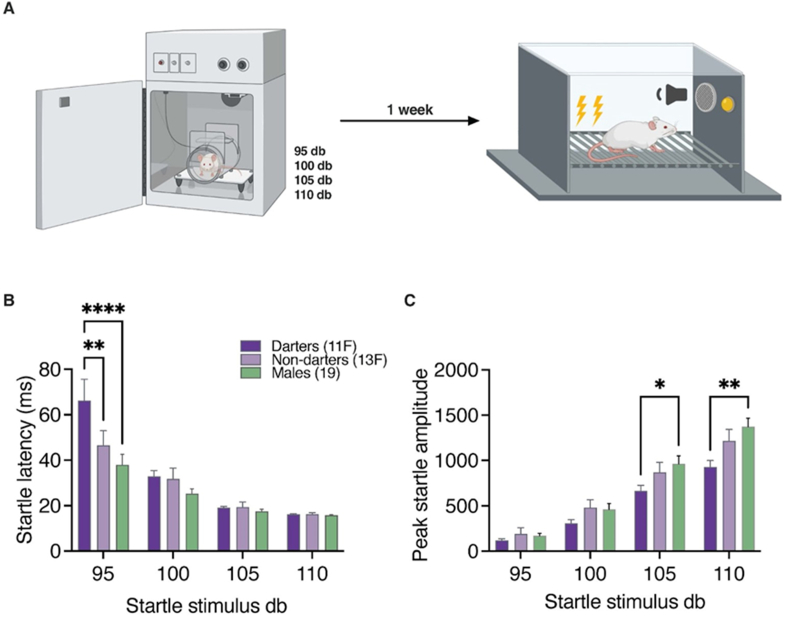
Fig. 2.
Darters do not exhibit enhanced startle responses. A. Experimental design consisted of the acoustic startle test where animals were exposed to a series of startle-evoking white noises at four different decibel levels presented in a random order for 1 h. One week later, animals ran through a 7 CS-US fear conditioning paradigm. B. Darter females have a greater latency to startle than non-darter females and males at 95 db stimuli. C. Males have a greater peak startle amplitude than darter females at 105 db and 110 db stimuli. (∗) p < 0.05, (∗∗) p < 0.01, (∗∗∗∗) p < 0.00001. Design figure in panel A created in Biorender.
3.2.1. Darters do not have greater startle responses than non-darters and males
A mixed model ANOVA comparing the between subject factor of Darter identity and the within subject factor of stimulus decibel on startle latency found significant main effects for both Darter identity (F(2, 160) = 6.582, p = 0.0018) and decibel (F(3, 160) = 51.48, p < 0.0001) (Fig. 2B). There was also a significant interaction between Darter identity and decibel (F(6, 160) = 3.164, p = 0.0058). Dunnett's multiple comparisons test found significant differences between female Darters and Non-darters (p = 0.0013) and female Darters and males (p < 0.0001) only at the 95 dB stimulus. In the acoustic startle test, at lower (95 dB) decibels, female Darters had a longer latency to startle than female Non-darters and males, but at higher decibels (100 dB, 105 dB, 110 dB), female Darters did not differ from female Non-darters and males. Together, these data show that at lower decibels, Darters are slower to startle than Non-darters and males.
A two-way ANOVA comparing the between subject factor of Darter identity and the within subject factor of stimulus decibel on peak startle amplitude found significant main effects for decibel (F (1.737, 69.46) = 290.1, p < 0.0001), a trending main effect for Darter identity (F(2, 40) = 2.748, p = 0.07), and a significant interaction between Darter identity and decibel (F(6, 120) = 3.822, p = 0.0016) (Fig. 2C). Dunnett's multiple comparisons test found significant differences between males and female Darters at 105 dB (p = 0.027) and 110 dB (p = 0.002). The lack of a difference between Darters' and Non-darters’ peak startle suggests that Darters are not merely hyperreactive and more sensitive to a broad range of aversive stimuli compared to Non-darters.
3.3. Experiment 3
A graphical representation for experiment 3 can be found in Fig. 3A. Sixty-two females (26 0.3 mA, 20 1 mA and 16 CS-only) and 59 males (22 0.3 mA, 21 1 mA, and 16 CS-only) went through the hot plate assay followed by fear conditioning approximately one week later and then cFos analysis. Twenty-four males and 22 females were included in the final hot plate analysis and the rest were excluded due to a video quality issue that prevented accurate scoring. For the cFos portion of the experiment, each region has different N's for males and females due to timing effects of staining.
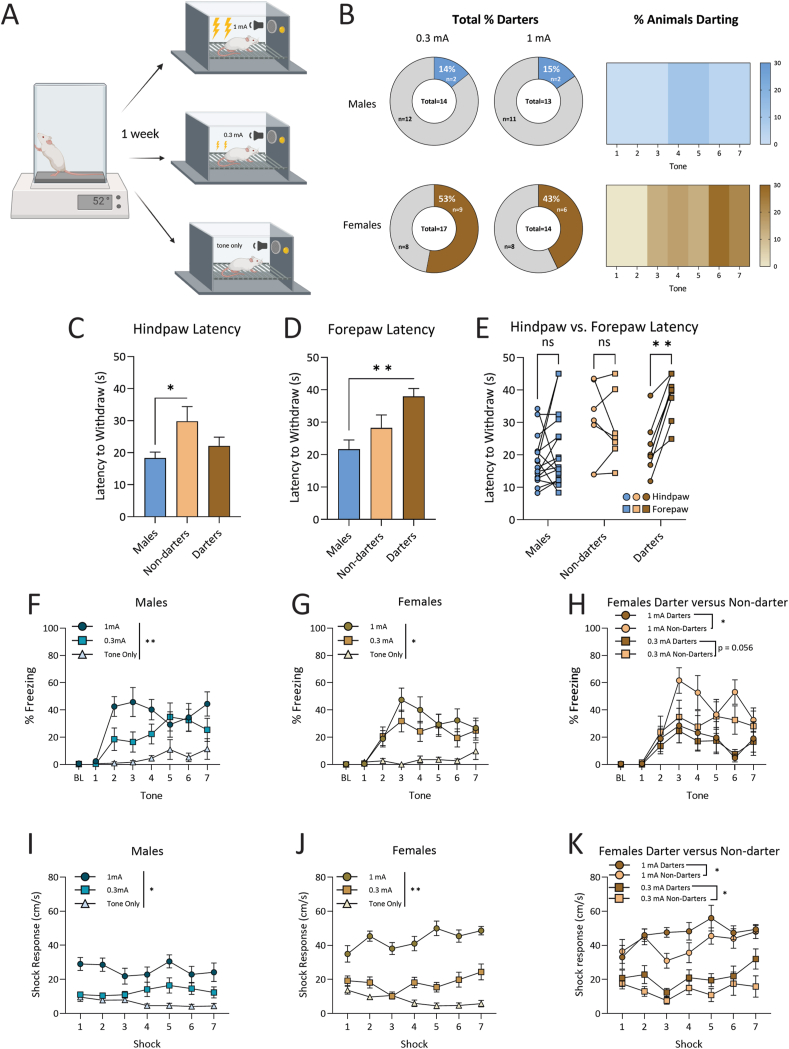
Fig. 3.
Behavior results for Experiment 3. A. Experimental design consisted of one day of the hot plate assay to obtain baseline thermal pain threshold. One week following exposure to the hot plate, animals ran through a 7 CS-US fear conditioning paradigm in one of three groups: 1 mA shock, 0.3 mA shock, or CS-only control groups. B. Proportion of Darters in males and females across 0.3 and 1 mA shock intensity groups and heatmaps for both sexes showing prevalence of conditioned darting across fear conditioning tone C. Female Non-darters have a longer latency to withdraw their hind paw than males. D. Female Darters have a longer latency to withdraw their forepaw than males. E. Darters consistently withdraw their hind paws before their forepaws, a pattern not seen in Males or non-Darters. F. Percent of time spent freezing increased because of increasing shock intensity in males G. Percent of time spent freezing increased with higher shock intensity in females. H. In females, non-Darters froze more than Darters, regardless of shock intensity. I. Shock response increased with higher shock intensity in males J. Shock response increased with higher shock intensity in females. K. In Females, Darters showed higher shock response than non-Darters exposed to the same shock intensity … (∗) p < 0.05, (∗∗) p < 0.01. Design figure in panel A created in Biorender.
3.3.1. Behavior comparisons across hot plate and fear conditioning paradigms
3.3.1.1. There is a relationship between nociceptive behavior and Darter identity
During fear conditioning, females darted more than males, as shown by a significant chi-squared test (χ2(1) = 7.384, p = 0.003) and darted to the later tones (Fig. 3B). Non-darter females had a longer latency to withdraw their hind paws than males (Fig. 3C). One-way ANOVA revealed a significant effect of Darter identity on hind paw withdrawal (F(2, 28) = 4.17, p = 0.03), and Dunnett's multiple comparison tests resulted in a significant difference between males and Non-darters (p = 0.02), but not males and Darters (p = 0.59). A one-way ANOVA similarly revealed a significant effect of sex on forepaw attending latency (F(2,28) = 6.60, p = 0.005) (Fig. 3D). Dunnett's multiple comparisons revealed that males still showed the quickest latency to attend, but only in comparison to female Darters (p = 0.003), who had the longest latency to attend, and that males were not different than not Non-darters (p = 0.36). Finally, we examined within-subjects latencies between hind and fore paw. A two-way ANOVA revealed a main effect of Darter identity (F(2,56) = 7.699, p = 0.001), paw (hind vs. fore) (F(1,56) = 5.048, p = 0.029) and a significant interaction between Darter identity and paw (F(2,56) = 3.47, p = 0.038). Darters were the only group to show a significant within group difference between fore and hind paw latency, consistently withdrawing their hind paw before their fore paw (p = 0.005), as identified by a Sidak's multiple comparisons test (Fig. 3E). Therefore, unlike males and Non-darters, Darters exhibit a predictable pattern of paw withdrawal order in a static hot plate test.
3.3.1.2. Conditioned and unconditioned responses during fear conditioning differ by shock intensity and sex
Both males and females froze more to higher shock intensities, and female Darters froze less than their Non-darter counterparts. A 3-way ANOVA of sex, shock intensity, and tone revealed a significant main effect of shock intensity on freezing (F(2, 79) = 18.834, p < 0.001), in that males and females in either shock group froze more than CS-only animals (p < 0.05 for both sexes when 0.3 and 1 mA are compared to CS-only) (Fig. 3F and G). When females were split by Darter identity, a 3-way ANOVA of shock intensity, Darter identity and tone revealed a significant main effect of Darter identity on freezing (F(1,31) = 10.15, p = 0.003): 1 mA Darters froze less than their Non-darter counterparts (p = 0.018), and 0.3 mA Darters trended towards freezing less than their Non-darter counterparts (p = 0.056) (Fig. 3H).
Both males and females reached higher maximum shock velocities in response to higher shock intensities, and female Darters in both shock intensities reached higher shock velocity than their Non-darter counterparts. A 3-way ANOVA revealed that animals exposed to higher shock intensities reached higher maximum shock velocities (F(2,79) = 66.37, p < 0.001), regardless of sex (females and males, all p < 0.05) (Fig. 3I and J). There was also a main effect of sex (F(1,79) = 14.91, p < 0.001), and females had overall higher shock response than males at the 1 mA shock intensity (p < 0.001). When split by Darter identity, a 3-way ANOVA revealed that in females there was a significant effect of shock intensity (F(1, 31) = 84.23, p < 0.001) and Darter identity (F(1,31) = 9.28, p = 0.005) on shock response. Female Darters at both 0.3 and 1 mA shock intensities reached higher maximum shock velocities than their Non-darter counterparts (p < 0.05 for both shock intensities) (Fig. 3K).
3.3.2. cFos expression in key regions following fear conditioning
The experimental design for all c-fos assays is shown in Fig. 4A. Animals were excluded from specific regions if tissue from that region was too damaged to accurately count.
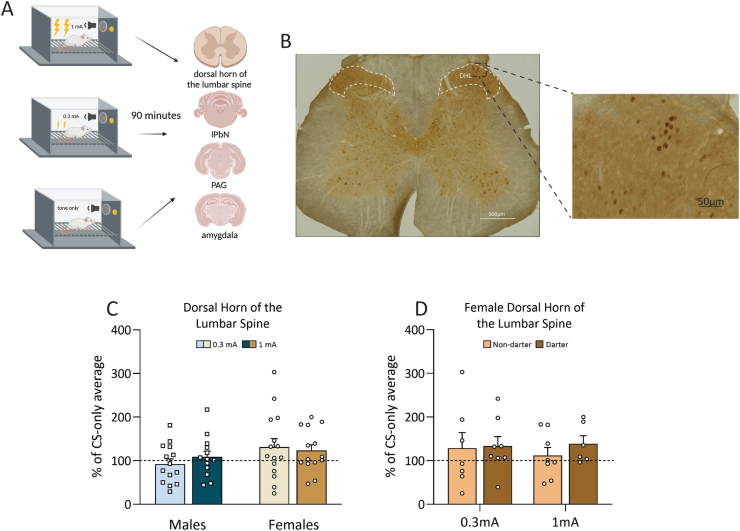
Fig. 4.
cFos activity in the dorsal horn of the lumbar spine following fear conditioning. A. Experimental design for cFos portion of the experiment. B. Representative image of L3 of the Dorsal Horn of the Lumbar stained with DAB for cFos. C. There were no differences in cFos expression across shock intensities or sexes. D. There were no differences in cFos expression across Darter identity. Dotted line indicates CS-only animal cFos levels. Design figure in panel A created in Biorender.
3.3.2.1. cFos expression in the DHL did not differ based on sex, shock intensity, or Darter identity
Thirty-nine female (16 0.3 mA, 14 1 mA, 9 CS-only) and 37 male (13 0.3 mA, 14, 1 mA, 10 CS-only) rats were included in the DHL analyses. Fig. 4B shows a representative image of cFos DAB staining in the DHL. A two-way ANOVA revealed that there were no significant effects of shock intensity (F(1,52) = 0.085, p = 0.77), or sex on cFos expression, although females trended towards more cFos expression, regardless of shock intensity (F(1,52) = 3.161, p = 0.08) (Fig. 4C). When females were separated by Darter identity, a two-way ANOVA revealed no effect of Darter identity (F(1,25) = 0.379, p = 0.53) or shock (F(1,25) = 0.174, p = 0.82) (Fig. 4D), suggesting that the potential increase in cFos expression seen in females is not due to any differences between Darters and Non-darters in the DHL.
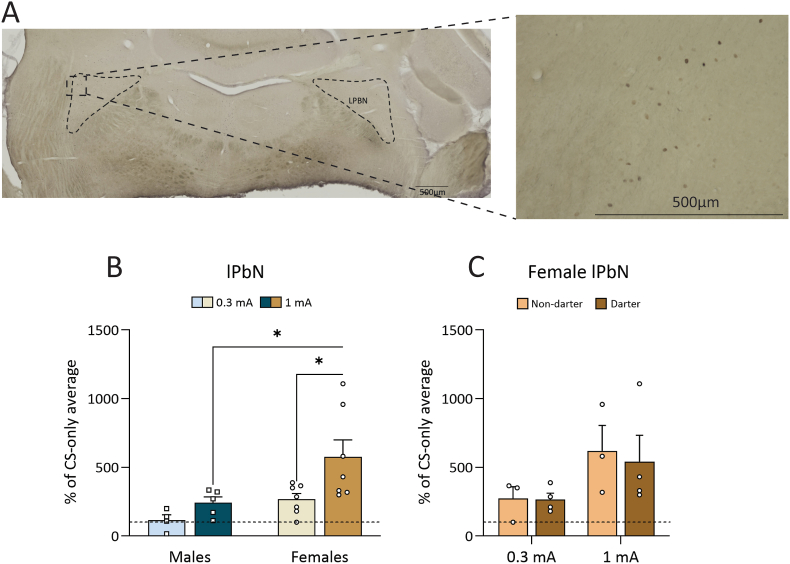
3.3.2.2. cFos expression in the lPbN is sex and shock intensity-dependent
Twenty-two female (7 0.3 mA, 8 1 mA, 7 CS-only) and 23 male (7 0.3 mA, 8 1 mA, 8 CS-only) rats were included in the lPbN analyses. Fig. 5A shows a representative image of cFos DAB staining in the lPbN. A two-way ANOVA revealed a significant main effect of shock intensity (F(1,19) = 6.199, p = 0.022), and Sidak's multiple comparisons post hoc test revealed that this was driven by females' heightened expression in the 1 mA group compared to the 0.3 mA group (p = 0.011) (Fig. 5B). There was also a significant main effect of sex on cFos expression (F(1,19) = 7.843, p = 0.011) and Sidak's multiple comparisons post hoc test revealed that this was driven by the 1 mA group, where females showed significantly more expression than males (p = 0.011) (Fig. 6B). When females were grouped by Darter identity, a two-way ANOVA showed that the effect of shock intensity almost reached significance (F(1,10) = 4.653, p = 0.056), but there was no effect of Darter identity (F(1,10) = 0.082, p = 0.78) (Fig. 5C). The increase in cFos expression in the 1 mA females compared to the 0.3 mA females was not due to Darter identity.
Fig. 5.
cFos activity in the lateral parabrachial nucleus following fear conditioning. A. Representative image of the lPbN following staining. B. There was as significant effect of shock in females only, and a significant effect of sex, driven by the 1 mA animals. C. There were no differences in cFos expression across Darter identity. Dotted line indicates CS-only animal cFos levels. (∗) p < 0.05. Dotted line indicates CS-only animal cFos levels.
Fig. 6.
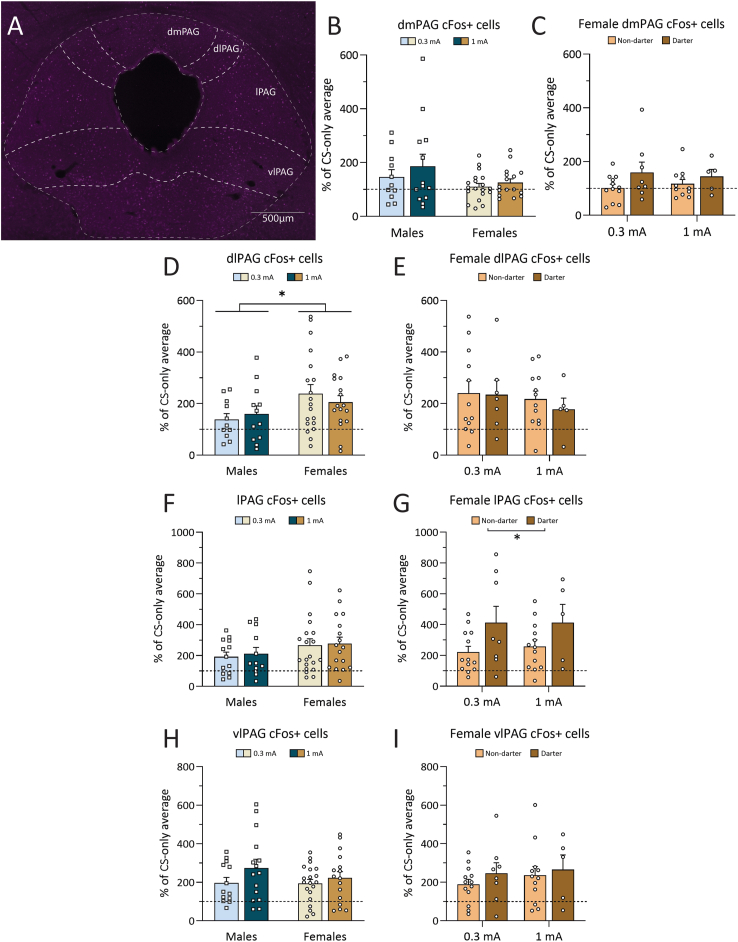
cFos analyses from the columns of the periaqueductal gray following fear conditioning. A. Representative image of the immunofluorescent staining for cFos in the PAG. Image has been edited for this figure to make cells more visible to the naked eye. B-C. cfos + cells in the dmPAG, by sex and for females only by Darter identity. No differences were found between any groups. D. There was a significant effect of sex in the dlPAG: Females had more cFos expression than males. E. There was no significant effect of shock or Darter identity in the dlPAG. F. No sex or shock effect was found in the lPAG. G. There was a significant effect of Darter identity in the lPAG: Darters showed increased cFos + cells in the lPAG compared to Non-darters, regardless of shock intensity. H. There was no effect of shock or sex in the vlPAG. I. There was no effect of Darter identity in the vlPAG. (∗) p < 0.05. Dotted line indicates CS-only animal cFos levels.
3.3.2.3. cFos expression in the PAG following fear conditioning varies depending on column
Fig. 6A is a representative image of each column of the PAG. Fifty-one female (24 0.3 mA, 19 1 mA, 8 CS-only) and 35 male (13 0.3 mA, 14, 1 mA, 8 CS-only) rats were included in the PAG analyses. The PAG was analyzed based on sub columns, as each column has unique roles in pain and fear responding (Deng et al., 2016; Keay and Bandler, 2015; Reis et al., 2023; Vianna et al., 2001; Watson et al., 2016).
A two-way ANOVA showed no effect of shock intensity (F(1,55) = 1.193, p = 0.28) in the dorsomedial (dmPAG), but there was a trending effect of sex (F(1,55) = 3.639, p = 0.062), with males appearing to have higher cFos expression than females (Fig. 6B). A two-way ANOVA showed no effect of Darter identity in females (F(1,32) = 0.0005, p = 0.98) (Fig. 6C). In the dorsolateral (dlPAG), a two-way ANOVA showed no significant effect of shock intensity (F(1,55) = 0.024, p = 0.85), but there was a significant effect of sex (F(1,55) = 5.047, p = 0.029) and Sidak's multiple comparisons post hoc test revealed a trending effect for females in the 0.3 mA group compared to males in the 0.3 mA group (p = 0.067) (Fig. 6D). When females were split by Darter identity, a two-way ANOVA showed no effect of Darter identity (F(1, 32) = 0.670, p = 0.42) (Fig. 6E). These results from the dorsal columns of the PAG suggest that the dmPAG is not heavily recruited by either sex during fear conditioning and that the dlPAG is recruited in females, but not in males.
A two-way ANOVA revealed that there was no significant effect of shock intensity in the lPAG (F(1,58) = 0.144, p = 0.70), or of sex F(1,58) = 2.74, p = 0.10), (Fig. 6F). When separated by Darter identity, a two-way ANOVA showed that there was a significant effect of Darter identity on cFos expression (F(1,35) = 6.236, p = 0.02). This failed to reach significance in post-hoc analyses, but 0.3 mA Darters trended to have more expression than their Non-darter counterparts, regardless of shock intensity (p = 0.08) (Fig. 6G). Overall, this suggests that recruitment of the lPAG is higher in Darters compared to Non-darters. No differences were found across sexes or Darter identity in the vlPAG. As analyzed in a two-way ANOVA, the vlPAG did not show a significant effect of shock intensity (F(1,60) = 2.69, p = 0.11) or sex (F(1,60) = 0.711, p = 0.40) (Fig. 6H). No effect was found when females were analyzed by Darter identity via two-way ANOVA (F(1,34) = 0.484, p > 0.05) (Fig. 6I). It is important to note that cFos expression in the dlPAG, lPAG and vlPAG in males and females at both shock intensities was well above the CS-only mean (dotted lines on all graphs).
3.3.2.4. cFos expression in the lateral, central and basolateral amygdala varies based on shock intensity and Darter identity
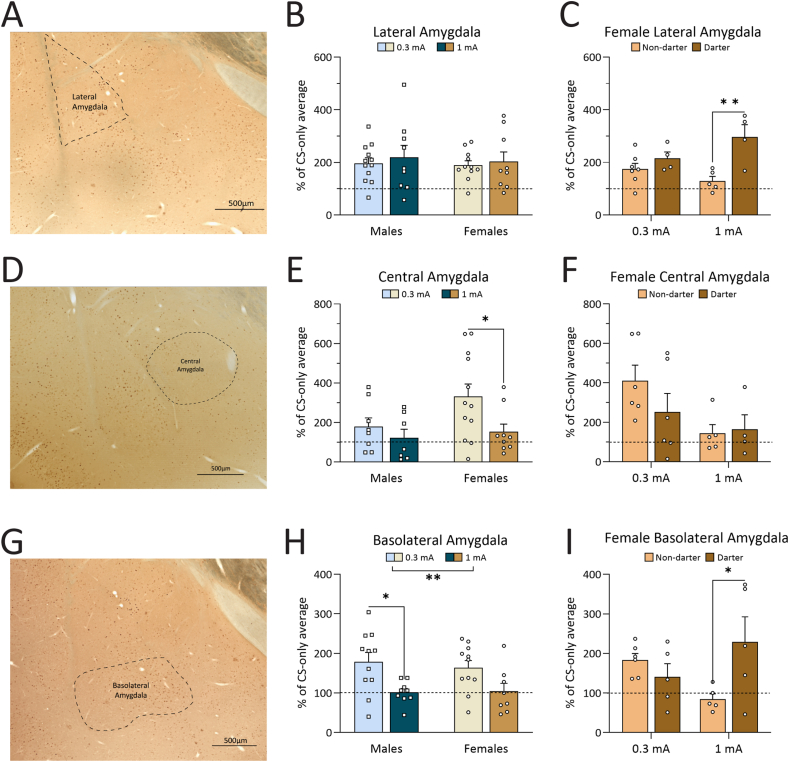
Thirty-four female (14 0.3 mA, 11 1 mA, 9 CS-only) and 37 male (13 0.3 mA, 14, 1 mA, 10 CS-only) rats were included in the amygdala analyses. Fig. 7A is a representative image of DAB cFos staining in the LA. A two-way ANOVA revealed no significant effect of shock intensity (F(1,37) = 0.378, p = 0.54) or sex (F(1,37) = 0.154, p = 0.70) (Fig. 7B). When females were separated by Darter identity, there was a significant shock intensity x Darter identity interaction (F(1,16) = 5.06, p = 0.04) and significant main effect of Darter identity (F(1,16) = 13.69, p = 0.002) (Fig. 7C). Sidak's multiple comparisons post-hoc test revealed that Darters had higher cFos expression than Non-darters in the 1 mA condition (p = 0.002).
Fig. 7.
cFos analyses from the lateral, central, and basolateral amygdala. A. Representative image of DAB cFos staining in the lateral amygdala. −2.76 from Bregma. B. There was no main effect of shock intensity or sex in the lateral amygdala. C. There was a significant effect of shock intensity and a significant interaction between Darter identity and shock intensity in females: At higher shock intensities, female Darters had more cFos expression in the lateral amygdala than Non-darters. D. Representative image of DAB cFos staining in the central amygdala. −2.40 from Bregma. E. No effects of shock intensity or sex were found in the central amygdala. F. There was a significant shock x Darter identity interaction in females: Female Non-darters had significantly less cFos expression in the central amygdala at higher shock intensities; Darter did not follow this pattern. G. Representative image of DAB cFos staining in the basolateral amygdala. −3.00 from Bregma. H. There was a significant effect of shock intensity in the BLA, although post-hoc tests did not reach significance. I. There was a significant shock x Darter identity interaction in females: Non-darters decreased cFos expression at higher shock intensities while Darters increased. (∗) p < 0.05, (∗∗) p < 0.01, (∗∗∗) p < 0.001. Dotted line indicates CS-only animal cFos levels.
Fig. 7D is a representative image of DAB cFos staining in the CeA. A 2-way ANOVA revealed a significant main effect of shock intensity (F(1,32) = 4.651, p = 0.039), and Sidak's multiple comparison tests revealed that the 0.3 mA females had significantly more expression than the 1 mA females (p = 0.033). There was no significant effect of sex on cFos expression (F(1,32) = 3.866, p = 0.10) (Fig. 7E). When females were separated by Darter identity there was a significant effect of shock intensity (F(1,17) = 4.95, p = 0.0399) (Fig. 7F). Sidak's multiple comparisons test revealed that Female Non-darters were just shy of having significantly more cFos expression at 0.3 mA than 1 mA (p = 0.051), and Darters did not differ in their expression (p = 0.70).
Fig. 7G is a representative image of the BLA with DAB cFos staining. A two-way ANOVA revealed no significant main effect of sex (F(1,35) = 0.09995, p = 0.75), but a significant main effect of shock intensity (F(1,35) = 12.58, p = 0.001) (Fig. 7H), with both males and Non-darter females showing increased cFos expression in response to 0.3 mA shocks. Sidak's multiple comparisons revealed that 0.3 mA males had significantly more cFos expression than 1 mA males (p = 0.014) and the same effect was trending in females (p = 0.075). When females were separated by Darter identity, a significant shock intensity x Darter identity interaction was found (F(1,17) = 6.87, p = 0.018) (Fig. 7I). Sidak's multiple comparisons post hoc test revealed that, similar to the LA, Darters had higher cFos expression than Non-darters at 1 mA (p = 0.02).
4. Discussion
The purpose of these studies was to determine if Darters exhibited increased responding and sensitivity to aversive stimuli, and if this could explain Darters’ heightened unconditioned responses. We compared CORT levels following fear conditioning, behavioral responding in the acoustic startle test, paw withdrawal latency in the hot plate test, and fear conditioning-induced brain activity across Darters, Non-darters, and male rats. Overall, we found that potential differences in glucocorticoid response during fear conditioning, startle reactivity, and thermal pain sensitivity do not explain the heightened shock response found in Darters compared to Non-darters and males, but examination of differences in cFos expression in regions related to pain and fear processing identified promising areas of future research.
Experiments 1 and 2 found that Darters do not have higher CORT responses to fear conditioning than Non-Darters, and that their propensity for escape-like movements during fear conditioning does not translate to an increased startle amplitude. In fact, it appears that Darters are slower to startle and have a lower startle amplitude than Non-darters and males. Taken together, these results suggest that Darters’ choice of escape-like movement during fear conditioning is not due to heightened glucocorticoid responses or exaggerated acoustic startle response compared to Non-darters.
Experiment 3 furthers our understanding of the relationship between conditioned responses and aversive stimuli and asked if thermal pain was predictive of behavior during fear conditioning. The hot plate test is typically used as a measurement of thermal pain sensitivity in rodents (Espejo and Mir, 1993; Gunn et al., 2011). We replicated previously found results that males have quicker latency to withdraw hind paw than females, (Gunn et al., 2011). Results from the hot plate test also revealed that there might be some differences in sensitivity between fore paw and hind paw in Darters compared to males and Non-darters. Although fore paw withdrawal has been used to indicate pain sensitivity (Espejo and Mir, 1993), it is not as common as hind paw withdrawal due to the other behaviors a rodent engages in that require the removal of the front paws from the hot plate (e.g. rearing, exploring) (Espejo and Mir, 1993). We chose to examine both fore and hind paw withdrawal because we noticed that some animals withdrew their hind paw first, while others withdrew their fore paw first. We included this data because of the striking differences between Darters, Non-darters and males. Future studies should examine how the fore and hind paws differentially respond to aversive stimuli, and what this difference between groups might indicate about aversive stimuli responding. It is important to note that the hot plate produces thermal pain, and the foot shock does not, so relationships between behaviors on these assays should be interpreted as exploring individual differences between Darters, Non-darters and males across a range of aversive stimuli, and not as a general indication of Darters' pain sensitivity. To gain a deeper understanding of the relationship between Darter identity and pain sensitivity, future studies could compare behavior on fear conditioning to behaviors on other commonly used measurements of pain sensitivity, like the Von Frey test examining mechanical sensitivity (Deuis et al., 2017; Minett et al., 2011; Modi et al., 2023), Complete Freund's Adjuvant for inflammatory pain (Minett et al., 2011; Yu et al., 2021), or in models of chronic pain (Gupta et al., 2017; Kuner and Kuner, 2021; Minett et al., 2011; Raver et al., 2020).
The dorsal horn of the lumbar spine is the primary site of nociceptive input (Coghill et al., 1991; D'Mello and Dickenson, 2008; Wang et al., 2022) and the lack of significant cFos activity based on shock, sex, or Darter identity at the level of the DHL suggests that the fear conditioning paradigm used in this study was not sufficient to activate the canonical peripheral pain pathways beginning at the DHL (Millan, 2002; Westlund and Willis, 2015). This might be due to experimental limitations rather than a true lack of effect. Although cFos in the spinal cord increases following other models on pain induction, such as neuropathy and sciatic nerve stimulation (Bojovic et al., 2015, 2016; Jongen et al., 2005; Landry et al., 2006), it is possible that other immediate early genes (e.g. Arc) are more responsive to an acute stimulus such as a foot shock. The foot shock used in this experiment is 0.5 s in duration and each animal is exposed to the shock a total of seven times, which might be too transient of a stimulus to activate cFos expression in the spinal cord. The DHL responds to noxious stimuli (D'Mello and Dickenson, 2008; Watkins et al., 1984), defined as anything potentially damaging or harmful to tissue (Todd, 2010). The shock intensities used in this experiment therefore might not be sufficiently noxious to activate the DHL neurons to protect the limbs from tissue damage. In contrast, the shock intensity effect in the lPbN in females suggests that this region can detect foot shock intensity and that the effect increases as shock intensity increases, but that its activity is not dependent on or responsible for differences seen between female Darters and Non-darters. These results suggest that the DHL is not involved in processing the foot shock, but the primary supraspinal target of nociceptive transmission from the DHL, the lPbN, (Chiang et al., 2019) might be.
The PAG plays a critical role in pain and threat/fear processing. Following fear conditioning, others have shown that each column of the PAG shows an increase in cFos expression (Carrive et al., 1997). Even though the role of the vlPAG in conditioned freezing behavior is thought to be well-established (De Oca et al., 1998; Keay and Bandler, 2015; Liu et al., 2022; Reis et al., 2023; Vianna et al., 2001; T. C. Watson et al., 2016), we did not find that 1 mA animals had more cFos expression than 0.3 mA animals, even though our 1 mA animals froze more than our 0.3 mA animals (Fig. 3F and G). It is important to note that both 0.3 mA and 1 mA shock intensity groups did have higher levels of cFos than CS-only baseline (dotted line in Fig. 6H), so perhaps the vlPAG simply was not able to differentiate between shock intensities. Somewhat surprisingly, the dmPAG and dlPAG showed little to no differences in expression in response to different shock intensities, across sexes, or across Darter identity. The dmPAG and dlPAG are known to be involved in escape-like responses (Deng et al., 2016; Kim et al., 2013; Lefler et al., 2020; Reis et al., 2021; Vianna et al., 2001; Watson et al., 2016), so we expected to see differences between Darter identity groups in these areas. Although, because of the dmPAG's known role in pain processing (Butler et al., 2011; Linnman et al., 2012), its lack of involvement further suggests that pain from the foot shock is not a motivating driver of the differences seen between males and females and Darters and Non-darters during fear conditioning. The lack of effect found here might also be due to our choice of slice (−8.04 from bregma) for cFos analysis. The PAG in the rat is known to have different functions depending on the rostral/caudal location (Depaulis et al., 1992; Keay and Bandler, 2015). Regions more rostral or caudal to the bregma point that we chose might have more of an influence. Indeed, many papers do not list their bregma points used for immunohistochemistry procedures (Canteras and Goto, 1999; Comoli et al., 2003; Samineni et al., 2017), or chose sections that were either rostral (De Oca et al., 1998) or caudal (de Mello Rosa et al., 2022) to ours, or included a range of vlPAG coordinates (Borelli et al., 2005). We chose this point because it is close to the anatomical midpoint of the PAG (Loyd and Murphy, 2009), and because of previous studies' use of this bregma point when investigating pain (Loyd et al., 2007) and fear responses (de Andrade Rufino et al., 2019; Vianna et al., 2001; Wright and McDannald, 2019). The most intriguing and promising column for follow-up studies is the lPAG, in which we observed greater activity in Darters compared to Non-darters. The lPAG is associated with escape and flight behavior, and, in particular, post-encounter and circa-strike defensive behavior, both of which are evasive threat responses (Motta et al., 2017; Zhang et al., 2024). Because of the lPAG's role in escape behavior in response to threats, it is perhaps unsurprising that we see an effect of conditioned response here, suggesting it may be involved in driving darting as a conditioned response. Higher intensity stimuli have been shown to evoke escape-like responses (Bolles and Fanselow, 1980). It is possible that Darters find the shock to be more aversive or threatening, but not necessarily more painful, which leads to their heightened shock response and escape-like response and therefore heightened cFos expression in the lPAG. Further research into the role of conditioned darting and the lPAG is needed.
Connections between the LA, CeA and BLA are critical for processing both pain and fear and their behavioral expressions (Almeida et al., 2004; Hogri et al., 2022a; Lindsay et al., 2021; Peirs and Seal, 2016). Only the BLA showed a significant effect of shock intensity, highlighting its role in learning the CS-US association and in the processing of aversive stimuli. We would expect more cFos expression following exposure to a 1 mA shock because of the increase in shock intensity and resulting increase in freezing, but this was not the case. This suggests that there are other regions responsible for interpreting and communicating the aversive nature of increasing foot shock. In the LA and BLA, there was an increase in cFos expression in the 1 mA female Darters compared to the 1 mA female Non-darters. The LA is involved in the auditory processes involved in formation of the CS-US pairing (Janak and Tye, 2015) and the BLA contains a distinct neural assembly that encodes the negative affective valence of pain (Corder et al., 2019). This supports the hypothesis that Darters might find the foot shock more aversive, leading to a greater recruitment of these areas critical for fear learning and processing the affective side of aversive stimuli. If the stimulus is interpreted as more aversive, Darters might be more motivated to avoid future exposure to it. These results highlight the differential recruitment of brain regions that may lead to similar behavioral outcomes in males and female Non-darters.
One aspect of fear conditioning that this study did not examine was fear-conditioned analgesia. Animals who express conditioned freezing are hypothesized to experience fear-conditioned analgesia, characterized by a reduction in pain sensitivity (Fanselow, 1984). Females do not show the same fear-conditioned analgesia as males, with studies either finding less fear-conditioned analgesia or none in females (Llorente-Berzal et al., 2022; Stock et al., 2001). Darters could represent a subset of females in which fear-induced analgesia wholly fails, resulting in the interpretation of the foot shock as more painful and subsequent heightened shock responses. Future studies will examine this possibility.
A potential limitation of this study is the use of cFos as a proxy for neural activity. Although cFos is widely used and accepted as an indicator of neuronal activation (Bullitt, 1990), it is not temporally specific. As a result, we might be unable to detect significant differences across the sexes, Darter identities, and shock intensities. It is possible that some regions are more active earlier in the fear conditioning paradigm than other regions (such as the LA, involved in learning the auditory CS-US association), and that is why we do not see significant effects of shock intensity. Although methods such as fiber photometry would allow more specific mapping of activation, the methods used in this study allowed us to examine a broad range of regions and networks involved in fear conditioning within individual animals. In addition, the use of cFos did not allow us to specify what types of cells are being activated during fear conditioning. Each of the regions examined are heterogenous with respect to cell-type specificity (Chiang et al., 2020; Ge et al., 2022; Keay and Bandler, 2015; McPherson and Ingram, 2022; Swanson and Petrovich, 1998; Watanabe et al., 2017), and we look forward to addressing the question of cell-type specific activation of these regions in future studies.
5. Conclusions
This work furthers our understanding about the differences between Darters, Non-darters and males. The overall results from this research allow us to conclude that Darters are not overall simply more sensitive to aversive stimuli, nor that hyperarousal is what leads to the escape-like conditioned responses and heightened unconditioned responses. This adds to our previous body of work, identifying that the sex-biased nature of darting is not dependent on the estrous cycle (Gruene et al., 2015) or weight of the animals (Gruene et al., 2015; Mitchell et al., 2022). This research further emphasizes the importance of looking beyond freezing as a conditioned response (Chu et al., 2024), especially when female subjects are included. It provides novel insights into the regions that are activated and involved in conditioned fear responses, as well as an understanding of how an animal's conditioned fear responses might or might not be indicative of their behavior in response to other stimuli. Future studies should explicitly investigate other fear responses aside from freezing, as well as the circuits underlying conditioned and unconditioned fear responses.
Funding sources
This work was supported by NIH grant R01-MH123803 awarded to RMS.
CRediT authorship contribution statement
Julia R. Mitchell: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Lindsay Vincelette: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Samantha Tuberman: Visualization, Investigation, Data curation. Vivika Sheppard: Investigation, Data curation. Emmett Bergeron: Investigation, Data curation. Roberto Calitri: Validation, Supervision. Rose Clark: Investigation, Data curation. Caitlyn Cody: Investigation, Formal analysis, Data curation. Akshara Kannan: Investigation, Data curation. Jack Keith: Investigation, Data curation. Abigail Parakoyi: Investigation, Data curation. MaryClare Pikus: Investigation. Victoria Vance: Investigation, Data curation. Leena Ziane: Investigation, Data curation. Heather Brenhouse: Supervision, Resources, Conceptualization. Mikaela A. Laine: Writing – review & editing, Supervision, Formal analysis, Conceptualization. Rebecca M. Shansky: Writing – review & editing, Supervision, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We extend our deepest gratitude to Eliza Greiner for assistance in editing this manuscript and with statistical analyses, and to Larry Deng and Esther Ofielu for helping with Experiment 1. We also extend our thanks to Anne Murphy (GSU) for help with the spinal cord and PAG portion of Experiment 3. Experimental design figures were created using BioRender.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2024.100675.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Almeida T.F., Roizenblatt S., Tufik S. Afferent pain pathways: a neuroanatomical review. Brain Res. 2004;1000(1–2):40–56. doi: 10.1016/j.brainres.2003.10.073. [DOI] [PubMed] [Google Scholar]
- Bartley E.J., Fillingim R.B. Sex differences in pain: a brief review of clinical and experimental findings. Br. J. Anaesth. 2013;111(1):52–58. doi: 10.1093/bja/aet127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch E.E. 2012. Periaqueductal Gray an Interface for Behavioral Control. [DOI] [PubMed] [Google Scholar]
- Berkley K.J. Sex differences in pain. Behav. Brain Sci. 1997;20(3):371–380. doi: 10.1017/S0140525X97221485. [DOI] [PubMed] [Google Scholar]
- Blanton H.L., Barnes R.C., McHann M.C., Bilbrey J.A., Wilkerson J.L., Guindon J. Sex differences and the endocannabinoid system in pain. Pharmacol. Biochem. Behav. 2021;(August 2020):202. doi: 10.1016/j.pbb.2021.173107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojovic O., Bramham C.R., Tjølsen A. Stimulation-induced expression of immediate early gene proteins in the dorsal horn is increased in neuropathy. Scandinavian Journal of Pain. 2016;10(1):43–51. doi: 10.1016/j.sjpain.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Bojovic O., Panja D., Bittins M., Bramham C.R., Tjølsen A. Time course of immediate early gene protein expression in the spinal cord following conditioning stimulation of the sciatic nerve in rats. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0123604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles R.C., Fanselow M.S. A perceptual-defensive-recuperative model of fear and pain. Behav. Brain Sci. 1980;3(2):291–301. doi: 10.1017/S0140525X0000491X. [DOI] [Google Scholar]
- Borelli K.G., Ferreira-Netto C., Coimbra N.C., Brandão M.L. Fos-like immunoreactivity in the brain associated with freezing or escape induced by inhibition of either glutamic acid decarboxylase or GABAA receptors in the dorsal periaqueductal gray. Brain Res. 2005;1051(1–2):100–111. doi: 10.1016/j.brainres.2005.05.068. [DOI] [PubMed] [Google Scholar]
- Borkar C.D., Dorofeikova M., Le Q.S.E., Vutukuri R., Vo C., Hereford D., Resendez A., Basavanhalli S., Sifnugel N., Fadok J.P. Sex differences in behavioral responses during a conditioned flight paradigm. Behav. Brain Res. 2020;389 doi: 10.1016/j.bbr.2020.112623. [DOI] [PubMed] [Google Scholar]
- Bullitt E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J. Comp. Neurol. 1990;296(4):517–530. doi: 10.1002/cne.902960402. [DOI] [PubMed] [Google Scholar]
- Butler R.K., Nilsson-Todd L., Cleren C., Léna I., Garcia R., Finn D.P. Molecular and electrophysiological changes in the prefrontal cortex-amygdala-dorsal periaqueductal grey pathway during persistent pain state and fear-conditioned analgesia. Physiol. Behav. 2011;104(5):1075–1081. doi: 10.1016/j.physbeh.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Canteras N.S., Goto M. Fos-like immunoreactivity in the periaqueductal gray of rats exposed to a natural predator. Neuroreport. 1999;10(2):413–418. doi: 10.1097/00001756-199902050-00037. [DOI] [PubMed] [Google Scholar]
- Carrive P., Leung P., Harris J., Paxinos G. Conditioned fear to context is associated with increased Fos expression in the caudal ventrolateral region of the midbrain periaqueductal gray. Neuroscience. 1997;78(1):165–177. doi: 10.1016/S0306-4522(97)83047-3. [DOI] [PubMed] [Google Scholar]
- Carter R.B. Differentiating analgesic and non-analgesic drug activities on rat hot plate: effect of behavioral endpoint. Pain. 1991;47 doi: 10.1016/0304-3959(91)90207-E. [DOI] [PubMed] [Google Scholar]
- Chiang M.C., Bowen A., Schier L.A., Tupone D., Uddin O., Heinricher M.M. Parabrachial complex: a hub for pain and aversion. J. Neurosci. 2019;39(42):8225–8230. doi: 10.1523/JNEUROSCI.1162-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang M.C., Nguyen E.K., Canto-Bustos M., Papale A.E., Oswald A.M.M., Ross S.E. Divergent neural pathways emanating from the lateral parabrachial nucleus mediate distinct components of the pain response. Neuron. 2020;106(6):927–939.e5. doi: 10.1016/j.neuron.2020.03.014. [DOI] [PubMed] [Google Scholar]
- Choi S., Hachisuka J., Brett M.A., Magee A.R., Omori Y., Iqbal N., ul A., Zhang D., DeLisle M.M., Wolfson R.L., Bai L., Santiago C., Gong S., Goulding M., Heintz N., Koerber H.R., Ross S.E., Ginty D.D. Parallel ascending spinal pathways for affective touch and pain. Nature. 2020;587(7833):258–263. doi: 10.1038/s41586-020-2860-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu A., Gordon N.T., DuBois A.M., Michel C.B., Hanrahan K.E., Williams D.C., Anzellotti S., McDannald M.A. A fear conditioned cue orchestrates a suite of behaviors in rats. Elife. 2024;13 doi: 10.7554/eLife.82497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S., Herry C., Grenier F., Wolff S.B.E., Letzkus J.J., Vlachos I., Ehrlich I., Sprengel R., Deisseroth K., Stadler M.B., Müller C., Lüthi A. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468(7321):277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Coghill R.C. The distributed nociceptive system: a framework for understanding pain. Trends Neurosci. 2020;43(10):780–794. doi: 10.1016/j.tins.2020.07.004. Elsevier Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill R.C., Price D.D., Hayes R.L., Mayer D.J. Spatial distribution of nociceptive processing in the rat spinal cord. JOURNALOF NEUROPHYSIOLOGY. 1991;65(Issue 1) doi: 10.1152/jn.1991.65.1.133. [DOI] [PubMed] [Google Scholar]
- Colom-Lapetina J., Li A.J., Pelegrina-Perez T.C., Shansky R.M. Behavioral diversity across classic rodent models is sex-dependent. Front. Behav. Neurosci. 2019;13(March):1–8. doi: 10.3389/fnbeh.2019.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoli E., Ribeiro-Barbosa E.R., Canteras N.S. Predatory hunting and exposure to a live predator induce opposite patterns of Fos immunoreactivity in the PAG. Behav. Brain Res. 2003;138(1):17–28. doi: 10.1016/S0166-4328(02)00197-3. [DOI] [PubMed] [Google Scholar]
- Corder G., Ahanonu B., Grewe B.F., Wang D., Schnitzer M.J., Scherrer G. An amygdalar neural ensemble that encodes the unpleasantness of pain. Science. 2019;363(6424):276–281. doi: 10.1126/science.aap8586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Andrade Rufino R., Mota-Ortiz S.R., De Lima M.A.X., Baldo M.V.C., Canteras N.S. The rostrodorsal periaqueductal gray influences both innate fear responses and acquisition of fear memory in animals exposed to a live predator. Brain Struct. Funct. 2019;224(4):1537–1551. doi: 10.1007/s00429-019-01852-6. [DOI] [PubMed] [Google Scholar]
- de Mello Rosa G.H., Ullah F., de Paiva Y.B., da Silva J.A., Branco L.G.S., Corrado A.P., Medeiros P., Coimbra N.C., Franceschi Biagioni A. Ventrolateral periaqueductal gray matter integrative system of defense and antinociception. Pflueg. Arch. Eur. J. Physiol. 2022;474(4):469–480. doi: 10.1007/s00424-022-02672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Oca B.M., DeCola J.P., Maren S., Fanselow M.S. Distinct regions of the periaqueductal gray are involved in the acquisition and expression of defensive responses. J. Neurosci. 1998;18(9):3426–3432. doi: 10.1523/jneurosci.18-09-03426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H., Xiao X., Wang Z. Periaqueductal gray neuronal activities underlie different aspects of defensive behaviors. J. Neurosci. 2016;36(29):7580–7588. doi: 10.1523/JNEUROSCI.4425-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depaulis A., Keay KevinA., Bandler R. Longitudinal neuronal organization of defensive reactions in the midbrain periaqueductal gray region of the rat. Exp. Brain Res. 1992;90(2) doi: 10.1007/BF00227243. [DOI] [PubMed] [Google Scholar]
- Deuis J.R., Dvorakova L.S., Vetter I. Methods used to evaluate pain behaviors in rodents. Front. Mol. Neurosci. 2017;10 doi: 10.3389/fnmol.2017.00284. Frontiers Media S.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Mello R., Dickenson A.H. Spinal cord mechanisms of pain. Br. J. Anaesth. 2008;101(1):8–16. doi: 10.1093/bja/aen088. Oxford University Press. [DOI] [PubMed] [Google Scholar]
- Espejo E.F., Mir D. Structure of the rat's behaviour in the hot plate test. Behav. Brain Res. 1993;56:171–176. doi: 10.1016/0166-4328(93)90035-o. [DOI] [PubMed] [Google Scholar]
- Fanselow M. What is conditioned fear? Trends Neurosci. 1984;7(12):460–462. doi: 10.1016/S0166-2236(84)80253-2. [DOI] [Google Scholar]
- Fillingim R.B. Sex-related influences on pain: a review of mechanisms and clinical implications. Rehabil. Psychol. 2003;48(3):165–174. doi: 10.1037/0090-5550.48.3.165. [DOI] [Google Scholar]
- Fullerton E.F., Doyle H.H., Murphy A.Z. Impact of sex on pain and opioid analgesia: a review. Current Opinion in Behavioral Sciences. 2018;23:183–190. doi: 10.1016/j.cobeha.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale G.D., Anagnostaras S.G., Godsil B.P., Mitchell S., Nozawa T., Sage J.R., Wiltgen B., Fanselow M.S. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J. Neurosci. 2004;24(15):3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge J., Cai Y., Pan Z.Z. Synaptic plasticity in two cell types of central amygdala for regulation of emotion and pain. Front. Cell. Neurosci. 2022;16 doi: 10.3389/fncel.2022.997360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard-Tremblay L., Auclair V., Daigle K., Léonard G., Whittingstall K., Goffaux P. Sex differences in the neural representation of pain unpleasantness. J. Pain. 2014;15(8):867–877. doi: 10.1016/j.jpain.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Gore F., Schwartz E.C., Brangers B.C., Aladi S., Stujenske J.M., Likhtik E., Russo M.J., Gordon J.A., Salzman C.D., Axel R. Neural representations of unconditioned stimuli in basolateral amygdala mediate innate and learned responses. Cell. 2015;162(1):134–145. doi: 10.1016/j.cell.2015.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granata L., Parakoyi A., Brenhouse H.C. Age- and sex-specific effects of maternal separation on the acoustic startle reflex in rats: early baseline enhancement in females and blunted response to ambiguous threat. Front. Behav. Neurosci. 2022;16 doi: 10.3389/fnbeh.2022.1023513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregus A.M., Levine I.S., Eddinger K.A., Yaksh T.L., Buczynski M.W. Sex differences in neuroimmune and glial mechanisms of pain. Pain. 2021;162(8):2186–2200. doi: 10.1097/j.pain.0000000000002215. Lippincott Williams and Wilkins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner E.M., Müller I., Norris M.R., Ng K.H., Sangha S. Sex differences in fear regulation and reward-seeking behaviors in a fear-safety-reward discrimination task. Behav. Brain Res. 2019;368(April) doi: 10.1016/j.bbr.2019.111903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruene T.M., Flick K., Stefano A., Shea S.D., Shansky R.M. Sexually divergent expression of active and passive conditioned fear responses in rats. Elife. 2015;4:1–9. doi: 10.7554/elife.11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn A., Bobeck E.N., Weber C., Morgan M.M. The influence of non-nociceptive factors on hot-plate latency in rats. J. Pain. 2011;12(2):222–227. doi: 10.1016/j.jpain.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Mayer E.A., Fling C., Labus J.S., Naliboff B.D., Hong J.Y., Kilpatrick L.A. Sex-based differences in brain alterations across chronic pain conditions. J. Neurosci. Res. 2017;95(Issues 1–2):604–616. doi: 10.1002/jnr.23856. John Wiley and Sons Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogri R., Teuchmann H.L., Heinke B., Holzinger R., Trofimova L., Sandkühler J. GABAergic CaMKIIa1 amygdala output attenuates pain and modulates emotional-motivational behavior via parabrachial inhibition. J. Neurosci. 2022;42(27):5373–5388. doi: 10.1523/JNEUROSCI.2067-21.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak P.H., Tye K.M. From circuits to behaviour in the amygdala. Nature. 2015;517(7534):284–292. doi: 10.1038/nature14188. Nature Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston A.L., File S.E. Sex differences in animal tests of anxiety. Physiol. Behav. 1991;49(2):245–250. doi: 10.1016/0031-9384(91)90039-Q. [DOI] [PubMed] [Google Scholar]
- Jongen J.L.M., Haasdijk E.D., Sabel-Goedknegt H., van der Burg J., Vecht Ch J., Holstege J.C. Intrathecal injection of GDNF and BDNF induces immediate early gene expression in rat spinal dorsal horn. Exp. Neurol. 2005;194(1):255–266. doi: 10.1016/j.expneurol.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Keay K.A., Bandler R. The Rat Nervous System. fourth ed. 2015. Periaqueductal gray; pp. 207–221. i. [DOI] [Google Scholar]
- Kim E.J., Horovitz O., Pellman B.A., Tan L.M., Li Q., Richter-Levin G., Kim J.J. Dorsal periaqueductal gray-amygdala pathway conveys both innate and learned fear responses in rats. Proc. Natl. Acad. Sci. U.S.A. 2013;110(36):14795–14800. doi: 10.1073/pnas.1310845110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokras N., Dalla C. Sex differences in animal models of psychiatric disorders. Br. J. Pharmacol. 2014;171(20):4595–4619. doi: 10.1111/bph.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner R., Kuner T. Cellular circuits in the brain and their modulation in acute and chronic pain. Physiol. Rev. 2021;101(1):213–258. doi: 10.1152/physrev.00040.2019. [DOI] [PubMed] [Google Scholar]
- Laine M.A., Mitchell J.R., Rhyner J., Clark R., Kannan A., Keith J., Pikus M.C., Bergeron E., Ravaglia I., Ulgenturk E., Shinde A., Shansky R.M. Sounding the alarm: sex differences in rat ultrasonic vocalizations during Pavlovian fear conditioning and extinction. ENeuro. 2022;9(6):1–48. doi: 10.1523/ENEURO.0382-22.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry E.S., Rouillard C., Levesque D., Guertin P.A. Profile of immediate early gene expression in the lumbar spinal cord of low-thoracic paraplegic mice. Behav. Neurosci. 2006;120(6):1384–1388. doi: 10.1037/0735-7044.120.6.1384. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23(1):155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lefler Y., Campagner D., Branco T. The role of the periaqueductal gray in escape behavior. Curr. Opin. Neurobiol. 2020;60:115–121. doi: 10.1016/j.conb.2019.11.014. [DOI] [PubMed] [Google Scholar]
- Lindsay N.M., Chen C., Gilam G., Mackey S., Scherrer G. Brain circuits for pain and its treatment. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abj7360. https://www.science.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C., Beucke J.C., Jensen K.B., Gollub R.L., Kong J. Sex similarities and differences in pain-related periaqueductal gray connectivity. Pain. 2012;153(2):444–454. doi: 10.1016/j.pain.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Li S., Ren L., Liu X., Li X., Wang Z. Different coding characteristics between flight and freezing in dorsal periaqueductal gray of mice during exposure to innate threats. Animal Models and Experimental Medicine. 2022;5(6):491–501. doi: 10.1002/ame2.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente-Berzal A., McGowan F., Gaspar J.C., Rea K., Roche M., Finn D.P. Sexually dimorphic expression of fear-conditioned analgesia in rats and associated alterations in the endocannabinoid system in the periaqueductal grey. Neuroscience. 2022;480:117–130. doi: 10.1016/j.neuroscience.2021.11.005. [DOI] [PubMed] [Google Scholar]
- Lopez-Aumatell R., Guitart-Masip M., Vicens-Costa E., Gimenez-Llort L., Valdar W., Johannesson M., Flint J., Tobeña A., Fernandez-Teruel A. Fearfulness in a large N/Nih genetically heterogeneous rat stock: differential profiles of timidity and defensive flight in males and females. Behav. Brain Res. 2008;188(1):41–55. doi: 10.1016/j.bbr.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Loyd D.R., Morgan M.M., Murphy A.Z. Morphine preferentially activates the periaqueductal gray-rostral ventromedial medullary pathway in the male rat: a potential mechanism for sex differences in antinociception. Neuroscience. 2007;147(2):456–468. doi: 10.1016/j.neuroscience.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd D.R., Murphy A.Z. Vol. 2009. Hindawi Publishing Corporation; 2009. The role of the periaqueductal gray in the modulation of pain in males and females: are the anatomy and physiology really that different? (Neural Plasticity). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano Nieves G., Bravo M., Bath K.G. Early life adversity ablates sex differences in active versus passive threat responding in mice. Stress. 2023;26(1) doi: 10.1080/10253890.2023.2244598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S., Quirk G.J. Neuronal signalling of fear memory. Nat. Rev. Neurosci. 2004;5(11):844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- McPherson K.B., Ingram S.L. Vol. 16. Frontiers Media S.A; 2022. Cellular and circuit diversity determines the impact of endogenous opioids in the descending pain modulatory pathway. (Frontiers in Systems Neuroscience). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan M.J. Descending control of pain. Prog. Neurobiol. 2002;66 doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Minett M.S., Quick K., Wood J.N. Behavioral measures of pain thresholds. Curr. Protoc. Mol. Biol. 2011;1(3):383–412. doi: 10.1002/9780470942390.mo110116. [DOI] [PubMed] [Google Scholar]
- Mitchell J.R., Trettel S.G., Li A.J., Wasielewski S., Huckleberry K.A., Fanikos M., Golden E., Laine M.A., Shansky R.M. Darting across space and time: parametric modulators of sex-biased conditioned fear responses. Learn. Mem. 2022;29(7):171–180. doi: 10.1101/lm.053587.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi A.D., Parekh A., Pancholi Y.N. Vol. 446. Elsevier B.V; 2023. Evaluating pain behaviours: widely used mechanical and thermal methods in rodents. (Behavioural Brain Research). [DOI] [PubMed] [Google Scholar]
- Mogil J.S., Chanda M.L. The case for the inclusion of female subjects in basic science studies of pain. Pain. 2005;117(Issues 1–2):1–5. doi: 10.1016/j.pain.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Motta S.C., Carobrez A.P., Canteras N.S. Vol. 76. Elsevier Ltd; 2017. The periaqueductal gray and primal emotional processing critical to influence complex defensive responses, fear learning and reward seeking; pp. 39–47. (Neuroscience and Biobehavioral Reviews). [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. seventh ed. 2013. Paxinos and Watson's the Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- Peirs C., Seal R.P. Neural circuits for pain: recent advances and current views. Science. 2016;354(6312):578–584. doi: 10.1126/science.aaf8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellman B.A., Schuessler B.P., Tellakat M., Kim J.J. Sexually dimorphic risk mitigation strategies in rats. ENeuro. 2017;4(1):1–11. doi: 10.1523/ENEURO.0288-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusator D.K., Greenwood-Van Meerveld B. Amygdala-mediated mechanisms regulate visceral hypersensitivity in adult females following early life stress: importance of the glucocorticoid receptor and corticotropin-releasing factor. Pain. 2017;158(2):296–305. doi: 10.1097/j.pain.0000000000000759. [DOI] [PubMed] [Google Scholar]
- Raver C., Uddin O., Ji Y., Li Y., Cramer N., Jenne C., Morales M., Masri R., Keller A. An amygdalo-parabrachial pathway regulates pain perception and chronic pain. J. Neurosci. 2020;40(17):3424–3442. doi: 10.1523/JNEUROSCI.0075-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis F.M.C.V., Liu J., Schuette P.J., Lee J.Y., Maesta-Pereira S., Chakerian M., Wang W., Canteras N.S., Kao J.C., Adhikari A. Shared dorsal periaqueductal gray activation patterns during exposure to innate and conditioned threats. J. Neurosci. 2021;41(25):5399–5420. doi: 10.1523/JNEUROSCI.2450-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis F.M.C.V., Mobbs D., Canteras N.S., Adhikari A. Vol. 228. Elsevier Ltd; 2023. Orchestration of innate and conditioned defensive actions by the periaqueductal gray. (Neuropharmacology). [DOI] [PubMed] [Google Scholar]
- Samineni V.K., Grajales-Reyes J.G., Copits B.A., O'Brien D.E., Trigg S.L., Gomez A.M., Bruchas M.R., Gereau R.W. Divergent modulation of nociception by glutamatergic and GABAergic neuronal subpopulations in the periaqueductal gray. ENeuro. 2017;4(2) doi: 10.1523/ENEURO.0129-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock H.S., Caldarone B., Abrahamsen G., Mongeluzi D., Wilson M.A., Rosellini R.A. Sex differences in relation to conditioned fear-induced enhancement of morphine analgesia. Physiol. Behav. 2001;72(3):439–447. doi: 10.1016/S0031-9384(00)00426-1. [DOI] [PubMed] [Google Scholar]
- Swanson L.W., Petrovich G.D. What is the amygdala? Trends Neurosci. 1998;21(8):323–331. doi: 10.1016/S0166-2236(98)01265-X. [DOI] [PubMed] [Google Scholar]
- Todd A.J. Neuronal circuitry for pain processing in the dorsal horn. Nat. Rev. Neurosci. 2010;11(12):823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toufexis D.J., Lipatova O., Johnson A.C., Abizaid A. Food-restriction lowers the acoustic startle response in both male and female rats, and, in combination with acute ghrelin injection, abolishes the expression of fear-potentiated startle in male rats. J. Neuroendocrinol. 2016;28(11) doi: 10.1111/jne.12436. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A., Mcgaugh J.L. 1999. Basolateral Amygdala Is Involved in Modulating Consolidation of Memory for Classical Fear Conditioning. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna D.M.L., Landeira-Fernandez J., Brandão M.L. Dorsolateral and ventral regions of the periaqueductal gray matter are involved in distinct types of fear. Neurosci. Biobehav. Rev. 2001;25(7–8):711–719. doi: 10.1016/S0149-7634(01)00052-5. [DOI] [PubMed] [Google Scholar]
- Wang L.H., Ding W.Q., Sun Y.G. Spinal ascending pathways for somatosensory information processing. Trends Neurosci. 2022;45(8):594–607. doi: 10.1016/j.tins.2022.05.005. Elsevier Ltd. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Dymecki S.M., Chirila A.M., Springel M.W., Toliver A.A., Zimmerman A.L., Orefice L.L., Bai L., Song B.J., Bashista K.A., O'Neill T.G., Zhuo J., Tsan C., Hoynoski J., Ginty D.D. The cellular and synaptic architecture of the mechanosensory dorsal horn. Cell. 2017;168(1–2):295–310.e19. doi: 10.1016/j.cell.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins L.R., Johannessen J.N., Kinscheck I.B., Mayer D.J. The neurochemical basis of footshock analgesia: the role of spinal cord serotonin and norepinephrine. Brain Res. 1984;290(1):107–117. doi: 10.1016/0006-8993(84)90740-6. [DOI] [PubMed] [Google Scholar]
- Watson T.C., Cerminara N.L., Lumb B.M., Apps R. Neural correlates of fear in the periaqueductal gray. J. Neurosci. 2016;36(50):12707–12719. doi: 10.1523/JNEUROSCI.1100-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlund K.N., Willis W.D. The Rat Nervous System. fourth ed. 2015. Pain system; pp. 703–731. [DOI] [Google Scholar]
- Wiesenfeld-Hallin Z. 2005. Sex Differences in Pain Perception. [DOI] [PubMed] [Google Scholar]
- Wright K.M., McDannald M.A. Ventrolateral periaqueductal gray neurons prioritize threat probability over fear output. Elife. 2019;8:1–28. doi: 10.7554/eLife.45013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Pati D., Pina M.M., Schmidt K.T., Boyt K.M., Hunker A.C., Zweifel L.S., McElligott Z.A., Kash T.L. Periaqueductal gray/dorsal raphe dopamine neurons contribute to sex differences in pain-related behaviors. Neuron. 2021;109(8):1365–1380.e5. doi: 10.1016/j.neuron.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambetti P.R., Schuessler B.P., Kim J.J. Sex differences in foraging rats to naturalistic aerial predator stimuli. iScience. 2019;16:442–452. doi: 10.1016/j.isci.2019.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Zhu Z., Ma W.-X., Kong L.-X., Yuan P.-C., Bu L.-F., Han J., Huang Z.-L., Wang Y.-Q. The contribution of periaqueductal gray in the regulation of physiological and pathological behaviors. Front. Neurosci. 2024;18 doi: 10.3389/fnins.2024.1380171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.