Abstract
Data on antimicrobial use were collected for the 2016 and 2017 calendar years from swine producers in the United States. Nine large systems, collectively producing over 20 million market pigs annually, voluntarily provided data to advance understanding of antimicrobial use in the industry and to support antimicrobial stewardship initiatives. The scope of the study was limited to growing pigs, and the granularity of data varied across the systems. Data were summarized both qualitatively and quantitatively by antimicrobial class, active ingredient and route of administration (injection, water and feed). Data on the purpose of administration, doses and durations of administration were not available, but some information was provided by the responsible veterinarians. Aggregate data were similar both qualitatively and quantitatively in 2016 and 2017, although marked changes between years were evident within systems for some antimicrobials. Antimicrobial use (by weight) was dominated by the tetracycline class (approximately 60% of total use). Antimicrobials in classes categorized as critically important constituted 4.5% and 5.3% of total use in 2016 and 2017, respectively. In both years, fluoroquinolone (0.23%, 0.46%) and 3rd generation cephalosporin (0.15%, 0.11%) use collectively accounted for <1% of total use. Administration was predominantly oral in feed and water, and injection comprised approximately 2% of use overall, but around 12% for critically important antimicrobials. There was considerable variability among systems in patterns of antimicrobial use. This pilot project demonstrates the feasibility of acquiring antimicrobial use data via voluntary sharing. It is currently being expanded among larger swine production systems, and further efforts to enable confidential data sharing and benchmarking for smaller producers are being pursued by the swine industry. Recognized biases in the data caution against over-interpretation of these data as an index of national use.
Keywords: antimicrobial use, growing pigs, resistance, stewardship
1 |. INTRODUCTION
The spectre of antimicrobial-resistant infections has loomed over human and veterinary medicine since antimicrobials became widely available in the 1940s, and clinically problematic resistance has reached crisis status in human medicine over the last 20 years (Anderson et al., 2019; Aslam et al., 2018). This has rightly brought scrutiny of how antimicrobials are used across all prescribing professions, along with efforts to define ‘best practices’ for antimicrobial use and how these practices should be disseminated and implemented (Lesho & Laguio-Vila, 2019; Lloyd & Page, 2018). It is both generally accepted, and perhaps self-evident, that negative consequences of antimicrobial use in animals may relate to both animal health (reduced therapeutic efficacy due to resistance in animal pathogens) and human health due to transmission of resistant organisms from animals to people (Morley et al., 2005). However, antimicrobial resistance (AMR) in animal pathogens remains relatively unimportant as a clinical problem, particularly in food animals. Certainly, in some settings, emerging resistance is a concern for particular animal diseases and pathogens (Coetzee et al., 2019; Hampson et al., 2019; Loeffler & Lloyd, 2018), but to date falls far short of a crisis. It is typical that reviews of antimicrobial use and resistance in veterinary medicine and ‘One Health’ perspectives include little or no discussion of resistance as a clinical problem impacting animal health (McEwen & Collignon, 2018; Palma et al., 2020). The impetus for greater regulatory restrictions on antimicrobial use and for better antimicrobial stewardship in veterinary medicine is driven over-whelmingly by potential implications for public health.
Because of longstanding concerns about AMR in major enteric foodborne pathogens such as Salmonella and Campylobacter (Anderson, 1968; McCrackin et al., 2016), food animal populations have understandably garnered the most attention. In the United States (US), this is reflected in both longstanding and more recent legal and regulatory restrictions on antimicrobial use in food animals (U.S. Food & Drug Administration, 1994, 2020). In contrast, veterinarians are essentially unconstrained in their choices for administering antimicrobials to treat companion animals, including horses. However, it is increasingly recognized that companion animals are also reservoirs of resistant zoonotic and commensal bacteria that can serve as sources of human infections (Leonard et al., 2012; Montgomery et al., 2018; Rendle & Page, 2018). The likely contributions to human risk of exposures to resistant bacteria in environmental reservoirs add further layers of complexity to the challenges of understanding and effectively managing AMR at a societal level (Graham et al., 2019).
It is axiomatic that reliable measurement is necessary, although not sufficient, to effectively address complex problems. Enhancing surveillance of antimicrobial use and resistance in both human and veterinary medicine has been a unanimous recommendation of groups charged with formulating plans to address the global AMR crisis. Initiatives to collect data on antimicrobial use in food animals continue to be implemented in many developed countries (Collineau et al., 2016; Werner et al., 2018), along with efforts to assess use globally (Van Boeckel et al., 2015; World Organization for Animal Health, 2020). A systematic review of English language studies of measurement of antimicrobial use in pigs included 25 papers, 22 of which were published since 2010 (Lekagul et al., 2019). The predominant data sources were national sales data and farm surveys, and the authors identified ten metrics used to quantify use. These were categorized into four general approaches that measured use as (a) milligrams of active substance per animal weight; (b) daily doses per weight at treatment; (c) daily doses per treatment period; or (d) daily doses per period-at-risk of treatment. The absence of a ‘gold standard’ metric for antimicrobial use remains an obstacle to harmonization that would be necessary for any meaningful comparison of antimicrobial use in different geographical and industry settings (Collineau et al., 2016; Merle & Meyer-Kühling, 2019; Schrag et al., 2020).
There is a direct relationship between the cost and difficulty of acquiring data and the granularity and scope of the data collected. National sales data for antimicrobials in animals are relatively easily acquired, but have recognized limitations (Bondt et al., 2013; Collineau et al., 2016; Merle & Meyer-Kühling, 2019). Sales data typically lack granularity, and few nations can accurately parse these data at species or sector (e.g. beef vs. dairy) level. National sales data primarily have utility for monitoring gross temporal trends within defined populations, but are unsuitable for comparisons across heterogeneous populations (Bondt et al., 2013; Merle & Meyer-Kühling, 2019). In the United States, the Center for Veterinary Medicine (CVM) of the Food and Drug Administration (FDA) has published annual summaries of the amounts of antimicrobial drugs sold or distributed for food-producing species since 2009 (U.S. Food & Drug Administration, 2019). The data are described by antimicrobial class, category of medical importance and route of administration, but to date have not included a defined denominator for population size. Estimates of sales by species, based on estimates from distributors, have been included since 2016, and future biomass denominators have been proposed to provide greater context regarding changes in livestock and poultry populations. In January 2017, the FDA implemented substantial changes to regulatory oversight of antimicrobials in the United States (U.S. Food & Drug Administration, 2020). These included the withdrawal of medically important antimicrobials for purposes of production enhancement in food animals, and removal of over-the-counter availability of all medically important antimicrobials approved for administration in feed or water. In 2016, CVM funded two cooperative agreements to collect more granular and species-specific data of antimicrobial use in major food animal species over 5 years. This study reports the methods employed in the initial efforts to acquire data for the swine industry, and a descriptive analysis of the data for the calendar years of 2016 and 2017.
2 |. MATERIALS AND METHODS
2.1 |. Definitions
The term ‘antimicrobial’, rather than antibiotic, is employed throughout the manuscript in line with the prevailing norm of the literature in this field. However, the set of active ingredients included for this study was limited to antimicrobials used to treat, control or prevent bacterial infections, or used for growth promotion. These include compounds of synthetic (e.g. sulphonamides) or natural (e.g. penicillin) origin that were administered either orally (in feed or water) or parenterally to swine. No antiviral or antifungal compounds were included in the study as none are approved for use in swine in the USA, and fungal infections are rare, and typically self-limiting, in modern pig production. Other compounds that are active against bacteria and may be used topically or in farm environments, including antiseptic drugs, antibacterial soaps and chemical disinfectants were beyond the scope of the study.
The classification of the medical importance of antimicrobial classes follows Appendix A of Guidance for Industry #152 (GFI #152) of the FDA published in 2003 (U.S. Food & Drug Administration, 2003). Active ingredients and classes not listed in GFI #152 were deemed ‘not medically important’ in line with FDA guidelines for reporting sales of antimicrobials in food animals in the United States (U.S. Food & Drug Administration, 2019). In reporting these data, FDA includes ionophores (not medically important), whereas ionophore use is excluded from reports of antimicrobial use in many other jurisdictions. Avilamycin, an orthosomycin antibiotic, was licensed subsequent to GFI #152 and classified as not medically important, as this class is not used in humans.
2.2 |. Overview of the swine industry and veterinary services
The inventory of the commercial swine industry of the United States as of March 2016 was 67.6 million pigs, of which approximately 61.7 million (>91.3%) were growing (weaning to market) pigs (U.S. Department of Agriculture, 2020). In March 2018, the corresponding figures were 72.9 million total inventory (66.7 million growing pigs; 91.5%), and expansion of the industry has continued through December 2019 (77.3 million total; 70.9 million growing pigs) (U.S. Department of Agriculture, 2020). Swine production is distributed across more than 60,000 farms, and annual production of market hogs was 118 million in 2016 and 121 million in 2017 (U.S. Department of Agriculture, 2018). The industry is moderately concentrated, being less concentrated than poultry industries but more concentrated than the bovine industries. The largest 40 swine producers account for approximately two-thirds of total production. A feature of the United States industry, and particularly for larger producers, is the widespread use of multiple site production in which weaned pigs are transported from breeding farms to geographically separate facilities for rearing until market (Davies, 2012). These ‘off site’ facilities for growing pigs are a mix of specialized nursery farms (typically for 6–8 weeks post-weaning), specialized finishing farms (receiving pigs from nursery farms for rearing until market age) and wean-to-finish farms (rearing pigs from weaning until market). Veterinary services, including oversight of antimicrobial use, are provided by both salaried employees in larger companies, and by independent veterinarians. Farms are legally required to keep specific records of antimicrobial administration to food animals for up to 2 years. Generally, treatment records are held on paper at farms and, therefore, are not accessible electronically.
2.3 |. Recruitment of producers and data collection
The project was guided by a preliminary assessment of options for measuring antimicrobial use in the United States swine industry (Davies, 2017) and was developed and executed in consultation with an advisory group convened by the National Pork Board. The group includes specialist swine veterinarians representing both integrated systems and clinics servicing independent farms, and swine producers. Initial discussions addressed likely hurdles to establishment of a voluntary system for reporting antimicrobial use and identified priority issues such as the feasibility of different data collection options, establishing processes to assure confidentiality, industry communication and collaboration, and metrics. The scope for the initial study was to collect data on antimicrobial use in growing pigs (weaning to market) from a convenience sample of production systems that were willing to share data voluntarily. No financial or other incentives were provided to participants, including any compensation for staff time needed to aggregate and communicate data. Individual systems did receive feedback about their patterns of use in relation to other systems, although the identity of other systems was also not revealed to participants.
Seventeen swine production companies were approached via direct personal contacts, mostly veterinarians, associated with the companies. All expressed interest in the project, and 11 indicated willingness to participate in the initial phase of the project. In-person meetings were held to explain the goals of the project and to discuss potential concerns and logistics. Ten systems formalized non-disclosure agreements to ensure the confidentiality of the proprietary data. One system subsequently withdrew, but confirmed interest in participating at a later date. Relevant personnel in each collaborating system were provided with a description of the data needs with respect to antimicrobial use (weight of active ingredients by route in growing pigs), the target population denominator (live weight of hogs marketed, or carcass weight) and time frames (calendar years from 2016). With one exception, data presented in this report were sourced from accounting records of distribution of antimicrobials to farms within companies, which has several limitations. Firstly, the data do not include details on the indication (disease or clinical syndrome) or purpose (treatment, control, prevention, growth promotion) for which the antimicrobials were used. Secondly, although some systems reported use separately for nursery and finishing phases, there is limited information on age of administration, and details of dose and duration of administration are not captured for accounting purposes.
Recognizing the diversity of accounting or recording systems used across companies, companies were asked to provide the data in formats that fulfilled the needs listed above with least cost and disruption to staff. As such, the level of granularity in the data varied broadly across systems, including (a) aggregate annual use across entire systems; (b) use by phase of growth (e.g. nursery, finishing); (c) use attributed to individual lots (groups) of animals, including animal weight marketed by lot; and (d) data by lot derived from administration records (one system only). With the exception of that last system, the data represent amounts delivered to farms and are therefore maximal estimates of amounts administered to animals. Furthermore, amounts allocated for accounting purposes to particular lots of animals may or may not be used in their entirety in those groups, with potential carry over between groups. The following section outlines the granularity of data provided at a system level.
System A: Amounts of antimicrobials used in water or by injection were provided at the group (lot) level for groups closed out (i.e. marketed) in the respective calendar year. Antimicrobials included in feed could not be tracked at the lot level but were obtained at the system level by month. Data for the 12 months (January–December) of the respective calendar year were used as the annual estimate of in feed antimicrobial use. Aggregate data of use by groups closed out in the respective calendar year were used to estimate antimicrobial use in water and by injection, and the live weight marketed from those groups was the denominator.
System B: Amounts of antimicrobials by all routes were provided at a lot level for both nursery and finishing groups and were derived from actual administration records. Carcass weights were provided for finishing groups closed out in each year, which were converted to live weights using a divisor of 0.75 (average industry carcass yield). Antimicrobial use in nursery groups closed out in the respective calendar year was used to estimate nursery use and combined with finishing use to derive total estimates for growing pigs.
System C: Data were provided as aggregate weights (active ingredient) of antimicrobials allocated to growing pigs in each calendar year, and the live weight of pigs marketed. For 2016 data, route of administration could not be definitively determined for some antimicrobials (0.3% of total use across systems).
System D: Amounts of antimicrobials by all routes were provided at a group level for both nursery, finishing and wean-to-finish groups. Live weights were provided for finishing and wean-to-finish groups closed out in each year. Antimicrobial use in finishing (and wean-to-finish) groups closed out in the respective year was determined. Antimicrobial use in nursery groups closed out in the respective calendar year was added to derive total estimates.
System E: Data were provided as aggregate weights of active ingredients used in pigs in each calendar year, but included some antimicrobials used in feed on sow farms, due to inability to readily separate the two phases. Therefore, use is overestimated in this system.
System F: Data were provided at a site level (i.e. farm level, combining multiple lots per year) for use by water and injection, and at a system level for use in feed. Aggregate amounts used in growing pigs in each calendar year were calculated at a system level based on month of use (January to December). Live weight marketed per year was used as the denominator.
Systems G, H: Data were provided as aggregate amounts used in growing pigs in each calendar year, and live weight of pigs marketed in the corresponding year was used as the denominator.
System I: Data were provided as aggregate amounts used in pigs in each calendar year. Use by water and injection in growing pigs could be separated from breeding herd use. However, in feed use could not be readily separated by phase and included some medications used in sow farms. Therefore, use was overestimated in this system and overall. Live weight marketed per year was used as the denominator.
Data from each system were processed to populate standardized fields (system, year, active ingredient, route, weight of active ingredient; live weight marketed) for aggregation and analysis. Data were screened for potential inconsistencies (e.g. product name and active ingredient not congruent) and outliers (e.g. lots of market pigs with unlikely average weight), and confirmation or corrections were sought from the participants. Active ingredients were also classified into antimicrobial classes, and classes into categories of medical importance as defined by FDA Guidance #152 as detailed above. In cases where use of combination products was reported as a single amount, this was parsed into the weight of respective active ingredients based on the product labels.
2.4 |. Descriptive analyses of data
The aggregate data were summarized both qualitatively (relative amounts of antimicrobials used by drug and route) and quantitatively. Analysis was conducted for individual active ingredients, by antimicrobial class, and by medical importance. The qualitative analysis aggregated data across all systems and was not weighted by system size. In the qualitative analysis, some data on use are displayed using codes for individual systems (A through I) to display the variability in the relative amounts among systems (e.g. by class and medical importance) and changes within systems between years.
Quantitative analysis used the weight of active ingredient(s) as the numerator and estimated live weight of pigs marketed as the denominator. Weights provided in pounds were converted to kilograms using a multiplier of 2.20. The use of weight as an aggregate measure of antimicrobial use across different compounds is problematic due to the variable potency of different compounds. For this reason, the analysis was structured to report the patterns of use (number of systems using the ingredient; median and mean use in mg/kg of live weight) of individual active ingredients at an enterprise level across the aggregate population, and not by aggregating data across systems. Also, as indicated above, in two systems data from breeding farms could not be readily separated from growing pig data, leading to some overestimation for those systems and overall. In the quantitative description, variability in use of individual active ingredients among systems is displayed in ranked order (1 through n) of use without designating individual systems. Measures of central tendency were derived from the means and medians of mg of active ingredient/kg live weight marketed for each system for the subsets of systems using each respective antimicrobial.
2.5 |. Supplementary information on indication and administration of antimicrobials
To address some of the limitations of the available data sources, supplementary information was requested via an on-line survey from veterinarians with oversight of antimicrobial use in the participating systems. The information collected, for each active ingredient and route, was the most common indication for use, the perceived distribution of use across age groups (early nursery, late nursery, early finishing, late finishing), and the most common protocols (dose, duration) of administration for antimicrobials administered by water or by injection. Protocols for in feed administration were not requested as these are specified by approved labels for each product. This survey information was not explicitly supported by data on antimicrobial use, but constitutes expert opinion based on the direct practical experiences of senior veterinarians working with these systems.
3 |. RESULTS
3.1 |. Qualitative analysis of antimicrobial use by class, route and active ingredient
The nine participating systems sold over 20 million market pigs in 2016 and 2017, of the order of 20% of the national production. Tetracyclines comprised around 60% of all antimicrobial use by weight (Table 1). Eight classes of antimicrobials (tetracyclines, lincosamides, pleuromutilins, beta lactams, aminoglycosides, macrolides, quinolones and cephalosporins) were used in all nine collaborating systems. The six (2016) and seven (2017) most used classes comprised over 90% of total use.
TABLE 1.
Relative use (% of total weight of active ingredients of antimicrobial classes (ranked by use in 2016), and number of the nine production systems using each class in 2016 and 2017
| 2016 | 2017 | |||
|---|---|---|---|---|
| Antimicrobial class | % of use | N systems | % of use | N systems |
| Tetracycline | 61.9 | 9 | 57.6 | 9 |
| Lincosamide | 8.2 | 9 | 9.0 | 9 |
| Beta Lactam | 6.3 | 9 | 7.8 | 9 |
| Polypeptidea | 5.6 | 5 | 6.0 | 2 |
| Pleuromutilina | 5.3 | 9 | 4.7 | 9 |
| Macrolide | 3.3 | 9 | 3.2 | 9 |
| Aminoglycoside | 3.0 | 9 | 4.1 | 9 |
| Ionophorea | 2.8 | 2 | 3.4 | 1 |
| Streptogramin | 1.2 | 3 | 0.0001 | 1 |
| Quinoxalinea | 1.0 | 6 | 1.1 | 7 |
| Sulphonamide | 0.6 | 2 | 0.8 | 3 |
| Quinolone | 0.23 | 9 | 0.46 | 9 |
| Potentiated Sulphonamide | 0.2 | 2 | 0.75 | 5 |
| Cephalosporin | 0.15 | 9 | 0.11 | 9 |
| Aminocyclitol | 0.13 | 2 | 0.003 | 1 |
| Orthosomycina | 0.06 | 3 | 0.94 | 4 |
| Phenicol | 0.002 | 5 | 0.014 | 4 |
| Bambermycinsa | 0.001 | 1 | N/A | N/A |
Not medically important.
Across all systems in both 2016 and 2017, the majority of antimicrobials (by weight) were administered in feed (70.5%, 67.8% respectively). The bulk of the balance was administered in water (27.5%, 30.1% of total use) and a relatively small proportion (1.9%, 2.1%) by injection. With respect to categories of medical importance, use of critically important classes comprised 4.5% and 5.3% of total use in 2016 and 2017, respectively, predominantly comprised of macrolide antimicrobials (Table 2). Antimicrobial classes categorized as highly important comprised the majority of use (80.7% and 78.4% of total use in 2016 and 2017, respectively), and were predominantly tetracyclines. Antimicrobials deemed not medically important (14.8%, 16.2% of total use) were administered exclusively in feed apart from the pleuromutilin tiamulin which was administered in both feed and water.
TABLE 2.
Relative use (% of total weight of active ingredients) of antimicrobial classes within categories of medical importance (FDA Guidance for Industry #152) in 2016 and 2017
| Category of medical importance | 2016 | 2017 |
|---|---|---|
| Critically important | 4.5% of total use | 5.3% of total use |
| % within category | % within category | |
| Macrolide | 74.0% | 60.5% |
| Sulphonamide | 13.0% | 14.9% |
| Potentiated Sulphonamide | 4.5% | 14.0% |
| Quinolone | 5.1% | 8.6% |
| Cephalosporin | 3.3% | 2.0% |
| Total | 100% | 100% |
| Highly important | 80.7% of total use | 78.4% of total use |
| % within category | % within category | |
| Tetracycline | 76.7% | 73.4% |
| Lincosamide | 10.2% | 11.5% |
| Beta Lactam | 7.8% | 9.9% |
| Aminoglycoside | 3.7% | 5.2% |
| Streptogramin | 1.5% | 0.0001% |
| Aminocyclitol | 0.16% | 0.004% |
| Phenicol | 0.003% | 0.02% |
| Total | 100% | 100% |
| Not medically important | 14.8% of total use | 16.2% of total use |
| % within category | % within category | |
| Pleuromutilin | 37.5% | 29.3% |
| Polypeptide | 36.0% | 36.9% |
| Ionophore | 19.0% | 21.0% |
| Quinoxaline | 7.1% | 7.0% |
| Orthosomycin | 0.39% | 5.8% |
| Bambermycin | 0.01% | n/a |
| Total | 100% | 100% |
Considerable variability was found among systems with respect to the distribution of antimicrobial classes used, and also within systems between years (Figure 1). This was similarly apparent when viewed by categories of medical importance (Figure 2).
FIGURE 1.
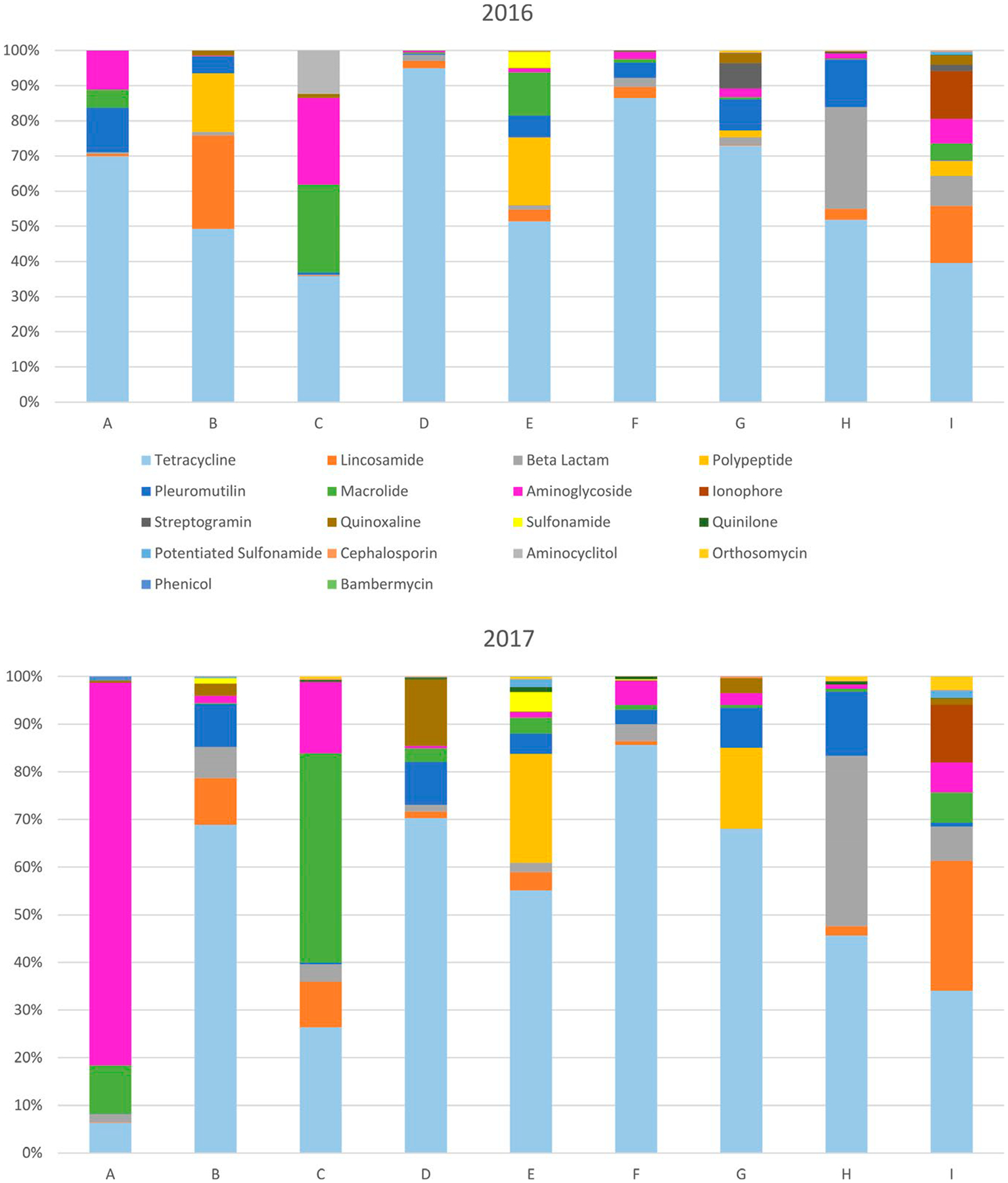
Relative use of antimicrobial classes among production systems (A through I) in 2016 and 2017
FIGURE 2.
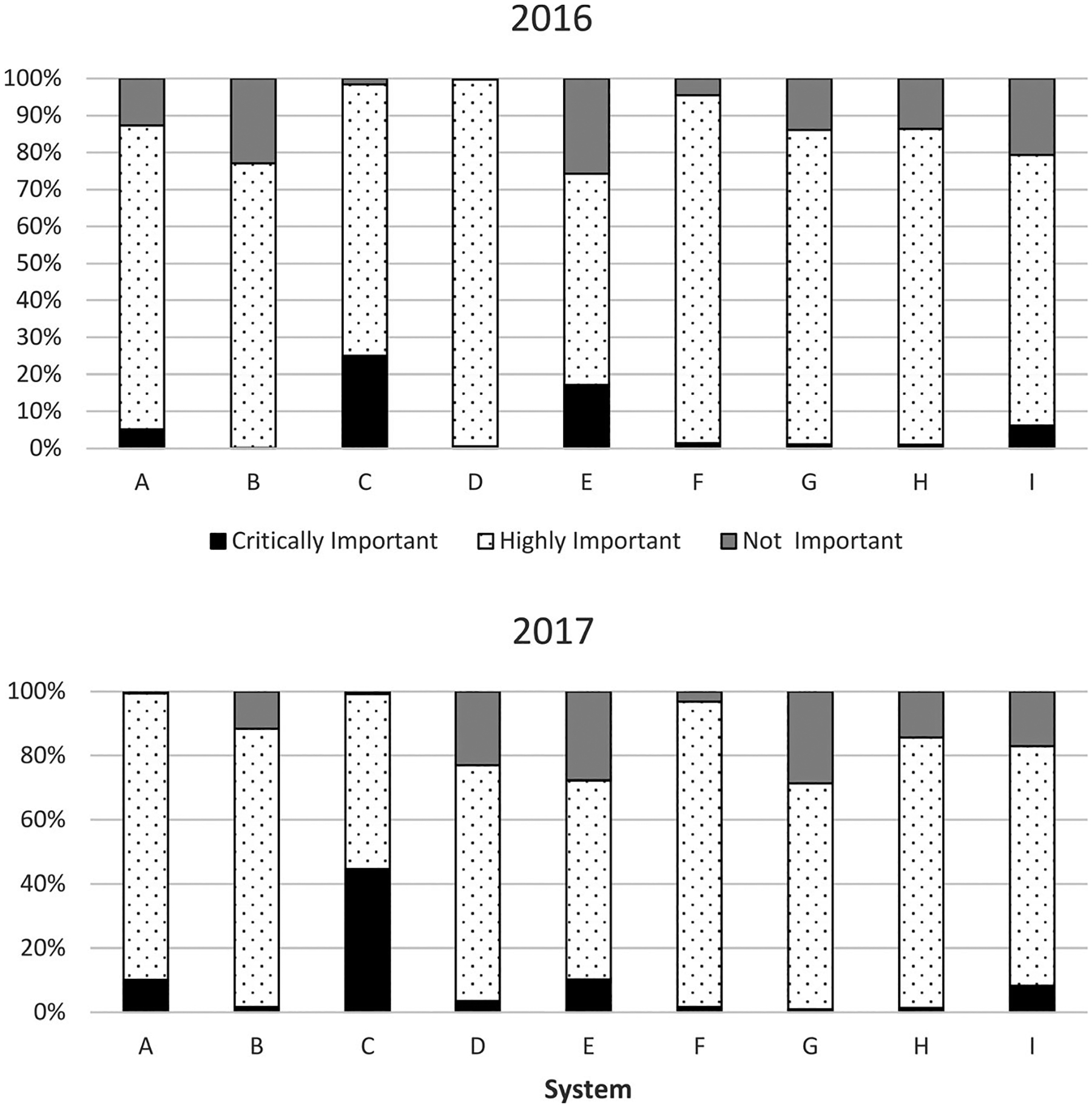
Relative use (% of total weight of active ingredients) of antimicrobial classes by category of medical importance among production systems (A through I) in 2016 and 2017
Antimicrobials deemed critically important for human medicine were proportionally administered more by injection (12.7%, 11.3% of use in 2016 and 2017 respectively) than were antimicrobials in other categories (1.7% across both years). Critically important antimicrobials were used in similar amounts for both feed (49.9%, 48.1% for 2016 and 2017, respectively) and water (37.4%, 40.5%). The quinolones (enrofloxacin) and cephalosporins (ceftiofur) and the macrolide tulathromycin were administered solely by injection. Other macrolides were mostly administered in feed (55.6%) and water (43.7%), with some tylosin used by injection (0.8%). Sulphonamides (91.2%) were predominantly used in feed, and potentiated sulphonamides (sulphadiazine/trimethoprim) were solely used in water.
Among highly important classes, tetracyclines were predominantly used in feed (89.6%, 75.4% in 2016 and 2017, respectively) and less so in water (10.1%, 23.9%), and by injection (0.3%, 0.7%). Lincosamides (lincomycin) were also used in feed (59.9%, 73.4%, respectively), in water (30.9%, 20.3%) and by injection (9.2%, 7.8%). Aminoglycosides (96.0%, 76.7% in 2016 and 2017, respectively) and beta lactams (96.7%) were mostly used in water, while streptogramins were solely used in feed. Phenicols were used in both by injection (74%) and in water (26%) across both years.
3.2 |. Reported use by antimicrobial active ingredient
At the level of individual antimicrobial active ingredients, chlortetracycline alone accounted for 49.3% of use in 2016, and 40.9% in 2017 (Table 3). The six most used antimicrobials (chlortetracycline, oxytetracycline, lincomycin, penicillin, bacitracin, tiamulin) accounted for 86.8% of total weight used in 2016, and 84.8% in 2017. In contrast, the six least used antimicrobials (sulphadimethoxine, tetracycline, florfenicol, ampicillin, bambermycins and sulphamethoxazole) collectively accounted for only 0.09% of use across both years.
TABLE 3.
Relative use (% of total weight) of active ingredients of antimicrobials used in nine swine productions systems in 2016 and 2017
| Active ingredient | 2016 | 2017 |
|---|---|---|
| Chlortetracycline | 49.3% | 40.9% |
| Oxytetracycline | 12.5% | 16.6% |
| Lincomycin | 8.2% | 9.0% |
| Penicillin | 5.8% | 7.5% |
| Bacitracin | 5.6% | 6.0% |
| Tiamulin | 5.3% | 4.7% |
| Neomycin | 2.9% | 4.0% |
| Narasin | 2.8% | 3.4% |
| Tylosin | 1.6% | 0.20% |
| Virginiamycin | 1.2% | 0.0001% |
| Carbadox | 1.0% | 1.1% |
| Tylvalosin | 0.85% | 0.86% |
| Tilmicosin | 0.68% | 2.2% |
| Sulphamethazine | 0.58% | 0.70% |
| Amoxicillin | 0.49% | 0.28% |
| Enrofloxacin | 0.23% | 0.46% |
| Trimethoprim/Sulphadiazine | 0.20% | 0.75% |
| Tulathromycin | 0.17% | 0.01% |
| Ceftiofur | 0.15% | 0.11% |
| Spectinomycin | 0.13% | 0.003% |
| Gentamicin | 0.10% | 0.06% |
| Avilamycin | 0.06% | 0.94% |
| Tetracycline | 0.03% | 0.03% |
| Sulphadimethoxine | 0.005% | 0.09% |
| Ampicillin | 0.002% | 0.0002% |
| Florfenicol | 0.002% | 0.01% |
| Bambermycins | 0.001% | n/a |
| Sulphamethoxazole | 0.00004% | n/a |
3.3 |. Quantitative analysis of antimicrobial use across participating systems
The quantitative analysis expresses the use of individual antimicrobials (mg active ingredient) in relation to the biomass denominator of live weight of pigs marketed in a year from each system. Different antimicrobials have different potencies (i.e. relationships between weight and dose required), and therefore combining weights of multiple and different active ingredients is uninformative. For this reason, the quantitative analysis is reported for each individual antimicrobial and not in aggregate (i.e. combining data for different antimicrobials). For each year, the summary information indicates the number of systems using each antimicrobial, and the mean and median use per kg marketed across the set of systems using that antimicrobial (Table 4).
TABLE 4.
Mean and median use (mg of active ingredients per kg of liveweight marketed) in 2016 and 2017 across systems using the respective antimicrobials
| 2016 | 2017 | |||||
|---|---|---|---|---|---|---|
| Active ingredient | # systems | Mean | Median | # systems | Mean | Median |
| Amoxicillin | 4 | 4.0 | 1.9 | 5 | 2.2 | 0.73 |
| Ampicillin | 2 | 0.04 | 0.04 | 1 | 0.006 | 0.006 |
| Avilamycin | 3 | 0.39 | 0.04 | 4 | 1.76 | 0.81 |
| Bacitracin | 5 | 50.1 | 6.6 | 2 | 38.2 | 38.2 |
| Carbadox | 6 | 5.0 | 2.1 | 7 | 3.7 | 2.3 |
| Ceftiofur | 9 | 0.20 | 0.06 | 9 | 0.15 | 0.10 |
| Chlortetracycline | 9 | 139.4 | 73.4 | 9 | 70.4 | 55.1 |
| Enrofloxacin | 9 | 0.29 | 0.27 | 9 | 0.49 | 0.26 |
| Flavomycin | 1 | 0.014 | 0.014 | 0 | — | — |
| Florfenicol | 5 | 0.013 | 0.007 | 4 | 0.07 | 0.05 |
| Gentamicin | 6 | 0.21 | 0.20 | 7 | 0.12 | 0.11 |
| Lincomycin | 9 | 42.5 | 3.8 | 9 | 10.1 | 2.3 |
| Narasin | 2 | 10.7 | 10.7 | 1 | 23.3 | 23.3 |
| Neomycin | 9 | 5.2 | 3.8 | 9 | 5.9 | 3.9 |
| Oxytetracycline | 7 | 15.0 | 4.8 | 6 | 21.9 | 3.8 |
| Penicillin | 9 | 6.6 | 1.7 | 9 | 8.4 | 2.6 |
| Spectinomycin | 2 | 2.3 | 2.3 | 1 | 0.03 | 0.03 |
| Sulphadimethoxine | 1 | 0.05 | 0.05 | 1 | 0.86 | 0.86 |
| Sulphamethazine | 1 | 6.0 | 6.0 | 3 | 3.04 | 2.97 |
| Sulphmethoxazole | 1 | 0.004 | 0.004 | 0 | — | — |
| Tetracycline | 3 | 0.52 | 0.61 | 2 | 0.63 | 0.63 |
| Tiamulin | 9 | 14.5 | 8.4 | 9 | 8.8 | 6.0 |
| Tilmicosin | 8 | 1.34 | 0.74 | 7 | 3.6 | 0.56 |
| Trimethoprim/Sulphadiazine | 2 | 0.76 | 0.76 | 5 | 1.3 | 0.59 |
| Tulathromycin | 7 | 0.87 | 0.01 | 7 | 0.02 | 0.002 |
| Tylosin | 9 | 1.89 | 0.22 | 8 | 0.45 | 0.13 |
| Tylvalosin | 6 | 1.6 | 1.1 | 5 | 1.9 | 1.8 |
| Virginiamycin | 3 | 6.8 | 2.8 | 1 | 0.0004 | 0.0004 |
The quantitative use of antimicrobials varied widely among systems and between years in some systems. In particular, in 2016 use was markedly higher in single systems for chlortetracycline and lincomycin, but was substantially reduced (>70% reduction for chlortetracycline; >90% for lincomycin) in those same systems in 2017. In contrast, oxytetracycline use (over two-fold) and penicillin use (>20%) increased in 2017 in the highest using systems compared to 2016. Due to the observation that use was often not normally distributed, median use is a preferable indicator of central tendency rather than mean use for most antimicrobials.
For critically important antimicrobials, much lower amounts were used relative to the highly important category. However, marked variability among systems was evident for all antimicrobials, and substantial variability in use within systems between years was observed for some antimicrobials (Figure 4). Ceftiofur use was generally stable within systems for both years, although use approximately halved in three systems in 2017. Similar use between years was also evident for enrofloxacin, apart from one system in which use increased substantially (approximately seven-fold) in 2017. Use of tylosin was markedly higher in one system than all others in 2016, but reduced by over 95% to be similar to other systems in 2017. In contrast, substantial increases (>3-fold) in tilmicosin use were observed for the two systems that most used that antimicrobial in 2016.
FIGURE 4.
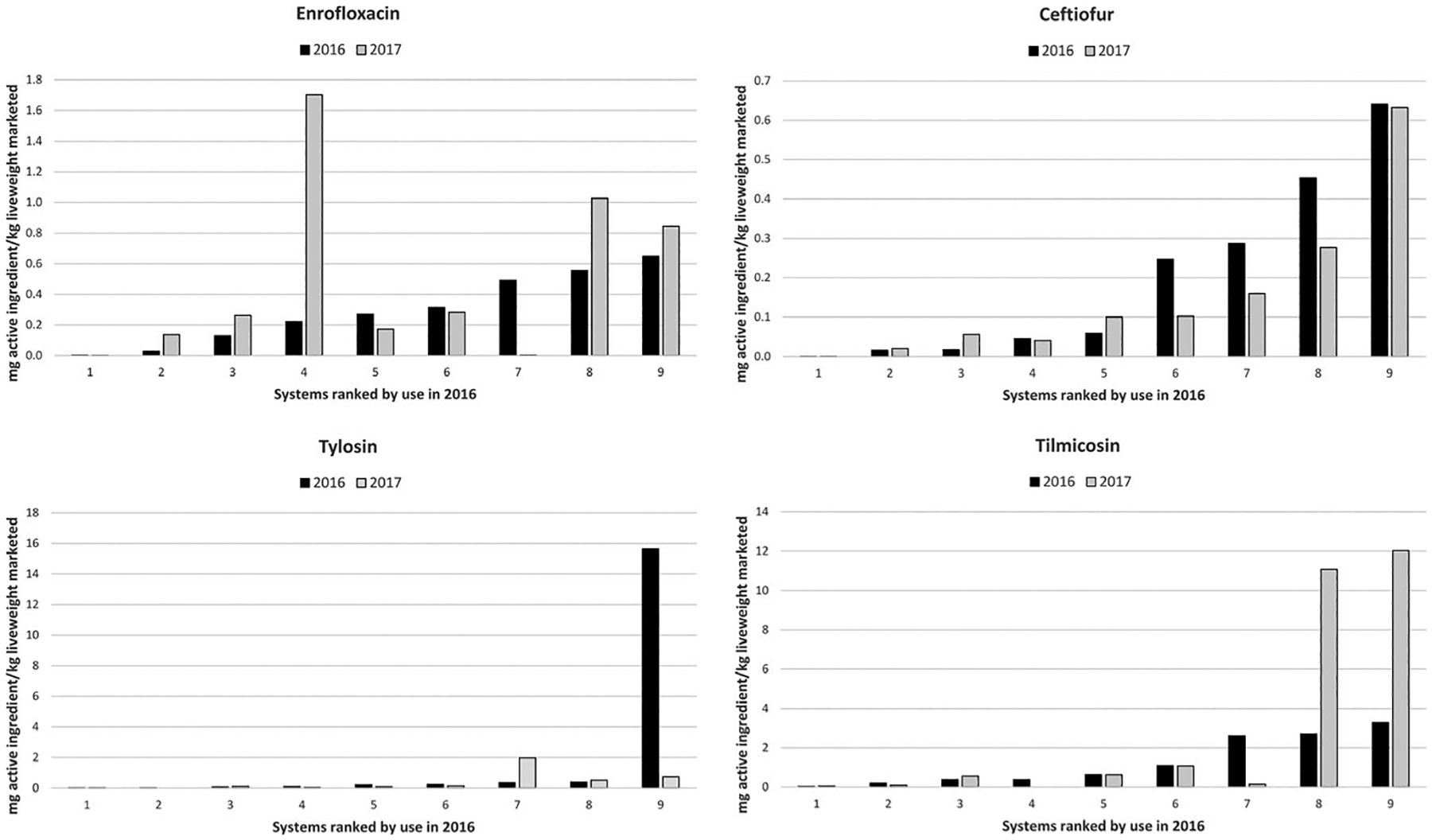
Use (mg of active ingredient/kg liveweight marketed) for critically important antimicrobials across systems using enrofloxacin, ceftiofur, tylosin and tilmicosin. For each antimicrobial, systems are ordered by use in 2016, and vertical scales are varied by graph
3.4 |. Veterinary opinions of patterns of administration
Veterinarians from seven of the nine systems completed the survey requesting information regarding indications, timing (age) and protocols for administering antimicrobials. As indicated in the methods, this information constitutes expert opinion based on the practical experiences of the veterinarians in the participating systems and was not derived through analysis of data.
With respect to age of administration, across all routes of administration the early nursery phase (Table 5) appears to be the phase where antimicrobial use is concentrated, comprising approximately 40% of perceived distribution (both medically and not medically important).
TABLE 5.
Overall perceived distribution (% of use by production phase) of antimicrobial use by production phase (early nursery EN; late nursery LN; early finishing EF; late finishing LF)
| Route | EN | LN | EF | LF | Nursery | Finishing |
|---|---|---|---|---|---|---|
| Feed | 37 | 18 | 29 | 16 | 55 | 45 |
| Injection | 38 | 20 | 18 | 23 | 59 | 41 |
| Water | 42 | 19 | 20 | 19 | 60 | 40 |
| ALL | 40 | 19 | 22 | 20 | 59 | 41 |
Note: Totals may not sum to 100% due to rounding.
Perceived age distributions differed widely across individual active ingredients (Table 6), and 11 of the 19 antimicrobials included were predominantly (75% or more) allocated to nursery pigs (~3–10 weeks of age), and particularly in the early nursery period following weaning. In contrast, only five of the 19 were predominantly used in finishing pigs, two of which (bacitracin and narasin) are not medically important, with the others being lincomycin and two macrolide antimicrobials (tylosin and tylvalosin).
TABLE 6.
Overall perceived distributions of antimicrobial use (% of use of active ingredient) by production phase (EN—early nursery; LN—late nursery; EF—early finishing; LF—later finishing) for medically important and non-medically important antimicrobials
| Antibiotic | EN | LN | EF | LF | All nursery | All finishing |
|---|---|---|---|---|---|---|
| Medically important | ||||||
| Amoxicillin | 78 | 22 | 0 | 0 | 100 | 0 |
| Florfenicol | 100 | 0 | 0 | 0 | 100 | 0 |
| Tulathromycin | 100 | 0 | 0 | 0 | 100 | 0 |
| Penicillin | 68 | 27 | 6 | 0 | 94 | 6 |
| Gentamicin | 77 | 16 | 7 | 0 | 93 | 7 |
| Enrofloxacin | 71 | 19 | 8 | 3 | 90 | 10 |
| Tilmicosin | 50 | 38 | 13 | 0 | 88 | 13 |
| Neomycin | 83 | 4 | 14 | 0 | 86 | 14 |
| Ceftiofur | 51 | 28 | 5 | 16 | 79 | 21 |
| Sulphonamides | 48 | 28 | 25 | 0 | 75 | 25 |
| Chlortetracycline | 28 | 31 | 35 | 7 | 59 | 41 |
| Oxytetracycline | 4 | 26 | 51 | 18 | 31 | 69 |
| Tylvalosin | 3 | 15 | 34 | 49 | 18 | 83 |
| Tylosin | 0 | 9 | 15 | 76 | 9 | 91 |
| Lincomycin | 0 | 3 | 32 | 65 | 3 | 97 |
| Non-medically important | ||||||
| Carbadox | 66 | 34 | 0 | 0 | 100 | 0 |
| Tiamulin | 27 | 19 | 46 | 7 | 46 | 54 |
| Narasin | 0 | 0 | 40 | 60 | 0 | 100 |
| Bacitracin | 0 | 0 | 30 | 70 | 0 | 100 |
All respondents indicated that aminoglycosides (neomycin, gentamicin) were used exclusively for enteric diseases, particularly Escherichia coli and Salmonella enteritis. Similarly, carbadox use was limited to enteric diseases, including Brachyspira spp. In contrast, beta lactam antimicrobials (penicillin and amoxicillin) were used predominantly for Streptococcus suis infections, with other stated indications being Glaesserella (Haemophilus) parasuis infections and wound infections. Lincomycin was administered to treat lameness/arthritis and respiratory disease, with most respondents specifically stating mycoplasmas (M. hyopneumoniae, M. hyosynoviae) as the target pathogens. For enrofloxacin, the major indications varied among systems but aligned with label approvals to treat respiratory pathogens, S. suis and E. coli, predominantly in nursery pigs. Ceftiofur use was also concentrated in the nursery phase in most systems, and for a range of indications, particularly pneumonia and septicaemia (S. suis). However, one system used ceftiofur solely in the finishing phase to treat Actinobacillus pleuropneumoniae. This marked difference in administration patterns with respect to both age and indication would lead to highly misleading comparisons of use between systems with metrics that assume a common indication and standard weight at treatment.
4 |. DISCUSSION
A fundamental goal of this study was to assess the feasibility of obtaining data on antimicrobial use in the United States swine industry through sharing of proprietary records. The voluntary participation by nine large swine producers demonstrated the potential for acquiring data through collaboration across the industry and provided the first substantial description of antimicrobial use in United States swine beyond annual sales and distribution data reported by pharmaceutical companies to the FDA (U.S. Food & Drug Administration, 2019). The collaborating systems are widely distributed among major swine producing states and collectively reared approximately 20% of national production. However, the number of systems enrolled in this initial phase of the project was small, and considerable variability in patterns of use among systems was evident both qualitatively and quantitatively. Furthermore, the data aggregated for the qualitative analysis were not weighted by size of systems and therefore are biased towards usage patterns of the largest participants. Other features of the study that limit the representativeness of the data as an index of the overall industry include the use of convenience sampling and the voluntary nature of participation. The data likely more closely reflect the integrated sector of the industry that is responsible for the majority of national production. However, given the diversity seen in patterns of use among the nine systems and between years within systems, more robust sampling of this sector is necessary to achieve more reliable estimates. The absence of small enterprises (the smallest system had over 15,000 sows) and farrow-to-finish herds is likely a significant bias with respect to overall industry use of antimicrobials. Another factor associated with farm size is the source of veterinary services. All but one system in this study employed veterinarians within the enterprise rather than use external veterinary services. This is common (but not universal) among large swine companies in the United States, but not among smaller operations. The extent to which the source of veterinary services could influence antimicrobial use is unknown. However, it may be substantial, particularly over the period of this study in which over-the-counter access (i.e. available without veterinary oversight if used according to label) to most antimicrobials ceased in January, 2017.
Another distinction of the study was that it focused solely on growing pig production, rather than the entire industry. This parallels the approaches adopted in the corresponding projects on beef cattle, broilers and turkeys (Hope et al., 2020; Singer et al., 2020a, 2020b). Variability in age susceptibility is a recognized characteristic of the epidemiology of most swine diseases, and infectious diseases are generally more problematic in younger animals than in adult breeding stock (Lekagul et al., 2019). Therefore, the different age demographics and infectious disease challenges in breeding herds create different needs for antimicrobial use than in growing pig populations. Reports from other countries typically show much lower use of antimicrobials in sow and piglet populations than in growing pigs and particularly weaned pigs (DANMAP, 2019). The participating veterinarians in this study indicated that most antimicrobials were used predominantly in the nursery (weaned pig) populations (Tables 5 and 6), a pattern that is consistent with reports in other countries and reflects the relative vulnerability of recently weaned pigs to infectious diseases (Lekagul et al., 2019). In the United States, growing pigs comprise over 91% of the commercial swine inventory and constituted 97.2% of the federally inspected swine slaughtered in 2017 (U.S. Department of Agriculture, 2020). Therefore, in the context of the overall pork supply, use in breeding herds is arguably of minor importance. However, some participating systems have expressed interest in sharing breeding herd data in the future. If pursued, it would be essential to stratify rather than combine the analysis of the two sectors to gain fuller understanding of patterns of use and improve utility for benchmarking and informing stewardship.
Some aspects of the study that complement the national sales data are greater granularity with respect to active ingredients (vs. antimicrobial classes reported in sales data), a defined population denominator for quantitative estimates, and confidence that all amounts reported were distributed only for use in swine. Furthermore, the data documented the use of some active ingredients (amoxicillin, ampicillin, trimethoprim/sulphadiazine) that are not captured in the sales data for swine as they have no approved label claim. Antimicrobials, with some specific exceptions, may be used legally (by injection or in water) in food-producing animals in an extra-label manner under the supervision of a licensed veterinarian as stipulated by the Animal Medicinal Drug Use Clarification Act (AMDUCA) of 1994 (U.S. Food & Drug Administration, 1994). The flexibility provided by AMDUCA is an essential element of antimicrobial stewardship that enables veterinarians to more effectively utilize antimicrobial susceptibility testing to select appropriate antimicrobials. Notably, the combined use of these antimicrobials comprised only 0.68% and 1.0% of total use in 2016 and 2017, respectively, and varied within companies between years. The small amounts and variable pattern of use within systems would be consistent with veterinarians targeting extra-label use of these products to address specific problems. Penicillin, which is solely approved in swine for injection to treat Erysipelothrix rhusopathiae infections, was predominantly administered extra-label in water to treat S. suis infections. Streptococcus suis is among the most important bacterial diseases of swine and can cause sudden outbreaks with high mortality (Gottschalk, 2012). Internationally, amoxicillin is recognized as a first choice antimicrobial for treatment and control of S. suis, and has remained efficacious despite long-term use in swine medicine (Burch & Sperling, 2018; Gottschalk, 2012). However, in the United States, there are no labelled approvals for amoxicillin in any food animal species, apart from one intramammary product for bovine mastitis. However, the critically important antimicrobials enrofloxacin and ceftiofur are labelled for treatment of S. suis. Although it is legally permissible to use amoxicillin in swine under AMDUCA, some US veterinarians and production systems have eliminated amoxicillin as a treatment option for S. suis due to concerns of public perception surrounding the use in pigs of products that are licensed for human but not animal use (personal communications). For treatment and control of S. suis infections in the United States, antimicrobial stewardship (in this case the ability to choose the most appropriate antimicrobial for a specific pathogen) may be compromised by public commentary surrounding antimicrobial use in food animals.
Acknowledging the caveats associated with the data, some observations merit elaboration. Firstly, although some substantial changes were evident between years within individual systems for particular antimicrobials, the summary data were generally similar in 2016 and 2017 both qualitatively (Table 1) and quantitatively (Table 4). This contrasts with the FDA sales data for 2016 and 2017 in which aggregate use (by weight) in the swine industry was estimated to decline by 37% between these years, which spanned the introduction of the regulatory changes in January 2017. This discordance between the two sources of data may in part be attributable to characteristics of the study participants. Discussions with the associated veterinarians indicated that any adjustments necessary to therapeutic protocols because of the impending regulatory changes (announced in December 2013 and implemented in January 2017) were made well in advance of the implementation date (personal communications). Thus, 2016 is likely not the optimal baseline for assessing the impact of those regulatory changes in larger swine systems. Sales data across all food animals show a decline from peak sales in 2015 (U.S. Food & Drug Administration, 2019) despite industry expansion, which is consistent with some segments of producers implementing changes in anticipation of, rather than concurrent with, the regulatory changes. In addition, all participating systems had long established veterinary–client–patient relationships and oversight. This obviated the need for over-the-counter access to antimicrobials which was phased out in January 2017. In contrast, one would anticipate the impact of removing over-the-counter antimicrobials to be greatest for producers having the least veterinary involvement in their operations, and that changes in that sector were more likely to be delayed until the regulations came into effect.
The three medically important antimicrobials with the greatest absolute reductions in mean use across the nine systems were chlortetracycline (69.0 mg/kg live weight reduction, 49% relative reduction), lincomycin (32.4 mg/kg live weight reduction, 76% relative reduction) and tylosin (1.44 mg/kg live weight reduction, 76%). For all three, use in the systems with the highest quantitative use in 2016 was several fold higher (3.7× for chlortetracycline; 13× for lincomycin; 40× for tylosin) than in the second ranked system (Figures 3 and 4). However, use of these antimicrobials was reduced markedly in the respective systems in 2017. In the case of tylosin, the reduction was due to system-wide implementation of a vaccination program to control Lawsonia intracellularis in place of control using tylosin (personal communication). There were other instances where veterinarians stated that changes observed within systems between years were attributable to strategic decisions taken to combat-specific diseases (e.g. tulathromycin to control M. hyopneumoniae).
FIGURE 3.
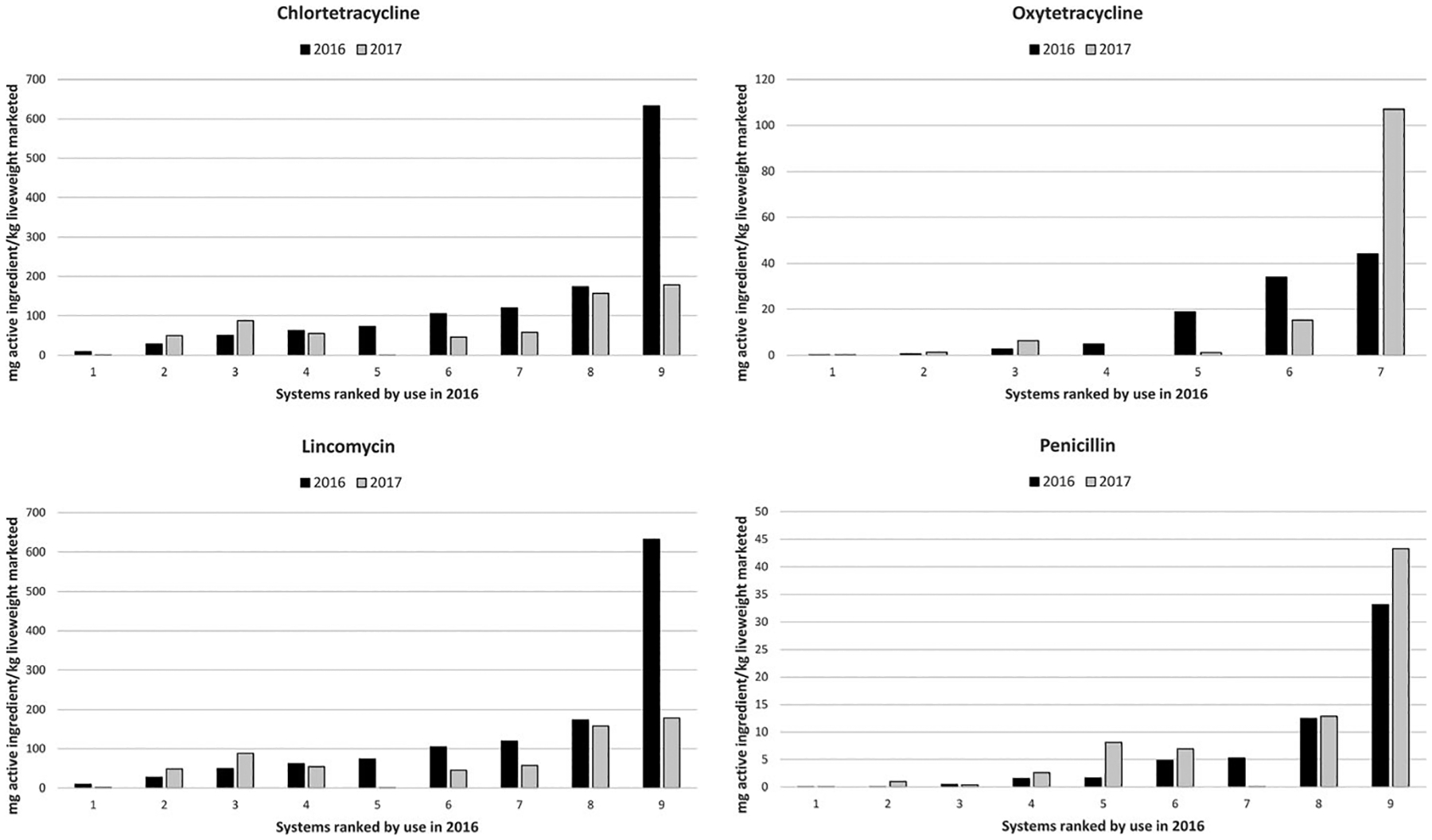
Use (mg of active ingredient/kg liveweight marketed) for highly important antimicrobials across systems using chlortetracycline, oxytetracycline, lincomycin and penicillin. For each antimicrobial, systems are ordered by use in 2016, and vertical scales are varied by graph
The dominance of the tetracycline class is consistent with FDA sales data and is a feature among most reports of antimicrobial use in swine and other food animals species internationally (Lekagul et al., 2019; Van Boeckel et al., 2015; World Organization for Animal Health, 2020). Tetracyclines were among the earliest antimicrobials used in food animal production (Jukes, 1985) and have multiple approved label claims for administration via feed, water and injection to swine in the United States. Although tetracycline resistance (phenotypically and genotypically) is prevalent in some pathogens and commensal bacteria of swine (Hayer et al., 2020a, 2020b; Holmer et al., 2019), this class appears to remain clinically valuable and widely used, even in herds where tetracycline resistance genes are abundant across the microbiome (Pollock et al., 2020). With respect to critically important antimicrobials, most concern regarding human health has surrounded the use in food animals of fluoroquinolones (e.g. enrofloxacin) and third generation cephalosporins (e.g. ceftiofur). In some countries, these antimicrobials have been either not licensed for use in food animal or have been withdrawn. In the United States, extra-label drug use in food animals is illegal for fluoroquinolones, and enrofloxacin was withdrawn from use in the poultry industries in 2005 due to emergence of fluoroquinolone-resistant Campylobacter (Nelson et al., 2007). For third generation cephalosporins, extra-label use is restricted (permissible only for extra-label indications, but not for altered route, dose or duration), and use for disease prevention is illegal. Although enrofloxacin and ceftiofur were used in all participating systems, they collectively accounted for <1% of total use by weight in both years and were predominantly used in younger pigs that would not be marketed until several months following treatment.
Meaningful interpretation of antimicrobial use data demands, at a minimum, detailed comprehension of the metrics used to quantify antimicrobial amounts, estimate population denominators and define the period for collection. Pig production approximates a steady-state production flow, with a breeding to market interval of approximately 10 months, a growth period (birth to market) of approximately 6 months, and unequal antimicrobial use across that growth period. In this study, the temporal associations between antimicrobial amounts and population denominators were not uniform due to variability in accounting practices. For systems that recorded use at a lot level, the study populations constituted those lots of animals that were marketed in each calendar year. This defines a 12-month calendar year population denominator, but means that lots marketed in early January 2016 would have received all their antimicrobial exposure during 2015. Other systems provided contemporaneous data on antimicrobial amounts and pigs marketed. This clearly demarcates concurrent 12-month periods of antimicrobial distribution and live weight marketed, but the numerators and denominators are not directly linked (i.e. hogs marketed in January would not have been exposed to the antimicrobials allocated in January). However, assuming relative stability in production and health, both approaches would be expected to yield reasonable, though non-equivalent, estimates of use over periods of 12 months. At a more granular level, recording use separately in nursery and finishing lots should also yield similar, but non-equivalent, estimates to those recorded for pigs managed in wean-to-finish lots. Noting that the overall industry expansion over the period of the study was of the order of 8%, these subtle differences in recording would be amplified by changes in the age demographics of an expanding (or contracting) pig population. That is, the incremental antimicrobial use (concentrated in weaned pigs) associated with an expanding breeding population will occur several months prior to the associated increment in the market pig population. These rather esoteric nuances are unlikely to substantially distort analyses of high-level data aggregated over large segments of animal time (e.g. millions of pigs over 1 year), but become increasingly problematic for more refined comparisons, such as comparison of individual farms.
A dizzying array of metrics has emerged for quantifying antimicrobial use in food animals, and no ‘gold standard’ approach exists (Collineau et al., 2016; Lekagul et al., 2019; Werner et al., 2018). This reflects the non-trivial nature of identifying a measure of antimicrobial use that is truly biologically informative (Schrag et al., 2020). Ideally, the measure should predict the collective ‘selection pressure’ for phenotypic and/or genotypic resistance that is clinically relevant to public health and/or animal health. However, in the absence of any scientific consensus of what constitutes ‘resistance that is clinically relevant to public health and/or animal health’, no substantive evidence base exists from which to derive a clinically informative metric. Furthermore, the expectation that any single measure could provide informative comparisons across biological systems that are profoundly different (e.g. broiler vs. swine vs. dairy vs. beef production) is unrealistic and arguably futile.
In the quest to minimize the human health impact of antimicrobial use in food animals, yet meet the needs for animal health and welfare, the definition of success must lie in some measurable impact on human health. The most resource-intensive initiatives to monitor antimicrobial use in food animals have defined success by reduction in use per se, without reference to outcomes that are clinically relevant to human or animal health (DANMAP, 2019; More, 2020; Pozio & Zarlenga, 2013; Speksnijder et al., 2015; Stege et al., 2003). Reduction of antimicrobial use per se (by any metric) in animal production is an intervention that has a logical foundation, but has yet to be clearly linked to any demonstrable benefit for human health. This point is not made to diminish such initiatives, which are motivated by the need to ‘do something’ to address a major public health problem, or at least to appease public or market concerns. However, the presumption that measurable benefits to public health will accrue is far from certain (Bennani et al., 2020).
Because the state of the art is so distant from the ideal (Knight et al., 2019), it is pragmatic to suggest that different metrics be selected based on the specific and defined purpose (Collineau et al., 2016). It follows that extracting value from investments made to acquire data requires identification of the most fruitful purpose. We posit that the greatest value will come from information that is relevant to specific industries and environments. Information of high specificity, but perhaps minimal generalizability, will best support education and stewardship in any given context. As such, preferred metrics and data needs are likely variable across different settings. Recently, it was proposed that the dairy industry in the United Kingdom employs UK-specific metrics using UK-specific medicine dose and course regimens and cattle weights in order to monitor trends nationally (Mills et al., 2018). The value of customized metrics is reinforced by the difficulty in defining any single preferred metric even within a national industry (Schrag et al., 2020). The value of pursuing ‘harmonization’ in measuring antimicrobial use to compare fundamentally different biological systems (e.g. different species, or beef vs. dairy production), or even different geographies, is questionable.
We elected to describe antimicrobial use by weight of active ingredient as the simplest approach that would meet the goals of the study. These were to assess the feasibility of acquiring data via voluntary sharing of proprietary data by industry and to conduct a descriptive analysis of patterns of antimicrobial use at a broad level. The major shortcoming of weight as a metric is the variable potency (weight of active ingredient corresponding to a unit of treatment or dose) of different active ingredients (Jensen et al., 2004). For this reason, weight is a meaningless metric if aggregated across diverse ingredients, classes and routes of administration. Consequently, we used the qualitative analysis to describe variability in use across systems and between years, and the quantitative description (mg/kg live weight) on an ingredient-by-ingredient basis, without aggregation. A preferred option in some European countries is to use potency-adjusted measures (e.g. ‘defined daily doses’) that are then applied across herds using general assumptions regarding the doses and weights of animals treated (Jensen et al., 2004; Postma et al., 2015; Speksnijder et al., 2015). However, the generalized assumptions on administered dose (assumed constant regardless of indication) and weight at administration (using opinion-based assumptions of when animals are most likely treated) are problematic and can misclassify herds in relation to actual administration (Kasabova et al., 2019; Waret-Szkuta et al., 2019). A simple example from the current study illustrates this dilemma. One system used ceftiofur solely for treatment of porcine pleuropneumonia (APP) in late finishing pigs, whereas all others used this drug predominantly in weaned pigs. Assuming an approximately 15-fold difference in bodyweight at treatment (e.g. 7 kg vs. 105 kg), the weight of ceftiofur used to treat a fixed number of pigs would be 15-fold greater in the system treating APP and would be administered in animals approaching market age. In terms of actual used doses, the two scenarios would be deemed equivalent in antimicrobial use. If a defined daily dose approach were employed, it would likely overestimate use (on a dose basis) in the older pigs and underestimate use in the younger pigs, to a degree that would depend on the assumed standard weight at treatment. The question as to which of the three comparisons might best indicate the relative likelihood of selection for AMR of human health relevance remains unanswerable. We observe analogous problems in broiler and turkey production in which generalizations about indications and weight at treatment confound interpretation of antimicrobial use data (Singer et al., 2020a, 2020b).
The origin and implementation of this project reflect recognition of the need to advance antimicrobial stewardship in the United States swine industry. The general support, even without immediate participation, of most systems approached is a foundation for expansion of the project to obtain more representative information across the industry based on voluntary participation and confidential sharing of data. The National Pork Board is currently exploring options to develop a tool for independent producers to input and benchmark data on antimicrobial use.
Impacts.
Demonstrates the feasibility of obtaining data on antimicrobial use in the United States swine industry through sharing of proprietary records within industry.
Provides the first substantial description of antimicrobial use in United States swine beyond annual sales and distribution data.
Indicates that specific metrics of antibiotic use that focus on conditions within individual industries will be most useful to advance stewardship.
ACKNOWLEDGEMENTS
Funding for this project was made possible by the U.S. Food and Drug Administration through grant U01FD005878 and support from the National Pork Board. Views expressed in this publication do not necessarily reflect the official policies of the Department of Health and Human Services, nor does any mention of trade names, commercial practices or organization imply endorsement by the United States Government. Valued input on the development and execution of the project was received from Drs. Mike Apley, Nora Schrag, and Katie Hope, and also Dr. Kathe Bjork and colleagues at the Center for Epidemiology and Animal Health, United States Department of Agriculture. The authors are indebted to the ownership and management of the participating companies who voluntarily shared proprietary data on antimicrobial use in their businesses. We are equally indebted to the veterinarians and staff in those operations who invested time and effort to acquire the data reported in the study. The members on the advisory group convened by the National Pork Board to facilitate industry input are also gratefully acknowledged, particularly Dr. Jennifer Wishnie and Dr. Heather Fowler as coordinators of the group.
Funding information
U.S. Food and Drug Administration, Grant/Award Number: U01FD005878
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- Anderson ES (1968). Drug resistance in Salmonella typhimurium and its implications. British Medical Journal, 3, 333–339. 10.1136/bmj.3.5614.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M, Clift C, Schulze K, Sagan A, Nahrgang S, Ait Ouakrim D, & Mossialos E (2019). Averting the AMR crisis: What are the avenues for policy action for countries in Europe? Copenhagen, Denmark: European Observatory on Health Systems and Policies. https://europepmc.org/article/med/31287637 [PubMed] [Google Scholar]
- Aslam B, Wang W, Arshad MI, Khurshid M, Muzammil S, Rasool MH, … Baloch Z (2018). Antibiotic resistance: A rundown of a global crisis. Infection and Drug Resistance, 11, 1645–1658. 10.2147/IDR.S173867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennani H, Mateus A, Mays N, Eastmure E, Stark KDC, & Hasler B (2020). Overview of evidence of antimicrobial use and antimicrobial resistance in the food chain. Antibiotics-Basel, 9, 49. 10.3390/antibiotics9020049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondt N, Jensen VF, Puister-Jansen LF, & van Geijlswijk IM (2013). Comparing antimicrobial exposure based on sales data. Preventive Veterinary Medicine, 108, 10–20. 10.1016/j.prevetmed.2012.07.009 [DOI] [PubMed] [Google Scholar]
- Burch DGS, & Sperling D (2018). Amoxicillin—current use in swine medicine. Journal of Veterinary Pharmacology and Therapeutics, 41, 356–368. 10.1111/jvp.12482 [DOI] [PubMed] [Google Scholar]
- Coetzee JF, Magstadt DR, Sidhu PK, Follett L, Schuler AM, Krull AC, … O’Connor AM (2019). Association between antimicrobial drug class for treatment and retreatment of bovine respiratory disease (BRD) and frequency of resistant BRD pathogen isolation from veterinary diagnostic laboratory samples. PLoS One, 14(12), e0219104. 10.1371/journal.pone.0219104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collineau L, Belloc C, Stark KD, Hemonic A, Postma M, Dewulf J, & Chauvin C (2016). Guidance on the selection of appropriate indicators for quantification of antimicrobial usage in humans and animals. Zoonoses and Public Health, 64, 165–184. 10.1111/zph.12298 [DOI] [PubMed] [Google Scholar]
- DANMAP 2018 (2019). Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. Retrieved from https://www.danmap.org/-/media/arkiv/projekt-sites/danmap/danmap-reports/danmap-2018/dan-map_2018.pdf?la=en
- Davies PR (2012). One world, one health: The threat of emerging swine diseases. A north American perspective. Transboundary and Emerging Diseases, 59(SUPPL. 1), 18–26. 10.1111/j.1865-1682.2012.01312.x [DOI] [PubMed] [Google Scholar]
- Davies P (2017). Assessment of alternatives for monitoring antimicrobial use in the swine industry and design and implementation of a pilot system. Retrieved from https://www.pork.org/wp-content/uploads/2018/02/15-186-DAVIES-final-report.pdf
- Gottschalk M (2012). Streptococcosis. In Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ & Stevenson GW (Eds.), Diseases of swine (12th ed., pp. 841–855). Chichester, UK: Wiley-Blackwell. [Google Scholar]
- Graham DW, Bergeron G, Bourassa MW, Dickson J, Gomes F, Howe A, … Wittum TE (2019). Complexities in understanding antimicrobial resistance across domesticated animal, human, and environmental systems. Annals of the New York Academy of Sciences, 1441, 17–30. 10.1111/nyas.14036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson DJ, Lugsomya K, La T, Phillips ND, Trott DJ, & Abraham S (2019). Antimicrobial resistance in Brachyspira – An increasing problem for disease control. Veterinary Microbiology, 229, 59–71. 10.1016/j.vetmic.2018.12.019 [DOI] [PubMed] [Google Scholar]
- Hayer SS, Rovira A, Olsen K, Johnson TJ, Vannucci F, Rendahl A, … Alvarez J (2020a). Prevalence and trend analysis of antimicrobial resistance in clinical Escherichia coli isolates collected from diseased pigs in the USA between 2006 and 2016. Transboundary and Emerging Diseases. 10.1111/tbed.13528 [DOI] [PubMed] [Google Scholar]
- Hayer SS, Rovira A, Olsen K, Johnson TJ, Vannucci F, Rendahl A, … Alvarez J (2020b). Prevalence and time trend analysis of antimicrobial resistance in respiratory bacterial pathogens collected from diseased pigs in USA between 2006–2016. Research in Veterinary Science, 128, 135–144. 10.1016/j.rvsc.2019.11.010 [DOI] [PubMed] [Google Scholar]
- Holmer I, Salomonsen CM, Jorsal SE, Astrup LB, Jensen VF, Høg BB, & Pedersen K (2019). Antibiotic resistance in porcine pathogenic bacteria and relation to antibiotic usage. BMC Veterinary Research, 15, 449. 10.1186/s12917-019-2162-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope KJ, Apley MD, Schrag NFD, Lubbers BV, & Singer RS (2020). Antimicrobial use in 22 U.S. beef feedyards: 2016–2017. Zoonoses and Public Health, 67(Suppl. 1): 94–110. 10.1111/zph.12775 [DOI] [PubMed] [Google Scholar]
- Jensen VF, Jacobsen E, & Bager F (2004). Veterinary antimicrobial-usage statistics based on standardized measures of dosage. Preventive Veterinary Medicine, 64, 201–215. 10.1016/j.prevetmed.2004.04.001 [DOI] [PubMed] [Google Scholar]
- Jukes TH (1985). Some historical notes on chlortetracycline. Reviews of Infectious Diseases, 7, 702–707. 10.1093/clinids/7.5.702 [DOI] [PubMed] [Google Scholar]
- Kasabova S, Hartmann M, Werner N, Käsbohrer A, & Kreienbrock L (2019). Used daily dose vs. defined daily dose-contrasting two different methods to measure antibiotic consumption at the farm level. Frontiers in Veterinary. Science, 6(116). 10.3389/fvets.2019.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight GM, Davies NG, Colijn C, Coll F, Donker T, Gifford DR, … Atkins KE (2019). Mathematical modelling for antibiotic resistance control policy: Do we know enough? BMC Infectious Diseases, 19, 1011. 10.1186/s12879-019-4630-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekagul A, Tangcharoensathien V, & Yeung S (2019). Patterns of antibiotic use in global pig production: A systematic review. Veterinary and Animal Science, 7, 100058. 10.1016/j.vas.2019.100058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard EK, Pearl DL, Finley RL, Janecko N, Reid-Smith RJ, Peregrine AS, & Weese JS (2012). Comparison of antimicrobial resistance patterns of Salmonella spp. and Escherichia coli recovered from pet dogs from volunteer households in Ontario (2005–06). Journal of Antimicrobial Chemotherapy, 67, 174–181. 10.1093/jac/dkr430 [DOI] [PubMed] [Google Scholar]
- Lesho EP, & Laguio-Vila M (2019). The slow-motion catastrophe of antimicrobial resistance and practical interventions for all prescribers. Mayo Clinic Proceedings, 94, 1040–1047. 10.1016/j.mayocp.2018.11.005 [DOI] [PubMed] [Google Scholar]
- Lloyd DH, & Page SW (2018). Antimicrobial stewardship in veterinary medicine. In Schwarz S, Cavaco L & Shen J (Eds.), Antimicrobial resistance in bacteria from livestock and companion animals (pp. 675–697). Washington, DC: ASM Press. 10.1128/microbiolspec.ARBA-0023-2017 [DOI] [Google Scholar]
- Loeffler A, & Lloyd DH (2018). What has changed in canine pyoderma? A narrative review. Veterinary Journal, 235, 73–82. 10.1016/j.tvjl.2018.04.002 [DOI] [PubMed] [Google Scholar]
- McCrackin MA, Helke KL, Galloway AM, Poole AZ, Salgado CD, & Marriott BP (2016). Effect of antimicrobial use in agricultural animals on drug-resistant foodborne campylobacteriosis in humans: A systematic literature review. Critical Reviews in Food Science and Nutrition, 56, 2115–2132. 10.1080/10408398.2015.1119798 [DOI] [PubMed] [Google Scholar]
- McEwen SA, & Collignon PJ (2018). Antimicrobial resistance: A one health perspective. In Schwarz S, Cavaco L & Shen J (Eds.), Resistance in bacteria from livestock and companion animals (pp. 521–547). Washington, DC: ASM Press. 10.1128/microbiolspec.ARBA-0009-2017 [DOI] [Google Scholar]
- Merle R, & Meyer-Kühling B (2019). Sales data as a measure of antibiotics usage: Concepts, examples and discussion of influencing factors. Veterinary Medicine and Science, 6, 154–163. 10.1002/vms3.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills HL, Turner A, Morgans L, Massey J, Schubert H, Rees G, … Reyher KK (2018). Evaluation of metrics for benchmarking antimicrobial use in the UK dairy industry. Veterinary Record, 182, 379. 10.1136/vr.104701 [DOI] [PubMed] [Google Scholar]
- Montgomery MP, Robertson S, Koski L, Salehi E, Stevenson LM, Silver R, … Laughlin ME (2018). Multidrug-resistant campylobacter jejuni outbreak linked to puppy exposure - United States, 2016–2018. Morbidity and Mortality Weekly Report, 67, 1032–1035. 10.15585/mmwr.mm6737a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- More SJ (2020). European perspectives on efforts to reduce antimicrobial usage in food animal production. Irish Veterinary Journal, 73, 2. 10.1186/s13620-019-0154-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley PS, Apley MD, Besser TE, Burney DP, Fedorka-Cray PJ, Papich MG, … Weese JS (2005). Antimicrobial drug use in veterinary medicine. Journal of Veterinary Internal Medicine, 9, 617–629. 10.1892/0891-6640(2005)19[617:ADUIVM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nelson JM, Chiller TM, Powers JH, & Angulo FJ (2007). Fluoroquinolone-resistant campylobacter species and the with drawal of fluoroquinolones from use in poultry: A public health success story. Clinical Infectious Diseases, 44, 977–980. 10.1086/512369 [DOI] [PubMed] [Google Scholar]
- Palma E, Tilocca B, & Roncada P (2020). Antimicrobial resistance in veterinary medicine: An overview. International Journal of Molecular Sciences, 21, E1914. 10.3390/ijms21061914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock J, Muwonge A, Hutchings MR, Mainda G, Bronsvoort BM, Gally DL, & Corbishley A (2020). Resistance to change: AMR gene dynamics on a commercial pig farm with high antimicrobial usage. Scientific Reports, 10, 1708. 10.1038/s41598-020-58659-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma M, Sjolund M, Collineau L, Losken S, Stark KD, Dewulf J & Consortium M (2015). Assigning defined daily doses animal: A European multi-country experience for antimicrobial products authorized for usage in pigs. The Journal of Antimicrobial Chemotherapy, 70, 294–302. 10.1093/jac/dku347 [DOI] [PubMed] [Google Scholar]
- Pozio E, & Zarlenga DS (2013). New pieces of the Trichinella puzzle. International Journal for Parasitology, 43, 983–997. 10.1016/j.ijpara.2013.05.010 [DOI] [PubMed] [Google Scholar]
- Rendle DI, & Page SW (2018). Antimicrobial resistance in companion animals. Equine Veterinary Journal, 50, 147–152. 10.1111/evj.12785 [DOI] [PubMed] [Google Scholar]
- Schrag NFD, Godden SM, Apley MD, Singer RS, & Lubbers BV (2020). Antimicrobial use quantification in adult dairy cows – Part 3 – Use measured by standardized regimens and grams on 29 dairies in the United States. Zoonoses and Public Health, 67(Suppl. 1), 82–93. 10.1111/zph.12773. [DOI] [PubMed] [Google Scholar]
- Singer RS, Porter LJ, Schrag NFD, Davies PR, Apley MD, & Bjork K (2020a). Estimates of on-farm antimicrobial usage in broiler chicken production in the United States, 2013–2017. Zoonoses and Public Health, 67(Suppl. 1), 22–35. 10.1111/zph.12764 [DOI] [PubMed] [Google Scholar]
- Singer RS, Porter LJ, Schrag NFD, Davies PR, Apley MD, & Bjork K (2020b). Estimates of on-farm antimicrobial usage in turkey production in the United States, 2013–2017. Zoonoses and Public Health, 67(Suppl. 1), 36–50. 10.1111/zph.12763 [DOI] [PubMed] [Google Scholar]
- Speksnijder DC, Mevius DJ, Bruschke CJM, & Wagenaar JA (2015). Reduction of veterinary antimicrobial use in the Netherlands. The Dutch Success Model. Zoonoses and Public Health, 62, 79–87. 10.1111/zph.12167 [DOI] [PubMed] [Google Scholar]
- Stege H, Bager F, Jacobsen E, & Thougaard A (2003). VETSTAT-the Danish system for surveillance of the veterinary use of drugs for production animals. Preventive Veterinary Medicine, 57, 105–115. 10.1016/s0167-5877(02)00233-7 [DOI] [PubMed] [Google Scholar]
- U. S. Food and Drug Administration (2019). 2018 Summary report on antimicrobials sold or distributed for use in food producing animals. Retrieved from https://www.fda.gov/media/133411/download
- U.S. Department of Agriculture (2018). Livestock slaughter 2017 summary. Retrieved from https://downloads.usda.library.cornell.edu/usda-es-mis/files/r207tp32d/cn69m6457/pc289m639/LiveSlauSu-04-18-2018.pdf
- U.S. Department of Agriculture (2020). Hog inventory. Retrieved from https://www.nass.usda.gov/Surveys/Guide_to_NASS_Surveys/Hog_Inventory/index.php. Accessed March 23, 2020.
- U.S. Food and Drug Administration (1994). Animal medicinal drug use clarification act. Retrieved from https://www.fda.gov/animal-veterinary/acts-rules-regulations/animal-medicinal-drug-use-clarification-act-1994-amduca
- U.S. Food and Drug Administration (2003). Guidance for Industry #152. Evaluating the safety of antimicrobial new animal drugs with regard to their microbiological effects on bacteria of human health concern. Retrieved from https://www.fda.gov/media/69949/download
- U.S. Food and Drug Administration (2020). Antimicrobial resistance guidances. Retrieved from https://www.fda.gov/animal-veterinary/guidance-industry/antimicrobial-resistance-guidances. Accessed March 23, 2020.
- Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, Robinson TP, Laxminarayan R (2015). Global trends in antimicrobial use in food animals. Proceedings of the National Academy of Sciences of the United States of America, 112(18), 5649–5654. 10.1073/pnas.1503141112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waret-Szkuta A, Coelho V, Collineau L, Hémonic A, Buy C, Treff M, & Raboisson D (2019). How input parameters and calculation rules influence on-farm antimicrobial use indicators in animals. Frontiers in Veterinary Science, 6, 438. 10.3389/fvets.2019.00438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner N, McEwen S, & Kreienbrock L (2018). Monitoring antimicrobial drug usage in animals: Methods and applications. microbiology. Spectrum, 6, ARBA-0015–2017. 10.1128/microbiolspec.arba-0015-2017 [DOI] [PubMed] [Google Scholar]
- World Organization for Animal Health (OIE) (2020). Annual report on antimicrobial agents intended for use in animals. Paris, France. Retrieved from https://www.oie.int/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/A_Fourth_Annual_Report_AMU.pdf. [Google Scholar]


