Abstract
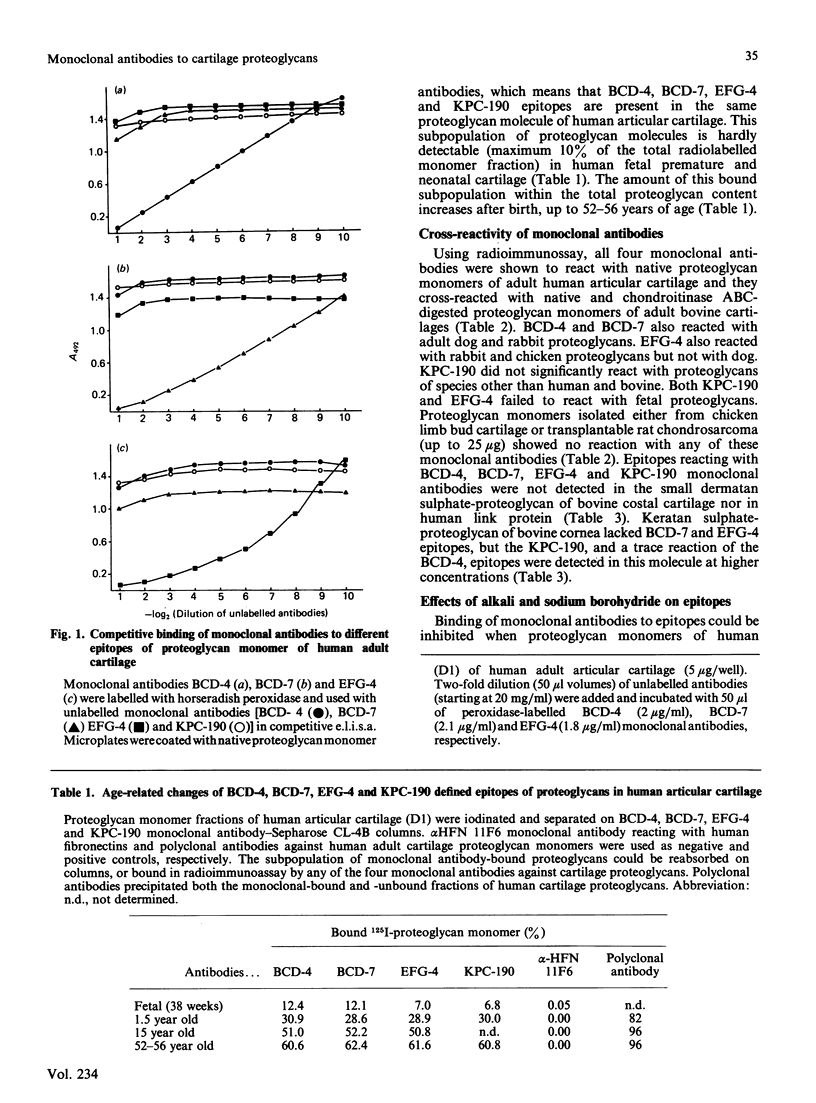
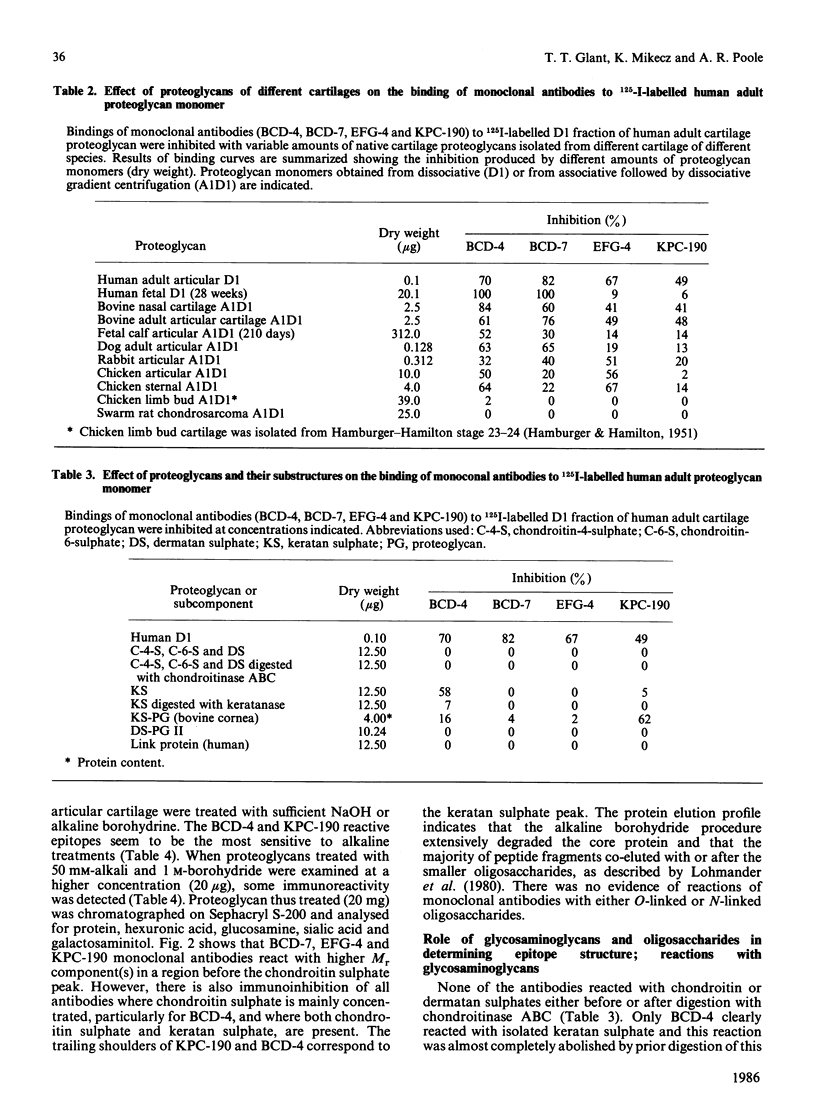
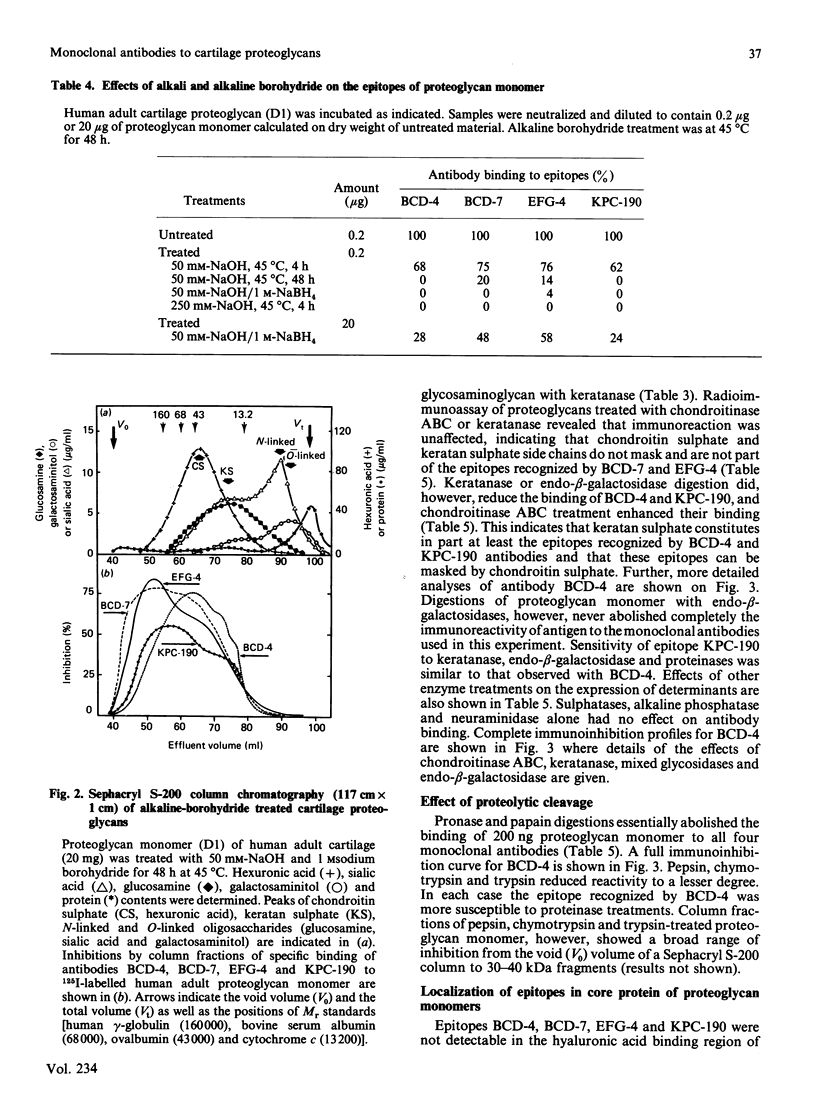
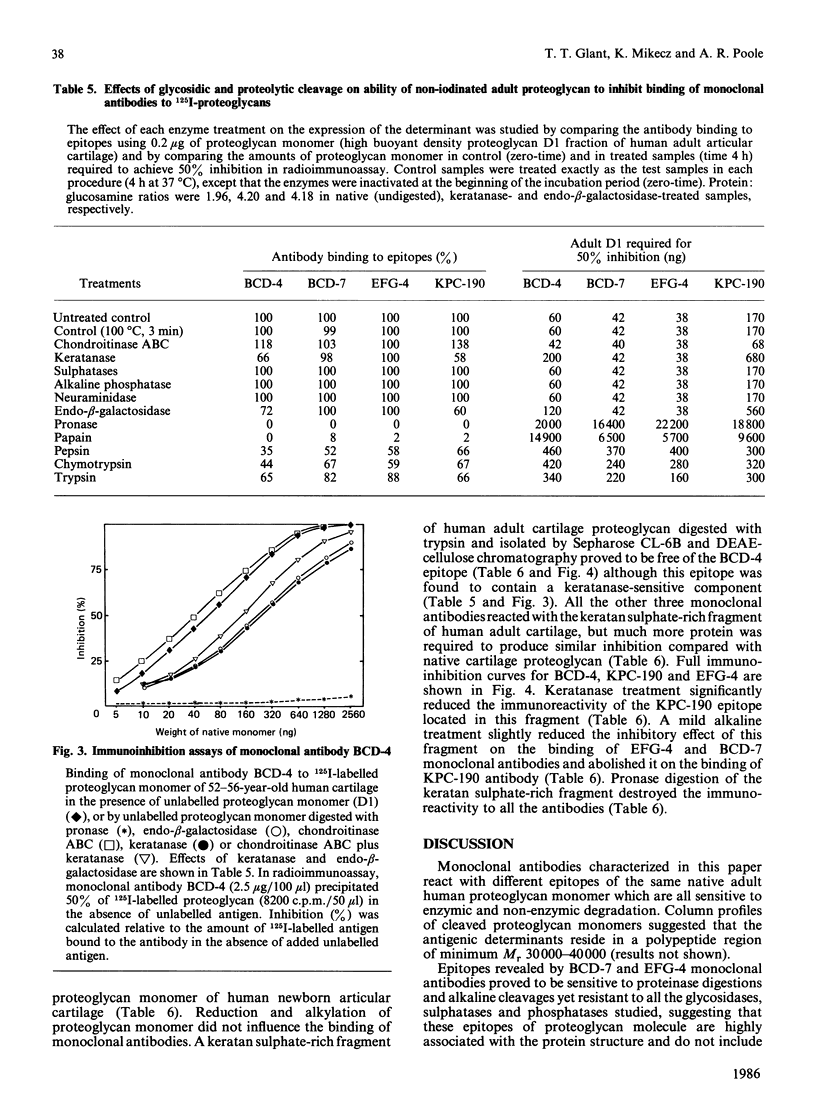
Monoclonal antibodies produced against chondroitinase-treated human adult cartilage proteoglycans were selected for their ability to recognize epitopes on native proteoglycans. Binding analyses revealed that four of these monoclonal antibodies (BCD-4, BCD-7, EFG-4 and KPC-190) each recognized a different epitope on the same proteoglycan molecule which represents a subpopulation of a high buoyant density (D1) fraction of human articular cartilage proteoglycans (10, 30, 50 and 60% in fetal-newborn, 1.5 years old, 15 years old and 52-56 years old cartilages, respectively). Analysis of epitope specificities revealed that BCD-7 and EFG-4 monoclonal antibodies recognized epitopes on proteoglycan monomer which are associated with the protein structure in that they are sensitive to cleavage by Pronase, papain and alkali treatment and do not include keratan sulphate, chondroitin sulphate or oligosaccharides. The BCD-4 and KPC-190 epitopes also proved to be sensitive to Pronase or papain digestion or to alkali treatment, but keratanase or endo-beta-galactosidase also reduced the immunoreactivity of these epitopes. These observations indicate that the BCD-4 and KPC-190 epitopes represent peptides substituted with keratan sulphate or keratan sulphate-like structures. The BCD-4 epitope is, however, absent from a keratan sulphate-rich fragment of human adult proteoglycan, while the other three epitopes were detected in this fragment. None of these four epitopes were detected in the link proteins of human cartilage, in the hyaluronic acid-binding region of human newborn cartilage proteoglycan, in Swarm rat chondrosarcoma proteoglycan, in chicken limb bud proteoglycan monomer and in the small dermatan sulphate-proteoglycan of bovine costal cartilage. EFG-4 and KPC-190 epitopes were not detected in human fetal cartilage proteoglycans, although fetal molecules contained trace amounts of epitopes reactive with BCD-4 and BCD-7 antibodies.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- BOAKE W. C., MUIR H. The non-antigenicity of chondroitin sulphate. Lancet. 1955 Dec 10;269(6902):1222–1223. doi: 10.1016/s0140-6736(55)93005-1. [DOI] [PubMed] [Google Scholar]
- Buckwalter J. A., Poole A. R., Reiner A., Rosenberg L. C. Immunoferritin binding to proteoglycan monomers. An electron microscopic study. J Biol Chem. 1982 Sep 25;257(18):10529–10532. [PubMed] [Google Scholar]
- Carlson D. M. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J Biol Chem. 1968 Feb 10;243(3):616–626. [PubMed] [Google Scholar]
- Caterson B., Christner J. E., Baker J. R. Identification of a monoclonal antibody that specifically recognizes corneal and skeletal keratan sulfate. Monoclonal antibodies to cartilage proteoglycan. J Biol Chem. 1983 Jul 25;258(14):8848–8854. [PubMed] [Google Scholar]
- Champion B. R., Reiner A., Roughley P. J., Poole A. R. Age-related changes in the antigenicity of human articular cartilage proteoglycans. Coll Relat Res. 1982 Jan;2(1):45–60. doi: 10.1016/s0174-173x(82)80040-x. [DOI] [PubMed] [Google Scholar]
- Christner J. E., Caterson B., Baker J. R. Immunological determinants of proteoglycans. Antibodies against the unsaturated oligosaccharide products of chondroitinase ABC-digested cartilage proteoglycans. J Biol Chem. 1980 Aug 10;255(15):7102–7105. [PubMed] [Google Scholar]
- Conrad G. W., Ager-Johnson P., Woo M. L. Antibodies against the predominant glycosaminoglycan of the mammalian cornea, keratan sulfate-I. J Biol Chem. 1982 Jan 10;257(1):464–471. [PubMed] [Google Scholar]
- Couchman J. R., Caterson B., Christner J. E., Baker J. R. Mapping by monoclonal antibody detection of glycosaminoglycans in connective tissues. Nature. 1984 Feb 16;307(5952):650–652. doi: 10.1038/307650a0. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Matsumura G. Endo-beta-galactosidase of Escherichia freundii. Hydrolysis of pig colonic mucin and milk oligosaccharides by endoglycosidic action. Biochem Biophys Res Commun. 1975 May 19;64(2):465–471. doi: 10.1016/0006-291x(75)90344-7. [DOI] [PubMed] [Google Scholar]
- Funderburgh J. L., Stenzel-Johnson P. R., Chandler J. W. Monoclonal antibodies to rabbit corneal keratan sulfate proteoglycan. Curr Eye Res. 1982;2(11):769–776. doi: 10.3109/02713688209020010. [DOI] [PubMed] [Google Scholar]
- Glant T. T. Concanavalin A-binding link protein in the proteoglycan aggregate of hyaline cartilage. Biochem Biophys Res Commun. 1982 May 14;106(1):158–163. doi: 10.1016/0006-291x(82)92071-x. [DOI] [PubMed] [Google Scholar]
- Glant T. T., Hadházy C., Mikecz K., Sipos A. Appearance and persistence of fibronectin in cartilage. Specific interaction of fibronectin with collagen type II. Histochemistry. 1985;82(2):149–158. doi: 10.1007/BF00708199. [DOI] [PubMed] [Google Scholar]
- Glant T., Hadházy C., Csernyánszky H. Species-common antigen of connective tissues. Acta Biol Acad Sci Hung. 1975;26(3-4):197–208. [PubMed] [Google Scholar]
- Glant T. Induction of cartilage degradation in experimental arthritis produced by allogeneic and xenogeneic proteoglycan antigens. Connect Tissue Res. 1982;9(3):137–144. doi: 10.3109/03008208209160254. [DOI] [PubMed] [Google Scholar]
- Glant T., Lévai G., Hadházy C. The localization of proteoglycans and glycoproteins in the hyaline cartilage. Histochemistry. 1977 Sep 22;53(4):291–299. doi: 10.1007/BF00509246. [DOI] [PubMed] [Google Scholar]
- Glant T., Lévai G. Localization of antigenic components in proteoglycan aggregate of bovine nasal cartilage. Histochemistry. 1983;77(2):217–232. doi: 10.1007/BF00506565. [DOI] [PubMed] [Google Scholar]
- HUGHES R. C., JEANLOZ R. W. THE EXTRACELLULAR GLYCOSIDASES OF DIPLOCOCCUS PNEUMONIAE. II. PURIFICATION AND PROPERTIES OF A BETA-N-ACETYLGLUCOSAMINIDASE. ACTION ON A DERIVATIVE ON THE ALPHA-1-ACID GLYCOPROTEIN OF HUMAN PLASMA. Biochemistry. 1964 Oct;3:1543–1548. doi: 10.1021/bi00898a026. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. The specific interaction of hyaluronic acid with cartillage proteoglycans. Biochim Biophys Acta. 1972 Sep 15;279(2):401–405. doi: 10.1016/0304-4165(72)90160-2. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. I. The role of hyaluronic acid. J Biol Chem. 1974 Jul 10;249(13):4232–4241. [PubMed] [Google Scholar]
- Hascall V. C., Kimura J. H. Proteoglycans: isolation and characterization. Methods Enzymol. 1982;82(Pt A):769–800. doi: 10.1016/0076-6879(82)82102-2. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Heinegård D., Axelsson I. Distribution of keratan sulfate in cartilage proteoglycans. J Biol Chem. 1977 Mar 25;252(6):1971–1979. [PubMed] [Google Scholar]
- Heinegård D. Extraction, fractionation and characterization of proteoglycans from bovine tracheal cartilage. Biochim Biophys Acta. 1972 Nov 28;285(1):181–192. doi: 10.1016/0005-2795(72)90190-0. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Paulsson M., Inerot S., Carlström C. A novel low-molecular weight chondroitin sulphate proteoglycan isolated from cartilage. Biochem J. 1981 Aug 1;197(2):355–366. doi: 10.1042/bj1970355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinegård D. Polydispersity of cartilage proteoglycans. Structural variations with size and buoyant density of the molecules. J Biol Chem. 1977 Mar 25;252(6):1980–1989. [PubMed] [Google Scholar]
- Hopwood J. J., Robinson H. C. Studies on the polydispersity and heterogeneity of cartilage proteoglycans. Identification of 3 proteoglycan structures in bovine nasal cartilage. Biochem J. 1975 Dec;151(3):581–594. doi: 10.1042/bj1510581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins R. B., Hall T., Dorfman A. Chondroitin 6-sulfate oligosaccharides as immunological determinants of chick proteoglycans. J Biol Chem. 1981 Aug 25;256(16):8279–8282. [PubMed] [Google Scholar]
- Jourdian G. W., Dean L., Roseman S. The sialic acids. XI. A periodate-resorcinol method for the quantitative estimation of free sialic acids and their glycosides. J Biol Chem. 1971 Jan 25;246(2):430–435. [PubMed] [Google Scholar]
- Keiser H., DeVito J. Immunochemical studies of fragments of bovine nasal cartilage proteoglycan subunit. Connect Tissue Res. 1974;2(4):273–282. doi: 10.3109/03008207409152256. [DOI] [PubMed] [Google Scholar]
- Kimura J. H., Thonar E. J., Hascall V. C., Reiner A., Poole A. R. Identification of core protein, an intermediate in proteoglycan biosynthesis in cultured chondrocytes from the Swarm rat chondrosarcoma. J Biol Chem. 1981 Aug 10;256(15):7890–7897. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loewi G., Muir H. The antigenicity of chondromucoprotein. Immunology. 1965 Aug;9(2):119–127. [PMC free article] [PubMed] [Google Scholar]
- Lohmander L. S., De Luca S., Nilsson B., Hascall V. C., Caputo C. B., Kimura J. H., Heinegard D. Oligosaccharides on proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1980 Jul 10;255(13):6084–6091. [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Newsome D. A., Nilsson B., Hascall V. C., Hassell J. R. Purification of keratan sulfate proteoglycan from monkey cornea. J Biol Chem. 1983 May 25;258(10):6051–6055. [PubMed] [Google Scholar]
- Pacifici M., Soltesz R., Thal G., Shanley D. J., Boettiger D., Holtzer H. Immunological characterization of the major chick cartilage proteoglycan and its intracellular localization in cultured chondroblasts: a comparison with Type II procollagen. J Cell Biol. 1983 Dec;97(6):1724–1736. doi: 10.1083/jcb.97.6.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A. R., Pidoux I., Reiner A., Tang L. H., Choi H., Rosenberg L. Localization of proteoglycan monomer and link protein in the matrix of bovine articular cartilage: An immunohistochemical study. J Histochem Cytochem. 1980 Jul;28(7):621–635. doi: 10.1177/28.7.6156200. [DOI] [PubMed] [Google Scholar]
- Poole A. R., Reiner A., Mort J. S., Tang L. H., Choi H. U., Rosenberg L. C., Caputo C. B., Kimura J. H., Hascall V. C. Cartilage link proteins. Biochemical and immunochemical studies of isolation and heterogeneity. J Biol Chem. 1984 Dec 10;259(23):14849–14856. [PubMed] [Google Scholar]
- Poole A. R., Reiner A., Roughley P. J., Champion B. Rabbit antibodies to degraded and intact glycosaminoglycans which are naturally occurring and present in arthritic rabbits. J Biol Chem. 1985 May 25;260(10):6020–6025. [PubMed] [Google Scholar]
- Poole A. R., Reiner A., Tang L. H., Rosenberg L. Proteoglycans from bovine nasal cartilage. Immunochemical studies of link protein. J Biol Chem. 1980 Oct 10;255(19):9295–9305. [PubMed] [Google Scholar]
- Ratcliffe A., Fryer P. R., Hardingham T. E. The distribution of aggregating proteoglycans in articular cartilage: comparison of quantitative immunoelectron microscopy with radioimmunoassay and biochemical analysis. J Histochem Cytochem. 1984 Feb;32(2):193–201. doi: 10.1177/32.2.6363519. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. C., Choi H. U., Tang L. H., Johnson T. L., Pal S., Webber C., Reiner A., Poole A. R. Isolation of dermatan sulfate proteoglycans from mature bovine articular cartilages. J Biol Chem. 1985 May 25;260(10):6304–6313. [PubMed] [Google Scholar]
- Roughley P. J., Poole A. R., Mort J. S. The heterogeneity of link proteins isolated from human articular cartilage proteoglycan aggregates. J Biol Chem. 1982 Oct 25;257(20):11908–11914. [PubMed] [Google Scholar]
- Roughley P. J., White R. J. Age-related changes in the structure of the proteoglycan subunits from human articular cartilage. J Biol Chem. 1980 Jan 10;255(1):217–224. [PubMed] [Google Scholar]
- Sandson J., Rosenberg L., White D. The antigenic determinants of the protein-polysaccharides of cartilage. J Exp Med. 1966 May 1;123(5):817–828. doi: 10.1084/jem.123.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda S., Schlamowitz M. Studies of 125I trace labeling of immunoglobulin G by chloramine-T. Immunochemistry. 1970 Nov;7(11):885–898. doi: 10.1016/0019-2791(70)90051-0. [DOI] [PubMed] [Google Scholar]
- Stanescu V., Maroteaux P., Sobczak E. Proteoglycan populations of baboon (Papio papio) articular cartilage. Biochem J. 1977 Apr 1;163(1):103–109. doi: 10.1042/bj1630103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanescu V., Stanescu R. The distribution of proteoglycans of high electrophoretic mobility in cartilages from different species and of different ages. Biochim Biophys Acta. 1983 Jun 9;757(3):377–381. doi: 10.1016/0304-4165(83)90065-x. [DOI] [PubMed] [Google Scholar]
- Stevens J. W., Oike Y., Handley C., Hascall V. C., Hampton A., Caterson B. Characteristics of the core protein of the aggregating proteoglycan from the Swarm rat chondrosarcoma. J Cell Biochem. 1984;26(4):247–259. doi: 10.1002/jcb.240260405. [DOI] [PubMed] [Google Scholar]
- Tang L. H., Rosenberg L., Reiner A., Poole A. R. Proteoglycans from bovine nasal cartilage. Properties of a soluble form of link protein. J Biol Chem. 1979 Oct 25;254(20):10523–10531. [PubMed] [Google Scholar]
- Upholt W. B., Vertel B. M., Dorfman A. Translation and characterization of messenger RNAs in differentiating chicken cartilage. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4847–4851. doi: 10.1073/pnas.76.10.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upholt W. B., Vertel B. M., Dorfman A. Translation and characterization of messenger RNAs in differentiating chicken cartilage. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4847–4851. doi: 10.1073/pnas.76.10.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venn G., Mason R. M. Absence of keratan sulphate from skeletal tissues of mouse and rat. Biochem J. 1985 Jun 1;228(2):443–450. doi: 10.1042/bj2280443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Ishii T., Nariuchi H. Fractionation of IgG, IgG2a, IgG2b and IgG3 immunoglobulins from mouse serum by protein A-sepharose column chromatography. Jpn J Exp Med. 1981 Feb;51(1):65–70. [PubMed] [Google Scholar]
- Wieslander J., Heinegård D. Immunochemical analysis of cartilage proteoglycans. Antigenic determinants of substructures. Biochem J. 1979 Apr 1;179(1):35–45. doi: 10.1042/bj1790035. [DOI] [PMC free article] [PubMed] [Google Scholar]


