Abstract
BACKGROUND
Responsive neurostimulation (RNS) has emerged as an effective neuromodulatory intervention for patients with medically refractory epilepsy who are not candidates for resective or ablative surgery. However, in patients with multifocal seizures arising from a widely distributed network, optimizing lead placement can be challenging.
OBSERVATIONS
Here, the authors present the case of a patient with drug-resistant multifocal, nonlateralizing seizures and multiple developmental brain lesions who underwent phase II monitoring with stereoelectroencephalography electrodes targeting the lesion and surrounding cortex as well as the centromedian thalamus. Neurophysiological signals observed during recorded events implicated a seizure network within the left perisylvian polymicrogyria, involving the left parietal operculum, insula, and centromedian thalamic regions rather than a single focus.
LESSONS
Using a regional RNS approach to modulate this network, the patient improved from 5 seizures a day to freedom from disabling seizures shortly after lead implantation despite low stimulation parameters. This has implications for understanding the timescale of adaptive mechanisms that occur in response to stimulation and supports the use of RNS as a surgical treatment for drug-resistant epilepsy.
Keywords: responsive neurostimulation, neuromodulation, centromedian nucleus, intracranial electroencephalography, thalamus, regional approach
ABBREVIATIONS: ASM = antiseizure medication, CMT = centromedian nucleus of the thalamus, DBS = deep brain stimulation, DRE = drug-resistant epilepsy, EEG = electroencephalography, EMU = epilepsy monitoring unit, MRI = magnetic resonance imaging, PSYL = posterior sylvian fissure, RNS = responsive neurostimulation, SEEG = stereo-EEG, SOZ = seizure onset zone.
Epilepsy is a highly prevalent disorder, affecting more than 45 million people worldwide.1 Antiseizure medications (ASMs) effectively control seizures and allow for an improved quality of life for approximately two-thirds of patients. For the remaining patients with medically refractory epilepsy, surgery may be the best way to reduce or eliminate seizures.2 Resective or ablative procedures, such as anterior temporal lobectomy and laser amygdalohippocamptomy, have shown marked efficacy over medication management.3 However, patients with multifocal epilepsy or who are otherwise considered high risk for resection require alternative treatment options.4–7
Responsive neurostimulation (RNS) is a neuromodulatory intervention that allows for the treatment of epileptogenic zones through closed-loop detection of ictal activity and subsequent stimulation to terminate seizures.8, 4 Because closed-loop detection and stimulation are thought to be most effective when delivered at the seizure focus, RNS leads are typically targeted toward the hypothesized seizure onset zone (SOZ).8 However, in patients in whom seizures arise from a widely distributed network, optimal lead placement can be challenging. One emerging strategy in these cases is to position the RNS leads to target nodes within the seizure network through a regional approach.9, 10
In the following case report, we present an example of a patient with drug-resistant multifocal, nonlateralizing seizures and multiple developmental brain lesions. This patient underwent phase II monitoring with stereoelectroencephalography (SEEG) electrodes targeting the lesion and the surrounding cortex as well as the centromedian thalamus. Neurophysiological signals observed during recorded events implicated a seizure network within the left perisylvian polymicrogyria, involving the left parietal operculum, insula, and centromedian thalamic regions rather than a single focus. Using a regional RNS approach to modulate this network, the patient improved from 5 seizures a day to freedom from disabling seizures.
Illustrative Case
A 25-year-old female with a history of global developmental delay, multiple congenital malformations, and drug-resistant epilepsy (DRE) was referred to the neurology service by a community provider to assess whether her seizures were psychogenic nonepileptic or epileptic. Her history of seizures began at the age of 6 months as focal aware seizures consisting of left-sided clonic activity. She was started on ASMs, eventually reaching seizure freedom intervals of 1–2 years with levetiracetam, clonazepam, and oxcarbazepine. She was previously trialed on phenobarbital and lamotrigine during childhood and had no family history of seizures.
At the age of 25 years, during a period of increased family and life stressors, she developed a new semiology, described as a sudden fall with preserved awareness lasting seconds. These seizures occurred between 1 and 5 times a day and resulted in numerous head strikes and falls, impacting her quality of life. There was no change in frequency with increases in her ASM or the addition of new agents (e.g., lacosamide and cenobamate). Due to family concerns and a history of head strikes and falls, she was referred to the epilepsy monitoring unit (EMU) for diagnostic evaluation of nonepileptic versus epileptic events.
Diagnostic EMU admission revealed delta-theta slowing in the right temporal region and multifocal discharges over the left frontal region and independently over the right and left temporal regions. Five very brief (< 4 seconds) stereotyped clinical events during her stay demonstrated right lower-extremity flexion, left head version, and right-hand dystonia. Although there was no electroencephalography (EEG) correlate, given the semiology and multiple possible epileptic foci, these events were thought to be scalp-negative seizures.
Magnetic resonance imaging (MRI) demonstrated scattered abnormalities, including an absent corpus callosum, callosal dysgenesis from prior lipoma, bilateral perisylvian polymicrogyria, gray matter heterotopias, and focal cortical dysplasia. Hippocampi were symmetric and normal in size bilaterally with intact interior architecture. Positron emission tomography imaging demonstrated mild asymmetric diminished activity in the left mid-temporoparietal region. Magnetoencephalography showed frequent interictal epileptiform activity clustering in the left temporoparietal cortex and right temporal lobe. Neuropsychological testing showed notable impairments in executive learning and memory as well as overall moderate to severe impairment across all measured domains in a pattern suggestive of stronger right-hemisphere dysfunction. However, this lateralizing pattern was difficult to attribute to a precise seizure focus due to the presence of multiple structural brain abnormalities.
Given the patient’s asymmetry of semiology and nonlateralizing seizures with multifocal interictal abnormalities on EEG, the seizures were hypothesized to represent asymmetric tonic seizures, with a deep frontal or perisylvian onset. To more precisely characterize the seizure focus, a bilateral SEEG lead implantation strategy targeting the multiple lesions, frontal region, and perisylvian region was developed.
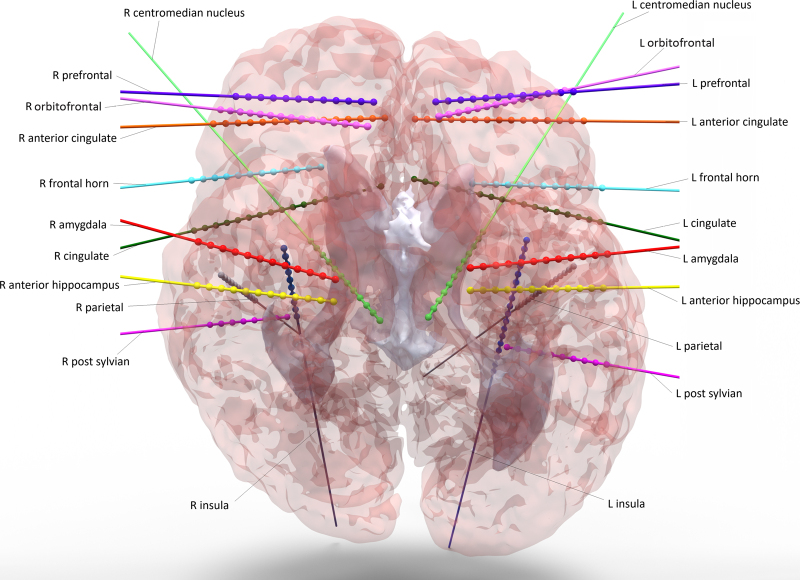
The patient underwent MRI and CT-guided placement of 22 depth leads (with a total of 305 contacts) bilaterally in the following locations (Fig. 1): insula, anterior cingulate cortex, frontal horn, prefrontal cortex, parietal region, cingulate, centromedian nucleus of the thalamus (CMT), amygdala, anterior hippocampus, orbitofrontal cortex, and posterior sylvian fissure (PSYL). Postoperatively, the patient was admitted to the EMU and recovered without complication.
FIG. 1.
Reconstructed SEEG electrodes using preoperative MRI and postoperative CT. L = left; R = right.
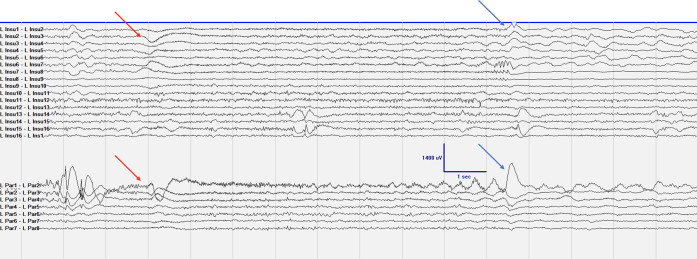
During her EMU stay, the patient had 24 typical seizures, each lasting < 10 seconds, with bilateral lower-extremity adduction and left head version, with variable right upper-extremity elevation or figure 4 posturing. These seizures were preceded by a DC shift of 500 ms prior to electrographic onset followed by continued rhythmic activity within the left insular, parietal, and PSYL leads (Fig. 2). In approximately half the seizures, the left CMT also demonstrated high gamma frequency activity simultaneously with the onset of seizure activity on SEEG. Stimulation of the left parietal region and left PSYL contacts during seizure stimulation testing most consistently led to the wave sensation over the patient’s legs and sensation of head turn or leg elevation (without the corresponding actions). These findings suggested potential spread to the SOZ, which was postulated to lie between these two electrodes. Given these findings and the lack of language involvement in the patient’s seizure semiology, no language mapping or functional MRI studies were performed. After acquiring sufficient evidence, all depth electrodes were explanted on postoperative day 7. The patient recovered without complications and was discharged the next day on her typical ASMs.
FIG. 2.
Typical seizure with LPar1–2 sentinel spike, followed by a large DC shift in LPar1–2 and LInsul2–7 (red arrow). Low-voltage fast activity evolving into delta slowing is seen primarily in LPar1–2. The blue arrows demonstrate an abrupt offset with postictal slowing. LInsul = left insula; LPar = left parietal (operculum).
Based on the data collected during phase II monitoring, there was no single focus; the patient’s asymmetric tonic seizures were thought to arise from a network that included the left perisylvian polymicrogyria and frequently the left CMT. Given the multifocal nature of the patient’s seizure semiology, the comprehensive epilepsy team recommended an RNS system targeting the left parietal operculum and left insula over deep brain stimulation (DBS) of the anterior nucleus of the thalamus or CMT. This choice of RNS over DBS was also motivated by the consistently observed activity in the left parietal operculum and left insular nodes versus the variable involvement of the left CMT during seizures. The patient agreed to proceed with this intervention and underwent an uncomplicated RNS device placement with depth electrodes (Fig. 3). The device was turned on 3 months after the initial implant to low bipolar settings (left insula: 200 Hz, 160 μs, 100 ms, 0.3 mA; left operculum: 200 Hz, 160 μs, 100 ms, 0.5 mA) and later up-titrated (left insula: 0.6 mA, 200 Hz, 160 μs, 100 ms; left operculum: 1.0 mA, 200 Hz, 160 μs, 100 ms). After the second increase, roughly 3 months after stimulation activation, she was completely seizure free for 7 months. At the 18-month follow-up, she continued to have no typical seizures with left head version, bilateral lower-extremity adduction, or right lower-extremity extension with variable right upper-extremity extension and right-hand dystonia. However, a new semiology occurred, consisting of a seconds-long head drop with right upper-extremity extension and right-hand dystonia. As there was no involvement of the lower extremities, she continued to remain free of falls and injuries. RNS parameters were modified to the left insula (1 mA, 200 Hz, 160 μs, 100 ms, 1 μC/cm2) and left operculum (2 mA, 200 Hz, 160 μs, 100 ms, 2 μC/cm2). At the most recent follow-up, it became clear that overstimulation of the insula was likely causing an increase in seizure activity, so the patient’s settings were changed (left insula: 0.8 mA, 200 Hz, 160 μs, 170 ms, 0.8 μC/cm2; left operculum: 1.5 mA, 200 Hz, 160 μsc, 100 ms, 1.5 μC/cm2). There was no reduction in ASMs throughout this period.
FIG. 3.
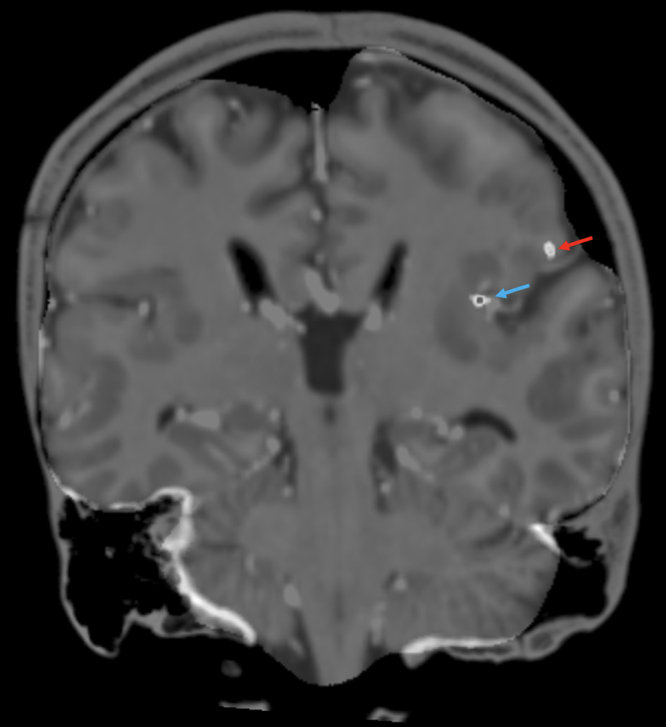
Coronal T1-weighted MRI coregistered with postoperative CT showing RNS contacts in the left insula (blue arrow) and left parietal operculum (red arrow).
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
Observations
Circuit-based neuromodulation for the treatment of DRE has become central to epilepsy surgery. Neuromodulatory devices such as those for RNS and DBS have become increasingly valuable tools for treating patients with regional seizure onset or SOZs in eloquent areas of the brain.11–13 To effectively treat patients using this approach, identification of nodes within a patient’s seizure network is critical14, 15 but can often be challenging.16 Here, we present the case of a patient with drug-resistant multifocal, nonlateralizing seizures and multiple developmental brain lesions. Findings from invasive monitoring implicated a seizure network involving the left parietal, insular, and centromedian thalamic regions rather than a single focus. Using an RNS approach to modulate regional nodes within this network, the patient improved from 5 focal aware seizures a day to an Engel class IA outcome within 3 months of stimulation activation (6 months after implantation) and Engel class IB at the 18-month outcome.
Previous work has demonstrated that in patients with drug-resistant regional neocortical epilepsy, RNS is a feasible treatment option with long-term outcomes comparable to those of other interventions.9 The initial seizure freedom seen in the present case supports this finding and further suggests that RNS may be the optimal treatment strategy for the management of seizures originating from a broad network. Notably, our patient had a bipolar electrode configuration compared to the lead-to-lead configuration as in the work by Ma and colleagues, demonstrating that this stimulation pattern may also be effective for treating multifocal seizures.9 The RNS settings for this patient were also remarkably low (initially 0.3 μC/cm2 and maximum 1 μC/cm2 at the most recent follow-up) compared to those in prior studies. Further work to investigate the impact of RNS settings and lead configurations on outcomes is necessary.
Although the precise effects of brain stimulation on epileptogenic networks remain unclear, it is hypothesized that the therapeutic benefit may come from disrupting or suppressing aberrant activity at the site of seizure onset to restore normal oscillatory patterns.17, 18 However, recent evidence has suggested that improvement in seizure outcomes may come from long-term, stimulation-induced changes in seizure networks rather than the target of single regions, as in DBS.19, 20 Importantly, the timescale (e.g., weeks versus months versus years) in which plasticity-dependent changes in network activity occur remains unclear. In the present case, an Engel IA outcome was achieved within 3 months, a shorter time frame than typically observed for adaptive changes to occur, and persisted for at least 7 months. Additionally, the electrocorticography onset detected by the RNS system preceded clinical symptoms by only 500 ms This time window is shorter than what prior work has suggested is needed to terminate a seizure before the onset of behavioral symptoms.19
These findings suggest that network modulation or modulation of specific nodes within a network leading to seizure reduction can, at least partially, operate on a shorter timescale and that effective seizure control in the regional RNS approach may cumulatively build through modulation over successive stimulation events.
Lessons
Here, we present a patient with drug-resistant multifocal epilepsy treated with RNS using a regional approach. Seizure freedom was achieved shortly after system implantation despite low stimulation parameters. This result has implications for our understanding of the timescale of adaptive mechanisms that occur in response to stimulation and supports the use of RNS as a surgical treatment for drug-resistant epilepsy.
Disclosures
Dr. Kimata reported previously owned shares of Neuropace outside the submitted work.
Author Contributions
Conception and design: all authors. Acquisition of data: Collins, Asaad, Ayub. Analysis and interpretation of data: Kimata, Ayub. Drafting the article: Kimata, Asaad. Critically revising the article: Kimata, Asaad, Ayub. Reviewed submitted version of manuscript: Kimata, Asaad, Ayub. Approved the final version of the manuscript on behalf of all authors: Kimata. Statistical analysis: Kimata. Administrative/technical/material support: Collins, Asaad, Ayub. Study supervision: Asaad, Ayub.
Correspondence
Anna R. Kimata: Rhode Island Hospital/Brown University, Providence, RI. anna_kimata@brown.edu.
References
- 1.GBD 2016 Epilepsy Collaborators. Global, regional, and national burden of epilepsy, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(4):357-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kobau R, Zahran H, Thurman DJ, et al. Epilepsy surveillance among adults–19 states, Behavioral Risk Factor Surveillance System, 2005. MMWR Surveill Summ. 2008;57(6):1-20. [PubMed] [Google Scholar]
- 3.Wiebe S, Blume WT, Girvin JP, Eliasziw M, Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345(5):311-318. [DOI] [PubMed] [Google Scholar]
- 4.Skarpaas TL, Jarosiewicz B, Morrell MJ. Brain-responsive neurostimulation for epilepsy (RNS® System). Epilepsy Res. 2019;153:68-70. [DOI] [PubMed] [Google Scholar]
- 5.Fisher R, Salanova V, Witt T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899-908. [DOI] [PubMed] [Google Scholar]
- 6.Beaudreault CP, Muh CR, Naftchi A, et al. Responsive neurostimulation targeting the anterior, centromedian and pulvinar thalamic nuclei and the detection of electrographic seizures in pediatric and young adult patients. Front Hum Neurosci. 2022;16:876204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burdette D, Mirro EA, Lawrence M, Patra SE. Brain-responsive corticothalamic stimulation in the pulvinar nucleus for the treatment of regional neocortical epilepsy: a case series. Epilepsia Open. 2021;6(3):611-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geller EB. Responsive neurostimulation: review of clinical trials and insights into focal epilepsy. Epilepsy Behav. 2018;88S:11-20. [DOI] [PubMed] [Google Scholar]
- 9.Ma BB, Fields MC, Knowlton RC, et al. Responsive neurostimulation for regional neocortical epilepsy. Epilepsia. 2020;61(1):96-106. [DOI] [PubMed] [Google Scholar]
- 10.Zheng B, Liu DD, Theyel BB, et al. Thalamic neuromodulation in epilepsy: a primer for emerging circuit-based therapies. Expert Rev Neurother. 2023;23(2):123-140. [DOI] [PubMed] [Google Scholar]
- 11.Kwon CS, Schupper AJ, Fields MC, et al. Centromedian thalamic responsive neurostimulation for Lennox-Gastaut epilepsy and autism. Ann Clin Transl Neurol. 2020;7(10):2035-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elder C, Friedman D, Devinsky O, Doyle W, Dugan P. Responsive neurostimulation targeting the anterior nucleus of the thalamus in 3 patients with treatment-resistant multifocal epilepsy. Epilepsia Open. 2019;4(1):187-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Velasco AL, Velasco F, Jiménez F, et al. Neuromodulation of the centromedian thalamic nuclei in the treatment of generalized seizures and the improvement of the quality of life in patients with Lennox-Gastaut syndrome. Epilepsia. 2006;47(7):1203-1212. [DOI] [PubMed] [Google Scholar]
- 14.Schaper FLWVJ, Nordberg J, Cohen AL, et al. Mapping lesion-related epilepsy to a human brain network. JAMA Neurol. 2023;80(9):891-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aghakhani Y, Rosati A, Olivier A, Gotman J, Andermann F, Dubeau F. The predictive localizing value of tonic limb posturing in supplementary sensorimotor seizures. Neurology. 2004;62(12):2256-2261. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda A, Sato T, Ohara S, et al. “Supplementary motor area (SMA) seizure” rather than “SMA epilepsy” in optimal surgical candidates: a document of subdural mapping. J Neurol Sci. 2002;202(1-2):43-52. [DOI] [PubMed] [Google Scholar]
- 17.Hess CW, Vaillancourt DE, Okun MS. The temporal pattern of stimulation may be important to the mechanism of deep brain stimulation. Exp Neurol. 2013;247:296-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heck CN, King-Stephens D, Massey AD, et al. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia. 2014;55(3):432-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kokkinos V, Sisterson ND, Wozny TA, Richardson RM. Association of closed-loop brain stimulation neurophysiological features with seizure control among patients with focal epilepsy. JAMA Neurol. 2019;76(7):800-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boddeti U, McAfee D, Khan A, Bachani M, Ksendzovsky A. Responsive neurostimulation for seizure control: current status and future directions. Biomedicines. 2022;10(11):2677. [DOI] [PMC free article] [PubMed] [Google Scholar]