Abstract
The mammalian Toll-like receptor 4, TLR4, is an important component in the innate immune response to gram-negative bacterial infection. The role of TLR4 in antiviral immunity has been largely unexplored. In this study, the in vivo immune responses to respiratory syncytial virus (RSV) and influenza virus infection were examined in TLR4-deficient (C57BL/10ScNCr) and TLR4-expressing (C57BL/10Sn) mice. TLR4-deficient mice challenged with RSV, but not influenza virus, exhibited impaired natural killer (NK) cell and CD14+ cell pulmonary trafficking, deficient NK cell function, impaired interleukin-12 expression, and impaired virus clearance compared to mice expressing TLR4. These findings suggest that Toll signaling pathways have an important role in innate immunity to RSV.
The mammalian Toll-like receptors (TLR), a family of proteins structurally related to Drosophila Toll protein, were identified as critical regulators of innate immunity to a variety of microbes, including gram-positive and -negative bacteria, mycobacteria, and fungi (7, 12, 13, 14, 23, 24). Several studies suggest that Toll-like receptor 2 (TLR2) is a signaling receptor for gram-positive bacteria and fungi (2, 7, 24, 27, 32). TLR4 has recently been shown to be the signal-transducing receptor activated by bacterial lipopolysaccharide (LPS); and mice in which the TLR4 gene is either mutated or missing are hyporesponsive to LPS and do not respond with shock to gram-negative bacterial infection (21, 22). The conserved nature of the TLR and their role in innate immunity suggest that other infectious pathogens, such as viruses, might also activate the innate immune response via the Toll signaling pathway. Several studies from our laboratory indicate that the innate immune response is an important component of respiratory syncytial virus (RSV) immunity (29, 30). Recent in vitro evidence that TLR4 and CD14 are involved in the innate immune responses to the RSV F glycoprotein prompted us to investigate the role of TLR4 in the in vivo immune response to RSV infection.
RSV is the single most important cause of lower respiratory tract disease in infants and young children worldwide and is a high priority for vaccine development. Unfortunately, a broad range of approaches toward RSV vaccine development has not yet produced a safe and effective vaccine. RSV is a member of the family Paramyxoviridae, existing as an enveloped virus containing a negative-sense, single-stranded RNA genome that encodes 11 proteins. Two major surface viral glycoproteins, the F (fusion) and G (attachment) glycoproteins, are associated with the induction of neutralizing antibodies and long-term protective immunity (4, 25). The F glycoprotein has been reported to induce primarily a Th1-type immune response, while the G glycoprotein induces primarily a Th2-type immune response contributing to both protective immunity and disease pathogenesis (6, 9, 20). We have recently reported that the G glycoprotein can alter pulmonary trafficking of natural killer (NK) cells and polymorphonuclear cells (PMNs), inhibit Th1-cytokine expression, and alter macrophage inflammatory protein 1α (MIP-1α), MIP-1β, MIP-2, monocyte chemoattractant protein 1 (MCP-1), and interferon-inducible protein 10 (IP-10) chemokine mRNA expression in bronchoalveolar cells (29, 30), suggesting that the innate immune response is important during RSV infection. It is anticipated that a better understanding of viral and host mechanisms that affect RSV immunity might facilitate vaccine development.
In this study, we examine the in vivo innate immune response in TLR4-deficient (TLR4null) C57BL/10ScNCr mice and wild-type C57BL/10Sn mice (TLR4wt) challenged with RSV or influenza virus to address the role of TLR4 in the innate immune response to a respiratory virus infection. We chose to compare RSV and influenza virus for several reasons. Influenza virus, like RSV, is a major respiratory pathogen, causing significant morbidity and mortality in young children, immunocompromised adults, and the elderly. A member of the Orthomyxoviridae family, influenza virus is an enveloped negative-stranded RNA virus, and like RSV, it primarily infects the respiratory epithelium, causing cytopathology and inflammation of the respiratory tract. The results of this study indicate that TLR4 is important for activation of the innate immune response to RSV infection and may be important to the pathogenesis of RSV disease.
MATERIALS AND METHODS
Animals
Specific-pathogen-free, 6-to-8-week-old, female C57BL/10ScNCr (TLR4null) (National Cancer Institute, Bethesda, Md.) and C57BL/10Sn (TLR4wt) (Jackson Laboratory, Bar Harbor, Maine) mice were examined. The C57BL/10ScNCr strain is homozygous for a null mutation of the TLR4 gene (21, 33, 34). A related mouse strain, C57BL/10ScCr (not used in these studies), has a reported defect in interleukin 12 (IL-12)-induced production of gamma interferon (IFN-γ) (16). However, the C57BL/1-ScNCr mice used in the present study were IL-12 responsive and expressed IFN-γ at levels similar to those of the wild type. The C57BL/10Sn strain was used as the control. All studies were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee.
Virus infection.
Mice were anesthetized by intraperitoneal administration of Avertin (2,2,2-tribromoethanol, 0.2 ml/g of body weight; Sigma-Aldrich, St. Louis, Mo.) and intranasally (i.n.) challenged with 106 PFU of the A2 strain of RSV or 240 HAU of a mouse-adapted strain of influenza A virus (HKx31) in Dulbecco's phosphate-buffered saline (PBS) (GIBCO Laboratories, Grand Island, N.Y.).
Viruses
The A2 strain of RSV was propagated in Vero cells (ATCC CCL 881) as previously described (29). The mouse-adapted HKx31 strain of influenza A virus was cultured in embryonated eggs and harvested as previously described (28).
Collection of BAL cells and preparation of NK cells.
Mice were anesthetized with Avertin and exsanguinated by severing the right caudal artery. Bronchoalveolar lavage (BAL) cells were harvested by lavaging the lungs with PBS containing 1% bovine serum albumin (Sigma). Natural killer cells (DX5+) were enriched from BAL cells using the MACS separation system (Miltenyi Biotech, Inc., Auburn, Calif.) according to the manufacturer's instructions. Viability was assessed by trypan blue exclusion. The purity of DX5+ cell populations ranged from 80 to 90% as determined by flow cytometry (Becton Dickinson, Mountain View, Calif.).
Fluorescence-activated cell sorter analysis.
The procedure used for extracellular staining of BAL cells was modified for microculture staining as described previously (30). Fluorescein isothiocyanate-conjugated or phycoerythrin-conjugated anti-CD3ɛ (145-2C11), anti-CD45R/B220 (RA3-6B2), anti-NK cell (2B4 and DX5), antineutrophil (RB6-8C5), and anti-CD14 (rmC5-3) monoclonal antibodies and isotype antibody controls were used (BD-PharMingen, San Diego, Calif.). Ten thousand events were collected and analyzed using a FACScan and Cell Quest software (Becton Dickinson, San Diego, Calif.). Intracellular cytokine staining was modified for microculture staining as described previously (30). Briefly, BAL cells were incubated in PBS containing Golgi Stop (PharMingen) for 3 h at 37°C to accumulate intracellular cytokines. The cells were washed in PBS, stained with anti-CD3, anti-CD4, or anti-CD8 antibody, fixed, and permeabilized in Cytofix/Cytoperm (PharMingen). Cells were washed and resuspended in an appropriate dilution of anti-IL-2 (JES6-5H4), anti-IL-4 (BVD4-1D11), anti-IL-5 (TRFK5), anti-IL-12 (C15.6), or anti-IFN-γ (XMG1.2) antibody diluted in PBS containing permeabilization buffer, stained, washed, and analyzed as described previously (30) (all from PharMingen). IL-12 expression presented in Table 1 was determined by subtracting the total IL-12 expression by ungated BAL cell populations from IL-12 expression by CD3+ BAL cell populations. The total cytokine-expressing CD4+ or CD8+ cell populations were determined by multiplying the percent cytokine-expressing CD4+ or CD8+ cells by the total BAL cell population.
TABLE 1.
Intracellular cytokine expression by BAL cells after primary infection with RSV or influenza virus
| Day | Phenotype/cytokine | Mean % positive IC cytokine expression of BAL cells ± SEMa
|
|||
|---|---|---|---|---|---|
| RSV (wt) | RSV (null) | FLU (wt) | FLU (null) | ||
| 5 | CD3+/IL-2 | 11 ± 2 | 10 ± 1 | 15 ± 1 | 25 ± 1b |
| CD3+/IL-4 | 12 ± 1 | 11 ± 1 | 20 ± 2 | 28 ± 1 | |
| CD3+/IL-5 | 10 ± 0 | 8 ± 1 | 15 ± 1 | 18 ± 2 | |
| CD3+/IFN-γ | 13 ± 1 | 11 ± 1 | 22 ± 2 | 31 ± 1c | |
| CD3−/IL-12 | 29 ± 6 | 7 ± 1d | 28 ± 2 | 6 ± 4e | |
| 7 | CD3+/IL-2 | 6 ± 2 | 8 ± 2 | 10 ± 2 | 8 ± 1 |
| CD3+/IL-4 | 8 ± 4 | 10 ± 0 | 18 ± 2 | 13 ± 2 | |
| CD3+/IL-5 | 6 ± 1 | 4 ± 1 | 21 ± 7 | 19 ± 2 | |
| CD3+/IFN-γ | 11 ± 3 | 9 ± 1 | 26 ± 1 | 18 ± 2 | |
| CD3−/IL-12 | 30 ± 1 | 21 ± 1f | 30 ± 3 | 39 ± 1g | |
| 11 | CD3+/IL-2 | 7 ± 1 | 7 ± 1 | 7 ± 1 | 4 ± 1 |
| CD3+/IL-4 | 8 ± 1 | 8 ± 0 | 9 ± 0 | 4 ± 1 | |
| CD3+/IL-5 | 13 ± 0 | 13 ± 2 | 9 ± 1 | 6 ± 1 | |
| CD3+/IFN-γ | 10 ± 2 | 11 ± 3 | 11 ± 1 | 5 ± 1 | |
| CD3−/IL-12 | 32 ± 6 | 15 ± 5h | 34 ± 4 | 35 ± 5 | |
C57BL/10ScNCr (null) and C57BL/10ScSn (wt) mice were i.n. infected with the A2 strain of RSV or the HKx31 influenza A (FLU) virus. BAL samples from three to five mice were examined at days 5, 7, and 11 p.i. The results of two separate experiments are shown. Data are represented as percent positive intracellular (IC) expression of BAL cells ± SEM. IL-2, -4, and -5, and IFN-γ expression by CD3+ cells and IL-12 expression by CD3− cell populations are presented. Bold type indicates significant differences between TLR4wt and TLR4null cytokine expression.
P value comparing FLU(wt) to FLU(null) is 0.014.
P value comparing FLU(wt) to FLU(null) is 0.029.
P value comparing RSV(wt) to RSV(null) is 0.034.
P value comparing FLU(wt) to FLU(null) is 0.050.
P value comparing RSV(wt) to RSV(null) is 0.011.
P value comparing FLU(wt) to FLU(null) is 0.103.
P value comparing RSV(wt) to RSV(null) is 0.093.
NK Cytotoxicity assays.
YAC-1 cells (ATCC TIB 160) were used as target cells. The cells were maintained in minimal essential medium (SMEM) (GIBCO Laboratories, Grand Island, N.Y.) containing 10% fetal bovine serum (FBS) (37°C, 8% CO2). Two different cytotoxicity assays were used to evaluate NK cytotoxicity. YAC-1 target cells were labeled with either 2 μl of 3 mM DIOC18 (Molecular Probes, Eugene, Ore.)(30 min at 37°C) or 200 μCi of 51Cr (Amersham Pharmacia Biotech, Quebec, Canada) (18 h at 37°C), washed twice with PBS, and resuspended in SMEM containing 10% FBS. BAL cell populations, unfractionated or purified for NK (DX5+) cells, were used as effector cells. Effector BAL cell populations, pooled from 5 to 10 mice, were harvested, and NK cytotoxicity tests were performed using a two-color fluorescence assay (L-7010; Molecular Probes) per the manufacturer's directions or by lysis of 51Cr-labeled target cells. Briefly, effector cells and 104 DIOC18-labeled targets were plated in a 96-well V-bottomed plate (Costar, Cambridge, Mass.) to yield effector-to-target ratios of 40:1, 20:1, 10:1, and 5:1 and incubated for 4 h at 37°C. The percent lysis was calculated as described previously (3). Spontaneous lysis was determined for DIOC18-labeled targets incubated in the absence of effectors. Spontaneous lysis ranged from 4 to 13%. NK cytotoxicity was also determined using a standard 51Cr release assay as described previously (5). Briefly, effector cells were incubated (4 h at 37°C) with 104 51Cr-labeled target cells, in triplicate wells, using 40:1, 20:1, 10:1, and 5:1 E/T ratios. As appropriate, EGTA-MgCl2 (5 and 10 mM, respectively; Sigma) was added to the corresponding wells. To address the effect of IL-12 on cytolysis, BAL cells were harvested from five mice per group, pooled, and cultured for 24 h with or without 2 ng of rmIL-12 (R&D Systems, Minneapolis, Minn.)/ml. The cultured cells were incubated with 51Cr-labeled YAC-1 target cells at 40:1, 20:1, 10:1, and 5:1 effector-to-target ratios for 4 h at 37°C. Spontaneous and maximum 51Cr releases were determined by incubating target cells with either medium or with 2% saponin in the absence of effector cells. The percent specific 51Cr release (percent cytotoxicity) was calculated as [(experimental cpm − spontaneous cpm)/(maximum cpm −spontaneous cpm)] × 100. Spontaneous lysis counts ranged from 317 to 550 cpm. The use of either assay yielded comparable results.
CTL assay.
Spleens from TLR4wt and TLR4null mice were harvested at days 5 and 11 post-RSV infection. Spleen cells were restimulated in vitro for 7 days with RSV-infected spleen cells, which had been infected with RSV for 3 h at 37°C in RPMI containing 10% FBS. Secondary in vitro cytolytic cell activity was measured using a standard 51Cr release assay. SVB6KHA target cells (H-2b) were incubated with RSV (multiplicity of infection, 100) and 51Cr (200 μCi) for 18 h at 37°C, washed two times in RPMI containing 10% FBS, and distributed in V-bottom 96-well plates (Costar) at a concentration of 104 cells/100 μl. Effector cells were added at an effector-to-target ratio of 50:1 and serially diluted to 2:1 in triplicate. Plates were centrifuged at 1,200 rpm for 2 min and then incubated at 37°C for 5 h. After incubation, 100 μl of the supernatant was removed, and radioactivity was measured in a gamma counter (Perkin-Elmer Life Sciences, Boston, Mass.). Spontaneous and maximal release was measured by incubating the target cells in media alone or in 10% Triton X-100 detergent, respectively. Specific release of 51Cr from target cells was calculated as follows: [(experimental cpm − spontaneous cpm)/(maximum cpm − spontaneous cpm)] × 100.
Virus titers in lung tissue.
Lungs were aseptically removed from three to five mice per group at days 3, 5, 7, and 11 post-RSV or influenza virus infection and stored at −70°C until the assay. Identical weights (∼0.1 g of tissue) of individual lung samples were homogenized in 1 ml of Dulbecco's PBS (GIBCO), and 10-fold serial dilutions of the lung homogenates were subsequently added to confluent Vero cell monolayers to detect RSV titers or confluent Madin-Darby Canine Kidney (MDCK) cell monolayers to detect influenza virus titers. Following adsorption for 1 to 2 h at 37°C, monolayers were overlaid with either Dulbecco's modified Eagle medium (GIBCO) containing 10% FBS for Vero cells or Dulbecco's modified Eagle medium containing 0.1% BSA (Sigma), 0.1 μg of trypsin (Sigma)/ml, 0.8% agar (BioWhittaker, Rockland, Maine) for MDCK cells. The monolayers were incubated at 37°C for 3 to 4 days, and RSV plaques were enumerated after immunostaining with monoclonal antibodies against the G and F glycoproteins (130-2G and 131-2A, respectively) as described previously (30).
Influenza virus plaques were enumerated after fixing MDCK monolayers with 80% methanol and staining them with 2% crystal violet in 10% ethanol.
Statistical analysis.
Statistical significance was determined using a Student t test, and a probability of < 0.05 was considered statistically significant.
RESULTS
Decreased NK and CD14+ pulmonary cell infiltration in RSV-infected TLR4null mice.
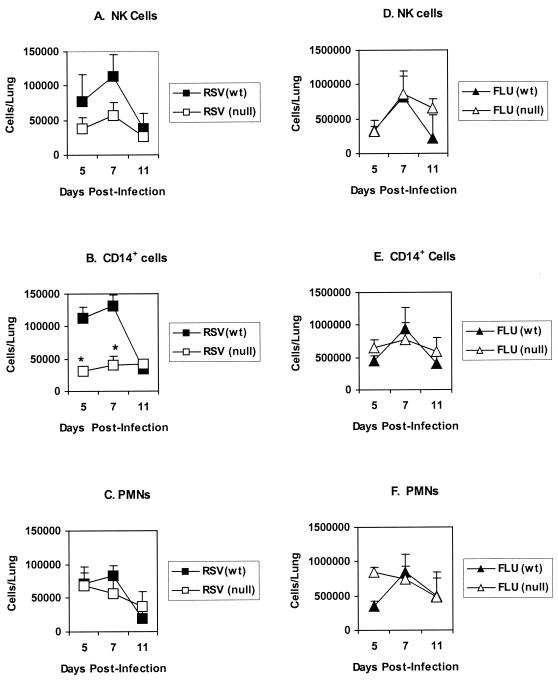
To investigate the importance of TLR4 in the immune response to respiratory viral infections, we first examined the phenotype of BAL cells infiltrating the lungs of TLR4-deficient (TLR4null) mice and TLR4-expressing (TLR4wt) control mice following i.n. challenge with RSV or influenza virus. Flow cytometry was used to identify NK cells (2B4+), monocytes and macrophages (CD14+), and PMN (RB6-8C5+) present in the BAL cell population at days 5, 7, and 11 postinfection (p.i.). Representative data from three separate experiments is presented in Fig. 1. For RSV-infected TLR4wt mice, the pulmonary influx of NK cells (Fig. 1A) and CD14+ cells (Fig. 1B) peaked on day 7 p.i. and subsequently declined by day 11 p.i., as was observed following influenza virus infection of TLR4wt mice (Fig. 1D and E). In contrast, RSV-infected TLR4null mice displayed a pattern of NK and CD14+ cell infiltration very different from that for TLR4wt mice (Fig. 1A and B). RSV-infected TLR4null mice exhibited decreased infiltration of pulmonary NK cells and CD14+ cells (P < 0.05) between days 5 and 7 p.i. with RSV (Fig. 1A and 1B); however, no significant differences in the total numbers of pulmonary BAL cells were detected between TLR4wt and TLR4null mice (1 × 106 to 6 × 105 cells/lung versus 0.85 × 106 to 5 × 105 cells/lung) throughout the period (days 5 to 11 p.i.) examined. In contrast to the case with RSV infection, the numbers of NK and CD14+ cells in the BAL of influenza virus-infected TLR4wt and TLR4null mice were comparable (Fig. 1D and E). The altered BAL infiltration by NK and CD14+ cells observed in RSV-infected TLR4null mice was not associated with altered numbers of PMN cells or T cells (CD3+) present in the BAL after infection with RSV or influenza virus (Fig. 1C and F), and no consistent differences in the percentages of these cell types were detected between TLR4wt and TLR4null mice. Of note, there was a small increase (3 to 5%) in B cells (B220+ CD45R+) in TLR4null mice compared to wild-type controls; however, this response may be inherent in TLR4null mice, since the small increase in B cells was detected after either RSV or influenza virus infection.
FIG. 1.
Decreased pulmonary infiltration of NK cells and CD14+ cells in RSV-infected TLR4null mice. Flow-cytometric analysis of BAL cell subsets from TLR4-deficient (TLR4null) and TLR4-expressing (TLR4wt) mice infected i.n. with the A2 strain of RSV virus (RSV) or the HKx31 influenza A virus (FLU). BAL samples were stained with antibodies against NK cells (A and D), CD14+ monocytes/macrophages (B and E), and PMNs (C and F). Data are presented as the mean number of cells/lung ± standard error of the mean at days 5, 7, and 11 p.i. from three independent experiments. Asterisks indicate a significant difference (P < 0.05) between TLR4null and TLR4wt mice.
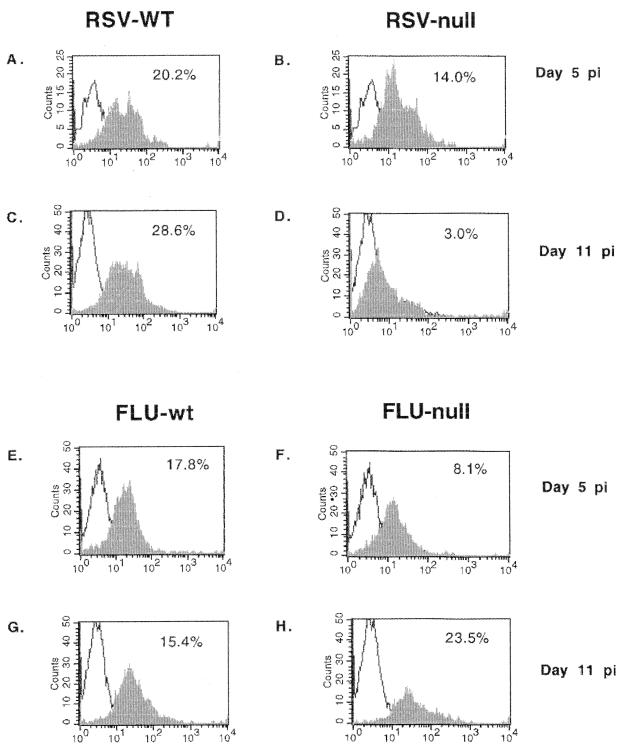
To address another aspect of TLR4 cell activation that may affect cell trafficking, we examined Th1 (IL-2, IFN-γ, and IL-12) and Th2 (IL-4 and IL-5) intracellular cytokine expression by BAL cells in TLR4null and TLR4wt mice infected with RSV or influenza virus (Table 1). Overall, the absence of TLR4 (TLR4null) was associated with reduced IL-12 expression by BAL cells from RSV-infected mice. For RSV-infected TLR4null mice, IL-12 expression by BAL cells was significantly decreased from that observed for TLR4wt mice on day 5 p.i. (7% versus 29%; P < 0.05) and day 7 p.i. (21% versus 30%; P < 0.05), and by day 11 p.i., IL-12 expression remained decreased but was not statistically different between TLR4null and TLR4wt mice. It is possible that decreased pulmonary CD14+ cell infiltration observed after RSV infection, and/or inadequate activation of these cells, may relate to the lowered IL-12 expression observed in TLR4null mice. Although TLR4null mice infected with influenza virus initially exhibited reduced IL-12 expression at day 5 p.i. (6% versus 28%, P < 0.05) compared to TLR4wt mice, there was no association of decreased IL-12 expression with decreased CD14+ infiltration (Fig. 1) or NK cytotoxicity (data not shown). By day 7 p.i., the level of IL-12 expression for influenza virus-infected TLR4null mice was comparable to that for TLR4wt mice (39% versus 30%) (Table 1) and remained comparable to that for TLR4wt mice throughout the time course (Table 1 and Fig. 2). Interestingly, the percentage of cells expressing Th1 and Th2 cytokines was higher for influenza virus-infected mice than for RSV-infected mice; however, this difference may reflect the increased sensitivity of mice to the mouse-adapted influenza virus infection (31) compared to human RSV infection. Of note, at day 5 p.i., levels of IFN-γ and IL-2 cytokine expression were increased in TLR4null mice relative to TLR4wt mice infected with influenza virus. The absence of TLR4 did not significantly alter Th2 cytokine expression by CD3+ cells following RSV or influenza virus infection (Table 1), and neither RSV nor influenza virus infection elicited a predominately Th1- or Th2-type cytokine response (Tables 1 and 2). Of the CD3+ BAL cells examined, CD4+ cells predominantly expressed both Th1 (IFN-γ and IL-2) and Th2 (IL-4 and IL-5) cytokines (Table 2).
FIG. 2.
Decreased expression of IL-12 by BAL cells after RSV infection. BAL samples from RSV- or influenza virus-infected TLR4wt and TLR4null mice were harvested and examined for intracellular IL-12 expression (x axis). IL-12 expression at day 5 post-RSV infection for TLR4wt and TLR4null samples is shown in panels A and B, respectively. IL-12 expression at day 5 post-influenza virus infection for TLR4wt and TLR4null samples is shown in panels E and F, respectively. IL-12 expression at day 11 post-RSV infection for TLR4wt and TLR4null is shown in panels C and D, respectively. IL-12 expression at day 11 post-influenza virus infection for TLR4wt and TLR4null samples is shown in panels G and H, respectively.
TABLE 2.
Intracellular cytokine expression by CD4+ and CD8+ BAL cells after primary infection with RSV or influenza virus
| Day | Phenotype/cytokine | IC cytokine expression by CD4+ and CD8+ BAL cells ± SEMa
|
|||
|---|---|---|---|---|---|
| RSV (wt) | RSV (null) | FLU (wt) | FLU (null) | ||
| 5 | CD4+/IL-2 | 18,000 ± 4,700 | 15,000 ± 5,000 | 65,000 ± 8,600 | 25,000 ± 5,000 |
| CD4+/IL-4 | 13,000 ± 3,000 | 20,000 ± 6,000 | 60,000 ± 7,800 | 50,000 ± 9,000 | |
| CD4+/IL-5 | 15,000 ± 4,000 | 19,000 ± 6,000 | 14,0000 ± 19,000 | 99,000 ± 2,000 | |
| CD4+/IFN-γ | 18,000 ± 5,000 | 25,000 ± 8,000 | 39,000 ± 8,000 | 30,000 ± 6,000 | |
| CD8+/IL-2 | 3,000 ± 900 | 8,000 ± 2,500 | 5,900 ± 800 | 8,000 ± 1,500 | |
| CD8+/IL-4 | 5,000 ± 1,300 | 6,000 ± 1,800 | 5,900 ± 800 | 8,000 ± 1,500 | |
| CD8+/IL-5 | 7,000 ± 1,800 | 8,000 ± 2,500 | 24,000 ± 3,000 | 58,000 ± 11,000 | |
| CD8+/IFN-γ | 8,000 ± 2,200 | 15,000 ± 5,000 | 4,600 ± 1,000 | 11,000 ± 2,000 | |
| 7 | CD4+/IL-2 | 3,000 ± 580 | 2,200 ± 840 | 22,800 ± 7,400 | 30,000 ± 3,900 |
| CD4+/IL-4 | 2,700 ± 500 | 1,800 ± 690 | 21,300 ± 7,000 | 28,000 ± 3,580 | |
| CD4+/IL-5 | 1,800 ± 350 | 2,600 ± 990 | 30,400 ± 10,000 | 38,000 ± 4,880 | |
| CD4+/IFN-γ | 3,300 ± 630 | 2,200 ± 840 | 35,000 ± 11,000 | 46,000 ± 5,850 | |
| CD8+/IL-2 | 900 ± 180 | 800 ± 300 | 9,000 ± 3,000 | 15,000 ± 1,950 | |
| CD8+/IL-4 | 900 ± 180 | 800 ± 300 | 7,600 ± 2,000 | 10,000 ± 1,300 | |
| CD8+/IL-5 | 600 ± 120 | 1,200 ± 460 | 11,000 ± 3,000 | 18,000 ± 2,280 | |
| CD8+/IFN-γ | 1,500 ± 290 | 1,000 ± 380 | 20,000 ± 6,400 | 28,000 ± 3,580 | |
| 11 | CD4+/IL-2 | 14,000 ± 4,400 | 7,400 ± 1,600 | 36,000 ± 12,000 | 30,500 ± 4,200 |
| CD4+/IL-4 | 10,000 ± 3,100 | 6,200 ± 1,300 | 30,000 ± 10,000 | 28,000 ± 3,900 | |
| CD4+/IL-5 | 10,000 ± 3,100 | 5,100 ± 1,100 | 33,000 ± 11,000 | 20,000 ± 3,000 | |
| CD4+/IFN-γ | 11,000 ± 3,500 | 6,200 ± 1,300 | 39,000 ± 13,000 | 33,000 ± 4,600 | |
| CD8+/IL-2 | 3,600 ± 1,000 | 4,000 ± 800 | 15,000 ± 5,000 | 11,100 ± 3,000 | |
| CD8+/IL-4 | 2,600 ± 1,000 | 2,300 ± 500 | 12,000 ± 4,000 | 11,100 ± 3,000 | |
| CD8+/IL-5 | 2,400 ± 800 | 2,300 ± 500 | 9,000 ± 3,000 | 11,100 ± 1,500 | |
| CD8+/IFN-γ | 5,400 ± 1,700 | 4,000 ± 800 | 15,000 ± 5,000 | 25,000 ± 3,400 | |
C57BL/10ScNCr (null) and C57BL/10ScSn (wt) control mice were i.n. infected with the A2 strain of RSV or the HKx31 influenza A (FLU) virus. BAL samples from three mice per group were taken at days 5, 7, and 11 p.i. Data represent total CD4+ and CD8+ BAL cell IL-2, -4, or -5 or IFN-γ expression ± SEM (as described in Materials and Methods). IC, intracellular.
Decreased NK cell cytotoxicity in TLR4null mice after RSV infection.
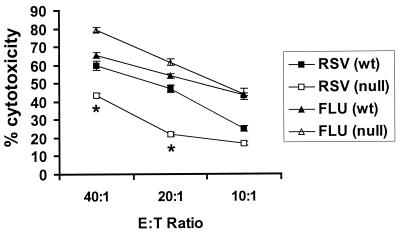
The decreased pulmonary NK cell trafficking in RSV-infected TLR4null mice observed on days 5 and 7 p.i. (Fig. 1A) suggested that differences in NK cytotoxicity may occur between TLR4wt and TLR4null mice; thus, we examined NK cell cytolysis at day 7 post-RSV or influenza virus infection of TLR4wt and TLR4null mice. Examination revealed that NK cells from RSV-infected TLR4null mice are deficient in their ability to lyse YAC-1 target cells compared to cells from RSV-infected TLR4wt mice (Fig. 3). Following RSV infection, the level of NK cytotoxicity in TLR4null mice was significantly diminished (43% at 40:1) from that in TLR4wt mice (60% at 40:1; P < 0.05). In contrast, levels of NK cytotoxicity were similar in TLR4null and TLR4wt mice infected with influenza virus (Fig. 3). These results suggest that the innate immune response to influenza virus infection may be less dependent of TLR4 activation, but it is possible that other TLR activation pathways may be involved in this response.
FIG. 3.
Impaired NK cell activation in TLR4null mice after RSV infection. BAL samples from TLR4null and TLR4wt mice were harvested 7 days post-RSV or -influenza virus (FLU) infection. NK lytic activity against YAC-1 target cells was determined. Results are expressed as the mean of three independent experiments ± the standard error of the mean. Asterisks indicate a significant difference (P < 0.05) between TLR4null and TLR4wt mice. E:T Ratio, effector-to-target ratio.
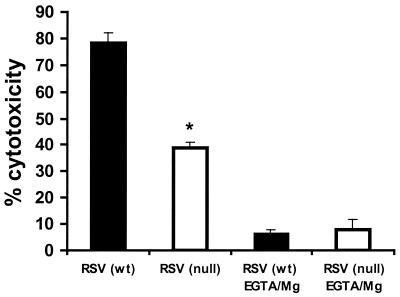
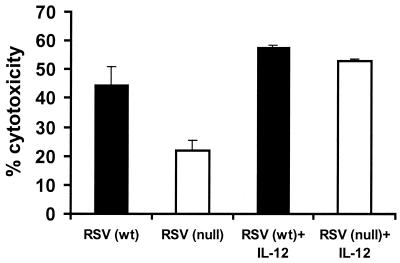
The impaired NK cytotoxicity observed for TLR4null mice infected with RSV might be explained by differences in NK cell numbers (Fig. 1A) and/or by a functional defect in NK cytotoxicity. To examine this possibility, NK cytotoxicity assays were performed using equal numbers of purified NK cells from the BAL of TLR4wt and TLR4null mice (Fig. 4). At day 7 p.i., RSV-infected TLR4null mice had significantly impaired NK cell function compared to TLR4wt mice (Fig. 4). To address one mechanism for this defect, differences in calcium-dependent perforin-mediated cytolysis were examined for NK cells from TLR4null mice using the calcium chelator EGTA-Mg2+ (Fig. 4). More than 79% of NK cytotoxicity was inhibited in the presence of EGTA-Mg2+, suggesting that target cell lysis was mediated primarily through a perforin-dependent mechanism. IL-12 has been shown to enhance NK activity, and RSV-infected TLR4null mice have altered IL-12 expression (Table 1 and Fig. 2). It has also been reported that a substrain of TLR4null mice (C57BL/10ScCr) that was originally derived from the C57BL/10ScNCr strain used in these studies, has a defect in IL-12 responsiveness (16, 33, 34). Therefore, we examined the effect of exogenous addition of 2 ng of IL-12/ml on NK cell cytotoxicity in TLR4null and TLR4wt responses (Fig. 5). NK cytotoxicity in RSV-infected TLR4null mice was restored to wild-type levels by the addition of IL-12 (Fig. 5). Thus, the C57BL/10ScNCr mice used in these studies are IL-12 responsive.
FIG. 4.
RSV-induced NK cytotoxicity is perforin dependent. Seven days p.i., BAL samples from TLR4null (open bar) and TLR4wt (closed bar) mice were examined following RSV infection. Samples were purified for NK cells (DX5+) (80 to 90% enrichment) by positive selection using the MACS separation system. NK lytic activity against YAC-1 target cells was assessed in the presence or absence of EGTA-MgCl2+ at effector-to-target ratios of 40:1 (presented), 20:1, 10:1, and 5:1 (data not shown). The asterisk indicates a significant difference (P < 0.05) between TLR4null and TLR4wt mice. The results are representative of two independent experiments.
FIG. 5.
Addition of IL-12 enhances NK-mediated cytotoxicity in RSV-infected TLR4null mice. BAL samples from TLR4null and TLR4wt mice were examined for cytotoxicity 7 days post-RSV infection. Effector BAL cells were cultured in the presence of 2 ng of IL-12/ml for 24 h. NK lytic activities against YAC-1 target cells at effector-to-target ratios of 40:1 (presented), 20:1, 10:1, and 5:1 (data not shown) were analyzed. A representative experiment is shown.
Delayed RSV clearance in TLR4null mice.
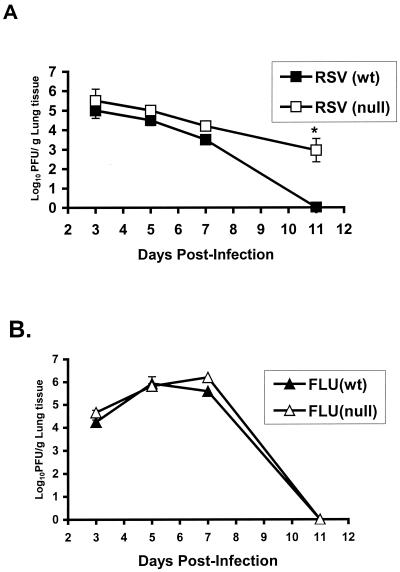
Since TLR4 appeared to be associated with IL-12 expression, as well as NK cell trafficking and cytotoxicity in RSV (but not influenza virus)-infected animals, we next examined whether the absence of TLR4 altered viral clearance (Fig. 6). Examination revealed that the diminished innate immune response (i.e., decreased IL-12 expression and decreased NK cytotoxicity) in TLR4null mice was associated with a compromised ability to clear acute RSV infection compared to that of RSV-infected TLR4wt mice. The lung titers of RSV in TLR4null mice were higher at all time points examined and were significantly higher at day 11 p.i. (P < 0.05) (Fig. 6A). In three of four experiments, mean titers of RSV virus in TLR4null mice had increased 100-to-1,000-fold over titers of virus in TLR4wt mice at day 11 p.i. (Fig. 6A). Notably, the delayed RSV viral clearance in TLR4null mice compared to that in TLR4wt mice correlated with their impaired NK activity (Fig. 3 and 4). In contrast, similar levels of virus clearance were observed for influenza virus-infected TLR4null and TLR4wt mice (Fig. 6B).
FIG. 6.
Delayed RSV clearance in TLR4null mice. The lungs of TLR4null and TLR4wt mice were harvested at days 3, 5, 7, and 11 p.i. with RSV (A) or influenza virus (FLU) (B). The asterisk indicates a significant difference (P < 0.01) between TLR4null and TLR4wt mice. The results are expressed as mean log10 PFU/g ± the standard error of the mean from four separate experiments. In each experiment, three to five mice per group at each time point were analyzed.
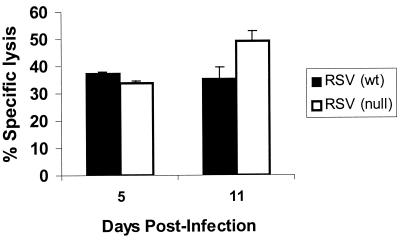
CD8+ CTL responses for RSV-infected TLR4null mice were comparable to those for TLR4wt mice at day 5 p.i., suggesting that early elevated RSV titers observed in TLR4null mice were not associated with impaired CD8+ CTL responses in these mice. Interestingly, the CTL activity in TLR4null mice at day 11 p.i. was modestly increased over the cytolysis observed for TLR4wt mice (Fig. 7). This slight increase in CTL activity may be associated with the clearance of RSV by TLR4null mice, which was observed at day 15 p.i. (data not shown).
FIG. 7.
Kinetics of CTL activity in RSV-infected TLR4null mice. TLR4wt and TLR4null mice were sacrificed on days 5 and 11 post-RSV infection. Data are values for percent specific lysis at an effector-to-target ratio of 50:1 and are representative of two independent experiments.
DISCUSSION
In this study, we examined the in vivo role of TLR4 in the innate immune response to two major respiratory viral pathogens, RSV and influenza virus. We demonstrated that TLR4 is important in the innate immune response to RSV infection but does not appear to be as important following influenza virus infection. Acute RSV infection in TLR4wt mice was similar to the pulmonary cell infiltration and pattern and magnitude of cytokine expression that we and others have previously observed in BALB/c mouse studies (8, 10, 17, 31). In contrast, associated with the absence of TLR4 is diminished IL-12 expression and diminished numbers of pulmonary NK cells and CD14+ cells in RSV-infected mice. In addition, the limited numbers of NK cells that traffic to the lungs in TLR4null mice in response to RSV infection have significantly impaired cytotoxicity, primarily through a defect in perforin-mediated lysis which is reflected by the decreased ability to clear RSV infection in these mice. It is possible that inadequate activation of CD14+ cells may contribute to the low IL-12 expression observed at day 5 p.i. for RSV- and influenza virus-infected TLR4null mice and to the delayed virus clearance observed in RSV-infected TLR4null mice (11). Although RSV and influenza virus infection induce similar host cell cytopathologies, these viruses likely induce different innate immune responses, since influenza virus-infected TLR4null mice did not display altered immune cell trafficking or impaired NK cytotoxicity compared to the case with TLR4wt mice, as was observed following RSV infection of these mice.
The apparent lack of similar deficiencies in the innate immune response observed for influenza virus infection of TLR4null mice, compared to RSV infection of TLR4null mice, suggests that the defects observed are not generalized but are specific to the response to RSV infection, suggesting that RSV stimulates the NK cell and CD14+ cell response through the TLR4 pathway. The major surface glycoproteins of RSV (G and F) are likely candidates for inducing TLR4 activation. Recent in vitro studies from our laboratory have demonstrated that the F glycoprotein can stimulate TLR4 activation in a CD14-dependent fashion (11). In contrast, the RSV G glycoprotein appears to suppress rather than promote NK and PMN activation, as indicated by decreased cell trafficking to the lungs of RSV-infected mice and decreased virus clearance (30). Members of our group have also demonstrated that the G glycoprotein alters MIP-1α, MIP-1β, MIP-2, MCP-1, and IP-10 mRNA expression during RSV infection (29). MIP-1α, MIP-1β, MIP-2, MCP-1, and IP-10 are chemokines that contribute to recruitment and trafficking of innate immune cells into the lung (29).
One possible explanation for the diminished NK cytotoxicity observed in RSV-infected TLR4null mice may be associated with a failure to activate the MyD88/IRAK/NF-κB signaling cascade (1, 15). TLR4, the IL-1 receptor, and the IL-18 receptor generate intracellular signaling by a shared molecular protein cascade involving sequential recruitment of MyD88 and IRAK to the receptors, phosphorylation of IRAK, and activation of Traf6. This ultimately leads to translocation of NF-κB to the nucleus and gene transcription. MyD88-deficient mice have impaired IL-18-mediated NK-cell activation (1). Also, IL-18 is a potent inducer of NK cells and upregulator of perforin-mediated NK activity (18, 19). Similar to MyD88 (−/−) mice, IL-18-deficient mice also display defects in NK cytotoxicity (26). Further, antibody blockade of IL-18 has been shown to result in diminished IFN-γ expression and lymphocyte infiltration of the lungs of mice challenged with another respiratory virus, adenovirus (35). In addition, decreased expression of IL-12 in the TLR4null mice infected with RSV, but not influenza virus (Table 1), may also contribute to altered NK cell trafficking and cytotoxicity. IL-12 enhances NK activity and has been shown to act synergistically with IL-18 in the activation of NK cells (26). Our experiments suggest that alteration of NK cell trafficking and NK effector function are more severe in RSV-infected TLR4null mice than in influenza virus-infected TLR4null mice. We hypothesize that TLR4null mice may fail to generate an effective NK cell response to RSV because of defects in generating both direct (MyD88/IRAK) and cytokine-mediated (IL-12) signals for NK cell activation. In addition, one study suggests that a related strain of TLR4null mice may also have a defect in IL-12 responsiveness (16). However, our experiments suggest that the C57BL/10ScNCr mice used in the present study are IL-12 responsive. In future studies, we will address the contributions of background genes, such as the IL-12 receptor, on TLR4 function in viral pathogenesis using congenic TLR4 knockout mouse strains.
The study presented here provides strong evidence for the involvement of TLR4 activation in the in vivo innate response to nonbacterial microbial pathogens: in particular, RSV. Based upon our in vitro studies that show that the RSV F glycoprotein can activate TLR4 (11), we hypothesize that the F glycoprotein is also important for TLR4 activation during the immune response to RSV infection and that TLR4 is an important contributor to the RSV innate immune response. Understanding the mechanisms that contribute to RSV innate immunity may allow new approaches for prevention and/or treatment of RSV-associated disease.
ACKNOWLEDGMENTS
This research was supported in part by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention, National Center for Infectious Diseases, Division of Viral and Rickettsial Diseases, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the Centers for Disease Control and Prevention.
We thank Jacqueline M. Katz, Jean L. Hu-Primmer, Thomas Rowe, Mary Renshaw, and Al Barskey (Centers for Disease Control and Prevention, Atlanta, Ga.) and Janice Riberdy (St. Jude's Children Research Hospital, Memphis, Tenn.) for their technical assistance.
REFERENCES
- 1.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 2.Aliprantis A O, Yang R B, Mark R B, Suggett S, Devaux B, Radolf J D, Klimpel G R, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 3.Chang L, Gusewitch G A, Chritton D B W, Folz J C, Lebeck L K, Nehlsen-Cannarella S L. Rapid flow-cytometric assay for the assessment of natural killer cell activity. J Immunol Methods. 1993;166:45–54. doi: 10.1016/0022-1759(93)90327-4. [DOI] [PubMed] [Google Scholar]
- 4.Connors M, Collins P L, Firestone C Y, Murphy B R. Respiratory syncytial virus (RSV) F, G, M2 (22K), and N proteins each induce resistance to RSV challenge, but resistance induced by M2 and N proteins is relatively short-lived. J Virol. 1991;65:1634–1637. doi: 10.1128/jvi.65.3.1634-1637.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duke R C. Methods of analyzing chromatin changes accompanying apoptosis of target cells in killer cell assays. In: Campbell K, Colonna M, editors. Natural killer cell protocols: cellular and molecular methods. Totowa, N.J: Humana Press; 2000. pp. 125–145. [DOI] [PubMed] [Google Scholar]
- 6.Hancock G E, Speelman D J, Heers K, Bortell E, Smith J, Cosco C. Generation of atypical pulmonary inflammatory responses in BALB/c mice after immunization with the native attachment (G) glycoproteins of respiratory syncytial virus. J Virol. 1996;70:7783–7791. doi: 10.1128/jvi.70.11.7783-7791.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirschfeld M, Kirschning C J, Schwandner R, Wesche H, Weis J H, Wooten J R M, Weis J J. Inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J Immunol. 1999;163:2382–2386. [PubMed] [Google Scholar]
- 8.Hussell T, Georgiou A, Sparer T E, Matthews S, Pala P, Openshaw P J M. Host genetic determinants of vaccine-induced eosinophilia during respiratory syncytial virus infection. J Immunol. 1998;161:6215–6222. [PubMed] [Google Scholar]
- 9.Johnson T R, Johnson J E, Roberts S R, Wertz G W, Parker R A, Graham B S. Priming with secreted glycoproteins G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J Virol. 1998;72:2871–2880. doi: 10.1128/jvi.72.4.2871-2880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimpen J L, Ogra P L. Mucosal T cells recovered from mice after infection with respiratory syncytial virus display a memory/activation phenotype. Pediatr Allergy Immunol. 1995;6:119–123. doi: 10.1111/j.1399-3038.1995.tb00270.x. [DOI] [PubMed] [Google Scholar]
- 11.Kurt-Jones E A, Popova L, Kwinn L, Haynes L M, Jones L P, Tripp R A, Walsh E E, Freeman M W, Golenbock D T, Anderson L J, Finberg R W. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 12.Means T, Wang K S, Lien E, Yoshimura A, Goldenbock D, Fenton M. Human Toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999;163:3920–3927. [PubMed] [Google Scholar]
- 13.Medzhitov R, Janeway C A. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 14.Medzhitov R, Preston-Hurlburt P, Janeway C A. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 15.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway C A. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 16.Merlin T, Sing A, Nielsen P J, Galanos C, Freudenberg M A. Inherited IL-12 unresponsiveness contributes to the high LPS resistance of the LPSd C57BL/10ScCr mouse. J Immunol. 2001;166:566–573. doi: 10.4049/jimmunol.166.1.566. [DOI] [PubMed] [Google Scholar]
- 17.Munoz J L, McCarthy C A, Clark M E, Hall C B. Respiratory syncytial virus infection in C57BL/6 mice: clearance of virus from the lungs with virus-specific cytotoxic T cells. J Virol. 1991;65:4494–4497. doi: 10.1128/jvi.65.8.4494-4497.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okamura H, Tsutsui H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K. Cloning of a new cytokine that induces IFNγ production by T cells. Nature. 1995;378:88–90. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 19.Okamura H, Tsuisi H, Kashiwamura S, Yoshimoto T, Nakanishi K. IL-18: a novel cytokine that augments both innate and acquired immunity. Adv Immunol. 1998;70:281–312. doi: 10.1016/s0065-2776(08)60389-2. [DOI] [PubMed] [Google Scholar]
- 20.Openshaw P J. Immunity and immunopathology to respiratory syncytial virus. The mouse model. Am J Respir Crit Care Med. 1995;152:S59–S62. doi: 10.1164/ajrccm/152.4_Pt_2.S59. [DOI] [PubMed] [Google Scholar]
- 21.Poltorak A, He X, Smirnova I, Liu M Y, Huffel C V, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 22.Qureshi S T, Lariviere L, Leveque G, Clermont S, Moore K J, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J Exp Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rock F L, Hardiman G, Timans J C, Kastelein R A, Bazan J F. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci USA. 1998;95:588–593. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning C J. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 25.Stott E J, Taylor G, Ball L A, Anderson K, Young K K, King A M, Wertz G W. Immune and histopathological responses in animals vaccinated with recombinant vaccinia viruses that express individual genes of human respiratory syncytial virus. J Virol. 1987;61:3855–3861. doi: 10.1128/jvi.61.12.3855-3861.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18 deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 28.Tripp R A, Hou S, McMickle A, Houston J, Doherty P C. Recruitment and proliferation of CD8+ T cells in respiratory virus infections. J Immunol. 1995;154:6013–6021. [PubMed] [Google Scholar]
- 29.Tripp R A, Jones L, Anderson L J. Respiratory syncytial virus G and/or SH glycoproteins modify CC and CXC chemokine mRNA expression in the BALB/c mouse. J Virol. 2000;74:6227–6229. doi: 10.1128/jvi.74.13.6227-6229.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tripp R A, Moore D, Jones L, Sullender W, Winter J, Anderson L J. Respiratory syncytial virus (RSV) G and/or SH proteins alter Th1 cytokines, natural killer cells and neutrophils responding to pulmonary infection in BALB/c mice. J Virol. 1999;73:7099–7107. doi: 10.1128/jvi.73.9.7099-7107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tripp R A, Sarawar S R, Doherty P C. Characteristics of the influenza virus-specific CD8+ T cell response in mice homozygous for disruption of the H-2lAb gene. J Immunol. 1995;155:2955–2959. [PubMed] [Google Scholar]
- 32.Underhill D M, Ozinsky A, Hajjar A, Stevens A, Wilson C B, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 33.Vogel S, Bhat N, Qureshi S, Malo D. Genetic control of endotoxin responsiveness: the Lps gene revisted. In: Brade H, Opal S, Vogel S, Morrison D, editors. Endotoxin in health and disease. New York, N.Y: Marcel Dekker, Inc; 1999. pp. 735–750. [Google Scholar]
- 34.Vogel S, Hansen C, Rosenstreich D. Characterization of a congenitally LPS-resistant, athymic mouse strain. J Immunol. 1979;122:619–622. [PubMed] [Google Scholar]
- 35.Xing Z, Zganiacz A, Wang J, Divangahi M, Nawaz F. IL-12-independent Th1-type immune responses to respiratory viral infection: requirement of IL-18 for IFNγ release in the lung but not for the differentiation of viral-reactive Th1-type lymphocytes. J Immunol. 2000;164:2575–2584. doi: 10.4049/jimmunol.164.5.2575. [DOI] [PubMed] [Google Scholar]