Abstract
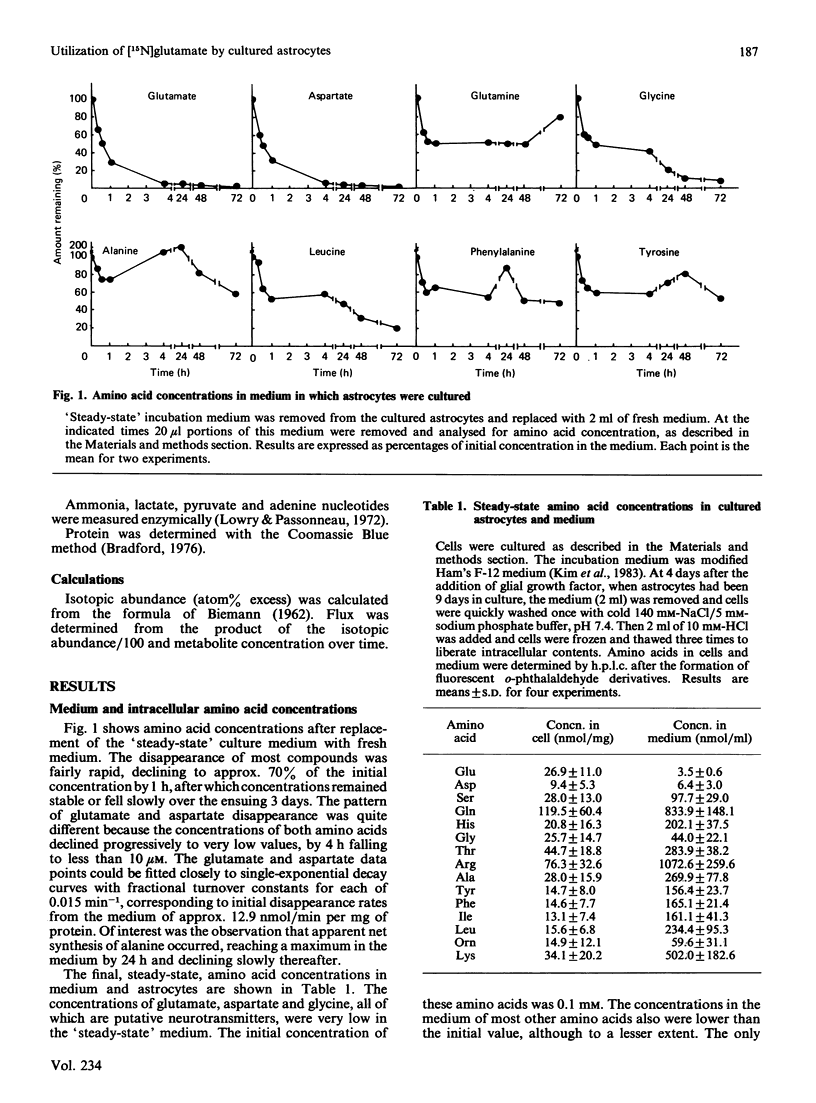
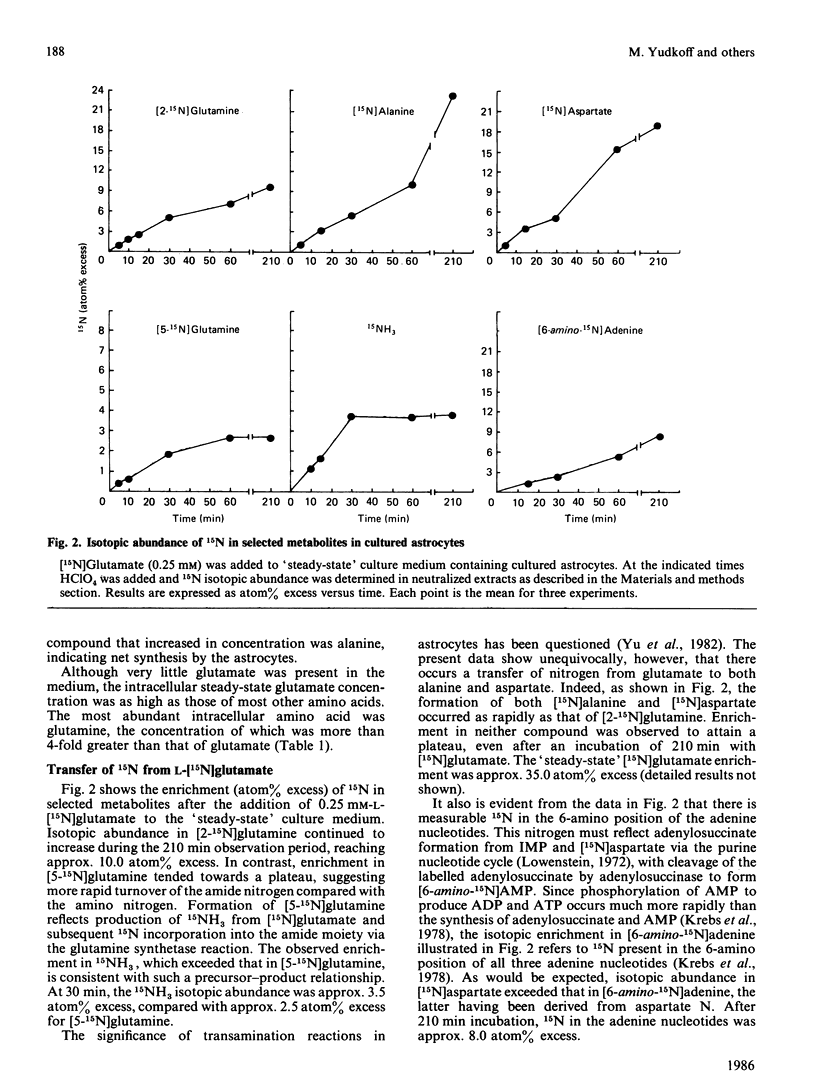
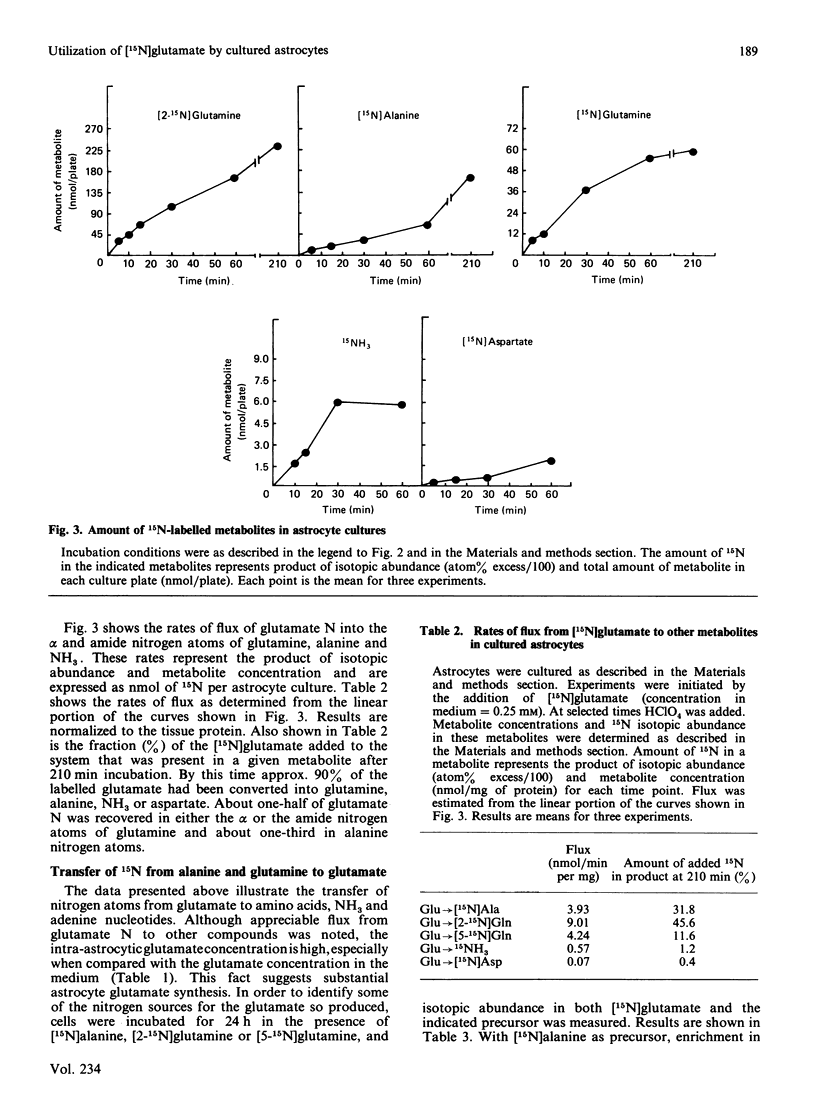
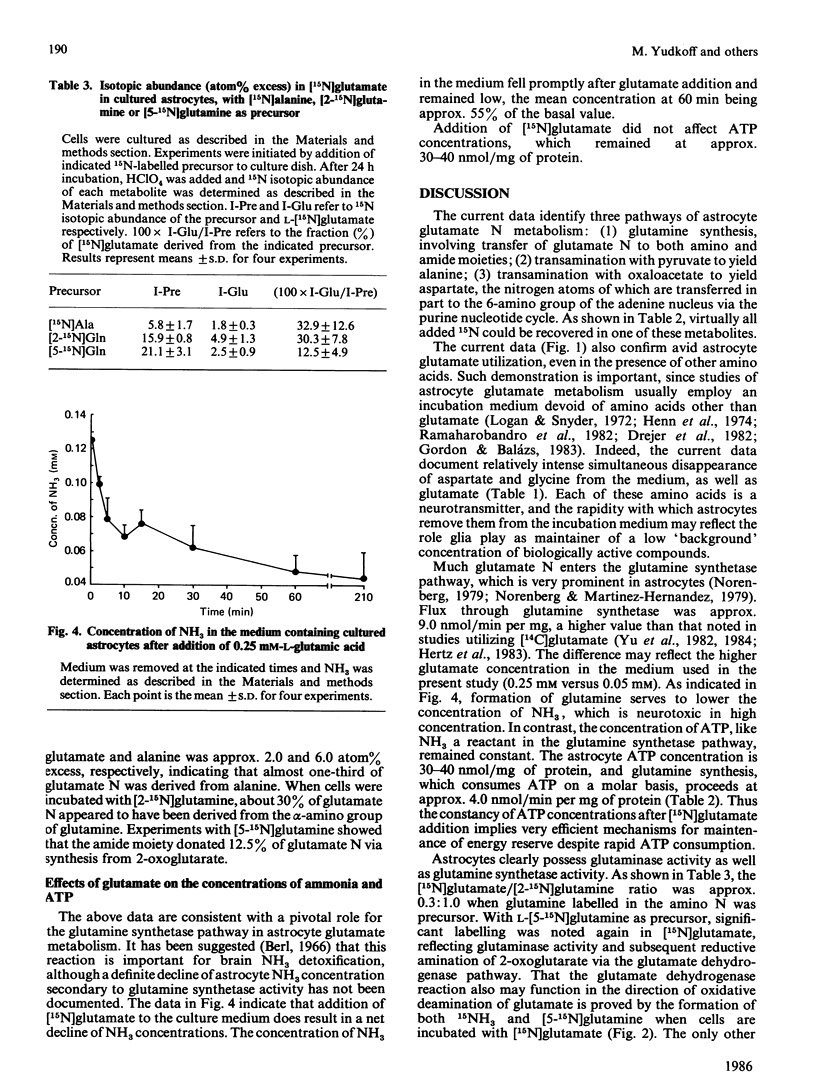
The metabolism of 0.25 mM-[15N]glutamic acid in cultured astrocytes was studied with gas chromatography-mass spectrometry. Almost all 15N was found as [2-15N]glutamine, [2-15N]glutamine, [5-15N]glutamine and [15N]alanine after 210 min of incubation. Some incorporation of 15N into aspartate and the 6-amino position of the adenine nucleotides also was observed, the latter reflecting activity of the purine nucleotide cycle. After the addition of [15N]glutamate the ammonia concentration in the medium declined, but the intracellular ATP concentration was unchanged despite concomitant ATP consumption in the glutamine synthetase reaction. Some potential sources of glutamate nitrogen were identified by incubating the astrocytes for 24 h with [5-15N]glutamine, [2-15N]glutamine or [15N]alanine. Significant labelling of glutamate was noted with addition of glutamine labelled on either the amino or the amide moiety, reflecting both glutaminase activity and reductive amination of 2-oxoglutarate in the glutamate dehydrogenase reaction. Alanine nitrogen also is an important source of glutamate nitrogen in this system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERL S., TAKAGAKI G., CLARKE D. D., WAELSCH H. Metabolic compartments in vivo. Ammonia and glutamic acid metabolism in brain and liver. J Biol Chem. 1962 Aug;237:2562–2569. [PubMed] [Google Scholar]
- Baldessarini R. J., Yorke C. Uptake and release of possible false transmitter amino acids by rat brain tissue. J Neurochem. 1974 Oct;23(4):839–848. doi: 10.1111/j.1471-4159.1974.tb04411.x. [DOI] [PubMed] [Google Scholar]
- Berl S. Glutamine synthetase. Determination of its distribution in brain during development. Biochemistry. 1966 Mar;5(3):916–922. doi: 10.1021/bi00867a016. [DOI] [PubMed] [Google Scholar]
- Bradford H. F., Ward H. K. On glutaminase activity in mammalian synaptosomes. Brain Res. 1976 Jun 25;110(1):115–125. doi: 10.1016/0006-8993(76)90212-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cooper A. J., McDonald J. M., Gelbard A. S., Gledhill R. F., Duffy T. E. The metabolic fate of 13N-labeled ammonia in rat brain. J Biol Chem. 1979 Jun 25;254(12):4982–4992. [PubMed] [Google Scholar]
- Drejer J., Larsson O. M., Schousboe A. Characterization of L-glutamate uptake into and release from astrocytes and neurons cultured from different brain regions. Exp Brain Res. 1982;47(2):259–269. doi: 10.1007/BF00239385. [DOI] [PubMed] [Google Scholar]
- Freed B. R., Gelbard A. S. Distribution of 13N following intravenous injection of [13N]ammonia in the rat. Can J Physiol Pharmacol. 1982 Jan;60(1):60–67. doi: 10.1139/y82-008. [DOI] [PubMed] [Google Scholar]
- Gordon R. D., Balázs R. Characterization of separated cell types from the developing rat cerebellum: transport of glutamate and aspartate by preparations enriched in Purkinje cells, granule neurones, and astrocytes. J Neurochem. 1983 Apr;40(4):1090–1099. doi: 10.1111/j.1471-4159.1983.tb08097.x. [DOI] [PubMed] [Google Scholar]
- Henn F. A., Goldstein M. N., Hamberger A. Uptake of the neurotransmitter candidate glutamate by glia. Nature. 1974 Jun 14;249(458):663–664. doi: 10.1038/249663a0. [DOI] [PubMed] [Google Scholar]
- Jones B. N., Gilligan J. P. o-Phthaldialdehyde precolumn derivatization and reversed-phase high-performance liquid chromatography of polypeptide hydrolysates and physiological fluids. J Chromatogr. 1983 Aug 26;266:471–482. doi: 10.1016/s0021-9673(01)90918-5. [DOI] [PubMed] [Google Scholar]
- Kim S. U., Stern J., Kim M. W., Pleasure D. E. Culture of purified rat astrocytes in serum-free medium supplemented with mitogen. Brain Res. 1983 Sep 5;274(1):79–86. doi: 10.1016/0006-8993(83)90522-x. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Hems R., Lund P., Halliday D., Read W. W. Sources of ammonia for mammalian urea synthesis. Biochem J. 1978 Dec 15;176(3):733–737. doi: 10.1042/bj1760733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S., Yudkoff M. Gas chromatographic-mass spectrometric determination of isotopic enrichment of 6-15NH2 in adenine nucleotides. Anal Biochem. 1985 Mar;145(2):354–361. doi: 10.1016/0003-2697(85)90374-4. [DOI] [PubMed] [Google Scholar]
- Lockwood A. H., McDonald J. M., Reiman R. E., Gelbard A. S., Laughlin J. S., Duffy T. E., Plum F. The dynamics of ammonia metabolism in man. Effects of liver disease and hyperammonemia. J Clin Invest. 1979 Mar;63(3):449–460. doi: 10.1172/JCI109322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan W. J., Snyder S. H. High affinity uptake systems for glycine, glutamic and aspaspartic acids in synaptosomes of rat central nervous tissues. Brain Res. 1972 Jul 20;42(2):413–431. doi: 10.1016/0006-8993(72)90540-9. [DOI] [PubMed] [Google Scholar]
- Nissim I., Yudkoff M., Lapidot A. Simultaneous determination of [2-15N]- and [5-15N]glutamine with gas chromatography-mass spectroscopy: applications to nitrogen metabolic studies. Anal Biochem. 1984 Nov 15;143(1):14–20. doi: 10.1016/0003-2697(84)90550-5. [DOI] [PubMed] [Google Scholar]
- Nissim I., Yudkoff M., Yang W., Terwilliger T., Segal S. Gas chromatography-mass spectrometry determination of [15N]ammonia enrichment in blood and urine. Anal Biochem. 1981 Jun;114(1):125–130. doi: 10.1016/0003-2697(81)90462-0. [DOI] [PubMed] [Google Scholar]
- Norenberg M. D. Distribution of glutamine synthetase in the rat central nervous system. J Histochem Cytochem. 1979 Mar;27(3):756–762. doi: 10.1177/27.3.39099. [DOI] [PubMed] [Google Scholar]
- Norenberg M. D., Martinez-Hernandez A. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res. 1979 Feb 2;161(2):303–310. doi: 10.1016/0006-8993(79)90071-4. [DOI] [PubMed] [Google Scholar]
- Ramaharobandro N., Borg J., Mandel P., Mark J. Glutamine and glutamate transport in cultured neuronal and glial cells. Brain Res. 1982 Jul 22;244(1):113–121. doi: 10.1016/0006-8993(82)90909-x. [DOI] [PubMed] [Google Scholar]
- Ruscák M., Orlický J., Zúbor V., Hager H. Alanine aminotransferase in bovine brain: purification and properties. J Neurochem. 1982 Jul;39(1):210–216. doi: 10.1111/j.1471-4159.1982.tb04720.x. [DOI] [PubMed] [Google Scholar]
- Schultz V., Lowenstein J. M. The purine nucleotide cycle. Studies of ammonia production and interconversions of adenine and hypoxanthine nucleotides and nucleosides by rat brain in situ. J Biol Chem. 1978 Mar 25;253(6):1938–1943. [PubMed] [Google Scholar]
- Shank R. P., Aprison M. H. Present status and significance of the glutamine cycle in neural tissues. Life Sci. 1981 Feb 23;28(8):837–842. doi: 10.1016/0024-3205(81)90044-8. [DOI] [PubMed] [Google Scholar]
- Swain J. L., Hines J. J., Sabina R. L., Harbury O. L., Holmes E. W. Disruption of the purine nucleotide cycle by inhibition of adenylosuccinate lyase produces skeletal muscle dysfunction. J Clin Invest. 1984 Oct;74(4):1422–1427. doi: 10.1172/JCI111553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A. C., Schousboe A., Hertz L. Influence of pathological concentrations of ammonia on metabolic fate of 14C-labeled glutamate in astrocytes in primary cultures. J Neurochem. 1984 Feb;42(2):594–597. doi: 10.1111/j.1471-4159.1984.tb02721.x. [DOI] [PubMed] [Google Scholar]
- Yu A. C., Schousboe A., Hertz L. Metabolic fate of 14C-labeled glutamate in astrocytes in primary cultures. J Neurochem. 1982 Oct;39(4):954–960. doi: 10.1111/j.1471-4159.1982.tb11482.x. [DOI] [PubMed] [Google Scholar]
- Yudkoff M., Nissim I., Kim S. U., Pleasure D., Segal S. Metabolism of 15NH3 in organotypic cerebellar explants and cultured astrocytes: studies with gas chromatography-mass spectrometry. J Neurochem. 1984 Jan;42(1):283–286. doi: 10.1111/j.1471-4159.1984.tb09731.x. [DOI] [PubMed] [Google Scholar]
- Yudkoff M., Nissim I., Kim S., Pleasure D., Hummeler K., Segal S. [15N] leucine as a source of [15N] glutamate in organotypic cerebellar explants. Biochem Biophys Res Commun. 1983 Aug 30;115(1):174–179. doi: 10.1016/0006-291x(83)90985-3. [DOI] [PubMed] [Google Scholar]


